Gold Migration and Precipitation as Collaurum in Orogenic Gold Deposits: Constrains from Microscopic Gold Particles Observed in the Alteration Zone in Shanggong Gold Ore, Henan, China
Abstract
1. Introduction
2. Geological Backround

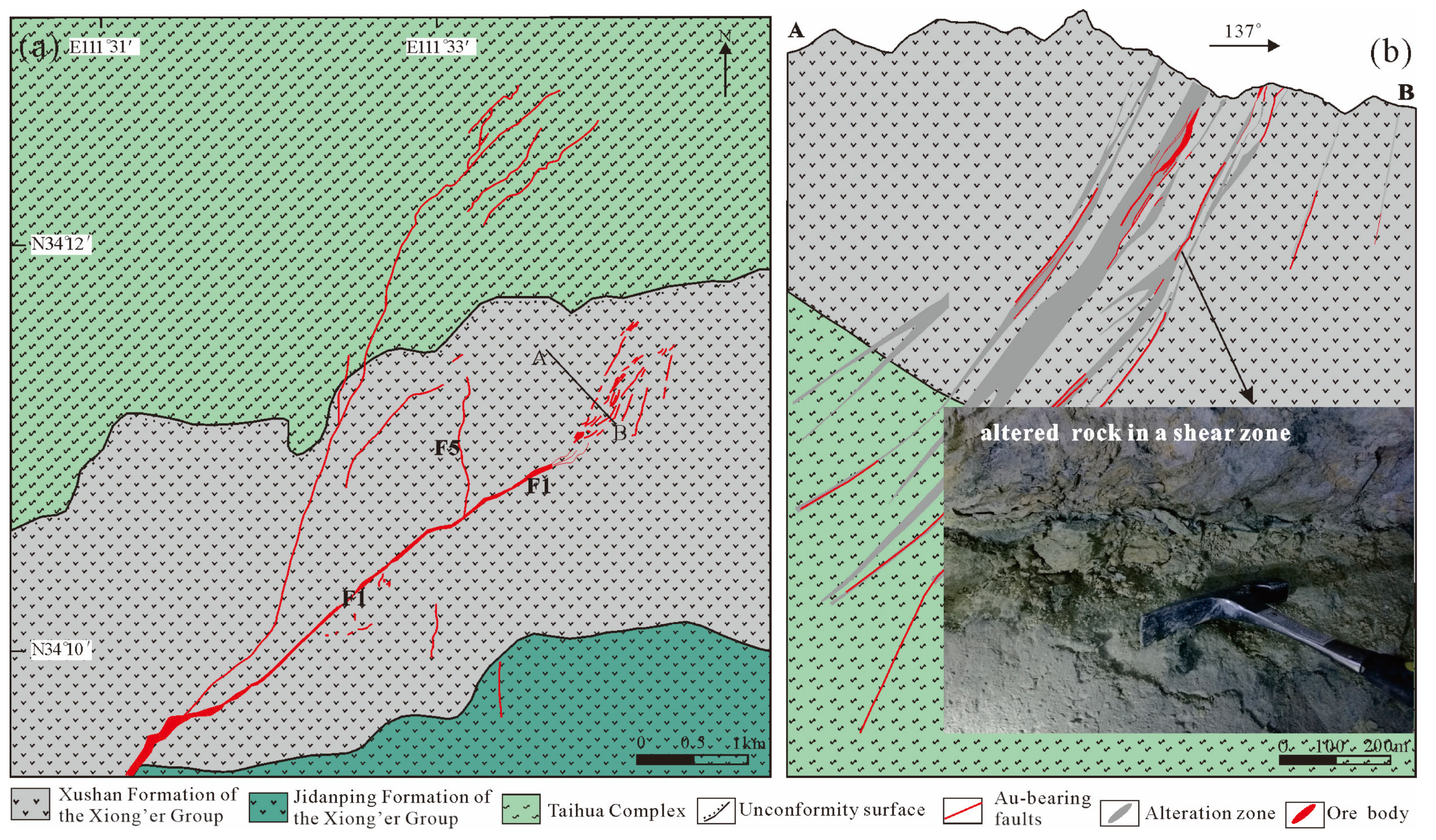
3. Sampling and Analysis Methods
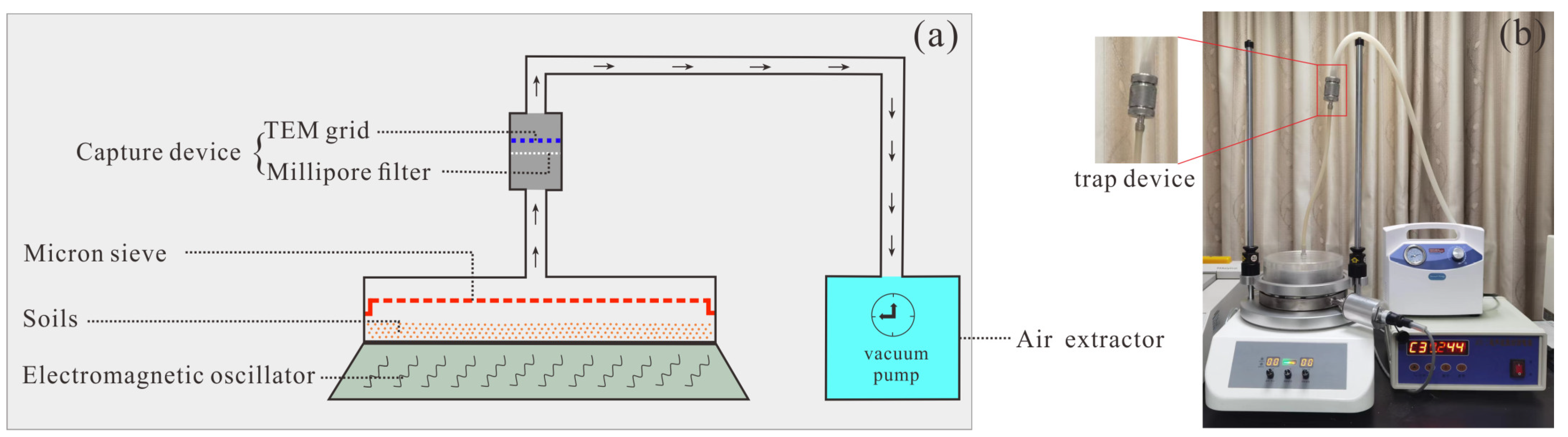
3.1. Sample Collection and Preparation
3.2. Analytical Methods
3.2.1. TEM Analysis
3.2.2. SEM Analysis
3.2.3. Chemical Analysis
4. Results
4.1. Chemical Composition
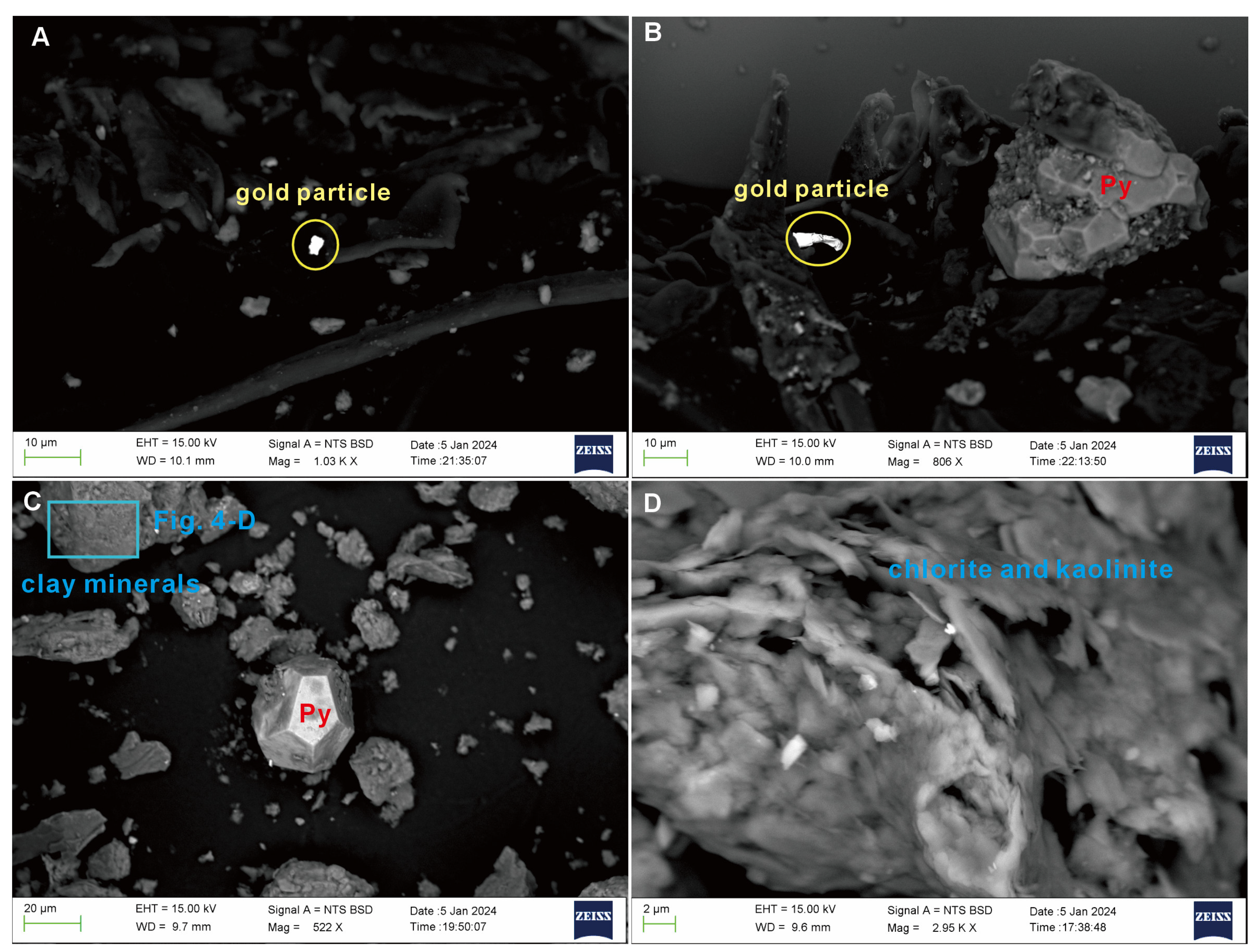
4.2. SEM Observation of the Altered Rock
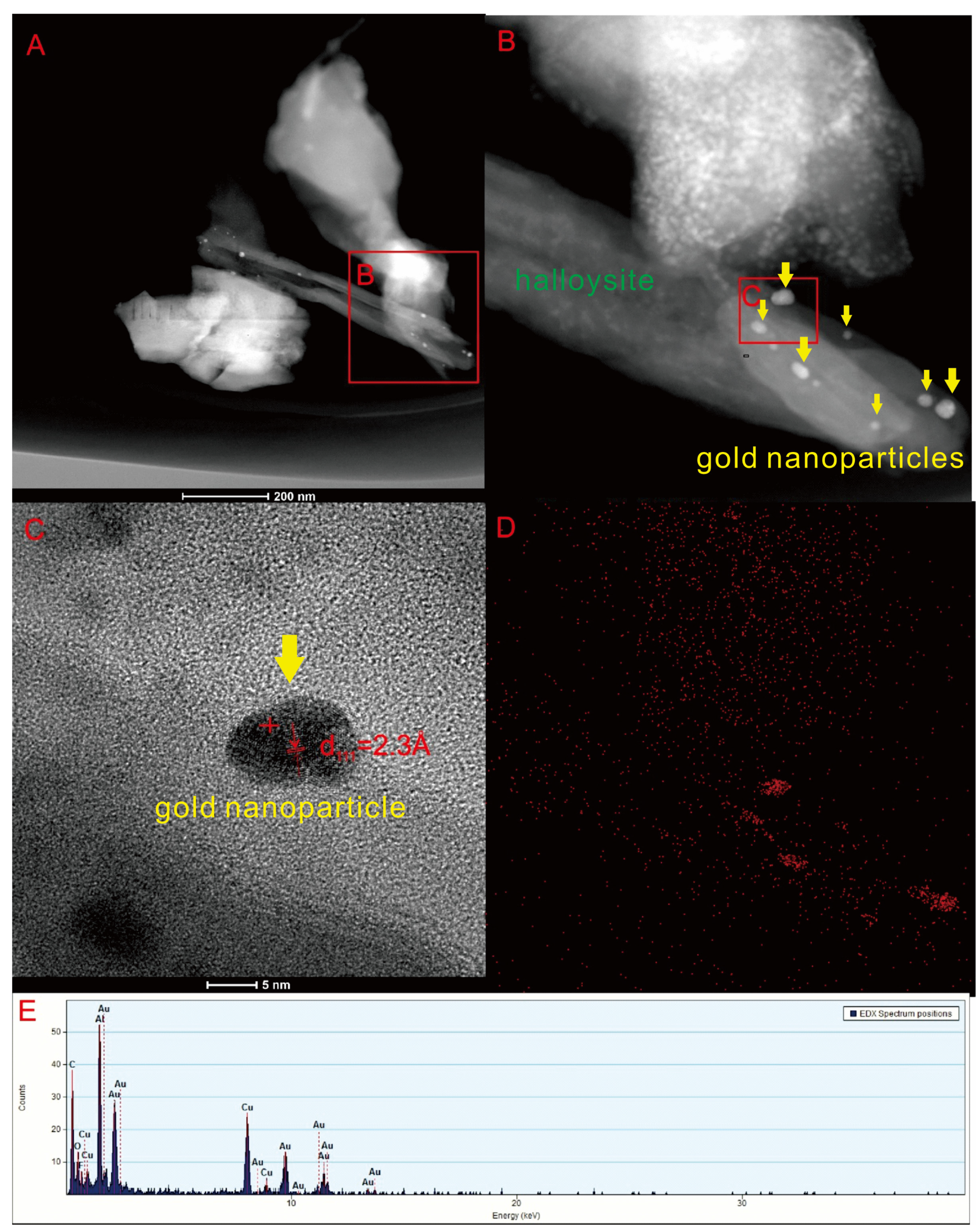
4.3. TEM Observation of the Altered Rock
5. Discussion
5.1. Gold May Migrate as Colloidal Gold in the Ore-Forming Fluid of Orogenic Gold Deposits
5.2. Precipitation and Occurrence of Gold in the Alteration Zone
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, M.G.; Wu, F.Y.; Hu, R.Z.; Jiang, S.Y.; Li, W.C.; Wang, R.C.; Wang, D.H.; Qi, T.; Qin, K.Z.; Wen, H.J. Critical metal mineral resources: Current research status and scientific issues. Bull. Natl. Nat. Sci. Found. China 2019, 33, 106–111, (In Chinese with English Abstract). [Google Scholar]
- Liu, W.; Etschmann, B.; Testemale, D.; Hazemann, J.; Rempel, K.; Müller, H.; Brugger, J. Gold transport in hydrothermal fluids: Competition among the Cl, Br, HS and NH3(aq) ligands. Chem. Geol. 2014, 376, 11–19. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Tagirov, B.R.; Schott, J.; Bazarkina, E.F.; Hazemann, J.L.; Proux, O. An in situ X-ray absorption spectroscopy study of gold-chloride complexing in hydrothermal fluids. Chem. Geol. 2009, 259, 17–29. [Google Scholar] [CrossRef]
- Seward, T.M. Thio complexes of gold and the transport of gold in hydrothermal ore solutions. Geochim. Cosmochim. Acta 1973, 37, 379–399. [Google Scholar] [CrossRef]
- Zezin, Y.D.; Migdisov, A.A.; Williams-Jones, A.E. The solubility of gold in H2O–H2S vapor at elevated temperature and pressure. Geochim. Cosmochim. Acta 2011, 75, 5140–5153. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Akinfiev, N.N.; Borisova, A.Y.; Zotoz, A.V.; Kouzmanov, K. Gold speciation and transport in geological fluids: Insights from experiments and physical-chemical modelling. Geol. Soc. Lond. Spec. Publ. 2014, 402, 9–70. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubessy, J. Stability and abundance of the trisulfur radical ion S3− in hydrothermal fluids. Earth Planet. Sci. Lett. 2015, 411, 298–309. