Adsorption Characteristics of Illite and Kerogen Oil Phase: Thermodynamics Experiments
Abstract
1. Introduction
2. Illite and Kerogen Oil Phase Adsorption Experiment
2.1. Samples
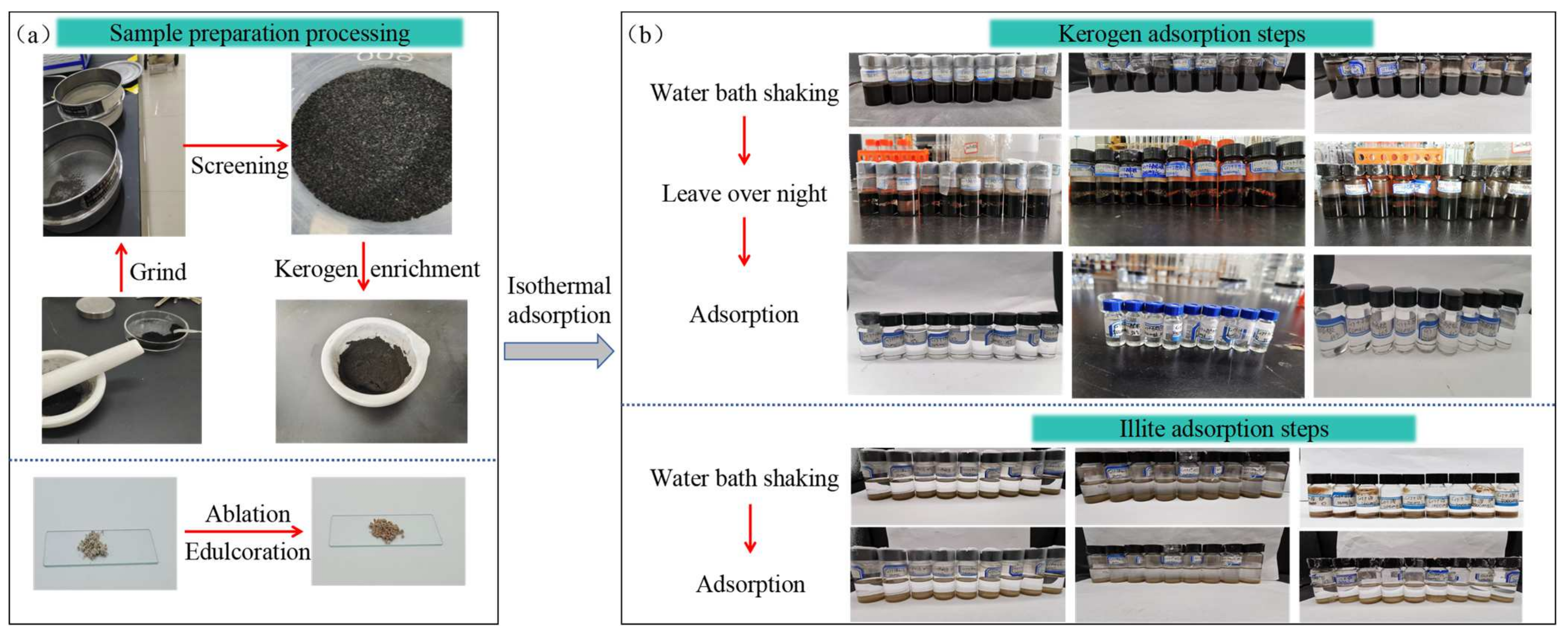
2.2. Experimental Methods and Steps
3. Experimental Results
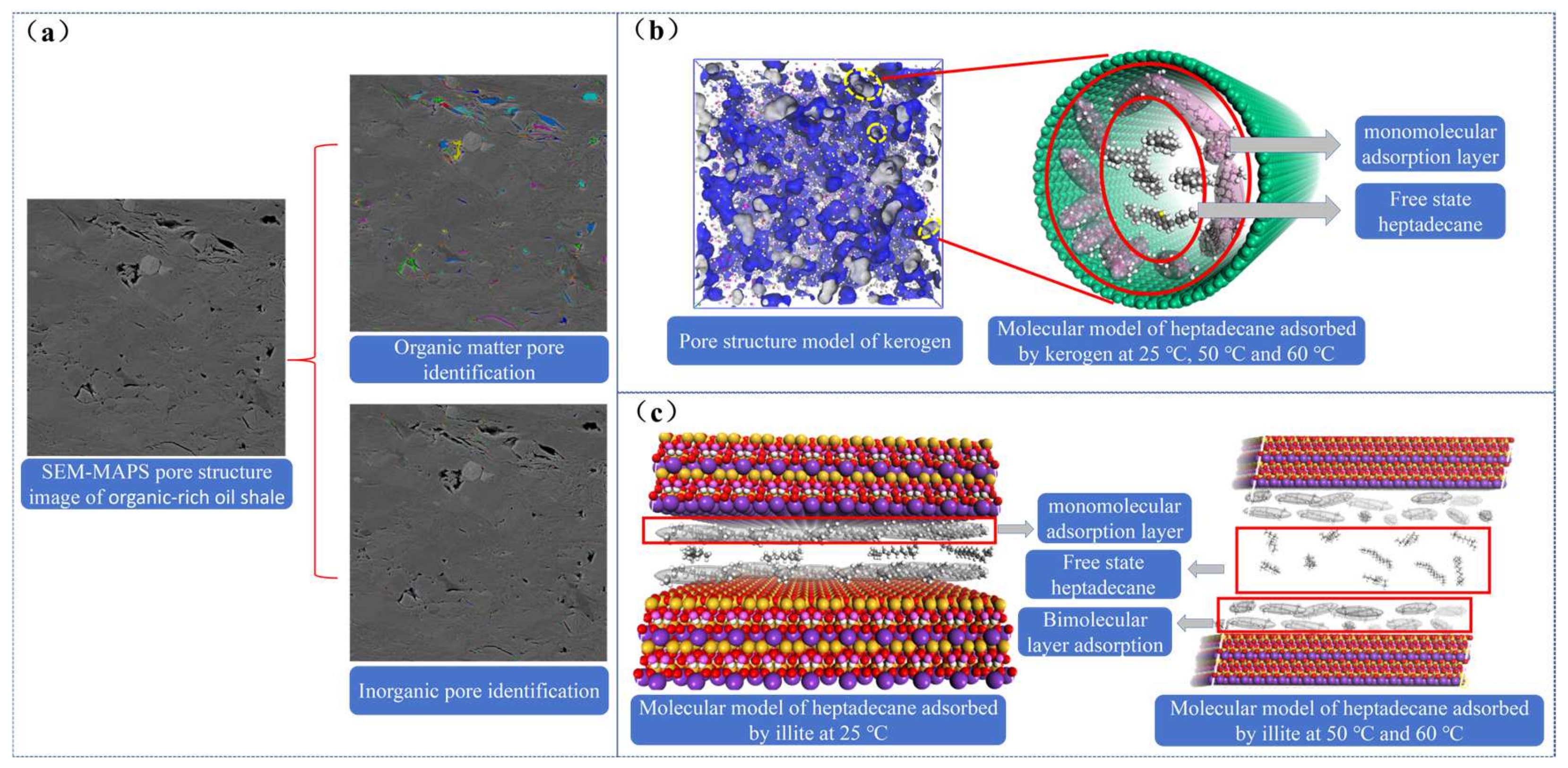
4. Analysis and Discussion
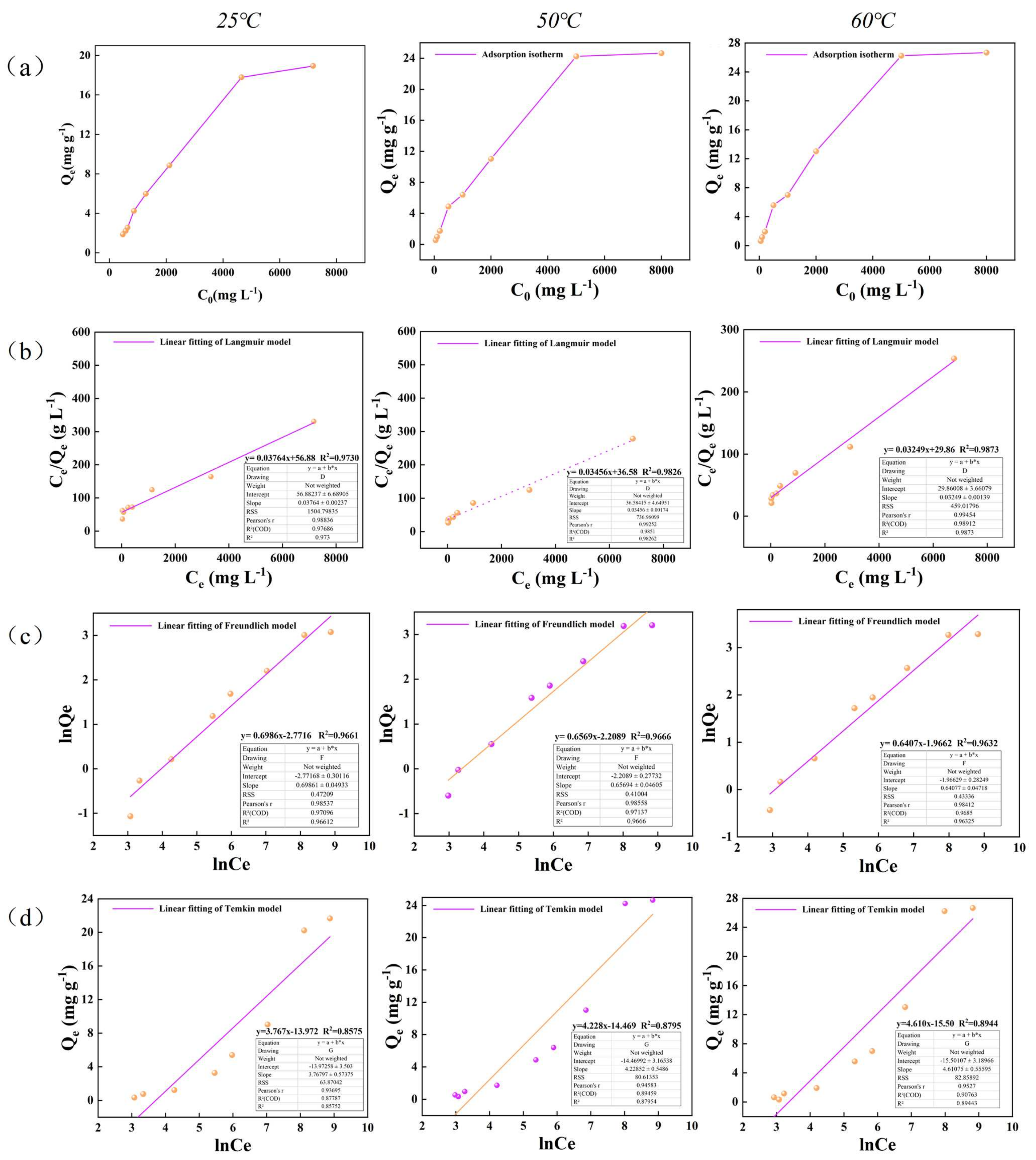
4.1. Evaluation of Adsorption Isotherm Model
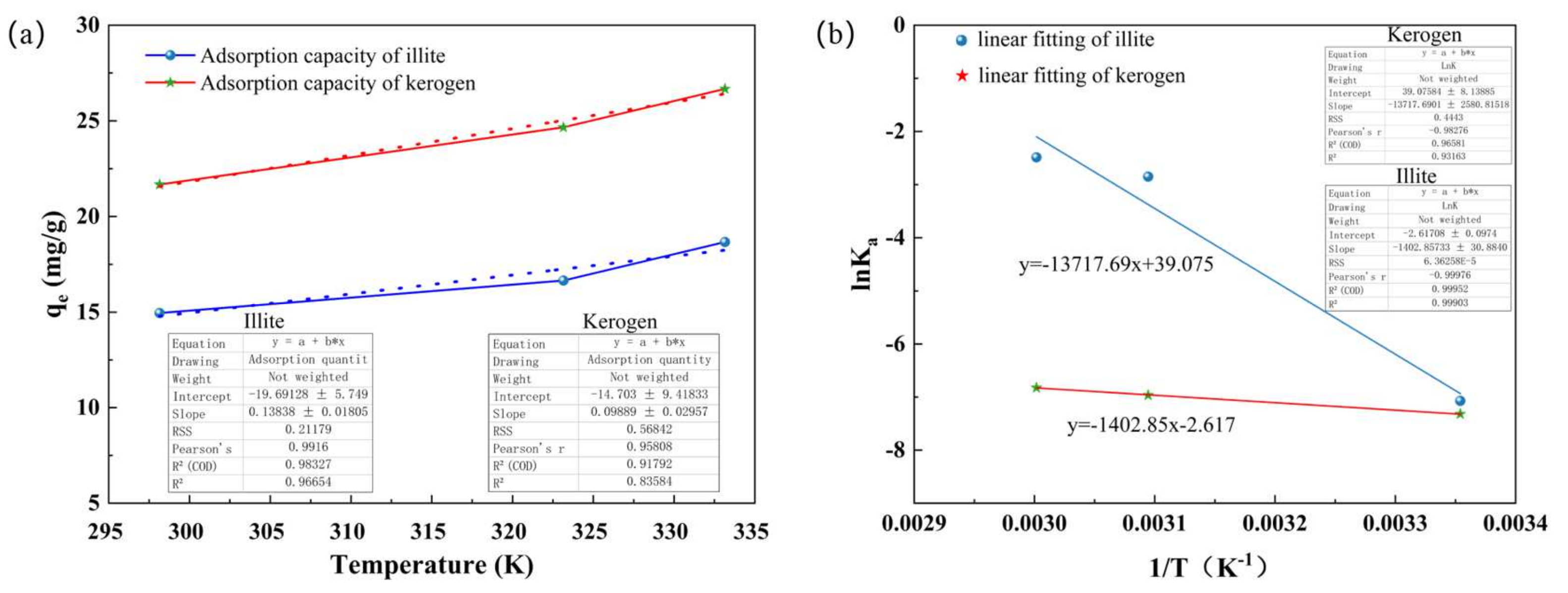
4.2. Thermodynamic Evaluation of Adsorption
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Xiang, Q.Y.; Ding, K.L. Development of oil shale at home and abroad. World Pet. Ind. 2024, 31, 16–25. [Google Scholar]
- Jin, Z.; Zhang, Q.; Zhu, R.; Dong, L.; Fu, J.; Liu, H.; Yun, L.; Liu, G.; Li, M.; Zhao, X.; et al. Classification of lacustrine shale oil reservoirs in China and its significance. Oil Gas Geol. 2023, 44, 801–819. [Google Scholar]
- Zhao, W.; Bian, C.; Li, Y.; Zhang, J.; He, K.; Liu, W.; Zhang, B.; Lei, Z.; Liu, C.; Zhang, J.; et al. Enrichment factors of movable hydrocarbons in lacustrine shale oil and exploration potential of shale oil in Gulong Sag, Songliao Basin, NE China. Pet. Explor. Dev. 2023, 50, 455–467. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhang, J.; Zhang, Y.; Liu, Z.; Luo, B.; Bian, C.; Li, J.; Wang, X.; Zhao, X.; et al. Evaluation of the compositions of lacustrine shale oil in China’s typical basins and its implications. Oil Gas Geol. 2023, 44, 1479–1498. [Google Scholar]
- Li, J.; Wang, M.; Jiang, C.; Lu, S.; Li, Z. Sorption model of lacustrine shale oil: Insights from the contribution of organic matter and clay minerals. Energy 2022, 260, 125011. [Google Scholar]
