Açaí Seed Biochar-Based Phosphate Fertilizers for Improving Soil Fertility and Mitigating Arsenic-Related Impacts from Gold Mining Tailings: Synthesis, Characterization, and Lettuce Growth Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Mining Tailings
2.2. Production and Characterization of Biochars
2.3. Experiment Conduction
2.4. Fertility Analyses
2.5. Inorganic P Fractionation
2.6. Nutrients and Arsenic in Plants
2.7. Arsenic Fractionation and Bioaccessibility
2.8. Evaluation of Risk to Human Health
2.9. Statistical Analysis
3. Results
3.1. Characterization of Tailings
3.2. Biochar Characterization
3.3. Effect of Biochars on Mining Tailings
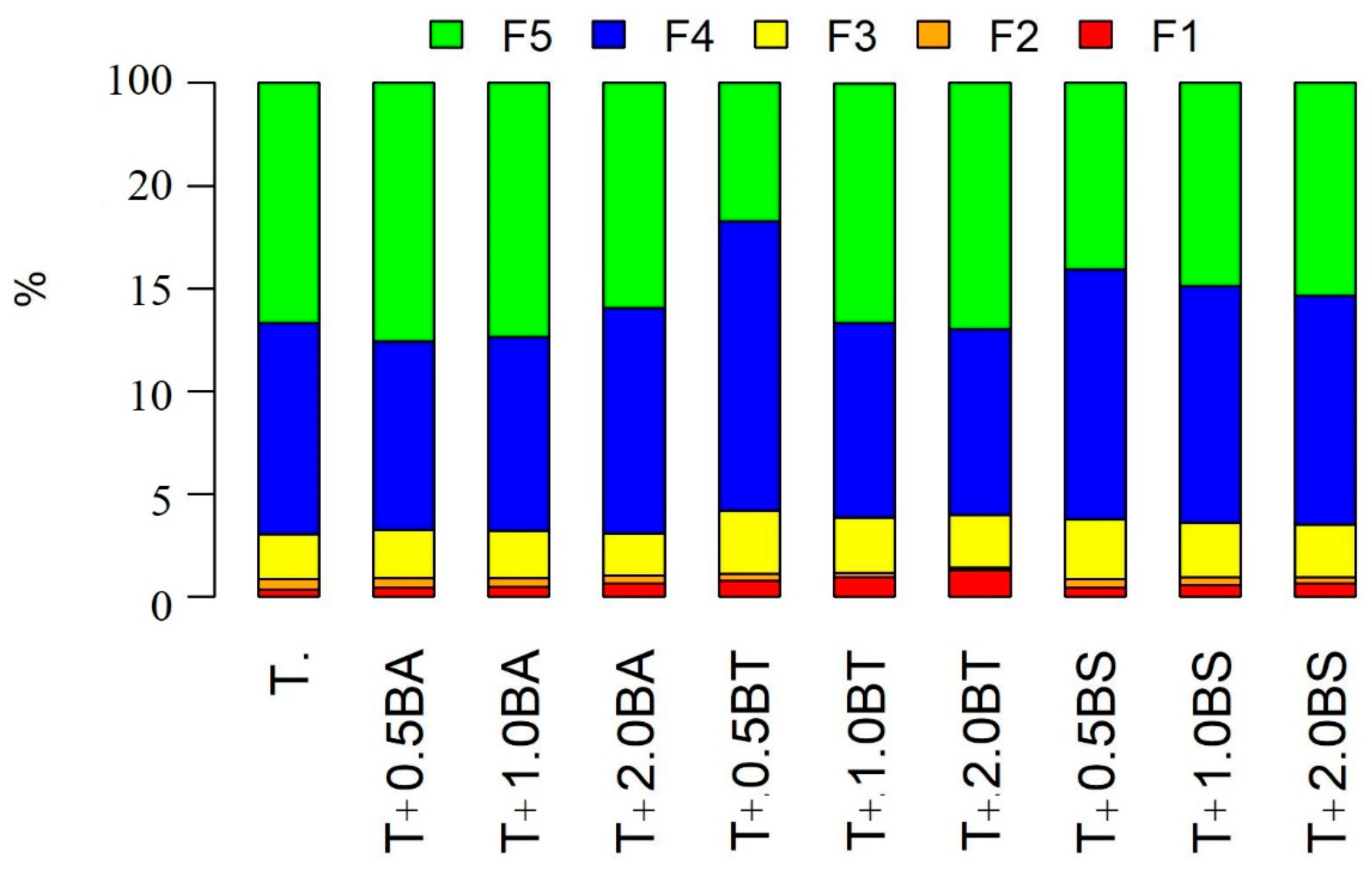
3.4. Inorganic P and As Fractionation
3.5. Arsenic-Plant Interaction
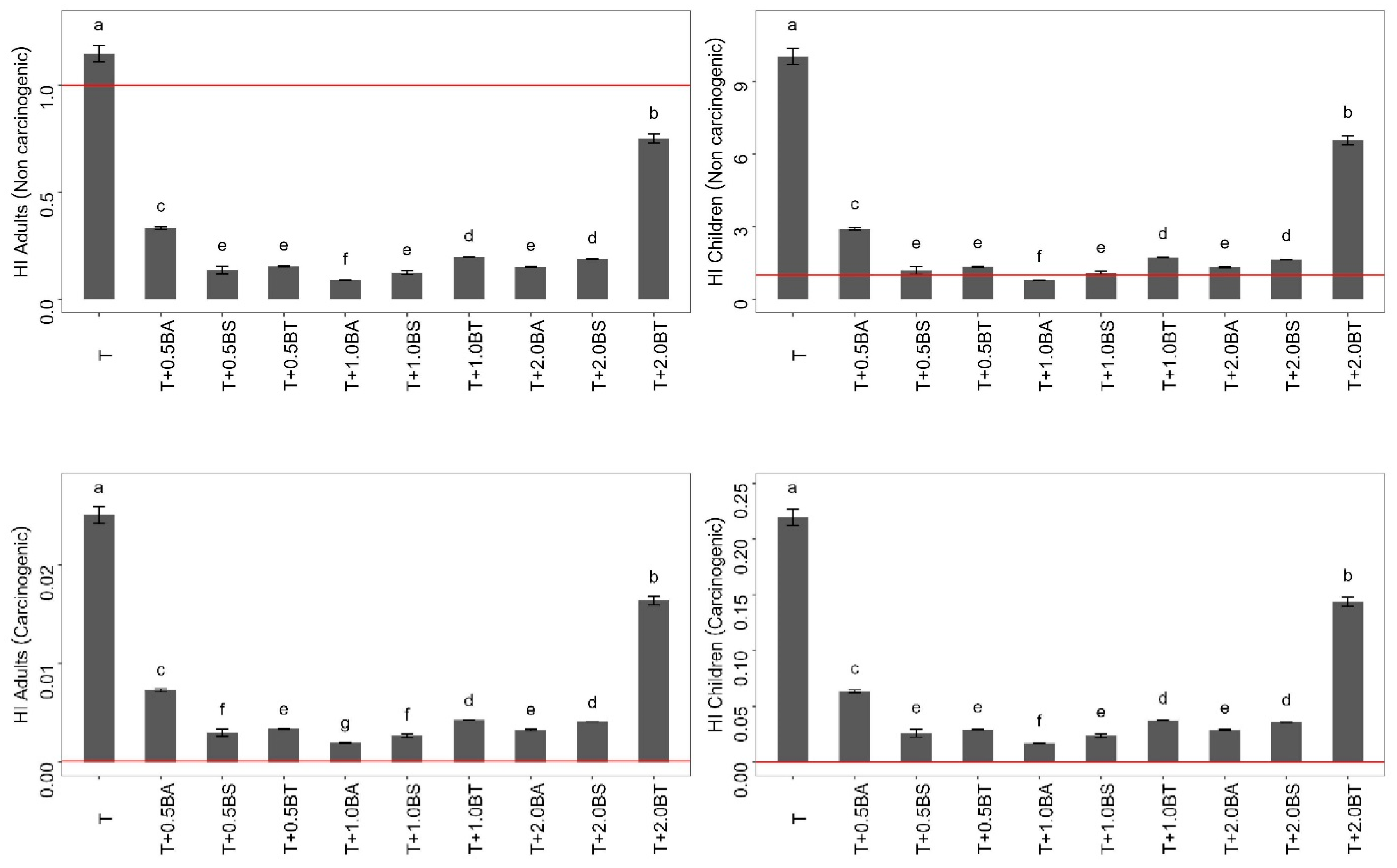
3.6. Human Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silveira Pereira, W.V.; Ramos, S.J.; Melo, L.C.A.; Dias, Y.N.; Martins, G.C.; Ferreira, L.C.G.; Fernandes, A.R. Human and Environmental Exposure to Rare Earth Elements in Gold Mining Areas in the Northeastern Amazon. Chemosphere 2023, 340, 139824. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Tun, A.Z.; Chotpantarat, S.; Siriwong, W. Related Health Risk Assessment of Exposure to Arsenic and Some Heavy Metals in Gold Mines in Banmauk Township, Myanmar. Sci. Rep. 2021, 11, 22843. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Substance Priority List; ATSDR: Atlanta, GA, USA, 2024. [Google Scholar]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sharma, P.; Mitra, S.; Mallick, I.; Ghosh, A. Arsenic Uptake and Bioaccumulation in Plants: A Review on Remediation and Socio-Economic Perspective in Southeast Asia. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100430. [Google Scholar] [CrossRef]
- Kofroňová, M.; Mašková, P.; Lipavská, H. Two Facets of World Arsenic Problem Solution: Crop Poisoning Restriction and Enforcement of Phytoremediation. Planta 2018, 248, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Roychoudhury, A. Recent Trend in Nanoparticle Research in Regulating Arsenic Bioaccumulation and Mitigating Arsenic Toxicity in Plant Species. J. Plant Biochem. Biotechnol. 2021, 30, 793–812. [Google Scholar] [CrossRef]
- De Souza Neto, H.F.; da Silveira Pereira, W.V.; Dias, Y.N.; de Souza, E.S.; Teixeira, R.A.; de Lima, M.W.; Ramos, S.J.; do Amarante, C.B.; Fernandes, A.R. Environmental and Human Health Risks of Arsenic in Gold Mining Areas in the Eastern Amazon. Environ. Pollut. 2020, 265, 114969. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.S.; Texeira, R.A.; da Costa, H.S.C.; Oliveira, F.J.; Melo, L.C.A.; do Carmo Freitas Faial, K.; Fernandes, A.R. Assessment of Risk to Human Health from Simultaneous Exposure to Multiple Contaminants in an Artisanal Gold Mine in Serra Pelada, Pará, Brazil. Sci. Total Environ. 2017, 576, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.J.; de Oliveira, S.S.H.; Ribeiro, P.G.; Martins, G.C.; da Silva, E.C., Jr.; Gastauer, M.; Caldeira, C.F.; Pereira, W.V.D.S.; Santos, D.C.; Sarmento, P.S.D.M.; et al. Combining Approaches for Environmental Assessment of Rehabilitated Gold-mining Areas in the Eastern Amazon. Land Degrad. Dev. 2024, 35, 2313–2325. [Google Scholar] [CrossRef]