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubrovinsky, L.S. The S3− Ion Is Stable in Geological Fluids at Elevated Temperatures and Pressures. Science 2011, 311, 1052–1054. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Guillaume, D.; Borisova, A.Y.; Gisquet, P.; Hazemann, J.-L.; Lahera, E.; Del Net, W.; Proux, O.; Testemale, D.; et al. Sulfur radical species form gold deposits on earth. Proc. Natl. Acad. Sci. USA 2015, 112, 13484. [Google Scholar] [CrossRef]
- Barré, G.; Truche, L.; Bazarkina, E.F.; Michels, R.; Dubessy, J. First evidence of the trisulfur radical ion S3− and other sulfur polymers in natural fluid inclusions. Chem. Geol. 2017, 462, 1–14. [Google Scholar] [CrossRef]
- Gopon, P.; Douglas, J.O.; Auger, M.A.; Hansen, L.; Wade, J.; Cline, J.S.; Robb, L.J.; Moody, M.P. A Nanoscale Investigation of Carlin-Type Gold Deposits: An Atom-Scale Elemental and Isotopic Perspective. Econ. Geol. 2019, 114, 1123–1133. [Google Scholar] [CrossRef]
- Saunders, J.A. Colloidal transport of gold and silica in epithermal precious-metal systems: Evidence from the Sleeper deposit, Nevada. Geology 1990, 18, 757–760. [Google Scholar] [CrossRef]
- Saunders, J.A.; Schoenly, P.A. Boiling, colloid nucleation and aggregation, and the genesis of bonanza Au-Ag ores of the sleeper deposit, Nevada. Miner. Depos. 1995, 30, 199–210. [Google Scholar] [CrossRef]
- Herrington, R.J.; Wilkinson, J.J. Colloidal gold and silica in mesothermal vein systems. Geology 1993, 21, 539–542. [Google Scholar] [CrossRef]
- Frondel, C. Stability of colloidal gold under hydrothermal conditions. Econ. Geol. 1938, 33, 1–20. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Hannington, M.; Hareardóttir, V.; Garbe-Schönberg, D.; Brown, K.L. Gold enrichment in active geothermal systems by accumulating colloidal suspensions. Nat. Geosci. 2016, 9, 299–302. [Google Scholar] [CrossRef]
- Taran, Y.A.; Bernard, A.; Gavilanes, J.C.; Africano, F. Native gold in mineral precipitates from high temperature volcanic gases of Colima volcano. Mexico. Appl. Geochem. 2000, 15, 337–346. [Google Scholar] [CrossRef]
- Larocque, A.; Stimac, J.A.; Siebe, C.; Greengrass, K.; Chapman, R.; Mejia, S.R. Deposition of a high-sulfidation Au assemblage from a magmatic volatile phase, Volcán Popocatépetl, Mexico. J. Volcanol. Geoth. Res. 2008, 170, 51–60. [Google Scholar] [CrossRef]
- Wang, X.Q.; Dong, B.M.; Lin, X.; Xu, S.F.; Yao, W.S.; Ye, R. Geochemical challenges of diverse regolith-covered terrains for mineral exploration in China. Ore Geol. Rev. 2016, 73, 417–431. [Google Scholar]
