Fluorite and Gibbsite Solubility Controls the Vertical Transport of Fluoride and Aluminum during Rainwater Percolation through Ashfall Deposits in La Palma (Canary Islands, Spain)
Abstract
1. Introduction
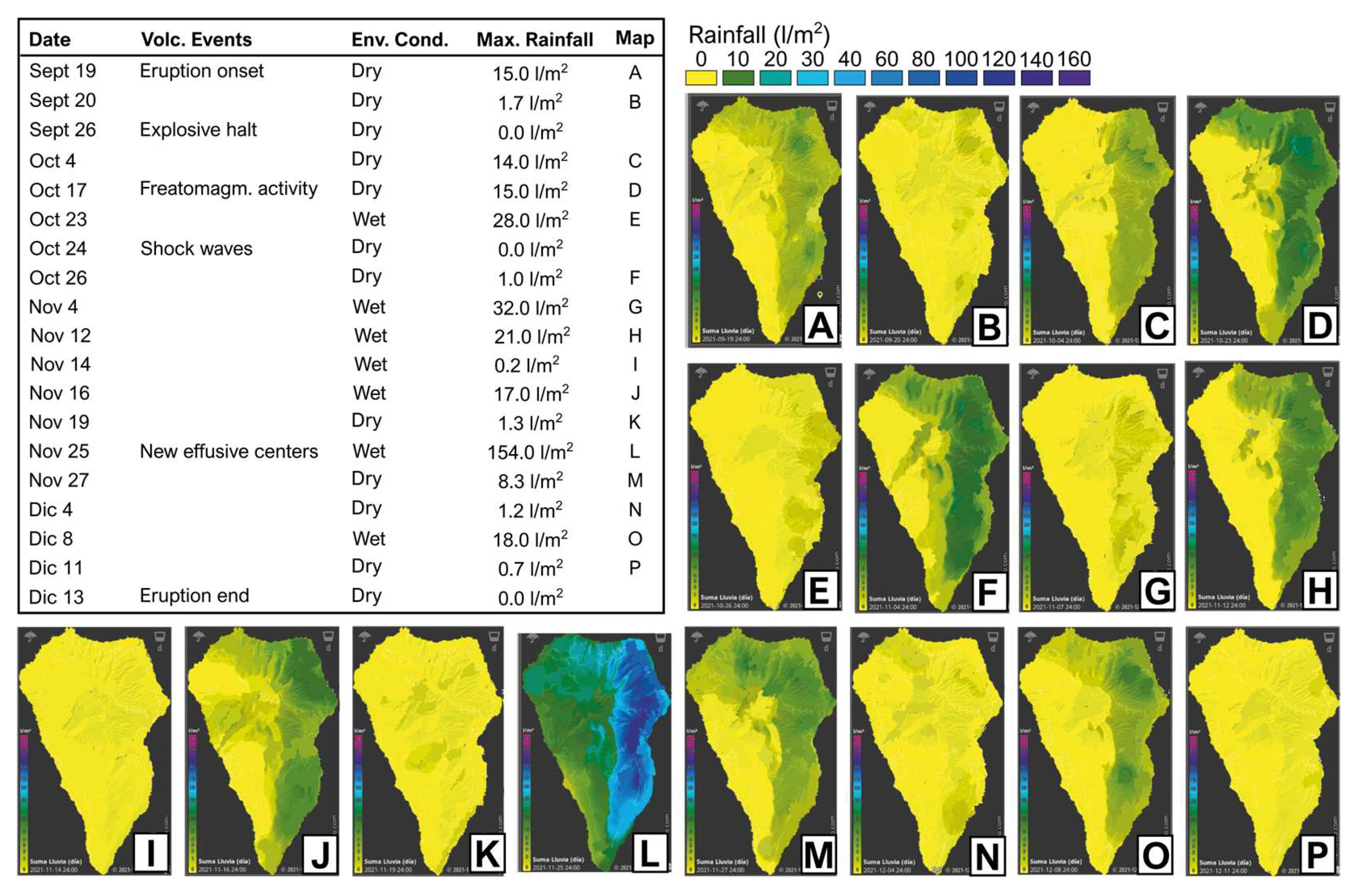