- Masud, M.M.; Baquy, M.A.-A.; Akhter, S.; Sen, R.; Barman, A.; Khatun, M.R. Liming Effects of Poultry Litter Derived Biochar on Soil Acidity Amelioration and Maize Growth. Ecotoxicol. Environ. Saf. 2020, 202, 110865. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, K.R.; Alharby, H.F.; Bamagoos, A.A.M.; Pirzadah, T.B. Biochar Promotes Arsenic (As) Immobilization in Contaminated Soils and Alleviates the As-Toxicity in Soybean (Glycine max (L.) Merr.). Chemosphere 2022, 292, 133407. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, L.; Song, D.; Zhang, S.; Wu, H.; Li, S.; Wang, X. High Adsorption Capacity of Mg–Al-Modified Biochar for Phosphate and Its Potential for Phosphate Interception in Soil. Chemosphere 2020, 259, 127469. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Yang, Y.; Xu, Z.; Qiu, H.; Yang, F.; Peijnenburg, W.J.G.M.; Zhang, W.; Qiu, R.; Wang, S. Two Years of Aging Influences the Distribution and Lability of Metal(Loid)s in a Contaminated Soil Amended with Different Biochars. Sci. Total Environ. 2019, 673, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, H.-Z.; Chen, G.-K.; Li, H.-S. Effects of Biochar and Crop Straws on the Bioavailability of Cadmium in Contaminated Soil. Sci. Rep. 2020, 10, 9528. [Google Scholar] [CrossRef]
- Gong, H.; Chi, J.; Ding, Z.; Zhang, F.; Huang, J. Removal of Lead from Two Polluted Soils by Magnetic Wheat Straw Biochars. Ecotoxicol. Environ. Saf. 2020, 205, 111132. [Google Scholar] [CrossRef]
- Tahir, A.H.F.; Al-Obaidy, A.H.M.J.; Mohammed, F.H. Biochar from Date Palm Waste, Production, Characteristics and Use in the Treatment of Pollutants: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 737, 012171. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Conradie, J.; Ohoro, C.R.; Amaku, J.F.; Oyedotun, K.O.; Maxakato, N.W.; Akpomie, K.G.; Okeke, E.S.; Olisah, C.; Malloum, A.; et al. Biochar from Coconut Residues: An Overview of Production, Properties, and Applications. Ind. Crop. Prod. 2023, 204, 117300. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Chatla, A.; Zakaria, Y.; Kochkodan, V.; Shanableh, A.; Laoui, T.; Atieh, M.A. Palm Leaves Based Biochar: Advanced Material Characterization and Heavy Metal Adsorption Study. Biomass Convers. Biorefinery 2022, 14, 14811–14830. [Google Scholar] [CrossRef]
- Díaz, L.C.; Pino, N.; Peñuela, G. Biochar from Oil Palm Waste as an Amendment for the Remediation of Soil Disturbed by Open-Cast Coal Mining. Glob. Adv. Res. J. Eng. Technol. Innov. 2016, 5, 17–22. [Google Scholar]
- Miranda, L.d.V.A.; Mochiutti, S.; da Cunha, A.C.; Cunha, H.F.A. Descarte e Destino Final de Caroços de Açaí Na Amazônia Oriental-Brasil. Ambient. Soc. 2022, 25, e01382. [Google Scholar] [CrossRef]
