Mineral Chemistry of Olivine, Oxy-Spinel, and Clinopyroxene in Lavas and Xenoliths from the Canary, Azores, and Cape Verde Islands (Macaronesia, North Atlantic Ocean): New Data and Comparisons with the Literature
Abstract
1. Introduction
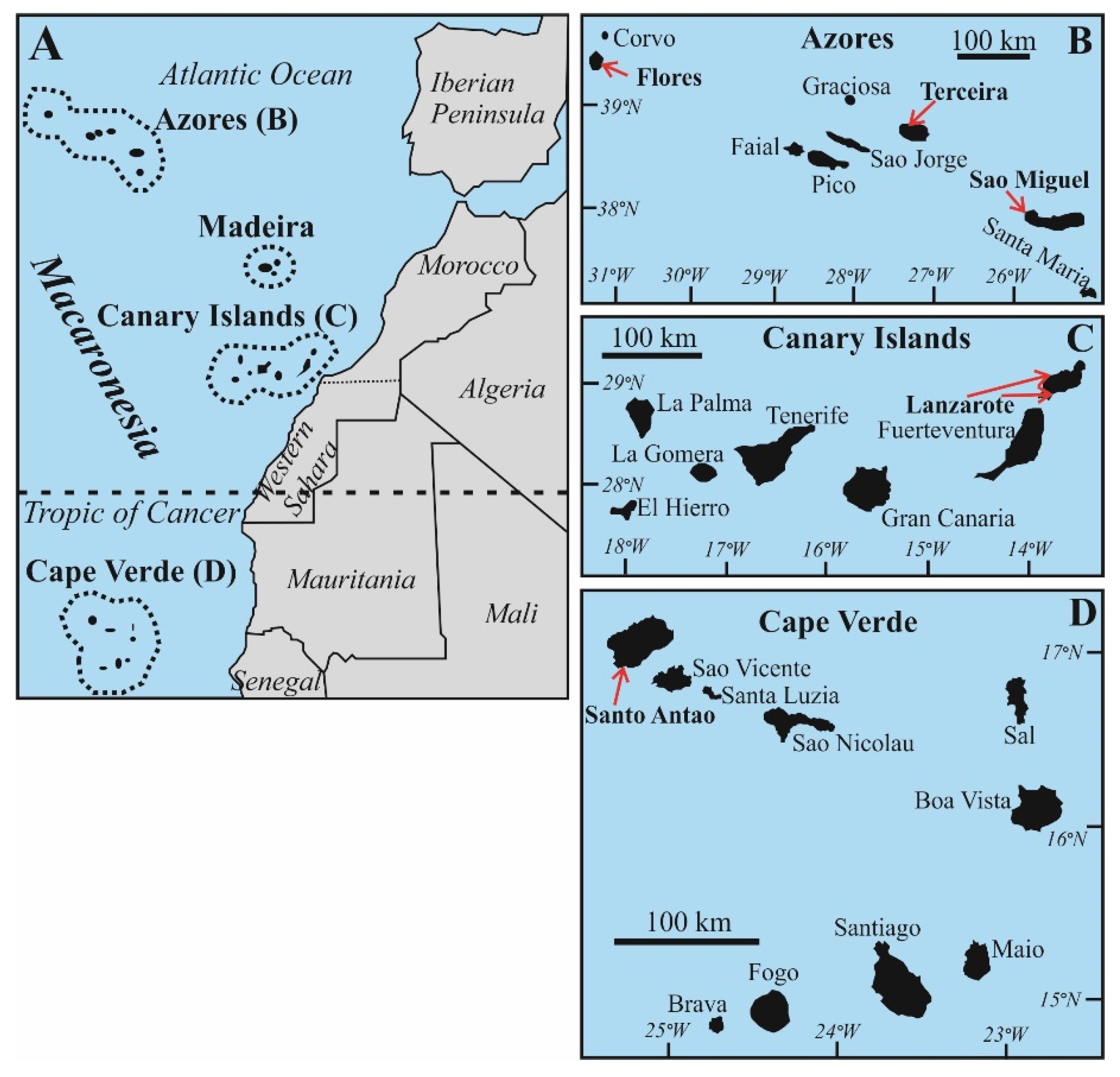
2. Provenance of the Studied Samples and Source of Literature Data
2.1. Geological Setting and Sample Selection from the Macaronesia Archipelagos
2.1.1. Azores Islands
2.1.2. The Canary Islands
2.1.3. The Cape Verde Islands
2.2. Selected Data from Literature
3. Analytical Techniques
4. Results of the Present Work and Comparison with Literature
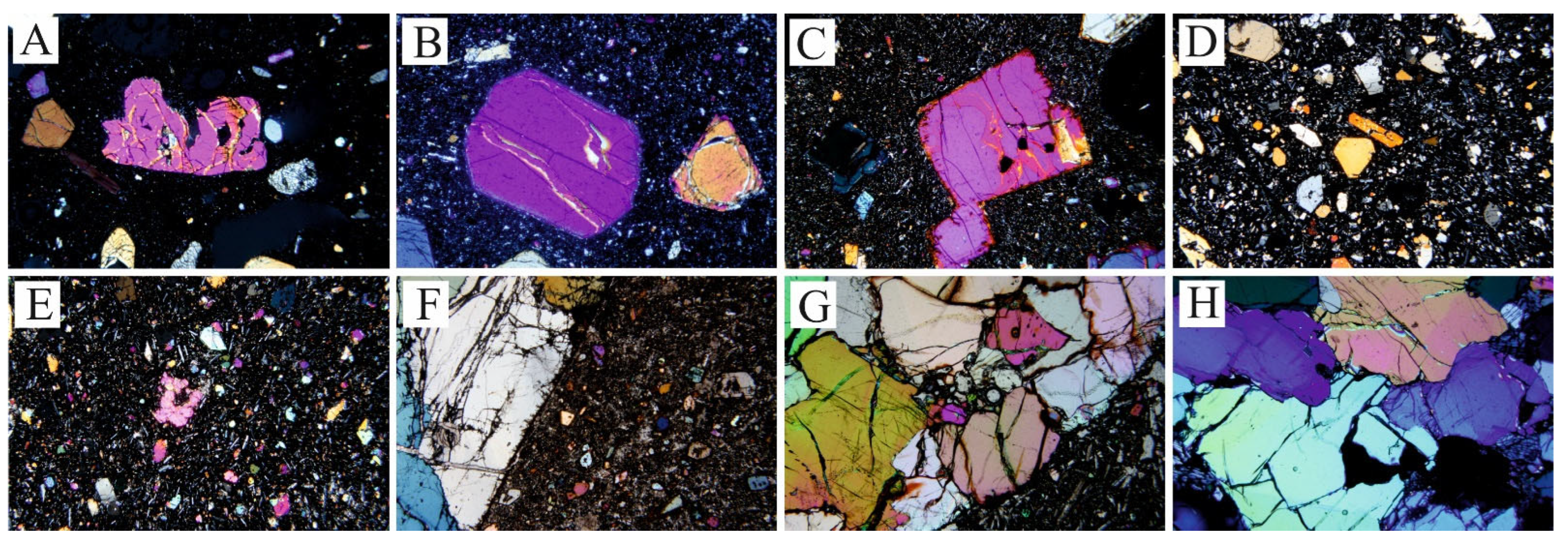
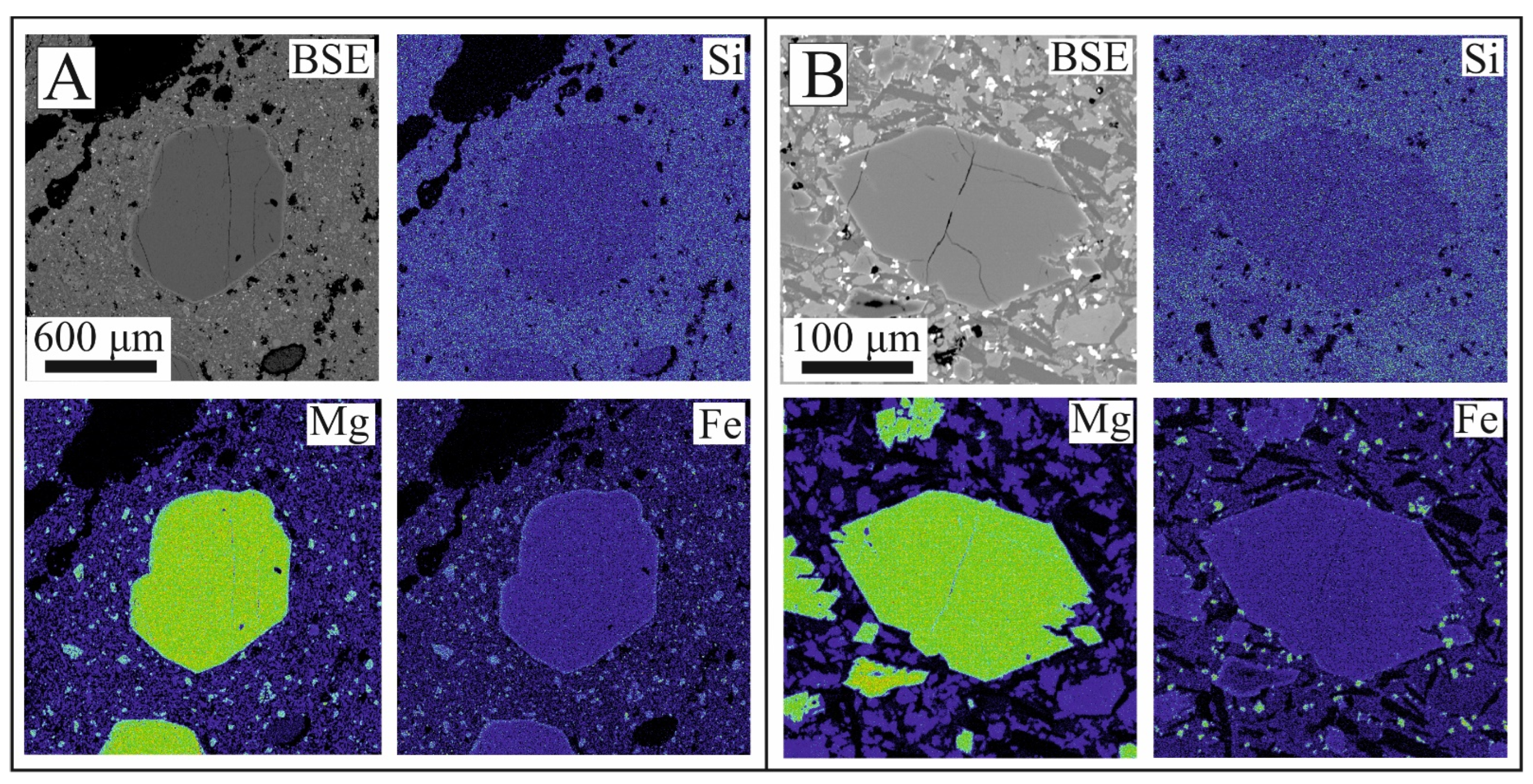
4.1. Texture and Composition of Olivine
4.2. Texture and Composition of Clinopyroxene
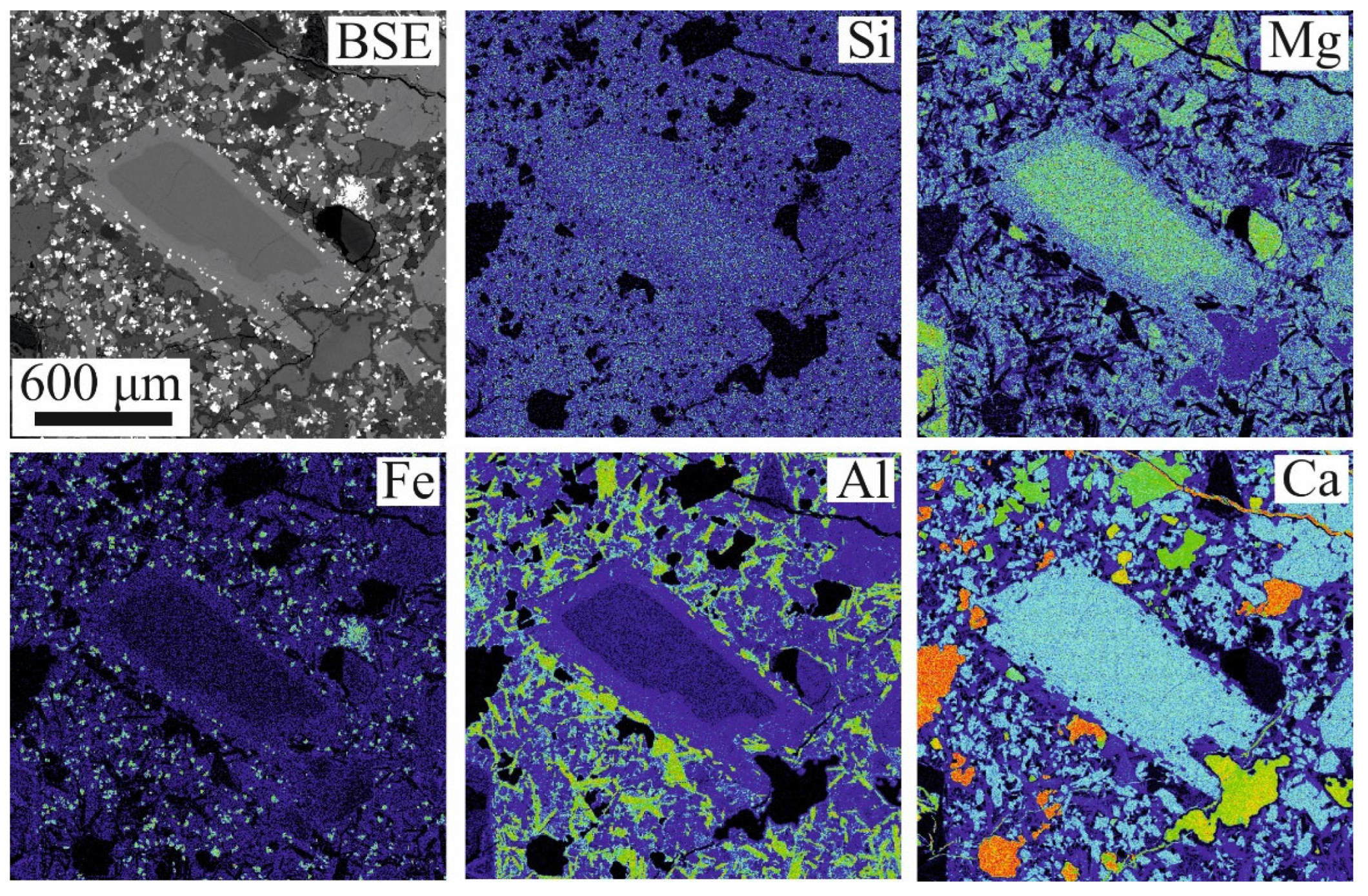
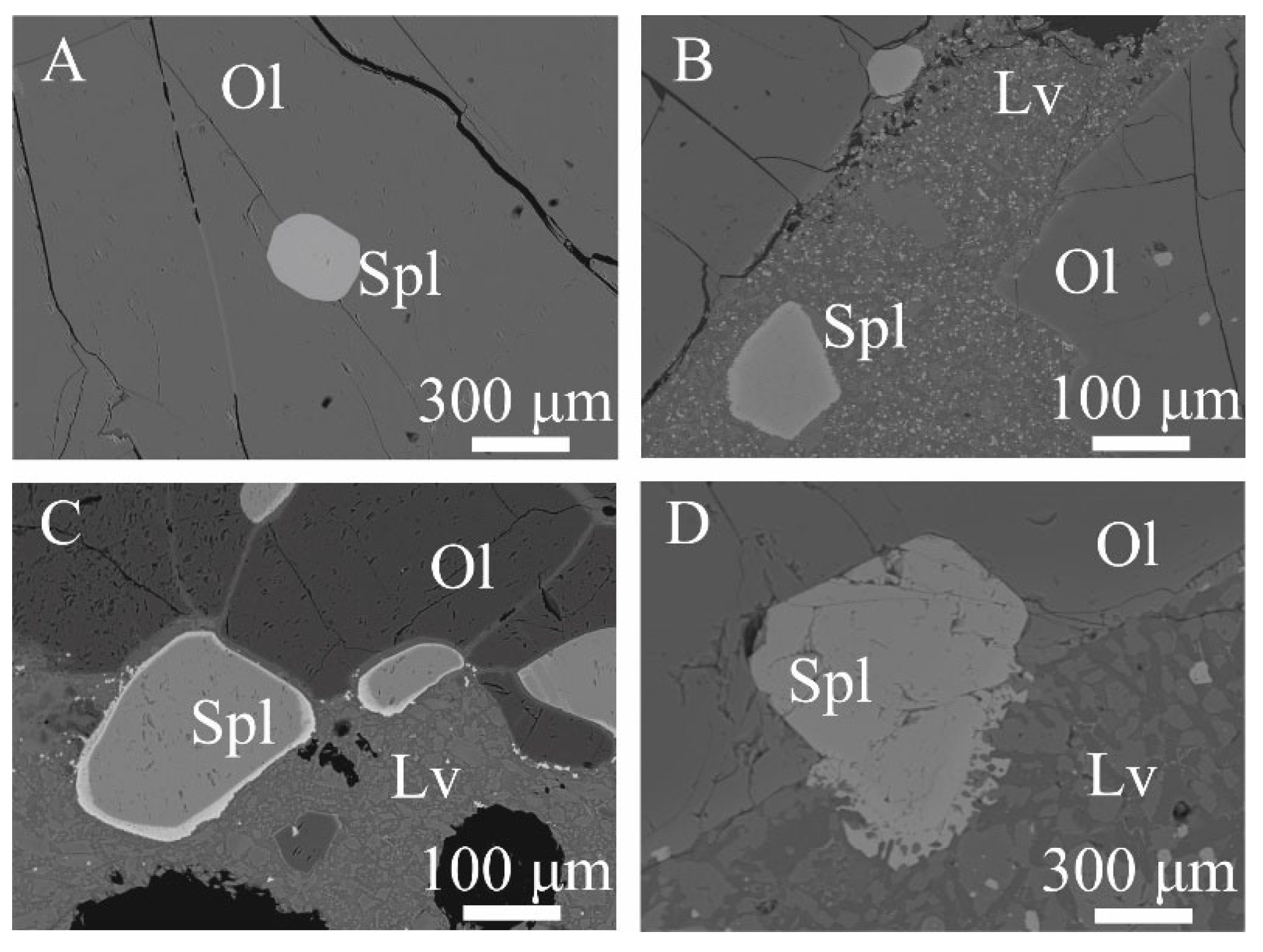
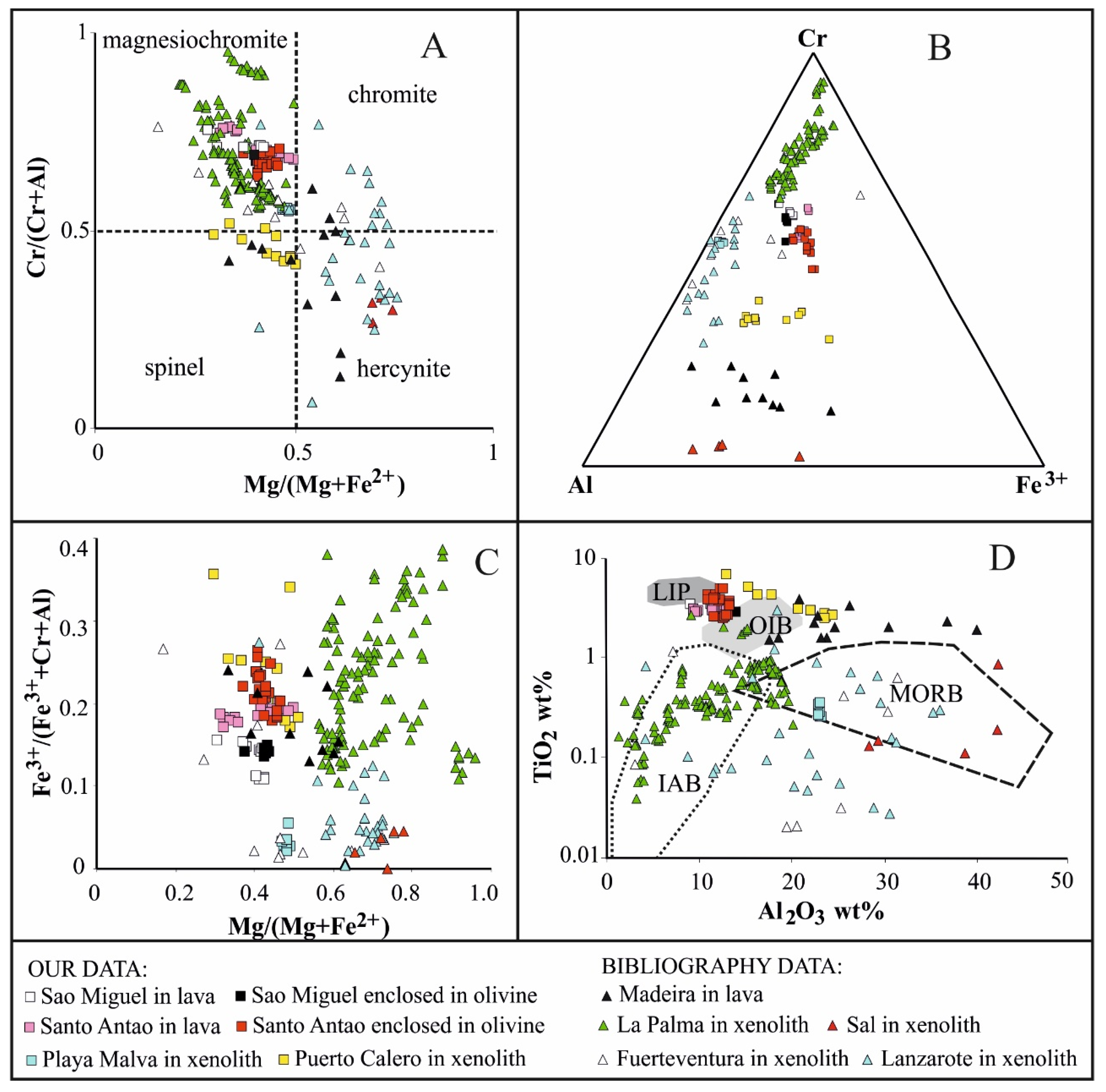
4.3. Texture and Composition of Oxy-Spinel
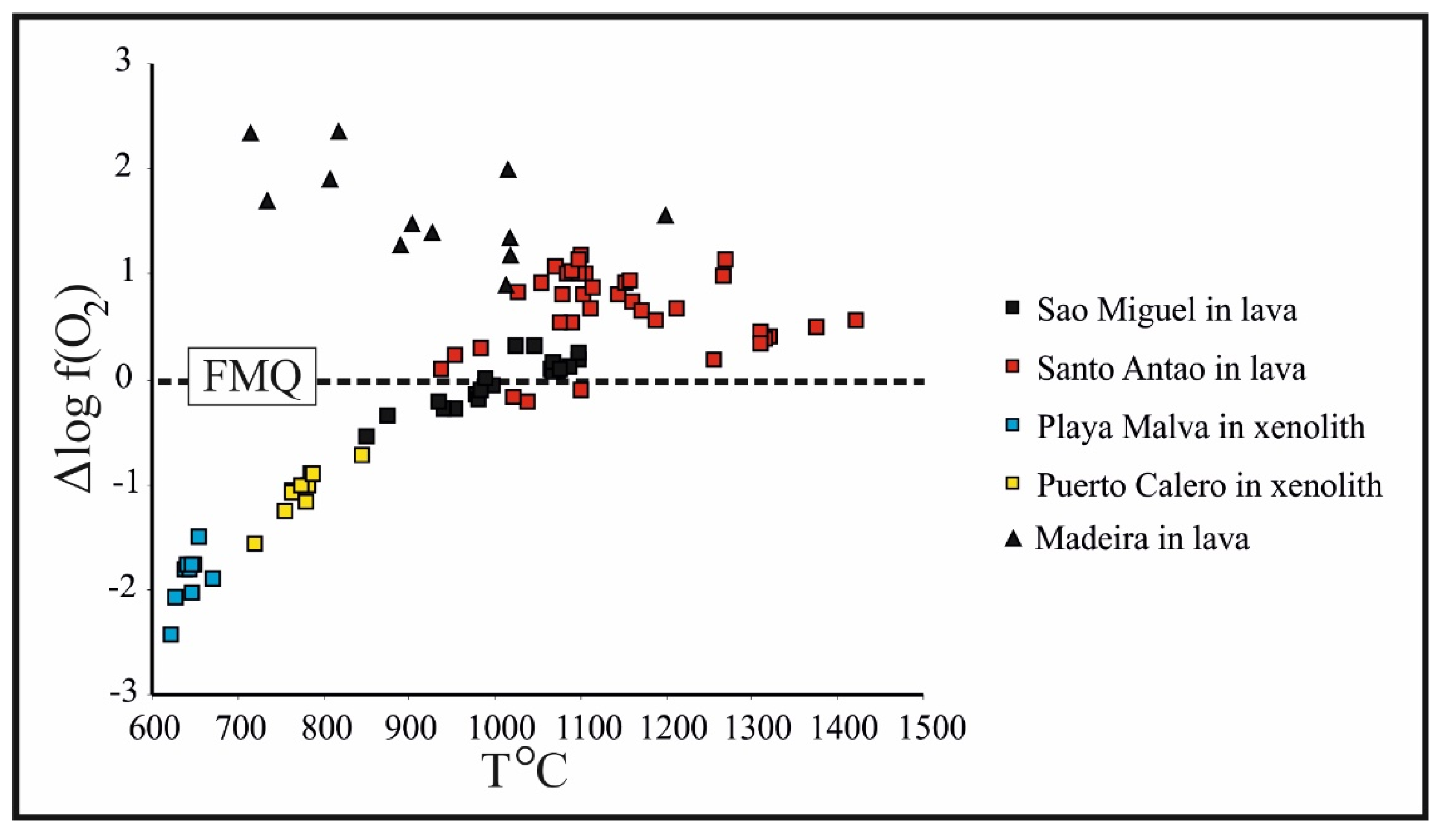
4.4. Oxy-Spinel and Olivine Thermometry and Oxygen Barometry
5. Discussion
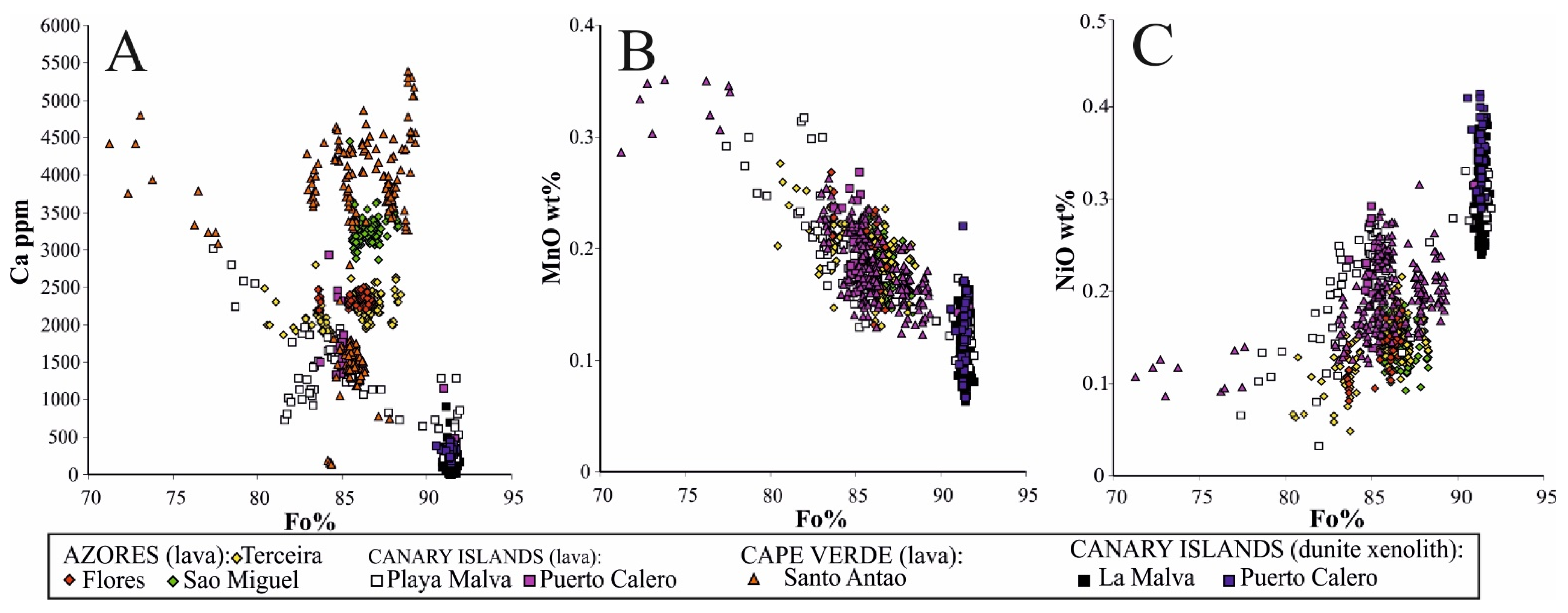
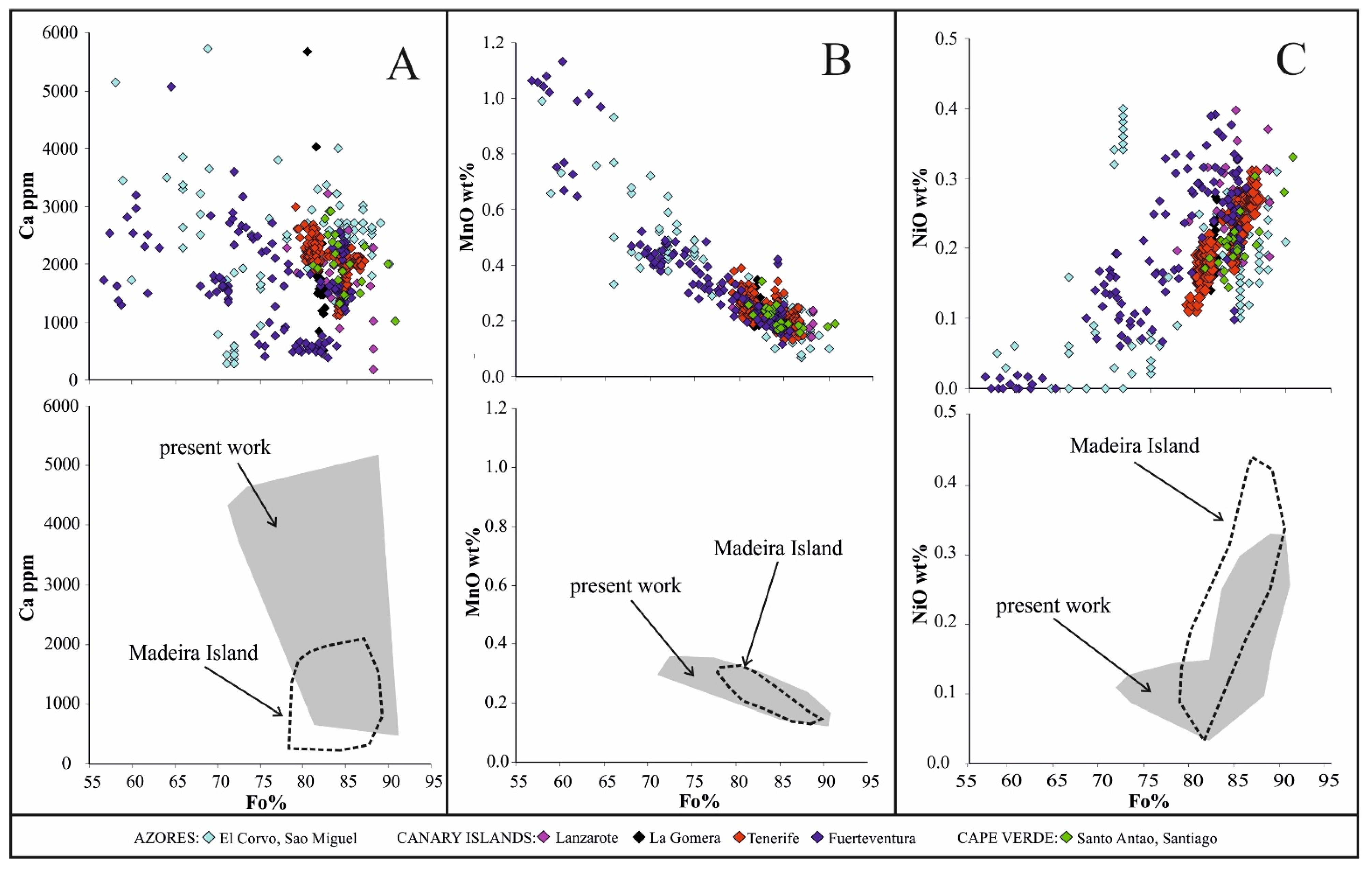
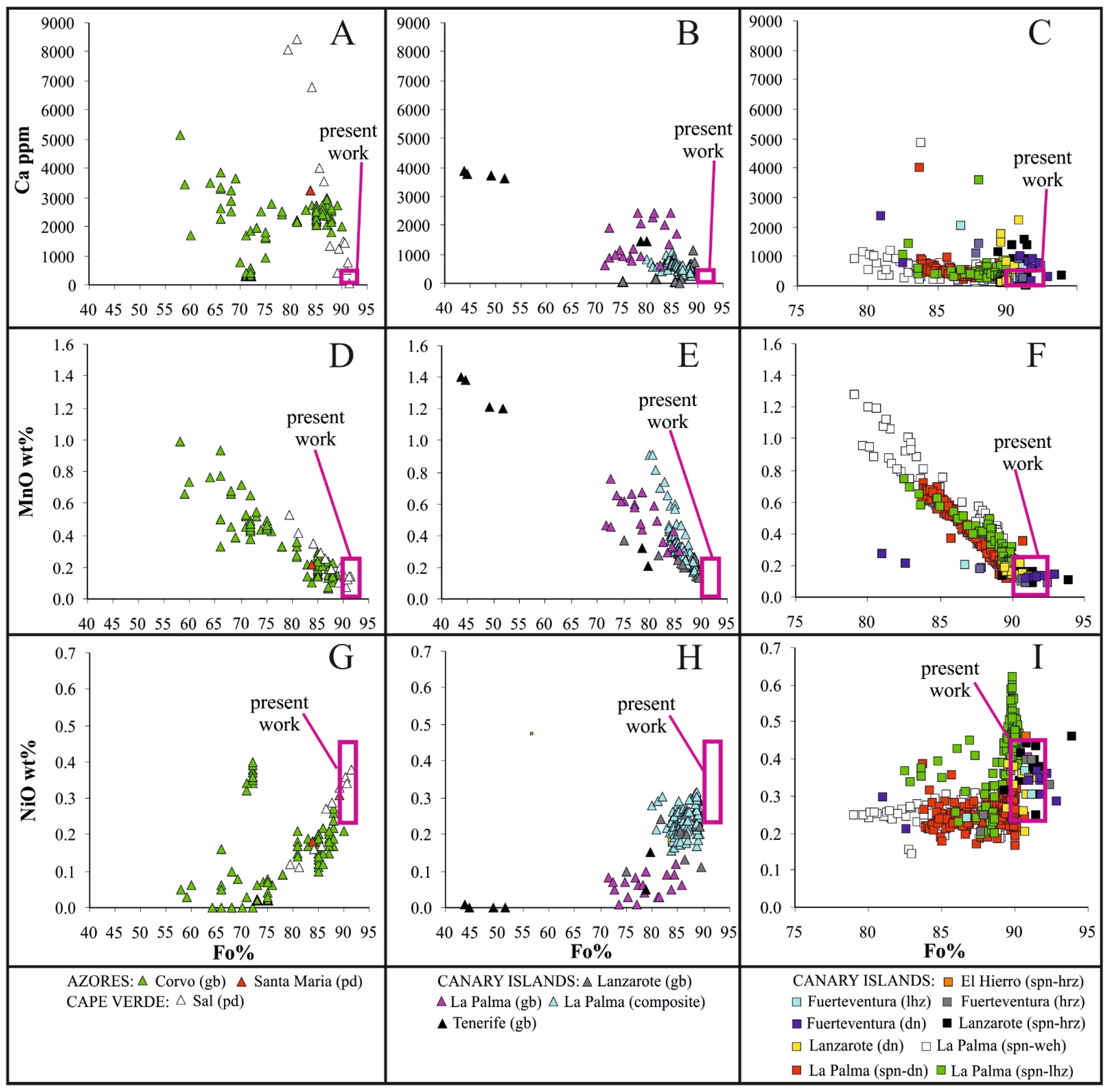
5.1. The Significance of Compositional Variations in Olivine
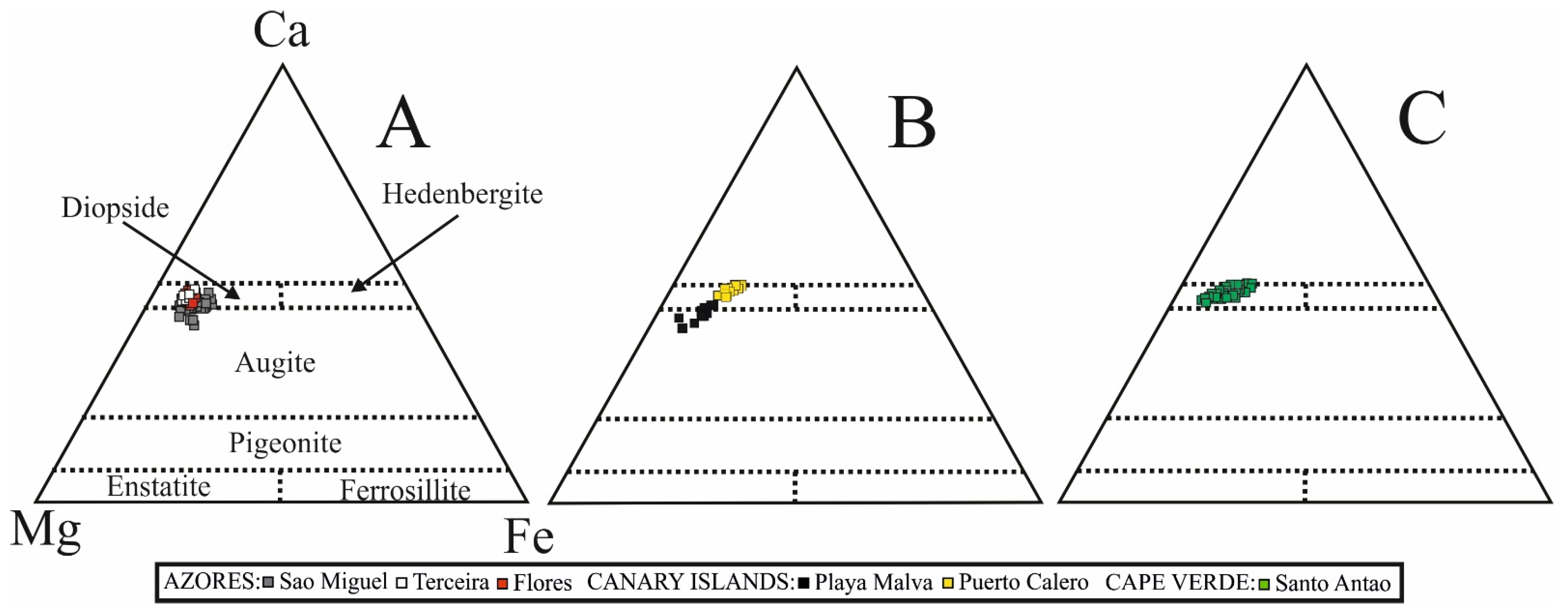
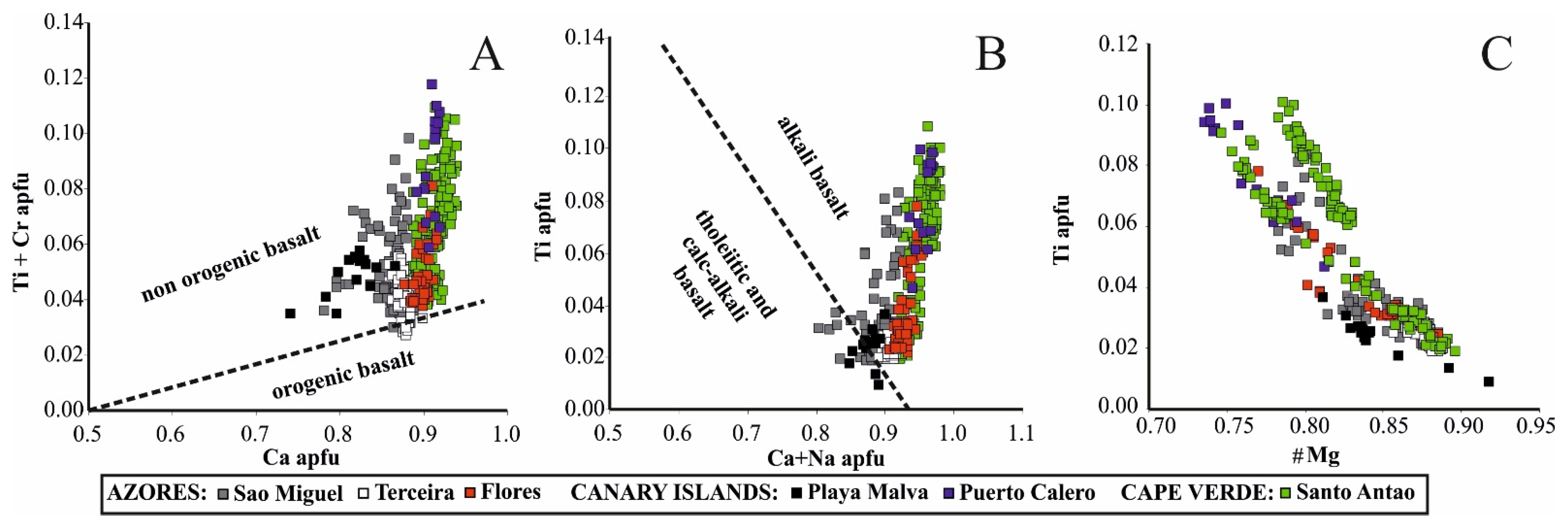
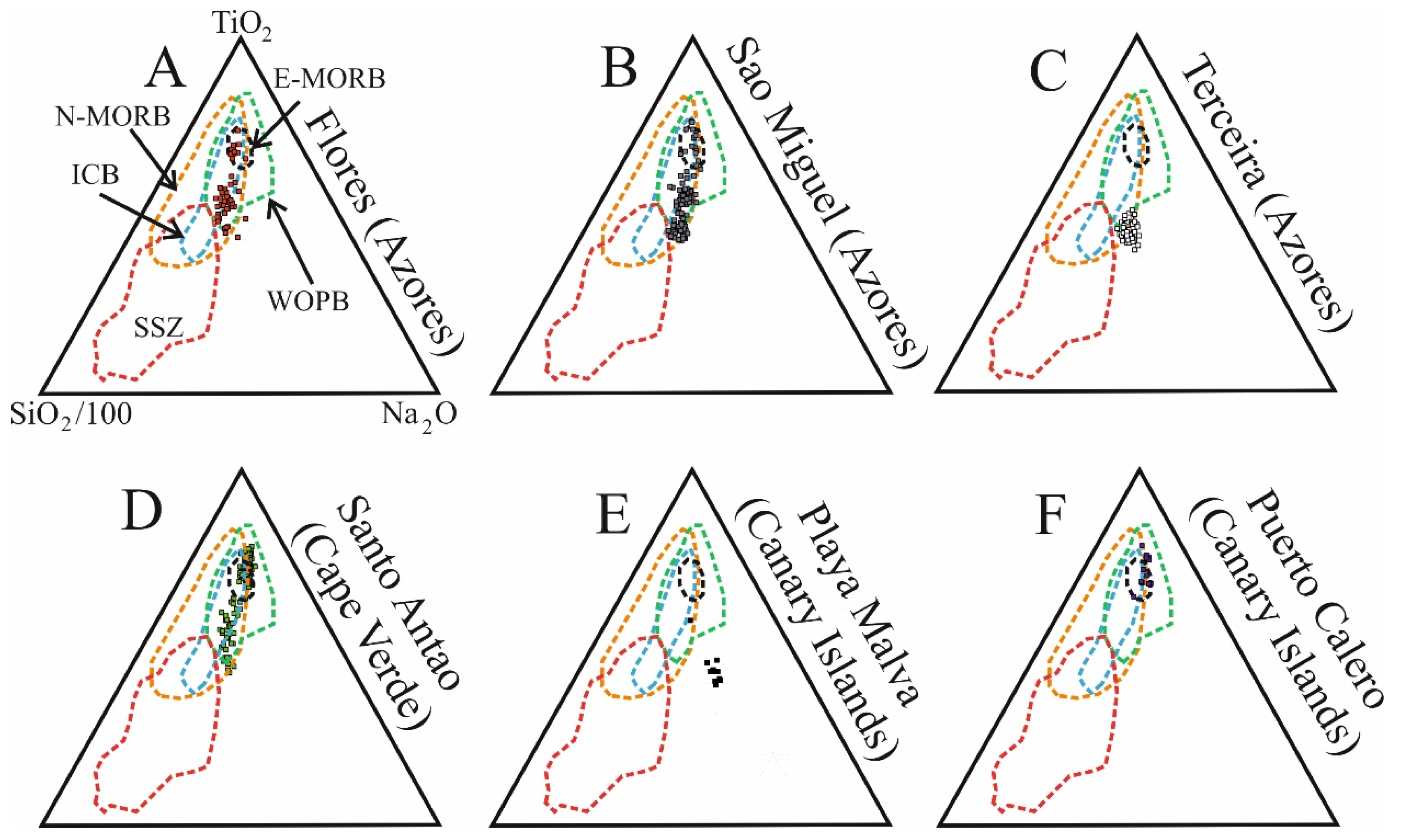
5.2. The Significance of Clinopyroxene Composition
5.3. The Significance of Oxy-Spinel Composition and Its Relationships with Co-Existing Olivine
6. Summary and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jollands, M.C.; Dohmen, R.; Padrón-Navarta, J.A. Hide and Seek—Trace Element Incorporation and Diffusion in Olivine. Elements 2023, 19, 144–150. [Google Scholar] [CrossRef]
- Garuti, G.; Pushakarev, E.V.; Thalhammer, O.A.; Zaccarini, F. Chromitites of the Urals (part 1): Overview of chromite mineral chemistry and geo-tectonic setting. Ofioliti 2012, 37, 27–53. [Google Scholar]
- Dick, H.J.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib. Mineral. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Nisbet, E.G.; Pearce, J.A. Clinopyroxene composition in mafic lavas from different tectonic settings. Contrib. Mineral. Petrol. 1977, 63, 149–160. [Google Scholar] [CrossRef]
- Klügel, A.; Albers, E.; Hansteen, T.H. Mantle and crustal xenoliths in a tephriphonolite from La Palma (Canary Islands): Implications for phonolite formation at Oceanic Island volcanoes. Front. Earth Sci. 2022, 10, 761902. [Google Scholar] [CrossRef]
