Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings
Abstract
1. Introduction
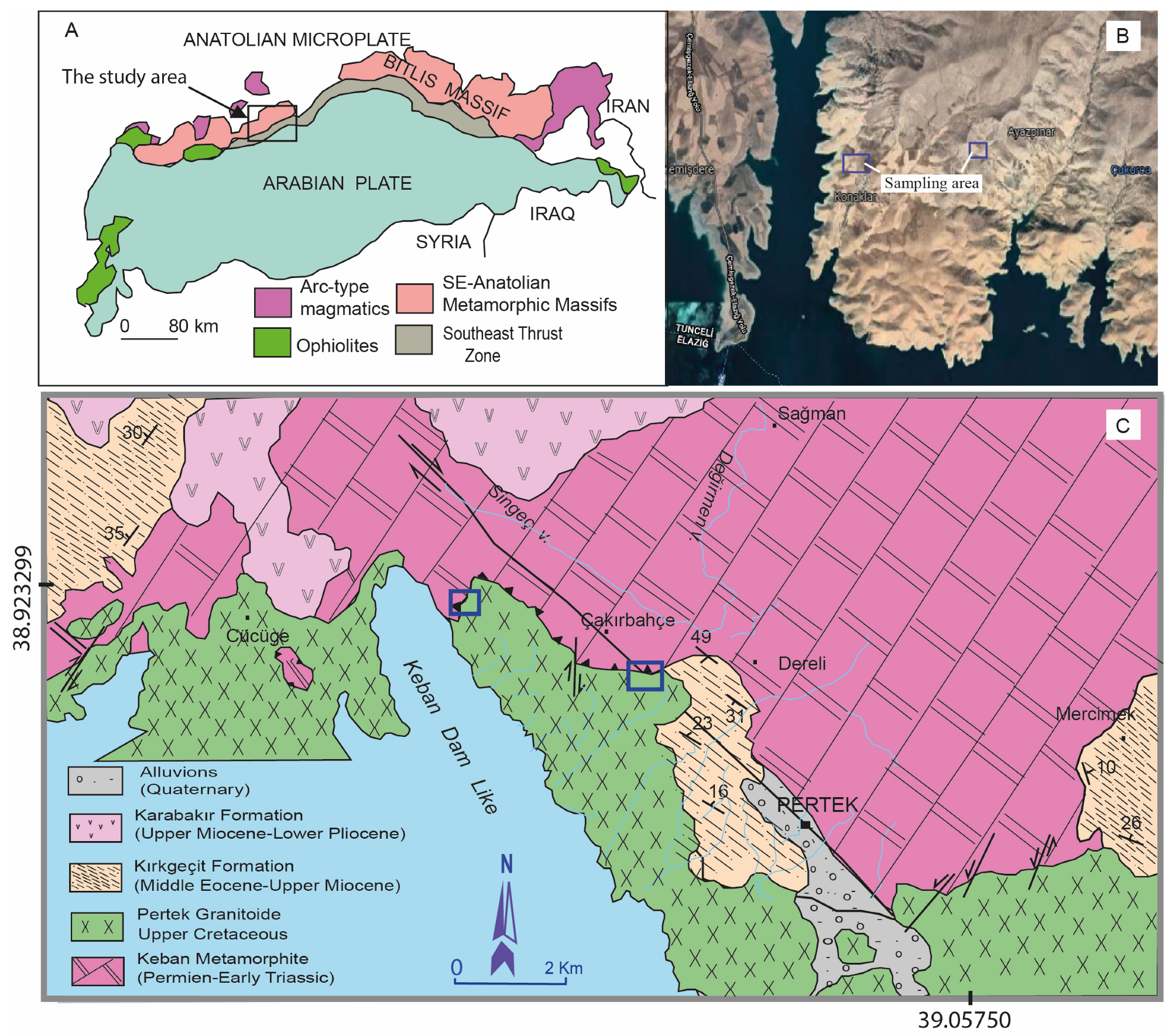
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Mineralization
4.2. Geochemistry
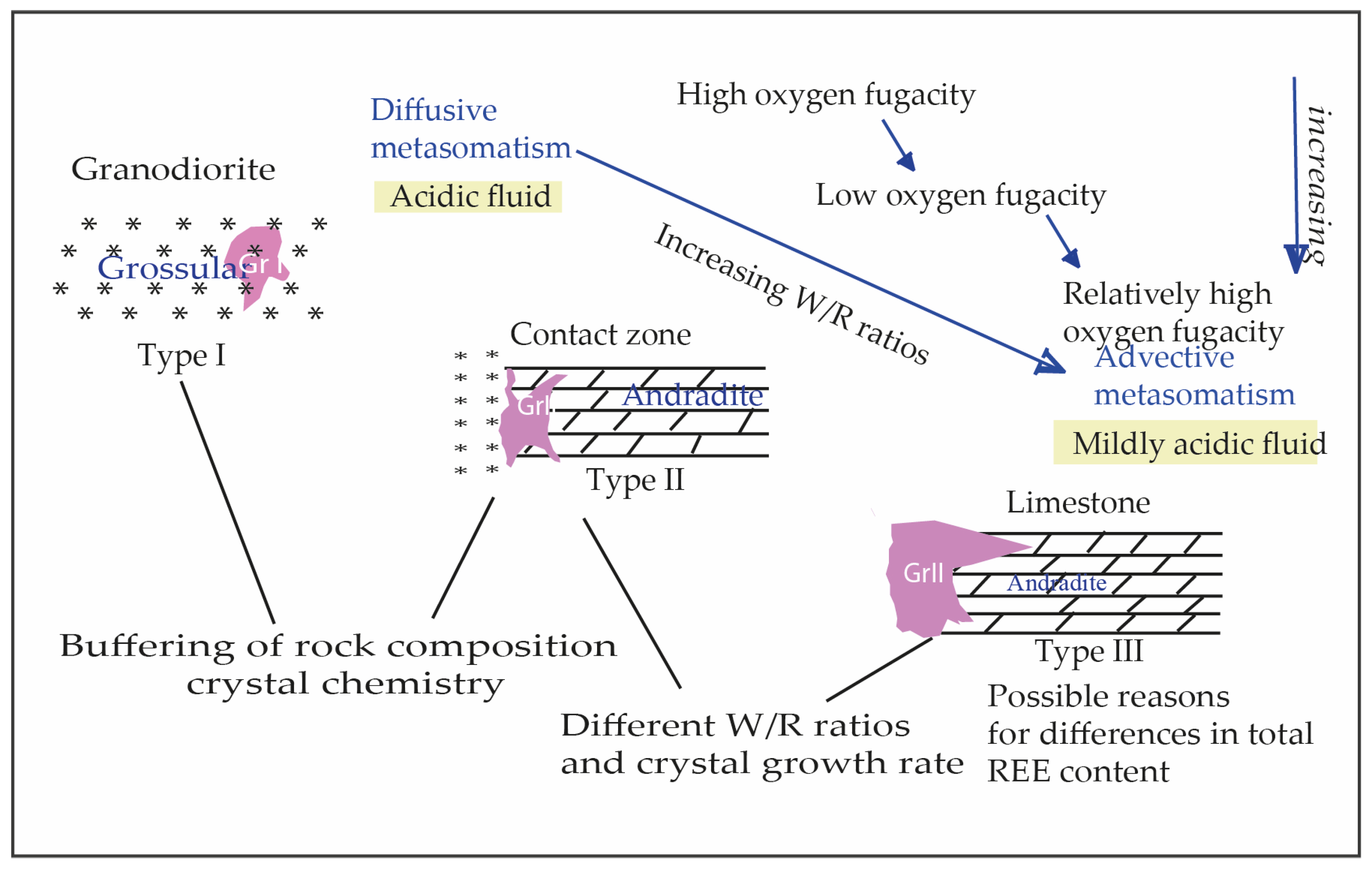
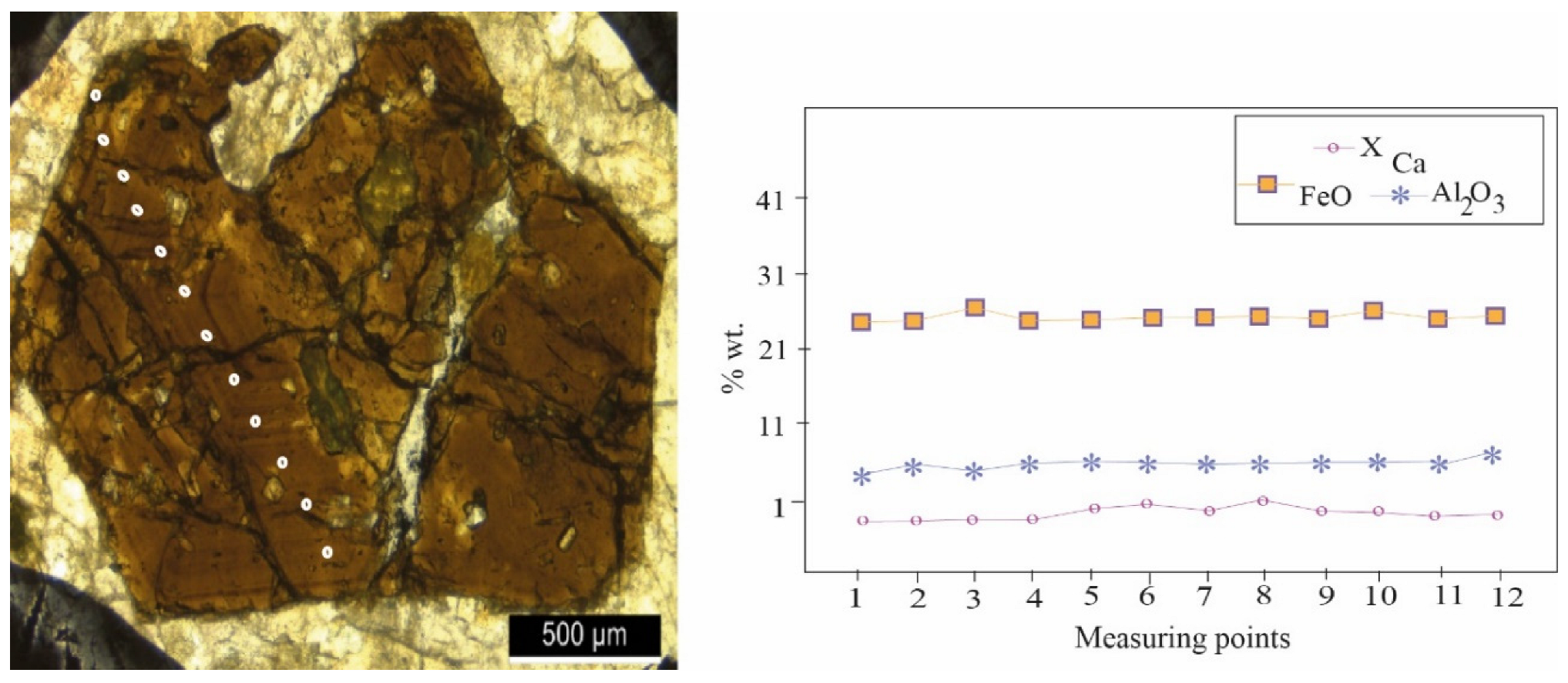
4.2.1. Zoning
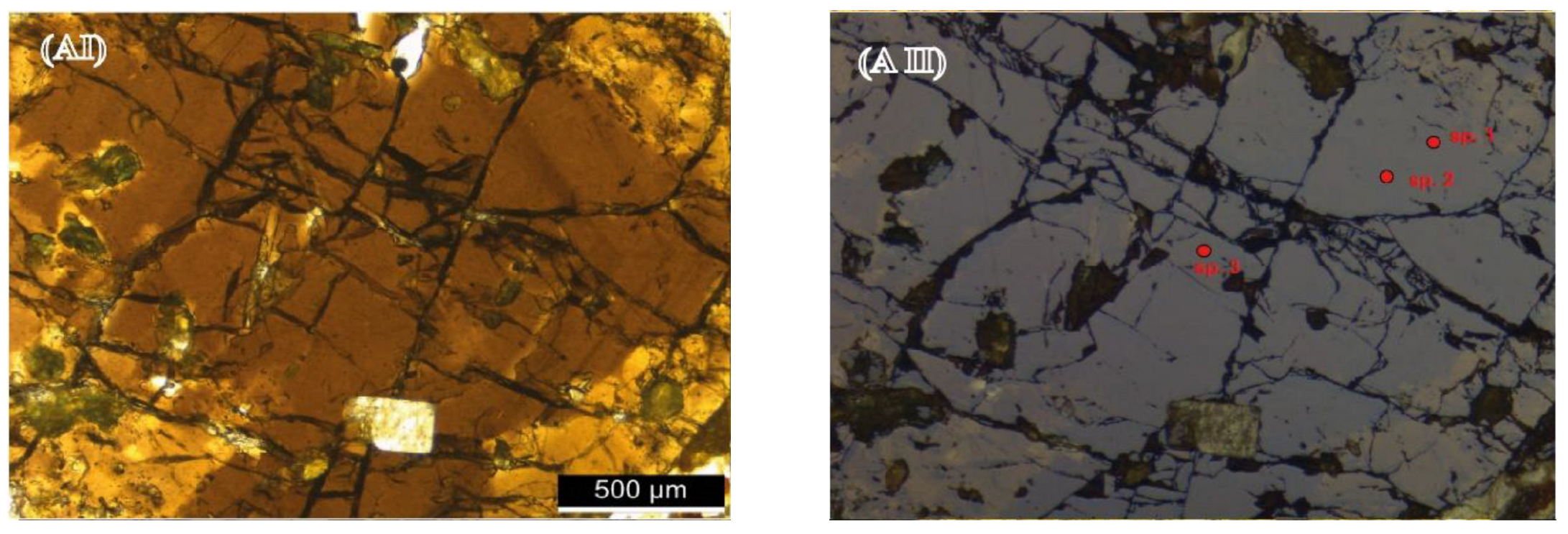
4.2.2. Inclusions
5. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basu, A.; Faggert, B.; Sharma, M. Sm–Nd isotopic study of wollastonite skarn and garnet amphibolite metamorphism in the Adirondack Mountains. Eos 1988, 69, 468. [Google Scholar]
- Lee, C.-T.A.; Shen, B.; Slotnick, B.S.; Liao, K.; Dickens, G.R.; Yokoyama, Y.; Lenardic, A.; Dasgupta, R.; Jellinek, M.; Lackey, J.S. Continental arc–island arc fluctuations, growth of crustal carbonates, and long-term climate change. Geosphere 2013, 9, 21–36. [Google Scholar] [CrossRef]
- Blackburn, T.J.; Stockli, D.F.; Walker, J.D. Magnetite (U–Th)/He dating and its application to the geochronology of intermediate to mafic volcanic rocks. Earth Planet. Sci. Lett. 2007, 259, 360–371. [Google Scholar] [CrossRef]
- Blackburn, T.J. Development of new applications in volcanic (U-Th)/He geochronology. Ph.D. Thesis, University of Kansas, Lawrence, KS, USA, 2006. [Google Scholar]
- Lal, N.; Nagpaul, K.; Sharma, K. Fission-track ages and uranium concentration in garnets from Rajasthan, India. Geol. Soc. Am. Bull. 1976, 87, 687–690. [Google Scholar] [CrossRef]
- Bowring, J.; McLean, N.M.; Bowring, S. Engineering cyber infrastructure for U-Pb geochronology: Tripoli and U-Pb Redux. Geochem. Geophys. Geosyst. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Chiaradia, M.; Schaltegger, U.; Spikings, R.; Wotzlaw, J.F.; Ovtcharova, M. How accurately can we date the duration of magmatic-hydrothermal events in porphyry systems?—An invited paper. Econ. Geol. 2013, 108, 565–584. [Google Scholar] [CrossRef]
- Caddick, M.J.; Konopásek, J.; Thompson, A.B. Preservation of garnet growth zoning and the duration of prograde metamorphism. J. Petrol. 2010, 51, 2327–2347. [Google Scholar] [CrossRef]
- Lee, C.-T.A.; Lackey, J.S. Global continental arc flare-ups and their relation to long term greenhouse conditions. Elements 2015, 11, 125–130. [Google Scholar] [CrossRef]
- Krogh, T. A low-contamination method for hydrothermal decomposition of zircon and extraction of U and Pb for isotopic age determinations. Geochim. Cosmochim. Acta 1973, 37, 485–494. [Google Scholar] [CrossRef]
- Li, J.; Deng, W.; Zhou, D.; Liu, M.F.; Zhao, Y.S.; Guo, X.-F. Laser ablation ICP-MS titanite U–Th–Pb dating of hydrothermal ore deposits: A case study of the Tonglusha. Chem. Geol. 2010, 270, 56–67. [Google Scholar] [CrossRef]