- Liu, X.; Wang, X.; Liu, Y.; Zhang, J.; Liu, J.; Liu, Q. Current status and development direction of seismic prospecting technology for continental shale oil in China. Acta Pet. Sin. 2023, 44, 2270–2285. [Google Scholar]
- Zou, C.; Yang, Z.; Zhu, R.; Zhang, G.; Hou, L.; Wu, S.; Tao, S.; Yuan, X.; Dong, D.; Wang, Y.; et al. Progress in Chinas Unconventional Oil & Gas Exploration and Development and Theoretical Technologies. Acta Geol. Sin. 2015, 89, 979–1007. [Google Scholar]
- Cong, Q.; Chen, J.; Lu, G.; Jiang, F.; Pang, B.; Shi, K.; Yang, X.; Wang, Y.; Pang, H. Review on influencing factors and microscopic mechanism of shale adsorption capacity by molecular dynamics simulation. J. Cent. S. Univ. Sci. Technol. 2022, 53, 3474–3489. [Google Scholar]
- Cai, L.; Yang, T.; Tian, J.; Yi, J.; Ren, Q. Advances in Studies of Development and Growth Mechanisms of Clay Minerals in Tight Sandstone Reservoirs. Acta Sedimen Tologica Sin. 2023, 41, 1859–1889. [Google Scholar]
- Dang, W.; Zhang, J.C.; Wang, F.Q.; Li, P.; Dan, Z.; Wang, R. Thermodynamics and kinetics of water vapor adsorption onto shale: A case study of the Permian Shanxi Formation, Ordos Basin. Oil Gas Geol. 2021, 42, 173–185. [Google Scholar]
- Huang, H.; Li, R.; Lyu, Z.; Cheng, Y.; Zhao, B.; Jiang, Z.; Zhang, Y.; Xiong, F. Comparative study of methane adsorption of Middle-Upper Ordovician marine shales in the western Ordos Basin, NW China: Insights into impacts of moisture on thermodynamics and kinetics of adsorption. Chem. Eng. J. 2022, 446, 137411. [Google Scholar] [CrossRef]
- Tao, W. Mechanism of Supercritical Methane Adsorption in Wufeng and Longmaxi Shales from the Dongxi Area, Sichuan Basin. Master’s Thesis, China University of Geosciences, Wuhan, China, 2023. [Google Scholar]