- Guedes, R.S.; Pinto, D.A.; Ramos, S.J.; Dias, Y.N.; Caldeira, C.F.; Gastauer, M.; e Souza, P.W.M.; Fernandes, A.R. Biochar and Conventional Compost Reduce Hysteresis and Increase Phosphorus Desorbability in Iron Mining Waste. Rev. Bras. Ciência Do Solo 2021, 45, e0200174. [Google Scholar] [CrossRef]
- Ramos, S.J.; Pinto, D.A.; Guedes, R.S.; Dias, Y.N.; Caldeira, C.F.; Gastauer, M.; Souza-Filho, P.W.; Fernandes, A.R. Açaí Biochar and Compost Affect the Phosphorus Sorption, Nutrient Availability, and Growth of Dioclea Apurensis in Iron Mining Soil. Minerals 2021, 11, 674. [Google Scholar] [CrossRef]
- Souza, E.S.; Dias, Y.N.; Costa, H.S.C.; Pinto, D.A.; Oliveira, D.M.; Souza Falção, N.P.; Teixeira, R.A.; Fernandes, A.R. Organic Residues and Biochar to Immobilize Potentially Toxic Elements in Soil from a Gold Mine in the Amazon. Ecotoxicol. Environ. Saf. 2019, 169, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Dias, Y.N.; Souza, E.S.; da Costa, H.S.C.; Melo, L.C.A.; Penido, E.S.; do Amarante, C.B.; Teixeira, O.M.M.; Fernandes, A.R. Biochar Produced from Amazonian Agro-Industrial Wastes: Properties and Adsorbent Potential of Cd2+ and Cu2+. Biochar 2019, 1, 389–400. [Google Scholar] [CrossRef]
- Dias, Y.N.; Pereira, W.V.d.S.; da Costa, M.V.; de Souza, E.S.; Ramos, S.J.; Amarante, C.B.D.; Campos, W.E.O.; Fernandes, A.R. Biochar Mitigates Bioavailability and Environmental Risks of Arsenic in Gold Mining Tailings from the Eastern Amazon. J. Environ. Manag. 2022, 311, 114840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Copyrolysis of Biomass with Phosphate Fertilizers To Improve Biochar Carbon Retention, Slow Nutrient Release, and Stabilize Heavy Metals in Soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Singh, B.; Arbestain, M.C.; Lehmann, J. Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Ito, H.; Kawaguchi, N.; Someya, S.; Munakata, T.; Miyazaki, N.; Ishida, M.; Nakano, A. Experimental Investigation of Electrolytic Solution for Anion Exchange Membrane Water Electrolysis. Int. J. Hydrogen Energy 2018, 43, 17030–17039. [Google Scholar] [CrossRef]
- Abreu, M.M.; Neves, O.; Marcelino, M. Yield and Uranium Concentration in Two Lettuce (Lactuca sativa L.) Varieties Influenced by Soil and Irrigation Water Composition, and Season Growth. J. Geochem. Explor. 2014, 142, 43–48. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Hussain, S.; Sharma, V.; Arya, V.M.; Sharma, K.R.; Rao, C.S. Total Organic and Inorganic Carbon in Soils under Different Land Use/Land Cover Systems in the Foothill Himalayas. CATENA 2019, 182, 104104. [Google Scholar] [CrossRef]
- Qian, T.-T.; Jiang, H. Migration of Phosphorus in Sewage Sludge during Different Thermal Treatment Processes. ACS Sustain. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Drahota, P.; Grösslová, Z.; Kindlová, H. Selectivity Assessment of an Arsenic Sequential Extraction Procedure for Evaluating Mobility in Mine Wastes. Anal. Chim. Acta 2014, 839, 34–43. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; Office of Solid Waste and Emergency Response: Washington, DC, USA, 2001.
