Experimental and Theoretical Study of Sc2O3 Nanoparticles Under High Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nano-Sc2O3
- Materials
- Method
2.2. Experimental Procedure
2.3. Simulations Details
3. Results
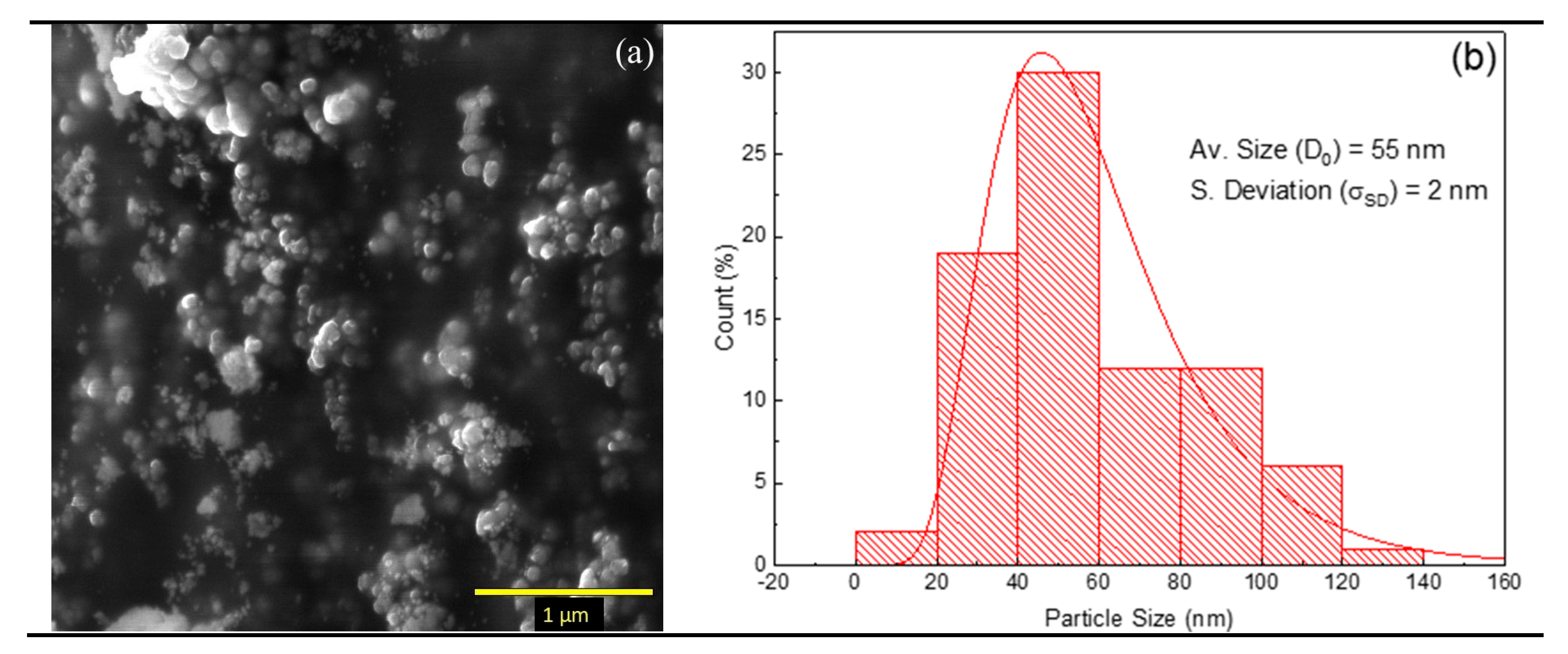
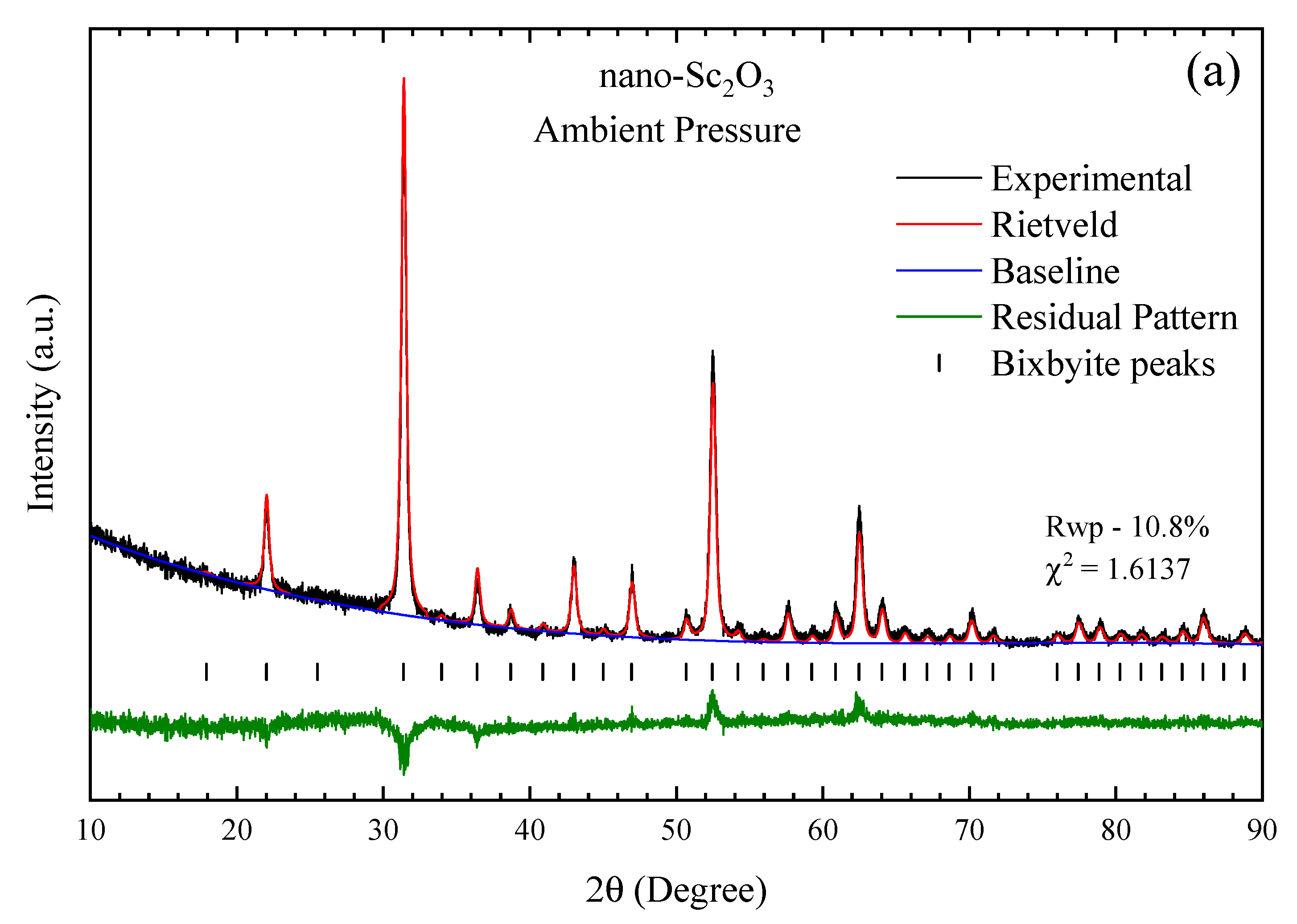
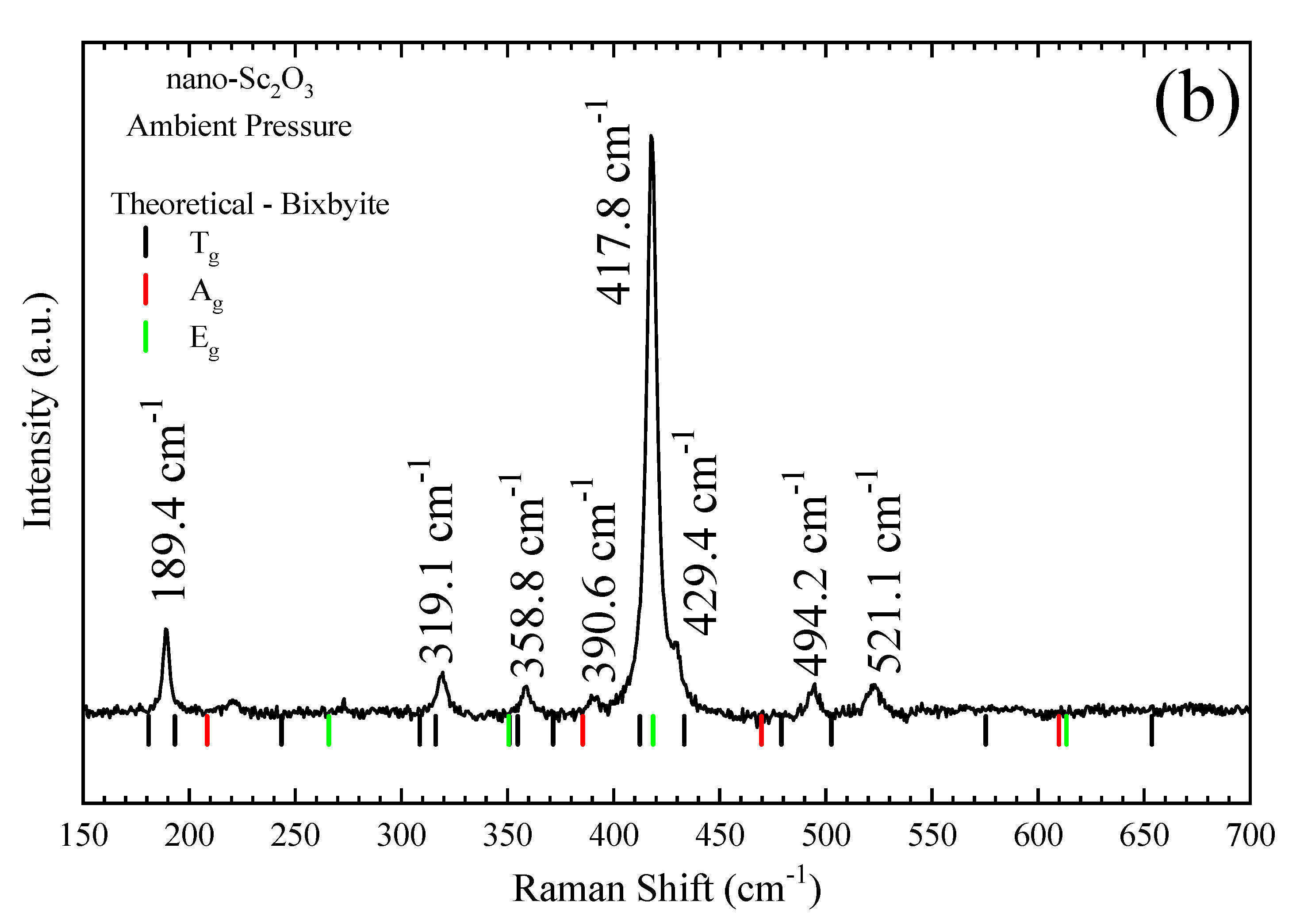
3.1. Ambient Pressure Characterization
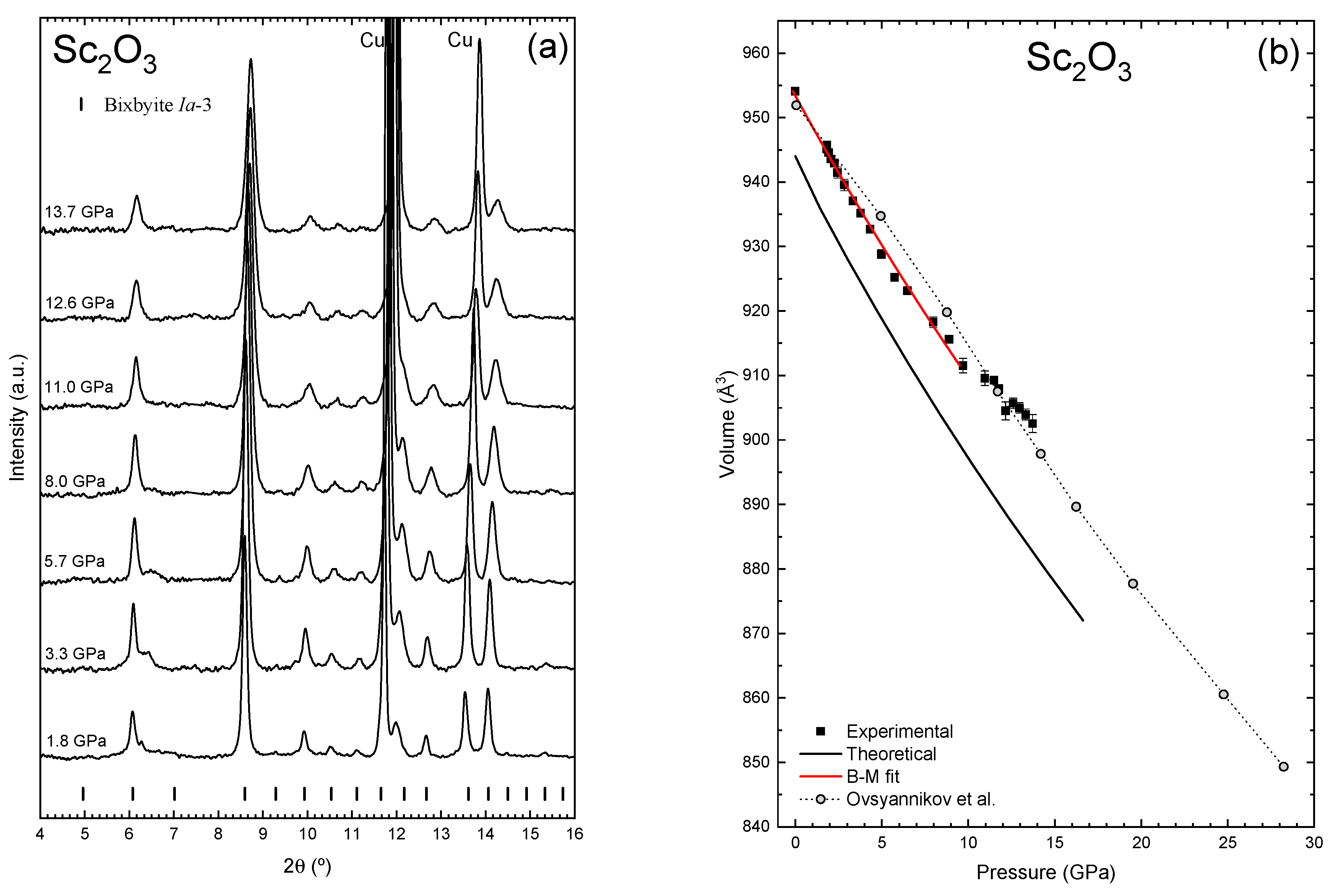
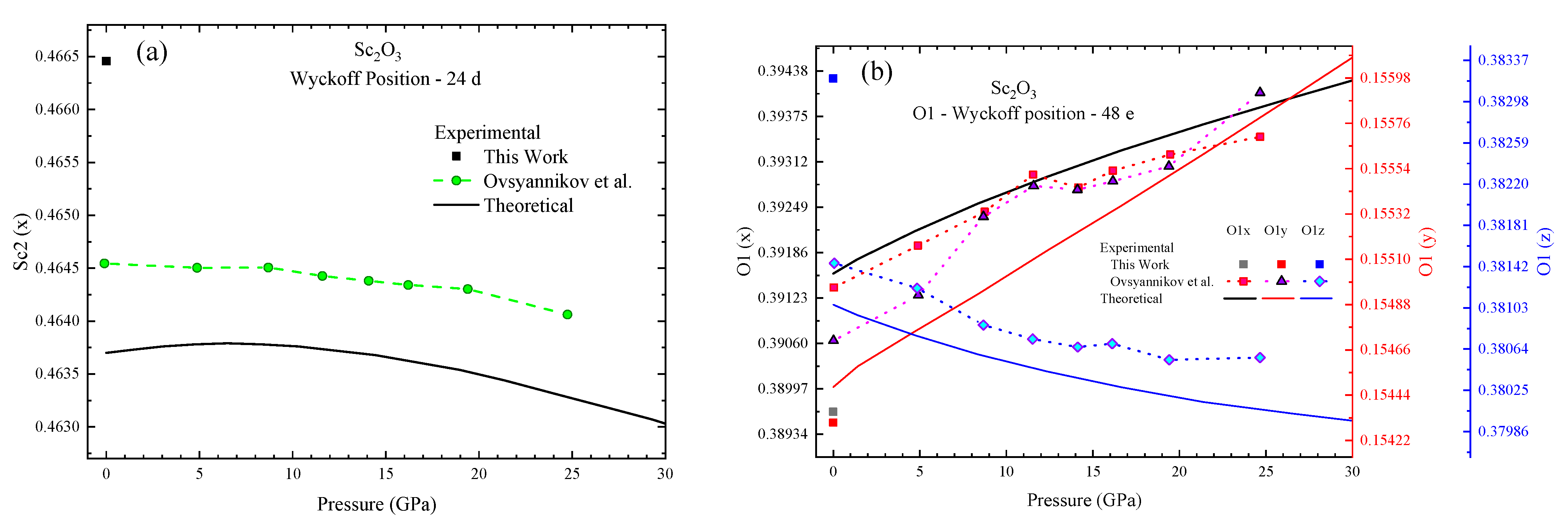
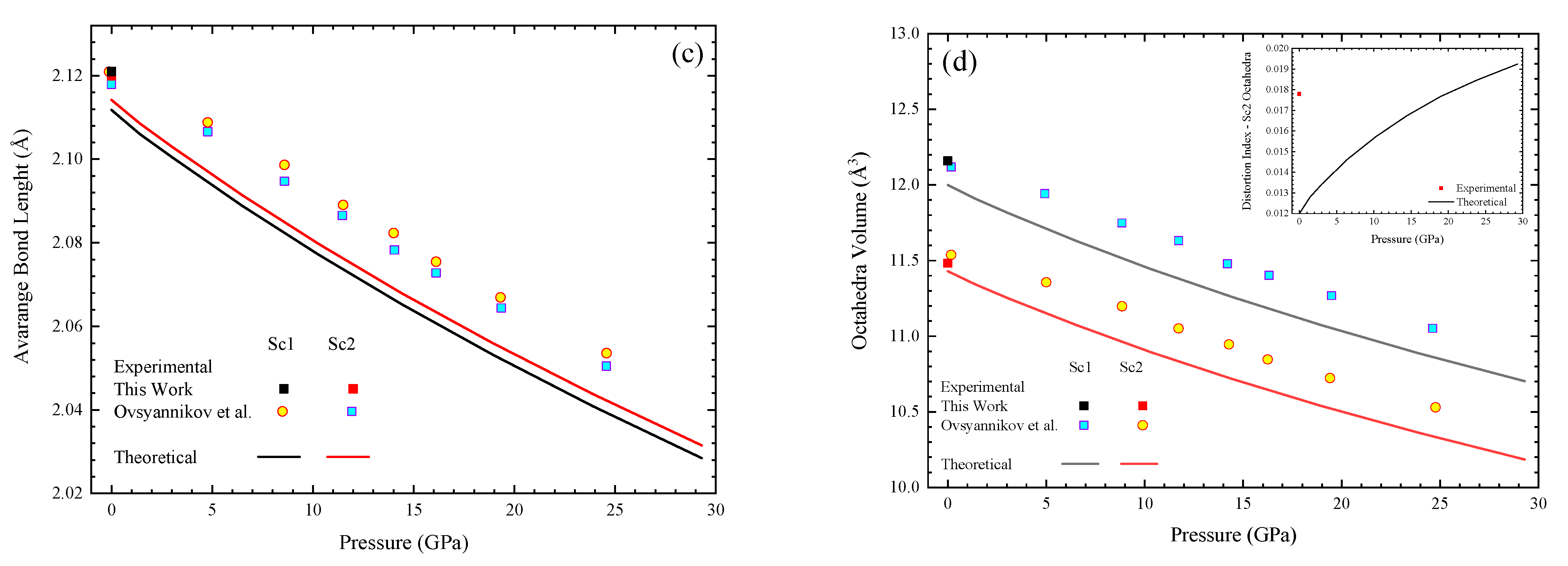
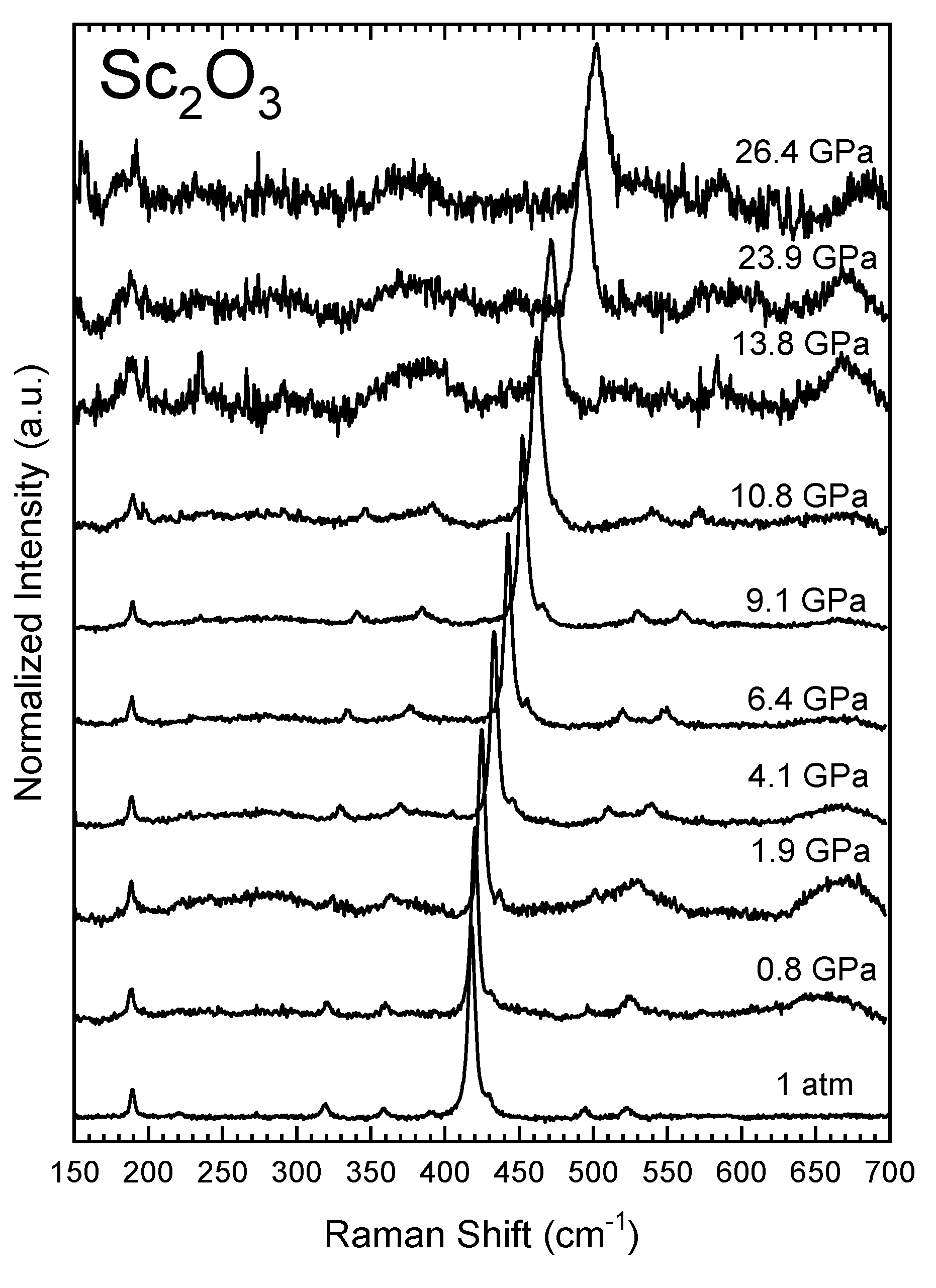
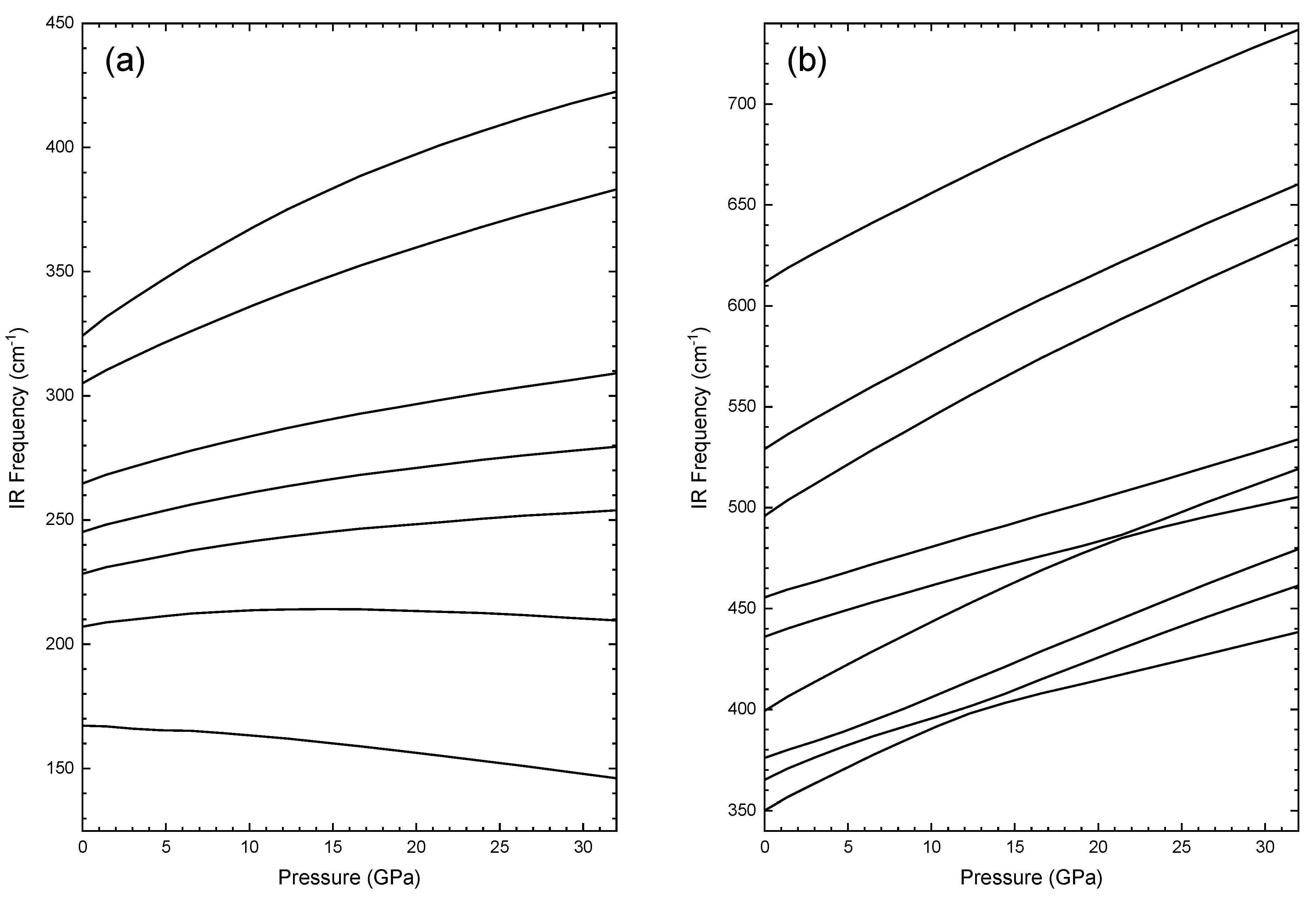
3.2. High-Pressure Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Lei, W.; Li, Y.; Ma, Y.; Hao, J.; Chen, X.; Jin, Y.; Liu, D.; Yu, S.; Cui, Q.; et al. High-pressure structural transitions of Sc2O3 by X-ray diffraction, raman spectra, and Ab initio calculations. Inorg. Chem. 2009, 48, 8251–8256. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikov, S.V.; Bykova, E.; Bykov, M.; Wenz, M.D.; Pakhomova, A.S.; Glazyrin, K.; Liermann, H.-P.; Dubrovinsky, L. Structural and vibrational properties of single crystals of Scandia, Sc2O3 under high pressure. J. Appl. Phys. 2015, 118, 165901. [Google Scholar] [CrossRef]