- Barbero, E.; Delavari, M.; Dolati, A.; Vahedi, L.; Langone, A.; Marroni, M.; Pandolfi, L.; Zaccarini, F.; Saccani, E. Early Cretaceous Plume–Ridge Interaction Recorded in the Band-e-Zeyarat Ophiolite (North Makran, Iran): New Constraints from Petrological, Mineral Chemistry, and Geochronological Data. Minerals 2020, 10, 1100. [Google Scholar] [CrossRef]
- Rogkala, A.; Petrounias, P.; Tsikouras, B.; Giannakopoulou, P.P.; Hatzipanagiotou, K. Mineralogical evidence for partial melting and melt-rock interaction processes in the mantle peridotites of Edessa ophiolite (North Greece). Minerals 2019, 9, 120. [Google Scholar] [CrossRef]
- Taracsák, Z.; Hartley, M.; Burgess, R.; Edmonds, M.; Iddon, F.; Longpré, M. High fluxes of deep volatiles from ocean island volcanoes: Insights from El Hierro, Canary Islands. Geochim. Cosmochim. Acta 2019, 258, 19–36. [Google Scholar] [CrossRef]
- Gómez-Ulla, A.; Sigmarsson, O.; Gudfinnsson, G.H. Trace element systematics of olivine from historical eruptions of Lanzarote, Canary Islands: Constraints on mantle source and melting mode. Chem. Geol. 2017, 449, 99–111. [Google Scholar] [CrossRef]
- Tornare, E.; Pilet, S.; Bussy, F. Magma differentiation in vertical conduits revealed by the complementary study of plutonic and volcanic rocks from Fuerteventura (Canary Islands). J. Petrol. 2016, 57, 2221–2250. [Google Scholar] [CrossRef]
- Albert, H.; Costa, F.; Martí, J. Timing of magmatic processes and unrest associated with mafic historical monogenetic eruptions in Tenerife Island. J. Petrol. 2015, 56, 1945–1966. [Google Scholar] [CrossRef]
- Barker, A.; Holm, P.M.; Troll, V. The role of eclogite in the mantle heterogeneity at Cape Verde. Contrib. Mineral. Petrol. 2014, 168, 1052. [Google Scholar] [CrossRef]
- Gurenko, A.A.; Geldmacher, J.; Hoernle, K.A.; Sobolev, A.V. A composite, isotopically-depleted peridotite and enriched pyroxenite source for Madeira magmas: Insights from olivine. Lithos 2013, 170, 224–238. [Google Scholar] [CrossRef]
- Larrea, P.; França, Z.; Lago, M.; Widom, E.; Galé, C.; Ubide, T. Magmatic processes and the role of antecrysts in the genesis of Corvo Island (Azores Archipelago, Portugal). J. Petrol. 2013, 54, 769–793. [Google Scholar] [CrossRef]
- Gurenko, A.A.; Bindeman, I.N.; Chaussidon, M. Oxygen isotope heterogeneity of the mantle beneath the Canary Islands: Insights from olivine phenocrysts. Contrib. Mineral. Petrol. 2011, 162, 349–363. [Google Scholar] [CrossRef]
