Till Geochemistry as a Vector to Metasomatic Iron and Alkali-Calcic Systems and Associated Deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada
Abstract
1. Introduction
2. Study Area
2.1. Geochemistry of MIAC Systems and Affiliated Deposits
2.2. Bedrock Geology of the Great Bear Magmatic Zone
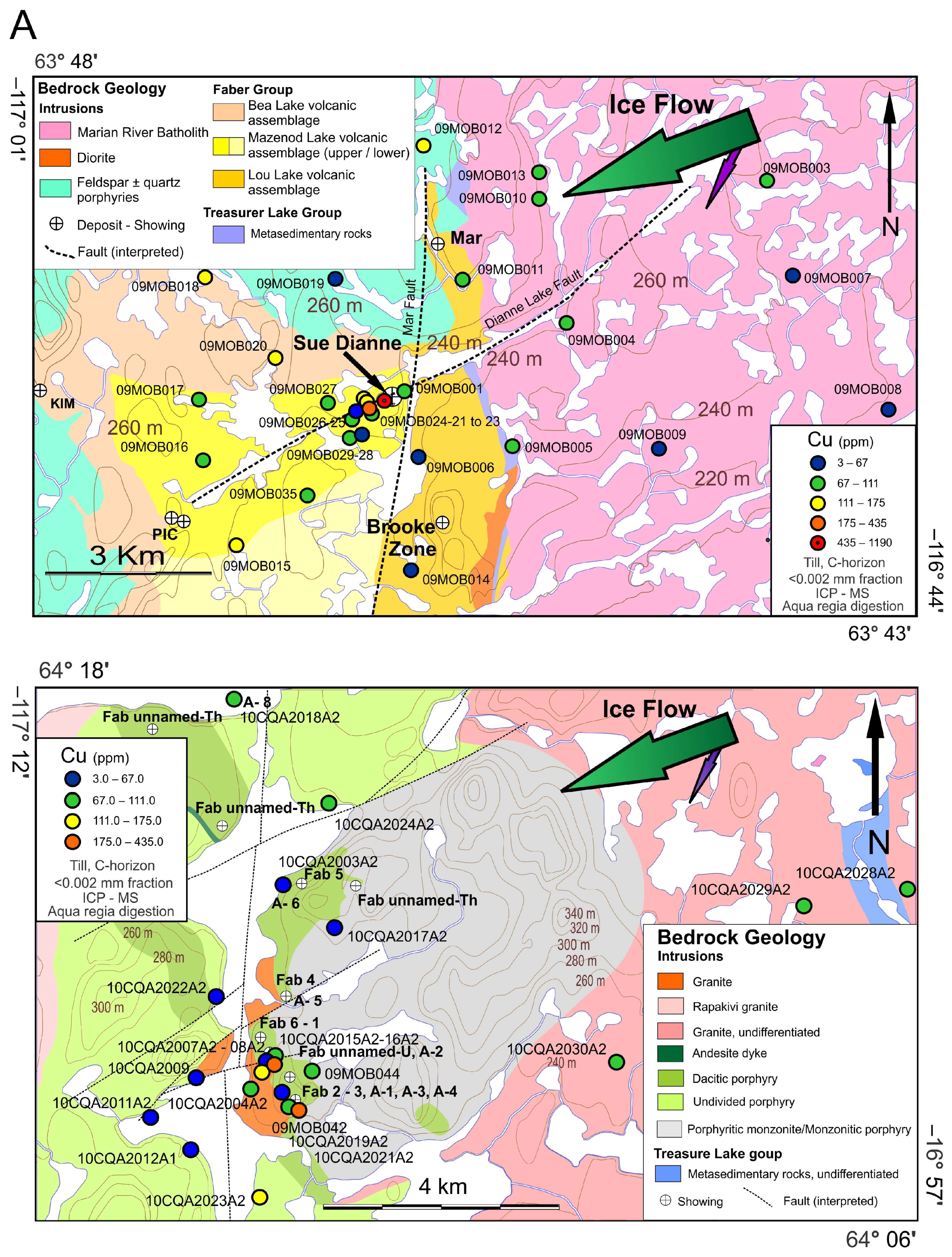
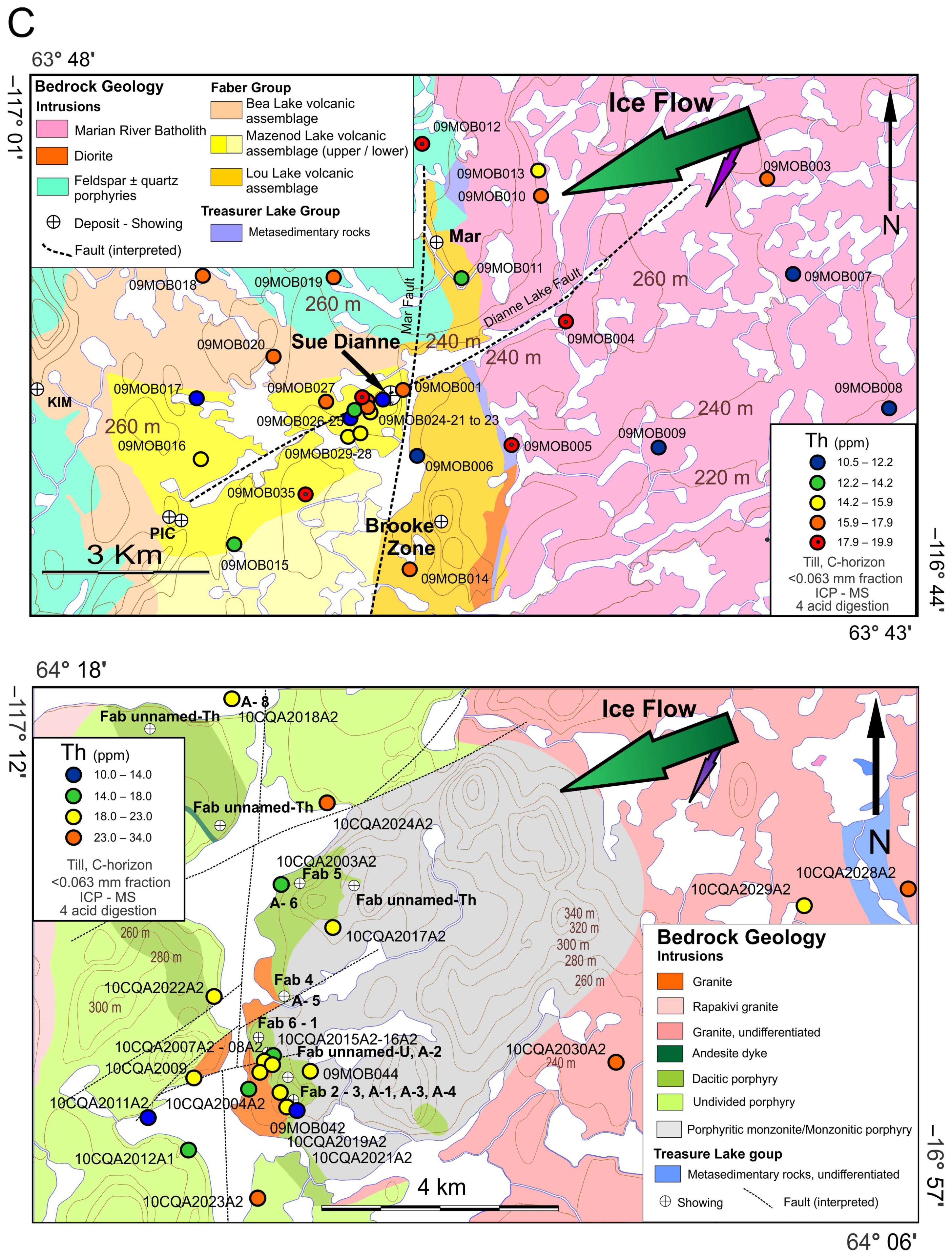
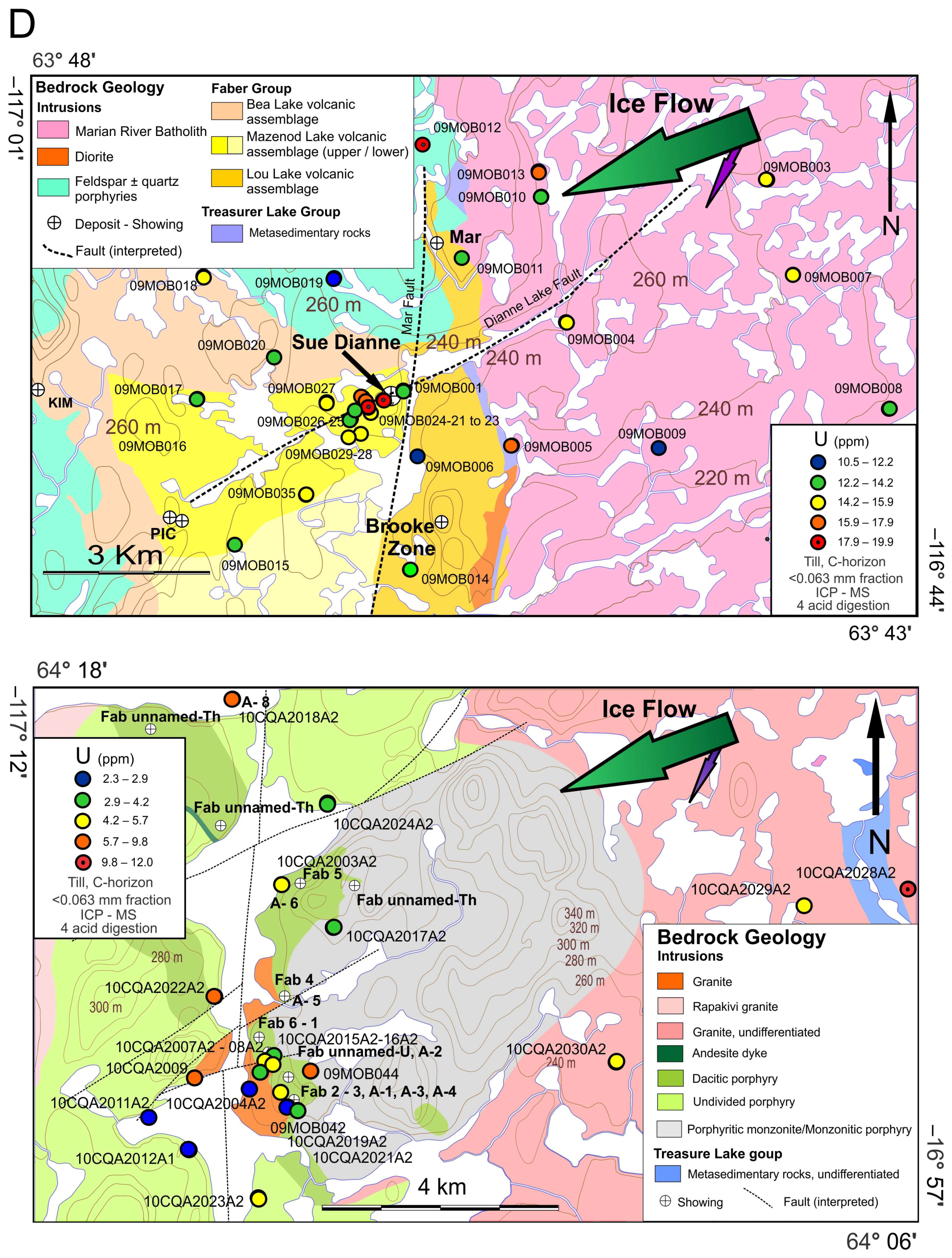
2.3. The Sue Dianne IOCG System

2.4. The Fab IOCG System
2.5. Quaternary Geology
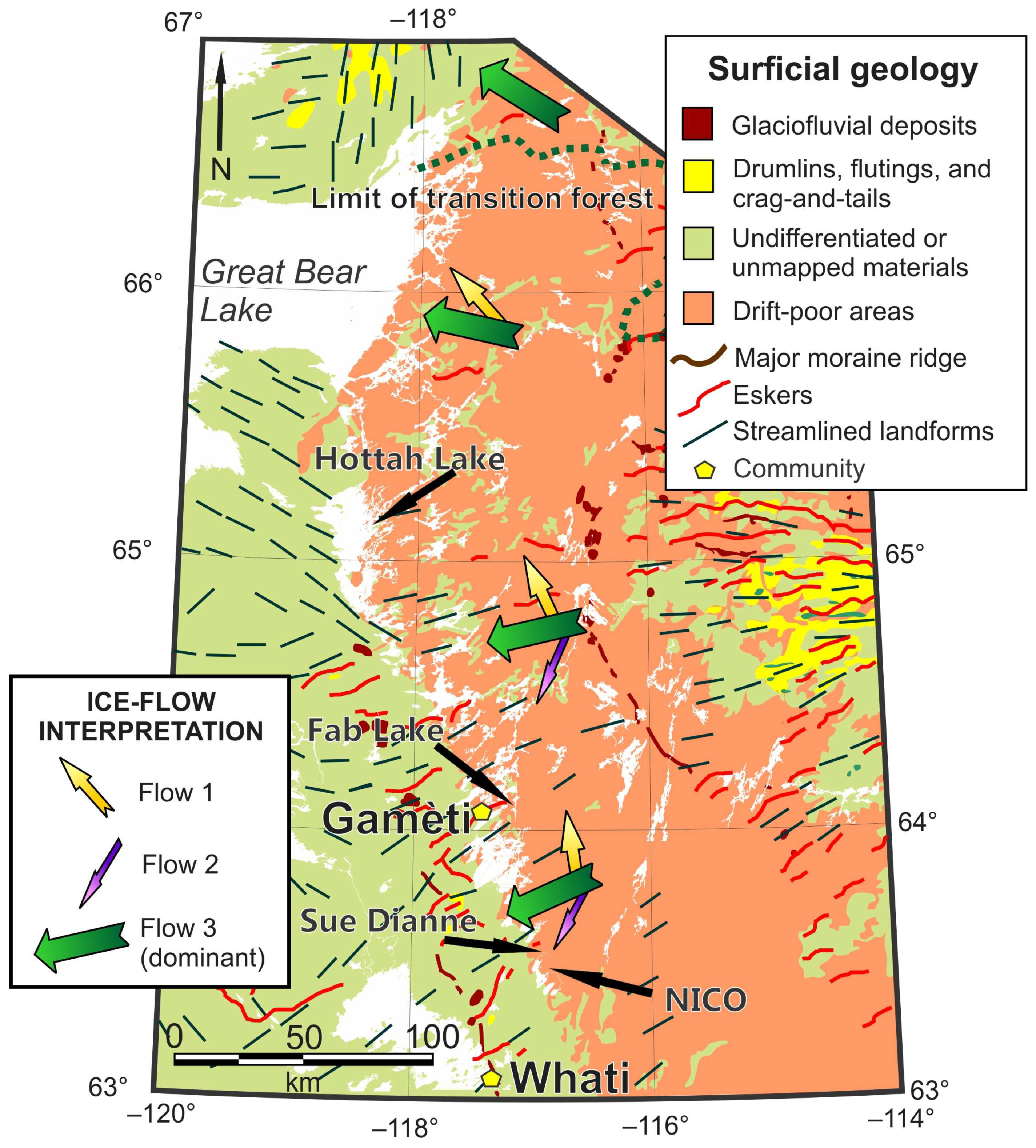
2.5.1. Physiography and Surficial Sediments
2.5.2. Glacial Geology and Glacial Transport
3. Methods
3.1. Sampling Procedures and Analytical Approach
3.2. Analytical Methods for Till Samples
3.3. Data Selection for Analyzed Till Samples
3.4. Till Geochemistry Data Preparation
4. Data Analysis and Results
4.1. Lithogeochemistry
4.2. Till Geochemistry
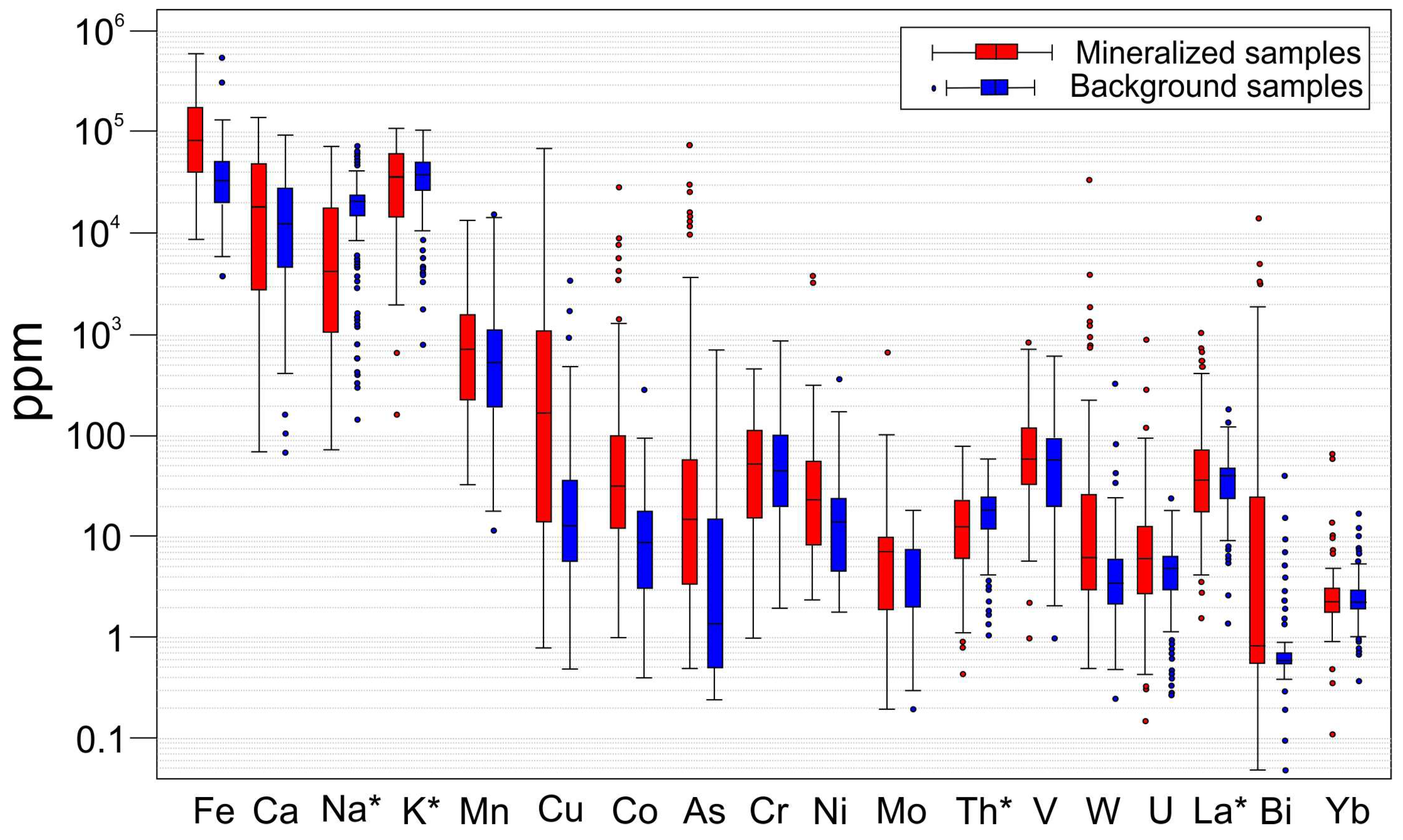
4.2.1. Elemental Distribution
4.2.2. Statistical Significance of Elemental Enrichments and Depletions
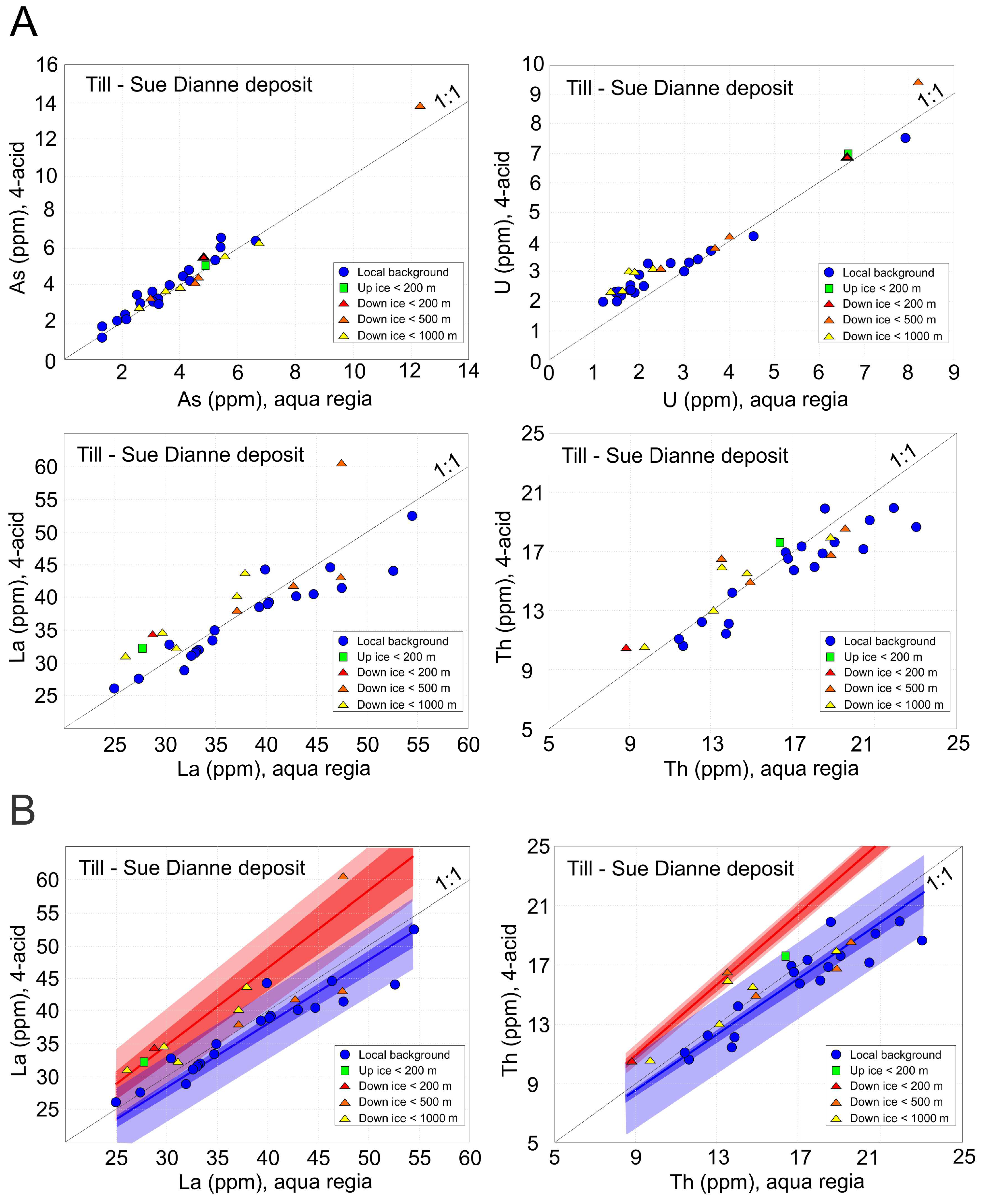
4.2.3. Comparison between Geochemical Dissolution Methods and Size Fractions
5. Discussion
5.1. Element Abundance in Till Associated with IOA/IOCG Mineralization and MIAC Systems
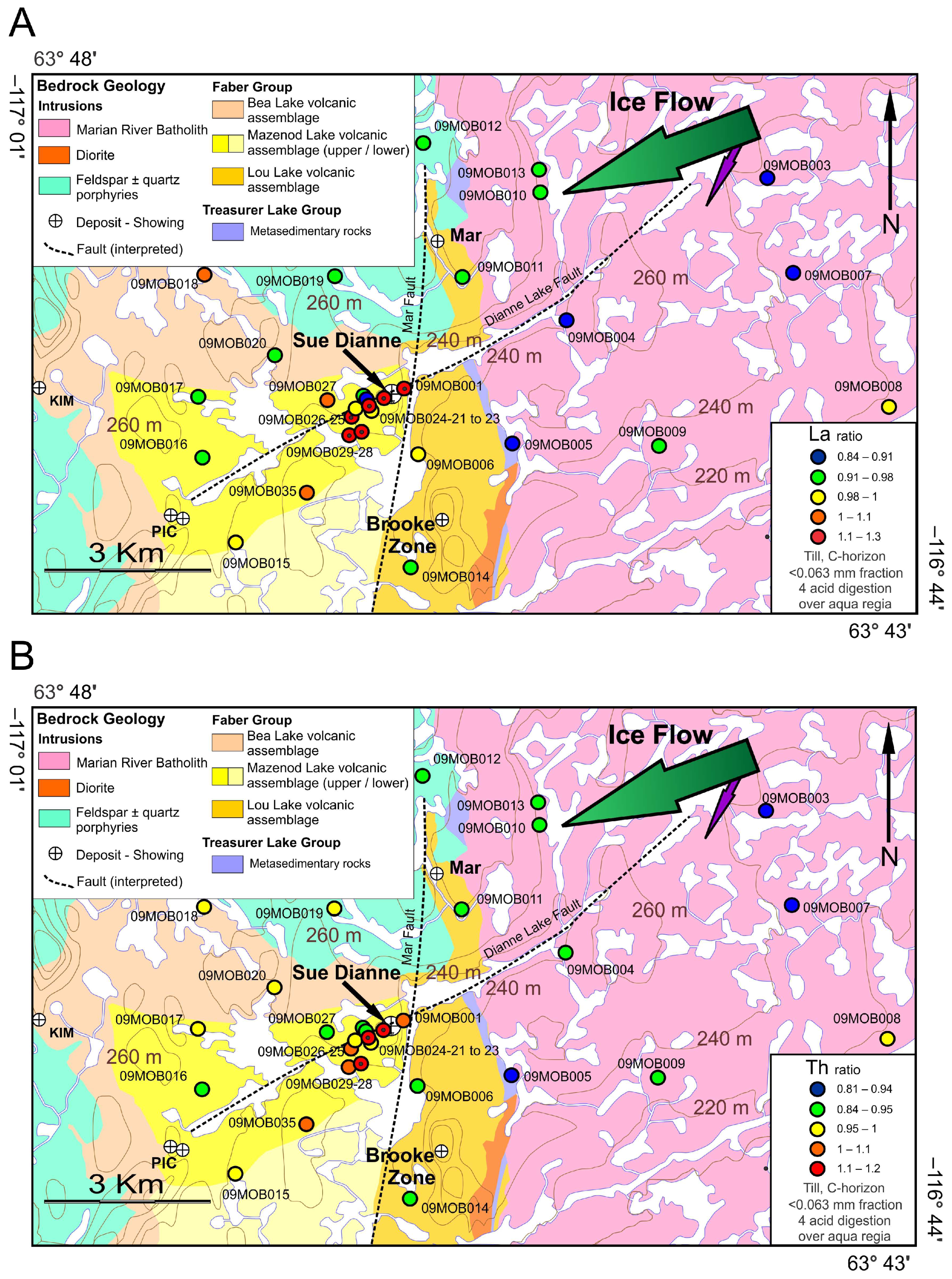
5.2. Digestion Method Ratios in Till with Respect to the Sue Dianne IOCG Deposit Alterations
5.3. Limitations of Till Geochemical Methods in the GBMZ