- Barzilai, S.; Halevy, I.; Yeheskel, O. Bulk modulus of Sc2O3: Ab initio calculations and experimental results. J. Appl. Phys. 2011, 110, 043532. [Google Scholar] [CrossRef]
- Yusa, H.; Tsuchiya, T.; Sata, N.; Ohishi, Y. High-pressure phase transition to the Gd2S3 Structure in Sc2O3: A new trend in dense structures in sesquioxides. Inorg. Chem. 2009, 48, 7537–7543. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Wu, X.; Qin, S. Phase stability and elasticity of Sc2O3 at high pressure. Eur. Phys. J. B 2011, 84, 11–16. [Google Scholar] [CrossRef]
- Wu, B.; Zinkevich, M.; Aldinger, F.; Wen, D.; Chen, L. Ab initio study on structure and phase transition of A- and B-type rare-earth sesquioxides Ln2O3 (Ln=La-Lu, Y, and Sc) based on density function theory. J. Solid State Chem. 2007, 180, 3280–3287. [Google Scholar] [CrossRef]
- Manjón, F.J.; Tresserras, J.A.S.; Ibáñez, J.; Pereira, A.L.J. Pressure-induced phase transitions in sesquioxides. Crystals 2019, 9, 630. [Google Scholar] [CrossRef]
- Bai, F.; Bian, K.; Huang, X.; Wang, Z.; Fan, H. Pressure Induced Nanoparticle Phase Behavior, Property, and Applications. Chem. Rev. 2019, 119, 7673–7717. [Google Scholar] [CrossRef]
- Yadav, D.; Bura, N.; Bhoriya, A.; Singh, J.; Srihari, V.; Poswal, H.K.; Sharma, N.D. Crystallographic structural variations in nano-crystalline Sc2O3 under pressure. Phys. Scr. 2023, 98, 045707. [Google Scholar] [CrossRef]