- Chew, D.; Petrus, J.; Kamber, J.B. U–Pb LA–ICPMS dating using accessory mineral standards with variable common Pb. Chem. Geol. 2014, 363, 185–199. [Google Scholar] [CrossRef]
- Clark, K.F.; Foster, C.T.; Damon, P.E. Cenozoic mineral deposits and subduction related magmatic arcs in Mexico. Geol. Soc. Am. Bull. 1982, 93, 533–544. [Google Scholar] [CrossRef]
- Clechenko, C.; Valley, J. Oscillatory zoning in garnet from the Willsboro Wollastonite Skarn, Adirondack Mts, New York: A record of shallow hydrothermal processes preserved in a granulite facies terrane. J. Metamorph. Geol. 2003, 21, 771–784. [Google Scholar] [CrossRef]
- Stowell, H.; Carlos, C.; Boyle, A.; Bulman, G. Garnet sector and oscillatory zoning linked with changes in crystal morphology during rapid growth, North Cascades, Washington. Am. Mineral. 2011, 96, 1354–1362. [Google Scholar] [CrossRef]
- Khon, M.J. Oscillatory and sector-zoned garnets record cyclic (?) rapid thrusting in central Nepal. Geochemistry. Geophys. Geosystems 2004, 5, 1–9. [Google Scholar] [CrossRef]
- Akizuki, M. Origin of optical variations in grosular-andradite garnet. Am. Mineral. 1984, 69, 328–338. [Google Scholar]
- Carlson, W.D. Scales of disequilibrium and rates of equilibration during metamorphism. Am. Mineral. 2002, 87, 185–204. [Google Scholar] [CrossRef]
- L’Heureux, I.; Jamtveit, B. A model of oscillatory zoning in solid solutions grown from aqueous solutions:Applications to the (Ba,Sr)SO4 system. Geochim. Cosmochim. Acta 2002, 66, 417–429. [Google Scholar] [CrossRef]
- Bersani, D.; Ando, S.; Vignola, P.; Moltifori, G.; Marino, I.G.; Lottici, P.P.; Diella, V. Micro-Raman spectroscopy as a routine tool for garnet analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 484–491. [Google Scholar] [CrossRef]
- Gaspar, M.; Knaack, C.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA–ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmotica Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Maschio, L.; Demichelis, R.; Orlando, R.; De La Pierre, M.; Mahmoud, A. The raman spectrum of grossular garnet. A quantum mechanical simulation of wavenumbers and intensities. J. Raman Spectrosc. 2014, 45, 710–715. [Google Scholar] [CrossRef]
- Jamtveit, B.; Agnarsdottir, K.V.; Wood, B.J. On the origin of zoned grossular-andradite garnets in hydrothermal systems. Eur. J. Mineral. 1995, 7, 1399–1410. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.S.; Lu, J.J.; Zhang, R.Q. Garnet and scheelite as indicators of multi-stage tungsten mineralization in the Huangshaping deposit, southern Hunan province, China. Ore Geol. Rev. 2018, 94, 193–211. [Google Scholar] [CrossRef]
