Process Optimization of an In-Situ Bioleaching Section with Associated Membrane Filtration in a Field Test Laboratory
Abstract
1. Introduction
2. Testing Environment and Evaluation of Optimization Potential
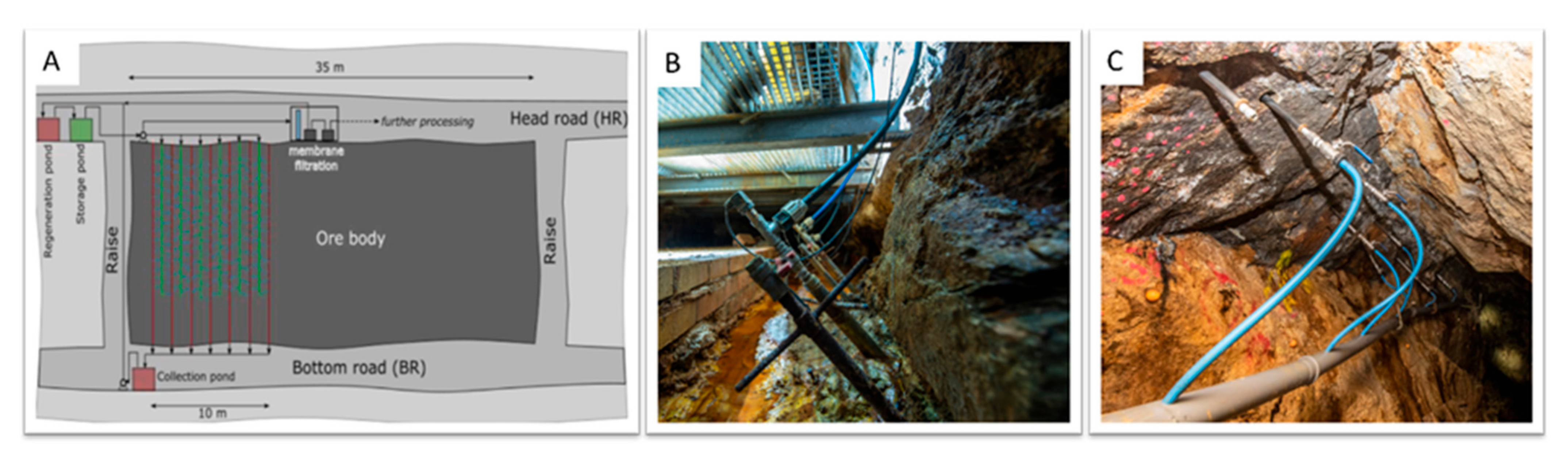
2.1. Geological and Hydrogeological Testing Site Conditions
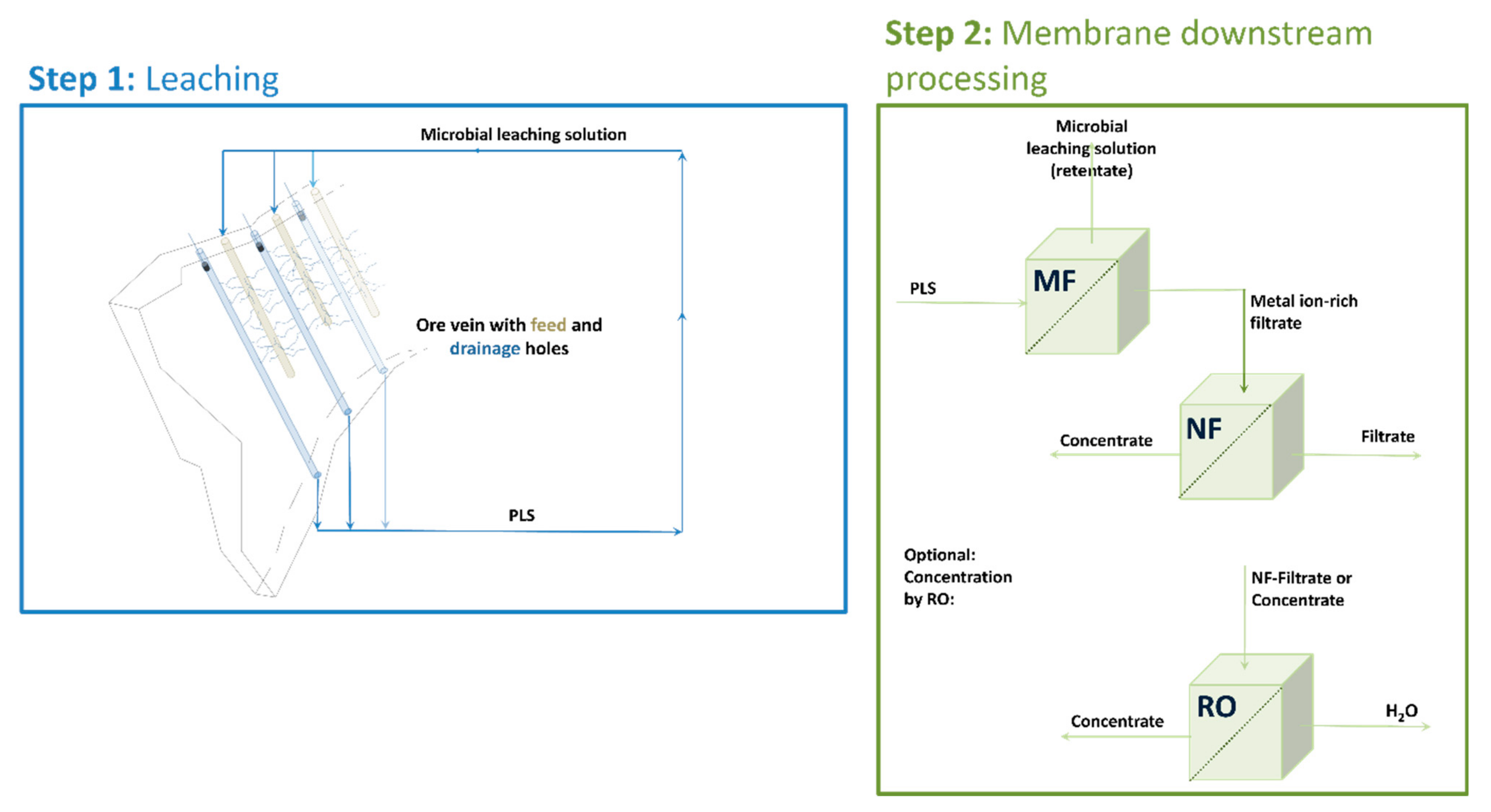
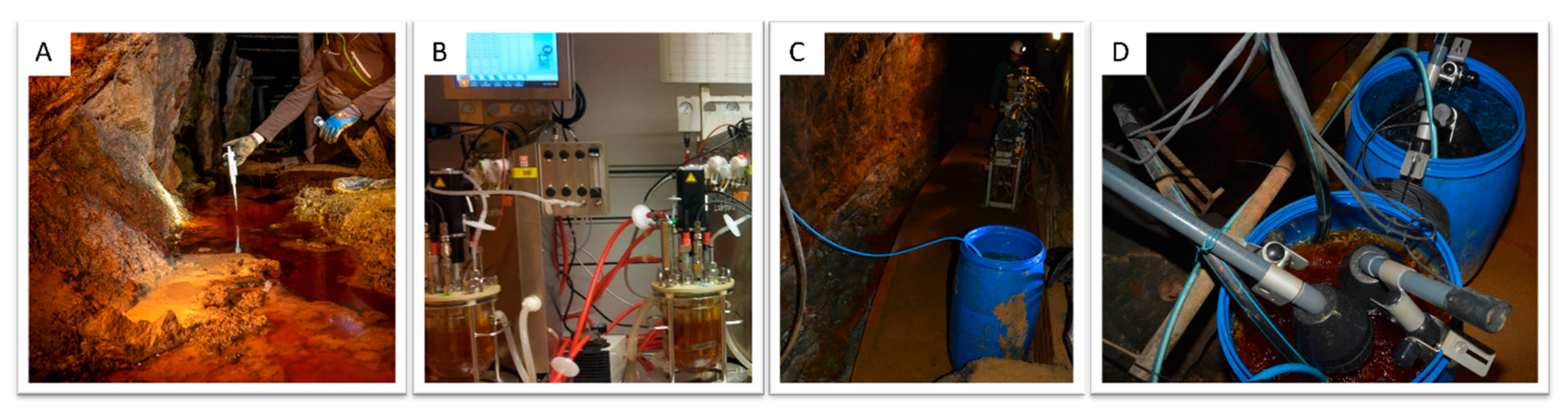
2.2. Bioleaching Setup
2.3. Microbiology
2.4. Membrane Downstream Processing
3. Materials and Methods
3.1. Solution Characteristics
3.2. Membrane Characterization Calculation Methods
4. Results and Discussion
4.1. Bioleaching Cycle
4.2. Microbiology
4.3. Bioleaching
4.4. Membrane Downstream Processing
4.4.1. NF Retention, Selectivity and CIP
4.4.2. MF Fouling
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Fathima, A.; Tang, J.Y.B.; Giannis, A.; Ilankoon, I.M.S.K.; Chong, M.N. Catalysing electrowinning of copper from E-waste: A critical review. Chemosphere 2022, 298, 134340. [Google Scholar] [CrossRef] [PubMed]
- Zarroca, M.; Roqué, C.; Linares, R.; Salminci, J.G.; Gutiérrez, F. Natural acid rock drainage in alpine catchments: A side effect of climate warming. Sci. Total Environ. 2021, 778, 146070. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Rodríguez, N.; Amils, R.; Sanz, J.L. Microbial Diversity in Anaerobic Sediments at Río Tinto, a Naturally Acidic Environment with a High Heavy Metal. Appl. Environ. Microbiol. 2011, 77, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Enders, M.S.; Knickerbocker, C.; Titley, S.R.; Southam, G. The Role of Bacteria in the Supergene Environment of the Morenci Porphyry Copper Deposit, Greenlee County, Arizona. Econ. Geol. 2006, 101, 59–70. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Younger, P.L.; Potter, A.B. Parys in Springtime: Hazard Management and Step towards Remediation of the UK’s Most Polluted Acidic Mine Discharge. In Proceedings of the 9th International Conference on Acid Rock Drainage (ICARD), Ottawa, ON, Canada, 20–26 May 2012; Available online: http://eprints.gla.ac.uk/69553/ (accessed on 17 December 2023).