- Abdulkareem, F.A.; Faugere, G.; Radman, A.; Irfan, S.A.; Sathiavelu, S.; Padmanabhan, E. Marcellus shale characteristics and CO2 adsorption: Equilibrium and kinetic modeling study. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1003. [Google Scholar]
- Wei, T. Study on the Adsorption Law of CH4, C2H6 and Their Binary Mixtures in Shale. Ph.D. Thesis, China University of Petroleum, Beijing, China, 2022. [Google Scholar]
- Zhan, C.; Younan, L. Study on shale adsorption in Fuxian area of Ordos Basin. Petrochem. Ind. Appl. 2023, 42, 83–90. [Google Scholar]
- Lian, P. Study on adsorption kinetics of nonionic surfactants in Bohai Oilfield. Unconv. Oil Gas 2023, 10, 61–67. [Google Scholar]
- Chen, L.; Zuo, L.; Jiang, Z.; Jiang, S.; Liu, K.; Tan, J.; Zhang, L. Mechanisms of shale gas adsorption: Evidence from thermodynamics and kinetics study of methane adsorption on shale. Chem. Eng. J. 2019, 361, 559–570. [Google Scholar]
- Chen, X. Study on the Kinetics of Adsorption of CH4 and CO2 on Porous Materials. Ph.D. Thesis, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Muath, A.; Qadeer, A.M.S.; Hamid, R. A sorption-kinetics coupled dual-porosity poromechanical model for organic-rich shales. Comput. Geotech. 2022, 147, 104755. [Google Scholar]
- Zhang, M.; Yang, M.; Jia, T.; Liu, H.; Gong, Z. Adsorption kinetic characteristics of anthracite in Longshan Mine. Nat. Gas Geosci. 2022, 33, 267–276. [Google Scholar]
- Xue, P.; Zhang, L.; Liang, Q.; Shi, Y.; Cao, C.; Tang, Y. Isothermal adsorption properties of supercritical methane on shale. Nat. Gas Geosci. 2020, 31, 1261–1270. [Google Scholar]
- Sun, Z. Productivity Difference of CO2 Rich Coalbed Methane Wells and Adsorption Thermodynamic Mechanism in the East of Haishiwan Coalfield. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2022. [Google Scholar]