- Azizi, M.R.; Abedeni, A.; Alipour, S.; Niroomand, S.; Sasmaz, A.; Talaei, B. Rare earth element geochemistry and tetrad effect in fluorites: A case study from the Qahr-Abad deposit, Iran. Neues Jahrb. Geol. Palaontol. Abh 2017, 283, 255–273. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19. Available online: https://journals.lib.unb.ca/index.php/GC/article/view/3773 (accessed on 10 May 2024).
- Tian, M.; Li, Y.; Miao, L.; Zhang, Y.; Gao, T.; Guo, J.; Xue, J.; He, B. Alteration and mineralization zoning, ore textures and ore-forming process of Yongping copper deposit, Jiangxi Province. Acta Petrol. Sin. 2019, 35, 1924–1938, (In Chinese with English Abstract). [Google Scholar]
- Park, C.; Song, Y.; Kang, I.-M.; Shim, J.; Chung, D.; Park, C.-S. Metasomatic changes during periodic fluid flux recorded in grandite garnet from the Weondong W-skarn deposit, South Korea. Chem. Geol. 2017, 451, 135–153. [Google Scholar] [CrossRef]
- Somarin, A.K. Garnet composition as an indicator of Cu mineralization: Evidence from skarn deposits of NW Iran. J. Geochem. Explor. 2004, 81, 47–57. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T.E.R.; Long, J.; Williams, C.T. The rare earth elements and uranium in garnets from the Beinn an Dubhaich aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, C.Q.; Mao, J.W.; Santosh, M.; Zhou, Y.M.; Hou, L. Garnets in porphyry–skarn systems: A LA–ICP–MS, fluid inclusion, and stable isotope study of garnets from the Hongniu-Hongshan copper deposit, Zhongdian area, NW Yunnan Province, China. J. Asian Earth Sci. 2015, 103, 229–251. [Google Scholar] [CrossRef]
- Sasmaz, A.; Ozkan, S.; Gursu, M.F.; Sasmaz, M. The hematological and biochemical changes in rats exposed to britholite mineral. Appl. Rad. Isot. 2017, 129, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Seman, S.; Stockli, D.F.; McLean, N.M. U-Pb geochronology of grossular-andradite garnet. Chem. Geol. 2017, 460, 106–116. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.F.; White, N.C.; Zhang, L.J.; Fan, Y.; Wang, F.Y.; Chen, X.F. The formation and trace elements of garnet in the skarn zone from the Xinqiao Cu-S-Fe- Au deposit, Tongling ore district, Anhui Province, Eastern China. Lithos 2018, 302, 467–479. [Google Scholar] [CrossRef]
- Mezger, K.; Hanson, G.; Bohlen, S. U-Pb systematics of garnet: Dating the growth of garnet in the Late Archean Pikwitonei granulite domain at Cauchon and Natawahunan Lakes, Manitoba, Canada. Contrib. Mineral. Petrol. 1989, 101, 136–148. [Google Scholar] [CrossRef]
- Meinert, L.D.; Nicolescu, S.; Mortensen, J.K.; Cornell, D.H. U-Pb Dating of Hydrothermal Garnets from Skarn Deposits: Implication for Petrogenesis and ore Deposits. In Proceedings of the GSA Annual Meeting, Boston, MA, USA, 5–8 November 2001. [Google Scholar]