- Gurenko, A.A.; Hoernle, K.A.; Sobolev, A.V.; Hauff, F.; Schmincke, H.U. Source components of the Gran Canaria (Canary Islands) shield stage magmas: Evidence from olivine composition and Sr–Nd–Pb isotopes. Contrib. Mineral. Petrol. 2010, 159, 689–702. [Google Scholar] [CrossRef]
- Gurenko, A.A.; Sobolev, A.V.; Hoernle, K.A.; Hauff, F.; Schmincke, H.U. Enriched, HIMU-type peridotite and depleted recycled pyroxenite in the Canary plume: A mixed-up mantle. Earth Planet. Sci. Lett. 2009, 277, 514–524. [Google Scholar] [CrossRef]
- Abu El-Rus, M.A.; Neumann, E.R.; Peters, V. Serpentinization and dehydration in the upper mantle beneath Fuerteventura (eastern Canary Islands): Evidence from mantle xenoliths. Lithos 2006, 89, 24–46. [Google Scholar] [CrossRef]
- Gurenko, A.; Hoernle, K.; Hauff, F.; Schmincke, H.U.; Han, D.; Miura, Y.; Kaneoka, I. Major, trace element and Nd–Sr–Pb–O–He–Ar isotope signatures of shield stage lavas from the central and western Canary Islands: Insights into mantle and crustal processes. Chem. Geol. 2006, 233, 75–112. [Google Scholar] [CrossRef]
- Holm, P.; Wilson, J.; Christensen, B.; Hansen, L.; Hansen, S.; Hein, K.; Mortensen, A.; Pedersen, R.; Plesner, S.; Runge, M. Sampling the Cape Verde mantle plume: Evolution of melt compositions on Santo Antão, Cape Verde Islands. J. Petrol. 2006, 47, 145–189. [Google Scholar] [CrossRef]
- Shaw, C.S.; Heidelbach, F.; Dingwell, D.B. The origin of reaction textures in mantle peridotite xenoliths from Sal Island, Cape Verde: The case for “metasomatism” by the host lava. Contrib. Mineral. Petrol. 2006, 151, 681–697. [Google Scholar] [CrossRef]
- Neumann, E.R.; Griffin, W.L.; Pearson, N.J.; O’Reilly, S.Y. The evolution of the upper mantle beneath the Canary Islands: Information from trace elements and Sr isotope ratios in minerals in mantle xenoliths. J. Petrol. 2004, 45, 2573–2612. [Google Scholar] [CrossRef]
- Mata, J.; Munhá, J. Madeira Island alkaline lava spinels: Petrogenetic implications. Mineral. Petrol. 2004, 81, 85–111. [Google Scholar] [CrossRef]
- Neumann, E.R.; Wulff-Pedersen, E.; Pearson, N.; Spencer, E. Mantle xenoliths from Tenerife (Canary Islands): Evidence for reactions between mantle peridotites and silicic carbonatite melts inducing Ca metasomatism. J. Petrol. 2002, 43, 825–857. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Crawford, A.J.; Meffre, S. Factors controlling chemistry of magmatic spinel: An empirical study of associated olivine, Cr-spinel and melt inclusions from primitive rocks. J. Petrol. 2001, 42, 655–671. [Google Scholar] [CrossRef]