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClenaghan, M.B.; Thorleifson, L.H.; DiLabio, R.N.W. Till geochemical and indicator mineral methods in mineral exploration. Ore Geol. Rev. 2000, 16, 145–166. [Google Scholar] [CrossRef]
- Klassen, R.A. A Quaternary geological perpective on geochemical exploration in glaciated terrain. Geol. Soc. Lond. Spec. Publ. 2001, 185, 1–17. [Google Scholar] [CrossRef]
- McClenaghan, M.B.; Kjarsgaard, B.A. Indicator mineral and geochemical methods for diamond exploration in glaciated terrain in Canada. Geol. Soc. Lond. Spec. Publ. 2001, 185, 83–123. [Google Scholar] [CrossRef]
- McMartin, I.; Dredge, L.A.; Grunsky, E.; Pehrsson, S. Till geochemistry in west-central Manitoba: Interpretation of provenance and mineralization based on glacial history and multivariate data analysis. Econ. Geol. 2016, 111, 1001–1020. [Google Scholar] [CrossRef]
- McClenaghan, B.M.; Paulen, R.C. Application of till mineralogy and geochemistry to mineral exploration. In Past Glacial Environment, 2nd ed.; Menzies, J., van der Meer, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 689–751. [Google Scholar]
- McClenaghan, B.M.; Spirito, W.A.; Plouffe, A.; McMartin, I.; Campbell, J.E.; Paulen, R.C.; Garrett, R.G.; Pelchat, P.; Gauthier, M.S. Geological Survey of Canada Till-Sampling and Analytical Protocols: From Field to Archive, 2020 Update; Open File 8591; Geological Survey of Canada: Ottawa, ON, Canada, 2020; 82p.