2. Climatic, Geographic and Geological Framework
3. Materials and Methods
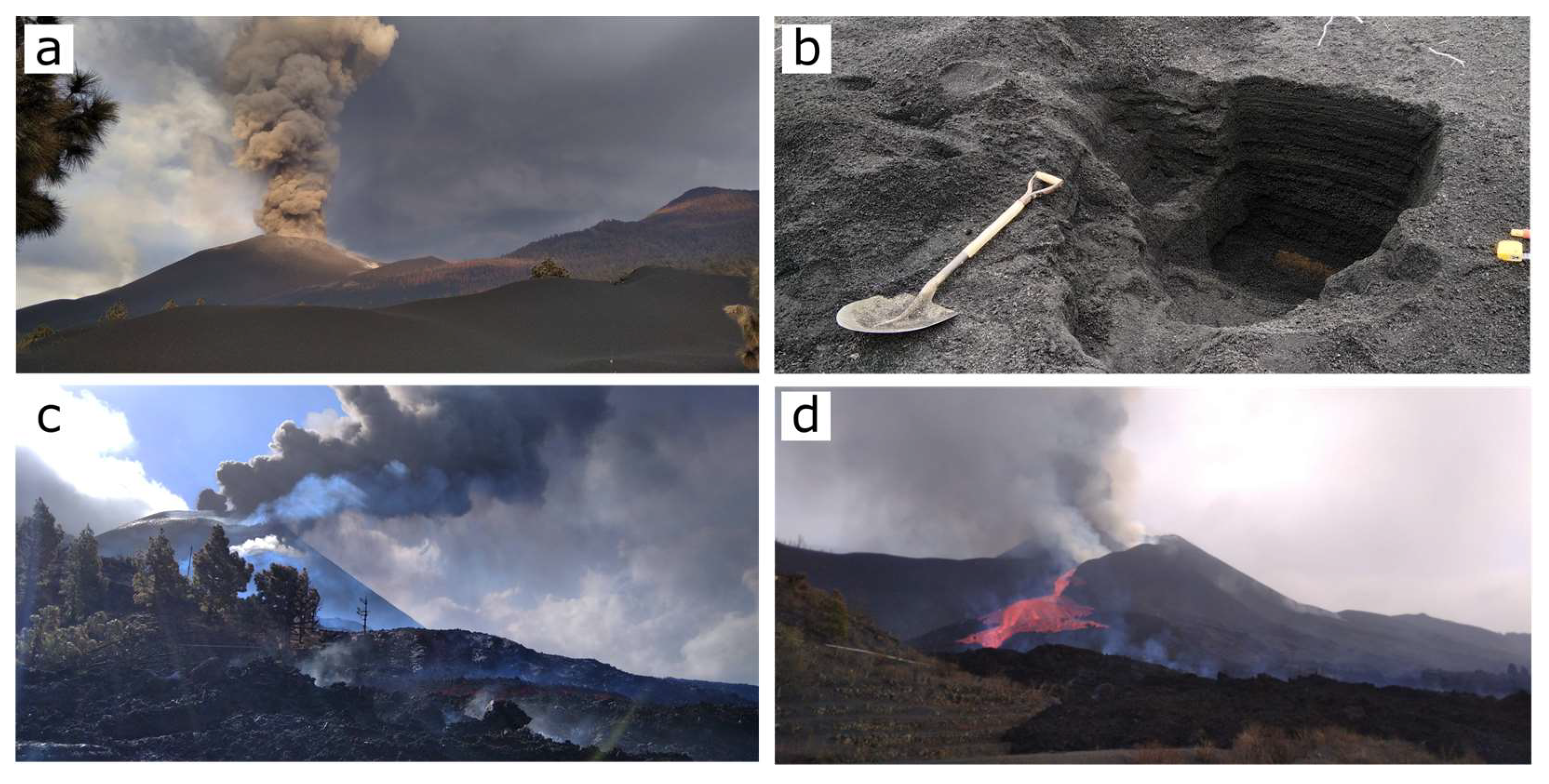
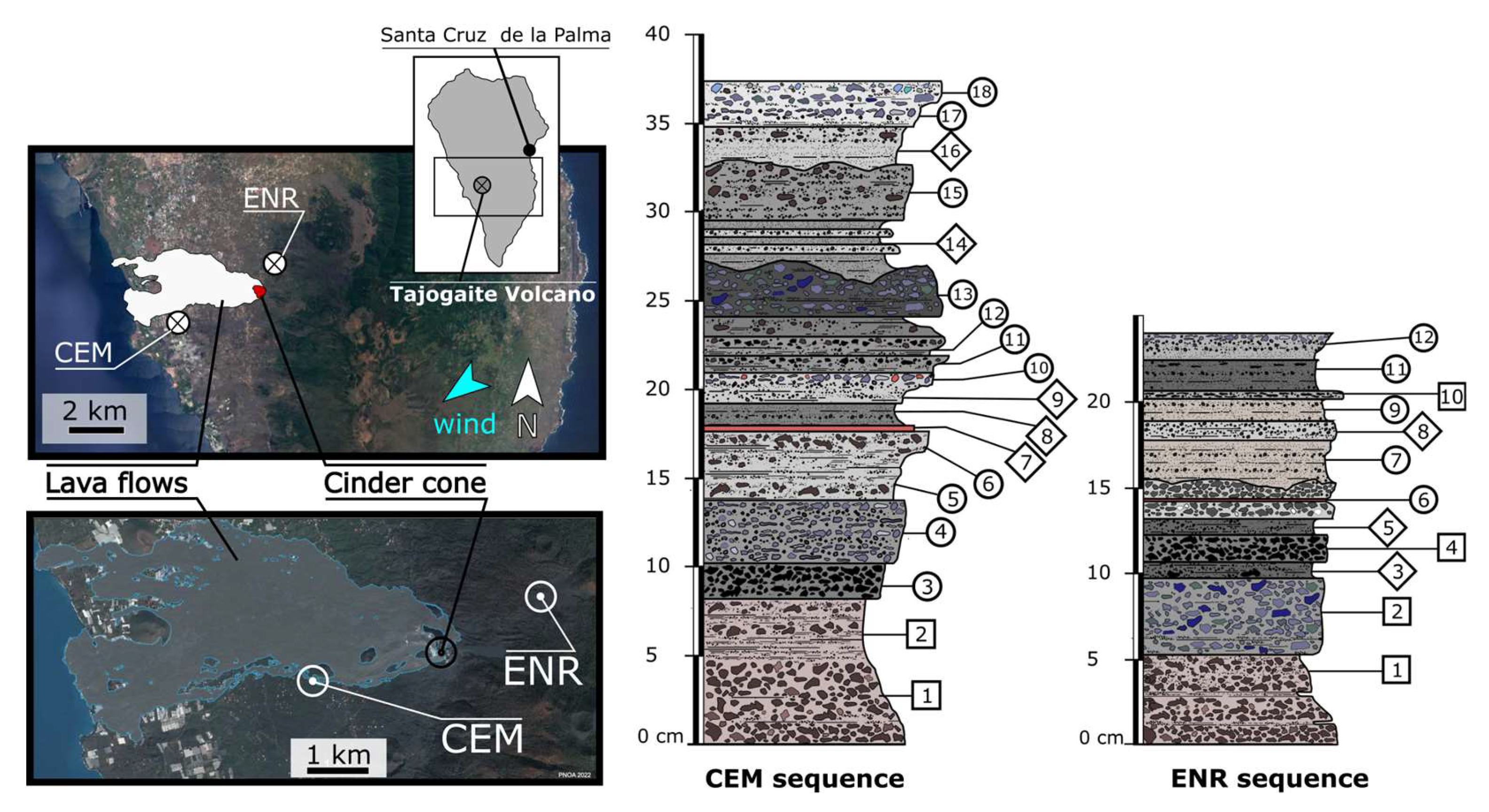
3.1. Sampling of Ashfall Deposits
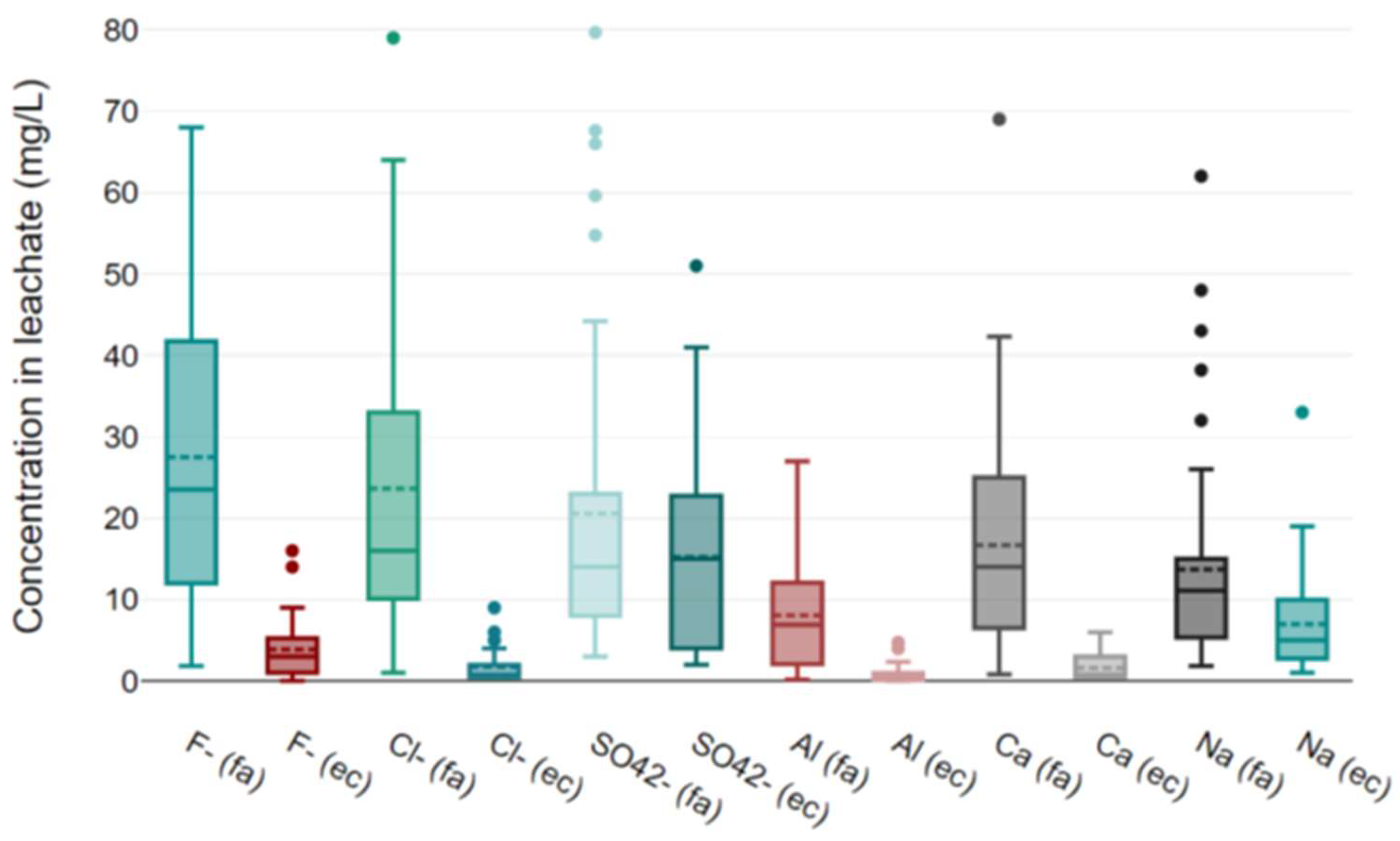
