Abstract
A detailed description of carbonate minerals of Triassic dolomites with different magnesium contents is presented in this article. Tests were carried out to determine geochemical and mineralogical characteristics. The following carbonate phases were identified: low-Mg calcite, high-Mg calcite, proto-dolomite, ordered dolomite, and huntite. The methods used were microscopic description, X-ray diffraction (XRD), X-ray fluorescence (XRF), and electron probe microanalysis (EMPA). Samples were collected from the Tarnowice Formation, which is the lower part of the profile of Upper Muschelkalk. On the basis of the obtained results, the chemical formulae of carbonate phases were calculated. The results indicate that Mg in low-Mg calcite ranges from 0.6 to 1.2% and in high-Mg calcite from 7.47 to 10.41%. In protodolomite, it ranges from 10.96 to 11.78%. In ordered dolomite, the Mg content is 13.18% on a stoichiometric basis. Due to the reduced Mg content in the identified huntite (in the range of 13.62% to 17.76), this carbonate phase is considered de-huntite.
1. Introduction
The area of Upper Silesia, including the areas of Bytom and Piekary Śląskie, is an area where Triassic carbonate rocks occur, including the Lower, Middle, and Upper Muschelkalk formations [1,2]. These rocks, in contrast to the limestone deposits of the area of the Opole Silesia [3,4,5,6,7,8,9,10,11,12,13,14,15,16], have not been thoroughly investigated in terms of the presence of carbonate phases with different magnesium contents. In the limestones of Opole Silesia, five carbonate phases were identified, characterized by different Mg contents: low-Mg calcite, high-Mg calcite, protodolomite, ordered dolomite, and huntite. The carbonate rocks rich in magnesium are used in many branches of industry [6,11,13,17,18,19,20].
The carbonate rocks can be used in different types of industry, for example, as crushed stones [18]. CaCO3 is used as the absorbent in the accelerated weathering of limestone (AWL) [19]. Carbonates with magnesium are also used in medicine. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization [20]. Knowledge of the Mg content in carbonate phases is very important for the application of rock as a sorbent in the desulphurization of flue gases in power plants and in lime production [6,13,18]. During the desulphurization process, the carbonate phases with higher Mg contents decompose at lower temperatures than the phases with low Mg contents or pure calcite without Mg substitution. This is due to the lower stabilization of some carbonate phases with Mg substitution, like high-Mg calcite [21,22,23,24,25,26]. Because of this, the desulphurization process starts earlier and is more effective [6,13].
The Middle Muschelkalk deposits, including the Tarnowice Unit [27,28], have not been the subject of detailed research in terms of identifying carbonate phases with different magnesium contents. However, due to the probability of the presence of carbonate phases with different amounts of Mg, mainly high-Mg calcite, which was identified in the rocks of the Lower Muschelkalk in the area of Opole Silesia, rocks of the Tarnowice Unit were chosen as the subject of the study.
During earlier studies of the Triassic carbonate rocks from the area of Opole Silesia, which is a formation of the Lower and partly Middle Muschelkalk, the presence of the following carbonate phases was identified: low-magnesium calcite, high-magnesium calcite, proto-dolomite, ordered dolomite, and huntite [6,9,13]. The presence of these phases made it possible to determine the conditions of sedimentation carbonate rocks and the diagenetic processes that these rocks underwent. Due to the probability of the presence of these mineral phases in the rocks of the Tarnowice Unit, which is a formation of the upper part of the Middle Muschelkalk [27,28], it is worth undertaking research that allowed new data to be obtained on the presence of carbonate phases with different magnesium contents and the geochemical composition of the carbonate rocks. Based on the research results, it was also possible to determine the sedimentation conditions under which the rocks of the Tarnowice Unit were formed and the diagenetic processes that influenced the final structure of the carbonate rocks.
The results of the research and the conclusions formed on the basis of the results will be a source of new data on the mineral composition and genesis of the rocks of the Tarnowice Unit.
2. Materials and Methods
2.1. Geology of the Studied Area
The subject of this article is carbonate rocks of the Tarnowice Unit (lower formation of Upper Muschelkalk–Middle Triassic) from selected areas of Upper Silesia (Silesian Voivodeship). The study zone includes the areas of Piekary Śląskie and Bytom (Figure 1a).
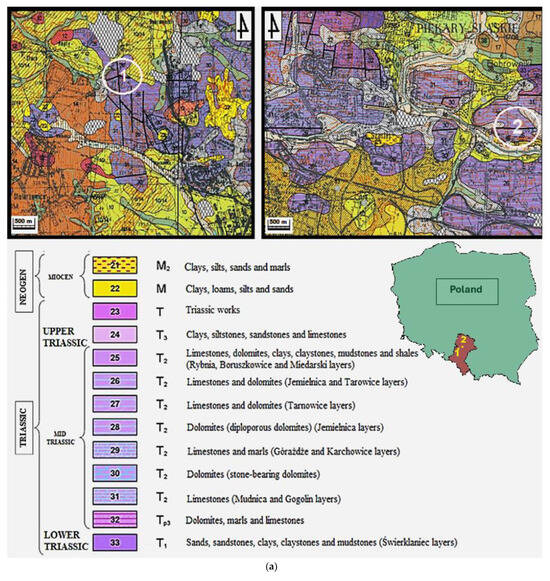
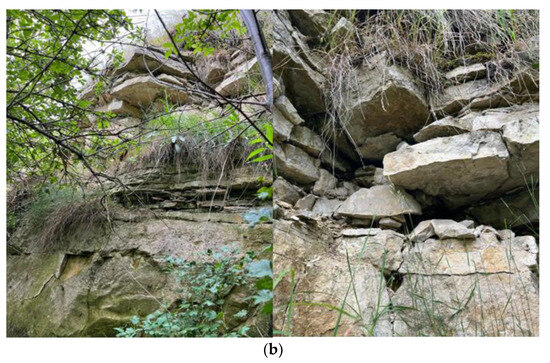
Figure 1.
(a) Location of the research area. Fragment of a detailed geological map of Poland [27]. Scale 1: 50,000. Sheet-910-Bytom (M-34-50-D). 1—Lazarówka Quarry (Lazarówka street-LZ), 2—Piekary Śląskie area (Karłowicz—PSK and Zawisza Czarny—PSZ streets).  —North. (b) Example of an outcrop of dolomites in the area of Karłowicz Street in Piekary Śląskie. This is an illustrative photo (Photo: Rafał Gajdzik).
—North. (b) Example of an outcrop of dolomites in the area of Karłowicz Street in Piekary Śląskie. This is an illustrative photo (Photo: Rafał Gajdzik).
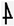 —North. (b) Example of an outcrop of dolomites in the area of Karłowicz Street in Piekary Śląskie. This is an illustrative photo (Photo: Rafał Gajdzik).
—North. (b) Example of an outcrop of dolomites in the area of Karłowicz Street in Piekary Śląskie. This is an illustrative photo (Photo: Rafał Gajdzik).