- Gu, H.; Yang, X.; Nie, Z.; Deng, J.; Duan, L.; Hu, Q.; Shakoor, M.A.; Gao, E.; Hafiz, A.A.J. Study of late-Mesozoic magmatic rocks and their related copper-gold-polymetallic deposits in the Guichi ore-cluster district, Lower Yangtze River Metallogenic Belt, East China. Int. Geol. Rev. 2018, 60, 1404–1434. [Google Scholar] [CrossRef]
- Mc Lelland, J.; Hamilton, M.; Selleck, B.; McLelland, J.; Walker, D.; Orrell, S. Zircon U-Pb geochronology of the Ottawan orogeny, Adirondack highlands, New York: Regional and tectonic implications. Precambrian Res. 2001, 109, 39–72. [Google Scholar] [CrossRef]
- Koesov, B.A.; Geiger, C.A. Raman spectra of silicate garnets. Physic Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Geiger, C.A.; Arnbruster, T. Mn3Al3Si3O12 Spessartine and Ca3Al2Si3O12 Grossular garnet: Structural dynamic and thermodynamic properties. Am. Mineral. 1997, 82, 571–581. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, T.F.; Fan, Y.; Xie, J.; Zhang, L.J. LA-ICP-MS in situ trace elements and FE-SEM analysis of pyrite from the Xinqiao Cu-Au-S deposit in Tongling Anhui and its constraints on the ore genesis. Acta Geosci. Sin. 2016, 2, 369–376. [Google Scholar]
- Sasmaz, A.; Kılıç, A.D.; Akgül, B.; Sasmaz, B. A spectral approach on mineralogy and geochemistry of garnet skarns in Arc-Type Granitoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122037. [Google Scholar] [CrossRef]
- Wang, L.J.; Shimazaki, H.; Wang, J.B.; Wang, Y.Z. Ore-forming fluids and mineralization of Fe-Sn deposit of skarn type in Huanggangliang. Sci. China (Ser. D) 2001, 31, 553–562. [Google Scholar]
- Zhang, S.Z.; Ling, Q.C. Characteristics on copper deposit of magmatic skarn type: An example from Dongshizishan copper deposit in Tongling county, Anhui Province. Earth Sci. J. China Univ. Geosci. 1993, 18, 801–813, (In Chinese with English Abstract). [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World Skarn Deposits (299–336). In Economic Geology; One Hundredth Anniversary, 1905–2005; Hedenquist, J.W., Thompson, J.F., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geology, Inc.: Littleton, CO, USA, 2005; pp. 1–1097. [Google Scholar]
- Burt, D.M. Skarn deposits-historical bibliography through 1970. Econ. Geol. 1982, 77, 755–763. [Google Scholar] [CrossRef]
- Sasmaz, A.; Önal, A.; Sagiroglu, A.; Önal, M. Origin and nature of the mineralizing fluids of thrust zone fluorites in Çelikhan (Adıyaman, Eastern Turkey): A geochemical approach. Geochem. J. 2005, 39, 131–139. [Google Scholar] [CrossRef]