3.2. Ashfall Leaching Tests
3.3. Chemical Analyses of Leachates
3.4. Geochemical Modelling
4. Results and Discussion
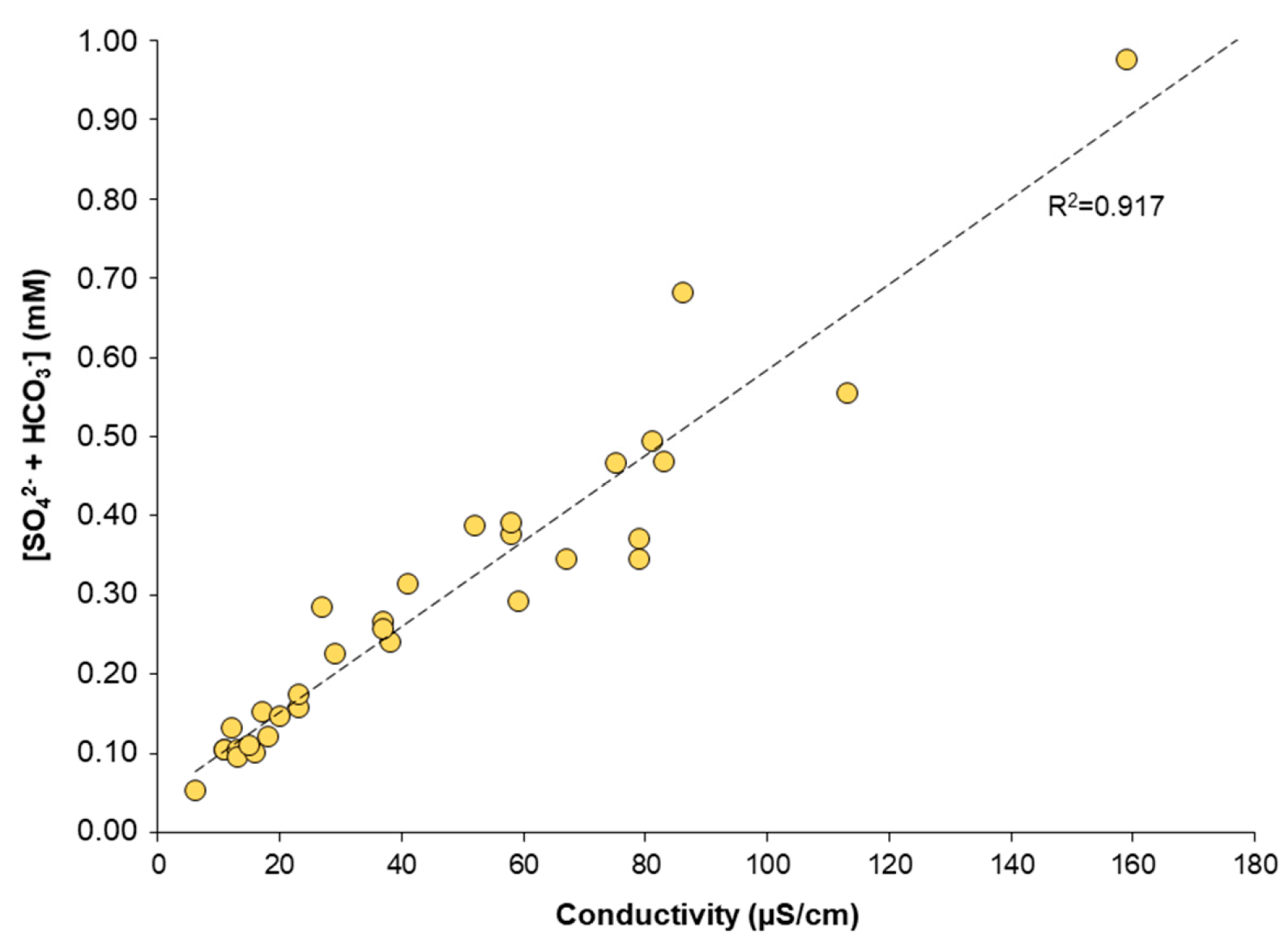
4.1. Vertical Profiles of pH, Sulfate, and Bicarbonate Concentration
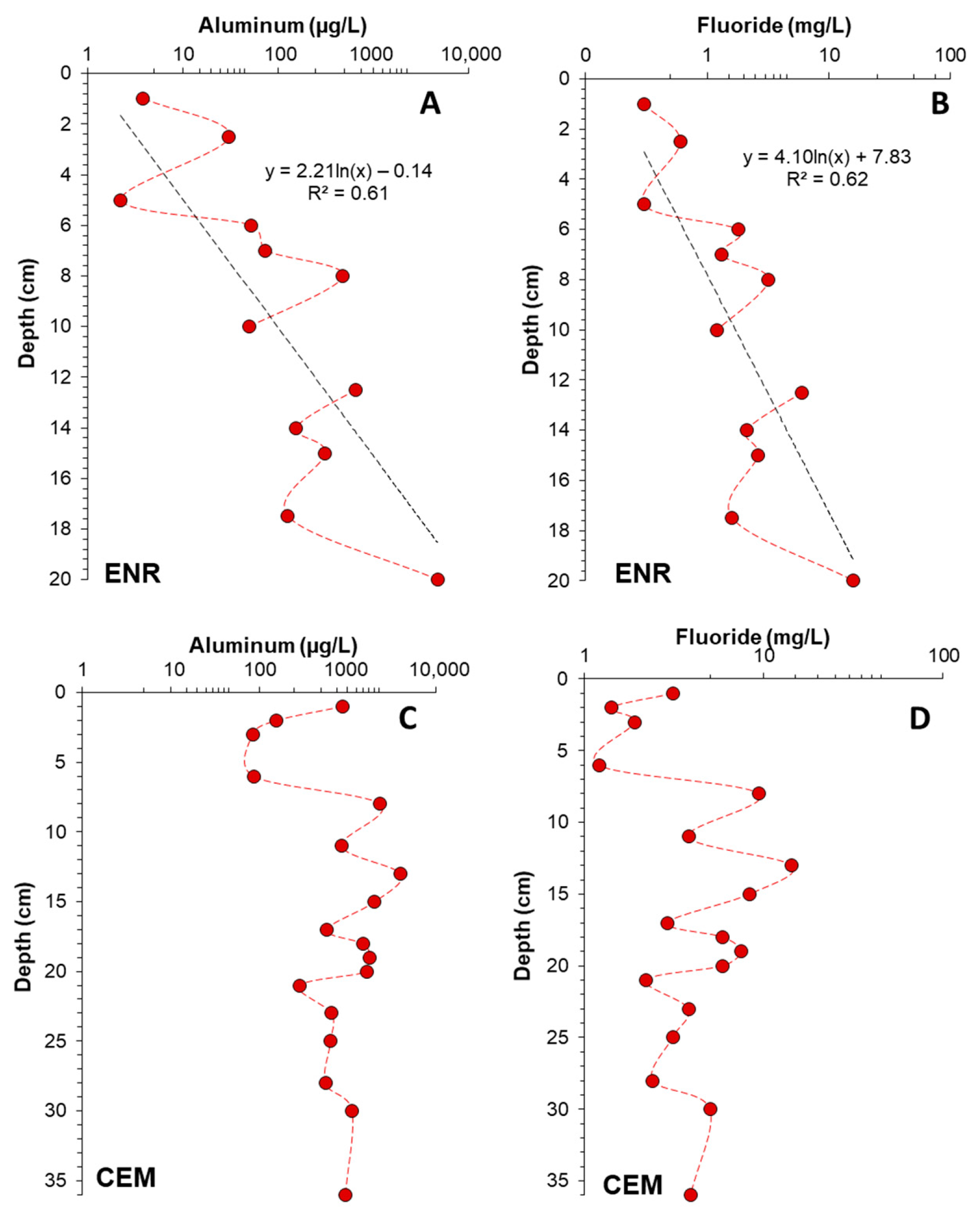