- Neumann, E.R.; Sørensen, V.; Simonsen, S.; Johnsen, K. Gabbroic xenoliths from La Palma, Tenerife and Lanzarote, Canary Islands: Evidence for reactions between mafic alkaline Canary Islands melts and old oceanic crust. J. Volcanol. Geotherm. Res. 2000, 103, 313–342. [Google Scholar] [CrossRef]
- Soesoo, A. A multivariate statistical analysis of clinopyroxene composition: Empirical coordinates for the crystallisation PT-estimations. GFF 1997, 119, 55–60. [Google Scholar] [CrossRef]
- Mattioli, M.; Upton, J.; Renzulli, A. Sub-volcanic crystallization at Sete Cidades volcano, São Miguel, Azores, inferred from mafic and ultramafic plutonic nodules. Mineral. Petrol. 1997, 60, 1. [Google Scholar] [CrossRef]
- Beccaluva, L.; Macciotta, G.; Piccardo, G.; Zeda, O. Clinopyroxene composition of ophiolite basalts as petrogenetic indicator. Chem. Geol. 1989, 77, 165–182. [Google Scholar] [CrossRef]
- Leterrier, J.; Maury, R.C.; Thonon, P.; Girard, D.; Marchal, M. Clinopyroxene composition as a method of identification of the magmatic affinities of paleo-volcanic series. Earth Planet. Sci. Lett. 1982, 59, 139–154. [Google Scholar] [CrossRef]
- Schweitzer, E.; Papike, J.; Bence, A. Statistical analysis of clinopyroxenes from deep-sea basalts. Am. Mineral. 1979, 64, 501–513. [Google Scholar] [CrossRef]
- Boone, G.M.; Fernandez, L.A. Phenocrystic olivines from the eastern Azores. Mineral. Mag. 1971, 38, 165–178. [Google Scholar] [CrossRef][Green Version]
- Pimentel, A.; Ramalho, R.S.; Becerril, L.; Larrea, P.; Brown, R.J. Ocean island volcanoes: Genesis, evolution and Impact. Front. Earth Sci. 2020, 8, 82. [Google Scholar] [CrossRef]
- Carracedo, J.C. Growth, structure, instability and collapse of Canarian volcanoes and comparisons with Hawaiian volcanoes. J. Volcanol. Geotherm. Res. 1999, 94, 1–19. [Google Scholar] [CrossRef]
- Carracedo, J.C.; Troll, V.R. North-East Atlantic Islands: The Macaronesian Archipelagos; Elsevier: Amsterdam, The Netherlands, 2021; pp. 674–699. ISBN 978-0-12-809663-5. [Google Scholar]
- Madeira, J.; Brum da Silveira, A.; Hipólito, A.; Carmo, R.; Gaspar, J.; Guest, J.; Duncan, A.; Barriga, F.; Chester, D. Active tectonics along the Eurasia-Nubia boundary: Data from the central and eastern Azores Islands. Volcan. Geol. São Miguel Isl. Azores Archipel. Geol. Soc. Lond. Mem. 2015, 44, 15–32. [Google Scholar]
- Miranda, J.; Luis, J.; Lourenço, N.; Goslin, J. Distributed deformation close to the Azores Triple “Point”. Mar. Geol. 2014, 355, 27–35. [Google Scholar] [CrossRef]