- Sinclair, L.; Thompson, J. In situ leaching of copper: Challenges and future prospects. Hydrometallurgy 2015, 157, 306–324. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining goes underground. Nat. Geosci. 2015, 8, 165–166. [Google Scholar] [CrossRef]
- MacGregor, R.A. Recovery of U3O8 by Underground Leaching. CIM Bulletin. 1966. Available online: https://store.cim.org/en/recovery-of-u3o8-by-underground-leaching (accessed on 17 December 2023).
- Ontario Geological Survey. Ontario Mineral Inventory Record: MDI41J07NE00025. Stanrock Mine–1956. Available online: http://www.geologyontario.mndm.gov.on.ca/mndmfiles/mdi/data/records/MDI41J07NE00025.html (accessed on 17 December 2023).
- Statistica Research Department. Uranium Production Worldwide from 2014 to 2022, by Mining Method (in Metric Tons). Available online: https://www.statista.com/statistics/202358/world-uranium-mine-production-by-method/ (accessed on 17 December 2023).
- Schippers, A.; Hedrich, S.; Vasters, J.; Drobe, M.; Sand, W.; Willscher, S. Biomining: Metal recovery from ores with microorganisms. Adv. Biochem. Eng. Biotechnol. 2014, 141, 1–47. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Brierley, C.L. How will biomining be applied in future? Trans. Nonferrous Met. Soc. China 2008, 18, 1302–1310. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of nickel-copper sulfides. Hydrometallurgy 2008, 91, 70–88. [Google Scholar] [CrossRef]
- Darling, P. SME Mining Engineering Handbook, 3rd ed.; Society for Mining Metallurgy and Exploration: Littleton, CO, USA, 2011; ISBN 978-0-87335-264-2. [Google Scholar]
- Henry, D. Extracting Copper from Ore in Place. Eng. Min. J. 1911, 91, 649. Available online: https://archive.org/details/sim_engineering-and-mining-journal_1911-05-27_91_21/page/1044/mode/2up (accessed on 17 December 2023).