- Ma, L. Experimental Investigation into Simultaneous Adsorption Process of Water Vapor and Methane onto Shales; China University of Petroleum: Beijing, China, 2021. [Google Scholar]
- Wang, M.; Lun, Z.; Zhao, C.; Wang, H.; Luo, C.; Fu, X.; Li, C.; Zhang, D. Influences of primary moisture on methane adsorption within Lower Silurian Longmaxi shales in the Sichuan Basin, China. Energy Fuels 2020, 34, 10810–10824. [Google Scholar] [CrossRef]
- Shan, H. The Characteristic and Influencing Factors of High-Pressure Methane Adsorption on Dry Coals. Master’s Thesis, China University of Petroleum, Beijing, China, 2020. [Google Scholar]
- Yu, Z.; Ding, K.; Han, C.; Wu, Y.; Zou, M.; Liu, Y.; Guan, F. Adsorption of diesel oil onto natural mud shale: From experimental investigation to thermodynamic and kinetic modelling. Int. J. Environ. Anal. 2023, 103, 3468–3482. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, D.; Zhu, J.; Liu, G.; Chen, K.; Tong, C.; Zhang, D.; Xu, X.; Man, Y.; You, J.; et al. Hydrocarbon accumulation regularities, new fields and new types of exploration, and resource potentials in Beibuwan Basin. Acta Pet. Sin. 2024, 45, 202–225. [Google Scholar]
- Yang, C.; Jiang, D.; Zhou, X.; Huang, J.; Huang, S.; Zhang, B. Key Factors and Mode of Hydrocarbon Accumulation of Haizhong Sag in Beibu Gulf Basin. Offshore Oil 2023, 43, 41–45. [Google Scholar]
- Zou, C.; Zhu, R.; Dong, D.; Wu, S. Scientific and technological progress, development strategy and policy suggestion regarding shale oil and gas. Acta Pet. Sin. 2022, 43, 1675–1686. [Google Scholar]