- Hannington, M.; Garbe-Schnberg, D. Detection of Gold Nanoparticles in Hydrothermal Fluids. Econ. Geol. 2019, 114, 397–400. [Google Scholar] [CrossRef]
- Gartman, A.; Hannington, M.; Jamieson, J.W.; Peterkin, B.; Garbe-Schönberg, D.; Findlay, A.J.; Fuchs, S.; Kwasnitschka, T. Boiling-induced formation of colloidal gold in black smoker hydrothermal fluids. Geology 2018, 46, 39–42. [Google Scholar] [CrossRef]
- Reith, F.; Cornelis, G. Effect of soil properties on gold- and platinum nanoparticle mobility. Chem. Geol. 2017, 466, 446–453. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Robert, F.; Hart, C.J. Gold Deposits in Metamorphic Belts: Overview of Current Understanding, Outstanding Problems, Future Research, and Exploration Significance. Econ. Geol. 2003, 98, 1–29. [Google Scholar]
- Hou, Z.; Wang, Q.; Zhang, H.; Xu, B.; Yu, N.; Wang, R.; Groves, I.D.; Zheng, Y.; Han, S.; Gao, L.; et al. Lithosphere architecture characterized by crust–mantle decoupling controls the formation of orogenic gold deposits. Natl. Sci. Rev. 2023, 10, nwac257. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.L.; Fuzikawa, K. Origin of the CO2-only fluid inclusions in the Palaeoproterozoic Carará vein-quartz gold deposit, Ipitinga Auriferous District, SE-Guiana Shield, Brazil: Implications for orogenic gold mineralisation. Ore Geol. Rev. 2010, 37, 31–40. [Google Scholar] [CrossRef]
- Gorczyk, W.; Gonzalez, C.M.; Hobbs, B. Carbon dioxide as a proxy for orogenic gold source. Ore Geol. Rev. 2020, 127, 103829. [Google Scholar] [CrossRef]
- Prokofiev, V.Y.; Banks, D.A.; Lobanov, K.V.; Selektor, S.L.; Milichko, V.A.; Akinfiev, N.N.; Borovikov, A.A.; Lüders, V.; Chicherov, M.V. Exceptional Concentrations of Gold Nanoparticles in 1,7 Ga Fluid Inclusions from the Kola Superdeep Borehole, Northwest Russia. Sci. Rep. 2020, 10, 1108. [Google Scholar] [CrossRef]
- Banks, D.A.; Bozkaya, G.; Bozkaya, O. Direct observation and measurement of Au and Ag in epithermal mineralizing fluid. Ore Geol. Rev. 2019, 111, 102955. [Google Scholar] [CrossRef]
- Mcleish, D.F.; Williams-Jones, A.E.; Vasyukova, O.V.; Clark, J.R.; Board, W.S. Colloidal transport and flocculation are the cause of the hyperenrichment of gold in nature. Proc. Natl. Acad. Sci. USA 2021, 118, e2100689118. [Google Scholar] [CrossRef]
- Chen, Y.J.; Pirajno, F.; Qi, J.P. The Shanggong gold deposit, Eastern Qinling Orogen, China: Isotope geochemistry and implications for ore genesis. J. Asian Earth. Sci. 2008, 33, 52–266. [Google Scholar] [CrossRef]