- Li, J.; Wei, Y.; Zhao, L.; Zhang, J.; Shangguan, Y.; Li, F.; Hou, H. Bioaccessibility of Antimony and Arsenic in Highly Polluted Soils of the Mine Area and Health Risk Assessment Associated with Oral Ingestion Exposure. Ecotoxicol. Environ. Saf. 2014, 110, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.J.D.; da Silva, E.B.; Fontes, M.P.F.; Liu, X.; Ma, L.Q. Speciation, Bioaccessibility and Potential Risk of Chromium in Amazon Forest Soils. Environ. Pollut. 2018, 239, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Pruvot, C.; Bidar, G.; Fritsch, C.; De Vaufleury, A.; Scheifler, R.; Douay, F. Prediction of Extractable Cd, Pb and Zn in Contaminated Woody Habitat Soils Using a Change Point Detection Method. Pedosphere 2016, 26, 282–298. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 4 July 2024).
- CONAMA. Resolução No 420, de 28 de Dezembro de 2009; CONAMA: Brasilia, Brazil, 2009; Volume 249, 16p. Available online: https://cetesb.sp.gov.br/areas-contaminadas/wp-content/uploads/sites/17/2017/09/resolucao-conama-420-2009-gerenciamento-de-acs.pdf (accessed on 4 July 2024).
- Lawrinenko, M.; Jing, D.; Banik, C.; Laird, D.A. Aluminum and Iron Biomass Pretreatment Impacts on Biochar Anion Exchange Capacity. Carbon N. Y. 2017, 118, 422–430. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Yuan, M.; Cai, R.; Macazo, F.C.; Minteer, S.D. Modified Biochar for Phosphate Adsorption in Environmentally Relevant Conditions. Chem. Eng. J. 2020, 380, 122375. [Google Scholar] [CrossRef]
- Seyedsadr, S.; Šípek, V.; Jačka, L.; Sněhota, M.; Beesley, L.; Pohořelý, M.; Kovář, M.; Trakal, L. Biochar Considerably Increases the Easily Available Water and Nutrient Content in Low-Organic Soils Amended with Compost and Manure. Chemosphere 2022, 293, 133586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liao, W.; Zhou, X.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Coeffect of Pyrolysis Temperature and Potassium Phosphate Impregnation on Characteristics, Stability, and Adsorption Mechanism of Phosphorus-Enriched Biochar. Bioresour. Technol. 2022, 344, 126273. [Google Scholar] [CrossRef] [PubMed]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and Quantification of Biochar Alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Prakash, N.B. Effect of Different Biochars on Acid Soil and Growth Parameters of Rice Plants under Aluminium Toxicity. Sci. Rep. 2020, 10, 12249. [Google Scholar] [CrossRef] [PubMed]
- Piash, M.I.; Iwabuchi, K.; Itoh, T.; Uemura, K. Release of Essential Plant Nutrients from Manure- and Wood-Based Biochars. Geoderma 2021, 397, 115100. [Google Scholar] [CrossRef]
- Wali, F.; Naveed, M.; Bashir, M.A.; Asif, M.; Ahmad, Z.; Alkahtani, J.; Alwahibi, M.S.; Elshikh, M.S. Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance. Sustainability 2020, 12, 9528. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Penido, E.S.; Castro, P.P.; Silva, C.A.; Melo, L.C.A. Co-Pyrolysis of Poultry Litter and Phosphate and Magnesium Generates Alternative Slow-Release Fertilizer Suitable for Tropical Soils. ACS Sustain. Chem. Eng. 2017, 5, 9043–9052. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Ni, N.; Nkoh, J.N.; Dong, Y.; Zhao, W.-R.; Pan, X.-Y.; Li, J.-Y.; Xu, R.-K.; Qian, W. Biochar Retards Al Toxicity to Maize (Zea Mays L.) during Soil Acidification: The Effects and Mechanisms. Sci. Total Environ. 2020, 719, 137448. [Google Scholar] [CrossRef] [PubMed]