- Zhu, J.; Li, X.; Yin, H.; Guo, H.; Lu, Z.; Liao, F.; Shi, Y.; An, T.; Wei, X. Shale characteristics and shale oil and gas resource potential of Liushagang Formation in Fushan sag, Beibu Gulf basin. China Offshore Oil Gas 2022, 34, 65–79. [Google Scholar]
- Rontani, F.J.; Smik, L.; Divine, D.; Husum, K.; Belt, S.T. Gas chromatography-mass spectrometry selected ion monitoring and gas chromatography-tandem mass spectrometry selected reaction monitoring analyses of mono-, di- and tri-unsaturated Csub25/sub highly branched isoprenoid alkene biomarkers in sea ice and sediment samples: A comparative study. Rapid Commun. Mass Spectrom. RCM 2024, 38, e9704. [Google Scholar] [PubMed]
- Wang, N.; Liu, Z.; Yang, J.; Ma, S.; Sun, Y.; Liu, Y. Research and Application on the Determination of Hydrocarbon Molecular Composition in Middle Distillate Oil by GC-MS. Acta Pet. Sin. Pet. Process. Sect. 2024, 1–13. [Google Scholar]
- Xiong, S.; Chen, M.; Yu, H.; Zhang, W.; Hu, Q.; He, S.; Yang, R. Experimental investigation of thermodynamic and kinetic behaviors from water vapor adsorption on four typical clay minerals. Gas Sci. Eng. 2023, 111, 204933. [Google Scholar] [CrossRef]
- Chen, Y. Study on adsorption mechanism and thermodynamic model of shale gas in Micro-Nano pore. Master’s Thesis, Central South University, Changsha, China, 2022. [Google Scholar]
- Liu, S.; Li, X.; Yin, X.; Zhang, P.; Li, W. Optimal model of isothermal adsorption for shale gas under high temperature and high pressure. China Min. Mag. 2018, 27, 160–166. [Google Scholar]
- Qayyimah, A.A.M.; Farhah, D.M.; Belladonna, M.; Elraeis, K.A. Supercritical methane adsorption measurement on shale using the isotherm modelling aspect. RSC Adv. 2022, 12, 20530–20543. [Google Scholar]