- Burton, C.; Cowman, S.; Heffernan, J.; Thorne, B. In-situ bioleaching of sulphide ores at Avoca, Ireland. Part I. Development, characterization, and operation of a medium-scale (6000 t) experimental leach site. In Proceedings of the International Symposium on Biohydrometallurgy, Dublin, Ireland, 1 May 1983; pp. 213–241. [Google Scholar]
- Derry, R.; Whittemore, R.G. Modelling the in-situ bioleaching of zinc copper sulphide ore from Avoca, Ireland. In Proceedings of the International Symposium on Biohydrometallurgy, Dublin, Ireland, 1 May 1983; pp. 243–263. [Google Scholar]
- Gallagher, V.; O’Connor, P. The Avoca Mine Site. In Royal Irish Academy. Biology and Environment: Proceedings of the Royal Irish Academy; 1999; Volume 99B, No. 1 pp. 43–57. Available online: http://www.jstor.org/stable/20500045 (accessed on 17 December 2023).
- Noske, A. Mikrobielle Laugung von Armerzen. Deutsch-Rumänische Zusammenarbeit. Seminar 17.–18. April 1991; Berichte des Forschungszentrum Jülich: Jülich 1991; ISSN: 0366-0885. Available online: https://juser.fz-juelich.de/record/848314/files/J%C3%BCl_2504_Noske.pdf (accessed on 17 December 2023).
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hatch Associates Ltd. BIOMOre—An Alternative Mining Concept. A New Mining Concept for Extraction Metals from Deep Ore Deposits by Using Biotechnology. D4.4 PLS Pre-Concentration, Product Recovery and Effluent Treatment. Ref. Ares(2017)1742378-31/03/2017. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5b1693b29&appId=PPGMS (accessed on 17 December 2023).
- Bomberg, M.; Miettinen, H.; Wahlström, M.; Kaartinen, T.; Ahoranta, S.; Lakaniemi, A.M.; Kinnunen, P. Evaluation of Long-Term Post Process Inactivation of Bioleaching Microorganisms. SSP 2017, 262, 57–60. [Google Scholar] [CrossRef]
- Bomberg, M.; Miettinen, H.; Hajdu-Rahkama, R.; Lakaniemi, A.-M.; Anacki, W.; Witecki, K.; Puhakka, J.A.; Ineich, T.; Slabbert, W.; Kinnunen, P. Indirect in situ bioleaching is an emerging tool for accessing deeply buried metal reserves, but can the process be managed?—A case study of copper leaching at 1 km depth. Environ. Technol. Innov. 2023, 32, 103375. [Google Scholar] [CrossRef]
- Murr, L.E.; Brierley, J.A. The Use of Large-Scale Test Facilities in Studies of the role of Microorganisms in Commercial Leaching Operations. In Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena; Murr, L.E., Torma, A.E., Brierley, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1978; pp. 491–521. [Google Scholar] [CrossRef]
- Miller, P.C. Large Scale Bacterial Leaching of a Copper Zinc Ore in Situ. In Fundamental and Applied Biohydrometallurgy, Proceedings of the Sixth International Symposium of Biohydrometallurgy, Vancouver, LM, Canada, 21–24 August 1985; Lawrence, R., Branion, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 215–239. [Google Scholar]
- Rossi, G.; Trois, P.; Visca, P. In-Situ Pilot Semi-Commercial Bioleaching Test at the San Valentino di Predoi Mine (Northern Italy). In Fundamental and Applied Biohydrometallurgy, Proceedings of the Sixth International Symposium of Biohydrometallurgy, Vancouver, LM, Canada, 21–24 August 1985; Lawrence, R., Branion, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 173–189. [Google Scholar]
- Brauckmann, B.; Poppe, W.; Beyer, W.; Lerche, R.; Steppke, H.D. Investigations of increased biological in-situ leaching of the “Old Deposit” of Preussag Rammelsberg ore mine. In Biohydrometallurgy. Science and Technology Letters; Norris, P.R., Ed.; ACS: Kew, UK, 1988; pp. 521–523. [Google Scholar]
- McCready, R.G.L.; Gould, W.D. Bioleaching of uranium at Denison Mines. In Biohydrometallugry’89; Sally, J., McCready, R.G.L., Wichlacz, P.L., Eds.; CANMET Spec. Publ.: Centre for Mineral and Energy Technology: Ottawa, ON, Canada, 1989; pp. 477–483. [Google Scholar]
- Götze, K.; Haseneder, R.; Braeuer, A.S. Investigations on Strategic Element Recovery by an Underground Membrane Pilot Plant from In-Situ Extracted Bioleaching Solutions. Minerals 2022, 12, 46. [Google Scholar] [CrossRef]
- Seifert, T.; Sandmann, D. Mineralogy and geochemistry of indium-bearing polymetallic vein-type deposits: Implications for host minerals from the Freiberg district, Eastern Erzgebirge, Germany. Ore Geol. Rev. 2006, 28, 1–31. [Google Scholar] [CrossRef]
- Bauer, M.E.; Seifert, T. An Indium-Enriched Polymetallic Vein from Freiberg, Germany—A Perfect Site for Experimental In-Situ Bioleaching of Strategic Metals in Complex ores? In Proceedings of the Mineral Resources in a Sustainable World, SGA 13th SGA Biennial Meeting, Nacy, France, 24–27 August 2015; Volume 2. [Google Scholar]
- Preuß, J. Geoelektrische Bohrlochtomographie an ausgewählten Bohrlöchern im Bereich des Erzganges “Wilhelm Stehender Nord”. 2. Zwischenbericht. IFU GmbH Privates Institut für Umweltanalysen. 04. May 2018. (Internal).
