Towards a Circular Economy in the Mining Industry: Possible Solutions for Water Recovery through Advanced Mineral Tailings Dewatering
Abstract
1. Introduction
1.1. Challenges of Mine Site Water Availability in the Face of Climate Change
1.2. Persistent Tailings Challenges
1.3. Objectives of This Critical Review
2. Water Consumption in the Mining Industry
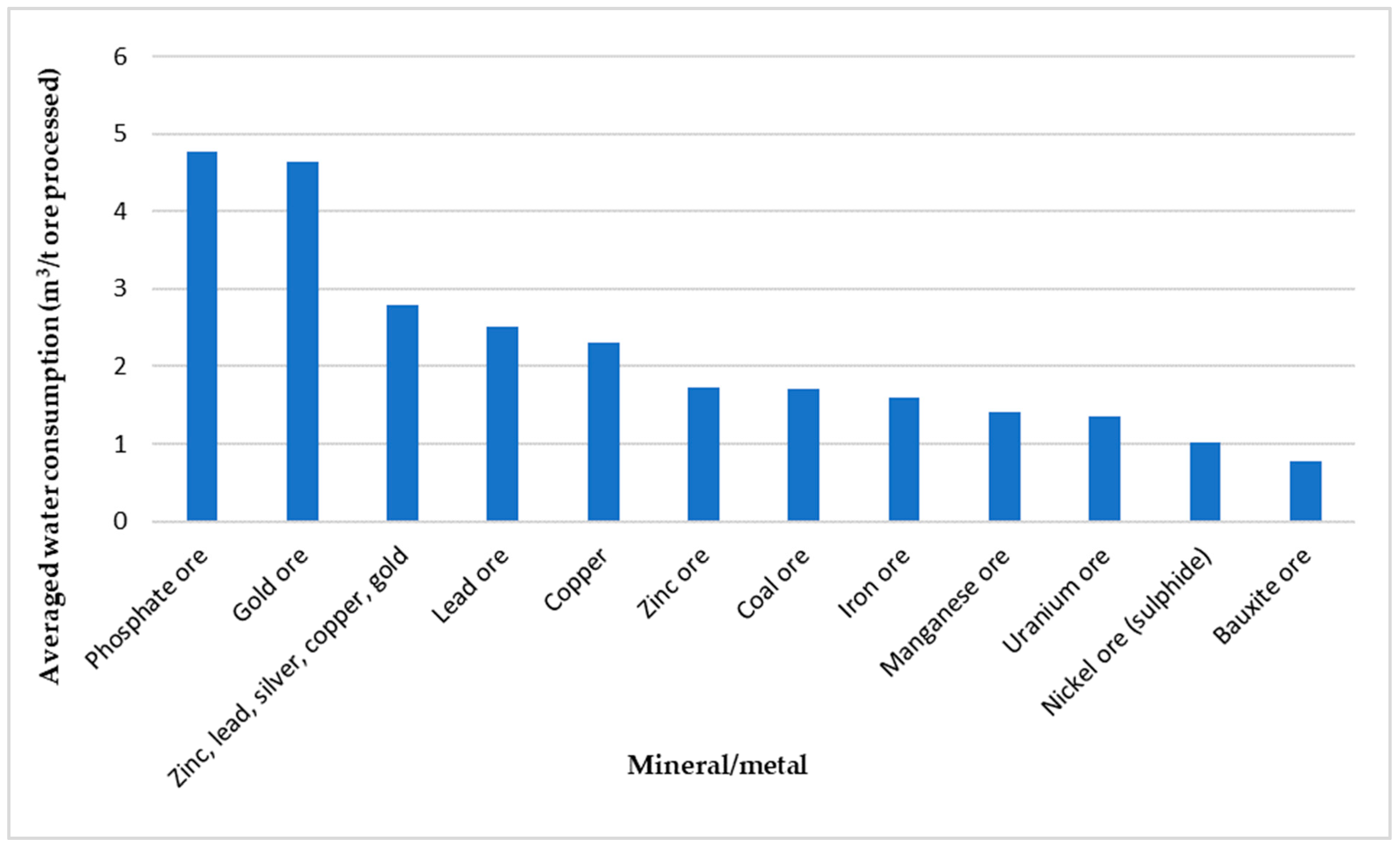
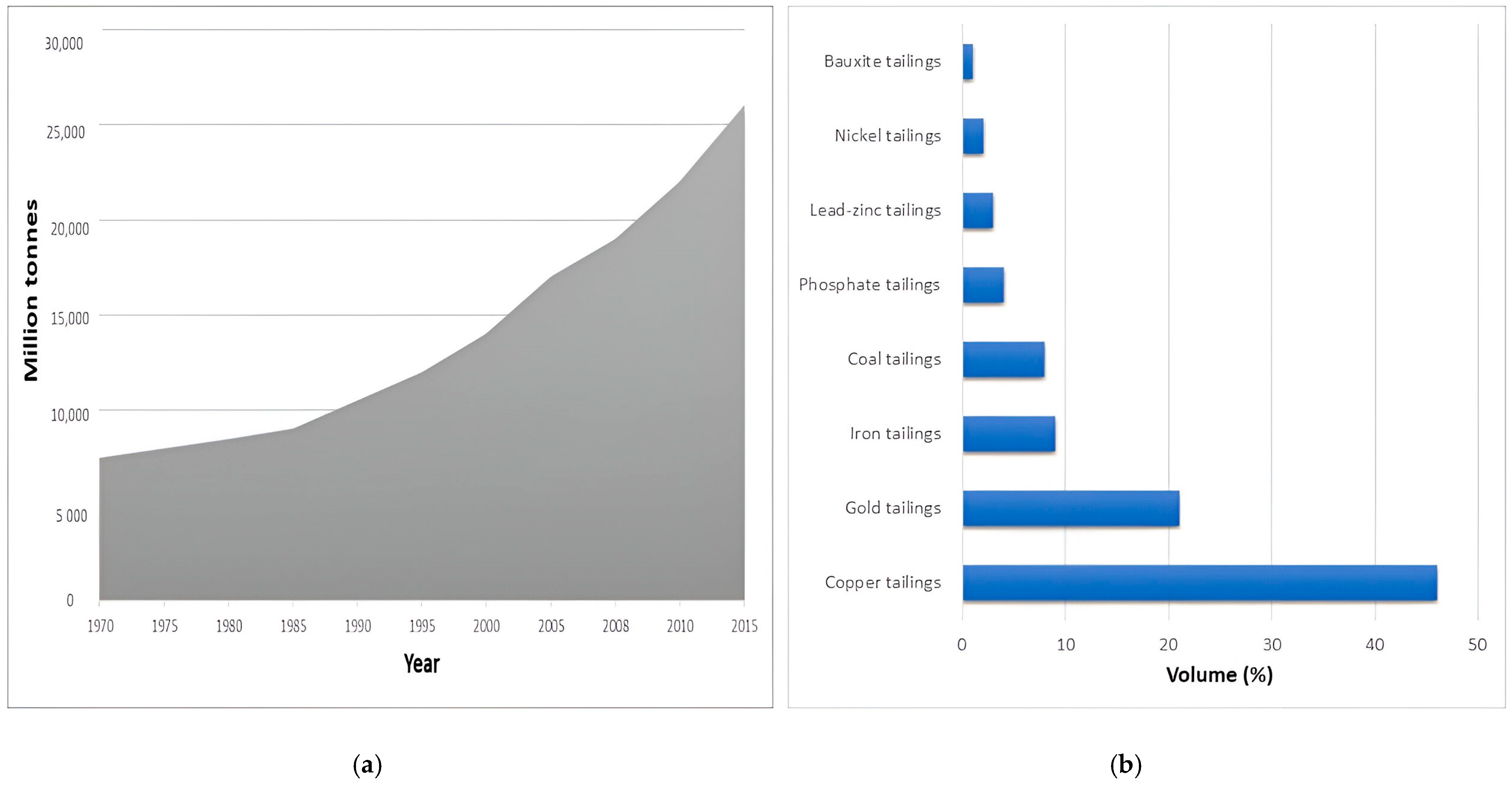
3. Mine Tailings Production: Overview and Industry Trends
4. Mine Tailings Characteristics
4.1. Physical Properties
4.2. Chemical and Mineralogical Properties
4.3. Geochemical Characteristics Environmental Issues from Accumulated Mine Tailings
| Type | Origin | Mineral Composition | Chemical Composition | Physical Propriety | Reference |
|---|---|---|---|---|---|
| Iron ore tailings | Sangan iron beneficiation plant | Major phases: hematite, quartz, calcite and, chlorite; minor phases: goethite and dolomite | Fe2O3 (31.33%), SiO2 (16.24%), CaO (14.98%), and MgO (12.33%) | D50 (µm) = 31.16 | [91] |
| Plomb tailings | Zaida abandoned mine site, Morocco | Quartz (50%), orthoclase (30%), albite(5.5%), chlorite (4%), barite (8%) and fluorite (2%). | SiO2 (68%) and Al2O3 (9%) | D50 (µm) = 230 Fine content (% < 63 μm) = 18.5 Specific gravity (g/cm3) = 2.7 Specific surface area (m2/g) = 1.484 | [64] |
| Zinc tailings | Boubeker abandoned mine site, Morocco | Dolomite content (87%) with a small content in terms of quartz (9%), smithsonite (3%) and albite (1%). | Cao (25%) and MgO (17%) | D50 (µm) = 120 Fine content (% < 63 µm) = 28 Specific gravity (g/cm3) = 2.9 Specific surface area (m2/g) = 1.573 | [64] |
| Nickel Rim tailings | Nickel rim, Sudbury, Canada | Gypsum, goethite, jarosite, and sulfides | Cu = 313 mg/g Ni = 294 mg/g Zn = 95 mg/g | Particle density (g/cm3) = 2.68, Bulk density (g/cm3) = 1.38 | [92] |