- Lourenço, N.; Miranda, J.; Luis, J.; Ribeiro, A.; Victor, L.M.; Madeira, J.; Needham, H. Morpho-tectonic analysis of the Azores Volcanic Plateau from a new bathymetric compilation of the area. Mar. Geophys. Res. 1998, 20, 141–156. [Google Scholar] [CrossRef]
- Ramalho, R.S.; Helffrich, G.; Madeira, J.; Cosca, M.; Thomas, C.; Quartau, R.; Hipólito, A.; Rovere, A.; Hearty, P.J.; Ávila, S.P. Emergence and evolution of Santa Maria Island (Azores)—The conundrum of uplifted islands revisited. Geol. Soc. Am. Bull. 2017, 129, 372–390. [Google Scholar] [CrossRef]
- Demande, J.; Fabriol, R.; Gerard, F.; Lundt, F.; Chovelon, P. Prospection Géothermique, Iles de Faial et de Pico (Açores). Rapport Géologique, Geochimique et Gravimétrique; BRGM: Orléans, France, 1982; 65p. [Google Scholar]
- Cannat, M.; Briais, A.; Deplus, C.; Escartın, J.; Georgen, J.; Lin, J.; Mercouriev, S.; Meyzen, C.; Muller, M.; Pouliquen, G.; et al. Mid-Atlantic Ridge–Azores hotspot interactions: Along-axis migration of a hotspot-derived event of enhanced magmatism 10 to 4 Ma ago. Earth Planet. Sci. Lett. 1999, 173, 257–269. [Google Scholar] [CrossRef]
- Gente, P.; Dyment, J.; Maia, M.; Goslin, J. Interaction between the Mid-Atlantic Ridge and the Azores hot spot during the last 85 Myr: Emplacement and rifting of the hot spot-derived plateaus. Geochem. Geophys. Geosyst. 2003, 4. [Google Scholar] [CrossRef]
- Storch, B.; Haase, K.; Romer, R.; Beier, C.; Koppers, A. Rifting of the oceanic Azores Plateau with episodic volcanic activity. Sci. Rep. 2020, 10, 19718. [Google Scholar] [CrossRef] [PubMed]
- Larrea, P.; França, Z.; Widom, E.; Lago, M. Petrology of the Azores Islands. In Volcanoes of the Azores—Revealing the Geological Secrets of the Central Northern Atlantic Islands; Active Volcanoes of the World Series; Kueppers, U., Beier, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 197–249. ISBN 978-3662585467. [Google Scholar]
- Andrade, M.; Pimentel, A.; Ramalho, R.; Kutterolf, S.; Hernández, A. The recent volcanism of Flores Island (Azores): Stratigraphy and eruptive history of Funda Volcanic System. J. Volcanol. Geotherm. Res. 2022, 432, 107706. [Google Scholar] [CrossRef]
- Self, S. The recent volcanology of Terceira, Azores. J. Geol. Soc. 1976, 132, 645–666. [Google Scholar] [CrossRef]
- Plesner, S.; Holm, P.; Wilson, J. 40Ar–39Ar geochronology of Santo Antão, Cape Verde Islands. J. Volcanol. Geotherm. Res. 2003, 120, 103–121. [Google Scholar] [CrossRef]