- Schlüter, R. Bestandsaufnahme und anstehende Arbeiten am untertägigen BHMZ Versuchsstand. Oral presentation. BHMZ Arbeitsgruppentreffen. 25 April 2016. (Internal).
- Mischo, H.; Schlüter, R. Untersuchung von Hydraulischen und Sprengtechnischen Konditionierungsmethoden zur In-Situ Laugung im Festgestein. Conference Paper-20. Kolloquium Bohr- und Sprengtechnik; Papierflieger: Clausthal-Zellerfeld, Germany, 2017; pp. 17–27. [Google Scholar]
- Schlüter, R. Statusbericht Teilprojekt 2: Design, Einrichtung und Durchführung eines in-situ Versuchsstandes zur mikrobiellen Laugung im Forschungsbergwerk und Lehrbergwerk. Freiberger Biohydrometallurgisches Zentrum–BHMZ. 2018. (Internal).
- Krichler, T. BHMZ Zwischenbericht-Teilprojekt unter Tage. Institut für Bergbau und Spezialbergbau, TU BAF, Freiberg. 9 November 2020. (Internal).
- Temple, K.L.; Colmer, A.R. The autotrophic oxidation of iron by a new bacterium, thiobacillus ferrooxidans. J. Bacteriol. 1951, 62, 605–611. [Google Scholar] [CrossRef]
- Sharma, P. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions. Hydrometallurgy 2003, 71, 285–292. [Google Scholar] [CrossRef]
- Schippers, A. Microorganisms Involved in Bioleaching and Nucleic Acid-Based Molecular Methods for Their Identification and Quantification. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–33. [Google Scholar] [CrossRef]
- Plumb, J.J.; Muddle, R.; Franzmann, P.D. Effect of pH on rates of iron and sulfur oxidation by bioleaching organisms. Miner. Eng. 2008, 21, 76–82. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Eisen, N.; Schlömann, M. TP6: Selectivity of microbial leaching of sphalerite in the presence of Indium and germanium. Institute of biosciences, TU BAF. Oral presentation. Holzhau, Germany. 20 May 2016. (Internal).
- Schopf, S. BHMZ-Teilabschlussbericht. 2nd May 2019. (internal).
- Schlüter, R. BHMZ-Arbeitsgruppentreffen: Bestandsaufnahme und anstehende Arbeiten am untertägigen Versuchsstand. Oral presentation. 8th May 2018. (Internal).
- Zhang, R.; Hedrich, S.; Ostertag-Henning, C.; Schippers, A. Effect of elevated pressure on ferric iron reduction coupled to sulfur oxidation by biomining microorganisms. Hydrometallurgy 2018, 178, 215–223. [Google Scholar] [CrossRef]
- Torma, A. Microbiological Extraction of Cobalt and Nickel from Sulfide Ores and Concentrates. Canadian Patent 960463, 7 January 1975. [Google Scholar]
- Werner, A. Entwicklung Eines Membranbasierten Trenn- und Anreicherungsverfahrens zur Selektiven Gewinnung von Indium und Germanium aus Laugungslösungen. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 13 August 2018. [Google Scholar] [CrossRef]
- Werner, A.; Haseneder, R.; Repke, J.-U. Design and Conception of a Membrane Pilot Plant for the In Situ Treatment of Bioleaching Solutions. Chem. Ing. Tech. 2019, 91, 145–150. [Google Scholar] [CrossRef]
- Aguiar, A.; Andrade, L.; Grossi, L.; Pires, W.; Amaral, M. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Sep. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.M.C.; Cornelissen, E.R.; Vrouwenvelder, J.S.; Verliefde, A.; van Loosdrecht, M.C.M.; Picioreanu, C. Cost of fouling in full-scale reverse osmosis and nanofiltration installations in the Netherlands. Desalination 2021, 500, 114865. [Google Scholar] [CrossRef]
- Zhao, D.; Qiu, L.; Song, J.; Liu, J.; Wang, Z.; Zhu, Y.; Liu, G. Efficiencies and mechanisms of chemical cleaning agents for nanofiltration membranes used in produced wastewater desalination. Sci. Total Environ. 2019, 652, 256–266. [Google Scholar] [CrossRef]
- Andrade, L.H.; Aguiar, A.O.; Pires, W.L.; Grossi, L.B.; Amaral, M.C.S. Comprehensive bench- and pilot-scale investigation of NF for gold mining effluent treatment: Membrane performance and fouling control strategies. Sep. Purif. Technol. 2017, 174, 44–56. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. [Google Scholar] [CrossRef]
- Beril Gönder, Z.; Arayici, S.; Barlas, H. Advanced treatment of pulp and paper mill wastewater by nanofiltration process: Effects of operating conditions on membrane fouling. Sep. Purif. Technol. 2011, 76, 292–302. [Google Scholar] [CrossRef]
- Sauser, B.; Verma, D.; Ramirez-Marquez, J.; Grove, R. From TRL to SRL: The Concept of Systems Readiness Levels. In Proceedings of the Conference on Systems Engineering Research (CSER), Los Angeles, CA, USA, 7–8 April 2006; Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=b501c19469fa5d11ada2858f310451b81b3dc61a (accessed on 17 December 2023).