| Chromite ore tailing sample | M/s. Tata steel, Sukinda, Odisha, India | Major phases: magnesioferrite, chromite, Goethite, gibbsite, and quartz; Minor phases: hematite and kaolinite | Fe (27.39%), Cr2O3 (17.95%) SiO2 (17.25%) and Al2O3 (12.39%). | D50 = 10.72 µm Particle density (g/cm3) = 3.02 BET surface area (m2/g) = 23.79 | [93] |
| Calamine processing mine tailings | Calamine hydrometallurgical processing plant, Morocco | Gypsum, quartz, and calcite | SiO2 (13.4 wt%), CaO (23.5 wt%) Fe2O3 (13.3 wt%) and SO3 (24.6 wt%) | 2–63 μm = 68% Specific gravity (g/cm3) = 2.66 Specific surface area (m2/g) = 18.65 | [94] |
| Phosphate Sludge | Gafsa, Tunisia | Fluoroapatite, Calcite, dolomite), and clays (i.e., Heulandite, vermiculite and palygorskite) | SiO2 (39 wt%), CaO (17 wt%), Al2O3 (7.3 wt%) and P2O5 (6.5 wt%) | D50 = 8.3 μm D80 = 17.2 μm | [4] |
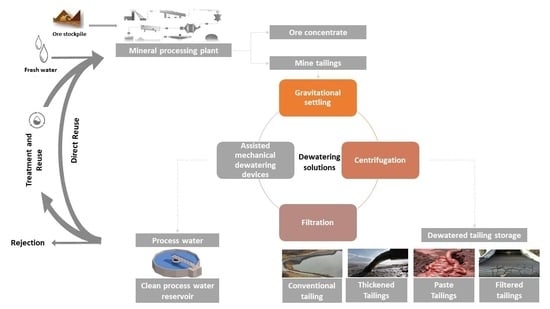
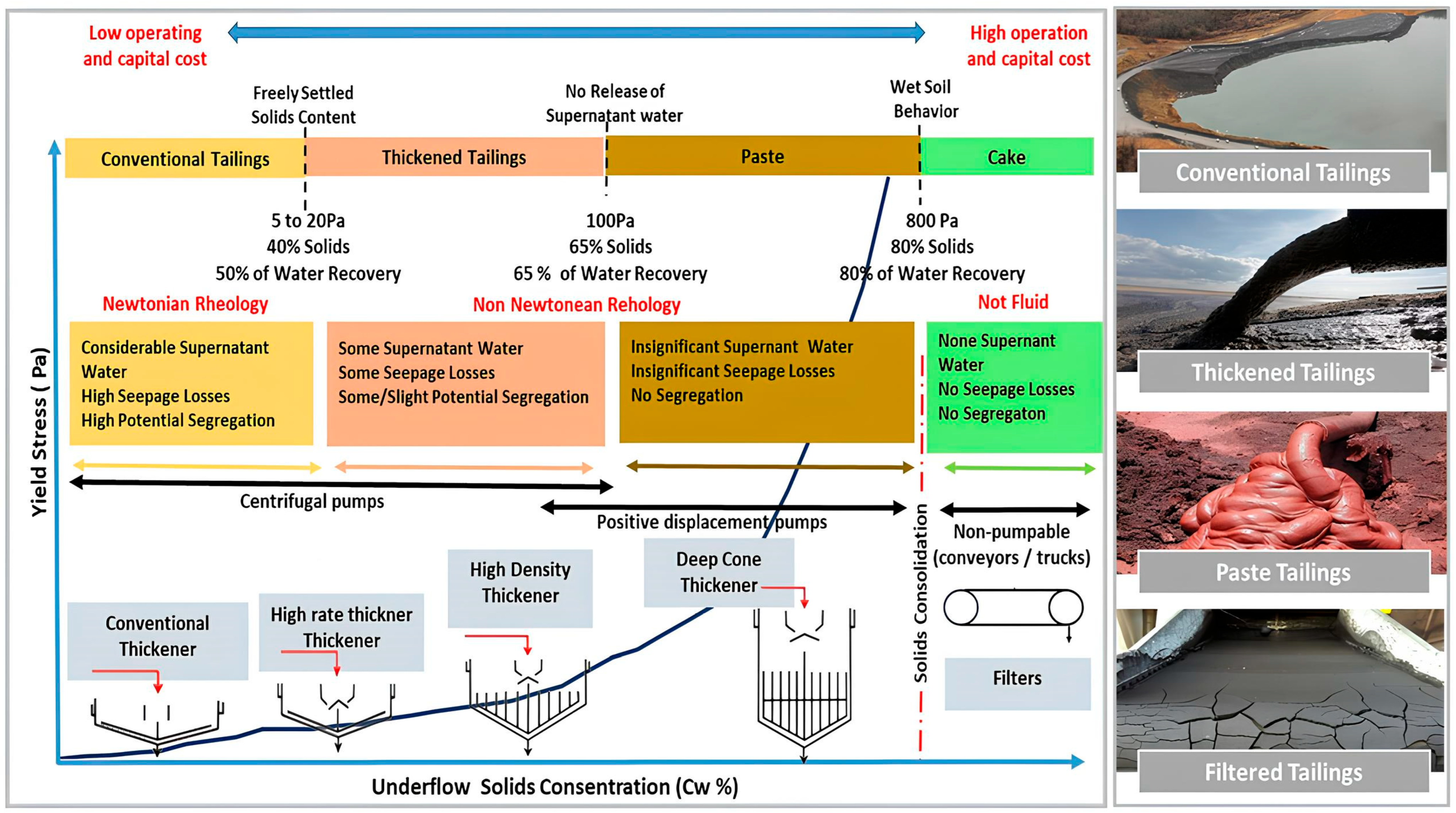
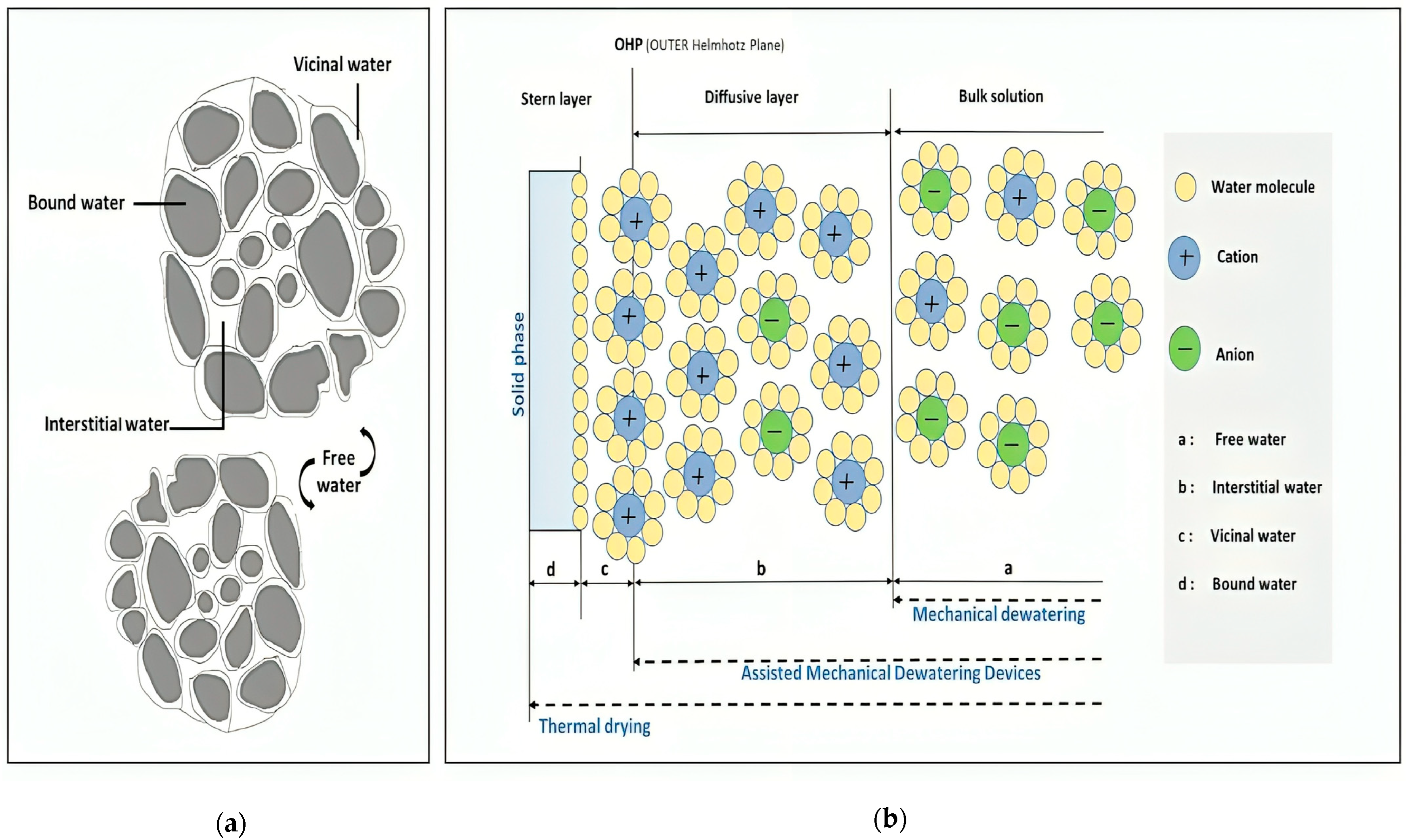
5. Dewatering Methods Classification
5.1. Mechanical Dewatering
5.1.1. Gravitational Settling
Settling Rate Enhancement
Thickener Types
5.1.2. Advanced Centrifugal Techniques for Sedimentation and Filtration
Hydrocyclones
Sedimenting Centrifuges
Filtering Centrifuges
Flocculant Considerations
5.1.3. Filtration Techniques
Vacuum Filtration
Pressure Filtration
Filtration and Centrifugation
Operational Considerations
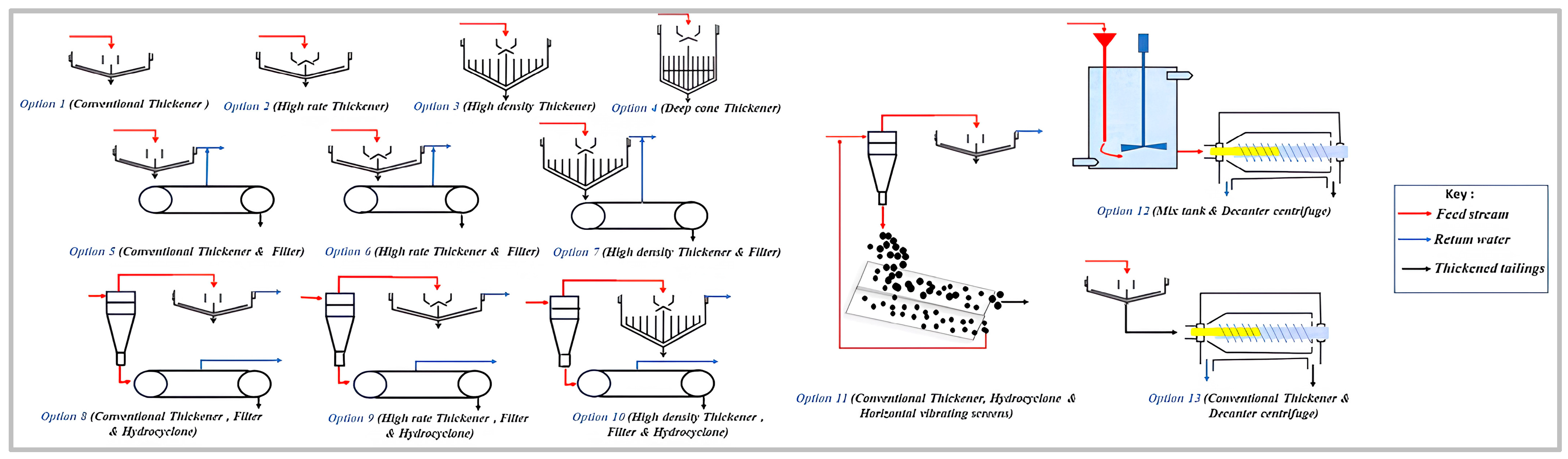
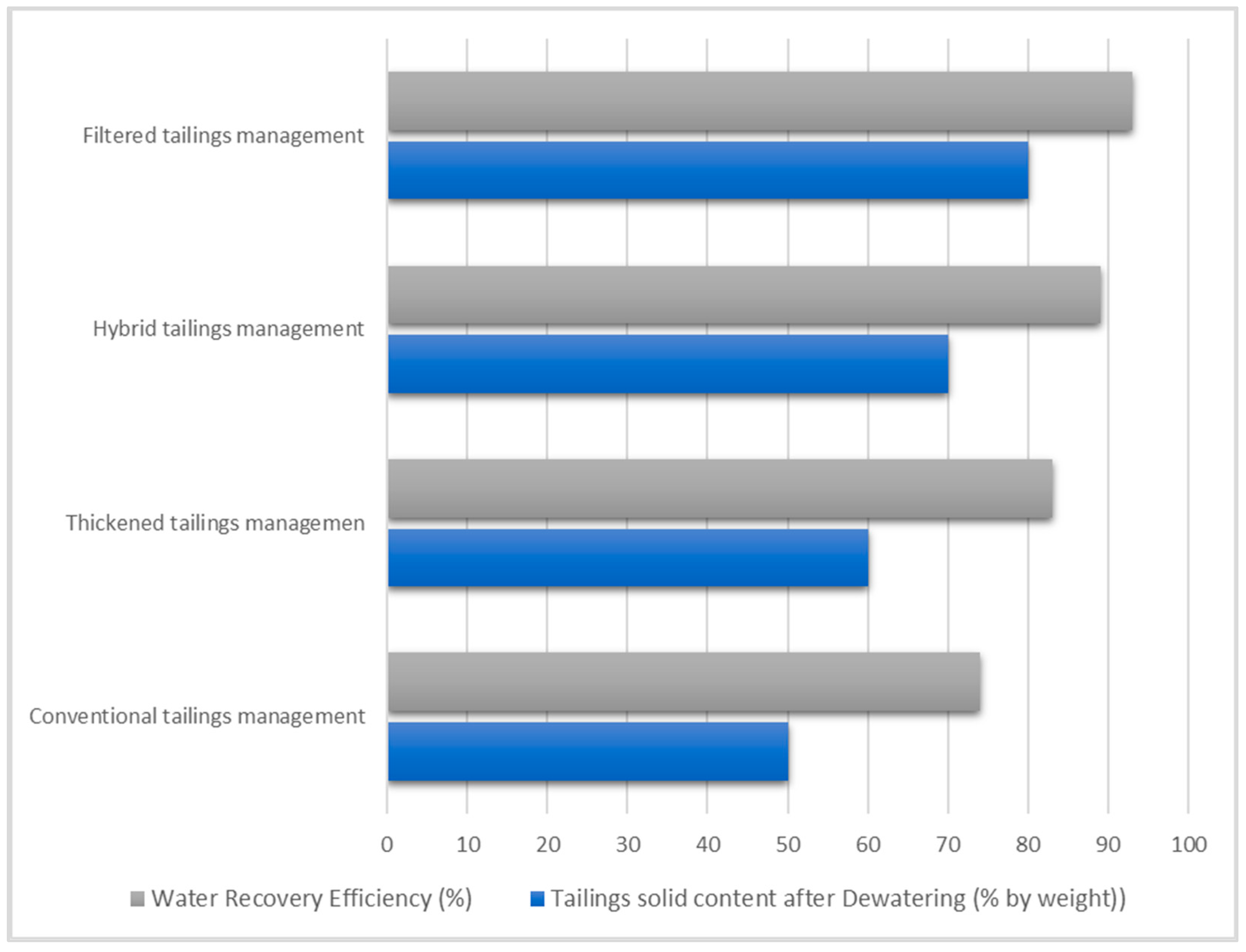
5.1.4. Hybrid Mechanical Dewatering Methods (Dewatering Circuit)
5.2. Innovative Techniques for Enhanced Tailings Dewatering (Assisted Mechanical Dewatering Devices)
5.2.1. Electrical Mechanical Dewatering
5.2.2. Thermal–Mechanical Dewatering and Thermal Electroosmosis Dewatering
5.2.3. Acoustic and Electroacoustic Dewatering (EAD)
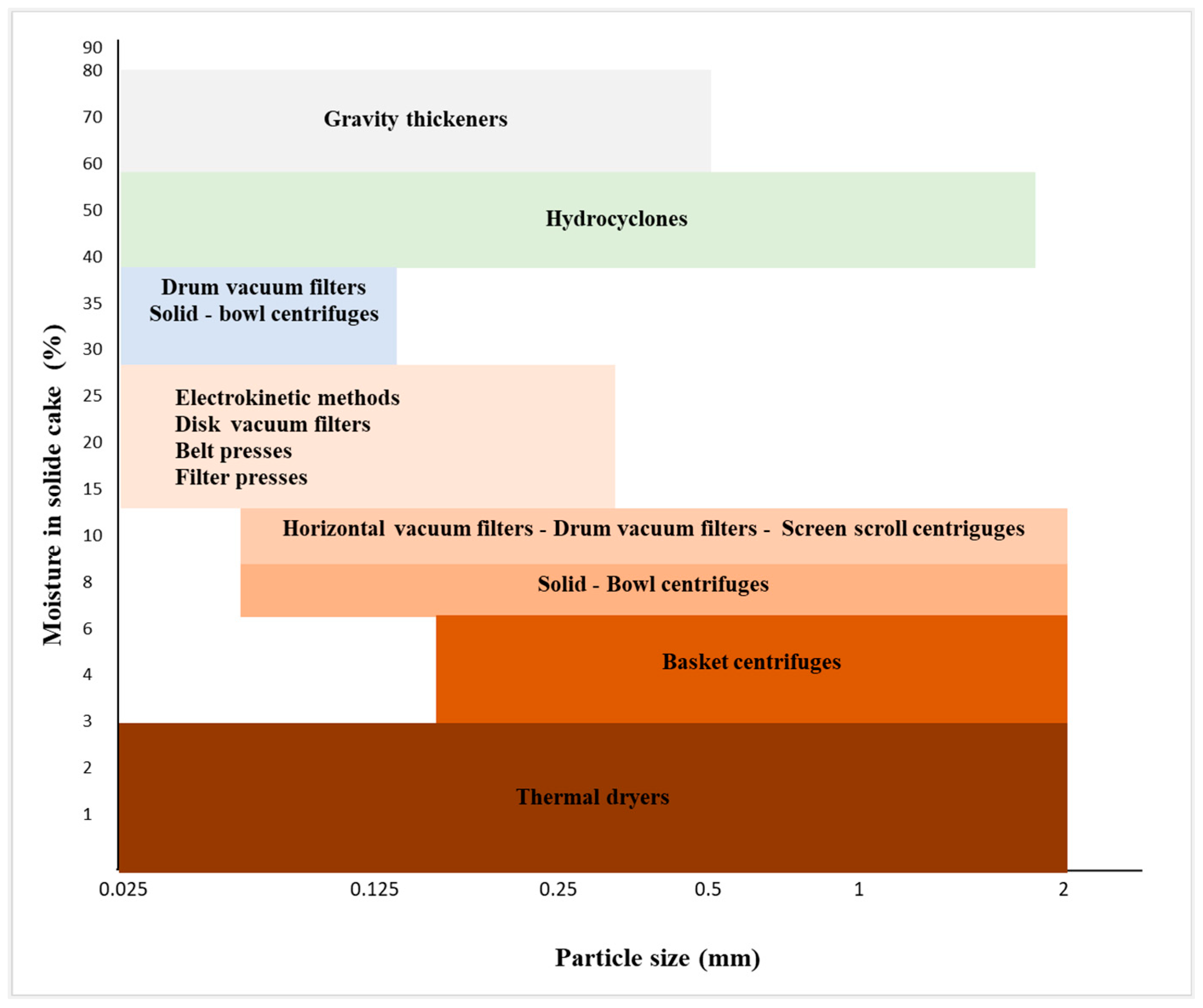
6. Tailings Dewatering Equipment Selection
7. Challenges Facing Water Recycling in Mining Industry
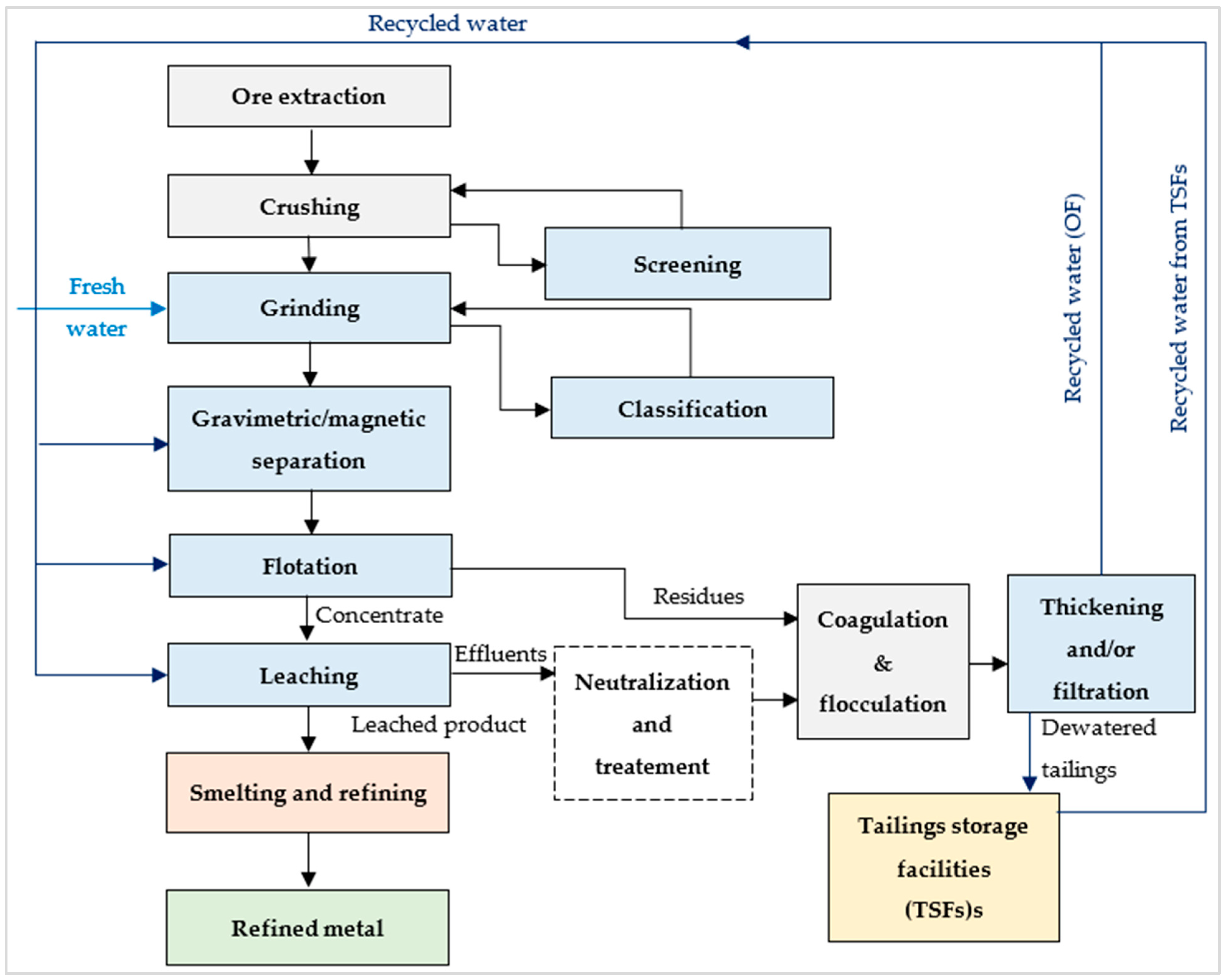
7.1. The Importance of Water Recycling in the Mining Industry
7.2. Examples of Mining Wastewater Treatment Processes
| Treatment Process Type | Method | Wastewater Type | Application | Reference |