- Ray, S.; León-Luis, S.F.; Manjón, F.J.; Mollar, M.A.; Gomis, Ó.; Rodríguez-Mendoza, U.R.; Agouram, S.; Muñoz, A.; Lavín, V. Broadband, site selective and time resolved photoluminescence spectroscopic studies of finely size-modulated Y2O3:Eu3+ phosphors synthesized by a complex based precursor solution method. Curr. Appl. Phys. 2014, 14, 72–81. [Google Scholar] [CrossRef]
- de Jesus Pereira, A.L.; Sans, J.Á.; Gomis, Ó.; Santamaría-Pérez, D.; Ray, S.; Godoy, A.; da Silva-Sobrinho, A.S.; Rodríguez-Hernández, P.; Muñoz, A.; Popescu, C.; et al. Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations. Nanomaterials 2024, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Fauth, F.; Peral, I.; Popescu, C.; Knapp, M. The new Material Science Powder Diffraction beamline at ALBA Synchrotron. Powder Diffr. 2013, 28, S360–S370. [Google Scholar] [CrossRef]
- Merrill, L.; Bassett, W.A. Miniature diamond anvil pressure cell for single crystal X-ray diffraction studies. Rev. Sci. Instrum. 1974, 45, 290–294. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 70, 094112. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Errandonea, D.; Muñoz, A.; Gonzalez-Platas, J. Comment on High-pressure x-ray diffraction study of YBO3/Eu3+, GdBO3, and EuBO3: Pressure-induced amorphization in GdBO3. J. Appl. Phys. 2014, 115, 113–116. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-Principles Determination of the Soft Mode in Cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Permin, D.A.; Gavrishchuk, E.M.; Klyusik, O.N.; Egorov, S.V.; Sorokin, A.A. Self-propagating high-temperature synthesis of Sc2O3 nanopowders using different precursors. Adv. Powder Technol. 2016, 27, 2457–2461. [Google Scholar] [CrossRef]
- Todorov, N.D.; Abrashev, M.V.; Marinova, V.; Kadiyski, M.; Dimowa, L.; Faulques, E. Raman spectroscopy and lattice dynamical calculations of Sc2O3 single crystals. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 87, 2–6. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Todorov, N.D.; Geshev, J. Raman spectra of R2O3 (R—Rare earth) sesquioxides with C-type bixbyite crystal structure: A comparative study. J. Appl. Phys. 2014, 116, 103508. [Google Scholar] [CrossRef]
- Schaack, G.; Koningstein, J.A. Phonon and Electronic Raman Spectra of Cubic Rare-Earth Oxides and Isomorphous Yttrium Oxide. J. Opt. Soc. Am. 1970, 60, 1110–1115. [Google Scholar] [CrossRef]
- Repelin, Y.; Proust, C.; Husson, E.; Beny, J.M. Vibrational Spectroscopy of the C-Form of Yttrium Sesquioxide. J. Solid State Chem. 1995, 118, 163–169. [Google Scholar] [CrossRef]