- Fan, H.R.; Xie, Y.H.; Wang, Y.L. Fluid-Rock Interaction during Mineralization of the Shanggong Structure-Controlled Alteration-Type Gold Deposit in Western Henan Province, Central China. Acta. Petrol. Sin. 1998, 14, 529–541, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.D.; Ren, Y.Z.; Du, X.Y.; Xia, W.Z.; Yue, H.; Sun, C.Y.; Sun, L.F. Characteristics of ore-forming fluids of Shanggong gold deposit in western Henan. Gold 2022, 43, 19–25, (In Chinese with English Abstract). [Google Scholar]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Yang, L.; Zhang, B.; Liu, Q.; Liu, D. The characteristic of microstructural deformation of gold bearing pyrite in Jiaodong: The links between nanoscale gold enrichment and crystal distortion. Ore Geol. Rev. 2020, 122, 103495. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, B.; Wu, H.; Liu, H.; Qiao, Y.; Zhang, S. Microscopic characterization of metallic nanoparticles in ore rocks, fault gouge and geogas from the Shanggong gold deposit, China. J. Geochem. Explor. 2020, 217, 106562. [Google Scholar] [CrossRef]
- Chen, Y.J.; Franco, P.; Qi, J.P.; Li, J.; Wang, H.H. Ore geology, fluid geochemistry and genesis of the Shanggong gold deposit, eastern Qinling Orogen, China. Resour. Geol. 2006, 56, 99–116. [Google Scholar] [CrossRef]
- Meng, L.; Lan, C.; Zhan, Q.; Wu, Q.; Zhao, T. Origin of the Shanggong gold deposit, the southern margin of the North China Craton: Constraints from Rb-Sr ages of sericite, and trace elements and sulfur isotope of pyrite. Ore Geol. Rev. 2022, 142, 104728. [Google Scholar] [CrossRef]
- Hu, X.L.; He, M.C.; Yao, S.Z. New understanding of the source of ore-forming material and fluid in the Shangong gold deposit, east Qinling. Acta. Geol. Sin. 2013, 87, 91–100, (In Chinese with English Abstract). [Google Scholar]
- Li, J.W.; Bi, S.J.; Selby, D.; Chen, L.; Vasconcelos, P.; Thiede, D.; Zhou, M.F.; Zhao, X.F.; Li, Z.K.; Qiu, H.N. Giant Mesozoic gold provinces related to the destruction of the North China Craton. Earth Planet. Sci. Lett. 2012, 349–350, 26–37. [Google Scholar] [CrossRef]
- Tang, K.F. Characteristics, Genesis, and Geodynamic Setting of Representative Gold Deposits in the Xiong’ershan District, Southern Margin of the North China Craton; China University of Geosciences: Wuhan, China, 2014. (In Chinese) [Google Scholar]
- Ren, Z.Y.; Li, J.W. Mineralization characteristics and metallogenic age of Shanggong gold deposit in western Henan Province. Miner. Depos. 2010, 29, 987–988. (In Chinese) [Google Scholar]
- Mao, J.W.; Xie, G.Q.; Zhang, Z.H.; Li, X.F.; Wang, Y.T.; Zhang, C.Q.; Li, Y.F. Mesozoic large-scale metallogenic pulses in North China and corresponding geodynamic settings. Acta Petrol. Sin. 2005, 21, 171–190, (In Chinese with English Abstract). [Google Scholar]