Similar to the sediments of the area of the Opole Silesia, the rocks of Upper Silesia (Tarnowice Unit) are also sediments of the Upper Muschelkalk (Middle Triassic). Triassic carbonate from the area of Upper Silesia is a sediment of the eastern part of the epicontinental Germanic Basin (southwestern part of Poland) [2,9,12,14,15]. This area is the eastern zone of the Central Europe Triassic intracratonic basin, of which the sequences of other parts lie in the terrains of Germany, Netherlands, Slovakia, Hungary, Austria, Italy, and Switzerland [14,15,24,25,26]. Variable carbonate phases with different Mg contents are present in limestones of all formations of the Lower Muschelkalk (area of Opole Silesia)—carbonate rocks of the Gogolin Unit, Górażdże Unit, Dziewkowice (Terebratula) Unit, and Karchowice Unit [5,6,7,8,9,10,11,12,13,14,15]. The formation names are regional and come from the names of the cities. The Triassic dolomites of the Tarnowice Unit—sediments of Upper Muschelkalk—occur in Upper Silesia [24,25,26,28]. The Lower Muschelkalk of Upper Silesia, the so-called Gogolin layers, is comprised of marine sediments (limestones, marly limestones, dolomite inserts, wavy limestones, lumpy limestones with clay, and at the bottom, limestones with faunal remains, i.e., mussels and crinoids). The next layers characterizing this period are the Karchowice layers, which are composed of crystalline and pelitic limestones with hornstones, in places with faunal remains, partly cavernous, while in the northern part, they are unlevelled. They also contain ore-bearing dolomites up to 60 m thick [28]. The Middle Muschelkalk also includes diplopore dolomite. Its structure and physical and chemical properties resemble ore-bearing dolomites. The fauna and flora in these rocks are numerous and very diverse; there are numerous remains of skeletons, impressions, and internal centers [1,2,28]. The most common are molluscs (mussels, snails), brachiopods, annelids, and reptile bones. There are also remains of echinoderms (sea urchins) and daylilies [1,2,28]. The Boruszowice, Tarnowice, and Wilkowice layers are composed of upper Muschelkalk, the thickness of which ranges from 8 to 18 m. They are composed of marls and clays interbedded with dolomites containing inserts of sandstones and limestones. These are layers typical of a drying inland sea [1,2,28].
An example of a dolomite outcrop in the area of Karłowicza Street in Piekary Śląskie is shown in Figure 1b.
2.2. Materials
The samples were given symbols depending on the sampling location: LZ—Lazarówka Quarry (area of Bytom—Sucha Góra city), PSK—Piekary Śląskie city, Mieczysław Karłowicz Street, PSZ—Piekary Śląskie city, Zawisza Czarny Street.
The samples were collected from the Tarnowice Formation. This is a lower formation of the Upper Muschelkalk (Middle Triassic). It is built of limestones and dolomites.
The limestones are lumpy, beige or gray-beige, while the dolomites are compact and brownish in color. The rocks have a sparitic or biomorphic texture. The structure is massive, in some areas cavernous, and disorderly [14,25].
During this project, two types of rocks were studied: lime dolomites and dolomites. Photographs of the samples are shown in the figure below (Figure 2). Samples taken from the Lazarówka Quarry are characterized by a dark brown color and low compactness, especially the LZ3 sample. This is probably the effect of oxidation or Fe staining. The rocks show a sparite texture and a chaotic, compact, and, in places, porous structure. The exception is the LZ3 sample—it is unconsolidated rock. Reaction with hydrochloric acid, either weak or occurring after pulverization, strongly indicates dolomite predominance. It can therefore be concluded that these are probably dolomitic limestones or lime dolomites. The data obtained by applying X-ray diffraction and X-ray fluorescence (XRF) will provide more detailed information about these rocks. In the case of rocks taken from the area of Piekary Śląskie, from Karłowicz (Figure 1b) and Zawisza Czarny Streets, the reaction with hydrochloric acid occurs after pulverization, which indicates the dolomite dominance. Thus, these rocks can be defined as dolomites. The rocks taken from the area along Zawisza Czarny Street present a brown or light brown color, a dolosparite texture, and a compact, chaotic structure. The rocks taken from the area of Karłowicz Street are characterized by a gray color, a dolosparite texture, and a compact, disordered structure (Figure 1b).

Figure 2.
Photographs of the rock samples. The rocks from Lazarówka Quarry (samples LZ1, LZ2, LZ3) are brownish in color. The color is probably due to the presence of Fe minerals. Concentrations of sparite with white calcite are visible in sample LZ2. Samples PSZ1, PSZ2, and PSZ3, from Piekary Śląskie are beige in color, but samples PSK1, PSK2, and PSK3 also taken from the area of Piekary Śląskie are gray in color. Therefore, it can be said that the rocks from Lazarówka Quarry contain a higher content of Fe minerals compared to rocks from Piekary Śląskie.
2.3. Methods
Microscopic observations under transmitted light were performed in the Laboratory of the Department of Applied Geology (Silesian University of Technology), using the Zeiss Axioscop 2 (Carl Zeiss AG, Oberkochen, Germany), in conjunction with the K 300 image analyzer. Thin sections were made from six carbonate rock samples. Observations were made at ×100 and ×200 magnifications. Phase identification by X-ray diffraction (XRD) was performed in the laboratory of the Solid State Department, Institute of Physics, Faculty of Mathematics, Physics and Chemistry of the University of Silesia. The tests were performed using an Empyrean powder diffractometer from PANalytical (Malvern Panalytical Ltd., Malvern, Worcestershire, UK) equipped with a goniometer in a vertical Θ-Θ arrangement, a Euler wheel with φ and χ rotation, x, y, and z stages (strain measurement, texture, and reflectometry), and detectors: scintillation and 3D-PIXcel. A Cu lamp equipped with a Ni filter was used. The XRF (X-ray fluorescence) analysis was also performed in the laboratory of the Solid State Department using an X-ray fluorescence spectrometer (XRF), model ZSX Primus II, by Rigaku (Rigaku Corporation, Tokyo, Japan), which uses wavelength dispersion for measurements. The maximum lamp voltage is 60 kV (rhodium lamp); the measuring range is Be-U. The detection limits for elements are posted in tables. Microprobe measurements were carried out at the Institute of Non-Ferrous Metals in Gliwice. Polished sections of three samples, PSK2, PSZ3, and LZ1, were prepared for analysis. The polished sections were sputtered with a gold layer. The contents of the following elements were measured: C, O, Mg, Al, Si, Ca, Mn, Fe, and S. The tests were performed by X-ray microanalysis using the JXA 8230 X-ray microprobe made by JEOL (JEOL Ltd., Tokyo, Japan) with an accelerating voltage of 15 kV and an electron beam current of 20 nA. Quantitative analyses of the chemical composition were carried out using WDS (wavelength-dispersive X-ray spectroscopy). The size of the analyzed area was 1 µm3. The element distribution maps were made using the EDS (energy dispersive spectroscopy) method. The standards used were pure metals for Fe, Mn, and Al oxide (Al2O3)—the standard for aluminum; calcium silicate (CaSiO3)—the standard for silicon and calcium; iron sulfide (Fe2S)—the standard for sulfur (for sample LZ1); and magnesium oxide (MgO)—the standard for magnesium.
3. Results
3.1. Mineralogical and Petrographic Characteristics of Samples
On the basis of the macroscopic description, six samples of investigated carbonate rocks were chosen for microscopic examination, and six thin sections of chosen samples were prepared (Figure 3).
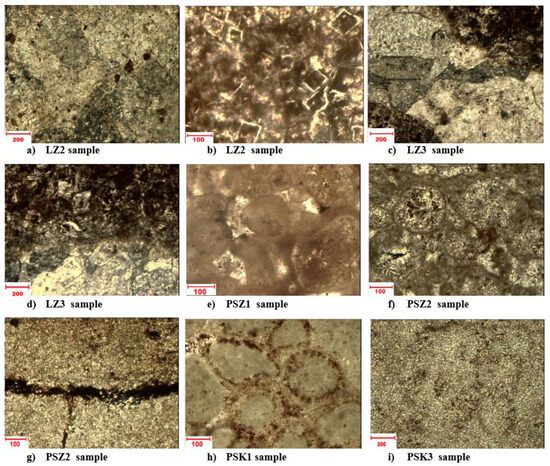
Figure 3.