|---|---|---|---|---|
| Physical (Adsorption on solid or at an interface) | Electrocoagulation | Wastewater from iron ore processing |
| [55] |
| Coagulation/flocculation | Wastewater |
| [214,234] | |
| Coagulation–sedimentation | Tannery wastewater |
| [235] | |
| Coagulation with electrooxidation | Wastewater |
| [214,236] | |
| Membrane technologies | Saline wastewater |
| [215] | |
| Filtration and ultrafiltration | Mine wastewater |
| [208] | |
| Ionic resin softening method (ion exchange) | Mine wastewater |
| [208] | |
| Adsorption method | Wastewater from acid mine drainage |
| [217] | |
| Reverse osmosis membrane | Mining wastewater |
| [237] | |
| Reverse osmosis | Industrial wastewater |
| [216] | |
| Chemical | Neutralization | Wastewater from acid mine drainage |
| [217] |
| Oxidation | Gold mining wastewater |
| [218] | |
| Oxidation | Acid Mine drainage wastewater |
| [238] | |
| Physicochemical | Dissolved air flotation (DAF) | Primary treated wastewater |
| [214] |
| Dissolved air flotation | Electroplating wastewater |
| [239] | |
| Dissolved air flotation (DAF) | Wastewater |
| [240] | |
| Electro-Fenton process | Acid mine wastewater |
| [241] | |
| Biological treatment (aerobic and anaerobic methods) |
| Saline wastewater |
| [215,242] |
| Algal photo-bioreactor + DAF |
| [214] | ||
| Active and passive biological treatments (active bioreactors, anaerobic bacteria, anaerobic wetland) | Acid mine drainage wastewater |
| [243] |
7.3. Economic Performance of Wastewater Treatment Processes
8. Conclusions and Research Needs
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amar, H.; Benzaazoua, M.; Elghali, A.; Hakkou, R.; Taha, Y. Waste rock reprocessing to enhance the sustainability of phos-phate reserves: A critical review. J. Clean. Prod. 2022, 381, 1–17. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Mineral Commodity Summaries 2020. pp. 1–240. Available online: https://pubs.usgs.gov/publication/mcs2020 (accessed on 10 February 2023).