- Wei, T.; Huiqin, L.; Qin, W.; Jing, W. A New Method for Calculating the Isosteric Heat of Adsorption of CH4 on Shale Under High Pressure. Contemp. Chem. Ind. 2022, 51, 1916–1922+1926. [Google Scholar]
- Wang, W.; Chen, X.; Yang, B. Calculation of Adsorption Thermodynamics Parameters for Adsorption on the Solid-Liquid Interface. Univ. Chem. 2021, 36, 233–240. [Google Scholar]
- Li, J.; Peng, L.; Guo, L.; Zhang, W. Solid-liquid Adsorption Isotherm Model and Thermodynamic Parameter Calculation. Leather Sci. Eng. 2023, 33, 36–43. [Google Scholar]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010–2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, W.; Liu, R.; Chen, Z.; Lu, N.; Guo, W. Insights into adsorption and diffusion behavior of shale oil in slit nanopores: A molecular dynamics simulation study. J. Mol. Liq. 2022, 359, 119322. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Feng, Y.; Dong, X.; Wang, Q.; Zhang, B. Adsorption Behavior of Heavy Oil on Montmorillonite Surface by Typical Surfactant: Molecular Dynamics Simulation. Chin. J. Comput. Phys. 2023, 40, 583–596. [Google Scholar]
- Jiaojiao, H. Effect of temperature on adsorption efficiency of activated carbon. Eng. Technol. Mag. 2021, 6, e0116. [Google Scholar]
- Wang, Y.; Wang, C.; Huang, X.; Zhang, Q.; Wang, T.; Guo, X. Guideline for modeling solid-liquid adsorption: Kinetics, isotherm, fixed bed, and thermodynamics. Chemosphere 2023, 349, 140736. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, A.S.; Gbadamosi, A.O.; Bode-Olajide, F.B.; Igbafe, A.I. Adsorption of methyl ester sulfonate surfactant on Berea sandstone: Parametric optimization, kinetics, isotherm and thermodynamics studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133363. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.F.; Hu, Q.H.; Jia, L.B.; Wang, X.M.; Gao, S.S.; Liu, L.F. Adsorption of methane onto mudstones under supercritical conditions: Mechanisms, physical properties and thermodynamic parameters. Pet. Sci. 2023, 20, 34–47. [Google Scholar] [CrossRef]