Microscopic photographs of carbonate rock samples. (a) sparitic, anhedral calcite crystals; Fe minerals in some areas, (b) dolomite rhombohedral crystals filled in iron oxides, (c) sparitic, anhedral calcite crystals; Fe oxides in some areas, (d) sparitic, anhedral calcite crystals; Fe oxides in some areas, (e) oval bioclasts bonded by microsparitic and porous cement; visible coatings filled with Fe oxides, (f) sparitic base rock mass, (g) oval bioclasts bonded by microsparitic contact or porous cement, (h) oval bioclasts bonded by microsparitic contact or porous cement, (i) microsparitic base rock mass; in the middle, stylolite filled with iron compounds.
The rocks from the Lazarówka Quarry in the microscopic images have a sparite texture (samples LZ2, LZ3—Figure 3a,c,d), in some places biomorphic (sample LZ2—Figure 3b). The structure is chaotic and compact. Sparite rocks contain veins built of coarse-crystalline calcite (Figure 3a,d), as well as euhedral, rhombohedral dolomite crystals (Figure 3c). Calcite presents visible variable relief. It is xenomorphic/anhedral in shape. Dolomite rhombohedra are often filled with iron compounds (Figure 3c), so they can be treated as dolomite pseudomorphs. Rocks with a biomorphic texture include oval bioclasts, bonded with contact cement, with pores in some places (Figure 3b). In the mineral composition, dolomite and calcite as well as admixtures of Fe compounds were observed. In microscopic images, iron minerals are opaque and dark brown. The samples from Piekary Śląskie have a biomorphic (samples PSZ1, PSZ2, PSK1—Figure 3e–g) or sparite (samples PSZ2, PSK3—Figure 3g,i) texture. The structure is compact and chaotic. Samples with a biomorphic texture that are similar to rocks from the Lazarówka Quarry include oval or elongated bioclasts, bonded with microsparite or sparite cement (porous or contact cement; Figure 3c,d). Some bioclasts have a rim made of iron compounds (Fe oxides or hydrooxide–goethite) (Figure 3h). The interior of some bioclasts is filled with micrite or microsparite (Figure 3e,f,h). Rocks with a sparite texture are built of a rock mass. Concentrations of iron compounds are present here (Figure 3c,d,g), and stylolites filled with Fe compounds can also be observed (Figure 3g). In addition, single, fine grains of chalcedony and quartz were identified in some samples. In the mineral composition, carbonate minerals dominate. Iron compounds (oxides and hydrooxide–goethite), chalcedony, and quartz are present in smaller amounts.
3.2. Results of X-ray Diffraction
Figure 4, Figure 5, Figure 6 and Figure 7 present the results of X-ray diffraction of the examined carbonate rocks of the Tarnowice Unit. The results of X-ray diffraction show that dolomite dominates in the examined rocks.
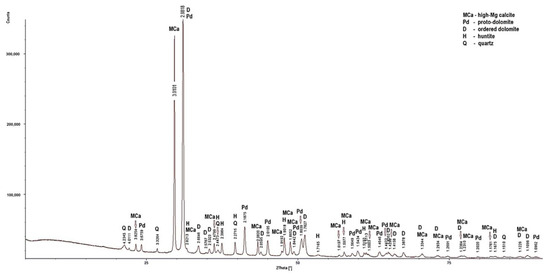
Figure 4.
X-ray diffraction patterns of sample LZ2.
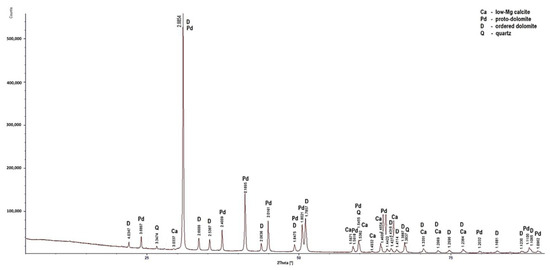
Figure 5.
X-ray diffraction patterns of sample PSK1.
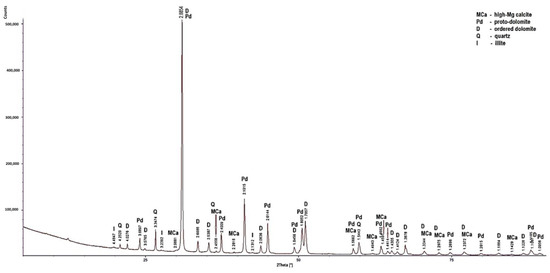
Figure 6.
X-ray diffraction patterns of sample PSK3.
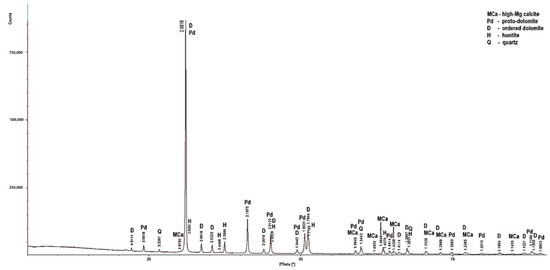
Figure 7.
X-ray diffraction patterns of sample PSZ1.
Low-magnesium calcite was determined only in the PSK1 sample (Figure 5). In this rock, apart from the dominant dolomite and the lesser amount of low-magnesium calcite, quartz was also determined. In the samples, apart from dolomite, high-magnesium calcite is present. Huntite was identified in the LZ2 and PSZ1 samples (Figure 4 and Figure 7). As for the non-carbonate minerals, quartz was determined in the diffraction patterns of all the samples (Figure 4), in addition to illite in sample PSK3 (Figure 6). The X-ray diffraction results indicate that the dominant calcite phase in the examined rocks is high-magnesium calcite. Based on the results of XRD tests, it can be concluded that the rocks from the Lazarówka Quarry can be described as calcareous dolomites, while the rocks from the area of Piekary Śląskie represent dolomites, with a small amounts of calcite or, in the case of some rocks, huntite.
3.3. Results of X-ray Fluorescence Analysis
By XRF analysis, the elements present in the studied carbonate rocks were determined. Because elemental and not oxide analysis was performed, LOI (Loss on Ignition) was not determined. The dolomites’ test results are given in Table 1, Table 2 and Table 3. The results of the study of the elemental composition indicate that the highest percentage presents the elements that constitute carbonate minerals, i.e., Ca, Mg, C, O (Table 1). These elements are part of the carbonate phases such as low-magnesium calcite, high-magnesium calcite, dolomite phases, and huntite. In smaller amounts, the following elements were determined: Na, Al, Si, K, Mn, Fe, F, P, S, Cl, Ti, Cr, Ni, Cu, Zn, Sr, Ba, Pb, and in some samples, Rb, Br, Co, As, and Cd.

Table 1.
Elemental composition of the studied samples.

Table 2.
Contents of major elements, oxides, MgCO3, and Ca/Mg ratio in analyzed samples.

Table 3.
Position of the analyzed rocks in classifications based on the Ca/Mg ratio and MgO and MgCO3 contents [%].
The content of individual elements, basic oxides included in the minerals of the examined rocks, the MgCO3 content, and the Ca to Mg ratio were calculated (Table 2).
Based on the obtained data, the position of the studied carbonate rocks was presented according to the classification of Chilingar [29] and Pettijohn [30] (Table 3).
The data presented in Table 4 indicate that the studied carbonate rocks represent lime dolomites, and some samples (LZ2, LZ3, and PSZ1), based on the classification of Pettijohn [30], represent calcareous dolomites. Therefore, these rocks, apart from the mineral dolomite, also contain different species of calcite, including high-magnesium calcite. This confirms the results of X-ray diffraction. The LZ2 sample, because of the high Fe2O3 content, can be considered Fe-dolomite.