- Wu, F.Y.; Lin, J.Q.; Wilde, S.A.; Yang, J.H. Nature and significance of the Early Cretaceous giant igneous event in eastern China. Earth Planet Sci. Lett. 2005, 233, 103–119. [Google Scholar] [CrossRef]
- Zhang, D.T.; Feng, J.Z.; Li, L.; Meng, X.F.; He, J.; Liu, Z.Y.; Xu, W.C. Discussion on Post-collision Lithospheric Evolution and Au-Mo Mineralization in the Southern Margin of the North China Craton. Geotecton. Metallog. 2015, 39, 300–314, (In Chinese with English Abstract). [Google Scholar]
- Wang, C.M.; Deng, J.; Zhang, S.T. Relationship between Huashan granite and gold mineralization in Xiong’ershan area, Henan. Geoscience 2006, 20, 315–321, (In Chinese with English Abstract). [Google Scholar]
- Chen, Y.J.; Zhai, M.G.; Jiang, S.Y. Significant achievements and open issuses in study of orogenesis and metallogenesis surrounding the North China continent. Acta Petrol. Sin. 2009, 25, 2695–2726, (In Chinese with English Abstract). [Google Scholar]
- Zhang, S.K.; Shi, B.T.; Wang, J.H.; Deng, H.L.; Tian, H.T.; Yan, Z.X.; Feng, S.P.; Zhang, H. Characteristics of isotopic compositions and metallogenic model of Jijiawa gold deposit in the western Henan. Miner. Explor. 2016, 7, 552–560, (In Chinese with English Abstract). [Google Scholar]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and Charging of Colloidal Silica Particles: Effect of Particle Size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Yang, Y.; Mei, Y.; Etschmann, B.; Brugger, J.; Johannessen, B. Colloidal gold in sulphur and citrate-bearing hydrothermal fluids: An experimental study. Ore Geol. Rev. 2019, 114, 103142. [Google Scholar] [CrossRef]
- Saunders, J.A. Silica and gold textures in bonanza ores of the Sleeper Deposit, Humboldt County, Nevada: Evidence for colloids and implications for epithermal ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Saunders, J.A.; Burke, M.; Brueseke, M.E. Scanning-electron-microscope imaging of gold (electrum) nanoparticles in middle Miocene bonanza epithermal ores from northern Nevada, USA. Miner. Depos. 2020, 55, 389–398. [Google Scholar] [CrossRef]
- Saunders, J.A.; Burke, M. Formation and aggregation of gold (electrum) nanoparticles in epithermal ores. Minerals 2017, 7, 163. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Alargova, R.G.; Deguchi, S.; Tsujii, K. Dispersion stability of colloids in sub- and supercritical water. J. Phys. Chem. B 2006, 110, 25901–25907. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ni, P.; Fan, H.R.; Lai, Y.; Su, W.C.; Zhang, H. Diagnostic fluid inclusions of different types hydrothermal gold deposits. Acta Petrol. Sin. 2007, 23, 2085–2108, (In Chinese with English Abstract). [Google Scholar]