- McClenaghan, B.M.; Spirito, W.A.; Day, S.J.A.; McCurdy, M.W.; McNeil, R.J.; Adcok, S.W. Overview of surficial geochemistry and indicator mineral surveys and case studies from the Geological Survey of Canada’s GEM Program. Geochem. Explor. Environ. Anal. 2022, 22, 1–70. [Google Scholar] [CrossRef]
- McClenaghan, B.M.; Paulen, R.C.; Smith, R.C.; Rice, J.M.; Plouffe, A.; McMartin, I.; Campbell, J.E.; Parsasadr, M.; Lethonen, M. Review of till geochemistry, indicator mineral, and boulder tracing methods for mineral exploration in glaciated terrain. Geochem. Explor. Environ. Anal. 2023, 23, 1–43. [Google Scholar] [CrossRef]
- McClenaghan, M.B. Till geochemistry in areas of thick drift and its application to gold exploration, Matheson area, northeastern Ontario. Explor. Min. Geol. 1994, 3, 17–30. [Google Scholar]
- Kaszycki, C.A.; Nielsen, E.; Gobert, G. Surficial geochemistry and response to volcanic-hosted massive sulphide mineralization in the Snow Lake region. In Extech I: A Multidisciplinary Approach to Massive Sulphide Research in the Rusty Lake-Snow Lake Greenstone Belts, Manitoba; Bonham-Carter, G.F., Galley, A.G., Hall, G.E.M., Eds.; Geological Survey of Canada: Bulletin, MB, Canada, 1996; Volume 426, pp. 139–154. [Google Scholar]
- Levson, V.M. Quaternary Geology and Till Geochemistry of the Babine Porphyry Copper Belt, British Columbia (NTS 93 L/9,16, M/1, 2, 7, 8); Bulletin 110; British Columbia Ministry of Energy and Mines, and Petroleum Resources: Victoria, UK, 2002; 278p.
