Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Methods
2.2.1. XRD Tests
2.2.2. Flotation Tests
2.2.3. Zeta Potential Analysis
2.2.4. Contact Angle Measurements
2.2.5. FT-IR Spectra Analysis
3. Results and Discussion
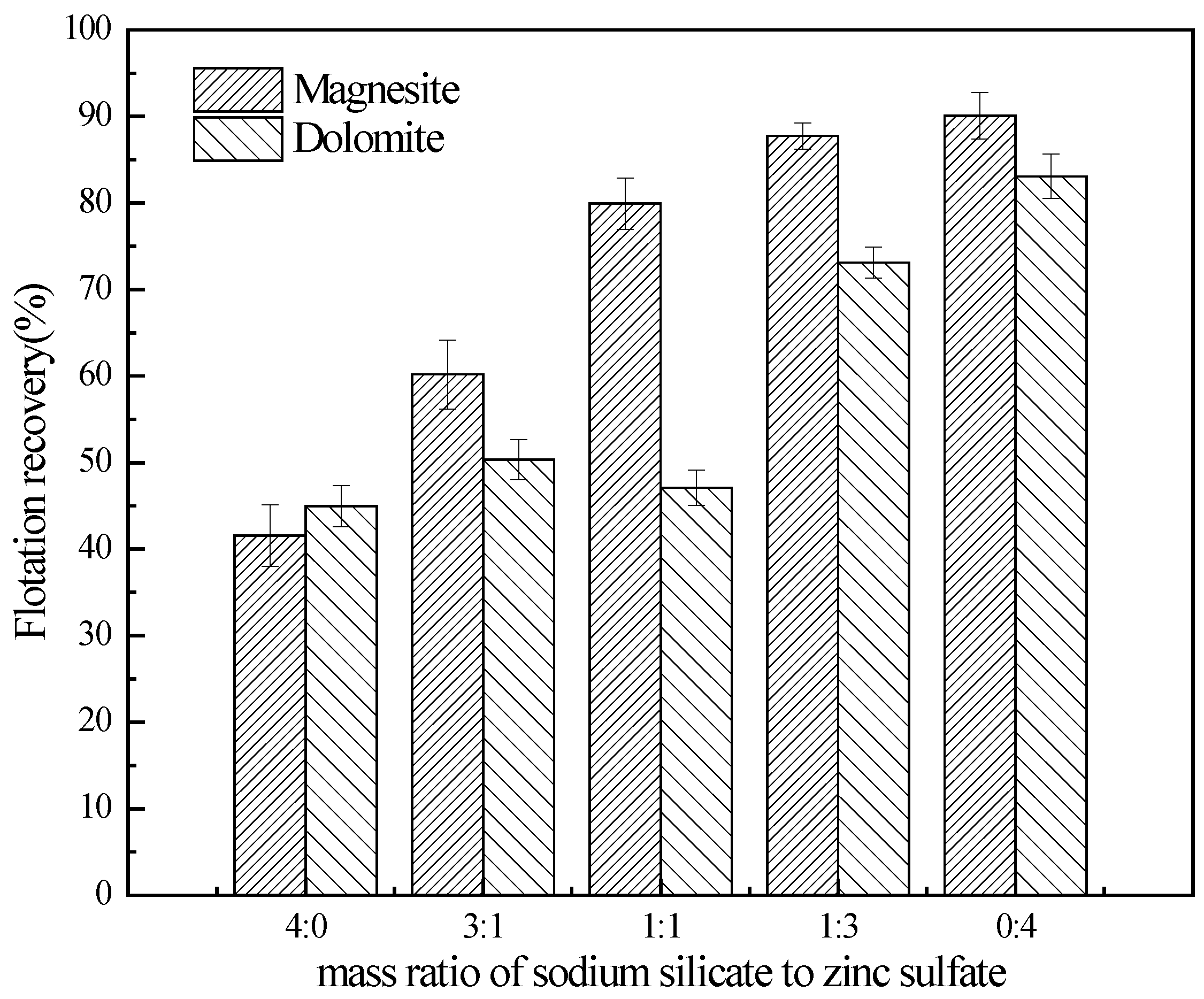
3.1. Flotation Studies
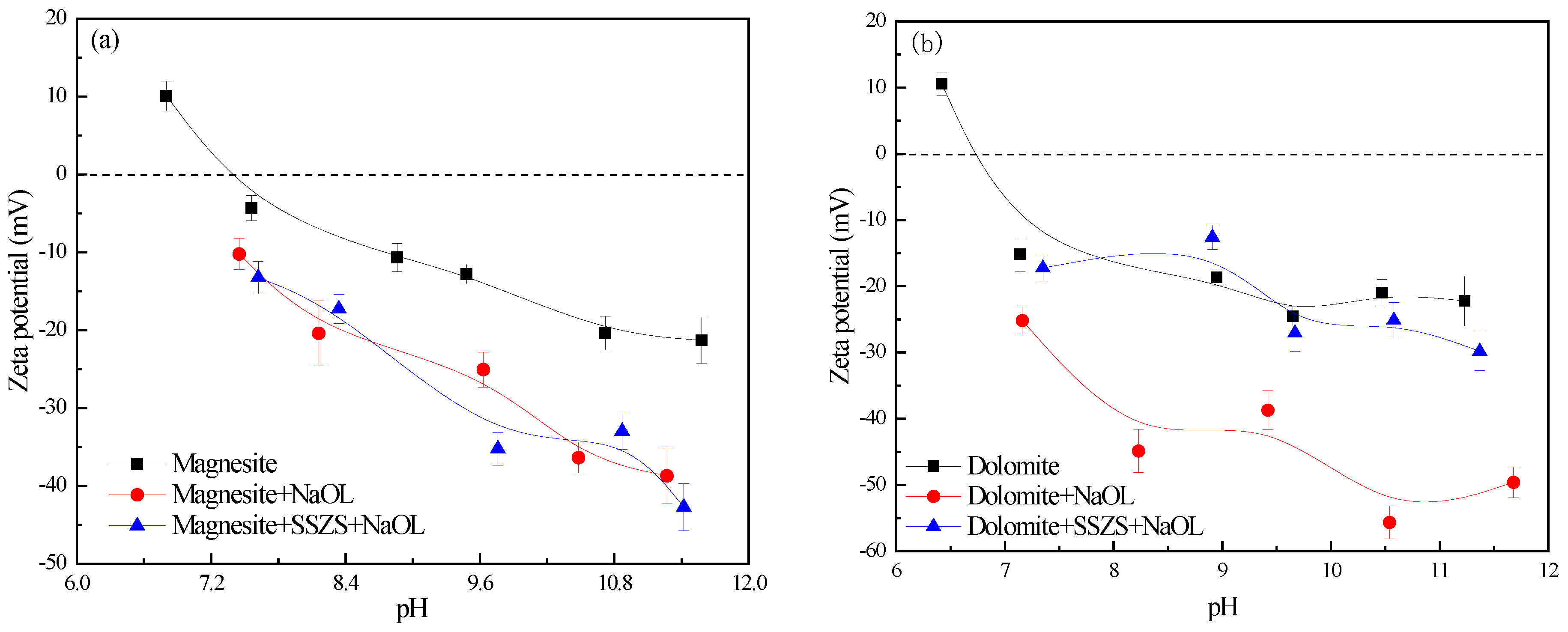
3.2. Zeta Potential Analysis
3.3. Contact Angle Measurements
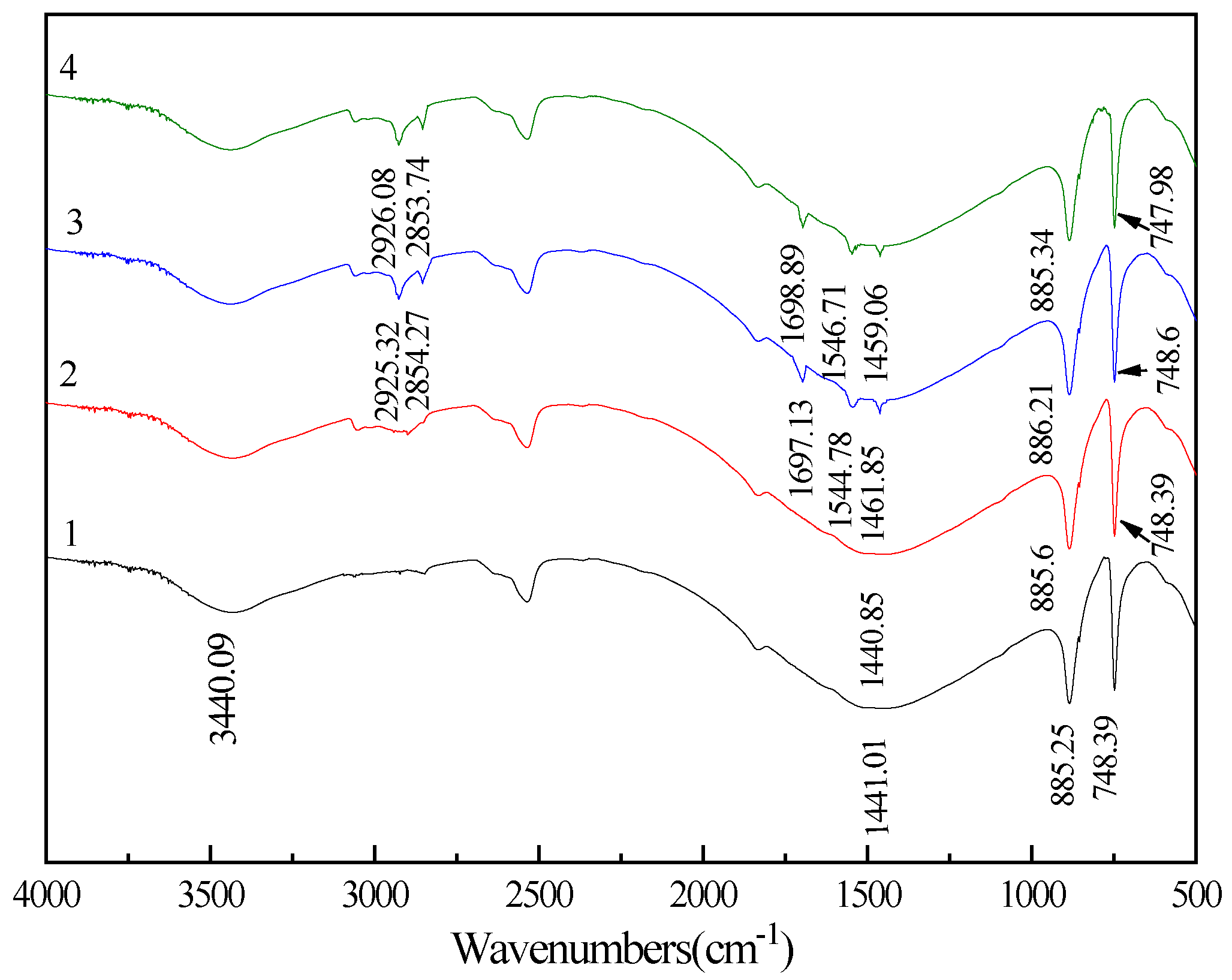
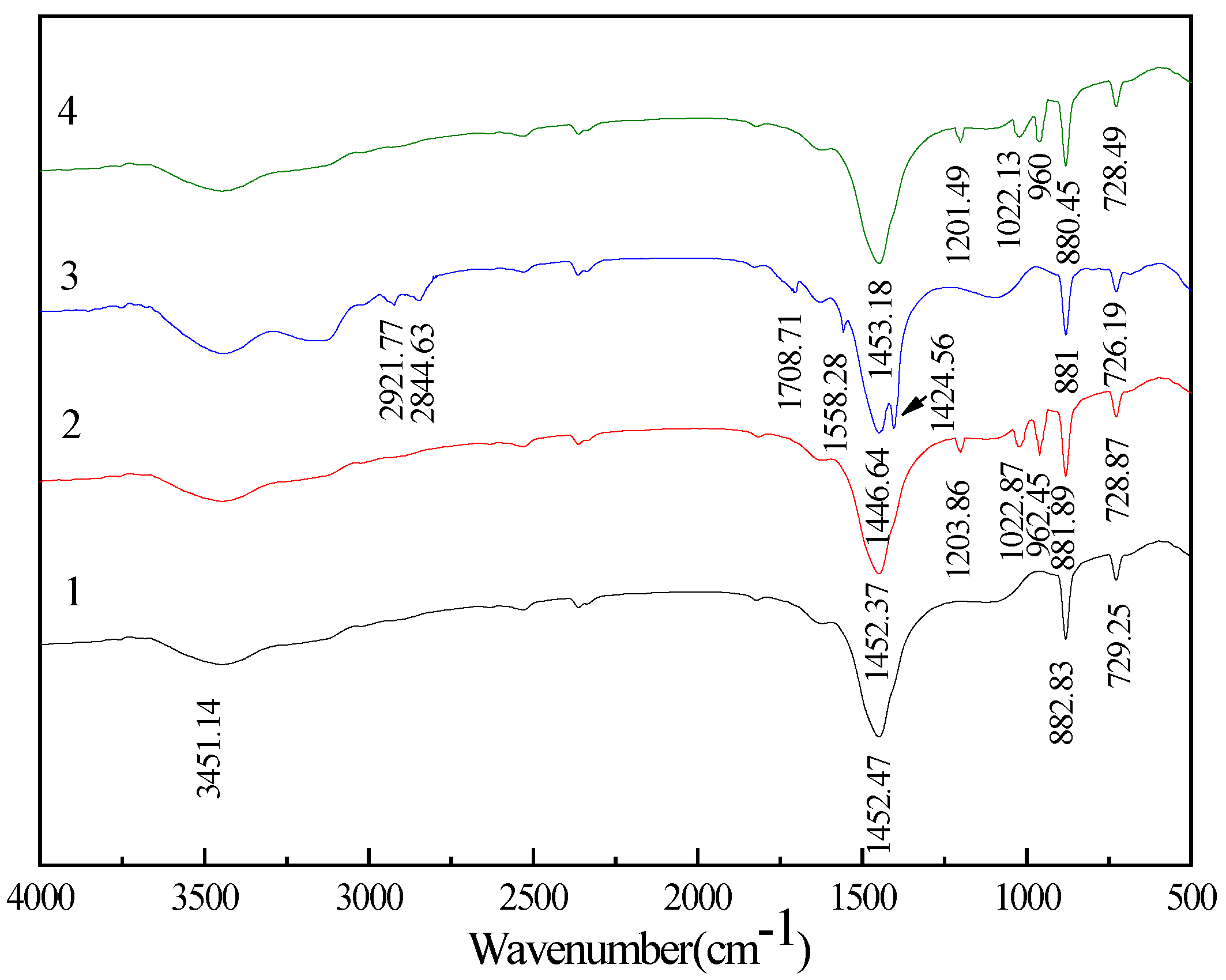
3.4. FT-IR Spectra Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tran, K.T.; Han, K.S.; Kim, S.J.; Kim, M.J.; Tran, T. Recovery of magnesium from Uyuni salar brine as hydrated magnesium carbonate. Hydrometallurgy 2016, 160, 106–114. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Hu, X.; Zhang, X.; Zheng, G. Flotation separation of quartz from magnesite using carboxymethyl cellulose as depressant. Trans. Nonferrous Met. Soc. China 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Peng, X.; Sun, W. Effect and mechanism of depressant amino trimethylene phosphonic acid on flotation separation of magnesite and dolomite. Conserv. Util. Miner. Resour. 