- Salhi, B. Mutations Socio-Spatiales et Environnementales du Bassin Minier de Gafsa (Sud Ouest de Tunisie): Apport des Outils Géomatiques. Ph.D. Thesis, Le Mans University, Mans, France, 2017. [Google Scholar]
- Ettoumi, M.; Jouini, M.; Neculita, C.M.; Bouhlel, S.; Coudert, L.; Haouech, I.; Benzaazoua, M. Characterization of Kef Shfeir phosphate sludge (Gafsa, Tunisia) and optimization of its dewatering. J. Environ. Manag. 2020, 254, 109801. [Google Scholar] [CrossRef] [PubMed]
- Meißner, S. The impact of metal mining on global water stress and regional carrying capacities—A GIS-based water impact assessment. Resources 2021, 10, 120. [Google Scholar] [CrossRef]
- Ettoumi, M.; Jouini, M.; Neculita, C.M.; Bouhlel, S.; Coudert, L.; Benzaazoua, M. Improvement of water recovery from phosphate sludge at the M’Dhilla Mine, Tunisia. Environ. Sci. Pollut. Res. 2022, 29, 68965–68975. [Google Scholar] [CrossRef] [PubMed]
- Mavis, J. Water Use in Industries of the Future: Mining Industry. Industrial Water Management: A Systems Approach, 2nd ed.; prepared by CH2M HILL for the Center for Waste Reduction Technologies; American Institute of Chemical Engineers: New York, NY, USA, 2003. Available online: https://www.energy.gov/eere/amo/articles/itp-mining-water-use-industries-future-mining-industry (accessed on 18 May 2023).
- Petit, O. La surexploitation des eaux souterraines: Enjeux et gouvernance. Nat. Sci. Soc. 2004, 12, 146–156. [Google Scholar] [CrossRef]
- Dlamini, N.; Senzanje, A.; Mabhaudhi, T. Assessing climate change impacts on surface water availability using the WEAP model: A case study of the Buffalo river catchment, South Africa. J. Hydrol. Reg. Stud. 2023, 46, 101330. [Google Scholar] [CrossRef]
- Tramblay, Y.; Llasat, M.C.; Randin, C.; Coppola, E. Climate change impacts on water resources in the Mediterranean. Reg. Environ. Chang. 2020, 20, 83. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Mata, L.; Arnell, N.W.; Döll, P.; Jimenez, B.; Miller, K.; Oki, T.; Şen, Z.; Shiklomanov, I. The implications of projected climate change for freshwater resources and their management. Hydrol. Sci. J. 2008, 53, 3–10. [Google Scholar] [CrossRef]
- Narsimlu, B.; Gosain, A.K.; Chahar, B.R. Assessment of Future Climate Change Impacts on Water Resources of Upper Sind River Basin, India Using SWAT Model. Water Resour. Manag. 2013, 27, 3647–3662. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Senent-Aparicio, J.; Martínez, C.; Pérez-Sánchez, J. Assessment of future hydrologic alteration due to climate change in the Aracthos River basin (NW Greece). Sci. Total Environ. 2020, 733, 139299. [Google Scholar] [CrossRef]
- Ericsson, M.; Löf, O. Mining’s contribution to national economies between 1996 and 2016. Miner. Econ. 2019, 32, 223–250. [Google Scholar] [CrossRef]
- Luo, T.; Young, R.; Reig, P. Aqueduct Projected Water Stress Country Rankings; Technical Note; World Resources Institute: Washington, DC, USA, 2015; pp. 1–16. Available online: https://www.wri.org/data/aqueduct-projected-water-stress-country-rankings (accessed on 26 May 2023).
- Araya, N.; Ramirez, Y.; Kraslawski, A.; Cisternas, L.A. Feasibility of re-processing mine tailings to obtain critical raw materials using real options analysis. J. Environ. Manag. 2021, 284, 112060. [Google Scholar] [CrossRef]
- Weng, R.; Chen, G.; Huang, X.; Tian, F.; Ni, L.; Peng, L.; Liao, D.; Xi, B. Geochemical Characteristics of Tailings from Typical Metal Mining Areas in Tibet Autonomous Region. Minerals 2022, 12, 697. [Google Scholar] [CrossRef]
- Wang, C.; Harbottle, D.; Liu, Q.; Xu, Z. Current state of fine mineral tailings treatment: A critical review on theory and practice. Miner. Eng. 2014, 58, 113–131. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A review of circular economy strategies for mine tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Zhang, L.; Zhang, P.; Wen, Q. Geotechnical Properties of Mine Tailings. J. Mater. Civ. Eng. 2017, 29, 04016220. [Google Scholar] [CrossRef]
- Pretel, A.F.; Vasconcelos, P.E.A.; Oliveira, R.C.R. Environmental Criminal Responsibility and Applicability of Brazilian Constitutional Principles. Int. J. Adv. Eng. Res. Sci. 2019, 6, 259–269. [Google Scholar] [CrossRef]
- Martí, J.; Riera, F.; Martínez, F. Interpretation of the failure of the Aznalcóllar (Spain) Tailings Dam. Mine Water Environ. 2021, 40, 189–208. [Google Scholar] [CrossRef]
- Demers, I.; Mbonimpa, M.; Benzaazoua, M.; Bouda, M.; Awoh, S.; Lortie, S.; Gagnon, M. Use of acid mine drainage treatment sludge by combination with a natural soil as an oxygen barrier cover for mine waste reclamation: Laboratory column tests and intermediate scale field tests. Miner. Eng. 2017, 107, 43–52. [Google Scholar] [CrossRef]
- Araya, N.; Ramírez, Y.; Cisternas, L.A.; Kraslawski, A. Use of real options to enhance water-energy nexus in mine tailings management. Appl. Energy 2021, 303, 117626. [Google Scholar] [CrossRef]
- Moreno Baqueiro Sansao, B.; Kellar, J.J.; Cross, W.M.; Romkes, A. Separation of particles of different surface energies through control of humidity. Miner. Eng. 2021, 160, 106680. [Google Scholar] [CrossRef]
- Luukkanen, S.; Tanhua, A.; Zhang, Z.; Mollehuara Canales, R.; Auranen, I. Towards waterless operations from mine to mill. Miner. Eng. 2022, 187, 107793. [Google Scholar] [CrossRef]
- Güney, E. Water footprint assessment of mining and processing of gold in Turkey. Int. J. Min. Reclam. Environ. 2024, 1–17. [Google Scholar] [CrossRef]
- Gunson, A.J. Quantifying, Reducing and Improving Mine Water Use. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Lin, S.; Liu, R.; Wu, M.; Hu, Y.; Sun, W.; Shi, Z.; Han, H.; Li, W. Minimizing beneficiation wastewater through internal reuse of process water in flotation circuit. J. Clean. Prod. 2020, 245, 118898. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Al-Zoubi, H. Purification of phosphate beneficiation wastewater: Separation of phosphate from Eshydia Mine (Jordan) by column-DAF flotation process. Int. J. Miner. Process. 2012, 110, 18–24. [Google Scholar] [CrossRef]
- Norgate, T.; Lovel, R. Water Use in Metal Production: A Life Cycle Perspective; Report no. DMR2505; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 2004; Available online: http://masters.donntu.ru/2012/feht/ponyka/library/ist.pdf (accessed on 10 February 2024).
- Northey, S.; Mohr, S.; Mudd, G.M.; Weng, Z.; Giurco, D. Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycl. 2014, 83, 190–201. [Google Scholar] [CrossRef]
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Ordóñez, J.I.; Jeldres, R.I.; Serna-Guerrero, R. Toward the Implementation of Circular Economy Strategies: An Overview of the Current Situation in Mineral Processing. Miner. Process. Extr. Metall. Rev. 2022, 43, 775–797. [Google Scholar] [CrossRef]
- Bureau of Mines. Minerals Yearbook: Metals, Minerals, and Fuels; Bureau of Mines: Washington, DC, USA, 1974; Volume 1, p. 1451. Available online: https://search.library.wisc.edu/digital/AET6LTJXWL7TNK8M (accessed on 5 July 2023).
- Al-Fariss, T.F.; Ozbelge, H.O.; El-Shall, H.S. On the Phosphate Rock Beneficiation for the Production of Phosphoric Acid in Saudi Arabia. J. King Saud Univ. Eng. Sci. 1992, 4, 13–31. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, E18507. [Google Scholar] [CrossRef]
- Bleiwas, D.I. Estimated Water Requirements for the Conventional Flotation of Copper Ores; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2012. Available online: https://pubs.usgs.gov/of/2012/1089/ (accessed on 5 July 2023).
- Dai, Q.; Kelly, J.; Elgowainy, A. Cobalt Life Cycle Analysis Update for the GREET Model; Argonne National Laboratory: Lemont, IL, USA, 2018; pp. 1–80. Available online: https://greet.anl.gov/files/update_cobalt (accessed on 5 July 2023).