- Klassen, R. The geochemical and physical properties of till, Bathurst Mining Camp, New Brunswick, Canada. Econ. Geol. Monogr. 2003, 11, 661–678. [Google Scholar]
- Lehtonen, M.; Marmo, J.; Nissinen, A.; Johanson, B.; Pakkanen, L. Glacial dispersal studies using indicator minerals and till geochemistry around two eastern Finland kimberlites. J. Geochem. Explor. 2005, 87, 19–43. [Google Scholar] [CrossRef]
- Campbell, J.E. Drift prospecting for uranium in the Athabasca Basin, Saskatchewan. In Application of till and Stream Sediment Heavy Mineral and Geochemical Methods to Mineral Exploration in Western and Northern Canada: Short Course Notes 18; Paulen, R., McMartin, I., Eds.; Geological Association of Canada: St. John’s, NB, Canada, 2009; Volume 111, pp. 2045–2072. [Google Scholar]
- McClenaghan, M.B.; Layton-Matthews, D.; Matile, G. Till geochemical signatures of magmatic Ni–Cu deposits, Thompson Nickel Belt, Manitoba, Canada. Geochem. Explor. Environ. Anal. 2011, 11, 145–159. [Google Scholar] [CrossRef]
- McClenaghan, B.M.; Peter, J. Till geochemical signatures of volcanogenic massive sulphide deposits: An overview of Canadian examples. Geochem. Explor. Environ. Anal. 2016, 1, 27–47. [Google Scholar] [CrossRef]
- Hashmi, S.; Ward, B.C.; Plouffe, A.; Leybourne, M.I.; Ferbey, T. Geochemical and mineralogical dispersal in till from the Mount Polley Cu-Au porphyry deposit, central British Columbia, Canada. Geochem. Explor. Environ. Anal. 2015, 15, 234–249. [Google Scholar] [CrossRef]
- Plouffe, A.; Ferbey, T.; Hashmi, S.; Ward, B.C. Till geochemistry and mineralogy: Vectoring towards Cu porphyry deposits in British Columbia, Canada. Geochem. Explor. Environ. Anal. 2016, 16, 213–232. [Google Scholar] [CrossRef]
- McClenaghan, B.M.; Paulen, R.C.; Orviatt, N.M. Geometry of indicator mineral and till geochemistry dispersal fans from the Pine Point Mississippi Valley-type Pb-Zn district, Northwest Territories, Canada. J. Geochem. Explor. 2018, 190, 69–86. [Google Scholar] [CrossRef]
- McClenaghan, B.M.; Ames, D.E.; Cabris, L.J. Indicator mineral and till geochemical signatures of the Broken Hammer Cu–Ni–PGE–Au deposit, North Range, Sudbury Structure, Ontario, Canada. Explor. Environ. Anal. 2020, 20, 337–356. [Google Scholar] [CrossRef]
- Hashmi, S.; Leybourne, M.I.; Hamilton, S.; Layton-Matthews, D.; McClenaghan, B.M. Suitability of surficial media for Ni–Cu–PGE exploration in an established mining camp: A case study from the South Range of the Sudbury Igneous Complex, Canada. Explor. Environ. Anal. 2022, 22, 1–22. [Google Scholar] [CrossRef]
- Plouffe, A.; Kjarsgaard, I.M.; Ferbey, T.; Wilton, D.H.C.; Petts, D.C.; Percival, J.B.; Kobylinski, C.H.; McNeil, R.J. Detecting buried porphyry Cu mineralization in a glaciated landscape: A case study from the Gibraltar Cu-Mo deposit, British Columbia, Canada. Econ. Geol. 2022, 117, 777–799. [Google Scholar] [CrossRef]
- Benavides, J.; Kyser, T.K.; Clark, A.H.; Stanley, C.; Oates, C. Application of molar element ratio analysis of lag talus composite samples to the exploration for iron oxide–copper–gold mineralization: Mantoverde area, northern Chile. Geochem. Explor. Environ. Anal. 2008, 8, 369–380. [Google Scholar] [CrossRef]
- Benavides, J.; Kyser, T.K.; Clark, A.H.; Stanley, C.; Oates, C. Exploration guidelines for copper-rich iron oxide–copper–gold deposits in the Mantoverde area, northern Chile: The integration of host-rock molar element ratios and oxygen isotope compositions. Geochem. Explor. Environ. Anal. 2008, 8, 343–367. [Google Scholar] [CrossRef]
- Fabris, A.J.; Halley, S.; Van Der Wilen, S.; Keeping, T.; Gordon, G. IOCG-style mineralisation in the central eastern Gawler Craton, SA. In Characterisation of Alteration, Geochemical Associations and Exploration Vectors; Report Book 2013/00014; Department of Innovation, Manufacturing, Trade, Resources and Energy: Adelaide, Australia, 2013. [Google Scholar]
- Fabris, A.J.; Van Der Wilen, S.; Keeping, T.; Gordon, G. Geochemicaal Footprint of IOCG Deposits Beneath Thick Cover: Insights from the Olympic Cu-Au Province, South Australia. In Proceedings of the 27th International Applied Geochemistry Symposium, Tucson, AZ, USA, 20–24 April 2015. [Google Scholar]
- Sadeghi, M.; Arvanitidis, N.; Landenbergger, A. Geochemistry of rare earth elements in bedrock and till, applied in the contrext of mineral potential in Sweden. Minerals 2020, 10, 365. [Google Scholar] [CrossRef]
- McMartin, I.; Corriveau, L.; Beaudoin, G. An orientation study of the heavy mineral signature of the NICO Co-Au-Bi deposit, Great Bear magmatic zone, NW Territories, Canada. Geochem. Explor. Environ. Anal. 2011, 11, 293–307. [Google Scholar] [CrossRef]
- Sappin, A.A.; Dupuis, C.; Beaudoin, G.; McMartin, I.; McClenaghan, M.B. Optimal ferromagnetic fraction representative of iron oxide compositional variations in till samples along ice-flow paths: Case studies from the Sue-Dianne IOCG and Thompson magmatic Ni-Cu deposits, Canada. Geochem. Explor. Environ. Anal. 2014, 14, 315–329. [Google Scholar] [CrossRef]
- Montreuil, J.-F.; Potter, E.G.; Corriveau, L.; Davis, W.J. Element mobility patterns in magnetite-group IOCG systems: The FAb IOCG system, Northwest Territories, Canada. Ore Geol. Rev. 2016, 72, 562–584. [Google Scholar] [CrossRef]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.; Blein, O.; De Toni, A.F. Mineral systems with IOCG and affiliated deposits: Part 3–metal pathways and ore deposit model. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits, Special Paper 52; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Geological Association of Canada: St. John’s, NB, Canada, 2022; pp. 205–245. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.; Ehrig, K.; Clark, J.; Mumin, A.H.; Williams, P.J. Mineral systems with IOCG and affiliated deposits: Part 1—Metasomatic footprints of alteration facies. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits, Special Paper 52; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Geological Association of Canada: St. John’s, NB, Canada, 2022; pp. 113–158. [Google Scholar]
- McMartin, I.; Corriveau, L.; Beaudoin, G. Heavy mineral and till geochemical signatures of the NICO Co-Au-Bi deposit, Great Bear magmatic zone, Northwest Territories, Canada. In Proceedings of the 24th International Applied Geochemistry Symposium (IAGS 2009), Fredericton, NB, Canada, 1–4 June 2009. [Google Scholar]
- Porter, T.M. Current understanding of iron oxide associated-alkali altered mineralized systems: Part 1—An overview; part 2—A review. In Hydrothermal Iron Oxide Copper-Gold and Related Deposits: A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2010; pp. 5–106. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Blein, O.; Ehrig, K.; Potter, E.G.; Fabris, A.J.; Clark, J. Mineral systems with IOCG and affiliated deposits: Part 2—geochemical footprints. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits, Special Paper 52; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Geological Association of Canada: St. John’s, NB, Canada, 2022; pp. 159–204. [Google Scholar]
- Williams, P.J.; Barton, M.D.; Johnson, D.A.; Fontboté, L.; De Haller, A.; Mark, G.; Oliver, N.H.; Marschik, R. Iron oxide copper-gold deposits: Geology, space-time distribution, and possible modes of origin. In Economic Geology, One Hundredth Anniversary Volume 1905–2005; Hedenquist, J., Thompson, F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 371–405. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.G. Alteration facies linkages among IOCG, IOA and affiliated deposits in the Great Bear magmatic zone, Canada. Econ. Geol. 2016, 111, 2045–2072. [Google Scholar] [CrossRef]
- Montreuil, J.-F.; Corriveau, L.; Grunsky, E. Compositional data analysis of hydrothermal alteration in IOCG systems, Great Bear magmatic zone, Canada: To each alteration type its own geochemical signature. Geochem. Explor. Environ. Anal. 2013, 13, 229–247. [Google Scholar] [CrossRef]
- Acosta-Góngora, G.P.; Potter, E.G.; Lawley, C.J.M. Geochemical characterization of the Central Mineral Belt U±Cu±Mo±V mineralization, Labrador, Canada: Evaluation of IOCG potential and application of unsupervised machine-learning. J. Geochem. Explor. 2022, 237, 106995. [Google Scholar] [CrossRef]
- Drejing-Carroll, D.; Hitzman, M.W.; Coller, D. Geology of the Nautanen North Cu-Au-Ag-(Mo) Deposit, Norrbotten, Sweden. Econ. Geol. 2023, 118, 1765–1794. [Google Scholar] [CrossRef]
- Corriveau, L.; Lauzière, K.; Montreuil, J.-F.; Potter, E.; Hanes, R.; Prémont, S. Dataset of Geochemical Data from Iron Oxide Alkali-altered Mineralising Systems of the Great Bear Magmatic Zone, Northwest Territories; Open File 7643; Geological Survey of Canada: Ottawa, ON, Canada, 2015; 24p.
- Barton, M.D.; Johnson, D.A. Footprints of Fe-Oxide (Cu-Au) Systems; Special Publication; Center for Global Metallogeny, University of Western Australia: Perth, Australia, 2004; pp. 112–116. [Google Scholar]
- Mumin, A.H. Echo Bay IOCG Thematic Map Series: Geology, Structure and Hydrothermal Alteration of a Stratovolcano Complex, Northwest Territories, Canada; Open File 7807; Geological Survey of Canada: Ottawa, ON, Canada, 2015; 19p.