| Sample Kind | Purity /Specification | Sample Source | |
|---|---|---|---|
| Adsorbent | Illite | 75–100 μm | Dehang Mineral Products Co., Ltd. |
| Kerogen | 90.51% | Shale core debris in Weixinan Sag, Beibuwan Basin | |
| Adsorbate | Heptadecane | AR | Chengdu Cologne Chemicals Co., Ltd. |
| Tetrachloroethylene | AR | Chengdu Cologne Chemicals Co., Ltd. | |
| Usage of the Experiment | Experiment Methods | Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drawing of standard curve | Infrared spectrophotometry | 20 | 40 | 60 | 80 | 100 | - | - | - |
| GC–MS | 10 | 10 | 10 | 10 | 10 | - | - | - | |
| Determination of adsorption capacity | Infrared spectrophotometry | 50 | 100 | 200 | 500 | 1000 | 2000 | 5000 | 8000 |
| GC–MS | 50 | 100 | 200 | 500 | 1000 | 2000 | 5000 | 10,000 | |
| Volume (Ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Adsorbent | Temperature /°C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL | KF | n | A | |||||||
| Illite | 25 | y = 0.05742x + 67.86 | y = 0.6792x − 2.9563 | y = 2.263x − 7.163 | ||||||
| 17.415 | 0.000849 | 0.981 | 0.0520 | 1.472 | 0.973 | 2.263 | −7.163 | 0.824 | ||
| 50 | y = 0.04855x + 84.86 | y = 0.6535x − 2.8480 | y = 2.3124x − 7.419 | |||||||
| 20.597 | 0.00572 | 0.903 | 0.0579 | 1.530 | 0.990 | 2.312 | −7.419 | 0.716 | ||
| 60 | y = 0.04375x + 65.95 | y = 0.6271x − 2.486 | y = 2.5725x − 7.747 | |||||||
| 22.857 | 0.000663 | 0.912 | 0.0832 | 1.594 | 0.981 | 2.572 | −7.747 | 0.698 | ||
| Kerogen | 25 | y = 0.03764x + 56.88 | y = 0.6986x − 2.7716 | y = 3.767x − 13.972 | ||||||