- Hanor, J.S. Origin of saline fluids in sedimentary basins. Geol. Soc. Lond. Spec. Publ. 1994, 78, 151–174. [Google Scholar] [CrossRef]
- Barton, I. The effects of temperature and pressure on the stability of mineral colloids. Am. J. Sci. 2019, 319, 737–753. [Google Scholar]
- Pamies, R.; Cifre, J.G.H.; Espín, V.F.; Collado-González, M.; Baños, F.G.D.; de la Torre, J.G. Aggregation behaviour of gold nanoparticles in saline aqueous media. J. Nanopart. Res. 2014, 16, 2376. [Google Scholar] [CrossRef]
- Groves, D.I. The crustal continuum model for late-Archaean lode-gold deposits of the Yilgarn Block, Western Australia. Miner. Depos. 1993, 28, 366–374. [Google Scholar] [CrossRef]
- Phillips, G.N.; Evans, K.A. Role of CO2 in the formation of gold deposits. Nature 2004, 429, 860–863. [Google Scholar] [CrossRef]
- Garofalo, P.S.; Ficker, M.B.; Gunther, D.; Bersani, D.; Lottici, P.P. Physical-chemical properties and metal budget of Au-transporting hydrothermal fluids in orogenic deposits. Geol. Soc. Lond. Spec. Publ. 2014, 402, 71–102. [Google Scholar] [CrossRef]
- Stefánsson, A.; Seward, T.M. Gold(I) complexing in aqueous sulfide solutions to 500 °C at 500 bar. Geochim. Cosmochim. Acta 2004, 68, 4121–4143. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Seward, T.M.; Barnes, H.L. Metal transport by hydrothermal ore fluids. In Geochemistry of Hydrothermal Ore Deposits., 3rd ed.; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1997; pp. 435–486. [Google Scholar]
- Wood, S.A.; Samson, I.M. Solubility of ore minerals and complexation of ore metals in hydrothermal solutions. Econ. Geol. 1998, 10, 33–80. [Google Scholar]
- Barton, P.B.; Chou, I.M. Refinement of the evaluation of the role of CO2 in modifying estimates of the pressure of epithermal mineralization. Econ. Geol. 1993, 88, 873–884. [Google Scholar] [CrossRef]
- Bakker, R.J. Package FLUIDS. Part 3: Correlations between equations of state, thermodynamics and fluid inclusions. Geofluids 2009, 9, 63–74. [Google Scholar] [CrossRef]
- Kokh, M.A.; Lopez, M.; Gisquet, P.; Lanzanova, A.; Candaudap, F.; Besson, P.; Pokrovski, G.S. Combined effect of carbon dioxide and sulfur on vapor-liquid partitioning of metals in hydrothermal systems. Geochim. Cosmochim. Acta 2016, 187, 311–333. [Google Scholar] [CrossRef]