- Hitzman, M.W.; Oreskes, N.; Einaudi, M.T. Geological characteristics and tectonic setting of proterozoic iron oxide (Cu U Au REE) deposits. Precam. Resear. 1992, 58, 241–287. [Google Scholar] [CrossRef]
- Barton, M.D.; Johnson, D.A. Evaporitic-source model for igneous-related Fe oxide–(REE-Cu-Au-U) mineralization. Geology 1996, 24, 259–262. [Google Scholar] [CrossRef]
- Montreuil, J.-F.; Corriveau, L.; Potter, E.G. On the relation between alteration signature and metal endowment of iron oxide alkali altered systems, southern Great Bear magmatic zone (Canada). Econ. Geol. 2016, 111, 2139–2168. [Google Scholar] [CrossRef]
- Hildebrand, R.S.; Hoffman, P.F.; Bowring, S.A. Tectono-magmatic evolution of the 1.9-Ga Great Bear magmatic zone, Wopmay Orogen, northwestern Canada. J. Volcan. Geotherm. Resear. 1987, 32, 99–118. [Google Scholar] [CrossRef]
- Hildebrand, R.S.; Hoffman, P.F.; Bowring, S.A. The Calderian orogeny in Wopmay orogen (1.9 Ga), northwestern Canadian Shield. Geol. Soc. Am. Bull. 2010, 122, 794–814. [Google Scholar] [CrossRef]
- Jackson, V.A.; van Breemen, O.; Ootes, L.; Bleeker, W.; Bennett, V.; Davis, W.J.; Ketchum, J.; Smar, L.; McFarlane, C. U–Pb zircon ages and field relationships of Archean basement and Proterozoic intrusions, south-central Wopmay Orogen, NWT: Implications for tectonic assignments 1, 2. Can. J. Earth Sc. 2013, 50, 979–1006. [Google Scholar] [CrossRef]
- Jackson, V.A.; Ootes, L.; Pierce, K.L.; Bennett, V.; Smar, L.; Mackay, D.; Sanderman, H.A. Geology of the South-Central Wopmay orogen, Northwest Territories (Parts of NTS 86B, 86C, and 86D); Results from the South Wopmay Bedrock Mapping Project; NWT Open File 2017-01; Northwest Territories Geological Survey: Yellowknife, NT, Canada, 2022; 103p.
- Hildebrand, R.S. Geological Synthesis of Northern Wopmay Orogen/Coppermine Homocline, Northwest Territories–Nunavut; Open File 6390, Open Report 2010-011; Northwest Territories Geological Survey: Yellowknife, NT, Canada; Geological Survey of Canada: Ottawa, ON, Canada, 2011.
- Jackson, V.A.; Irwin, D. Bedrock Geology of the Wopmay Orogen and Coppermine Homocline, Northwest Territories; NWT Open File 2021-02; Northwest Territories Geological Survey: Yellowknife, NT, Canada, 2022.
- Ootes, L.; Snyder, D.; Davis, W.J.; Acosta-Góngora, G.P.; Corriveau, L.; Mumin, A.H.; Montreuil, J.-F.; Gleeson, S.A.; Samson, I.A.; Jackson, V.A. A Paleoproterozoic Andean-type iron oxide copper-gold environment, the Great Bear magmatic zone, Northwest Canada. Ore Geol. Rev. 2017, 81, 123–139. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Prasad, N.; Charbonneau, B. Geological and Geophysical Signatures of a Large Polymetallic Exploration Target at Lou Lake, Southern Great Bear Magmatic Zone, Northwest Territories; Geological Survey of Canada: Ottawa, ON, Canada, 1996; pp. 147–158.
- Gandhi, S.S.; Potter, E.G.; Fayek, M. Polymetallic U-Ag Veins at Port Radium, Great Bear Magmatic Zone, Canada: Main Botryoidal Pitchblende Stage Cuts 1.74 Ga Diabase Dykes and Has REE Signatures Diagnostic of Unconformity-Type Deposits; Open File 7493; Geological Survey of Canada: Ottawa, ON, Canada, 2013; 1p.
- Potter, E.G.; Montreuil, J.-F.; DeToni, A. Geology and Hydrothermal Alteration of the Fab Lake Region, Northwest Territories; Open File 7339; Geological Survey of Canada: Ottawa, ON, Canada, 2013; 27p.
- Acosta-Góngora, G.P.; Gleeson, S.A.; Samson, I.M.; Ootes, L.; Corriveau, L. Trace element geochemistry of magnetite and its relationship to Cu-Bi-Co-Au-Ag-UW mineralization in the Great Bear magmatic zone, NWT, Canada. Econ. Geol. 2014, 109, 1901–1928. [Google Scholar] [CrossRef]
- DeToni, A.F. Systèmes à Oxydes de fer et Alterations en Élémeents Alcalins, Zone Magmatic du Grand Lac de l’Ours. Master’s Thesis, Centre–Eau Terre Environnement, Institut National de la Recherche Scientifique, Université du Québec, Québec, QC, Canada, 2016; 738p. [Google Scholar]
- Potter, E.; Acosta-Góngora, G.P.; Corriveau, L.; Montreuil, J.-F.; Yang, Z. Uranium enrichment processes in iron oxide and alkali-calcic alteration systems as revealed by uraninite trace element chemistry. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits, Special Paper 52; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Geological Association of Canada: St. John’s, NB, Canada, 2022; pp. 325–345. [Google Scholar]
- Goad, R.E.; Mumin, A.H.; Duke, N.; Neale, K.; Mulligan, D. Geology of the Proterozoic iron oxide-hosted, NICO cobalt-gold-bismuth, and Sue Dianne copper-silver deposits, southern Great Bear magmatic zone, Northwest Territories, Canada. In Hydrothermal Iron Oxide Copper-Gold and Related Deposits a Global Perspective Volume 1; Porter, T.M., Ed.; Porter Geoscience Consultancy Publishing: Adelaide, Australia, 2000; pp. 249–268. [Google Scholar]
- Goad, R.E.; Mumin, A.H.; Duke, N.A.; Neale, K.L.; Mulligan, D.L.; Camier, W.J. The NICO and Sue-Dianne Proterozoic, iron oxide-hosted, polymetallic deposits, Northwest Territories: Application of the Olympic Dam model in exploration. Explor. Min. Geol 2000, 9, 123–140. [Google Scholar] [CrossRef]
- Camier, W.J. The Sue-Dianne Fe-Oxide Cu-Ag-Au Breccia Complex, Southern Great Bear Magmatic Zone, Northwest Territories, Canada. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2002. [Google Scholar]
- Lypaczewski, P.; Normandeau, P.; Paquette, J.; McMartin, I. Petrographic and Cathodoluminescence Characterization of Apatite from the Sue-Dianne and Brooke IOCG Mineralization Systems, Great Bear Magmatic Zone, Northwest Territories; Open File 7319; Geological Survey of Canada: Ottawa, ON, Canada, 2013; 18p.
- Normandeau, P.X.; Harlov, D.E.; Corriveau, L.; Paquette, J.; McMartin, I. Characterization of fluorapatite within iron oxide alkali-calcic alteration systems of the Great Bear magmatic zone: A potential metasomatic process record. Can. Min. 2018, 56, 167–187. [Google Scholar] [CrossRef]
- Gandhi, S.S. Volcano-plutonic setting of U-Cu bearing magnetite veins of Fab claims, southern Great Bear magmatic zone, Northwest Territories. In Current Research, Paper 88-1C; Geological Survey of Canada: Ottawa, ON, Canada, 1988; pp. 177–188. [Google Scholar]
- Jackson, V.A. Preliminary Geologic Map of Part of the Southern Wopmay Orogen (Parts of NTS 86B and 86C; 2007 Updates); Descriptive Notes to Accompany 1:100 000 Scale Map; NWT Open Report 2008-007; Northwest Territories Geoscience Office: Yellowknife, NT, Canada, 2008.
- Normandeau, P.X.; McMartin, I. Composition of till and Bedrock across the Great Bear Magmatic Zone: Quaternary Field Database and Analytical Results from the GEM IOCG-Great Bear Project; Open File 7307; Geological Survey of Canada: Ottawa, ON, Canada, 2013; 22p.
- Lemmen, D.S.; Duk-Rodkin, A.; Bednarski, J.M. Late glacial drainage systems along the northwestern margin of the Laurentide Ice Sheet. Quat. Sci. Rev. 1994, 13, 805–828. [Google Scholar] [CrossRef]
- Smith, D.G. Glacial Lake McConnell: Paleogeography, age, duration, and associated river deltas, Mackenzie River basin, western Canada. Quat, Sci. Rev. 1994, 13, 829–843. [Google Scholar] [CrossRef]
- Aylsworth, J.M.; Shilts, W.W. Glacial Features around the Keewatin Ice Divide: Districts of Mackenzie and Keewatin; Paper 88-24; Geological Survey of Canada: Ottawa, ON, Canada, 1989; 21p.
- Fulton, R.J. Surficial Materials of Canada; Map 1880A, 1:5 000 000 scale; Geological Survey of Canada: Ottawa, ON, Canada, 1995; 1p.
- Dyke, A.S.; Prest, V.K. Late Wisconsinan and Holocene history of the Laurentide ice sheet. Géo. Phys. Quat. 1987, 41, 237–263. [Google Scholar] [CrossRef]
- Dyke, A.S.; Dredge, L. Quaternary geology of the northwestern Canadian Shield. In Geology of Canada Chapter 3 of Quaternary Geology of Canada and Greenland; Fulton, R.J., Ed.; Geological Survey of Canada: Ottawa, ON, Canada, 1989; Volume 1, pp. 189–214. [Google Scholar]
- Dyke, A.S. An outline of North American deglaciation with emphasis on central and northern Canada. Dev. Quat. Sci. 2004, 2, 373–424. [Google Scholar]
- Dalton, A.S.; Margold, M.; Stokes, C.R.; Tarasov, L.; Dyke, A.S.; Adams, R.S.; Allard, S.; Arends, H.E.; Atkinson, N.; Attig, J.W.; et al. An updated radiocarbon-based ice margin chronology for the last deglaciation of the North American Ice Sheet Complex. Quat. Sci. Rev. 2020, 234, 27. [Google Scholar] [CrossRef]
- Prest, V.K.; Grant, D.R.; Rampton, V.N. Glacial Map of Canada, Map 1253A; Geological Survey of Canada: Ottawa, ON, Canada, 1968.