3.4. Electron Microprobe Analysis (EMPA)
Three samples in the form of polished sections made of rocks were tested—LZ1, PSK2, and PSZ3. As part of the EDS microprobe analysis, maps of the distribution of elements were made. Moreover, quantitative analysis using the EDS method and quantitative analysis at points using the WDS method were performed. The results were normalized to 100%. On the basis of the measured Ca, Mg, and C, the contents of carbonate phases that constituted the examined dolomites were measured. Furthermore, the chemical formulas of the studied carbonate phases were calculated using spreadsheets. Other elements, such as Si, Al, Fe, and Mn, are probably bound in silicates and aluminosilicates. Fe and S could be bound in pyrite. Because these elements occur in small amounts, the formulae of the minerals they form have not been determined. The exception is the LZ2 sample, due to its relatively high iron content. In this sample, Fe is probably associated with Fe-dolomite. However, this sample was not studied by electron microprobe analysis.
3.4.1. Sample LZ1—Rock from the Lazarówka Quarry
In the first stage of microprobe analysis, the distribution of the following elements was determined: Mn, Ca, S, Si, Al, Mg, O, C, and Fe in the first micro-area of the LZ1 sample (Figure 8).
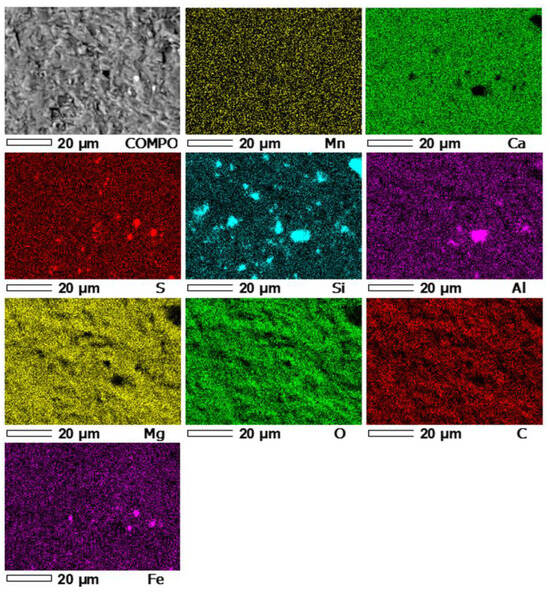
Figure 8.
Maps of the distribution of EDS elements in micro-area 1 of the LZ1 sample. COMPO—Image of the composition of micro-area 1 of sample LZ1.
The superposition of the Ca and Mg distribution images allows the determination of the presence of these elements in the same areas, which indicates the presence of calcium-magnesium carbonate phases. The superposition of the Fe and S distribution images allows the determination of the presence of iron sulfides–pyrite in the sample, and the occurrence of Si and Al in the same areas in the Si and Al images indicates the presence of aluminosilicates. The remaining Si is probably a silicate mineral—quartz.
In the second micro-area of the LZ1 sample, measurements of the elemental content were performed using the WDS method (Figure 9, Table 4).
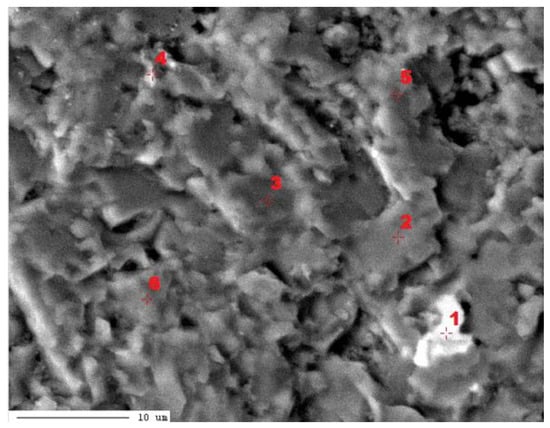
Figure 9.
Image of the composition of micro-area 2 of sample LZ1 with marked points used for quantitative analysis. 1–6-points of chemical analysis.

Table 4.
Results of the WDS analysis at the points of the LZ1 sample micro-area.
Table 4.
Results of the WDS analysis at the points of the LZ1 sample micro-area.
| Point Number | Element | Total [wt%] | MgO [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Si | Al | Ca | Fe | Mn | S | |||
| Content [wt%] | |||||||||||
| 1/low-Mg calcite (Ca0.95,Mg0.05)CO3 | 10.58 | 52.10 | 1.20 | 0.74 | 0.05 | 25.44 | 1.47 | 1.05 | 7.37 | 100.00 | 2.00 |
| 2/high-Mg calcite (Ca0.57,Mg0.43)CO3 | 10.93 | 54.19 | 10.37 | BDL | 0.07 | 20.96 | 2.87 | 0.60 | 0.01 | 100.00 | 17.28 |
| 3/high-Mg calcite (Ca0.69,Mg0.31)CO3 | 12.07 | 55.99 | 7.47 | 0.01 | 0.06 | 21.29 | 2.65 | 0.46 | BDL | 100.00 | 12.45 |
| 4/high-Mg calcite (Ca0.57,Mg0.43)CO3 | 11.90 | 53.47 | 10.41 | 0.05 | 0.05 | 21.96 | 1.89 | 0.25 | 0.02 | 100.00 | 17.35 |
| 5/protodolomite (Ca0.53,Mg0.47)CO3 | 11.20 | 53.49 | 11.39 | 0.05 | 0.08 | 20.92 | 2.25 | 0.61 | 0.01 | 100.00 | 18.98 |
| 6/protodolomite (Ca0.51,Mg0.49)CO3 | 11.29 | 53.44 | 11.71 | 0.01 | 0.11 | 20.69 | 2.17 | 0.56 | 0.02 | 100.00 | 19.52 |
BDL—below the detection limit.
The results of measurements in micro-area 2 of the LZ1 sample indicate the presence of three carbonate phases with different Mg contents: low-magnesium calcite (point 1), high-magnesium calcite (points 2, 3, and 4), and proto-dolomite (points 5 and 6) (Figure 9, Table 5). The proto-dolomite of sample LZ1 is characterized by a lower MgO content than the stoichiometric value for dolomite (MgO—21.86%, Mg—13.18%). It should be stated that in point 1 there is an increased content of sulfur. Therefore, there is a mixture of calcite and sulfur mineral.

Table 5.
Results of the EDS analysis at the points of micro-area 1 of the PSK2 sample.
3.4.2. Sample PSK2—Rock from the Area of Piekary Śląskie (Karłowicz Street)
In the first stage of the study of the PSK2 sample, in micro-area 1 of this sample, the distributions of the following elements were measured: Mn, Ca, Si, Al, Mg, O, C, and Fe (Figure 10).
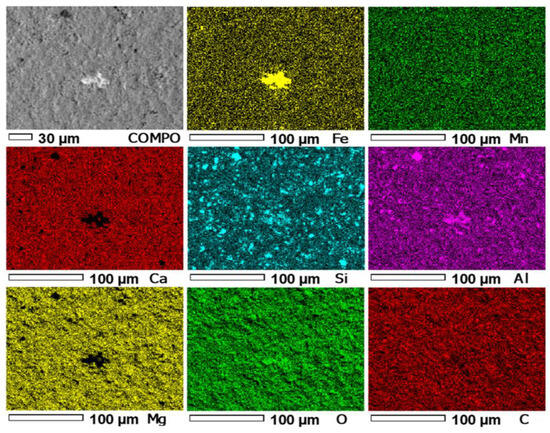
Figure 10.
Maps of the distribution of EDS elements in the micro-area 1 of PSK2. COMPO—Image of the composition of micro-area 1 of sample PSK2.