- Gunson, A.; Klein, B.; Veiga, M.; Dunbar, S. Reducing mine water requirements. J. Clean. Prod. 2012, 21, 71–82. [Google Scholar] [CrossRef]
- Mudd, B.G.M. Sustainability reporting and the platinum group metals: A global mining industry leader? Platin. Met. Rev. 2012, 56, 2–19. [Google Scholar] [CrossRef]
- Mudd, G.M. Sustainability reporting in the gold mining industry: The need for continual improvement. In Proceedings of the SSEE 2007 International Conference on Engineering Sustainability, Perth, Australia, 31 October–2 November 2007; pp. 267–275. [Google Scholar]
- Mudd, G.M.; Diesendorf, M. Sustainability of uranium mining and milling: Toward quantifying resources and eco-efficiency. Environ. Sci. Technol. 2008, 42, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Mudd, G.M. Sustainability reporting and water resources: A preliminary assessment of embodied water and sustainable mining. Mine Water Environ. 2008, 27, 136–144. [Google Scholar] [CrossRef]
- Silva, G.A.; Kulay, L.A. Application of life cycle assessment to the LCA case studies single superphosphate production. Int. J. Life Cycle Assess. 2003, 8, 209–214. [Google Scholar] [CrossRef]
- Ossa-Moreno, J.; McIntyre, N.; Ali, S.; Smart, J.C.R.; Rivera, D.; Lall, U.; Keir, G. The Hydro-economics of Mining. Ecol. Econ. 2018, 145, 368–379. [Google Scholar] [CrossRef]
- Zay Ya, K.; Otake, T.; Koide, A.; Sanematsu, K.; Sato, T. Geochemical characteristics of ores and surface waters for environmental risk assessment in the Pinpet iron deposit, southern Shan State, Myanmar. Resour. Geol. 2020, 70, 296–308. [Google Scholar] [CrossRef]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Beneficiation of Lead-Zinc Ores—A Review. Miner. Process. Extr. Metall. Rev. 2022, 43, 564–583. [Google Scholar] [CrossRef]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The use of seawater as process water at Las Luces copper–molybdenum beneficiation plant in Taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Mitchell, R.H. Primary and secondary niobium mineral deposits associated with carbonatites. Ore Geol. Rev. 2015, 64, 626–641. [Google Scholar] [CrossRef]
- Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. Assessing the performance of a novel pneumatic magnetic separator for the beneficiation of magnetite ore. Miner. Eng. 2020, 156, 106483. [Google Scholar] [CrossRef]
- Rankin, P.W.; Kelebek, S.; Di Feo, A.; Taylor, J.R. Water Recycling and Seasonal Water Quality Effects in Mineral Processing. In Proceedings of the 61st Conference of Metallurgists, COM 2022, Montréal, QC, Canada, 21–24 August 2023; pp. 967–977. [Google Scholar]
- Kirjavainen, V.; Heiskanen, K. Some factors that affect beneficiation of sulphide nickel–copper ores. Miner. Eng. 2007, 20, 629–633. [Google Scholar] [CrossRef]
- Wani, O.B.; Manzoor, S.; Molaei, N.; Shoaib, M.; Khan, S.; Zeng, H.; Bobicki, E.R. Beneficiation of Nickel from Ultramafic Ores: Using Sodium Citrate as a Green Processing Reagent. Resour. Conserv. Recycl. 2022, 186, 106496. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Treatment of iron ore beneficiation plant process water by electrocoagulation. Arab. J. Chem. 2021, 14, 102902. [Google Scholar] [CrossRef]
- Jing, G.; Meng, X.; Sun, W.; Kowalczuk, P.; Gao, Z. Recent advances in treatment and recycling of mineral processing wastewater. Environ. Sci. Water Res. Technol. 2023, 9, 1290–1304. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Gonzalez, E.S. Understanding the adoption of Industry 4.0 technologies in improving environmental sustainability. Sustain. Oper. Comput. 2022, 3, 203–217. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Forbes, E.; Verster, I.; Jokovic, V.; Awatey, B.; Parbhakar-Fox, A. Review on advances in mineral processing technologies suitable for critical metal recovery from mining and processing wastes. Clean. Eng. Technol. 2022, 7, 100451. [Google Scholar] [CrossRef]
- Nwaila, G.T.; Ghorbani, Y.; Zhang, S.E.; Frimmel, H.E.; Tolmay, L.C.K.; Rose, D.H.; Nwaila, P.C.; Bourdeau, J.E. Valorisation of mine waste—Part I: Characteristics of, and sampling methodology for, consolidated mineralised tailings by using Witwatersrand gold mines (South Africa) as an example. J. Environ. Manag. 2021, 295, 113013. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Lottermoser, B. Mine Wastes, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 304. ISBN 978-3-540-48629-9. [Google Scholar]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Ait-Khouia, Y.; Benzaazoua, M.; Demers, I. Environmental desulfurization of mine wastes using various mineral processing techniques: Recent advances and opportunities. Miner. Eng. 2021, 174, 107225. [Google Scholar] [CrossRef]
- Khalil, A.; Argane, R.; Benzaazoua, M.; Bouzahzah, H.; Taha, Y.; Hakkou, R. Pb–Zn mine tailings reprocessing using centrifugal dense media separation. Miner. Eng. 2019, 131, 28–37. [Google Scholar] [CrossRef]
- Baker, E.; Davies, M.; Fourie, A.; Mudd, G.; Thygesen, K. Chapter II. Mine Tailings Facilities: Overview and Industry Trends. In Towards Zero Harm: A Compendium of Papers Prepared for the Global Tailings Review; Oberle, B., Brereton, D., Mihaylova, A., Eds.; Global Tailings Review: London, UK, 2020; pp. 14–25. [Google Scholar]
- Araujo, F.S.; Taborda-Llano, I.; Nunes, E.B.; Santos, R.M. Recycling and reuse of mine tailings: A review of advancements and their implications. Geosciences 2022, 12, 319. [Google Scholar] [CrossRef]
- Vitti, C.; Arnold, B.J. The reprocessing and revalorization of critical minerals in mine tailings. Min. Metall. Explor. 2022, 39, 49–54. [Google Scholar] [CrossRef]
- Sarker, S.K.; Haque, N.; Bhuiyan, M.; Bruckard, W.; Pramanik, B.K. Recovery of strategically important critical minerals from mine tailings. J. Environ. Chem. Eng. 2022, 10, 107622. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Atencio, E. Past, Present, and Future of Copper Mine Tailings Governance in Chile (1905–2022): A Review in One of the Leading Mining Countries in the World. Int. J. Environ. Res. Public Health 2022, 19, 13060. [Google Scholar] [CrossRef] [PubMed]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chelgani, S.C.; Osbahr, I.; Möckel, R.; Peuker, U.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96, 94–98. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Shields, V.R.; Robshaw, T.J.; Porter, C.P.; Amphlett, J.T.; Hides, A.; Bruce, R.; Cordiner, J.; Ogden, M.D. Gold recovery from synthetic mine tailings leachate using chelating ion exchange resins with thiosulfate-thiourea lixiviant. Resour. Conserv. Recycl. Adv. 2023, 19, 200182. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, G.; Zhang, Q.; Zhao, W. Synergistic activation of sulfidized hemimorphite with copper-lead species for improving surface hydrophobicity and floatability. Sep. Purif. Technol. 2024, 332, 125854. [Google Scholar] [CrossRef]
- Montenegro, M.R.; Bruckard, W.J.; Gálvez, E.D.; Cisternas, L.A. Arsenic-rejection flotation circuit design and selection based on a multiple-objective evaluation. Miner. Eng. 2013, 45, 22–31. [Google Scholar] [CrossRef]
- Dimitrova, R.S.; Yanful, E.K. Factors affecting the shear strength of mine tailings/clay mixtures with varying clay content and clay mineralogy. Eng. Geol. 2012, 125, 11–25. [Google Scholar] [CrossRef]
- Sarsby, R.W. Environmental Geotechnics; Thomas Telford: London, UK, 2000; p. 584. ISBN 0-7277-2752-4. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay and Clay Minerals; IntechOpen: London, UK, 2021; Volume 24, pp. 1–29. ISBN 978-1-83969-564-3. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2013; p. 506. ISBN 978-0903056-33-5. [Google Scholar]
- Dash, M.; Dwari, R.K.; Biswal, S.K.; Reddy, P.S.R.; Chattopadhyay, P.; Mishra, B.K. Studies on the effect of flocculant adsorption on the dewatering of iron ore tailings. Chem. Eng. J. 2011, 173, 318–325. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Handbook of Clay Science; Intechopen: London, UK, 2006; pp. 1–18. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Fawell, P.; Berry, L. Improved water recovery: A review of clay-rich tailings and saline water interactions. Powder Technol. 2020, 364, 604–621. [Google Scholar] [CrossRef]
- Lawrence, S.; Davies, P. The Sludge Question: The Regulation of Mine Tailings in Nineteenth-Century Victoria. Environ. Hist. 2014, 20, 385–410. [Google Scholar] [CrossRef]
- Kalumba, D.; Mudenge, S. Review of the potential role of electrokinetics technology in tailings dewatering and minerals recovery. In Proceedings of the 22nd International Conference on Paste, Thickened and Filtered Tailings, Cape Town, South Africa, 8–10 May 2019; pp. 259–274. [Google Scholar]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Characterization of heavy metals in mine tailings and lake sediments: Implications on remediation. In Geo-Chicago 2016; ASCE: Reston, VA, USA, 2016; pp. 12–21. [Google Scholar]
- Jennings, S.; Neuman, D.; Blicker, P. Acid Mine Drainage and Effects on Fish, Health, and Ecology: A Review; Reclamation Research Group Publication: Bozeman, MT, USA, 2008. [Google Scholar]
- Jamieson, H.E. Geochemistry and Mineralogy of Solid Mine Waste: Essential Knowledge for Predicting Environmental Impact. Elements 2011, 7, 381–386. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Taha, Y.; Amar, H.; Ait-khouia, Y.; Bouzahzah, H.; Hakkou, R. Prediction of acid mine drainage: Where we are. Earth-Sci. Rev. 2023, 241, 104421. [Google Scholar] [CrossRef]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-rock hydrogeology and geochemistry. Appl. Geochem. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Arjmand, R.; Massinaei, M.; Behnamfard, A. Improving flocculation and dewatering performance of iron tailings thickeners. J. Water Process Eng. 2019, 31, 100873. [Google Scholar] [CrossRef]
- McGregor, R.; Blowes, D. The physical, chemical and mineralogical properties of three cemented layers within sulfide-bearing mine tailings. J. Geochem. Explor. 2002, 76, 195–207. [Google Scholar] [CrossRef]
- Dwari, R.K.; Angadi, S.I.; Tripathy, S.K. Studies on flocculation characteristics of chromite’s ore process tailing: Effect of flocculants ionicity and molecular mass. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 467–477. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Mansori, M.; Hakkou, R. Recycling Feasibility of Glass Wastes and Calamine Processing Tailings in Fired Bricks Making. Waste Biomass Valorization 2016, 8, 1479–1489. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F. Electrical field: A historical review of its application and contributions in wastewater sludge dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sanghi, R. Wastewater Reuse and Management; Springer: Berlin/Heidelberg, Germany, 2013; p. 502. ISBN 978-94-007-4941-2. [Google Scholar]
- Zhou, J.; Liu, Z.; She, P.; Ding, F. Water Removal from Sludge in a Horizontal Electric Field. Dry. Technol. 2007, 19, 627–638. [Google Scholar] [CrossRef]
- An-nori, A.; Ezzariai, A.; El Mejahed, K.; El Fels, L.; El Gharous, M.; Hafidi, M. Solar Drying as an Eco-Friendly Technology for Sewage Sludge Stabilization: Assessment of Micropollutant Behavior, Pathogen Removal, and Agronomic Value. Front. Environ. Sci. 2022, 10, 814590. [Google Scholar] [CrossRef]
- Burden, R.; Wilson, G.W. Commingling of Waste Rock and Tailings to Improve “Dry Stack” Performance: Design and Evaluation of Mixtures. Minerals 2023, 13, 295. [Google Scholar] [CrossRef]
- Cacciuttolo Vargas, C.; Marinovic Pulido, A. Sustainable Management of Thickened Tailings in Chile and Peru: A Review of Practical Experience and Socio-Environmental Acceptance. Sustainability 2022, 14, 10901. [Google Scholar] [CrossRef]
- Boger, D.V. Rheology of slurries and environmental impacts in the mining industry. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 239–257. [Google Scholar] [CrossRef]