- Arwenyo, B.; Varco, J.J.; Dygert, A.; Brown, S.; Pittman, C.U.; Mlsna, T. Contribution of Modified P-Enriched Biochar on PH Buffering Capacity of Acidic Soil. J. Environ. Manag. 2023, 339, 117863. [Google Scholar] [CrossRef] [PubMed]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A Review on Biochar Modulated Soil Condition Improvements and Nutrient Dynamics Concerning Crop Yields: Pathways to Climate Change Mitigation and Global Food Security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Korai, P.K.; Sial, T.A.; Pan, G.; Abdelrahman, H.; Sikdar, A.; Kumbhar, F.; Channa, S.A.; Ali, E.F.; Zhang, J.; Rinklebe, J.; et al. Wheat and Maize-Derived Water-Washed and Unwashed Biochar Improved the Nutrients Phytoavailability and the Grain and Straw Yield of Rice and Wheat: A Field Trial for Sustainable Management of Paddy Soils. J. Environ. Manag. 2021, 297, 113250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-Assisted Biomass Thermal Conversion: Reducing Carbon Loss and Improving Biochar Stability. PLoS ONE 2014, 9, e115373. [Google Scholar] [CrossRef] [PubMed]
- Canteral, K.F.F.; Dias, Y.N.; Fernandes, A.R. Biochars from Agro-Industrial Residues of the Amazon: An Ecological Alternative to Enhance the Use of Phosphorus in Agriculture. Clean Technol. Environ. Policy 2023, 25, 1119–1132. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual Relationships of Biochar and Soil PH, CEC, and Exchangeable Base Cations in a Model Laboratory Experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Masís-Meléndez, F.; Segura-Chavarría, D.; García-González, C.A.; Quesada-Kimsey, J.; Villagra-Mendoza, K. Variability of Physical and Chemical Properties of TLUD Stove Derived Biochars. Appl. Sci. 2020, 10, 507. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ibrahim, M.; Abd El-Rahman, L.; Khader, A. Effects of Biochar and Phosphorus Fertilizers on Phosphorus Fractions, Wheat Yield and Microbial Biomass Carbon in Vertic Torrifluvents. Commun. Soil Sci. Plant Anal. 2019, 50, 362–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Liao, Y.; Reid, B.J.; Chi, H.; Hou, Y.; Cai, C. Modest Amendment of Sewage Sludge Biochar to Reduce the Accumulation of Cadmium into Rice (Oryza sativa L.): A Field Study. Environ. Pollut. 2016, 216, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of Biochar Addition on the Abundance, Speciation, Availability, and Leaching Loss of Soil Phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef] [PubMed]
- Boorboori, M.R.; Gao, Y.; Wang, H.; Fang, C. Usage of Si, P, Se, and Ca Decrease Arsenic Concentration/Toxicity in Rice, a Review. Appl. Sci. 2021, 11, 8090. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, C.-H.; Syu, C.-H.; Jiang, P.-Y.; Huang, C.-C.; Lee, D.-Y. Effects of Phosphorous Application on Arsenic Toxicity to and Uptake by Rice Seedlings in As-Contaminated Paddy Soils. Geoderma 2016, 270, 60–67. [Google Scholar] [CrossRef]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and Biological Immobilization Mechanisms of Potentially Toxic Elements in Biochar-Amended Soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, S.-H.; Jeon, E.-K.; Kim, D.-H.; Tsang, D.C.W.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of Dissolved Organic Carbon from Sludge, Rice Straw and Spent Coffee Ground Biochar on the Mobility of Arsenic in Soil. Sci. Total Environ. 2018, 636, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; dos Santos, F.H.; Alleoni, L.R.F. Temporal Changes in Arsenic and Lead Pools in a Contaminated Sediment Amended with Biochar Pyrolyzed at Different Temperatures. Chemosphere 2022, 287, 132102. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.; González, V.; de Haro, S.; García, I. Are Soil Amendments Able to Restore Arsenic-Contaminated Alkaline Soils? J. Soils Sediments 2015, 15, 117–125. [Google Scholar] [CrossRef]
- Zhong, D.; Zhao, Z.; Jiang, Y.; Yang, X.; Wang, L.; Chen, J.; Guan, C.-Y.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. Contrasting Abiotic As(III) Immobilization by Undissolved and Dissolved Fractions of Biochar in Ca2+-Rich Groundwater under Anoxic Conditions. Water Res. 2020, 183, 116106. [Google Scholar] [CrossRef] [PubMed]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jiménez, E.; Schaller, J.; Knorr, K.-H. Review on the Interactions of Arsenic, Iron (Oxy)(Hydr)Oxides, and Dissolved Organic Matter in Soils, Sediments, and Groundwater in a Ternary System. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Kim, J.-G.; Kim, T.; Alessi, D.S.; Baek, K. Mobility of Arsenic in Soil Amended with Biochar Derived from Biomass with Different Lignin Contents: Relationships between Lignin Content and Dissolved Organic Matter Leaching. Chem. Eng. J. 2020, 393, 124687. [Google Scholar] [CrossRef]