- Krichler, T. Untersuchungen zur Echtzeitbetriebsüberwachung im Untertägigen Bergbau. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, 9 December 2021. [Google Scholar]

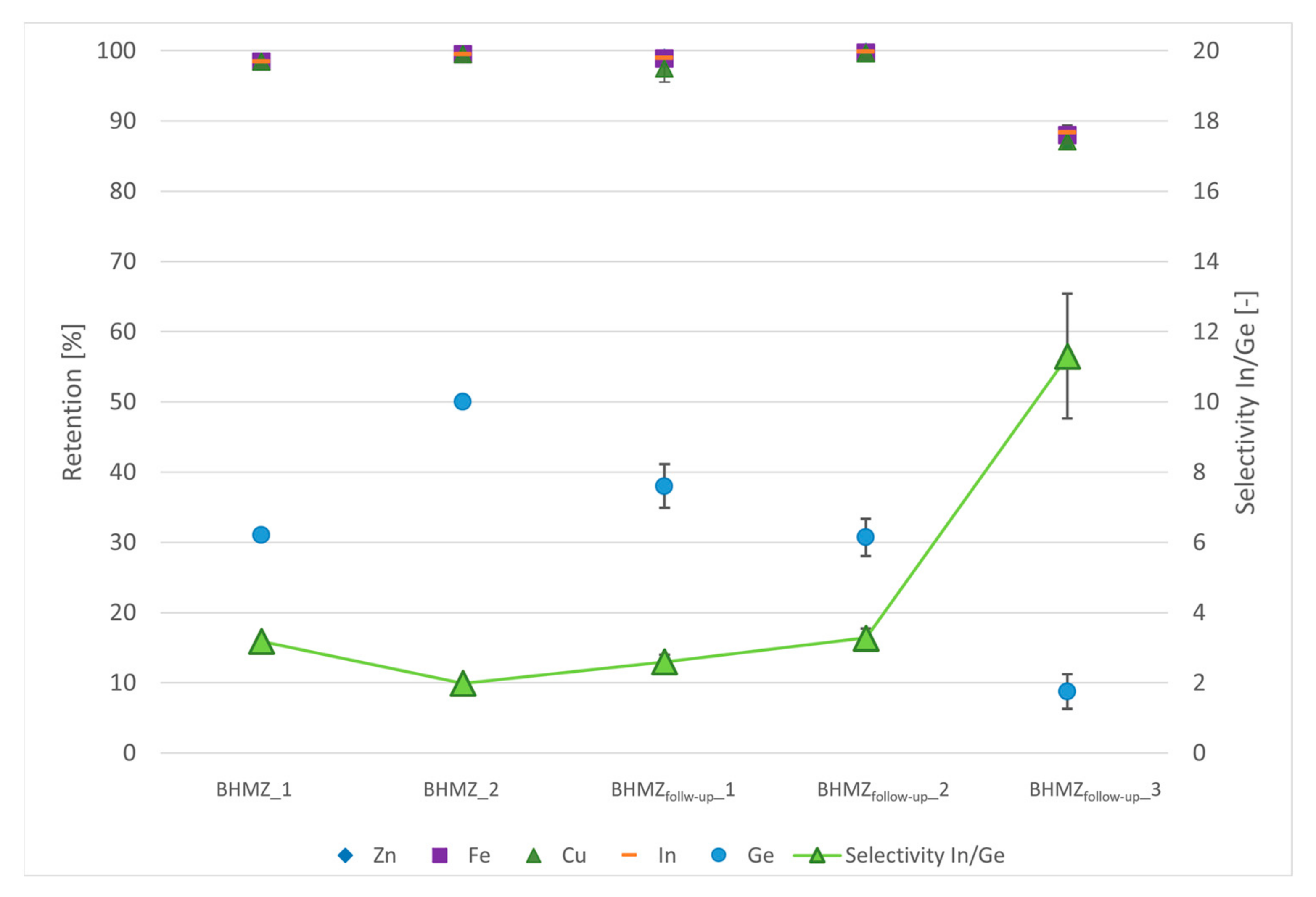

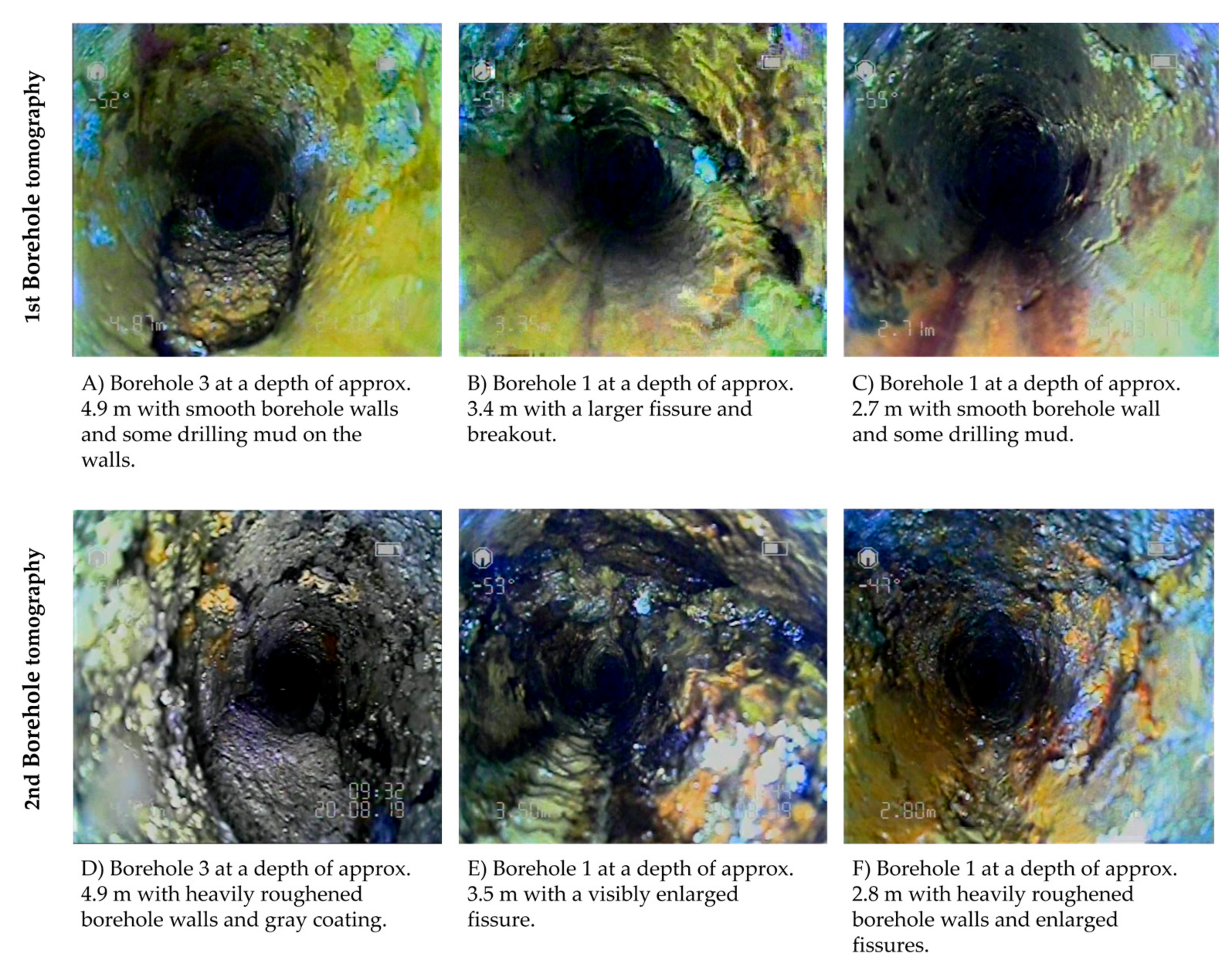
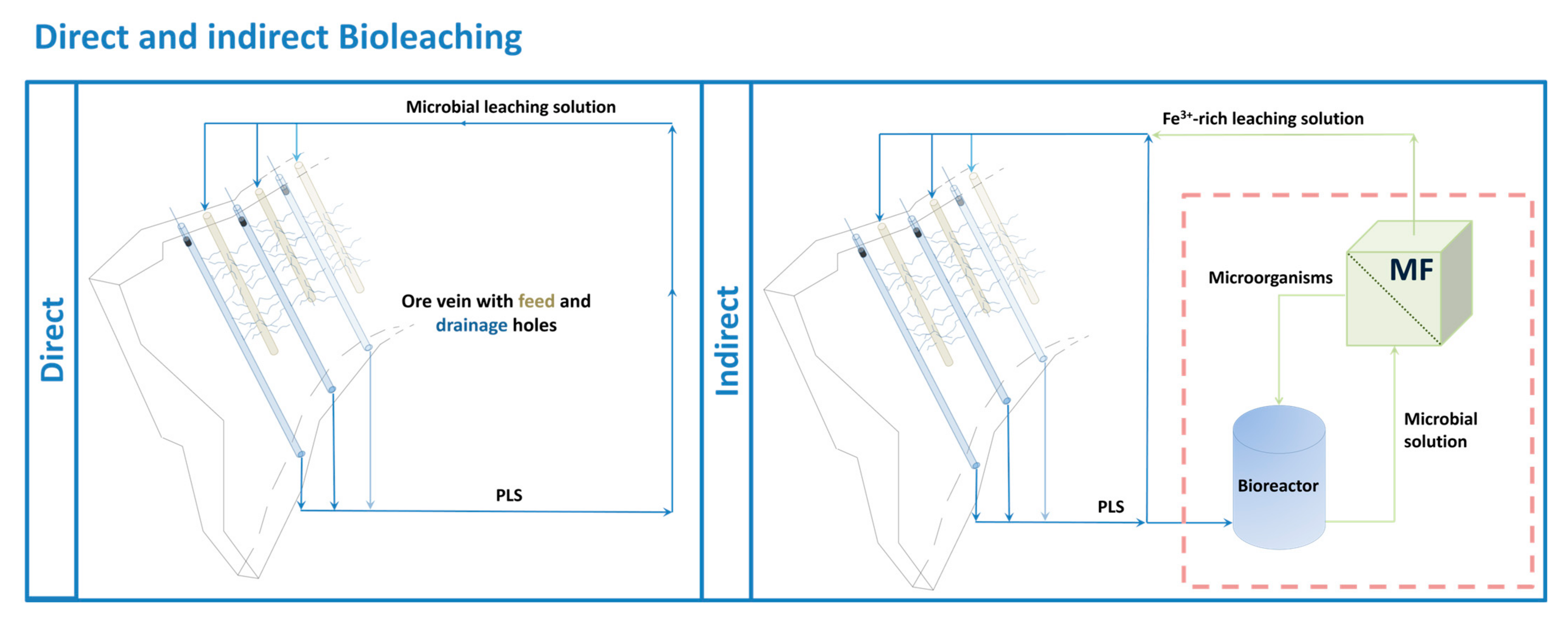


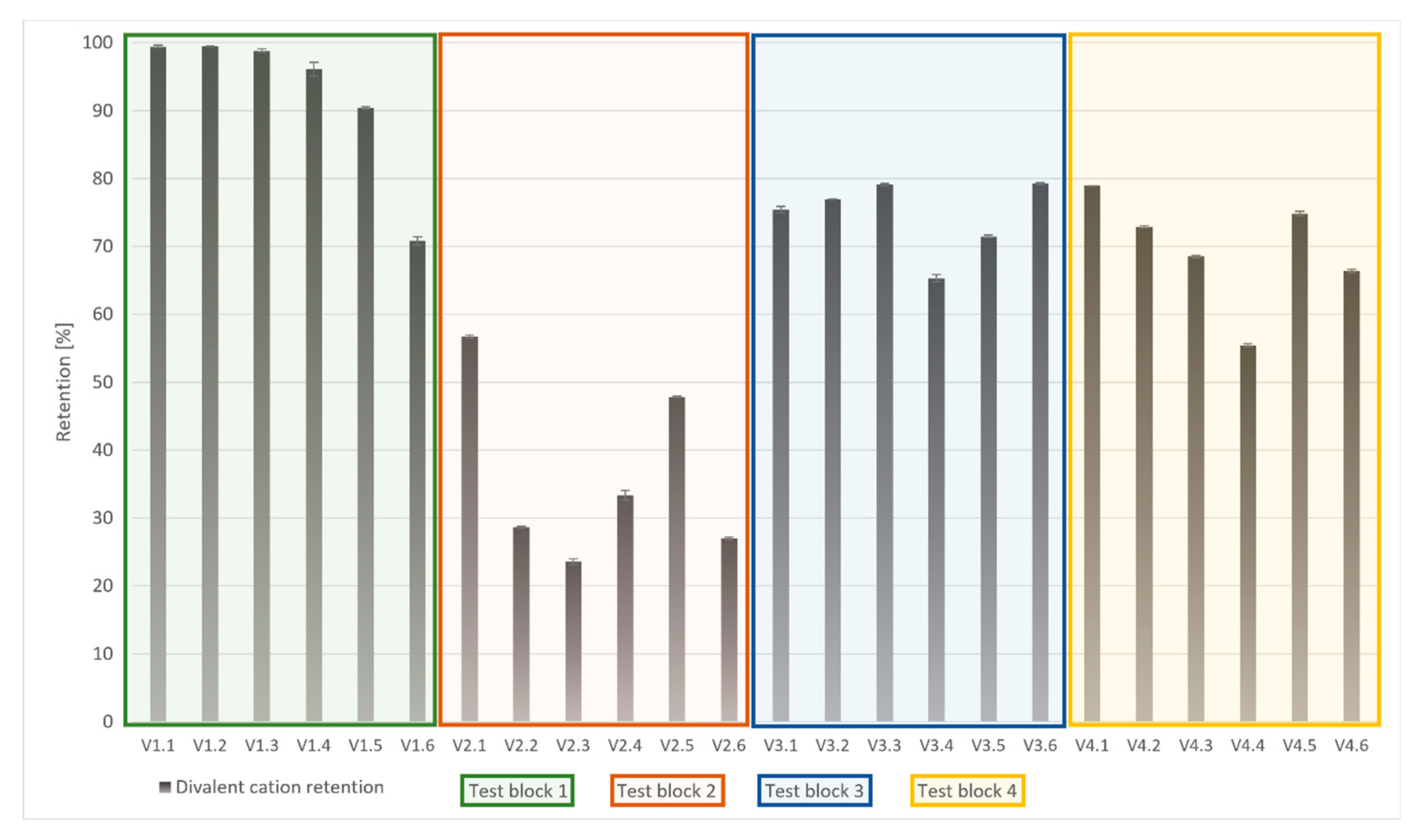
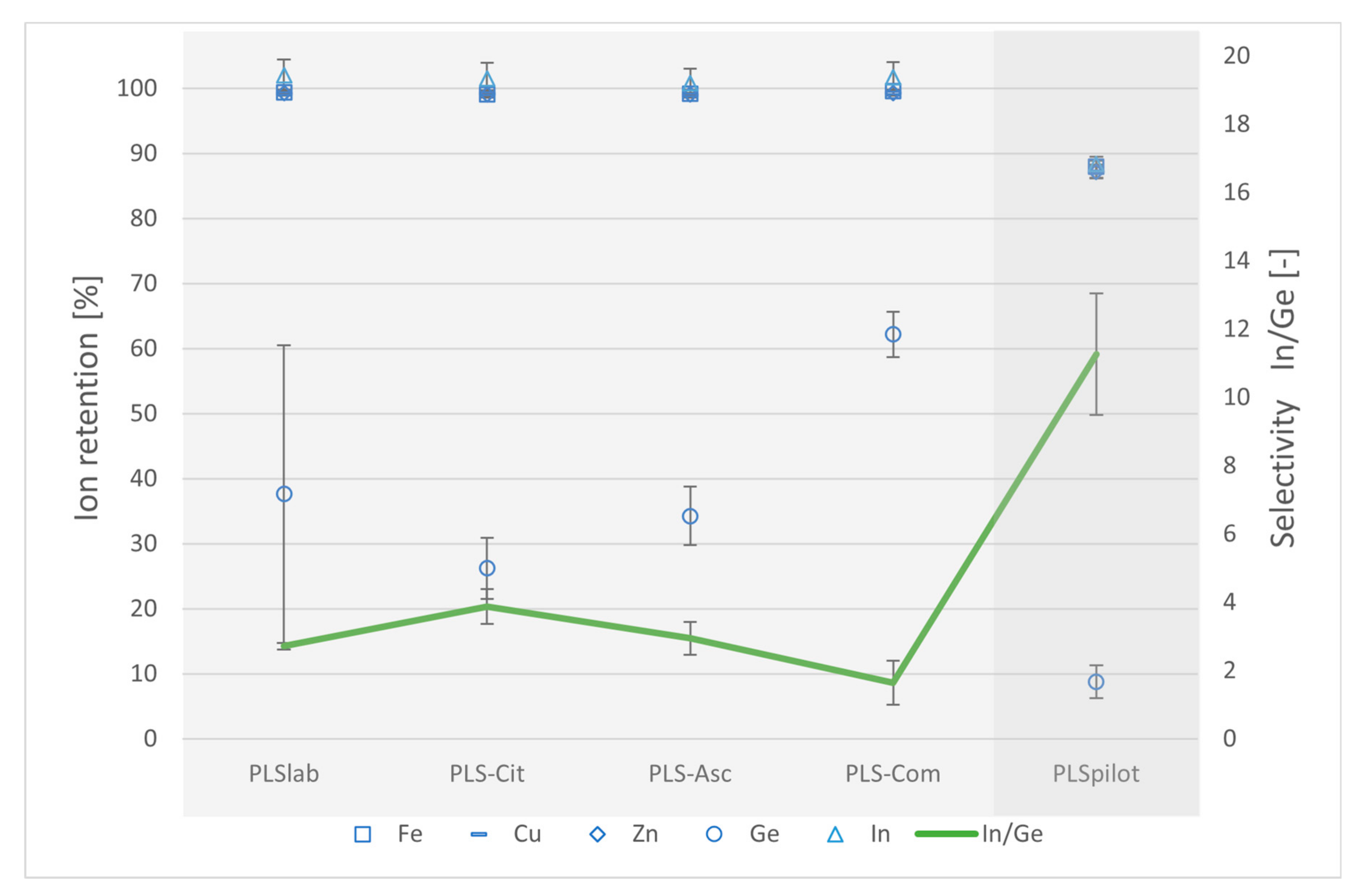

| Name_Test Section | Solution | Membrane | Pressure [bar] | Overflow Velocity [m s−1] | Recovery Rate [%] | Application Scale |
|---|---|---|---|---|---|---|
| BHMZ_1 [51] | PLSsynth | NF99HF | 15 | 2 | 11.5 | lab |
| BHMZ_2 [51] | PLSsynth | NF99HF | 15 | 2 | 60.0 | lab |
| BHMZfollow-up_1 [32] | PLSsynth | NF99HF | 15 | 1.25 | 27.5 | lab |
| BHMZfollow-up_2 [32] | PLSsynth | NF | 15 | 1.25 | 27.5 | lab |
| BHMZfollow-up_3 [32] | PLSreal | NF | 7.5 | 1.1 | 80.0 | pilot |
| pH | Conductivity | TOC | Turbidity | Elements | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | Zn | Fe | Cu | Ge | In | |||||
| (-) | (mS cm−1) | (mg L−1) | (FNU) | (mg L−1) | (µg L−1) | |||||
| PLSSynth. | 2.0 ± 0.1 | 12.7 ± 0.1 | <1 * | 0 | 2350 ± 139 | 256 ± 78 | 643 ± 22 | 19 ± 1 | 900 ± 20 | 960 ± 30 |