- Fernando Concha, A. Solid-Liquid Separation in the Mining Industry; Springer: Berlin/Heidelberg, Germany, 2014; p. 429. ISBN 978-3-319-02483-7. [Google Scholar]
- Bustos, M.C. Sedimentation and Thickening: Phenomenological Foundation and Mathematical Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; Volume 8, p. 285. ISBN 978-94-015-9327-4. [Google Scholar]
- Concha, F.; Bürger, R. Thickening in the 20 th century: A historical perspective. Min. Metall. Explor. 2003, 20, 57–67. [Google Scholar] [CrossRef]
- Li, C.-h.; Shi, Y.-q.; Liu, P.; Guo, N. Analysis of the Sedimentation Characteristics of Ultrafine Tailings Based on an Orthogonal Experiment. Adv. Mater. Sci. Eng. 2019, 2019, 5137092. [Google Scholar] [CrossRef]
- Spicer, P.T.; Pratsinis, S.E. Shear-induced flocculation: The evolution of floc structure and the shape of the size distribution at steady state. Water Res. 1996, 30, 1049–1056. [Google Scholar] [CrossRef]
- Malíková, P.; Thomas, J.; Chromíková, J.; Vidlář, J.; Kupka, J. Innovation in dewatering process of flotation tailings by study of particle interaction in colloidal environment. Perspect. Sci. 2016, 7, 171–177. [Google Scholar] [CrossRef]
- Khazaie, A.; Mazarji, M.; Samali, B.; Osborne, D.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Soldatov, A. A Review on Coagulation/Flocculation in Dewatering of Coal Slurry. Water 2022, 14, 918. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, T.; Lu, J.; Wang, D.; Yao, C. Characterization of kaolin flocs formed by polyacrylamide as flocculation aids. Int. J. Miner. Process. 2009, 91, 94–99. [Google Scholar] [CrossRef]
- Yan, Y.D.; Glover, S.M.; Jameson, G.J.; Biggs, S. The flocculation efficiency of polydisperse polymer flocculants. Int. J. Miner. Process. 2004, 73, 161–175. [Google Scholar] [CrossRef]
- Oladoja, N.A. Advances in the quest for substitute for synthetic organic polyelectrolytes as coagulant aid in water and wastewater treatment operations. Sustain. Chem. Pharm. 2016, 3, 47–58. [Google Scholar] [CrossRef]
- Neelakantan, R.; Vaezi G., F.; Sanders, R.S. Effect of shear on the yield stress and aggregate structure of flocculant-dosed, concentrated kaolinite suspensions. Miner. Eng. 2018, 123, 95–103. [Google Scholar] [CrossRef]
- Castillo, C.; Ihle, C.F.; Jeldres, R.I. Chemometric Optimisation of a Copper Sulphide Tailings Flocculation Process in the Presence of Clays. Minerals 2019, 9, 582. [Google Scholar] [CrossRef]
- Li, G.; Qiao, D.; Xie, J.; Ahmed, A. Optimization of Flocculation and Sedimentation Parameters of Total Tailing Filling Material Based on Response Surface Method. J. Nanomater. 2022, 2022, 4804721. [Google Scholar] [CrossRef]
- Shakeel, A.; Safar, Z.; Ibanez, M.; van Paassen, L.; Chassagne, C. Flocculation of Clay Suspensions by Anionic and Cationic Polyelectrolytes: A Systematic Analysis. Minerals 2020, 10, 999. [Google Scholar] [CrossRef]
- Bian, J.; Wang, H.; Xiao, C.; Zhang, D. An experimental study on the flocculating settling of unclassified tailings. PLoS ONE 2018, 13, e0204230. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Hou, Y.; Yang, S.; Chen, X. Study on static and dynamic flocculation settlement characteristics of fine tailings slurry and influence of flocculant on strength of fine tailings backfill. Case Stud. Constr. Mater. 2022, 17, e01525. [Google Scholar] [CrossRef]
- Jeldres, M.; Piceros, E.C.; Toro, N.; Torres, D.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Copper Tailing Flocculation in Seawater: Relating the Yield Stress with Fractal Aggregates at Varied Mixing Conditions. Metals 2019, 9, 1295. [Google Scholar] [CrossRef]
- Vaezi, G.F.; Sanders, R.S.; Masliyah, J.H. Flocculation kinetics and aggregate structure of kaolinite mixtures in laminar tube flow. J. Colloid. Interface. Sci. 2011, 355, 96–105. [Google Scholar] [CrossRef]
- Barrera, S.; Engels, J. High-density thickening for large production rates: Main challenges. In Paste 2018: Proceedings of the 21st International Seminar on Paste and Thickened Tailings; Australian Centre for Geomechanics: Perth, Australia, 2018; pp. 35–42. [Google Scholar]
- Dorr, J.V. LABORATORY AND PLANT: The Use of Hydrometallurgical Apparatus in Chemical Engineering. Ind. Eng. Chem. Res. 1915, 7, 119–130. [Google Scholar] [CrossRef]
- Svarovsky, L. Solid-Liquid Separation, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 166–190. ISBN 978-0-7506-4568-3. [Google Scholar]
- Watson, A.; Corser, P.; Garces Pardo, E.; Lopez Christian, T.; Vandekeybus, J. A comparison of alternative tailings disposal methods — the promises and realities. In Proceedings of the First International Seminar on the Reduction of Risk in the Management of Tailings and Mine Waste, Perth, Australia, 29 September–1 October 2010; pp. 499–514. [Google Scholar]
- Fourie, A. Perceived and realised benefits of paste and thickened tailings for surface deposition. In Proceedings of the 15th International Seminar on Paste and Thickened Tailings, Sun City, Pilansberg, South Africa, 16–19 April 2012; pp. 53–64. [Google Scholar]
- Miller, M. Thickener design, control and development. In Proceedings of the Conference: ALTA 2018, Perth, Australia, 19–26 May 2018. [Google Scholar]
- Tarleton, E.; Wakeman, R. Solid/liquid separation equipment. In Solid/Liquid Separation; ButterworthHeinemann: Oxford, UK, 2007; pp. 1–77. ISBN 1-85617-421-2. [Google Scholar]
- Zamorano, C.; Ramírez, S.; Sánchez, I.; Garrido, C. Technical and Economic Evaluation of Tailings Dewatering Circuits in the Largest Copper Mines. In Paste 2020: 23rd International Conference on Paste, Thickened and Filtered Tailings; Gecamin Publications: Santiago, Chile, 2020; pp. 1–13. [Google Scholar]
- Schoenbrunn, F.; Bach, M. The Development of Paste Thickening and Its Application to the Minerals Industry; An Industry Review. Berg-Hüttenmänn. Monatsh. 2015, 160, 257–263. [Google Scholar] [CrossRef]
- Schoenbrunn, F. Dewatering to higher densities—An industry review. In Proceedings of the 14th International Seminar on Paste and Thickened Tailings, Perth, Australia, 5–7 April 2011; pp. 19–23. [Google Scholar]
- Cacciuttolo, C.; Cano, D. Environmental Impact Assessment of Mine Tailings Spill Considering Metallurgical Processes of Gold and Copper Mining: Case Studies in the Andean Countries of Chile and Peru. Water 2022, 14, 3057. [Google Scholar] [CrossRef]
- Li, S.; Yu, Z.; Yu, H.; Wang, X. The Recent Progress China Has Made in High-Concentration Backfill. Sustainability 2022, 14, 2758. [Google Scholar] [CrossRef]
- Noble, A.; Luttrell, G.H. A review of state-of-the-art processing operations in coal preparation. Int. J. Min. Sci. Technol. 2015, 25, 511–521. [Google Scholar] [CrossRef]
- Klug, R.; Schwarz, N. Dewatering tailings: Rapid water recovery by use of centrifuges. In Proceedings of the 22nd International Conference on Paste, Thickened and Filtered Tailings, Cape Town, South Africa, 8–10 May 2019; pp. 369–383. [Google Scholar]
- Schaflinger, U. Centrifugal separation of a mixture. Fluid Dyn. Res. 1990, 6, 213–249. [Google Scholar] [CrossRef]
- Holdich, R.G.; Rushton, A.; Ward, A.S. Solid-Liquid Filtration and Separation Technology; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Yang, Q.; Li, Z.-m.; Lv, W.-j.; Wang, H.-l. On the laboratory and field studies of removing fine particles suspended in wastewater using mini-hydrocyclone. Sep. Purif. Technol. 2013, 110, 93–100. [Google Scholar] [CrossRef]
- Toprak, N.A.; Altun, O. Considering hydrocyclone operation for tailings dewatering purpose and its effects on product specifications of paste backfill operations. Miner. Eng. 2021, 173, 107176. [Google Scholar] [CrossRef]
- Van Ryssen, G.; Steenkamp, P. Thickening of tailings using hydrocyclones operating under vacuum conditions. In Proceedings of the 15th International Seminar on Paste and Thickened Tailings, Sun City, South Africa, 16–19 April 2012; pp. 225–231. [Google Scholar]
- Garmsiri, M.R.; Unesi, M. Challenges and opportunities of hydrocyclone-thickener dewatering circuit: A pilot scale study. Miner. Eng. 2018, 122, 206–210. [Google Scholar] [CrossRef]
- Ye, J.; Xu, Y.; Song, X.; Yu, J. Novel conical section design for ultra-fine particles classification by a hydrocyclone. Chem. Eng. Res. Des. 2019, 144, 135–149. [Google Scholar] [CrossRef]
- Sutherland, K.S.; Chase, G. Filters and Filtration Handbook, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 520. ISBN 978-1856174640. [Google Scholar]
- Meiring, S. Cake formation: Three tailings filtration technologies using pressure. In Proceedings of the Paste 2021: 24th International Conference on Paste, Thickened and Filtered Tailings, Perth, Australia, 21–23 September 2021; pp. 91–104. [Google Scholar]
- Mumbi, A.; Fengting, L.; Mwarania, F.; Uuganchimeg, B. An assessment of multi-plate screw press in dewatering process of sludge treatment (the best option?). Int. J. Adv. Res. 2017, 5, 740–747. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Nguyen, A.V.; Doi, A.; Dinh, E.; Nguyen, T.V.; Ejtemaei, M.; Osborne, D. Advanced solid-liquid separation for dewatering fine coal tailings by combining chemical reagents and solid bowl centrifugation. Sep. Purif. Technol. 2021, 259, 118172. [Google Scholar] [CrossRef]
- Woodruff, D.; MacNamara, L. Treatment of coal tailings. In The Coal Handbook: Towards Cleaner Production; Elsevier: Amsterdam, The Netherlands, 2013; pp. 529–559. [Google Scholar] [CrossRef]
- Masliyah, J.H.; Czarnecki, J.; Xu, Z. Handbook on Theory and Practice on Bitumen Recovery from Athabasca Oil Sands; Kingsley Publishing Services: Philadelphia, PA, USA, 2011. [Google Scholar]
- Bickert, G. Solid–liquid separation technologies for coal. In The Coal Handbook: Towards Cleaner Production: Coal Production; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 422–444. [Google Scholar] [CrossRef]
- Thompson, D.K.; Motta, F.L.; Soares, J.B.P. Investigation on the flocculation of oil sands mature fine tailings with alkoxysilanes. Miner. Eng. 2017, 111, 90–99. [Google Scholar] [CrossRef]
- Hahn, J. Tailings dewatering with increased filtration rates and lowest filter cake moisture for filtered tailings stacking. In Proceedings of the 22nd International Conference on Paste, Thickened and Filtered Tailings, Cape Town, South Africa, 8–10 May 2019; p. 245. [Google Scholar]
- Haldar, S.K. Mineral Exploration: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ulrich, B. Practical thoughts regarding filtered tailings. In Proceedings of the 22nd International Conference on Paste, Thickened and Filtered Tailings, Cape Town, South Africa, 8–10 May 2019; pp. 71–79. [Google Scholar]
- Oldecop, L.; Rodari, G. Unsaturated mine tailings disposal. Soils Rocks 2021, 44, 067421. [Google Scholar] [CrossRef]
- Asmatulu, R. Air Pressure-Assisted Centrifugal Dewatering of Concentrated Fine Sulfide Particles. Int. J. Rotating Mach. 2011, 2011, 131824. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operations: An Introduction; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Citeau, M.; Larue, O.; Vorobiev, E. Electric (Electro/Dielectro-Phoretic)—Force Field Assisted Separators. In Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 325–397. [Google Scholar]
- Keles, S.; Luttrell, G.; Yoon, R.-H.; Estes, T.; Schultz, W.; Bethell, P. Development of the Centribaric™ Dewatering Technology. Int. J. Coal Prep. Util. 2010, 30, 204–216. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Atencio, E. An Alternative Technology to Obtain Dewatered Mine Tailings: Safe and Control Environmental Management of Filtered and Thickened Copper Mine Tailings in Chile. Minerals 2022, 12, 1334. [Google Scholar] [CrossRef]
- Gomes, R.B.; De Tomi, G.; Assis, P.S. Iron ore tailings dry stacking in Pau Branco mine, Brazil. J. Mater. Process. Technol. 2016, 5, 339–344. [Google Scholar] [CrossRef]
- Muralidhara, H.; Parekh, B.; Senapati, N. Solid-Liquid Separation Process for Fine Particle Suspensions by an Electric and Ultrasonic Field. U.S. Patent 4,561,953, 31 December 1985. [Google Scholar]