- Kouzmanov, K.; Pokrovski, G.S. Hydrothermal controls on metal distribution in porphyry Cu(-Au-Mo) systems. SEG 2012, 16, 573–618. [Google Scholar]
- Walther, J.V.; Schott, J. The dielectric constant approach to speciation and ion pairing at high temperature and pressure. Nature 1988, 332, 635–638. [Google Scholar] [CrossRef]
- Kokh, M.A.; Akinfiev, N.N.; Pokrovski, G.S.; Salvi, S.; Guillaume, D. The role of carbon dioxide in the transport and fractionation of metals by geological fluids. Geochim. Cosmochim. Acta 2017, 197, 433–466. [Google Scholar] [CrossRef]
- Esumi, K.; Sarashina, S.; Yoshimura, T. Synthesis of gold nanoparticles from an organometallic compound in supercritical carbon dioxide. Langmuir 2004, 20, 5189–5191. [Google Scholar] [CrossRef]
- Ryoo, W.; Dickson, J.L.; Dhanuka, V.V.; Webber, S.E.; Bonnecaze, R.T.; Johnston, K.P. Electrostatic stabilization of colloids in carbon dioxide: Electrophoresis and dielectrophoresis. Langmuir 2005, 21, 5914–5923. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, B.; Xu, Z.; Yang, Z.; Yang, X. Solvation structure and dynamics for passivated Au nanoparticle in supercritical CO2: A molecular dynamic simulation. J. Colloid. Interf. Sci. 2011, 353, 22–29. [Google Scholar]
- Wilde, A.R.; Bierlein, F.P.; Pawlitschek, M. Lithogeochemistry of orogenic gold deposits in Victoria, SE Australia: A preliminary assessment for undercover exploration. J. Geochem. Explor. 2004, 84, 35–50. [Google Scholar] [CrossRef]
- Idrus, A.; Kolb, J.; Meyer, F.M. Mineralogy, Lithogeochemistry and Elemental Mass Balance of the Hydrothermal Alteration Associated with the Gold-rich Batu Hijau Porphyry Copper Deposit, Sumbawa Island, Indonesia. Resour. Geol. 2010, 59, 215–230. [Google Scholar] [CrossRef]
- Pentrák, M.; Czímerová, A.; Madejová, J.; Komadel, P. Changes in layer charge of clay minerals upon acid treatment as obtained from their interactions with methylene blue. Appl. Clay Sci. 2012, 55, 100–107. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Rakhila, Y.; Elmchaouri, A.; Mestari, A.; Korili, S.; Abouri, M.; Gil, A. Adsorption recovery of Ag(I) and Au(III) from an electronics industry wastewater on a clay mineral composite. Int. J. Min. Met. Mater. 2019, 26, 673–680. [Google Scholar] [CrossRef]
- Hong, H.; Tie, L. Characteristics of the minerals associated with gold in the Shewushan supergene gold deposit, China. Clay Clay Miner. 2005, 53, 162–170. [Google Scholar] [CrossRef]