- Cordon, G.; Iriel, A.; Cirelli, A.F.; Lagorio, M.G. Arsenic Effects on Some Photophysical Parameters of Cichorium Intybus under Different Radiation and Water Irrigation Regimes. Chemosphere 2018, 204, 398–404. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Arsenate and Arsenite: The Toxic Effects on Photosynthesis and Growth of Lettuce Plants. Acta Physiol. Plant. 2013, 35, 1201–1209. [Google Scholar] [CrossRef]
- Afloog, H. Environmental Geochemistry and Assessment of Pollution by Vanadium in Top Soil of Kirkuk, Northern Iraq. Iraqi Geol. J. 2020, 53, 74–95. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Fu, J.; Chen, Y.; Liu, Y.; Ma, L.Q. Arsenic-Induced Nutrient Uptake in As-Hyperaccumulator Pteris Vittata and Their Potential Role to Enhance Plant Growth. Chemosphere 2018, 198, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar-Based Metal Oxide Nanocomposites of Magnesium and Manganese Improved Root Development and Productivity of Safflower (Carthamus tinctorius L.) under Salt Stress. Rhizosphere 2021, 19, 100416. [Google Scholar] [CrossRef]
- Costa, H.S.C.; de Souza, E.S.; Dias, Y.N.; Melo, L.C.A.; Fernandes, A.R. Phytoremediator Potential of Ipomea asarifolia in Gold Mine Waste Treated with Iron Impregnated Biochar. Minerals 2022, 12, 150. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, S.-H.; Kim, J.-G. Evaluation of Factors Affecting Arsenic Uptake by Brassica Juncea in Alkali Soil after Biochar Application Using Partial Least Squares Path Modeling (PLS-PM). Chemosphere 2021, 275, 130095. [Google Scholar] [CrossRef] [PubMed]
- Zia, Z.; Bakhat, H.F.; Saqib, Z.A.; Shah, G.M.; Fahad, S.; Ashraf, M.R.; Hammad, H.M.; Naseem, W.; Shahid, M. Effect of Water Management and Silicon on Germination, Growth, Phosphorus and Arsenic Uptake in Rice. Ecotoxicol. Environ. Saf. 2017, 144, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Arco-Lázaro, E.; Pardo, T.; Clemente, R.; Bernal, M.P. Arsenic Adsorption and Plant Availability in an Agricultural Soil Irrigated with As-Rich Water: Effects of Fe-Rich Amendments and Organic and Inorganic Fertilisers. J. Environ. Manag. 2018, 209, 262–272. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Suchismita, D.; Gress, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic Uptake by Lettuce from As-Contaminated Soil Remediated with Pteris Vittata and Organic Amendment. Chemosphere 2017, 176, 249–254. [Google Scholar] [CrossRef] [PubMed]
| Treatments | Identification |
|---|---|
| Tailings | T |
| Tailings + 0.5% biochar without enrichment | T + 0.5BA |
| Tailings + 1% biochar without enrichment | T + 1.0BA |
| Tailings + 2% biochar without enrichment | T + 2.0BA |
| Tailings + 0.5% biochar enriched with TSP | T + 0.5BT |
| Tailings + 1% biochar enriched with TSP | T + 1.0BT |
| Tailings + 2% biochar enriched with TSP | T + 2.0BT |
| Tailings + 0.5% biochar enriched with SSP | T + 0.5BS |
| Tailings + 1% biochar enriched with SSP | T + 1.0BS |
| Tailings + 2% biochar enriched with SSP | T + 2.0BS |
| Element | Concentration | Prevention Value | Investigation Value | ||
|---|---|---|---|---|---|
| A | R | I | |||
| Fe (g kg−1) | 109.00 ± 2.40 | ||||
| Al (mg kg−1) | 8400.00 ± 453.60 | ||||
| As (mg kg−1) | 3000.00 ± 162.00 | 15 | 35 | 55 | 150 |
| Ca (mg kg−1) | 1800.00 ± 97.20 | ||||
| Co (mg kg−1) | 51.00 ± 1.12 | 25 | 35 | 65 | 90 |
| Cu (mg kg−1) | 215.00 ± 4.73 | 60 | 200 | 400 | 600 |
| K (mg kg−1) | 800.00 ± 12.40 | ||||