- Clark, C.D. Mega-scale glacial lineations and cross-cutting ice-flow landforms. Earth Surf. Process. Landforms. 1993, 18, 1–29. [Google Scholar] [CrossRef]
- Kerr, D.E.; O’Neill, H.B. Reconnaissance Surficial Geology, Hardisty Lake, Northwest Territories, NTS 86-C; Canadian Geoscience Map 337 (preliminary), scale 1:125,000; Geological Survey of Canada: Ottawa, ON, Canada, 2018.
- Kerr, D.E.; O’Neill, H.B. Reconnaissance Surficial Geology, Rivière Grandin, Northwest Territories, NTS 86-D; Canadian Geoscience Map 361, scale 1:125,000; Geological Survey of Canada: Ottawa, ON, Canada, 2018.
- Kerr, D.E.; O’Neill, H.B. Reconnaissance Surficial Geology, Calder River, Northwest Territories, NT S 86-F; Canadian Geoscience Map 389, scale 1:125 000; Geological Survey of Canada: Ottawa, ON, Canada, 2019.
- Kerr, D.E.; O’Neill, H.B. Reconnaissance Surficial Geology, Leith Peninsula, Northwest Territories, NTS 86-E; Canadian Geoscience Map 409, scale 1:125,000; Geological Survey of Canada: Ottawa, ON, Canada, 2019.
- Kerr, D.E. Reconnaissance Surficial Geology, Sloan River, Northwest Territories–Nunavut, NTS 86-K; Canadian Geoscience Map 450, scale 1:125 000; Geological Survey of Canada: Ottawa, ON, Canada, 2022.
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Compagny: Boston, MA, USA, 1977; 688p. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; p. 359. [Google Scholar]
- Grunsky, E.C. The interpretation of geochemical survey data. Geochem. Explor. Environ. Anal. 2010, 10, 27–74. [Google Scholar] [CrossRef]
- Cole, D.R.; Rose, A.W. Distribution and mode of occurrence of zinc and lead in glacial soils. J. Geochem. Explor. 1984, 20, 137–160. [Google Scholar] [CrossRef]
- Tarvainen, T. The geochemical correlation between coarse and fine fractions of till in southern Finland. J. Geochem. Explor. 1995, 54, 187–198. [Google Scholar] [CrossRef]
- Peuraniemi, V.; Aario, R.; Pulkkinen, P. Mineralogy and geochemistry of the clay fraction of till in northern Finland. Sed. Geol. 1997, 111, 313–327. [Google Scholar] [CrossRef]
- Klassen, R.A. The interpretation of background variation in regional geochemical surveys–an example from Nunavut, Canada. Geochem. Explor. Environ. Anal. 2001, 1, 163–173. [Google Scholar] [CrossRef]
- Shilts, W.W. Principles of geochemical exploration for sulphide deposits using shallow samples of glacial drift. Can. Inst. Min. Metall. Bull. 1975, 68, 73–80. [Google Scholar]
- Niskavaara, H. A Comprehensive Scheme of Analysis for Soils, Sediments, Humus and Plant Samples Using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES): Current Research; Geological Survey of Finland: Romaniemi, Finland, 1995; Volume 20, pp. 167–175. [Google Scholar]
- Koljonen, T.; Malisa, E. Solubility in aqua regia of selected chemical elements occurring in the fine fraction of till till. In Environmental Geochemistry in Northern Europe, Special Paper 9; Pulkkinen, E., Ed.; Geological Survey of Finland: Romaniemi, Finland, 1991; pp. 49–52. [Google Scholar]
- Shilts, W.W. Drift exploration. In Glacial Environments, Sediment Forms and Techniques; Menzies, J., Ed.; Butterworth Heinemann Ltd.: Oxford, UK, 1996; pp. 411–439. [Google Scholar]
- Foster, J.R. The efficiency of various digestion procedures on the extraction of metals from rocks and rock forming minerals. Can. Inst. Min. Bull. 1973, 66, 85–92. [Google Scholar]
- Snäll, S.; Liljefors, T. Leachability of major elements from minerals in strong acids. J. Geochem. Explor. 2000, 71, 1–12. [Google Scholar] [CrossRef]
- Dreimanis, A.; Vagners, U. The dependence of the composition of till upon the rule of bimodal composition. INQUA VIII Internat. Cong. Gener. Sess 1971, 787–789. [Google Scholar]
- Lahtinen, R.; Lestinen, P.; Savolainen, H. The Use of Total and Partial Dissolution till Geochemical Data in Delineating Favourable Areas for Ni Prospects: An Example from the Tampere–Hämeenlinna Area, Southern Finland; Special Paper 18; Geological Survey of Finland: Romaniemi, Finland, 1993; pp. 101–111. [Google Scholar]
- McMartin, I. Till Provenance across the Terminus of the Dubawnt Lake Ice Stream, Central Nunavut; Current Research 2017-1; Geological Survey of Canada: Ottawa, ON, Canada, 2017; 13p.
- Mäkinen, J. Effects of grinding and chemical factors on the generation and composition of the till fine fraction: An experimental study. J. Geochem. Explor. 1995, 54, 49–62. [Google Scholar]
- Sanford, R.F.; Pierson, C.T.; Crovelli, R.A. An objective replacement method for censored geochemical data. Mathem. Geol. 1993, 25, 59–80. [Google Scholar] [CrossRef]
- Thompson, M.; Howarth, R.J. Duplicate analysis in geochemical practice, part 1. Theoretical approach and estimation of analytical reproductubility. Analyst 1973, 101, 690–698. [Google Scholar] [CrossRef]
- R_Project. The R Project for Statistical Computing. 2016. Available online: www.r-project.org/ (accessed on 4 March 2011).
- Garrett, R. The GSC Applied Geochemistry EDA Package. Version 1.12. Available online: http://gsc.nrcan.gc.ca/dir/index_e.php?id=4961 (accessed on 4 March 2011).
- Garrett, R.G. The ‘rgr’ package for the R Open Source statistical computing and graphics environment-a tool to support geochemical data interpretation. Geochem. Explor. Environ. Anal. 2013, 13, 355–378. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks on one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2013; 828p. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Gibbons, J. Nonparametric Methods for Quantitative Analysis, 3rd ed.; Rinehart and Winston: New York, NY, USA, 1997; 853p. [Google Scholar]
- Kloke, J.D.; McKean, J.W. Rfit: Rank-based estimation for linear models. R J. 2012, 4, 57–64. [Google Scholar] [CrossRef]
- Turner, T.R. Estimating the propagation rate of a viral infection of potato plants via mixtures of regressions. J. R. Stat. Soc. Ser. C Appl. Stat. 2000, 49, 371–384. [Google Scholar] [CrossRef]
- Everitt, B.S.; Hand, D.J. Finite Mixture Distributions: Monographs on Applied Probability and Statistics; Chapman and Hall: London, UK, 1981; 85p. [Google Scholar]
- Titterington, D.M.; Smith, A.F.; Makov, U.E. Statistical Analysis of Finite Mixture Distributions; Wiley: New York, NY, USA, 1985; 243p. [Google Scholar]
- Harlov, D.E.; Förster, H.-J. Fluid-induced nucleation of (Y+ REE)-phosphate minerals within apatite: Nature and experiment. Part II. Fluorapatite. Am. Min. 2004, 88, 1209–1229. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Förster, H.-J. An experimental study of dissolution–reprecipitation in fluorapatite: Fluid infiltration and the formation of monazite. Contr. Min. Petro. 2005, 150, 268–286. [Google Scholar] [CrossRef]
- Budzyń, B.; Harlov, D.E.; Williams, M.L.; Jercinovic, M.J. Experimental determination of stability relations between monazite, fluorapatite, allanite, and REE-epidote as a function of pressure, temperature, and fluid composition. Am. Min. 2011, 96, 1547–1567. [Google Scholar] [CrossRef]