The superposition of the Ca and Mg distribution images (Figure 10) allows the determination of the presence of these elements in the same areas, which indicates the presence of calcium–magnesium carbonate phases. The superposition of the Ca, Mg, and Fe distribution images allows the determination of the presence of carbonate phases rich in Mg with the substitution of Fe (dolomite phases). The occurrence of Si and Al in the same areas of the Si and Al images indicates the presence of aluminosilicates. The remaining Si is similar to that in sample LZ1, i.e., probably due to quartz.
In micro-area 2 of the PSK2 sample, the contents of basic elements were measured using the EDS method (Figure 11, Table 5).
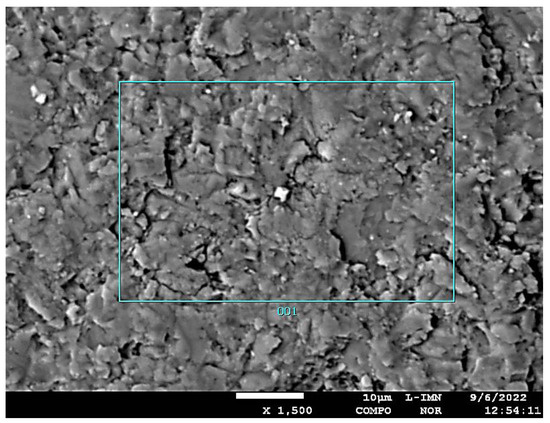
Figure 11.
Image of the composition of micro-area 2 (zone 1) of the PSK2 sample obtained by measurements using the EDS method.
The results of measurements using the EDS method in micro-area 1 of the PSK2 sample indicate the presence of a carbonate phase with an increased magnesium content, higher than that typical for dolomite, but lower than the stoichiometric value for huntite (MgO—34.25%, Mg—20.65%). This may indicate the presence of a huntite carbonate phase, transformed during the dehuntization process. During this process, part of the magnesium ions was removed from the huntite crystal, and therefore, the content of MgO is definitely lower than the stoichiometric value for huntite. Therefore, this mineral phase was named de-huntite.
In the third micro-area of the PSK2 sample, measurements of the elemental contents were performed using the WDS method (Figure 12, Table 6).

Figure 12.
Image of the composition of micro-area 3 of the PSK2 sample with marked points used for quantitative analysis. 1–6-points of chemical analysis.

Table 6.
The results of the WDS analysis of micro-area 2 of the PSK2 sample.
The results of measurements in micro-area 2 of the PSK2 sample indicate the presence of two carbonate phases with different Mg contents—high-magnesium calcite (points 3 and 4) and proto-dolomite (points 2, 4, 6) (Figure 12, Table 6). Slight variability in the Mg content was observed in high-Mg calcite. Proto-dolomite, similar to that in sample LZ1, is characterized by a lower MgO content than the stoichiometric value for dolomite. At point 1, increased contents of Si and Fe were measured. The increased contents of Si and Fe at point 1 indicate a mixture of chalcedony or other amorphous silica phase with the hydrous form of Fe. Therefore, there was a mixture of Fe oxyhydroxide and the amorphous form of silica with some minor contributions of carbonate to the beam: about 25% based on a comparison of the C concentration with pure carbonate spots. At point 3, an increased content of Al was measured that was probably bound in illite. This mineral was determined by X-ray diffraction.
3.4.3. PSZ3 Sample—Rock from the Area of Piekary Śląskie (Zawisza Czarny Street)
In the first stage of the study of the PSK2 sample, like in the case of previous samples, the distributions of the following elements were measured: Mn, Ca, S, Si, Al, Mg, O, C, and Fe in micro-area 1 of this sample (Figure 13).
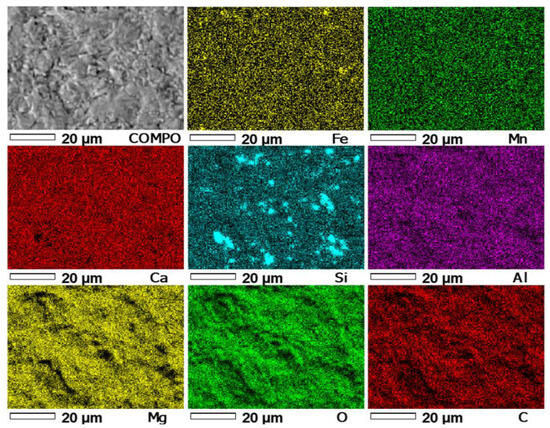
Figure 13.
Maps of the distribution of EDS elements in micro-area 1 of the PSZ3 sample. COMPO—Image of the composition of micro-area 1 of sample PSZ3.
The superposition of the Ca and Mg distribution images (Figure 13) allows the determination of the presence of these elements in the same areas, which indicates the presence of calcium–magnesium carbonate phases. The superposition of the Ca, Mg, and Fe distribution images allows the determination of the presence of carbonate phases rich in Mg with the substitution of Fe (dolomite phases) in some areas of the sample. The occurrence of Si and Al in the same areas of the Si and Al images indicate, similar to the previous samples, the presence of aluminosilicates (possibly illite). The remaining Si is probably quartz, similar to previous samples.
In micro-areas 1 and 2 of the PSZ3 sample, the contents of C, O, Mg, Al, Si, Ca, Mn, and Fe were measured using the EDS method (Figure 14, Table 7). The results of microprobe measurements made in micro-area 1 using the EDS method indicate the dominance of carbonate phases with an increased magnesium content, higher than that typical for dolomite, but slightly lower than the stoichiometric value of huntite. As in the case of the PSK2 sample, de-huntite is also present in this sample. Thus, the crystals of original huntite were probably transformed during dehuntization. As in the case of micro-area 1 of the PSZ3 sample, the de-huntite phase dominates in micro-area 2.
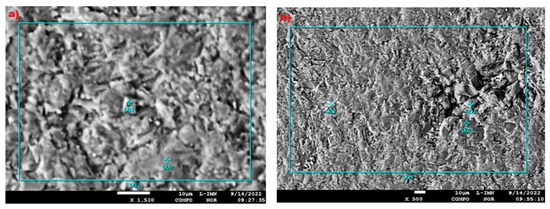
Figure 14.
Image of the composition of micro-areas of the PSZ3 sample with marked places of EDS quantitative analysis: (a) micro-area 1, (b) micro-area 2.

Table 7.
EDS analysis results for micro-areas 1 and 2 of the PSZ3 sample.
The results of microprobe measurements made in micro-area 1 using the EDS method indicate the dominance of carbonate phases with an increased magnesium content, higher than that typical for dolomite, but slightly lower than the stoichiometric value of huntite. As in the case of the PSK2 sample, de-huntite is also present in this sample. Thus, the crystals of original huntite were probably transformed during dehuntization. As in the case of micro-area 1 of the PSZ3 sample, the de-huntite phase dominates in micro-area 2.
In the third micro-area of the PSZ3 sample, measurements of the elemental content using the WDS method were performed (Figure 15, Table 8).
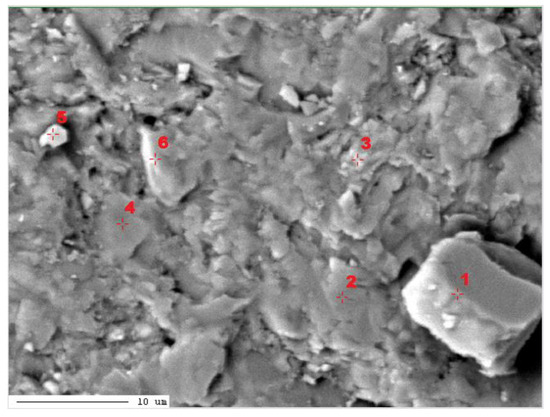
Figure 15.