4.2. Aluminum and Fluoride Profiles
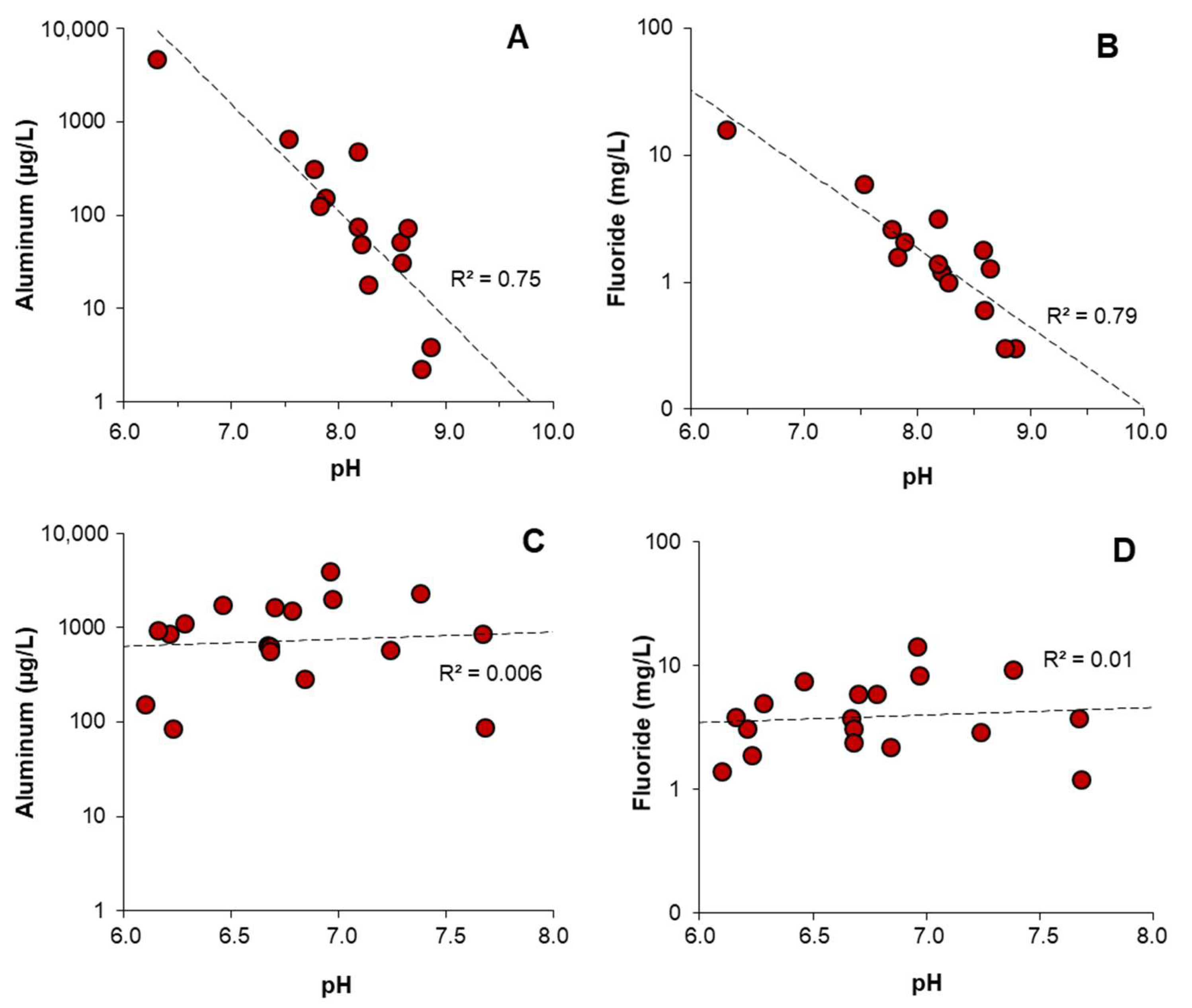
4.3. pH Control of Aluminum and Fluoride Concentrations
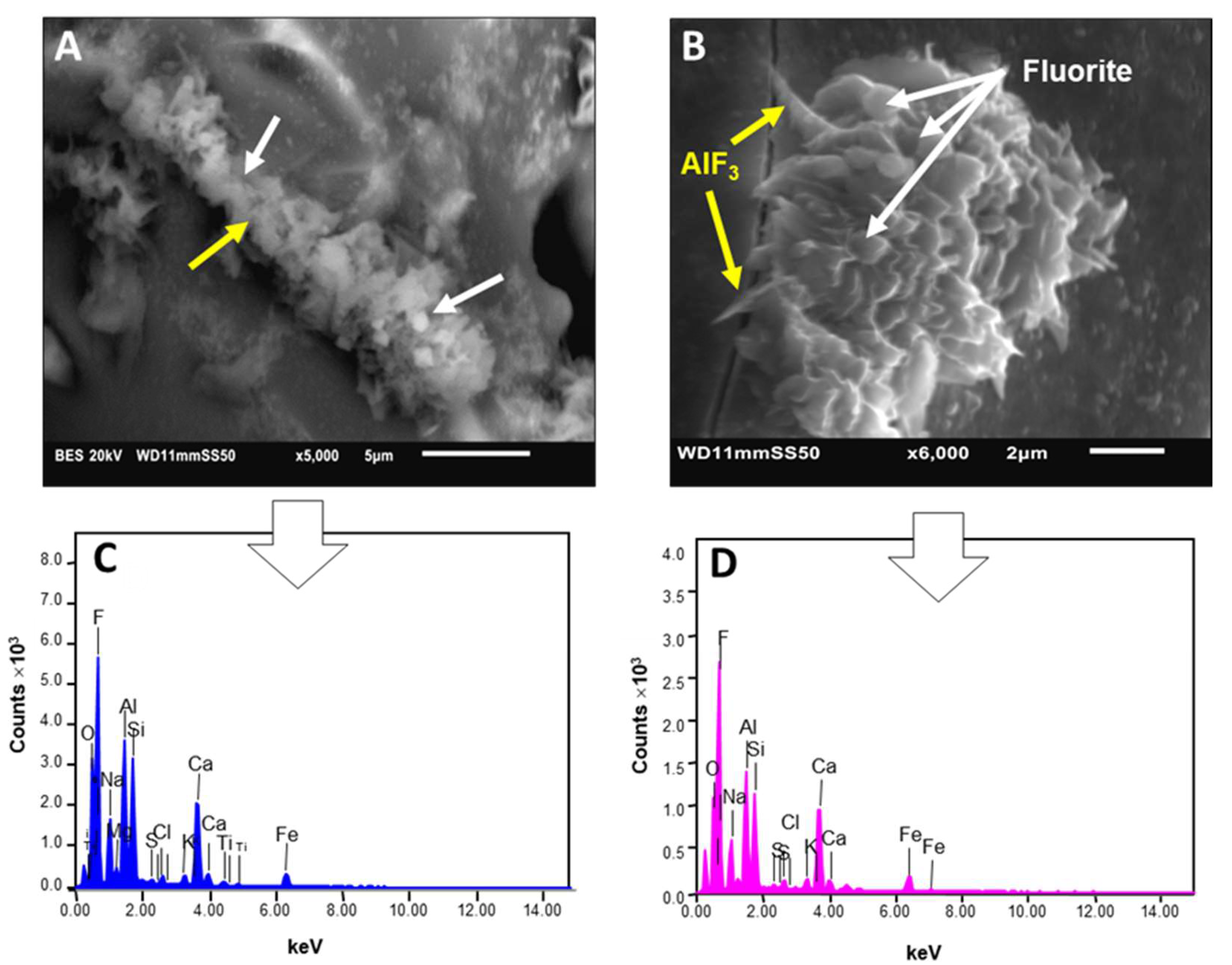
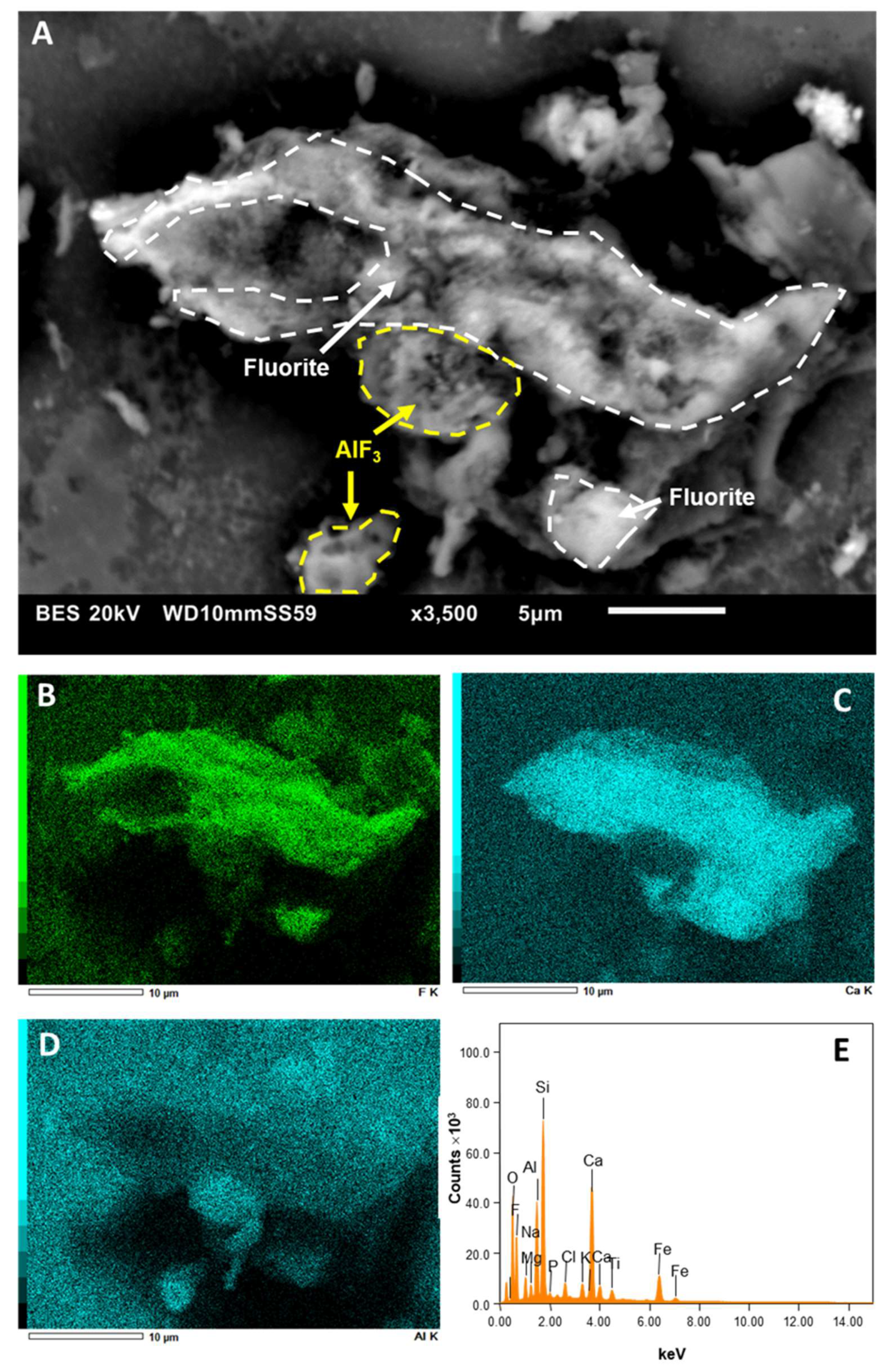