- Kwak, T.A.P.; Abeysinghe, P.B. Rare earth and uranium minerals present as daughter crystals in fluid inclusions, Mary Kathleen U-REE skarn, Queensland, Australia. Mineral. Mag. 1987, 51, 665–670. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, J.B.; Wang, Y.W.; Shimazaki, H. REE geochemistry of the Huangguangliang skarn Fe-Sn deposit, Inner Mongollia. Acta Petrol. Sin. 2002, 18, 575–584. [Google Scholar]
- Yang, F.Q.; Mao, J.W.; Xu, L.G.; Zhang, Y.; Liu, F.; Huang, C.L.; Zhou, G.; Liu, G.R.; Dai, J.Z. REE geochemistry of the Mengku iron deposit, Xinjiang, and its indication for iron mineralization. Acta Petrol. Sin. 2007, 23, 2443–2456, (In Chinese with English abstract). [Google Scholar]
- Einaudi, M.T.; Burt, D.M. Introduction-terminology, classification, and composition of skarn deposits. Econ. Geol. 1982, 77, 745–754. [Google Scholar] [CrossRef]
- Kılıç, A.D.; Ateş, C. Geochronology of the Late Cretaceous magmatism and metamorphism, Pütürge massif, Turkey. Yanshi Xuebao Acta Petrol. Sin. 2015, 31, 1485–1493. [Google Scholar]
- Kuşcu, İ. Skarns and Skarn Deposits of Turkey. In Mineral Resources of Turkey. Modern Approaches in Solid Earth Sciences; Pirajno, F., Ünlü, T., Dönmez, C., Şahin, M., Eds.; Springer: Cham, Germany, 2019; Volume 16. [Google Scholar]
- Rızaoğlu, T.; Parlak, O.; Höck, V.; Koller, F.; Hames, W.E.; Billor, Z. Andean Type Active Margin Formation in the Eastern Taurides: Geochemical and Geochronological Evidence from the Baskil Granitoid, SE Turkey. Tectonophysics 2009, 473, 188–207. [Google Scholar] [CrossRef]
- Robertson, H.F.; Parlak, O.; Rızaoğlu, T.; Ünlü, U.; Genç, C.; İnan, N.; Taşlı, K.; Ustaömer, T. Late Cretaceous-Mid Tertiary Tectonic Evolution of the Eastern Taurus Maountains and Southern Tethyan Ocean Evidence from the Elazığ Region SE Turkey. Geol. Soc. Lond. Spec. Publ. 2006, 272, 231–270. [Google Scholar] [CrossRef]
- Ural, M.; Sarı, B. New Planktonic Foraminifera Data from the Upper Cretaceous Pelagic Limestones of the Yüksekova Complex in the Maden Area (Southeast of Elazığ, Eastern Turkey). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 362, p. 121. [Google Scholar]
- Sar, A.; Ertürk, M.A.; Rizeli, M.E. Genesis of Late Cretaceous intra-oceanic arc intrusions in the Pertek area of Tunceli Province, eastern Turkey, and implications for the geodynamic evolution of the southern Neo-Tethys: Results of zircon U–Pb geochronology and geochemical and Sr-Nd isotopic analyses. Lithos 2019, 105263, 350–351. [Google Scholar]
- Lin, Y.C.; Chung, S.L.; Bingöl, A.F.; Beyarslan, M.; Lee, H.Y.; Yang, J.H. Petrogenesis of late Cretaceous Elazig magmatic rocks from SE Turkey: New age and geochemical and SrNd-Hf isotopic constraints. In Goldschmidt Conference Abstracts; European Association of Geochemistry: Prag, Czech Republic, 2015; p. 1869. [Google Scholar]