Image of the composition of micro-area 3 of the PSZ3 sample with marked points of quantitative analysis. 1–6-points of chemical analysis.

Table 8.
Results of WDS analysis in micro-area 3 of the PSZ3 sample.
The measurements carried out in micro-area 3 of the PSZ3 sample indicate the presence of three carbonate phases: low-magnesium calcite (point 1), high-magnesium calcite (point 3), and proto-dolomite (points 2, 4, 5, and 6) (Figure 15, Table 8). At point 3, an increased content of Fe was measured. Fe substitutes for Mg in high-Mg calcite crystal. It is possible that Fe likely forms a solid solution with ankerite or siderite [29,31,32]. However, these minerals were not identified by X-ray diffraction.
4. Discussion
The objective of this project was to study carbonate rocks of the Tarnowice Unit of selected areas of Upper Silesia (Silesian Voivodeship) to determine their geochemical and mineralogical properties. This topic was undertaken due to the probability of the presence of carbonate phases with different magnesium contents in these rocks, mainly the occurrence of high-magnesium calcite, which was identified in the rocks of the Lower Muschelkalk in the area of Opole Silesia. The Tarnowice Unit, which is the Middle Muschelkalk Formation, has not been studied in detail in this respect so far. The research zone includes the areas of Piekary Śląskie and Bytom. Samples of carbonate rocks were collected in the Lazarówka Quarry (area of Bytom city) and in the area of Piekary Śląskie, on Mieczysław Karłowicz Street and Zawisza Czarny Street.
4.1. Mineral Phases and the Trace Elements of the Triassic Dolomites
Macroscopically examined carbonate rocks differ slightly, mainly in terms of color. The intensity of the brownish color is probably associated with the presence of Fe minerals (oxides). The rocks from the Lazarówka Quarry show a brownish color, while the rocks from the area of Piekary Śląskie have colors from light brown to gray, a sparite texture, and a chaotic, compact, and in some areas, porous rock structure. Rocks from the Lazarówka Quarry are characterized by reduced compactness. A weak reaction with hydrochloric acid indicates dolomite dominance in these rocks. Microscopic images often reveal a biomorphic texture. The rocks consist of oval or elongated bioclasts bonded with contact or pore cement. Sparite, euhedral, and rhombohedral dolomite crystals have also been observed microscopically. Some crystals are filled with iron compounds. These crystals are probably pseudomorphs after dolomite.
Many interesting data were provided by the results of X-ray fluorescence (XRF). Based on the results of the analysis, the basic oxide composition of the rocks, the Ca to Mg ratio, and the MgCO3 content were calculated. Based on the obtained data, the position of the studied carbonate rocks in the classifications of Chilingar [29] and Pettijohn [30] was presented. The results of the study indicate that the carbonate rocks represent lime dolomites in the Chilingar [29] classification. According to Pettijohn [30], classification samples PSK1, PSK3, and PSZ2 represent lime dolomites and samples LZ2, LZ3, and PSZ1—calcareous dolomites. Therefore, these rocks, apart from the mineral dolomite, also contain calcite phases, including high-magnesium calcite. This was confirmed by the X-ray diffraction results.
Moreover, the results of the XRF analysis showed, apart from the high contents of elements such as Ca, Mg, C, and O, the presence, in trace amounts, of elements characteristic of minerals such as silicates, aluminosilicates, oxides, and sulfides, which, apart from Sr and Ba, are not basic components of carbonate rocks. These are Al, Si, Fe, K, Na, Ti, Cr, Mn, Zr, Co, Cd, Ni, Cu, Zn, Pb, As, Rb, Br, and Cl. These elements are most often supplied from the outside to the sea basin where carbonate sedimentation takes place. Some of them may occur in minerals formed during diagenetic processes. Manganese and iron can substitute Ca and Mg in carbonate minerals [31,32,33,34,35,36,37,38,39,40].
Strontium and barium are elements originally present in aragonite, an unstable phase of calcium carbonate, which, like high-magnesium calcite, is transformed into low-magnesium calcite during diagenesis. Sr and Ba often appear in the carbonate phases. According to literature data, Sr occurs in the skeletons of marine organisms [6,8,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Its presence in aragonite is associated with a larger ionic radius compared to the ionic radius of Ca; therefore, it easily enters the structure of aragonite, more similar to the structure of strontianite than to the structure of calcite. Therefore, aragonite contains a higher amount of Sr than calcite [8,31,32,33,34,35,36,39]. However, aragonite is an unstable phase of calcium carbonate, similar to high-magnesium calcite, and during diagenesis it is transformed into low-magnesium calcite. Thus, only the presence of strontium indicates that the primary phase of calcium carbonate was aragonite. Similar to Sr, Ba is found in the skeletons of marine organisms. Ba has a similar ionic radius to Sr [8,31,34,35,37], so it will be easier to enter the aragonite structure than calcite. Thus, only the presence of Sr and Ba indicates that the primary phase of calcium carbonate was aragonite.
Ni, K, Si, Al, as well as some Fe, are included in the aluminosilicates—mainly feldspars and clay minerals, but sulfides and arsenides cannot be formed [34,35,36]. S, Ti, Cr, some Fe, Ni, Cu, Zn, and Pb will probably be bound in sulfides and oxides. During the sedimentation processes, Ni is incorporated into the structures of clay minerals [31,34,35,36,40]. It can also form sulfides and arsenides. However, these minerals were not determined by X-ray diffraction. Ni can also appear together with Fe and Mn in smithsonite. Cd is usually found in zinc sulfides. It can also appear together with Fe, Mn, and Co in smithsonite [6,16,31,34,36]. Chlorine is bound in chlorides, and phosphorus is present in organic matter, but chlorides and organic matter were not studied in this project. Therefore, this is only a theoretical statement.
Some of the samples contain high Pb-Zn amounts. This supports the hypothesis that ore-forming and dolomitization fluids are related. Therefore, the similar processes of diagenesis that caused the mineralization with zinc and lead ores in the Śląsko-Krakowski Region probably also occurred there [2,28,44,45].
Detailed data were provided by the results of microprobe measurements. Based on the contents of elements, mainly Ca and Mg, determined during the measurements made in the micro-areas of the samples, the following carbonate phases with different Mg contents were determined: low-Mg calcite, high-Mg calcite, protodolomite, ordered dolomite, and huntite. On the basis of the microprobe measurements results, the chemical formulas of the carbonate phases were calculated. The results of studies in the sample micro-areas have shown that high-magnesium calcite has a Mg content higher than “pure” (low-Mg) calcite but lower than that of protodolomite [34,36,46]. The protodolomite is characterized by a lower content of magnesium in relation to the stoichiometric value for dolomite but higher than that of high-magnesium calcite [34,36,46]. Ordered dolomite has a content of Mg close to the stoichiometric value. Huntite determined by microprobe measurements is characterized by a lower Mg content than the stoichiometric value for this carbonate phase [34,36,46]. Therefore, this mineral phase was named de-huntite. The reduced magnesium content in de-huntite is probably the effect of the process of advanced-stage diagenesis—dehuntization [46]. This cannot exclude the theory, which assumes that the determined de-huntite phase may represent a huntite–dolomite solid solution or mixture.
The results of the microprobe measurements confirmed the data obtained by previous analytical methods.
The results of the executed tests show that dolomite phases (protodolomite and ordered dolomite—around 90%) dominate in the studied rocks. An increased content of high-Mg calcite (circa 8%) occurs in most rocks. Low-Mg calcite (about 5 to 8%) is present only in three rocks. Moreover, in three rocks, the huntite (around 5%) phase was also determined. The content of Mg allowed the naming of this phase as de-huntite. Quartz occurs in most rocks (about 1 to 2%). Illite was identified by X-ray diffraction in only one sample (PSK3). The estimated relative proportions of the mineral phases confirmed the classification of the studied rocks into the dolomite group.