4.4. Al- and Ca-Fluorides Observed by SEM-EDX
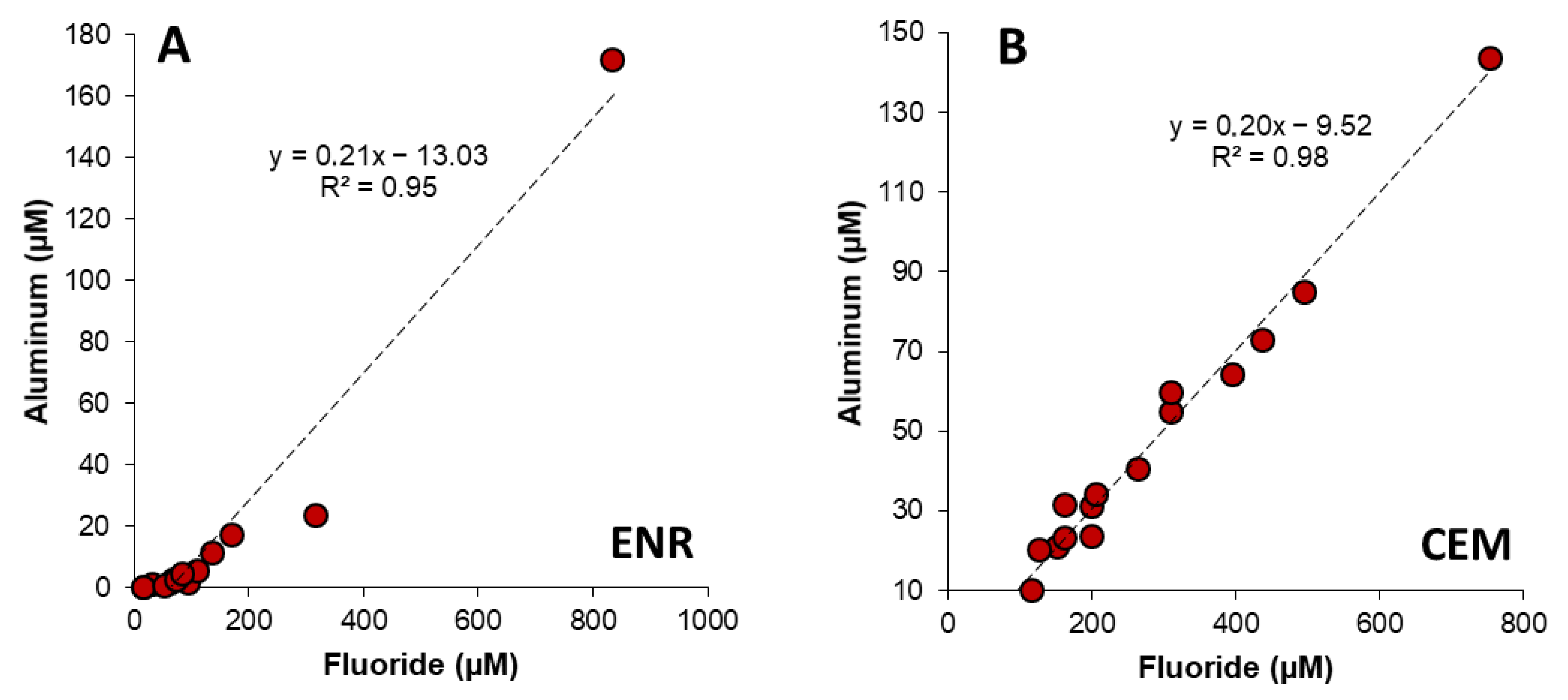
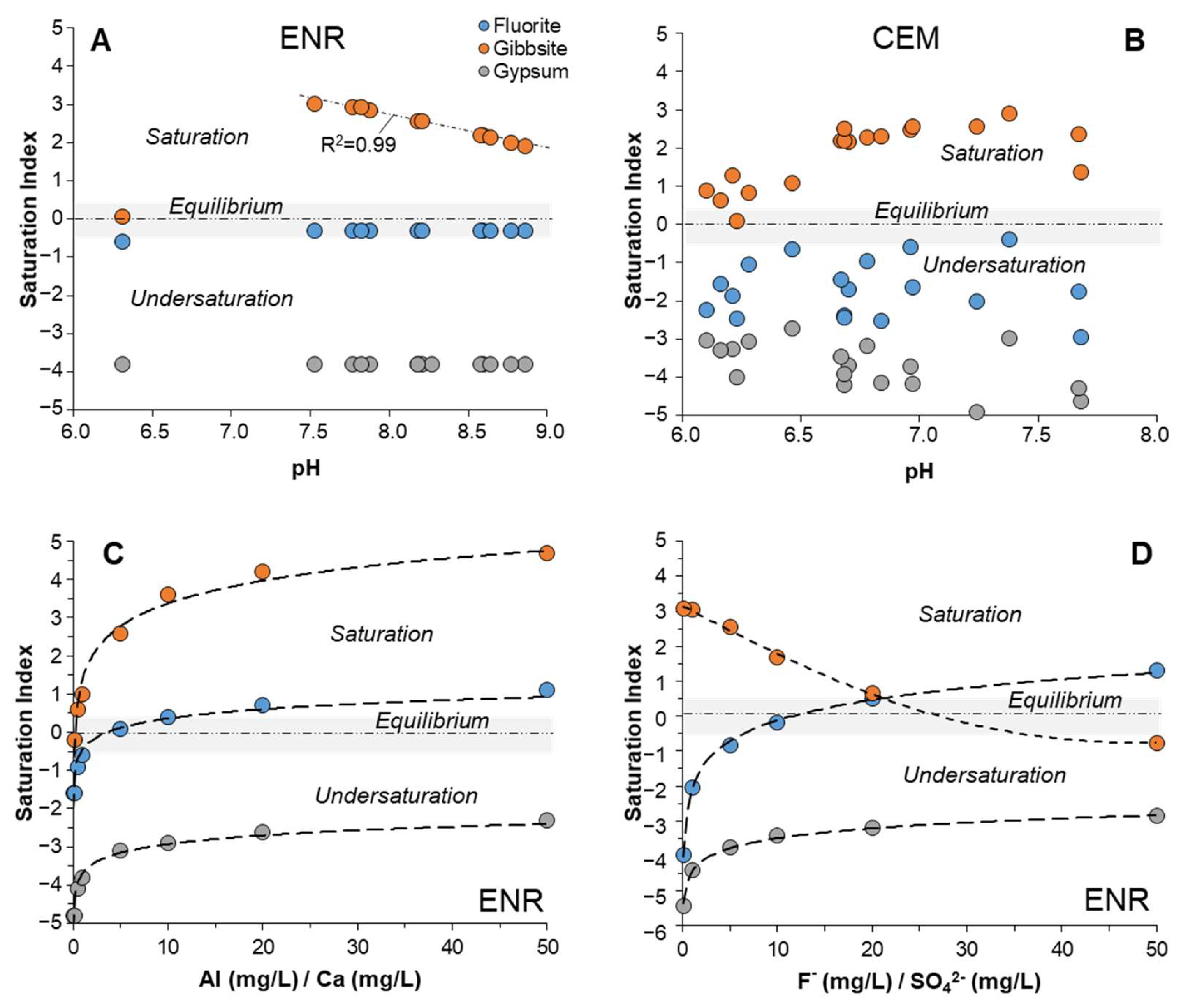
4.5. Geochemical Modeling of Solubility Equilibrium through the Ashfall Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PEVOLCA. Actualización de la Actividad Volcánica en Cumbre Vieja (La Palma). Informe del Comité Científico del PEVOLCA del 25 de diciembre de 2021. Available online: https://www.gobiernodecanarias.org/infovolcanlapalma/pevolca/ (accessed on 1 July 2022).