- Lee, J.E. Thermal Dewatering (TDW) to Reduce the Water Content of Sludge. Dry. Technol. 2006, 24, 225–232. [Google Scholar] [CrossRef]
- Clayton, S.A.; Wheeler, R.A.; Hoadley, A.F.A. Pore Destruction Resulting from Mechanical Thermal Expression. Dry. Technol. 2007, 25, 533–546. [Google Scholar] [CrossRef]
- Tuan, P.-A.; Mika, S.; Pirjo, I. Sewage sludge electro-dewatering treatment—A review. Dry. Technol. 2012, 30, 691–706. [Google Scholar] [CrossRef]
- Lockhart, N.C. Electro-dewatering of fine suspensions. In Advances in Solid-Liquid Separation; Springer: Berlin/Heidelberg, Germany, 1986; pp. 241–274. [Google Scholar]
- Shafaei, F.; Doulati Ardejani, F.; Bahroudi, A.; Hoseini, M.; Khakpour, M. Mechanical-Electrical dewatering (EDW) of mine Tailings: Influence of voltage level on water recovery and moisture reduction. Miner. Eng. 2022, 175, 107303. [Google Scholar] [CrossRef]
- Ferreira, L.D.; Gomes, R.C.; Ferreira, A.C. Electrokinetic dewatering of mine tailing: Influence of solid content and voltage level applied. Environ. Earth Sci. 2021, 81, 26. [Google Scholar] [CrossRef]
- Fourie, A.B.; Johns, D.G.; Jones, C.F. Dewatering of mine tailings using electrokinetic geosynthetics. Can. Geotech. J. 2007, 44, 160–172. [Google Scholar] [CrossRef]
- Chen, H.; Mujumdar, A.S.; Ragbaran, G.S.V. Laboratory Experiments on Electroosmotic Dewatering of Vegetable Sludge and Mine Tailings. Dry. Technol. 1996, 14, 2435–2445. [Google Scholar] [CrossRef]
- Loginov, M.; Citeau, M.; Lebovka, N.; Vorobiev, E. Electro-dewatering of drilling sludge with liming and electrode heating. Sep. Purif. Technol. 2013, 104, 89–99. [Google Scholar] [CrossRef]
- Bourgès-Gastaud, S.; Dolez, P.; Blond, E.; Touze-Foltz, N. Dewatering of oil sands tailings with an electrokinetic geocomposite. Miner. Eng. 2017, 100, 177–186. [Google Scholar] [CrossRef]
- Shang, J.; Lo, K. Electrokinetic dewatering of a phosphate clay. J. Hazard. Mater. 1997, 55, 117–133. [Google Scholar] [CrossRef]
- Larue, O.; Vorobiev, E. Sedimentation and water electrolysis effects in electrofiltration of kaolin suspension. AIChE J. 2004, 50, 3120–3133. [Google Scholar] [CrossRef]
- Larue, O.; Mouroko-Mitoulou, T.; Vorobiev, E. Pressurized Electroosmotic Dewatering in a Filter Cycle. Dry. Technol. 2007, 19, 2363–2377. [Google Scholar] [CrossRef]
- Larue, O.; Wakeman, R.J.; Tarleton, E.S.; Vorobiev, E. Pressure electroosmotic dewatering with continuous removal of electrolysis products. Chem. Eng. Sci. 2006, 61, 4732–4740. [Google Scholar] [CrossRef]
- Saveyn, H.; Pauwels, G.; Timmerman, R.; Van der Meeren, P. Effect of polyelectrolyte conditioning on the enhanced dewatering of activated sludge by application of an electric field during the expression phase. Water Res. 2005, 39, 3012–3020. [Google Scholar] [CrossRef]
- Lee, J.K.; Shang, J.Q.; Xu, Y. Electrokinetic dewatering of mine tailings using DSA electrodes. Int. J. Electrochem. Sci. 2016, 11, 4149–4160. [Google Scholar] [CrossRef]
- Rumky, J.; Deb, A.; Shim, M.J.; Laakso, E.; Repo, E. A review on the recent advances in electrochemical treatment technologies for sludge dewatering and alternative uses. J. Hazard. Mater. Adv. 2023, 11, 100341. [Google Scholar] [CrossRef]
- Yoshida, H.; Kitajyo, K.; Nakayama, M. Electroosmot1c Dewatering under A. C. Electric Field with Periodic Reversals of Electrode Polarity. Dry. Technol. 1999, 17, 539–554. [Google Scholar] [CrossRef]
- Kalumba, D.; Glendinning, S. A bench scale model for developing of an integrated in-situ remediation for heavy metals using EKG electrodes. In Advances in Unsaturated Soil, Seepage, and Environmental Geotechnics; ASCE: Reston, VA, USA, 2006; pp. 262–270. [Google Scholar]
- Ammami, M.; Song, Y.; Benamar, A.; Portet-Koltalo, F.; Wang, H. Electro-dewatering of dredged sediments by combined effects of mechanical and electrical processes: Influence of operating conditions. Electrochim. Acta 2020, 353, 136462. [Google Scholar] [CrossRef]
- Desabres, J.; Loginov, M.; Vorobiev, E. Model of electrofiltration in a filter press with anode flushing. Dry. Technol. 2017, 35, 1182–1194. [Google Scholar] [CrossRef]
- Yüksek, S. Electroosmotic Dewatering of Iron Ore Tailings: A Laboratory Study to Improve Geotechnical Properties. Adv. Civ. Eng. 2022, 2022, 7662997. [Google Scholar] [CrossRef]
- Cánovas, M.; Valenzuela, J.; Romero, L.; González, P. Characterization of electroosmotic drainage: Application to mine tailings and solid residues from leaching. J. Mater. Res. Technol. 2020, 9, 2960–2968. [Google Scholar] [CrossRef]
- Rumky, J.; Visigalli, S.; Turolla, A.; Gelmi, E.; Necibi, C.; Gronchi, P.; Sillanpaa, M.; Canziani, R. Electro-dewatering treatment of sludge: Assessment of the influence on relevant indicators for disposal in agriculture. J. Environ. Manag. 2020, 268, 110689. [Google Scholar] [CrossRef]
- Chaedir, B.A.; Kurnia, J.C.; Sasmito, A.P.; Mujumdar, A.S. Advances in dewatering and drying in mineral processing. Dry. Technol. 2021, 39, 1667–1684. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, R.; Cai, Y.; Fu, H.; Li, X.; Hu, X. Vacuum preloading and electro-osmosis consolidation of dredged slurry pre-treated with flocculants. Eng. Geol. 2018, 246, 123–130. [Google Scholar] [CrossRef]
- Mahmoud, A.; Fernandez, A.; Chituchi, T.M.; Arlabosse, P. Thermally assisted mechanical dewatering (TAMD) of suspensions of fine particles: Analysis of the influence of the operating conditions using the response surface methodology. Chemosphere 2008, 72, 1765–1773. [Google Scholar] [CrossRef]
- Hamidi, A.; Pashang, B.; Asadollahfardi, G.; Sheikhy, F. The efficiency of thermal electro-osmosis method in dewatering of alumina tailings. Arab. J. Geosci. 2023, 16, 228. [Google Scholar] [CrossRef]
- Peeters, B. Mechanical Dewatering and Thermal Drying of Sludge in a Single Apparatus. Dry. Technol. 2010, 28, 454–459. [Google Scholar] [CrossRef]
- Peuker, U.; Stahl, W. Steam pressure filtration: Mechanical-thermal dewatering process. Dry. Technol. 2001, 19, 807–848. [Google Scholar] [CrossRef]
- Du, J.; McLoughlin, R.; Smart, R.S.C. Improving thickener bed density by ultrasonic treatment. Int. J. Miner. Process. 2014, 133, 91–96. [Google Scholar] [CrossRef]
- Zhu, L.; Lyu, W.; Yang, P.; Wang, Z. Effect of ultrasound on the flocculation-sedimentation and thickening of unclassified tailings. Ultrason. Sonochem. 2020, 66, 104984. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.; Trampler, F.; Gröschl, M.; da Câmara Machado, A.; Laimer da Camara Machado, M. Use of an ultrasound cell retention system for the size fractionation of somatic embryos of woody species. Plant Cell Rep. 2000, 19, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Lo, S.-L.; Appels, L.; Dewil, R. Ultrasonic treatment of waste sludge: A review on mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1220–1288. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.; Tyagi, R.; Surampalli, R. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Aldrich, C.; Feng, D. Effect of ultrasonic preconditioning of pulp on the flotation of sulphide ores. Miner. Eng. 1999, 12, 701–707. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, P.; Wang, K.; Lyu, W. Efficient dewatering of unclassified tailings with flocculant: Role of ultrasound. Environ. Sci. Pollut. Res. Int. 2023, 30, 60354–60366. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Chen, Y. Ultrasonic enhancement of industrial sludge settling ability and dewatering ability. Tsinghua Sci. Technol. 2006, 11, 374–378. [Google Scholar] [CrossRef]
- Lippert, T.; Bandelin, J.; Schlederer, F.; Drewes, J.E.; Koch, K. Impact of ultrasound-induced cavitation on the fluid dynamics of water and sewage sludge in ultrasonic flatbed reactors. Ultrason. Sonochem. 2019, 55, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Önal, G.; Özer, M.; Arslan, F. Sedimentation of clay in ultrasonic medium. Miner. Eng. 2003, 16, 129–134. [Google Scholar] [CrossRef]
- Wakeman, R.; Smythe, M. Clarifying filtration of fine particle suspensions aided by electrical and acoustic fields. Chem. Eng. Res. Des. 2000, 78, 125–135. [Google Scholar] [CrossRef]
- Muralidhara, H.; Jirjis, B.; Senapati, N.; Menton, R.; Hsieh, P.; Chiang, S.; Cheng, Y.; Chauhan, S. Development of the Electroacoustic Dewatering (EAD) Process for Fine/Ultrafine Coal; Battelle Memorial Institute: Columbus, OH, USA, 1989. [Google Scholar]
- Abu-Orf, M.; Muller, C.D.; Park, C.; Novak, J.T. Innovative technologies to reduce water content of dewatered municipal residuals. J. Residuals Sci. Technol. 2004, 1, 83–91. [Google Scholar]
- Roe, C.H. Review of Desliming Methods and Equipment; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1984; Volume 8972.
- Carneiro, A.; Fourie, A. Assessing the impacts of uncertain future closure costs when evaluating strategies for tailings management. J. Clean. Prod. 2020, 247, 119173. [Google Scholar] [CrossRef]
- Kinnunen, P.; Obenaus-Emler, R.; Raatikainen, J.; Guignot, S.; Guimerà, J.; Ciroth, A.; Heiskanen, K. Review of closed water loops with ore sorting and tailings valorisation for a more sustainable mining industry. J. Clean. Prod. 2021, 278, 123237. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.J.; Vink, S. Quantitative Risk-Based Approach for Improving Water Quality Management in Mining. Environ. Sci. Technol. 2011, 45, 7459–7464. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.; Ji, H. Research on zero discharge treatment technology of mine wastewater. Energy Rep. 2022, 8, 275–280. [Google Scholar] [CrossRef]
- Le, T.M.K.; Mäkelä, M.; Schreithofer, N.; Dahl, O. A multivariate approach for evaluation and monitoring of water quality in mining and minerals processing industry. Miner. Eng. 2020, 157, 106582. [Google Scholar] [CrossRef]
- Pawar, N.D.; Harris, S.; Mitko, K.; Korevaar, G. Valorization of coal mine effluents—Challenges and economic opportunities. Water Resour. Ind. 2022, 28, 100179. [Google Scholar] [CrossRef]
- Masindi, V.; Chatzisymeon, E.; Kortidis, I.; Foteinis, S. Assessing the sustainability of acid mine drainage (AMD) treatment in South Africa. Sci. Total Environ. 2018, 635, 793–802. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Tong, L.; Liang, T.; Tian, Y.; Zhang, Q.; Pan, Y. Research progress on treatment of mine wastewater by bentonite composite. Arab. J. Geosci. 2022, 15, 681. [Google Scholar] [CrossRef]
- Badawi, A.K.; Ismail, B.; Baaloudj, O.; Abdalla, K.Z. Advanced wastewater treatment process using algal photo-bioreactor associated with dissolved-air flotation system: A pilot-scale demonstration. J. Water Process Eng. 2022, 46, 102565. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Trishitman, D.; Cassano, A.; Basile, A.; Rastogi, N.K. 9—Reverse osmosis for industrial wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–228. [Google Scholar]
- Tong, L.; Fan, R.; Yang, S.; Li, C. Development and Status of the Treatment Technology for Acid Mine Drainage. Min. Metall. Explor. 2021, 38, 315–327. [Google Scholar] [CrossRef]
- Coudert, L.; Bondu, R.; Rakotonimaro, T.V.; Rosa, E.; Guittonny, M.; Neculita, C.M. Treatment of As-rich mine effluents and produced residues stability: Current knowledge and research priorities for gold mining. J. Hazard. Mater. 2020, 386, 121920. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Zhang, Z.; Wang, X.; Wang, Q.; Wang, X.; Chen, C.; Jiang, L.; Li, X. Pilot-scale evaluation of the sustainability of membrane desalination systems for the concentrate volume minimization of coal chemical wastewater. Environ. Sci. Water Res. Technol. 2024, 10, 205–215. [Google Scholar] [CrossRef]
- Cui, M.; Jang, M.; Cho, S.-H.; Khim, J.; Cannon, F.S. A continuous pilot-scale system using coal-mine drainage sludge to treat acid mine drainage contaminated with high concentrations of Pb, Zn, and other heavy metals. J. Hazard. Mater. 2012, 215, 122–128. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Hayes, T.; Lokare, O.R.; Mittal, D.; Vidic, R.D. Laboratory and pilot-scale nanofiltration treatment of abandoned mine drainage for the recovery of products suitable for industrial reuse. Ind. Eng. Chem. Res. 2017, 56, 7355–7364. [Google Scholar] [CrossRef]