- Harlov, D.E.; Andersson, U.B.; Förster, H.-J.; Nyström, J.O.; Dulski, P.; Broman, C. Apatite–monazite relations in the Kiirunavaara magnetite–apatite ore, northern Sweden. Chem. Geol. 2002, 191, 47–72. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Hetherington, C.J. The relative stability of monazite and huttonite at 300–900 C and 200–1000 MPa: Metasomatism and the propagation of metastable mineral phases. Am. Min. 2007, 92, 1652–1664. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Davidson, G.J.; Mehrabi, B.; Meffre, S.; Ghazban, F. Significance of apatite REE depletion and monazite inclusions in the brecciated Se–Chahun iron oxide–apatite deposit, Bafq district, Iran: Insights from paragenesis and geochemistry. Chem. Geol. 2011, 281, 253–269. [Google Scholar] [CrossRef]
- Normandeau, P.X.; McMartin, I.; Paquette, J.; Corriveau, L.; Montreuil, J.-F. Geochemical signatures of IOCG mineralization and alteration in till. In Proceedings of the Goldschmidt 2012, Conference Abstracts, Montréal, QC, Canada, 23–28 June 2012. [Google Scholar]



| System | Till | Bedrock | |||||
|---|---|---|---|---|---|---|---|
| Down-Ice * | Up-Ice ** | Background | Total | Mineralized | Non-Mineralized | Total | |
| Sue Dianne | 10 | 1 | 19 | 30 | 25 | 63 | 88 |
| Fab | 13 | 2 | 8 | 23 | 10 | 20 | 30 |
| Other MIAC systems | 25 | - | - | 25 | 72 | 302 | 374 |
| GBMZ host rock | - | - | 9 | 9 | - | 215 | 215 |
| Slave Craton and Wopmay metamorphic zone | - | - | 5 | 5 | - | - | - |
| Population Tested | Na | K | Ca | V | Cr | Mn | Fe | Co | Ni | Cu | As | Mo | La | Yb | W | Bi | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineralized samples vs. GBMZ host rock | L | - | - | - | - | - | H | H | H | H | H | H | - | - | H | H | L | H |
| All of Sue Dianne system vs. GBMZ host rock | H | H | L | - | - | - | - | - | L | - | H | - | H | - | H | H | H | H |
| Mineralized vs. non-mineralized samples in the Sue Dianne system | L | - | - | - | - | - | H | H | - | H | - | H | - | L | H | H | L | H |
| All of Fab system samples vs. GBMZ host rock | L | - | H | - | - | - | - | - | - | - | H | - | H | H | - | - | H | H |
| Mineralized vs. non-mineralized samples in the Fab system | - | - | - | - | - | - | - | - | - | H | - | - | - | - | - | - | - | - |
| Setting | Sample(s) | Na | K | Ca | Fe | Co | La | Yb | Th | U | W * | Ni | Cu | As | Mo | Bi | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |||
| ICP-MS after a 4-Acid Digestion | ICP-MS after an Aqua Regia Digestion | ||||||||||||||||
| <0.063 mm Fractions | <0.002 mm Fraction | ||||||||||||||||
| MIAC showings within the GBMZ | Median (n = 25) | 20,100 | 25,600 | 10,400 | 21,800 | 8.5 | 37.4 | 1.7 | 15.6 | 3.8 | 0.70 | 43.4 | 81.3 | 8.6 | 1.2 | 1.9 | |
| Hottah Lake | median (n = 8) | 22,085 | 26,250 | 14,400 | 21,250 | 8.2 | 40.6 | 1.8 | 15.3 | 3.0 | 0.70 | 33.4 | 62.9 | 6.1 | 0.7 | 1.4 | |
| Stew Zone (NICO area) | 09MOB037 | 19,180 | 20,100 | 10,400 | 25,700 | 9.3 | 36.0 | 1.2 | 14.6 | 3.8 | 0.50 | 61.0 | 42.2 | 8.3 | 0.4 | 1.2 | |
| LCL | 09MOB043 | 23,390 | 22,400 | 12,200 | 19,200 | 12.2 | 30.3 | 1.4 | 13.7 | 2.8 | 0.50 | 85.3 | 85.2 | 25.7 | 1.0 | 1.5 | |
| UR | 09MOB041 | 20,420 | 22,200 | 10,000 | 18,500 | 5.6 | 31.3 | 2.0 | 22.9 | 7.1 | 0.90 | 27.2 | 55.3 | 12.6 | 3.4 | 2.4 | |
| Dry | 10CQA2061A2 | 14,520 | 25,600 | 8400 | 20,900 | 9.3 | 41.6 | 2.0 | 15.6 | 5.6 | 0.20 | 41.9 | 75.0 | 6.8 | 1.5 | 2.7 | |
| DV-3 | 10CQA2034A2 | 15,830 | 29,200 | 9600 | 49,400 | 16.1 | 69.9 | 2.7 | 30.6 | 6.4 | 0.50 | 49.0 | 82.1 | 19.7 | 1.9 | 1.0 | |
| DV-8 | 10CQA2031A2 | 18,480 | 29,800 | 10,500 | 36,600 | 12.6 | 77.2 | 2.4 | 29.6 | 6.9 | 0.90 | 53.7 | 124.6 | 29.5 | 4.0 | 2.0 | |
| Grouard Lake South | 10CQA2041A2 | 16,650 | 29,700 | 10,200 | 33,400 | 10.6 | 83.4 | 4.3 | 34.1 | 16.0 | 2.70 | 34.9 | 274.5 | 6.3 | 4.6 | 3.4 | |
| Jackpot-McPhoo | 10CQA2052A2 | 16,360 | 23,200 | 8700 | 36,500 | 14.0 | 33.7 | 1.4 | 12.2 | 3.8 | 0.20 | 54.2 | 44.8 | 16.4 | 1.7 | 2.1 | |
| Jackpot-McPhoo | 10CQA2055A2 | 18,230 | 22,700 | 9800 | 29,200 | 12.6 | 41.1 | 1.5 | 13.5 | 4.1 | 0.20 | 62.7 | 85.9 | 14.4 | 2.1 | 1.6 | |
| MRB | 09MOB032 | 20,340 | 23,500 | 11,100 | 16,700 | 5.8 | 26.6 | 1.3 | 10.6 | 2.3 | 0.40 | 61.0 | 81.3 | 11.7 | 0.6 | 0.9 | |
| HS-1 (NICO area) | 09MOB030 | 19,690 | 24,500 | 9800 | 29,900 | 13.0 | 36.1 | 1.2 | 18.9 | 2.5 | 0.90 | 83.1 | 161.2 | 42.1 | 0.7 | 2.6 | |
| OUR (NICO area) | 09MOB036 | 22,860 | 20,200 | 12,100 | 17,600 | 7.0 | 36.3 | 1.2 | 13.2 | 2.1 | 0.40 | 50.1 | 128.3 | 37.2 | 1.6 | 2.8 | |
| Lou Lake (NICO area) | 09MOB038 | 20,100 | 21,500 | 8600 | 20,200 | 6.0 | 29.1 | 1.1 | 11.8 | 2.3 | 0.20 | 30.9 | 36.7 | 12.0 | 0.9 | 1.7 | |
| Rainy Lake 1 (Terra mine area) | 10CQA2046A2 | 19,590 | 27,200 | 9700 | 25,800 | 9.0 | 32.0 | 1.7 | 13.0 | 3.0 | 0.80 | 49.3 | 175.2 | 25.9 | 2.4 | 3.5 | |
| UR | 09MOB040 | 18,870 | 22,000 | 8000 | 22,900 | 7.8 | 45.8 | 2.5 | 32.7 | 17.7 | 1.10 | 28.9 | 59.4 | 8.6 | 0.6 | 1.6 | |
| Torrie Lake-Unnamed | 10CQA2059A2 | 15,080 | 26,900 | 8100 | 29,200 | 10.6 | 56.5 | 2.5 | 20.4 | 8.9 | 0.50 | 47.2 | 170.3 | 6.9 | 1.2 | 2.8 | |
| Tatie | 09MOB045 | 17,230 | 25,600 | 8400 | 19,400 | 5.3 | 31.6 | 2.0 | 16.9 | 6.8 | 0.90 | 20.1 | 29.4 | 5.5 | 1.2 | 1.3 | |
| GBMZ background and other samples | |||||||||||||||||
| GBMZ background | Median (n = 9) | 18,840 | 24,900 | 11,400 | 25,700 | 8.7 | 41.0 | 2.0 | 16.0 | 3.3 | 0.60 | 38.2 | 76.0 | 8.8 | 1.1 | 1.6 | |
| Standard dev. | 2332 | 2819 | 1848 | 4268 | 2.1 | 5.6 | 0.3 | 2.5 | 2.4 | 0.30 | 9.2 | 24.8 | 3.8 | 1.7 | 0.3 | ||
| Slave Craton and east of the Wopmay fault zone | Median (n = 5) | 23,760 | 23,200 | 13,400 | 25,100 | 9.0 | 34.0 | 1.0 | 16.0 | 3.0 | 0.2 | 51.0 | 66.0 | 10.0 | 1.0 | 0.9 | |
| Sue Dianne deposits and local background | Median (n = 30) | 21,520 | 22,750 | 11,100 | 24,000 | 11.2 | 38.2 | 1.3 | 16.2 | 3.0 | 0.20 | 67.1 | 88.1 | 10.8 | 0.8 | 0.9 | |
| Down-ice (<200 m) | 09MOB002 | 7060 | 13,600 | 4100 | 28,100 | 5.4 | 34.3 | 1.3 | 10.5 | 6.9 | 0.30 | 20.0 | 1189.0 | 9.1 | 12.1 | 4.5 | |
| Down-ice (<500 m) | 09MOB021 | 21,580 | 25,500 | 12,400 | 31,800 | 15.0 | 43.0 | 1.3 | 16.7 | 3.8 | 0.20 | 77.4 | 114.9 | 8.6 | 0.7 | 0.7 | |
| Down-ice (<500 m) | 09MOB022 | 12,490 | 23,800 | 9100 | 52,200 | 43.2 | 60.4 | 1.7 | 16.5 | 9.4 | 0.60 | 93.8 | 347.9 | 14.9 | 2.3 | 2.8 | |
| Down-ice (<500 m) | 09MOB023 | 23,660 | 23,500 | 14,000 | 23,800 | 9.6 | 37.9 | 1.6 | 14.9 | 3.1 | 0.20 | 68.0 | 103.1 | 9.5 | 0.3 | 0.9 | |
| Down-ice (<500 m) | 09MOB024 | 21,790 | 24,000 | 12,600 | 30,600 | 14.9 | 41.7 | 1.3 | 18.5 | 4.2 | 0.20 | 73.5 | 173.1 | 9.5 | 0.6 | 1.0 | |
| Down-ice (<1000 m) | 09MOB025 | 22,590 | 23,500 | 14,100 | 24,000 | 11.0 | 32.1 | 1.4 | 13.0 | 2.3 | 0.20 | 48.8 | 51.4 | 7.9 | 0.6 | 1.0 | |
| Down-ice (<1000 m) | 09MOB026 | 18,400 | 21,800 | 9600 | 35,600 | 12.7 | 30.9 | 1.1 | 10.5 | 2.3 | 0.30 | 50.3 | 80.8 | 26.8 | 2.4 | 2.2 | |
| Down-ice (<1000 m) | 09MOB027 | 18,940 | 23,300 | 10,500 | 31,500 | 13.2 | 40.0 | 1.3 | 17.9 | 3.1 | 0.30 | 74.2 | 92.9 | 12.9 | 0.7 | 1.8 | |
| Down-ice (<1000 m) | 09MOB028 | 20,190 | 24,300 | 10,800 | 22,700 | 8.9 | 43.6 | 1.3 | 15.9 | 3.0 | 0.20 | 54.9 | 49.3 | 8.0 | 0.5 | 0.7 | |
| Down-ice (<1000 m) | 09MOB029 | 16,400 | 27,800 | 9000 | 33,500 | 15.0 | 34.4 | 1.4 | 15.5 | 3.0 | 0.40 | 74.6 | 95.5 | 13.5 | 2.0 | 1.1 | |
| Up-ice (<200 m) | 09MOB001 | 21,810 | 20,400 | 11,300 | 21,100 | 12.1 | 32.1 | 1.2 | 17.6 | 2.4 | 0.30 | 138.9 | 111.2 | 28.5 | 1.7 | 1.4 | |