| PLSreal | 2.1 ± 0.2 | 10.4 ± 1.9 | 4.0 ± 0.2 | 126.6 ± 10.6 | 2920 ± 75 | 727 ± 49 | 959 ± 76 | 22 ± 2 | 70 ± 30 | 40 ± 0 |
| MTW | 4.8 ± 0.1 | 1.9 ± 0.2 | 1.1 ± 0.2 | 3.7 ± 1.2 | 408 ± 51 | 90 ± 8 | 1 ± 0 | 1 ± 0 | * | * |
| MTWfiltrated | 3.7 ± 0.5 | 0.4 ± 0.1 | <1 * | - | 1606 ± 3 | 9 ± 3 | 1 ± 1 | 0 | 0 | 0 |
| Concentration [vol%] | Supplier | |
|---|---|---|
| Citric acid | 1 | © VWR International, LLC, Radnor, PA, USA |
| Caustic soda | 0.1 | VWR International, LLC, Radnor, PA, USA |
| Ascorbic acid | 1 | VWR International, LLC, Radnor, PA, USA |
| Commercial cleaning solution | 1–2 | Dr. Nähring Water Treatment GmbH, Dürnau, Germany |
| HCl | 7.3 | VWR International, LLC, Radnor, PA, USA |
| Tween 20 | 0.35 | Merck, Darmstadt, Germany |
| Duration [d] | Inlet Pressure [bar] | Initial Leaching Volume [L] | Feed Boreholes | Pump Interval [min] | Leaching Trials | ||
|---|---|---|---|---|---|---|---|
| Direct ISL | Initial test run | 21 | unknown | 17 | 1 | unknown | 1 |
| BHMZ | 15 | 2–3 | ≈180 | 5 | unknown | 2 | |
| BHMZ follow-up | 14–21 | 1 | ≈180 | 3–5 | 30 | 3 | |