- Castellanos-Alarcón, O.M.; Ríos-Reyes, C.A.; Mantilla-Figueroa, L.C. Occurrence of a skarn-type mineralogy found in Ciénaga Marbles, located in the NW foothills of the Santa Marta Massif (Colombia). Dyna 2015, 83, 69–79. [Google Scholar] [CrossRef]
- Ardila, R.; Carrascal, E.R.; Oyarzún, Y.J. Proposición de un modelo genético para los yacimientos cupríferos tipo skarn de San Antonio y Panulcillo, Región de Coquimbo, Chile. Congr. Geológico Chil. 1991, 1, 256–260. [Google Scholar]
- Kılıç, A.D.; Çakmak, B. Elemental Analysis of Ignimbirite by Raman Spectroscopy Methods and Instrumental Neutron Activation Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 2511, 119406. [Google Scholar]
- Sipahi, F.; Akpınar, İ.; Kaygusuz, A.; Vural, A.; Eker, Ç.S. Investigation of Karadağ Fe-Cu Skarn Deposit (Gümüshane, Turkey). In Proceedings of the III International Conference on Engineering and Natural Sciences, Budapest, Hungary, 3–7 May 2017. [Google Scholar]
- Pertoldova, J.; Tycova, P.; Verner, K.; Kosulicova, M.; Pertold, Z.; Kosler, J.; Konopasek, J.; Pudilova, M. Metamorphic history of skarns, origin of their protolith and implications for genetic interpretation; an example from three units of the Bohemian Massif. J. Geosci. 2009, 54, 101–134. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.Q.; Li, S.R.; Santosh, M.; Wang, J.Z.; Li, Q. Mineral chemistry of high-Mg diorites and skarn in the Han-Xing Iron deposits of South Taihang Mountains, China: Constraints on mineralization process. Ore Geol. Rev. 2015, 64, 200–214. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, F.; Jowitt, S.M.; Li, X.; Deng, Y.; Li, X.; Zhou, T. Garnet major and trace element evidence of the alteration and mineralizing processes associated with genesis of the Qiaomaishan skarn deposit, Xuancheng ore district, eastern China. Ore Geol. Rev. 2021, 137, 104304. [Google Scholar] [CrossRef]
- Scheibner, B.; Wörner, G.; Civetta, L.; Stosch, H.G.; Simon, K.; Kronz, A. Rare earth element fractionation in magmatic Ca-rich garnets. Contrib. Mineral. Petrol. 2007, 154, 55–74. [Google Scholar] [CrossRef]
- Ruan, C.-T.; Yu, X.-Y.; Su, S.-G.; Santosh, M.; Qin, L.-J. Anatomy of Garnet from the Nanminghe Skarn Iron Deposit, China: Implications for Ore Genesis. Minerals 2022, 12, 845. [Google Scholar] [CrossRef]
- Li, G.-Y.; Xue, C.-J.; Zhu, Q.; Yang, J.-W.; Zhao, X.-B. Identification of the Sedimentary Sources and Origin of Uranium for Zhiluo Formation of the Tarangaole U Deposit, Northeastern Ordos Basin. Minerals 2024, 14, 429. [Google Scholar] [CrossRef]