- Weis, F.A.; Skogby, H.; Troll, V.R.; Deegan, F.M.; Dahren, B. Magmatic water contents determined through clinopyroxene: Examples from the Western Canary Islands, Spain. Geochem. Geophys. Geosyst. 2015, 16, 2127–2146. [Google Scholar] [CrossRef]
- Neumann, E.R.; Wulff-Pedersen, E.; Johnsen, K.; Andersen, T.; Krogh, E. Petrogenesis of spinel harzburgite and dunite suite xenoliths from Lanzarote, eastern Canary Islands: Implications for the upper mantle. Lithos 1995, 35, 83–107. [Google Scholar] [CrossRef]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Am. Mineral. 1988, 73, 1123–1133. [Google Scholar]
- Ural, M. Major and Trace Element Compositions of Clinopyroxene Phenocrysts in Altered Basaltic Rocks from Yüksekova Complex within Bitlis Suture Zone (Elazığ, Eastern Turkey): Implications for the Tholeiitic to Calc-Alkaline Magmatism. Minerals 2023, 13, 266. [Google Scholar] [CrossRef]
- Bosi, F.; Biagioni, C.; Pasero, M. Nomenclature and classification of the spinel supergroup. Eur. J. Mineral. 2019, 31, 183–192. [Google Scholar] [CrossRef]
- Arai, S. Chemistry of chromian spinel in volcanic rocks as a potential guide to magma chemistry. Mineral. Mag. 1992, 56, 173–184. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.; Green, D. High-pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1994, 118, 109. [Google Scholar] [CrossRef]
- Nazzareni, S.; Barbarossa, V.; Skogby, H.; Zanon, V.; Petrelli, M. Magma water content of Pico Volcano (Azores Islands, Portugal): A clinopyroxene perspective. Contrib. Mineral. Petrol. 2020, 175, 87. [Google Scholar] [CrossRef]
- Batanova, V.G.; Sobolev, A.V.; Kuzmin, D.V. Trace element analysis of olivine: High precision analytical method for JEOL JXA-8230 electron probe microanalyser. Chem. Geol. 2015, 419, 149–157. [Google Scholar] [CrossRef]
- Jiang, P.; Perfit, M.; Foster, D.A.; Trucco, A. Accurate analyses of key petrogenetic minor and trace elements in olivine by electron microprobe. Chem. Geol. 2022, 614, 121199. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Hofmann, A.W.; Kuzmin, D.V.; Yaxley, G.M.; Arndt, N.T.; Chung, S.L.; Danyushevsky, L.V.; Elliott, T.; Frey, F.A.; Garcia, M.O.; et al. The amount of recycled crust in sources of mantle-derived melts. Science 2007, 316, 412–417. [Google Scholar] [CrossRef]
- Foley, S.F.; Prelevic, D.; Rehfeldt, T.; Jacob, D.E. Minor and trace elements in olivines as probes into early igneous and mantle melting processes. Earth Planet. Sci. Lett. 2013, 363, 181–191. [Google Scholar] [CrossRef]