- Heiderscheidt, E.; Leiviskä, T.; Campos Lopez, F.; Tesfamariam, A.; Postila, H. Suitability of natural and chemically modified peat as a sorbent material for mining water purification in small-scale pilot systems. Environ. Technol. 2022, 43, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.X.; Al-Abed, S.R.; Balz, D.A.; Butler, B.A.; Landy, R.B.; Smith, S.J. Bench-scale and pilot-scale treatment technologies for the removal of total dissolved solids from coal mine water: A review. Mine Water Environ. 2016, 35, 94–112. [Google Scholar] [CrossRef]
- Andrade, L.H.; Aguiar, A.O.; Pires, W.L.; Grossi, L.B.; Amaral, M.C.S. Comprehensive bench- and pilot-scale investigation of NF for gold mining effluent treatment: Membrane performance and fouling control strategies. Sep. Purif. Technol. 2017, 174, 44–56. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Nguyen, B.Q.; Duong, T.T.; Bui, A.T.K.; Nguyen, H.T.A.; Cao, H.T.; Mai, N.T.; Nguyen, K.M.; Pham, T.T.; Kim, K.-W. Pilot-Scale Removal of Arsenic and Heavy Metals from Mining Wastewater Using Adsorption Combined with Constructed Wetland. Minerals 2019, 9, 379. [Google Scholar] [CrossRef]
- Mulopo, J. Continuous pilot scale assessment of the alkaline barium calcium desalination process for acid mine drainage treatment. J. Environ. Chem. Eng. 2015, 3, 1295–1302. [Google Scholar] [CrossRef]
- Yim, G.; Ji, S.; Cheong, Y.; Neculita, C.M.; Song, H. The influences of the amount of organic substrate on the performance of pilot-scale passive bioreactors for acid mine drainage treatment. Environ. Earth Sci. 2015, 73, 4717–4727. [Google Scholar] [CrossRef]
- Grossi, L.B.; Magalhães, N.C.; Araújo, B.M.; de Carvalho, F.; Andrade, L.H.; Amaral, M.C.S. Water conservation in mining industry by integrating pressure-oriented membrane processes for nitrogen-contaminated wastewater treatment: Bench and pilot-scale studies. J. Environ. Chem. Eng. 2021, 9, 104779. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Tan, K.; Hou, S.; Cai, J.; Yuan, X.; Lan, Q.; Cao, J.; Yan, S. Treatment and recovery of iron from acid mine drainage: A pilot-scale study. J. Environ. Chem. Eng. 2022, 10, 106974. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Naji, O.; Alanezi, A.A.; Ghaffour, N.; Déon, S.; Subbiah, S.; Altaee, A. Development in forward Osmosis-Membrane distillation hybrid system for wastewater treatment. Sep. Purif. Technol. 2022, 286, 120498. [Google Scholar] [CrossRef]
- Bhojwani, S.; Topolski, K.; Mukherjee, R.; Sengupta, D.; El-Halwagi, M.M. Technology review and data analysis for cost assessment of water treatment systems. Sci. Total. Environ. 2019, 651, 2749–2761. [Google Scholar] [CrossRef]
- Owen, G.; Bandi, M.; Howell, J.A.; Churchouse, S.J. Economic assessment of membrane processes for water and waste water treatment. J. Membr. Sci. 1995, 102, 77–91. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Judd, S.J. An economic assessment of coagulant recovery from water treatment residuals. Desalination 2012, 287, 132–137. [Google Scholar] [CrossRef]
- Wang, Q.; Hyman, M.; Higgins, B.T. Factors impacting the effectiveness of biological pretreatment for the alleviation of algal growth inhibition on anaerobic digestate. Algal Res. 2021, 53, 102129. [Google Scholar] [CrossRef]
- Fettig, J.; Pick, V.; Oldenburg, M.; Phuoc, N.V. Treatment of tannery wastewater for reuse by physico-chemical processes and a membrane bioreactor. J. Water Reuse Desalin. 2016, 7, 420–428. [Google Scholar] [CrossRef]
- Young, P.; Taylor, M.J.; Buchanan, N.; Lewis, J.; Fallowfield, H.J. Case study on the effect continuous CO2 enrichment, via biogas scrubbing, has on biomass production and wastewater treatment in a high rate algal pond. J. Environ. Manag. 2019, 251, 109614. [Google Scholar] [CrossRef] [PubMed]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. Performance evaluation of reverse osmosis process in the post-treatment of mining wastewaters: Case study of Costerfield mining operations, Victoria, Australia. J. Water Proc. Eng. 2020, 34, 101116. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva, E. Enhanced Heavy Metal Removal from Acid Mine Drainage Wastewater Using Double-Oxidized Multiwalled Carbon Nanotubes. Molecules 2020, 25, 111. [Google Scholar] [CrossRef] [PubMed]
- Pooja, G.; Kumar, P.S.; Prasannamedha, G.; Varjani, S.; Vo, D.-V.N. Sustainable approach on removal of toxic metals from electroplating industrial wastewater using dissolved air flotation. J. Environ. Manag. 2021, 295, 113147. [Google Scholar] [CrossRef]
- Wang, L.K.; Wang, M.-H.S. A New Wave of Flotation Technology Advancement for Wastewater Treatment. In Environmental Flotation Engineering; Wang, L.K., Wang, M.-H.S., Shammas, N.K., Aulenbach, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 143–166. [Google Scholar]
- Chai, Y.; Qin, P.; Zhang, J.; Li, T.; Dai, Z.; Wu, Z. Simultaneous removal of Fe(II) and Mn(II) from acid mine wastewater by electro-Fenton process. Process Saf. Environ. Prot. 2020, 143, 76–90. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Kamel, M.M.; Fawzy, M.E. Biological treatment of saline wastewater using a salt-tolerant microorganism. Desalination 2010, 250, 1–5. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.-Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Yapıcıoğlu, P.; Yeşilnacar, M.İ. Energy cost optimization of groundwater treatment using biochar adsorption process: An experimental approach. Water Supply 2023, 23, 14–33. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Zhao, X.; Li, J. Review of the recent advances in the prevention, treatment, and resource recovery of acid mine wastewater discharged in coal mines. J. Water Proc. Eng. 2023, 52, 103555. [Google Scholar] [CrossRef]
- Merma, A.G.; Santos, B.F.; Rego, A.S.; Hacha, R.R.; Torem, M.L. Treatment of oily wastewater from mining industry using electrocoagulation: Fundamentals and process optimization. J. Mater. Process. Technol. 2020, 9, 15164–15176. [Google Scholar] [CrossRef]
- Micari, M.; Cipollina, A.; Tamburini, A.; Moser, M.; Bertsch, V.; Micale, G. Techno-economic analysis of integrated processes for the treatment and valorisation of neutral coal mine effluents. J. Clean. Prod. 2020, 270, 122472. [Google Scholar] [CrossRef]
- Yapıcıoğlu, P.; Yeşilnacar, M.İ. Investigating energy costs for a wastewater treatment plant in a meat processing industry regarding water-energy nexus. Environ. Sci. Pollut. Res. 2022, 29, 1301–1313. [Google Scholar] [CrossRef]
- Hart, B.; Boger, D. Tailings waste minimisation, rheology, and the triple bottom line. In Proceedings of the International Seminar on Paste and Thickened Tailings, Santiago, Chile, 20–22 April 2005; Australian Center for Geomechanics, University of Western Australia: Nedlands, Australia, 2005; pp. 5–27. [Google Scholar]

| Typical Use | Centrifuge Type | Centrifugal Force (g) | Rotational Speed (rpm) | Throughput of Solids (m3/h) | Particle Size (μm) | Feed Concentration |
|---|---|---|---|---|---|---|
| Sedimentation | Tubular bowl | 10,000–65,000 | Up to 50,000 | Up to 4 | 0.1–100 | <5% w/w. |
| Basket | Up to 1600 | Up to 3500 | 6–10 | 0.1–100 | <5% w/w. | |
| Disc stack or Disc bowl centrifuges | Up to 14,000 | Up to 10,000 | Up to 200 | 0.1–100 | 0.05%–10% w/w | |
| Scroll decanter | 2000–6000 | Up to 6000 | Up to 100–120 | 1–5000 | 4%–60% w/w. | |
| Filtration | Pusher | 500–1700 | N.A | Up to 80 | 40–7000 | 10%–40% w/w. |
| Peeler basket or horizontal axis basket centrifuge | 800–2200 | Up to 1500 | Up to 15 | 2–1000 | 4%–30% w/w | |
| Screen scroll centrifuge or Worm screen | 500–2600 | Up to 700 | Up to 150 | 60–5000 | 10%–40% w/w |
| Dewatering Technique | Advantages | Disadvantages |
|---|---|---|
| Gravitational settling | Simple and cost-effective, requires minimal equipment, suitable for preliminary dewatering before more intensive processes. | Limited solid capture, slower process, not suitable for achieving very low moisture content. |
| Decanter centrifuge | Continuous process, good for materials with varying solids content, efficient separation. | Higher initial investment and maintenance costs, higher energy consumption compared to other methods, some noise and vibration, Moderate to high polymer demand |
| Hydrocyclones | Can achieve efficient separation of fine particles, relatively compact design, useful for pre-dewatering or size classification. | Limited dewatering capacity, not suitable for high moisture reduction, may require additional steps for further dewatering. |
| Vibrating screens | Simple design, suitable for coarse dewatering or preliminary screening, minimal energy consumption. | Limited efficiency for fine particles, may require multiple passes or subsequent dewatering steps, larger space requirement for high-capacity operations. |
| Vacuum filtration | Efficient for materials with fine particles, lower energy consumption compared to other mechanical methods. | Slower process, potential clogging of filter medium, may require pre-treatment to prevent clogging. |
| Filter press | Good solids capture, efficient for materials with high water content, relatively dry cake output. | Slower process, batch operation, larger space requirement, more maintenance, higher manual labor involvement. |
| Belt press | Continuous process, relatively efficient for large-scale operations, lower energy consumption compared to centrifuges, low polymer consumption and simple operation | May require chemical additives, potential for belt wear and maintenance, limited to materials that can be effectively dewatered on a belt. |
| Screw presses | Continuous process, low operating costs, lower energy consumption, small footprint, automated, low noise level | low capacity, high polymer use, limited particle size range, material limitations: |
| Electro-dewatering | Efficient dewatering, relatively quick process, reduced energy consumption compared to thermal methods, potential for use with various materials. | May require specific conditions to optimize performance, initial setup costs, potential maintenance of electrodes, limited adoption in some industries. |
| Thermal drying | Efficient for high-water-content materials, produces a stable, dry product, reduces volume significantly. | High energy consumption, potential for emissions depending on the heating method, may require additional treatment of exhaust gases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamraoui, L.; Bergani, A.; Ettoumi, M.; Aboulaich, A.; Taha, Y.; Khalil, A.; Neculita, C.M.; Benzaazoua, M. Towards a Circular Economy in the Mining Industry: Possible Solutions for Water Recovery through Advanced Mineral Tailings Dewatering. Minerals 2024, 14, 319. https://doi.org/10.3390/min14030319
Hamraoui L, Bergani A, Ettoumi M, Aboulaich A, Taha Y, Khalil A, Neculita CM, Benzaazoua M. Towards a Circular Economy in the Mining Industry: Possible Solutions for Water Recovery through Advanced Mineral Tailings Dewatering. Minerals. 2024; 14(3):319. https://doi.org/10.3390/min14030319
Chicago/Turabian StyleHamraoui, Laila, Abdelilah Bergani, Mouna Ettoumi, Abdelmaula Aboulaich, Yassine Taha, Abdessamad Khalil, Carmen Mihaela Neculita, and Mostafa Benzaazoua. 2024. "Towards a Circular Economy in the Mining Industry: Possible Solutions for Water Recovery through Advanced Mineral Tailings Dewatering" Minerals 14, no. 3: 319. https://doi.org/10.3390/min14030319
APA StyleHamraoui, L., Bergani, A., Ettoumi, M., Aboulaich, A., Taha, Y., Khalil, A., Neculita, C. M., & Benzaazoua, M. (2024). Towards a Circular Economy in the Mining Industry: Possible Solutions for Water Recovery through Advanced Mineral Tailings Dewatering. Minerals, 14(3), 319. https://doi.org/10.3390/min14030319