| 26.567 | 0.00066 | 0.973 | 0.062 | 1.431 | 0.966 | 3.767 | −13.972 | 0.857 | ||
| 50 | y = 0.03456x + 36.58 | y = 0.6569x − 2.2089 | y = 4.228x − 14.469 | |||||||
| 28.935 | 0.00945 | 0.982 | 0.109 | 1.522 | 0.966 | 4.228 | −14.469 | 0.879 | ||
| 60 | y = 0.03249x + 29.86 | y = 0.6407x − 1.9662 | y = 4.610x − 15.50 | |||||||
| 30.778 | 0.00108 | 0.987 | 0.139 | 1.560 | 0.963 | 4.610 | −15.50 | 0.894 | ||
| Sample | Temperature (K) | ΔHθ (kJ/mol) | ΔSθ (J/(mol·K)) | ΔGθ (kJ/mol) |
|---|---|---|---|---|
| Illite | 298.15 | 114.049 | 324.869 | 18.145 |
| 323.15 | 7.654 | |||
| 333.15 | 6.887 | |||
| Kerogen | 298.15 | 11.663 | −21.757 | 18.146 |
| 323.15 | 18.711 | |||
| 333.15 | 18.899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xiong, J.; Zhu, Y.; He, R.; Chen, X.; Chen, Q.; Yan, Z.; Liu, C.; Ma, L. Adsorption Characteristics of Illite and Kerogen Oil Phase: Thermodynamics Experiments. Minerals 2024, 14, 579. https://doi.org/10.3390/min14060579
Tang X, Xiong J, Zhu Y, He R, Chen X, Chen Q, Yan Z, Liu C, Ma L. Adsorption Characteristics of Illite and Kerogen Oil Phase: Thermodynamics Experiments. Minerals. 2024; 14(6):579. https://doi.org/10.3390/min14060579
Chicago/Turabian StyleTang, Xin, Junjie Xiong, Yanming Zhu, Ruiyu He, Xiangru Chen, Qiuqi Chen, Zhangping Yan, Cheng Liu, and Litao Ma. 2024. "Adsorption Characteristics of Illite and Kerogen Oil Phase: Thermodynamics Experiments" Minerals 14, no. 6: 579. https://doi.org/10.3390/min14060579
APA StyleTang, X., Xiong, J., Zhu, Y., He, R., Chen, X., Chen, Q., Yan, Z., Liu, C., & Ma, L. (2024). Adsorption Characteristics of Illite and Kerogen Oil Phase: Thermodynamics Experiments. Minerals, 14(6), 579. https://doi.org/10.3390/min14060579