- Ubaldini, A.; Carnasciali, M.M. Raman characterisation of powder of cubic RE2O3 (RE=Nd, Gd, Dy, Tm, and Lu), Sc2O3 and Y2O3. J. Alloys Compd. 2008, 454, 374–378. [Google Scholar] [CrossRef]
- Ibáñez, J.; Sans, J.Á.; Cuenca-Gotor, V.; Oliva, R.; Gomis, Ó.; Rodríguez-Hernández, P.; Munõz, A.; Rodríguez-Mendoza, U.; Velázquez, M.; Veber, P.; et al. Structural and Lattice-Dynamical Properties of Tb2O3 under Compression: A Comparative Study with Rare Earth and Related Sesquioxides. Inorg. Chem. 2020, 59, 9648–9666. [Google Scholar] [CrossRef]
- Sans, J.A.; Manjón, F.J.; Popescu, C.; Cuenca-Gotor, V.P.; Gomis, O.; Muñoz, A.; Rodríguez-Hernández, P.; Contreras-García, J.; Pellicer-Porres, J.; Pereira, A.L.J.; et al. Ordered helium trapping and bonding in compressed arsenolite: Synthesis of As4O6·2He. Phys. Rev. B 2016, 93, 054102. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Gracia, L.; Santamaría-Pérez, D.; Vilaplana, R.; Manjón, F.J.; Errandonea, D.; Nalin, M.; Beltrán, A. Structural and vibrational study of cubic Sb2O3 under high pressure. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 174108. [Google Scholar] [CrossRef]


| V0 (Å3) | B0 | B0′ | ||
|---|---|---|---|---|
| Experimental (this work) | 953.4(5) | 194(4) | 4 (fixed) | |