- Putirka, K.; Tao, Y.; Hari, K.; Perfit, M.R.; Jackson, M.G.; Arevalo, R. The mantle source of thermal plumes: Trace and minor elements in olivine and major oxides of primitive liquids (and why the olivine compositions don’t matter). Am. Mineral. 2018, 103, 1253–1270. [Google Scholar] [CrossRef]
- De Hoog, J.C.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef]
- Shea, T.; Hammer, J.E.; Hellebrand, E.; Mourey, A.J.; Costa, F.; First, E.C.; Lynn, K.J.; Melnik, O. Phosphorus and aluminum zoning in olivine: Contrasting behavior of two nominally incompatible trace elements. Contrib. Mineral. Petrol. 2019, 174, 85. [Google Scholar] [CrossRef]
- Mavrogonatos, K.; Flemetakis, S.; Papoutsa, A.; Klemme, S.; Berndt, J.; Economou, G.; Pantazidis, A.; Baziotis, I.; Asimow, P. Phosphorus zoning from secondary olivine in mantle xenolith from Middle Atlas mountains (Morocco, Africa): Implications for crystal growth kinetics. Bull. Geol. Soc. Greece 2016, 50, 1923–1932. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.X.; Robinson, P.T.; Xiao, Y.; Bai, Y.; Liu, X.; Sakyi, P.A.; Jing, J.J.; Chen, C.; Liang, Z.; et al. Trace elements in olivine: Proxies for petrogenesis, mineralization and discrimination of mafic-ultramafic rocks. Lithos 2021, 388, 106085. [Google Scholar] [CrossRef]
- Jennings, E.S.; Gibson, S.A.; Maclennan, J. Hot primary melts and mantle source for the Paraná-Etendeka flood basalt province: New constraints from Al-in-olivine thermometry. Chem. Geol. 2019, 529, 119287. [Google Scholar] [CrossRef]
- Su, B.; Chen, Y.; Mao, Q.; Zhang, D.; Jia, L.H.; Guo, S. Minor elements in olivine inspect the petrogenesis of orogenic peridotites. Lithos 2019, 344, 207–216. [Google Scholar] [CrossRef]
- Back, M.E. Fleischer’s Glossary of Mineral Species; Mineralogical Record, Mineralogical Association of Canada: Québec, QC, Canada, 2018. [Google Scholar]
- Day, J.M.; Pearson, D.G.; Macpherson, C.G.; Lowry, D.; Carracedo, J.C. Pyroxenite-rich mantle formed by recycled oceanic lithosphere: Oxygen-osmium isotope evidence from Canary Island lavas. Geology 2009, 37, 555–558. [Google Scholar] [CrossRef]
- Day, J.M.; Pearson, D.G.; Macpherson, C.G.; Lowry, D.; Carracedo, J.C. Evidence for distinct proportions of subducted oceanic crust and lithosphere in HIMU-type mantle beneath El Hierro and La Palma, Canary Islands. Geochim. Cosmochim. Acta 2010, 74, 6565–6589. [Google Scholar] [CrossRef]
- Hart, S.R.; Davis, K.E. Nickel partitioning between olivine and silicate melt. Earth Planet. Sci. Lett. 1978, 40, 203–219. [Google Scholar] [CrossRef]