- Boynton, V. Cosmochemistry of the Rare Earth Elements: Meteorite Studies in Rare Earth Element Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 63–114. [Google Scholar]
- Tian, Z.D.; Leng, C.B.; Zhang, X.C.; Zafar, T.; Zhang, L.J.; Hong, W.; Lai, C.K. Chemical composition, genesis and exploration implication of garnet from the Hongshan Cu-Mo skarn deposit, SW China. Ore Geol. Rev. 2019, 112, 2–22. [Google Scholar] [CrossRef]
- Jamtveit, B. Oscillatory zonation patterns in hydrothermal grossular-andradite garnet: 413 Nonlinear dynamics in regions of immiscibility. Am. Mineral. 1991, 76, 1319–1327. [Google Scholar]
- Mirnejad, H.; Hasannejad, M.; Miller, N.; Hassanzadeh, J.; Bocchio, R.; Modabberi, S. Origin and Evolution of Oscillatory Zoned Garnet from Kasva Skarn, Northeast Tafresh, Iran. Can. Mineral. 2018, 56, 15–37. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Caro, C.; Sayagues, M.J.; Franco, V.; Conde, A.; Zaderenko, P.; Gámez, F. A hybrid silver-magnetite detector based on surface enhanced Raman scattering for differentiating organic compounds. Sens. Actuators B Chem. 2016, 228, 124–133. [Google Scholar] [CrossRef]
- Sasmaz, A. The Atbara porphyry gold–copper systems in the Red Sea Hills, Neoproterozoic Arabian–Nubian Shield, NE Sudan. J. Geochem. Expl. 2020, 214, 106539. [Google Scholar] [CrossRef]
- Boucherit, N.; Hugot-Le Goff, A.; Joiret, S. Raman Studies of Corrosion Films Grown on Fe and Fe-6Mo in Pitting Conditions. Corros. Sci. 1991, 32, 497–507. [Google Scholar] [CrossRef]
- Wang, Z.; Lazor, P.; Saxena, S.K.; Neil, C.O. High pressure Raman spectroscopy of ferrite MgFe2O4. Mater. Res. Bull. 2022, 37, 1589–1602. [Google Scholar] [CrossRef]
- Ray, G.E.; Dawson, G.L. Geology and Mineral Deposits of the Hedley Gold Skarn District, Southern British Columbia; Bulletin 87; British Columbia Ministry of Energy, Mines, and Petroleum Resources: Victoria, BC, Canada, 1994; p. 156. [Google Scholar]
- Zhai, D.G.; Liu, J.J.; Zhang, H.Y.; Wang, J.P.; Su, L.; Yang, X.-A.; Wu, S.H. Origin of oscillatory zoned garnets from the Xieertala Fe–Zn skarn deposit, northern China: In situ LA–ICP-MS evidence. Lithos 2014, 190, 279–291. [Google Scholar] [CrossRef]





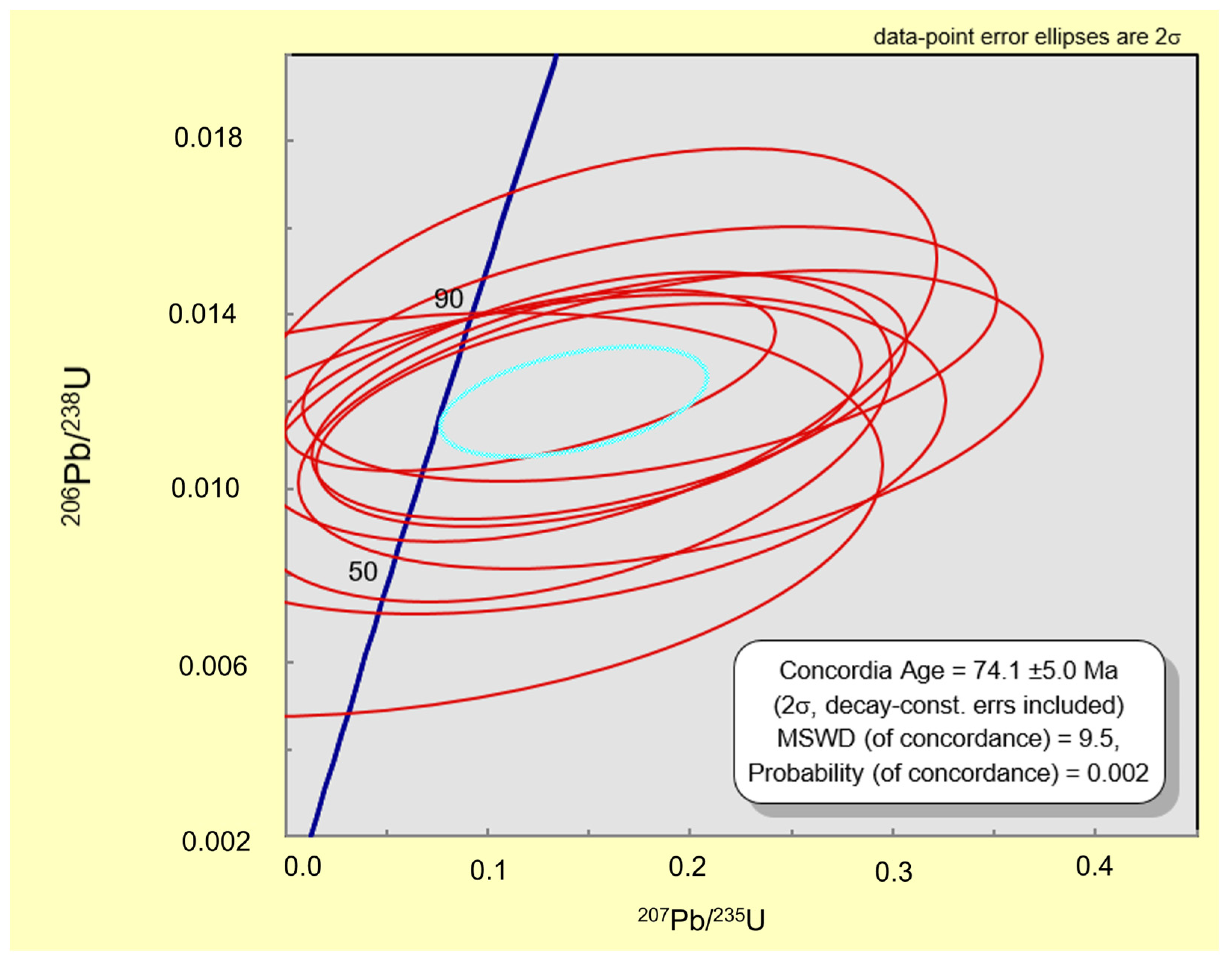
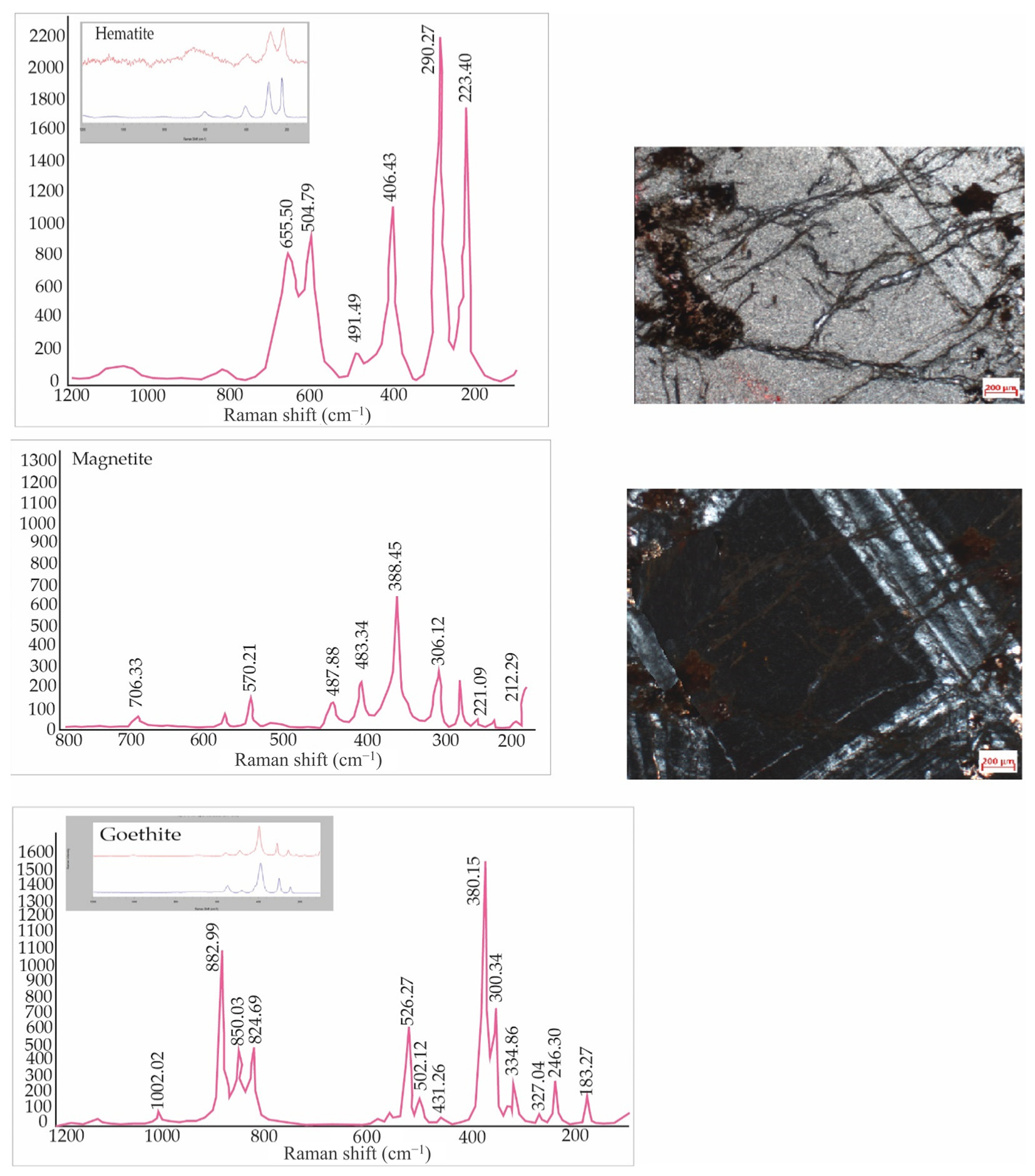
| Sample | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 36.1 | 35.7 | 35.6 | 35.5 | 35.5 | 35.4 | 35.3 | 36.5 | 36.4 | 35.1 | 35.5 | 35.4 |
| TiO2 | 2.35 | 3.01 | 2,41 | 2,62 | 2,40 | 2,44 | 2.21 | 2.34 | 2.44 | 2.66 | 2.77 | 3.13 |
| Al2O3 | 3.63 | 4.05 | 4.04 | 3.97 | 4.65 | 4.40 | 3.69 | 3.87 | 4.04 | 3.10 | 4.18 | 4.78 |
| MgO | 0.15 | 0.18 | 0.17 | 0.21 | 0.22 | 0.22 | 0.18 | 0.19 | 0.21 | 0.14 | 0.20 | 0.25 |
| MnO | 0.81 | 0.72 | 0.75 | 0.66 | 0.56 | 0.62 | 0.69 | 0.72 | 0.61 | 0.85 | 0.69 | 0.76 |