- Nadeau, O.; Harris, J. Remobilization and adsorption of colloidal gold on nano-particulate chlorite at the Hardrock Archean orogenic gold deposits: A new tool for gold exploration. J. Geochem. Explor. 2019, 204, 181–205. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Farrokhpay, S.; Bradshaw, D. Interactions of clay minerals in copper–gold flotation: Part 1—Rheological properties of clay mineral suspensions in the presence of flotation reagents. Miner. Eng. 2013, 50–51, 30–37. [Google Scholar] [CrossRef]
- Bakken, B.M.; Hochella, M.F.; Marshall, A.F.; Turner, A.M. High-resolution microscopy of gold in unoxidized ore from the Carlin mine, Nevada. Econ. Geol. 1989, 84, 171–179. [Google Scholar] [CrossRef]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal distribution of gold in pyrite and arsenopyrite: Examples from the Archean Boorara and Bardoc shear systems, Yilgarn craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” gold revealed: Direct imaging of gold nanoparticles in a Carlin-type deposit. Am. Miner. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
| Elements | S | Au | SiO2 | Al2O3 | TFe2O3 | MgO | CaO | Na2O | K2O | CIA |
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | μg/g | ng/g | % | % | % | % | % | % | % | |
| SGAR | 113 | 877 | 58.3 | 17.2 | 6.01 | 1.88 | 3.65 | 0.98 | 3.88 | 57.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Han, Z.; Zhang, B.; Wei, X.; Dong, C.; Liu, H. Gold Migration and Precipitation as Collaurum in Orogenic Gold Deposits: Constrains from Microscopic Gold Particles Observed in the Alteration Zone in Shanggong Gold Ore, Henan, China. Minerals 2024, 14, 327. https://doi.org/10.3390/min14030327
Qiao Y, Han Z, Zhang B, Wei X, Dong C, Liu H. Gold Migration and Precipitation as Collaurum in Orogenic Gold Deposits: Constrains from Microscopic Gold Particles Observed in the Alteration Zone in Shanggong Gold Ore, Henan, China. Minerals. 2024; 14(3):327. https://doi.org/10.3390/min14030327
Chicago/Turabian StyleQiao, Yu, Zhixuan Han, Bimin Zhang, Xiaocheng Wei, Chunfang Dong, and Hanliang Liu. 2024. "Gold Migration and Precipitation as Collaurum in Orogenic Gold Deposits: Constrains from Microscopic Gold Particles Observed in the Alteration Zone in Shanggong Gold Ore, Henan, China" Minerals 14, no. 3: 327. https://doi.org/10.3390/min14030327
APA StyleQiao, Y., Han, Z., Zhang, B., Wei, X., Dong, C., & Liu, H. (2024). Gold Migration and Precipitation as Collaurum in Orogenic Gold Deposits: Constrains from Microscopic Gold Particles Observed in the Alteration Zone in Shanggong Gold Ore, Henan, China. Minerals, 14(3), 327. https://doi.org/10.3390/min14030327