| Theoretical (this work) | 943.4(1) | 180.2(6) | 4 (fixed) | |
| Barzilai et al. (bulk) [3] | EDS | 953.1 | 198(12) | 4.8 |
| ADS | 954.6 | 191(11) | 4.8 | |
| DFT-GGA | 954.1 | 174.5(1) | 4.53 | |
| Liu et al. (bulk) [1] | 971.8(1) | 154(5) | 7 (Fixed) | |
| Ovsyannikov et al. (single crystal) [2] | 953.9(3) | 198.2(3) | 4 (Fixed) | |
| Yusa et al. (bulk) [4] | 955.7(2) | 189(7) | 4.0(6) | |
| Yadav et al. [9] (nanoparticles—31 nm) | 947(3) | 217(35) | 12(4) |
| Symmetry | Experimental | Theoretical | Ovsyannikov et al. [2] | |||||
|---|---|---|---|---|---|---|---|---|
| ω0 (cm–1) | dω/dP (cm–1/GPa) | ω0 (cm–1) | dω/dP (cm–1/GPa) | d2ω/dP2 (cm–1/GPa2) | ω0 (cm–1) | dω/dP (cm–1/GPa) | d2ω/dP2 (cm–1/GPa2) | |
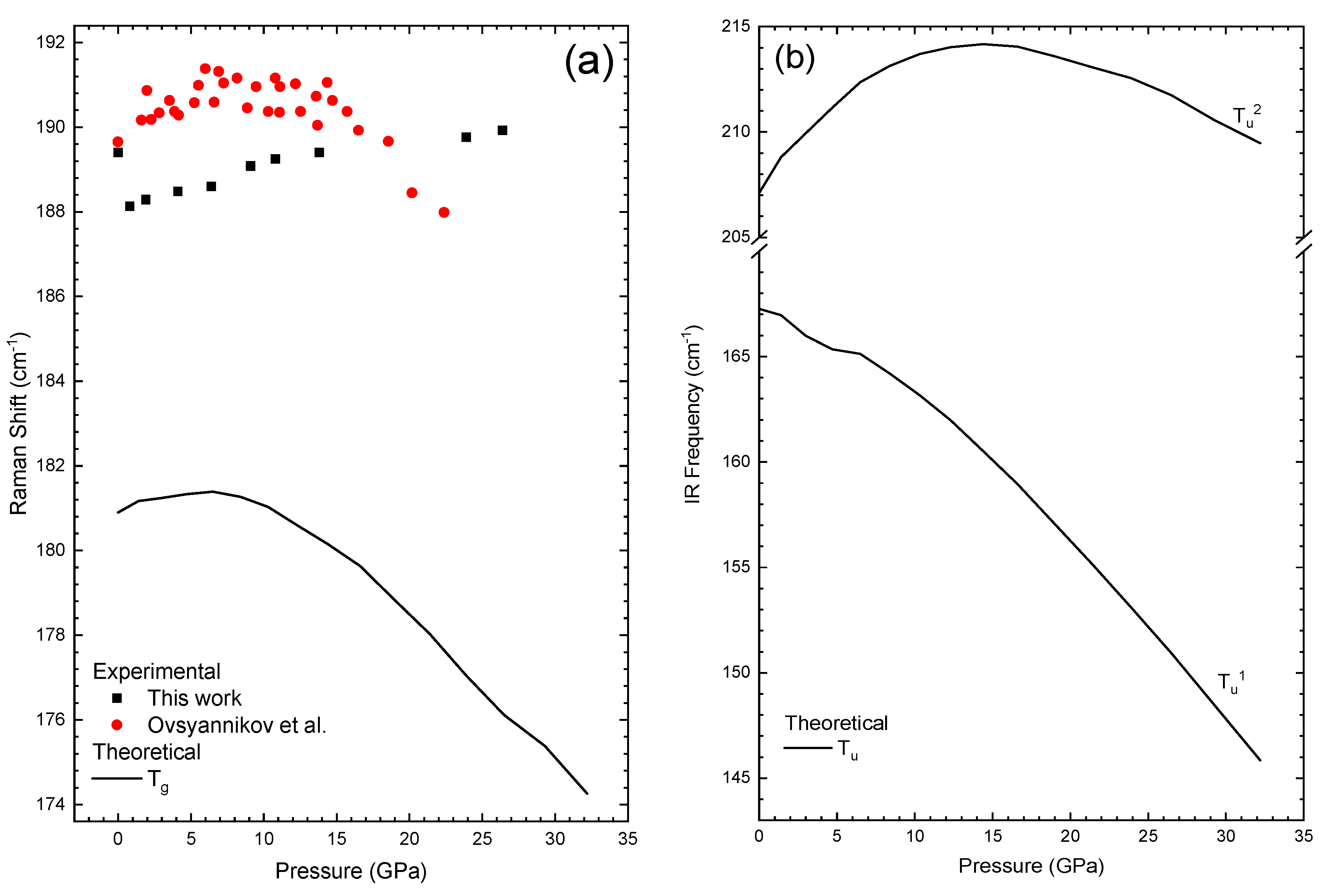
| Tg1 | 189.4 | 0.07(1) | 182.3 | 0.04 | −0.008 | 189.7 | 0.348 | −0.019 |
| Tg2 | 195.0 | 0.3 | ||||||
| Ag1 | 212.1 | 1.1 | 221 | 1.51 | 0.025 | |||
| Tg3 | 247.1 | 1.2 | 253 | 1.85 | −0.019 | |||
| Eg1 | 268.2 | 1.2 | 274 | 1.61 | 0.008 | |||
| Tg4 | 313.8 | 1.7 | ||||||
| Tg5 | 319.1 | 2.3 | 319.6 | 1.8 | 320 | 2.82 | −0.037 | |
| Eg2 | 353.2 | 2.5 | ||||||
| Tg6 | 355.0 | 2.3 | ||||||
| Tg7 | 358.8 | 2.9 | 357.0 | 2.5 | 359 | 3.05 | −0.015 | |
| Tg8 | 375.6 | 3.6 | 380 | 4.30 | −0.060 | |||