2022, 42, 91–99. [Google Scholar]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Luo, N.; Wei, D.; Shen, Y.; Han, C.; Zhang, C. Elimination of the adverse effect of calcium ion on the flotation separation of magnesite from dolomite. Minerals 2017, 7, 150. [Google Scholar] [CrossRef]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Yin, W.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Metall. Mater. 2020, 27, 571–583. [Google Scholar] [CrossRef]
- Sun, W.H.; Liu, W.G.; Dai, S.J.; Yang, T.; Duan, H.; Liu, W.B. Effect of Tween 80 on flotation separation of magnesite and dolomite using NaOL as the collector. J. Mol. Liq. 2020, 315, 113712. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Yin, W.; Chen, X. Influence of sodium phosphate salts with different chain length on the flotation behavior of magnesite and dolomite. Minerals 2020, 10, 1031. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W.; Yang, B.; Han, F. Simultaneous separation of quartz and dolomite from magnesite using monosodium phosphateas a regulator via reverse flotation. Miner. Eng. 2021, 172, 107185. [Google Scholar] [CrossRef]
- Sun, H.; Han, F.; Yin, W.; Hong, J.; Yang, B. Modification of selectivity in the flotation separation of magnesite from dolomite. Colloids Surf. A 2020, 606, 125460. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Anionic flotation of magnesium carbonates by modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.H.; Sun, W.; Zeng, X.B.; Fang, S.; Han, H.S.; Hong, K. Use of Al2(SO4)3 and acidiffed water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Sun, R.F.; Liu, D.; Liu, Y.B.; Wang, D.Q.; Wen, S.M. Pb-water glass as a depressant in the flotation separation of fluorite from calcite. Colloids Surf. A 2021, 629, 127447. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.L.; Zhang, M.; Cui, R.; Shi, J.; Ning, J.F. Effect of Zn2+ and its addition sequence on flotation separation of scheelite from calcite using water glass. Colloids Surf. A 2020, 588, 124394. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.M.; Forssberg, E. Mechanism of oleate interaction on salt-type minerals, part II. Adsorption and electrokinetic studies of apatite in the presence of sodium oleate and sodium metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.G.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Qi, G.W.; Parentich, A.; Little, L.H.; Warren, L.J. Selective flotation of apatite from iron oxides. Int. J. Miner. Process. 1992, 34, 83–102. [Google Scholar]
- Ding, K.; Laskowski, J.S. Application of a modified water glass in a cationic flotation of calcite and dolomite. Can. Metall. Quart. 2006, 45, 199–206. [Google Scholar] [CrossRef]
- Jin, J.; Gao, H.; Chen, X.; Peng, Y. The separation of kyanite from quartz by flotation at acidic pH. Miner. Eng. 2016, 92, 221–228. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Zhou, Q.; Lu, S. Acidized sodium silicate-an effective modifier in fluorite flotation. Miner. Eng. 1992, 5, 435–444. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, W.G.; Chen, X.D.; Wang, Z.H.; Liu, W.B.; Sun, W.H.; Shen, Y.B. The role of sodium tripolyphosphate in wet grinding process of magnesite. Colloids Surf. A 2023, 668, 131449. [Google Scholar] [CrossRef]
- Cao, Z.F.; Chen, P.; Yang, F.; Wang, S.; Zhong, H. Transforming structure of dolomite to enhance its ion-exchange capacity for copper(II). Colloids Surf. A 2018, 593, 201–208. [Google Scholar] [CrossRef]
- Sun, H.R.; Yin, W.Z. Selective flotation separation of magnesite from quartz by palmitoyl trimethylammonium chloride. Sep. Purif. Technol. 2022, 295, 121201. [Google Scholar] [CrossRef]
- Wright, K.; Cygan, R.T.; Slater, B. Structure of the (1014) surfaces of calcite, dolomite, and magnesite under wet and dry conditions. Phys. Chem. Chem. Phys. 2001, 3, 839–844. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, H.Q.; Xu, Y.B.; Shu, K.Q.; Fang, S.; Xu, L.H. The effect of dissolved calcite species on the flotation of bastnaesite using sodium oleate. Miner. Eng. 2020, 145, 106095. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G. Electrokinetic and flotation behaviors of hemimorphite in the presence of sodium oleate. Miner. Eng. 2015, 84, 74–76. [Google Scholar] [CrossRef]
- Solotchina, È.P.; Solotchin, P.A. Composition and structure of low-temperature natural carbonates of the calcite-dolomite series. J. Struct.Chem. 2014, 55, 779–785. [Google Scholar] [CrossRef]
- El-Nahrawy, A.M.; Ali, A.I.; Abou Hammad, A.B.; Youssef, A.M. Influences of Ag-NPs doping chitosan/calcium silicate nanocomposites for optical and antibacterial activity. Int. J. Biol. Macromol. 2016, 93, 267–275. [Google Scholar] [CrossRef]
- Spiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnasite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Liao, R.P.; Wen, S.M.; Liu, J.; Zuo, Q.; Zheng, Y.X.; Luo, D.Q. Flotation behavior and mechanism of ilmenite using ferrate as activator. Miner. Eng. 2022, 178, 107400. [Google Scholar] [CrossRef]