- Bennis, K.L.; Venzke, E. (Eds.) Report on La Palma (Spain). In Bulletin of the Global Volcanism Network; No. 2; Smithsonian Institution, Global Volcanism Program: Washington, WA, USA, 2022; Volume 47. [Google Scholar] [CrossRef]
- Taddeucci, J.; Scarlato, P.; Andronico, D.; Ricci, T.; Civico, R.; Del Bello, E.; Spina, L.; D’Auria, L.; Asensio-Ramos, M.; Calvo, D.; et al. The explosive activity of the 2021 Tajogaite eruption (La Palma, Canary Islands, Spain). Geochem. Geophys. Geosyst. 2023, 24, e2023GC010946. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Mata, M.P.; Vegas, J.; Lozano, G.; Mediato, J.; Martínez, J.; Galindo, I.; Sánchez, N.; del Moral, B.; Ordóñez, B.; et al. Leaching tests reveal fast aluminum fluoride release from ashfall accumulated in La Palma (Canary Islands, Spain) after the 2021 Tajogaite eruption. J. Volcanol. Geotherm. Res. 2023, 440, 107959. [Google Scholar] [CrossRef]
- Witham, C.S.; Oppenheimer, C.; Horwell, C.J. Volcanic ash-leachates: A review and recommendations for sampling methods. J. Volcanol. Geotherm. Res. 2005, 141, 299–326. [Google Scholar] [CrossRef]
- Bagnato, E.; Aiuppa, A.; Andronico, D.; Cristaldi, A.; Liotta, M.; Brusca, L.; Miraglia, L. Leachate analyses of volcanic ashes from Stromboli volcano: A proxy for the volcanic gas plume composition? J. Geophys. Res. 2011, 116, D17204. [Google Scholar] [CrossRef]
- Cabré, J.; Aulinas, M.; Rejas, M.; Fernandez-Turiel, J.L. Volcanic ash leaching as a means of tracing the environmental impact of the 2011 Grímsvötn eruption, Iceland. Environ. Sci. Pollut. Res. 2016, 23, 14338–14353. [Google Scholar] [CrossRef]
- Ruggieri, F.; Fernández-Turiel, J.-L.; Saavedra, J.; Gimeno, D.; Polanco, E.; Naranjo, J.A. Environmental geochemistry of recent volcanic ashes from Southern Andes. Environ. Chem. 2011, 8, 236–247. [Google Scholar] [CrossRef]
- Stewart, C.; Damby, D.E.; Tomašek, I.; Horwell, C.J.; Plumlee, G.S.; Armienta, M.A.; Hinojosa, M.G.; Appleby, M.; Delmelle, P.; Cronin, S.; et al. Assessment of leachable elements in volcanic ashfall: A review and evaluation of a standardized protocol for ash hazard characterization. J. Volcanol. Geotherm. Res. 2020, 392, 106756. [Google Scholar] [CrossRef]
- Cronin, S.J.; Manoharan, V.; Hedley, M.J.; Loganathan, P. Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed-pasture systems in New Zealand. N. Zeal. J. Agric. Res. 2000, 43, 295–321. [Google Scholar] [CrossRef]
- Cronin, S.J.; Nell, V.E.; Lecontre, J.A.; Hedley, M.J.; Loganathan, P. Environmental hazards of fluoride in volcanic ash: A case study from Ruapehu volcano, New Zealand. J. Volcanol. Geotherm. Res. 2003, 121, 271–291. [Google Scholar] [CrossRef]
- D’Alessandro, W. Human fluorosis related to volcanic activity: A review. WIT Trans. Biomed. Health 2006, 10, 21–30. [Google Scholar] [CrossRef]
- Oskarsson, N. The interaction between volcanic gases and tephra: Fluorine adhering to tephra of the 1970 Hekla eruption. J. Volcanol. Geotherm. Res. 1980, 8, 251–266. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Smedley, P.L. Fluoride in natural waters. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Blum, W.E.H. Fluorine speciation and mobility in F-contaminated soils. Soil Sci. 1992, 153, 357–364. [Google Scholar] [CrossRef]
- García-Herrera, R.; Gallego, D.; Henández, E.; Gimeno, L.; Ribera, P. Influence of the North Atlantic Oscillation on the Canary Islands Precipitation. J. Clim. 2001, 14, 3889–3903. [Google Scholar] [CrossRef]
- Troll, V.R.; Carracedo, J.C. (Eds.) The Geology of La Palma. In The Geology of the Canary Islands; Elsevier: Amsterdam, The Netherlands, 2016; pp. 101–180. [Google Scholar] [CrossRef]
- Carracedo, J.C.; Badiola, E.R.; Guillou, H.; de La Nuez, J.; Pérez-Torrado, F.J. Geology and volcanology of la Palma and El Hierro, Western Canaries. Estud. Geológicos 2001, 57, 175–273. [Google Scholar]
- Carracedo, J.C.; Rodríguez Badiola, E.; Guillou, H. Memoria de la Hoja nº 1085-III/IV (Isla de La Palma. El Pueblo), 1st ed.; Mapa Geológico de España E. 1:25.000, Segunda Serie; Insituto Geológico y Minero de España: Madrid, Spain, 2015; 170p. [Google Scholar]
- Carracedo, J.C.; Troll, V.R.; Day, J.M.D.; Geiger, H.; Aulinas, M.; Soler, V.; Deegan, F.M.; Perez-Torrado, F.J.; Gisbert, G.; Gazel, E.; et al. The 2021 Eruption of the Cumbre Vieja Volcanic Ridge on La Palma, Canary Islands. Geol. Today 2022, 38, 94–107. [Google Scholar] [CrossRef]
- Hernández-Pacheco, A.; Valls, M.C. The historic eruptions of La Palma Island (Canaries). Arquipélago. Série Ciências da Natureza 1982, 3, 83–94. [Google Scholar]
- Mata, M.P.; Ordóñez-Casado, B.; del Moral, B.; Mediato Arribas, J.F.; Bellido Martín, E.; Castillo, M.; Vegas, J.; Martínez Martínez, J.; Sánchez-España, J.; Pérez, R.; et al. Composición del material piroclástico de la erupción de 2021 en Cumbre Vieja (Isla de La Palma). Macla 2022, 26, 116–117. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reactions, one-dimensional transport, and inverse geochemical calculations. In Groundwater Book 6, Modeling Techniques; U.S. Geological Survey: Denver, CO, USA, 2013; p. 497. [Google Scholar]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, J. MINTEQA2/PRODEAFA2, A Geochemical Assessment Model for Environmental Systems: User Manual Supplement for Version 4.0.; U.S. EPA, NERL: Athens, GA, USA, 1999; 42p. [Google Scholar]
- Wolff-Boenish, D.; Gislason, S.R.; Elkers, E.H. The effect of fluoride on the dissolution rates of natural glasses at pH 4 and 25 °C. Geochim. Cosmochim. Acta 2004, 68, 4571–4582. [Google Scholar] [CrossRef]