| Mg (mg kg−1) | 1700.00 ± 91.80 | ||||
| Mn (mg kg−1) | 1140.00 ± 61.56 | ||||
| P (mg kg−1) | 330.00 ± 10.89 | ||||
| S (mg kg−1) | 100.00 ± 2.20 | ||||
| Zn (mg kg−1) | 76.00 ± 1.67 | 300 | 450 | 1000 | 2000 |
| Attribute | Biochar | ||
|---|---|---|---|
| BA | BT | BS | |
| pH (in water) | 6.71 | 3.02 | 4.25 |
| Ash content (%) | 3.62 | 23.62 | 15.77 |
| CEC a (cmolc kg−1) | 13.84 | 32.90 | 26.71 |
| AEC b (cmolc kg−1) | 11.14 | 18.14 | 14.27 |
| Al (g kg−1) | 0.11 | 3.24 | 0.92 |
| Ca (g kg−1) | 1.15 | 59.84 | 88.12 |
| Fe (g kg−1) | 0.13 | 3.91 | 3.51 |
| K (g kg−1) | 8.12 | 7.20 | 7.80 |
| Mg (g kg−1) | 1.13 | 5.02 | 1.41 |
| Total P (mg kg−1) | 2.91 | 15.02 | 10.05 |
| P-F1 c (mg kg−1) | 0.93 | 73.21 | 22.25 |
| P-F2 d (mg kg−1) | 0.14 | 3.13 | 1.26 |
| P-F3 e (mg kg−1) | 0.06 | 5.61 | 0.13 |
| P-F4 f (mg kg−1) | 1.23 | 85.57 | 51.18 |
| Treatment | pH | CEC | AEC | H + Al | Ca2+ | Mg2+ | Al3+ | K+ | P | OC | IC | TC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Water | cmolc dm−3 | mg kg−1 | ||||||||||||
| T | 6.46 e | 2.34 i | 2.17 e | 0.22 d | 0.94 e | 0.23 b | 0.11 c | 0.04 c | 17.67 h | 3.98 g | 5.32 c | 9.30 g | ||
| T + 0.5BA | 6.99 c | 5.01 h | 2.17 e | 0.11 e | 1.08 e | 0.01 d | 0.11 c | 0.06 c | 16.09 h | 5.60 f | 3.96 f | 9.56 g | ||
| T + 1.0BA | 7.17 b | 5.48 g | 2.18 e | 0.05 f | 0.84 f | 0.05 d | 0.11 c | 0.12 b | 18.37 h | 7.01 d | 4.79 e | 11.80 e | ||
| T + 2.0BA | 7.34 a | 8.30 c | 2.38 d | 0.01 f | 0.83 f | 0.34 a | 0.11 c | 0.16 b | 34.26 g | 8.43 b | 4.56 e | 12.99 c | ||
| T + 0.5BT | 6.99 c | 6.48 f | 2.32 d | 0.51 c | 1.12 e | 0.09 c | 0.11 c | 0.06 c | 110.50 e | 5.43 f | 5.25 c | 10.68 f | ||
| T + 1.0BT | 6.46 e | 7.72 d | 2.36 d | 0.81 b | 1.31 d | 0.10 c | 0.21 b | 0.09 c | 292.07 b | 6.87 e | 5.42 c | 12.29 d | ||
| T + 2.0BT | 6.09 f | 9.77 a | 2.42 c | 1.85 a | 2.33 b | 0.20 b | 0.52 a | 0.26 a | 608.21 a | 8.65 b | 5.31 c | 13.96 b | ||
| T + 0.5BS | 6.81 c | 7.06 e | 2.48 c | 0.26 d | 1.95 c | 0.05 d | 0.10 c | 0.04 c | 62.92 f | 6.14 e | 5.88 a | 12.02 d | ||
| T + 1.0BS | 6.98 c | 8.06 d | 2.57 b | 0.54 c | 2.05 c | 0.20 b | 0.11 c | 0.04 c | 122.90 d | 7.14 d | 5.59 b | 12.73 c | ||
| T + 2.0BS | 6.62 d | 8.53 b | 2.70 a | 0.81 b | 3.24 a | 0.20 b | 0.11 c | 0.09 c | 166.68 c | 9.88 a | 5.08 d | 14.96 a | ||
| Treatment | P-F1 | P-F2 | P-F3 | P-F4 |
|---|---|---|---|---|
| mg kg−1 | ||||
| T | 6.72 f | 20.83 g | 63.79 f | 2.39 f |
| T + 0.5BA | 1.47 g | 20.52 g | 68.36 f | 2.80 f |
| T + 1.0BA | 4.08 f | 19.49 g | 68.73 f | 3.30 f |
| T + 2.0BA | 10.02 e | 31.85 f | 94.32 e | 6.51 e |
| T + 0.5BT | 25.88 d | 82.54 d | 149.49 c | 54.31 c |
| T + 1.0BT | 129.72 b | 160.62 b | 211.30 b | 141.71 b |
| T + 2.0BT | 307.93 a | 251.27 a | 345.22 a | 368.05 a |
| T + 0.5BS | 8.53 f | 65.44 e | 105.41 e | 47.31 d |
| T + 1.0BS | 29.88 d | 89.13 d | 133.01 d | 58.02 c |
| T + 2.0BS | 70.55 c | 127.86 c | 153.20 c | 123.12 b |
| Variable | Unit | T | T + 0.5BA | T + 1.0BA | T + 2.0BA | T + 0.5BT | T + 1.0BT | T + 2.0BT | T + 0.5BS | T + 1.0BS | T + 2.0BS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | |||||||||||
| Biomass | g | 0.02 h | 0.02 h | 0.04 g | 0.09 e | 0.17 d | 0.08 f | 0.01 i | 0.26 c | 0.30 b | 0.52 a |
| As | g plant−1 | 0.34 a | 0.09 c | 0.02 e | 0.03 e | 0.03 e | 0.05 d | 0.22 b | 0.03 e | 0.02 e | 0.04 d |
| Ca | 0.01 h | 0.02 h | 0.05 g | 0.08 f | 0.26 d | 0.13 e | 0.01 h | 0.31 c | 0.33 b | 0.47 a | |
| Mg | 0.01 g | 0.01 g | 0.03 f | 0.05 e | 0.22 b | 0.12 d | 0.01 g | 0.15 c | 0.22 b | 0.39 a | |
| K | 0.33 i | 1.88 h | 5.07 g | 13.88 d | 12.00 e | 8.40 f | 1.29 h | 20.36 c | 26.59 b | 58.20 a | |
| P | 0.04 h | 0.05 h | 0.12 g | 0.33 f | 0.94 c | 0.74 d | 0.09 g | 0.68 e | 1.15 b | 2.26 a | |
| Fe | 0.07 c | 0.01 g | 0.01 f | 0.03 e | 0.05 d | 0.03 e | 0.03 e | 0.06 c | 0.10 b | 0.23 a | |