| Reconstruction | 20 | ca. 2 | ≈180 | 3 | 30 | 1 | |
| Indirect ISL | InISL | 7–22 | ca. 2 | 200–530 | 3 | 30 | 2 |
| InISLfast | 7 | ca. 2 | 200–530 | 3 | 3 | 2 | |
| Pressure [bar] | Overflow Velocity [m s−1] | Test ID | |||
|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 4 | ||
| 10 | 0.9 | V1.6 | V2.6 | V3.5 | V4.5 |
| 1.1 | V1.3 | V2.4 | V3.3 | V4.3 | |
| 15 | 1 | V1.1; V1.5 | V2.1; V2.5 | V3.1; V3.6 | V4.1; V4.6 |
| 20 | 0.9 | V1.2 | V2.2 | V3.2 | V4.2 |
| 1.1 | V1.4 | V2.3 | V3.4 | V4.4 | |
| Method | Concentration [Ma%] | Procedure | Time of Each CIP-Cycle | pH [-] | |
|---|---|---|---|---|---|
| Acid—base—acid | Citric acid-Caustic soda-Citric acid (PLS-Cit) | Citric acid: 1 Caustic soda: 0.05 Ascorbic acid: 1 | Acid— Clean water— Base— Clean water— Acid— Clean water | 20 min plus 10 min intermediate clean water flush | Acid: ~2.5 Base: 12 |
| Ascorbic acid-Caustic soda-Ascorbic acid (PLS-Asc) | |||||
| Commercial cleaner | Commercial cleaning solution (PLS-Com) | 1–2 | Commercial cleaner—Clean water | 60 to 180 min | ~7 |
| Pump | Pressure [bar] | Overflow Velocity [m s−1] | Feed |
|---|---|---|---|