| FeO | 24.4 | 23.4 | 23.6 | 23.5 | 20.3 | 23.0 | 23.7 | 24.3 | 22.3 | 25.8 | 23.5 | 25.0 |
| CaO | 32.9 | 33.3 | 32.9 | 31.8 | 32.2 | 33.4 | 32.8 | 33.3 | 31.0 | 34.2 | 34.0 | 36.5 |
| P2O5 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.07 | 0.02 | 0.01 | 0.02 | 0.06 |
| Sum | 100.4 | 100.4 | 99.5 | 98.3 | 95.8 | 99.5 | 98.7 | 100.2 | 97.1 | 100 | 100.3 | 100 |
| Th | 8 | 8 | 7 | 7 | 6 | 5 | 8 | 8 | 6 | 16 | 9 | 10 |
| U | 12 | 13 | 10 | 11 | 9 | 8 | 13 | 12 | 9 | 25 | 13 | 14 |
| Y | 347 | 507 | 450 | 366 | 451 | 485 | 300 | 302 | 414 | 795 | 313 | 359 |
| Sr | 17 | 19 | 17 | 15 | 16 | 17 | 17 | 20 | 15 | 24 | 17 | 17 |
| Sm | 60 | 72 | 64 | 54 | 59 | 58 | 51 | 54 | 53 | 79 | 54 | 59 |
| Zr | 1453 | 1405 | 1296 | 1380 | 1152 | 1225 | 1578 | 1398 | 1124 | 1855 | 1451 | 1546 |
| V | 445 | 671 | 638 | 634 | 739 | 739 | 465 | 514 | 715 | 744 | 543 | 557 |
| Sn | 17 | 19 | 17 | 15 | 16 | 17 | 17 | 20 | 15 | 24 | 17 | 17 |
| W | 53 | 80 | 66 | 77 | 73 | 66 | 112 | 59 | 71 | 52 | 78 | 83 |
| XCa | 0.97 | 0.97 | 0.97 | 0.97 | 0.98 | 0.98 | 0.97 | 0.97 | 0.98 | 0.97 | 0.98 | 0.97 |
| XFe | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Iso Ratios | Uncert | Iso Ratios | Uncert | Age (Ma) | Age uncert | ||
|---|---|---|---|---|---|---|---|
| 207Pb/235U | 207Pb/235U | 206Pb/238U | 206Pb/238U | cor cef | 206Pb/238U | 206Pb/238U | |
| Spot1 | 0.150 | 0.1100 | 0.0117 | 0.0021 | 0.45 | 75 | 13 |
| Spot3 | 0.180 | 0.1400 | 0.0131 | 0.0024 | 0.42 | 84 | 15 |
| Spot1 | 0.150 | 0.1400 | 0.0133 | 0.0037 | 0.44 | 84 | 23 |
| Spot3 | 0.190 | 0.1500 | 0.0116 | 0.0028 | 0.42 | 74 | 18 |
| Spot1 | 0.160 | 0.1200 | 0.0121 | 0.0023 | 0.48 | 77 | 15 |
| Spot2 | 0.140 | 0.1300 | 0.0112 | 0.0031 | 0.44 | 72 | 20 |
| Spot3 | 0.130 | 0.1600 | 0.0108 | 0.0030 | 0.34 | 69 | 19 |
| Spot1 | 0.121 | 0.0990 | 0.0125 | 0.0017 | 0.54 | 80 | 11 |
| Spot2 | 0.050 | 0.2000 | 0.0094 | 0.0038 | 0.25 | 60 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilic, A.D.; Konakci, N.; Sasmaz, A. Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings. Minerals 2024, 14, 539. https://doi.org/10.3390/min14060539
Kilic AD, Konakci N, Sasmaz A. Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings. Minerals. 2024; 14(6):539. https://doi.org/10.3390/min14060539
Chicago/Turabian StyleKilic, Ayşe Didem, Nevin Konakci, and Ahmet Sasmaz. 2024. "Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings" Minerals 14, no. 6: 539. https://doi.org/10.3390/min14060539
APA StyleKilic, A. D., Konakci, N., & Sasmaz, A. (2024). Garnet Geochemistry of Pertek Skarns (Tunceli, Turkey) and U-Pb Age Findings. Minerals, 14(6), 539. https://doi.org/10.3390/min14060539