| Variable | Unit | T | T + 0.5BA | T + 1.0BA | T + 2.0BA | T + 0.5BT | T + 1.0BT | T + 2.0BT | T + 0.5BS | T + 1.0BS | T + 2.0BS |
| Roots | |||||||||||
| Biomass | g | 0.01 h | 0.01 i | 0.02 g | 0.05 e | 0.05 d | 0.03 f | 0.01 h | 0.08 c | 0.15 b | 0.20 a |
| As | g plant−1 | 0.15 b | 0.26 a | 0.14 b | 0.11 c | 0.13 b | 0.11 c | 0.12 c | 0.12 c | 0.12 c | 0.25 a |
| Ca | 0.01 g | 0.00 g | 0.01 f | 0.02 d | 0.02 d | 0.02 e | 0.01 f | 0.09 c | 0.11 b | 0.20 a | |
| Mg | 0.00 f | 0.00 f | 0.01 e | 0.03 c | 0.02 d | 0.01 d | 0.00 f | 0.04 b | 0.04 b | 0.08 a | |
| K | 0.06 g | 0.10 g | 0.32 g | 2.37 d | 3.69 c | 2.36 d | 0.70 f | 1.83 e | 9.48 b | 16.00 a | |
| P | 0.02 i | 0.01 i | 0.04 h | 0.14 f | 0.28 d | 0.30 c | 0.08 g | 0.19 e | 0.33 b | 0.77 a | |
| Fe | 0.03 h | 0.01 i | 0.05 g | 0.19 c | 0.08 f | 0.09 e | 0.01 j | 0.11 d | 0.29 b | 0.66 a |
| Treatment | BCFr | BCFs | TRF | TI |
|---|---|---|---|---|
| T | 0.49 c | 0.96 a | 1.95 a | - |
| T + 0.5BA | 0.75 b | 0.27 c | 0.35 d | 0.80 i |
| T + 1BA | 0.82 a | 0.10 e | 0.30 d | 1.86 g |
| T + 2BA | 0.32 e | 0.05 f | 0.07 g | 4.35 e |
| T + 0.5BT | 0.39 d | 0.09 e | 0.24 e | 7.05 d |
| T + 1BT | 0.32 e | 0.14 d | 0.45 c | 3.60 f |
| T + 2BT | 0.73 b | 0.62 b | 0.85 b | 0.86 h |
| T + 0.5BS | 0.35 e | 0.09 e | 0.25 e | 10.61 c |
| T + 1BS | 0.34 e | 0.08 e | 0.22 e | 14.34 b |
| T + 2BS | 0.73 b | 0.12 d | 0.17 f | 22.17 a |
| Equations | p-Value | NRMSE | R2adj |
|---|---|---|---|
| L-As (BA) = (3.176 × 10−4) − (6.5000 × As.F1) − (0.42640 × As.F2) + (2.8850 × OC) − (3.0340 × Ca) + (0.4323 × P) | 2.23 × 10−5 | 0.00185 | 0.916 |
| L-As (BT) = (4.599 × 10−5) + (0.6899 × As.F5) + (0.890 × K) − (1.4190 × P) | 3.77 × 10−4 | 0.01275 | 0.914 |
| L-As (BS) = (2.255 × 10−4) + (1.07 × As.F1) − (0.2515 × OC) − (1.29 × Ca) − (1.479 × Mg) − (0.0484 × P) | 2.20 × 10−5 | 0.00040 | 0.903 |
| R-As (BA) = (−1.477 × 10−10) + (0.2157 × Ca) − (0.7912 × P.F2) | 9.46 × 10−4 | 0.01386 | 0.908 |
| R-As (BT) = (6.591 × 10−11) − (0.3525 × Al(soil)) − (0.2865 × Ca) + (0.2925 × Ca(soil)) − (0.5222 × Mg) + (0.6453 × P.F1) − (0.3063 × P.F3) − (0.05161 × P.F4) − (0.01515 × pH) | 2.02 × 10−6 | 0.00008 | 0.906 |
| R-As (BS) = (−3.444 × 10−11) + (0.9222 × Ca(soil)) + (0.6485 × K(soil)) − (0.58260 × P.F4) | 1.54 × 10−3 | 0.01403 | 0.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, Y.N.; Pereira, W.V.d.S.; Caldeira, C.F.; Ramos, S.J.; de Souza, E.S.; Ribeiro, P.G.; Fernandes, A.R. Açaí Seed Biochar-Based Phosphate Fertilizers for Improving Soil Fertility and Mitigating Arsenic-Related Impacts from Gold Mining Tailings: Synthesis, Characterization, and Lettuce Growth Assessment. Minerals 2024, 14, 732. https://doi.org/10.3390/min14070732
Dias YN, Pereira WVdS, Caldeira CF, Ramos SJ, de Souza ES, Ribeiro PG, Fernandes AR. Açaí Seed Biochar-Based Phosphate Fertilizers for Improving Soil Fertility and Mitigating Arsenic-Related Impacts from Gold Mining Tailings: Synthesis, Characterization, and Lettuce Growth Assessment. Minerals. 2024; 14(7):732. https://doi.org/10.3390/min14070732
Chicago/Turabian StyleDias, Yan Nunes, Wendel Valter da Silveira Pereira, Cecílio Frois Caldeira, Sílvio Junio Ramos, Edna Santos de Souza, Paula Godinho Ribeiro, and Antonio Rodrigues Fernandes. 2024. "Açaí Seed Biochar-Based Phosphate Fertilizers for Improving Soil Fertility and Mitigating Arsenic-Related Impacts from Gold Mining Tailings: Synthesis, Characterization, and Lettuce Growth Assessment" Minerals 14, no. 7: 732. https://doi.org/10.3390/min14070732
APA StyleDias, Y. N., Pereira, W. V. d. S., Caldeira, C. F., Ramos, S. J., de Souza, E. S., Ribeiro, P. G., & Fernandes, A. R. (2024). Açaí Seed Biochar-Based Phosphate Fertilizers for Improving Soil Fertility and Mitigating Arsenic-Related Impacts from Gold Mining Tailings: Synthesis, Characterization, and Lettuce Growth Assessment. Minerals, 14(7), 732. https://doi.org/10.3390/min14070732