| 1 | 1 | 7.3 | Microbial leaching solution |
| 2 | 5.7 | ||
| 2 | 1 | 0.7 | |
| 2 | |||
| 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Götze, K.; Hedrich, S.; Braeuer, A.S.; Haseneder, R. Process Optimization of an In-Situ Bioleaching Section with Associated Membrane Filtration in a Field Test Laboratory. Minerals 2024, 14, 308. https://doi.org/10.3390/min14030308
Götze K, Hedrich S, Braeuer AS, Haseneder R. Process Optimization of an In-Situ Bioleaching Section with Associated Membrane Filtration in a Field Test Laboratory. Minerals. 2024; 14(3):308. https://doi.org/10.3390/min14030308
Chicago/Turabian StyleGötze, Katja, Sabrina Hedrich, Andreas Siegfried Braeuer, and Roland Haseneder. 2024. "Process Optimization of an In-Situ Bioleaching Section with Associated Membrane Filtration in a Field Test Laboratory" Minerals 14, no. 3: 308. https://doi.org/10.3390/min14030308
APA StyleGötze, K., Hedrich, S., Braeuer, A. S., & Haseneder, R. (2024). Process Optimization of an In-Situ Bioleaching Section with Associated Membrane Filtration in a Field Test Laboratory. Minerals, 14(3), 308. https://doi.org/10.3390/min14030308