| Ag2 | 390.6 | - | 389.0 | 2.2 | 391 | 4.22 | −0.034 | |
| Tg9 | 417.8 | 3.1 | 416.5 | 3.2 | 420 | 3.96 | −0.025 | |
| Eg3 | 429.4 | 4.0 | 423.9 | 3.5 | 431 | 4.37 | −0.038 | |
| Tg10 | 439.9 | 3.0 | 445 | 5.72 | −0.084 | |||
| Ag3 | 474.4 | 3.7 | ||||||
| Tg11 | 494.2 | 4.2 | 481.1 | 3.6 | 496 | 4.59 | −0.042 | |
| Tg12 | 521.1 | 4.4 | 505.0 | 3.9 | 524 | 4.71 | −0.039 | |
| Tg13 | 577.5 | 3.2 | ||||||
| Ag4 | 612.8 | 3.7 | 625 | 4.77 | −0.060 | |||
| Eg4 | 617.1 | 3.7 | ||||||
| Tg14 | 658.5 | 3.9 | 669 | 4.82 | −0.034 | |||
| 709 | ||||||||
| Symmetry | Theoretical | ||
|---|---|---|---|
| ω0 (cm–1) | dω/dP (cm–1/GPa) | d2ω/dP2 (cm–1/GPa2) | |
| Tu1 | 167.26 | −0.67(2) | - |
| Tu2 | 207.10 | 0.75(4) | −0.0225(1) |
| Tu3 | 228.39 | 0.77(4) | - |
| Tu4 | 245.20 | 1.05(4) | - |
| Tu5 | 264.76 | 1.35(5) | - |
| Tu6 | 305.10 | 2.42(6) | - |
| Tu7 | 324.27 | 4.65(4) | −0.051(2) |
| Tu8 | 350.10 | 4.23(14) | −0.049(4) |
| Tu9 | 365.11 | 2.97(2) | - |
| Tu10 | 375.96 | 3.14(7) | - |
| Tu11 | 399.22 | 5.16(8) | −0.056(3) |
| Tu12 | 436.02 | 2.49(4) | - |
| Tu13 | 455.51 | 2.425(7) | - |
| Tu14 | 495.88 | 4.29(6) | - |
| Tu15 | 528.97 | 4.09(6) | - |