- Wolff-Boenish, D.; Gislason, S.R.; Elkers, E.H.; Putnis, C.V. The dissolution rates of natural glasses as a function of their composition at pH 4 and 10.6, and temperatures from 25 to 74 °C. Geochim. Cosmochim. Acta 2004, 68, 4843–4858. [Google Scholar] [CrossRef]
- Huerta, P.; Rodríguez-Berriguete, A.; Martín-García, R.; Martín-Pérez, A.; La Iglesia, A.; Alonso-Zarza, A.M. The role of climate and aeolian dust input in calcrete formation in volcanic islands (Lanzarote and Fuerteventura, Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 66–79. [Google Scholar] [CrossRef]
- Pokrovsky, L.S.; Golubev, S.V.; Schott, J.; Castillo, A. Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins. Chem. Geol. 2009, 265, 20–32. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.; Mediato, J.F.; Mata, M.P.; Ordóñez, B.; del Moral, B.; Bellido, E.; Pérez-López, R.; Rodríguez-Pascua, M.A.; Vegas, J.; Lozano, G.; et al. Early fumarolic minerals from the Tajogaite volcanic eruption (La Palma, 2021). J. Volcanol. Geotherm. Res. 2023, 435, 107771. [Google Scholar] [CrossRef]
- Kockum, P.C.F.; Herbert, R.B.; Gislason, S.R. A diverse ecosystem response to volcanic aerosols. Chem. Geol. 2006, 231, 57–66. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Ball, J.W. The geochemical behavior of aluminum in acidified surface waters. Science 1986, 232, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Rotteveel, L. Trends, Patterns, and Drivers of Freshwater Aluminium Concentrations: Revisiting the Conceptual Model of Freshwater Acidification. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2020. [Google Scholar]
- Botté, A.; Zaidi, M.; Guery, J.; Fichet, D.; Leignel, V. Aluminium in aquatic environments: Abundance and ecotoxicological impacts. Aquat. Ecol. 2022, 56, 751–773. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Schecher, W.D. The chemistry of aluminum in the environment. Environ. Geochem. Health 1990, 12, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Postek, K.M. The chemistry of aluminum in surface waters. In The Environmental Chemistry of Aluminum, 2nd ed.; Sposito, G., Ed.; CRC Press: London, UK, 1996; p. 56. [Google Scholar]
- Sánchez-España, J. The behavior of iron and aluminum in acid mine drainage: Speciation, Mineralogy, and Environmental Significance. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 137–149. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Gray, J.; Burgos, W.D. Geochemistry of dissolved aluminum at low pH: Extent and significance of Al-Fe(III) coprecipitation below pH 4.0. Geochim. Cosmochim. Acta 2016, 175, 128–149. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Burgos, W.D. Geochemistry of dissolved aluminum at low pH: Hydrobasaluminite formation and interaction with trace metals, silica and microbial cells under anoxic conditions. Chem. Geol. 2016, 441, 124–137. [Google Scholar] [CrossRef]
- Ayris, P.M.; Delmelle, P.; Pereira, B.; Maters, E.C.; Damby, D.E.; Durant, A.J.; Dingwell, D.B. Spatial analysis of Mount St. Helens tephra leachate compositions: Implications for future sampling strategies. Bull. Volcanol. 2015, 77, 60. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, W.; Bellomo, S.; Parello, F. Fluorine adsorption by volcanic soils at Mt. Etna, Italy. Appl. Geochem. 2012, 27, 1179–1188. [Google Scholar] [CrossRef]
- Loganathan, P.; Hedley, M.J.; Wallace, G.C.; Roberts, A.H.C. Fluoride accumulation in pasture forages and soils following longterm applications of phosphorous fertilisers. Environ. Pollut. 2001, 115, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, C.L.; Grossi, P.R.; Bugbee, B.G. Biogeochemistry of fluoride in a plant-solution system. J. Environ. Qual. 2003, 32, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- VROM. Aluminum Fluoride: Summary Risk Assessment Report; CAS No 7784-18-1, EEC 793/93, Ministry of Housing, Spatial Planning and the Environment (VROM): Bilthoven, The Netherlands, 2008; 12p. [Google Scholar]
- Exley, C.; Clarkson, E. Aluminum in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, R.W.; Playle, R.C. The bioavailability and toxicity of aluminium in aquatic environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-España, J.; Castillo, A.M.N.; Mata, M.P.; Martínez-Martínez, J.; Mediato, J.F. Fluorite and Gibbsite Solubility Controls the Vertical Transport of Fluoride and Aluminum during Rainwater Percolation through Ashfall Deposits in La Palma (Canary Islands, Spain). Minerals 2024, 14, 338. https://doi.org/10.3390/min14040338
Sánchez-España J, Castillo AMN, Mata MP, Martínez-Martínez J, Mediato JF. Fluorite and Gibbsite Solubility Controls the Vertical Transport of Fluoride and Aluminum during Rainwater Percolation through Ashfall Deposits in La Palma (Canary Islands, Spain). Minerals. 2024; 14(4):338. https://doi.org/10.3390/min14040338
Chicago/Turabian StyleSánchez-España, Javier, Ana M. Nieto Castillo, M. Pilar Mata, Javier Martínez-Martínez, and Jose F. Mediato. 2024. "Fluorite and Gibbsite Solubility Controls the Vertical Transport of Fluoride and Aluminum during Rainwater Percolation through Ashfall Deposits in La Palma (Canary Islands, Spain)" Minerals 14, no. 4: 338. https://doi.org/10.3390/min14040338
APA StyleSánchez-España, J., Castillo, A. M. N., Mata, M. P., Martínez-Martínez, J., & Mediato, J. F. (2024). Fluorite and Gibbsite Solubility Controls the Vertical Transport of Fluoride and Aluminum during Rainwater Percolation through Ashfall Deposits in La Palma (Canary Islands, Spain). Minerals, 14(4), 338. https://doi.org/10.3390/min14040338