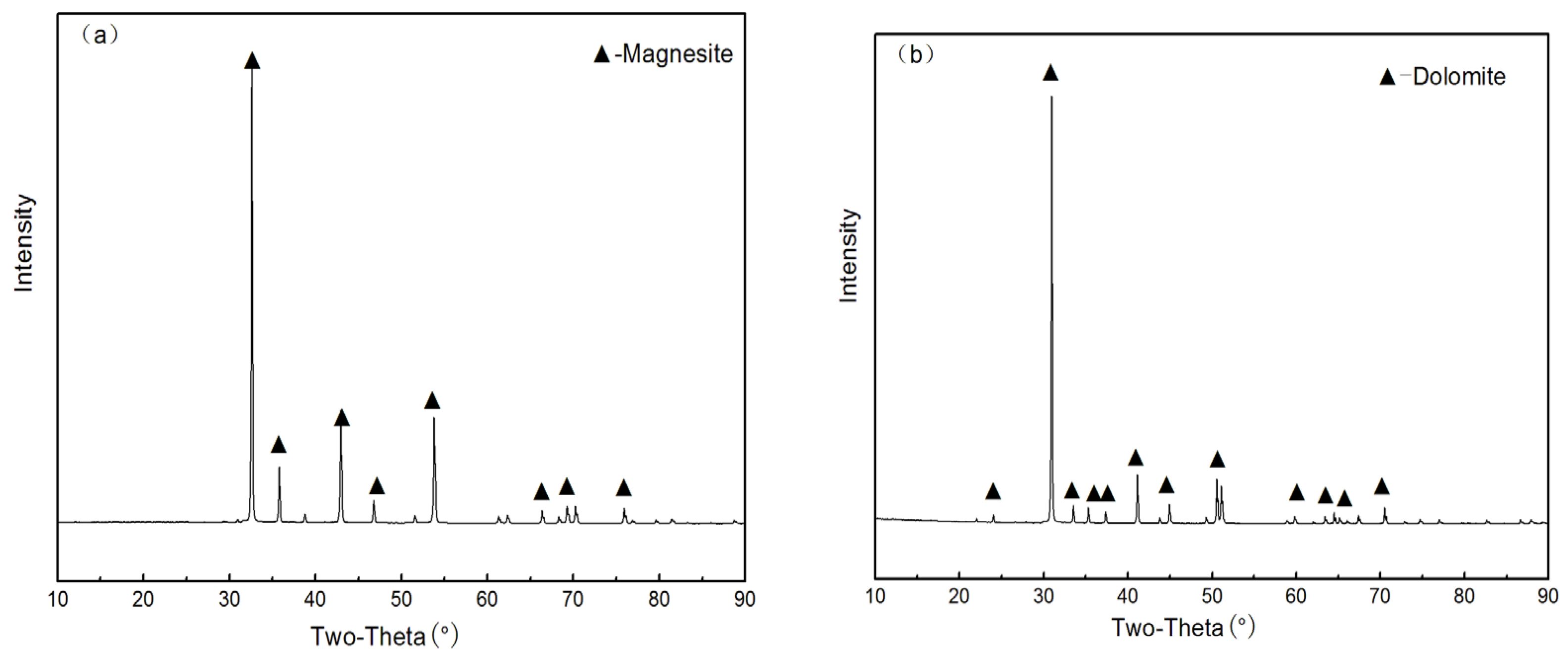
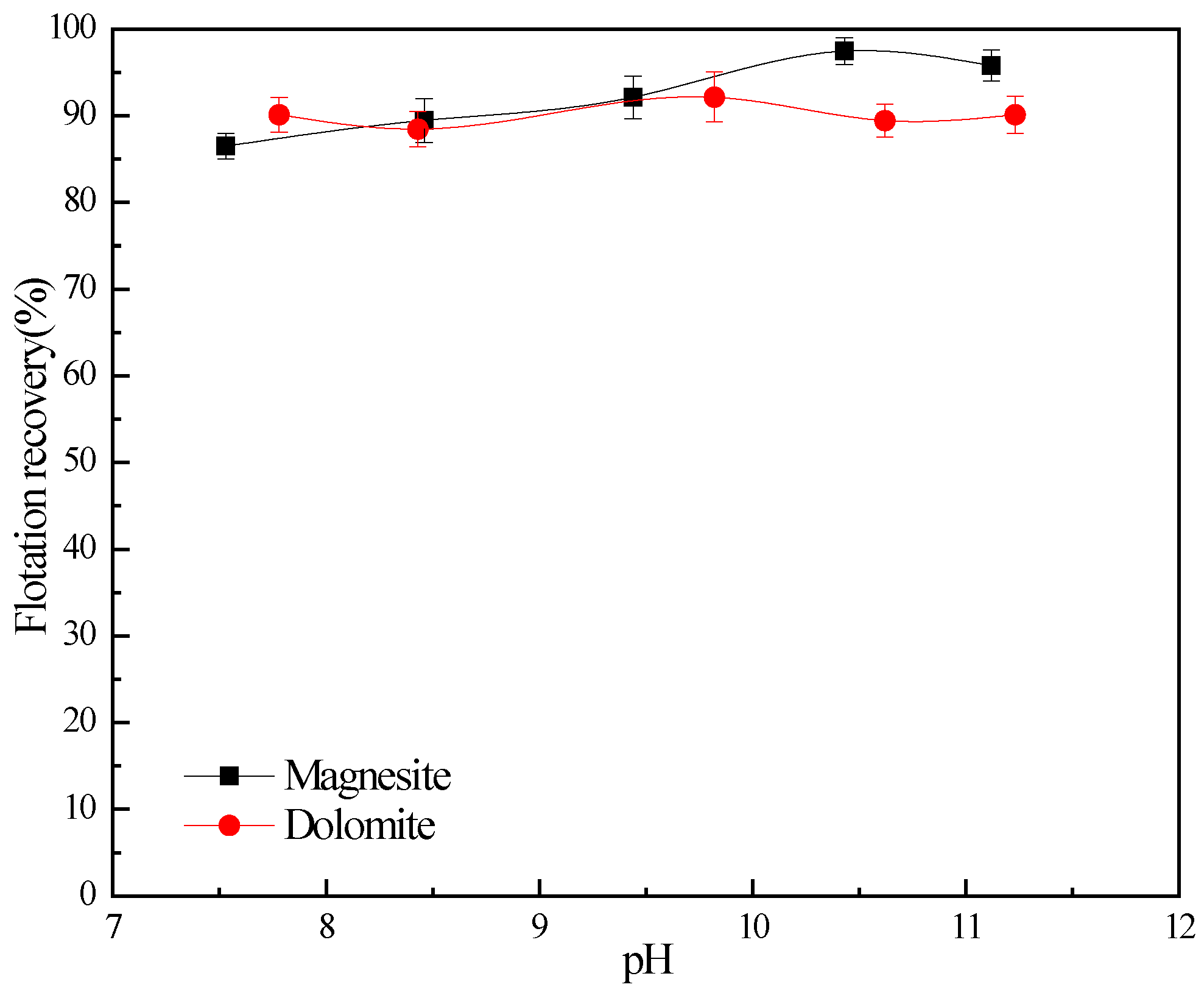
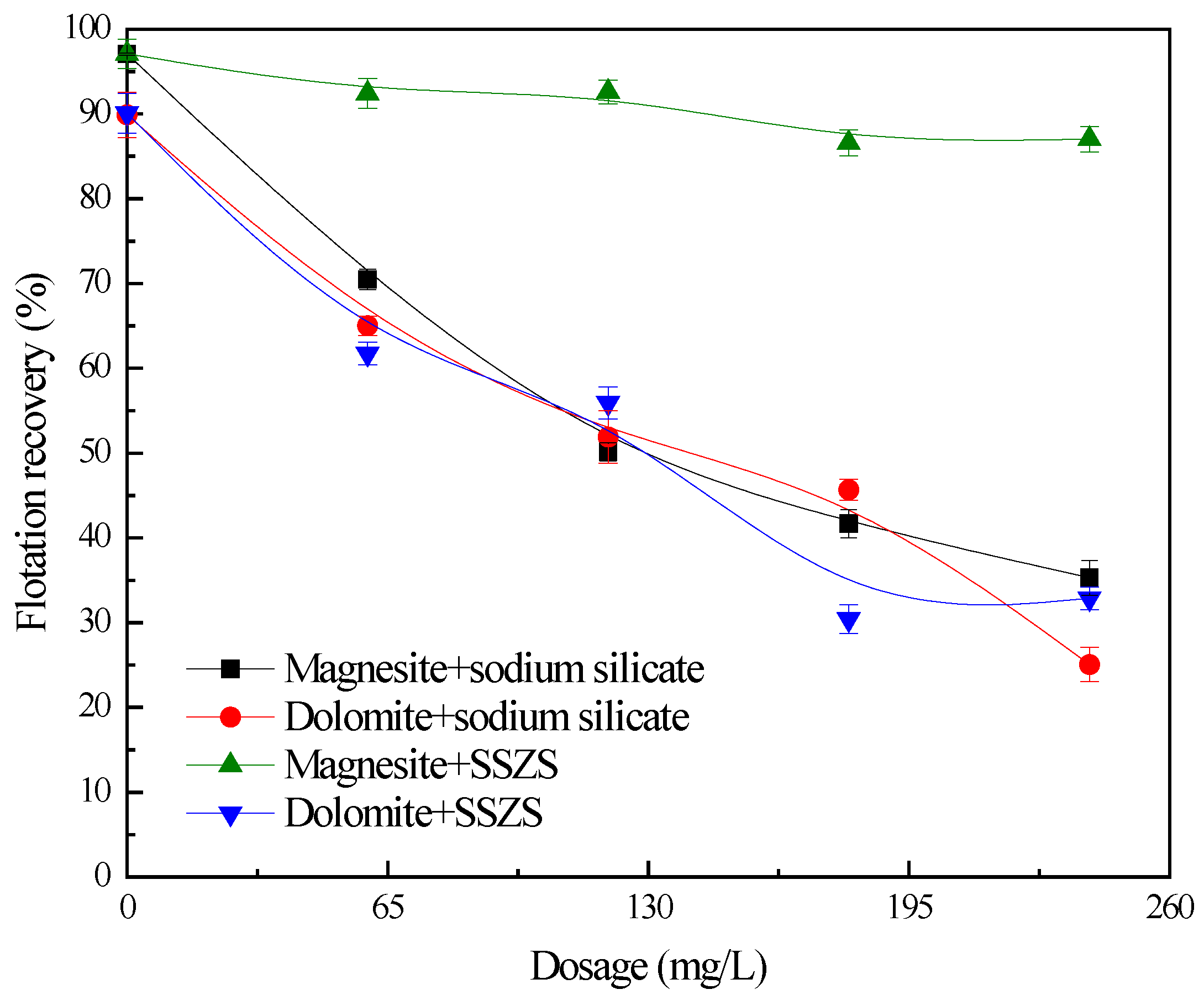
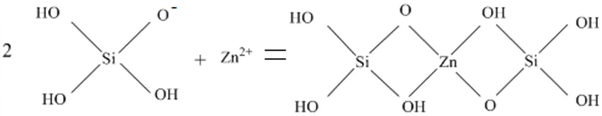
| Samples | MgO | CaO | SiO2 | Al2O3 | FeO |
|---|---|---|---|---|---|
| Magnesite | 47.24 | 0.17 | 0.19 | / | 0.17 |
| Dolomite | 21.52 | 30.13 | 0.21 | 0.10 | / |
| SSZS Dosage (mg/L) | Product | Weight Recovery (%) | Magnesite | Dolomite | ||
|---|---|---|---|---|---|---|
| Grade (%) | Recovery (%) | Grade (%) | Recovery (%) | |||
| 0 | concentrate | 97.45 | 49.85 | 97.43 | 50.15 | 97.46 |
| tailing | 2.55 | 50.19 | 2.57 | 49.81 | 2.54 | |
| raw ore | 100 | 49.86 | 100 | 50.14 | 100 | |
| 120 | concentrate | 68.12 | 62.13 | 83.63 | 37.87 | 52.23 |
| tailing | 31.88 | 25.99 | 16.37 | 74.01 | 47.77 | |
| raw ore | 100 | 50.61 | 100 | 49.39 | 100 | |
| 180 | concentrate | 59.13 | 75.21 | 87.99 | 24.79 | 29.64 |
| tailing | 40.87 | 14.85 | 12.01 | 85.15 | 70.36 | |
| raw ore | 100 | 50.54 | 100 | 49.46 | 100 | |
| Test System | Magnesite | Dolomite |
|---|---|---|
| Without reagent | 34.8° | 31.5° |
| With NaOL | 68.8° | 60.2° |
| With SSZS + NaOL | 67.4° | 33.5° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Shi, J.; Yan, B.; Wang, X. Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals 2024, 14, 355. https://doi.org/10.3390/min14040355
Luo N, Shi J, Yan B, Wang X. Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals. 2024; 14(4):355. https://doi.org/10.3390/min14040355
Chicago/Turabian StyleLuo, Na, Jingyang Shi, Baobao Yan, and Xiaoping Wang. 2024. "Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant" Minerals 14, no. 4: 355. https://doi.org/10.3390/min14040355
APA StyleLuo, N., Shi, J., Yan, B., & Wang, X. (2024). Flotation Separation of Magnesite from Dolomite Using Sodium Silicate Modified with Zinc Sulfate as a Selective Depressant. Minerals, 14(4), 355. https://doi.org/10.3390/min14040355