| Tu16 | 611.72 | 3.89(5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus Pereira, A.L.; Sans, J.A.; Vilaplana, R.; Ray, S.; Tadge, P.; Godoy, A., Jr.; Horta, I.M.; da Silva-Sobrinho, A.S.; Rodríguez-Hernández, P.; Muñoz, A.; et al. Experimental and Theoretical Study of Sc2O3 Nanoparticles Under High Pressure. Minerals 2025, 15, 21. https://doi.org/10.3390/min15010021
de Jesus Pereira AL, Sans JA, Vilaplana R, Ray S, Tadge P, Godoy A Jr., Horta IM, da Silva-Sobrinho AS, Rodríguez-Hernández P, Muñoz A, et al. Experimental and Theoretical Study of Sc2O3 Nanoparticles Under High Pressure. Minerals. 2025; 15(1):21. https://doi.org/10.3390/min15010021
Chicago/Turabian Stylede Jesus Pereira, André Luis, Juan Angel Sans, Rosario Vilaplana, Sudeshna Ray, Prachi Tadge, Armstrong Godoy, Jr., Isabela M. Horta, Argemiro S. da Silva-Sobrinho, Plácida Rodríguez-Hernández, Alfonso Muñoz, and et al. 2025. "Experimental and Theoretical Study of Sc2O3 Nanoparticles Under High Pressure" Minerals 15, no. 1: 21. https://doi.org/10.3390/min15010021
APA Stylede Jesus Pereira, A. L., Sans, J. A., Vilaplana, R., Ray, S., Tadge, P., Godoy, A., Jr., Horta, I. M., da Silva-Sobrinho, A. S., Rodríguez-Hernández, P., Muñoz, A., Popescu, C., & Manjón, F. J. (2025). Experimental and Theoretical Study of Sc2O3 Nanoparticles Under High Pressure. Minerals, 15(1), 21. https://doi.org/10.3390/min15010021