4.2. Formation of Dolomites during Sedimentary and Diagenetic Processes
On the basis of the research results, a theory about the rocks of the Tarnowice Beds formation and the diagenetic processes that influenced the final structure of these rocks was formed.
During the Upper Muschelkalk in the Triassic Basin, the development of fauna was observed, which stopped during the Middle Muschelkalk [1,14,15,25,26]. The development of the fauna was a result of wide connections with the Alpine Sea during the sea transgression. The East Carpathian Gate was probably active then, and further into the West, the Rhine and Burgundy Gates. At the beginning of the transgression, the sea bottom was inhabited by crinoids, whose skeletons formed the trochite layers. These layers created the lower part of the Upper Muschelkalk. Layers with Myophoria Transversa Bornemann and Pecten discites (Schlotheim) are also characteristic here [40,41,42,44,45]. There are also limestones with glauconite. On the basis of the fauna that occurs here, it is possible to distinguish these deposits from the formations of the Middle Muschelkalk. In the upper part there are limestones with a greater content of marls, locally claystones, dolomites, and sometimes sandy limestones and calcareous sandstones. The Tarnowice Unit was deposited above the Diplopora dolomites. They are also called lamellar dolomites [8]. They were formed during the marine regressions of the Upper Muschelkalk when another complex of limestone deposits was formed [28]. This was mainly due to uplift movements, which led to the shallowing of the sea and then the emergence of the Silesian–Cracow area [6,44,45]. Accordingly, mixed carbonate-clastic deposits of the Upper Muschelkalk were formed at that time [44,45]. There was also the erosion of older sediments, which lasted until the beginning of the Late Triassic, i.e., the Carnian [28].
Thus, the Tarnowice Unit consists of typical sediments of a drying, shallow sea [42,43]. The sediments of the Tarnowice Unit were probably originally limestones, which were analogous to the deposits and the Middle Muschelkalk dolomitized during diagenetic processes. The dolomites in the Śląsko-Krakowski Region were mineralized with zinc and lead ores during the diagenesis. They were the subject of exploitation. The dolomites of the Tarnowice Unit could probably have been formed in the same way as the ore-bearing dolomites as a result of metasomatic processes, and because of this, they may include the carbonate phases with different magnesium contents.
4.3. Summary
To summarize the above discussion, it should be stated on the basis of the research results that the rocks of the Tarnowice Unit consist not only of the dolomite phases but also of other carbonate phases with different Mg contents. Among them, high-magnesium calcite dominates, which has not previously been studied in detail in this formation. It is an unstable phase of calcium carbonate, which is usually not preserved in older sediments such as the Triassic but is usually transformed like aragonite into low-magnesium calcite during diagenetic processes. Another novelty is the presence of huntite, a carbonate phase with a magnesium content higher than that of dolomite. It can be defined as magnesium–calcium carbonate. This carbonate phase has not previously been identified in the rocks of the Tarnowice Unit. This carbonate phase is characterized by a lower Mg content than the stoichiometric value for huntite; consequently, this phase was named de-huntite.
5. Conclusions
Based on the obtained test results and their interpretation, the following conclusions were formulated:
- The studied carbonate rocks are lime dolomites according to the Chilingar classification. Three of them in the classical classification of carbonate rocks by Pettijohn represent calcareous dolomites.
- The analyzed rocks contain the following carbonate phases: protodolomite, ordered dolomite, high-magnesium calcite, low-magnesium calcite, and lower amounts of huntite. High-magnesium calcite has a Mg content higher than that of “pure” (low-magnesium) calcite but lower than that of protodolomite. Protodolomite is characterized by a lower magnesium content compared to the stoichiometric value for dolomite but is higher than that of high-magnesium calcite, while ordered dolomite has a Mg content close to the stoichiometric value. During microprobe measurements, the magnesium–calcium carbonate phase was determined. The Mg content of this phase is higher than that of ordered dolomite but lower than that typical for huntite. This mineral was named de-huntite. The reduced Mg content in this phase is probably the effect of dehuntization—the process of advanced-stage diagenesis.
- Results of X-ray fluorescence showed the presence of elements, not only those typical of carbonate minerals, but also accessory elements: Al, Si, Fe, K, Na, Ti, Cr, Cd, Br, Cr, As, Mn, Zr, Co, Ni, Cu, Zn, Pb, and Cl. Some of them are part of silicates or aluminosilicates, sulfades or oxides. Some may occur in minerals formed during diagenetic processes.
- On the basis of the obtained data, a theory associated with the formation of the studied rocks was formed. According to the data, the rocks of the Tarnowice Unit are the sediments of the drying, shallow, epicontinental sea. They were re-flooded by seawater during the regressions of the Upper Muschelkalk period. The original sediments of the Tarnowice Unit were probably limestones that were dolomitized during metasomatic processes. This may be evidenced by the presence of carbonate phases that differ in terms of the magnesium content.
- The achievement of the article is the identification of carbonate phases rich in magnesium, which have not been studied so far in the formation of the Tarnowice Unit, such as high-magnesium calcite, protodolomite, ordered dolomite, and de-huntite. The test results therefore provide new data on the mineral composition and geochemistry of the Tarnowice Unit. The most important data are the type of carbonate phases rich in Mg. This information will be used for further studies of Triassic carbonate rocks, in particular in terms of the possibility of their practical use, especially, for example, as a sorbent in the desulphurization of flue gases in power plants.
Author Contributions
Conceptualization, K.J.S.-P. and R.J., methodology, K.J.S.-P. and R.J., investigations, K.J.S.-P., analysis of the results, K.J.S.-P., writing—original draft preparation K.J.S.-P. and R.J., writing—review and editing, R.J., visualization, K.J.S.-P., supervision, R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this manuscript can be found in the cited articles and in the authors’ database.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matysik, M. Reefal environments and sedimentary processes of the Anisian Karchowice Beds in Upper Silesia, Southern Poland. Ann. Soc. Geol. Pol. 2010, 80, 123–145. [Google Scholar]
- Senkowiczowa, H. Possibilities of formalizing the lithostratigraphic division of the Middle and Upper Triassic of the Silesian-Cracow Upland. Geol. Q. 1980, 24, 787–804. [Google Scholar]
- Niedźwiedzki, R. Lithostratigraphy of the Górażdże Formation of the Dziewkowice Information in Opole Silesia; Geological and Mineralogical Works of Wrocław University LXXI: Wroclaw, Poland, 2000. [Google Scholar]
- Niedźwiedzki, R.; Szulc, J.; Zarankiewicz, M.; Stone treasures of the Saint Anne Land. Geological Guide 2013. Available online: www.annaland.pl (accessed on 24 March 2023).