| Sue Dianne deposit local background | |||||||||||||||||
| Marian River Batholith | Median (n = 7) | 21,700 | 22,000 | 12,000 | 22,500 | 8.4 | 31.9 | 1.2 | 15.7 | 3.0 | 0.20 | 62.7 | 69.3 | 9.6 | 0.8 | 0.8 | |
| Undifferentiated porphyry | Median (n = 9) | 21,640 | 22,600 | 10,900 | 23,300 | 9.8 | 34.9 | 1.2 | 16.5 | 2.5 | 0.20 | 57.1 | 85.3 | 10.9 | 0.8 | 1.0 | |
| Undifferentiated volcanic | Median (n = 3) | 21,460 | 21,500 | 12,100 | 23,300 | 11.3 | 44.0 | 1.6 | 18.7 | 3.3 | 0.30 | 70.9 | 90.6 | 14.8 | 1.0 | 0.9 | |
| Fab system and local background | Median (n = 23) | 21,520 | 22,750 | 11,100 | 24,000 | 11.2 | 38.2 | 1.3 | 16.2 | 3.0 | 0.50 | 62.4 | 78.2 | 9.6 | 2.0 | 1.0 | |
| Down-ice (<200 m) | 09MOB042 | 11,780 | 13,400 | 10,200 | 51,600 | 13.9 | 40.2 | 1.8 | 17.8 | 8.1 | 1.30 | 27.6 | 57.1 | 17.0 | 17.4 | 3.0 | |
| Down-ice (<250 m) | 10CQA2016A2 | 18,430 | 26,000 | 11,900 | 34,500 | 13.3 | 56.8 | 1.9 | 21.5 | 5.1 | 0.50 | 65.4 | 435.4 | 12.4 | 2.7 | 1.1 | |
| Down-ice (<250 m) | 10CQA2019A2 | 8250 | 13,300 | 5000 | 22,800 | 7.5 | 44.9 | 1.5 | 19.4 | 9.8 | 0.90 | 24.2 | 94.1 | 4.8 | 4.9 | 0.9 | |
| Down-ice (<250 m) | 10CQA2021A2 | 5320 | 11,800 | 3700 | 11,700 | 2.9 | 26.2 | 1.1 | 9.5 | 4.2 | 1.40 | 6.7 | 318.1 | 3.4 | 7.7 | 0.7 | |
| Down-ice (<300 m) | 10CQA2007A2 | 17,300 | 27,000 | 10,800 | 28,300 | 8.6 | 49.6 | 1.9 | 18.8 | 4.6 | 0.30 | 48.4 | 61.5 | 7.0 | 7.9 | 0.8 | |
| Down-ice (<500 m) | 10CQA2003A2 | 17,110 | 24,300 | 10,900 | 28,800 | 9.7 | 40.1 | 1.4 | 15.7 | 5.7 | 0.40 | 43.3 | 43.9 | 4.6 | 5.6 | 1.1 | |
| Down-ice (<500 m) | 10CQA2004A2 | 17,580 | 23,100 | 10,800 | 32,900 | 13.9 | 33.8 | 1.2 | 13.9 | 2.9 | 0.50 | 67.8 | 78.2 | 19.4 | 3.4 | 1.3 | |
| Down-ice (<500 m) | 10CQA2008A2 | 20,810 | 28,100 | 13,900 | 29,600 | 11.7 | 51.3 | 2.0 | 19.0 | 3.6 | 0.70 | 69.0 | 130.3 | 16.8 | 3.9 | 2.2 | |
| Down-ice (<500 m) | 10CQA2015A2 | 9410 | 14,600 | 7200 | 62,500 | 35.7 | 31.5 | 1.1 | 15.1 | 3.8 | 1.30 | 36.8 | 68.6 | 9.2 | 14.7 | 2.8 | |
| Down-ice (<2000 m) | 10CQA2009 | 16,060 | 25,600 | 12,950 | 43,800 | 19.9 | 53.0 | 1.6 | 22.5 | 8.9 | 0.50 | 62.4 | 46.9 | 6.2 | 0.9 | 0.6 | |
| Down-ice (<2000 m) | 10CQA2011A2 | 18,500 | 24,100 | 11,100 | 28,700 | 9.8 | 31.6 | 1.3 | 12.5 | 2.4 | 0.40 | 65.6 | 46.8 | 14.8 | 2.3 | 1.1 | |
| Down-ice (<2000 m) | 10CQA2012A1 | 20,540 | 27,000 | 14,300 | 25,900 | 11.4 | 36.9 | 1.6 | 15.5 | 2.9 | 0.50 | 51.8 | 27.2 | 5.2 | 2.0 | 0.7 | |
| Down-ice (<2000 m) | 10CQA2022A2 | 16,370 | 29,400 | 13,100 | 37,100 | 18.0 | 65.0 | 2.2 | 22.8 | 9.2 | 0.30 | 64.8 | 57.8 | 5.3 | 0.5 | 0.6 | |
| Up-ice (<500 m) | Average (n = 2) | 20,595 | 24,850 | 12,700 | 29,000 | 12.3 | 59.1 | 2.2 | 19.4 | 4.5 | 0.45 | 56.2 | 66.0 | 8.2 | 0.9 | 0.8 | |
| Fab system local background | |||||||||||||||||
| Undifferentiated porphyry | Median (n = 3) | 19,390 | 25,600 | 13,900 | 26,900 | 11.1 | 71.2 | 2.1 | 27.3 | 5.6 | 0.60 | 61.1 | 111.7 | 17.0 | 2.0 | 1.2 | |
| Undifferentiated granite | Median (n = 5) | 17,760 | 28,000 | 10,500 | 39,600 | 17.3 | 51.7 | 1.6 | 22.1 | 5.3 | 0.30 | 77.1 | 96.3 | 10.2 | 1.5 | 1.0 | |
| Population Tested | Digestion Method and Size Fraction | Na | K | Ca | Fe | Co | Ni | Cu | As | Mo | La | Yb | W | Bi | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample collected down-ice of all showings or deposits (from <200 m to <2000 m), tested against all background samples | aqua regia, <0.063 mm | na | H | - | - | - | - | - | na | - | - | H | H | - | - | |
| 4 acid, <0.063 mm | L | - | - | - | - | - | - | - | na | - | - | na | - | - | H | |
| aqua regia, <0.002 mm | - | - | H | - | L | L | - | - | - | - | na | na | H | L | - | |
| Samples collected down-ice of the Sue Dianne deposit (from <200 m to <1000 m), tested against the local background | aqua regia, <0.063 mm | na | H | - | H | - | - | H | H | na | - | - | - | H | - | - |
| 4 acid, <0.063 mm | L | H | - | H | - | - | H | - | na | - | - | na | na | - | - | |
| aqua regia, <0.002 mm | L | H | H | H | - | - | - | - | - | - | na | na | H | L | - | |
| Samples collected down-ice of the Fab system (from <500 m to <2000 m, tested against the local background | aqua regia, <0.063 mm | na | - | - | - | - | - | - | - | na | L | L | - | - | L | - |
| 4 acid, <0.063 mm | - | - | - | - | - | - | - | - | na | L | - | na | na | L | - | |
| aqua regia, <0.002 mm | L | L | - | - | L | L | L | L | - | L | na | na | - | L | L |
| Rank Based Regression Analysis | K | Ca | Fe | Co | Ni | Cu | As | La | Th | U | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| All samples (n = 92) | <0.60 | >0.99 | 0.66 | >0.99 | 0.99 | >0.99 | 0.99 | >0.99 | 0.98 | >0.99 | 0.99 | >0.99 | 0.88 | >0.99 | 0.91 | >0.99 | 0.88 | >0.99 | 0.97 | >0.99 |
| GBMZ background (n = 9) | <0.60 | <0.95 | <0.6 | <0.95 | 1.00 | >0.99 | 1.00 | >0.99 | 0.95 | >0.99 | 0.95 | >0.99 | 0.83 | >0.99 | 0.78 | >0.99 | 0.92 | >0.99 | 0.98 | >0.99 |
| Within other MIAC system (n = 25) | <0.60 | 0.99 | 0.61 | >0.99 | 0.98 | >0.99 | 0.97 | >0.99 | 0.94 | >0.99 | 0.98 | >0.99 | 0.77 | >0.99 | 0.96 | >0.99 | 0.97 | >0.99 | 0.98 | >0.99 |
| Sue Dianne deposit local background (n = 19) | <0.60 | 0.99 | 0.84 | >0.99 | 0.97 | >0.99 | 0.98 | >0.99 | 0.98 | >0.99 | 0.99 | >0.99 | 0.95 | >0.99 | 0.94 | >0.99 | 0.91 | >0.99 | 0.96 | >0.99 |
| Down-ice of Sue Dianne deposit (n = 10) | 0.62 | 0.97 | <0.6 | <0.95 | 0.98 | >0.99 | 0.96 | >0.99 | 1.00 | >0.99 | 1.00 | >0.99 | 1.00 | >0.99 | 0.92 | >0.99 | 0.87 | >0.99 | 0.99 | >0.99 |
| Fab system local background (n = 8) | 0.62 | <0.95 | 0.99 | >0.99 | 1.00 | >0.99 | 0.96 | >0.99 | 1.00 | >0.99 | 0.99 | >0.99 | 0.95 | >0.99 | 0.95 | >0.99 | 0.88 | >0.99 | 0.99 | >0.99 |
| Down-ice of Fab system (n = 13) | 0.83 | >0.99 | 0.85 | >0.99 | 0.98 | >0.99 | 0.96 | >0.99 | 0.98 | >0.99 | 0.93 | >0.99 | 0.86 | >0.99 | 0.97 | >0.99 | 0.86 | >0.99 | 1.00 | >0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by His Majesty the King in Right of Canada, as presented by the Minister of Natural Resources. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Normandeau, P.X.; McMartin, I.; Corriveau, L. Till Geochemistry as a Vector to Metasomatic Iron and Alkali-Calcic Systems and Associated Deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada. Minerals 2024, 14, 547. https://doi.org/10.3390/min14060547
Normandeau PX, McMartin I, Corriveau L. Till Geochemistry as a Vector to Metasomatic Iron and Alkali-Calcic Systems and Associated Deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada. Minerals. 2024; 14(6):547. https://doi.org/10.3390/min14060547
Chicago/Turabian StyleNormandeau, Philippe X., Isabelle McMartin, and Louise Corriveau. 2024. "Till Geochemistry as a Vector to Metasomatic Iron and Alkali-Calcic Systems and Associated Deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada" Minerals 14, no. 6: 547. https://doi.org/10.3390/min14060547
APA StyleNormandeau, P. X., McMartin, I., & Corriveau, L. (2024). Till Geochemistry as a Vector to Metasomatic Iron and Alkali-Calcic Systems and Associated Deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada. Minerals, 14(6), 547. https://doi.org/10.3390/min14060547