- Stanienda, K. Effects of Dolomitization Processes in the Triassic Limestone of Tarnów Opolski Deposit; Silesian University of Technology Press: Gliwice, Poland, 2011; ISBN 978-83-7880-071-2. [Google Scholar]
- Stanienda, K. Diagenesis of the Triassic limestone from the Opole Silesia in the Aspect of Magnesian Calcite Presence; Silesian University of Technology Press: Gliwice, Poland, 2013; ISBN 978-83-7335-872-0. [Google Scholar]
- Stanienda, K. Huntite in the Triassic limestones of Opolski Silesia. Miner. Resour. Manag. 2013, 9, 79–98. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Gogolin Beds from the area of Opole Silesia. Miner. Resour. Manag. 2014, 30, 17–42. [Google Scholar] [CrossRef]
- Stanienda, K. Carbonate phases rich in magnesium in the Triassic limestones of the East part of Germanic Basin. Carbonates Evaporites 2016, 31, 387–405. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Górażdże Beds from the area of Opole Silesia. Miner. Resour. Manag. 2016, 32, 67–92. [Google Scholar] [CrossRef][Green Version]
- Stanienda-Pilecki, K. Carbonate minerals with magnesium in Triassic Terebratula limestone in the term of limestone with magnesium application as a sorbent in desulfurization of flue gases. Arch. Min. Sci. 2017, 62, 459–482. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Magnesium calcite in Muschelkalk limestones of the Polish part of the Germanic Basin. Carbonates Evaporites 2018, 33, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Stanienda-Pilecki, K. The Use of Limestones Built of Carbonate Phases with Increased Mg Content in Processes of Flue Gas Desulfurization. Minerals 2021, 11, 1044. [Google Scholar] [CrossRef]
- Szulc, J. International Workshop—Field Seminar the Muschelkalk-Sedimentary Environments, Facies and Diagenesis-Excursion Guidebook and Abstracts; Opole: Kraków, Poland, 1990; pp. 1–32. [Google Scholar]
- Szulc, J. Early alpinie tectonics and lithofacies succesion in the Silesian part of the Muschelkalk Basin. In Muschelkalk; Hagdorn, H., Seilacher, A., Eds.; Goldschneck: Stuttgart, Germany, 1993; pp. 19–28. [Google Scholar]
- Shogenova, A.; Kleesment, A.; Shogenov, V. Chemical composition and physical properties of the rock. Est. Geol. Sect. Bull. 2005, 6, 31–38. Available online: https://kirjandus.geoloogia.info/reference/2546 (accessed on 25 January 2024).
- Santos, H.S.; Nguyen, H.; Venâncio, F.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- Lunger, W.H. Construction Materials: Crushed Stone, Sand, and Gravel. In Encyclopedia of Materials: Science and Techology; Elsevier: Philadelphia, PA, USA, 2001. [Google Scholar]
- Ngu, H. Carbon Capture Technologies in Reference Module in Earth Systems and Environmental Science; Elsevier Inc.: Malaysia, 2022. [Google Scholar]
- Li, Q.; Larusso, J.; Grand-Pierre, A.E.; Uitto, J. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6(-/-) mice-potential for treatment of pseudoxanthoma elasticum. Clin. Transl. Sci. 2009, 2, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.A.; Mackenzie, F.T.; Bishop, F.C.; Bischoff, W.D. Influence of temperature on the stability of magnesian calcite. Am. Mineral. 1991, 76, 1889–1896. Available online: http://www.minsocam.org/ammin/am76/am76_1889.pdf (accessed on 20 February 2020).
- Zhang, Y.; Dave, R.A. Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem. Geol. 2000, 163, 129–138. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Konishi, H.; Roden, E.E. A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. Am. Mineral. 2010, 95, 1650–1656. [Google Scholar] [CrossRef]
- Szulc, J. Middle triassic evolution of the Northern Peri-Tethys area is influenced by early opening of the Tethys Ocean. Ann. Soc. Geol. Pol. 2000, 70, 1–48. [Google Scholar]
- Szulc, J. Stratigraphy and correlation with Tethys and other Germanic subbasins. International Workshop on the Triassic of Southern Poland. In Proceedings of the Pan-European Correlation of Epicontinental Triassic 4th Meeting, Fieldtrip Guide, Zakopane, Poland, 3–8 September 2007; pp. 26–28. [Google Scholar]
- Szulc, J. Triassic of the Silesia-Cracow area. In Proceedings of the Materials of the 42nd Speleological Symposium, Kraków, Poland, 3–8 September 2007. [Google Scholar]
- Biernat, S. Reamble: Wilanowski S., Lewandowski J. (2016) Detailed Geological Map of Poland. Sheet 910—Bytom (M-34-50-D); Polish Geological Institute. National Research Institute: Warsaw, Poland, 1954. [Google Scholar]
- Bujoczek, M.; Gładysz, K.; Stępień, A. Tarnowskie Góry Underground—A Living Organism on a Selected Fragment of the Mine of the Fryderyk Mine (Blachówka—Western—Urban Glückhilfe); Project Report; Tarnowskie Góry Cave Mountain Club: Tarnowskie Góry, Poland, 2013. [Google Scholar]
- Chilingar, G.V. Classification of limestones and dolomites on basis of Ca/Mg ratio. J. Sed. Petrol. 1957, 27, 187–189. [Google Scholar]
- Pettijohn, F.J. Sedimentary Rocks, 3rd ed.; Cbs: New York, NY, USA, 1975. [Google Scholar]
- Boggs, S., Jr. Petrology of Sedimentary Rocks, 2nd ed.; Cambridge University Press: London, UK, 2010. [Google Scholar]
- Mackenzie, F.T.; Andersson, A.J. The Marine carbon system and ocean acidification during Phanerozoic Time. Geochem. Perspect. 2013, 2, 1–3. [Google Scholar] [CrossRef]
- Mavromatis, V.; Götschl, K.E.; Grengg, C.; Konrad, F.; Purgstaller, B.; Dietzel, M. Barium partitioning in calcite and aragonite as a function of growth rate. Geochim. Cosmochim. Acta 2018, 237, 65–78. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates; Elsevier: Amsterdam, The Netherlands, 1990; Volume 33, p. 707. [Google Scholar]
- Morse, J.W.; Andersson, A.J.; Mackenzie, F.T. Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and ‘‘ocean acidification’’: Role of high Mg-calcites. Geochim. Cosmochim. Acta 2006, 70, 5814–5830. [Google Scholar] [CrossRef]
- Polański, A. Basics of Geochemistry; Geological Publishing House: Warsaw, Poland, 1988. [Google Scholar]
- Tucker, M.E.; Wright, V.P. Carbonate Sedimentology; Blackwell Scientific Publications: Oxford, UK; Edinburgh Boston Melbourne: London, UK, 1990; pp. 366–372. [Google Scholar]
- Zhang, S.; Zhou, R.; DePaolo, D.J. The Seawater Sr/Ca Ratio in the Past 50 Myr from Bulk Carbonate Sediments Corrected for Diagenesis; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Zhang, S.; DePaolo, D.J. Equilibrium calcite-fluid Sr/Ca partition coefficient from marine sediment and pore fluids. Geochim. Cosmochim. Acta 2020, 289, 33–46. [Google Scholar] [CrossRef]
- Zhong, S.; Mucci, A. Calcite and aragonite precipitation from seawater solutions of various salinities: Precipitation rates and overgrowth compositions. Chem. Geol. 1989, 78, 283–299. [Google Scholar] [CrossRef]
- Makowski, H. Historical Geology; Geological Publishing House: Warsaw, Poland, 1977. [Google Scholar]
- Senkowiczowa, H.; Szyperko-Śliwczyńska, A. Stratigraphy and palaeography of the Triassic. Geol. Inst. 1972, 252. [Google Scholar]
- Czermiński, J. (Ed.) Geological Structure of Poland, Volume II, Catalog of Fossils, Part 2, Mesozoic; Geological Publishing House: Warsaw, Poland, 1970. [Google Scholar]
- Migaszewski, Z. Origin of the dolomites. Geol. Q. 1990, 34, 202. [Google Scholar]
- Migaszewski, Z. Current views on the genesis of dolomites. Geol. Q. 1990, 3, 111–117. [Google Scholar]
- Stanienda-Pilecki, K. Crystals structutres of carbonate phases with Mg in Triassic rocks, mineral formation and transitions. Sci. Rep. 2023, 13, 18759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).