- Beattie, P.; Ford, C.; Russell, D. Partition coefficients for olivine-melt and orthopyroxene-melt systems. Contrib. Mineral. Petrol. 1991, 109, 212–224. [Google Scholar] [CrossRef]
- Wang, Z.; Gaetani, G.A. Partitioning of Ni between olivine and siliceous eclogite partial melt: Experimental constraints on the mantle source of Hawaiian basalts. Contrib. Mineral. Petrol. 2008, 156, 661–678. [Google Scholar] [CrossRef]
- Li, C.; Thakurta, J.; Ripley, E.M. Low-Ca contents and kink-banded textures are not unique to mantle olivine: Evidence from the Duke Island Complex, Alaska. Mineral. Petrol. 2012, 104, 147–153. [Google Scholar] [CrossRef]
- Day, J.M. Noble gas isotope systematics in the Canary Islands and implications for refractory mantle components. Geochim. Cosmochim. Acta 2022, 331, 35–47. [Google Scholar] [CrossRef]
- Simon, N.S.; Neumann, E.R.; Bonadiman, C.; Coltorti, M.; Delpech, G.; Grégoire, M.; Widom, E. Ultra-refractory domains in the oceanic mantle lithosphere sampled as mantle xenoliths at ocean islands. J. Petrol. 2008, 49, 1223–1251. [Google Scholar] [CrossRef]
- Nazzareni, S.; Morgavi, D.; Petrelli, M.; Bartoli, O.; Perugini, D. Magmatic processes at Euganean Hills (Veneto Volcanic Province, Italy): Clinopyroxene investigation to unravel magmatic interactions. Geosciences 2022, 12, 108. [Google Scholar] [CrossRef]
- Capedri, S.; Venturelli, G. Clinopyroxene composition of ophiolitic metabasalts in the Mediterranean area. Earth Planet. Sci. Lett. 1979, 43, 61–73. [Google Scholar] [CrossRef]
- Engi, M. Equilibria involving Al-Cr spinel: Mg-Fe exchange with olivine. Experiments, thermodynamic analysis, and consequences for geothermometry. Am. J. Sci. 1983, 283, 29–71. [Google Scholar] [CrossRef]
- Bussolesi, M.; Grieco, G.; Tzamos, E. Olivine–spinel diffusivity patterns in chromitites and dunites from the Finero Phlogopite-Peridotite (Ivrea-Verbano Zone, Southern Alps): Implications for the thermal history of the massif. Minerals 2019, 9, 75. [Google Scholar] [CrossRef]
- Donaldson, C.H. The rates of dissolution of olivine, plagioclase, and quartz in a basalt melt. Min. Mag. 1985, 49, 683–693. [Google Scholar] [CrossRef]
- Costa, F.; Dungan, M. Short time scales of magmatic assimilation from diffusion modeling of multiple elements in olivine. Geology 2005, 33, 837–840. [Google Scholar] [CrossRef]
- Arculus, R.J.; Delano, J.W. Intrinsic oxygen fugacity measurements: Techniques and results for spinels from upper mantle peridotites and megacryst assemblages. Geochim. Cosmochim. Acta 1981, 45, 899–913. [Google Scholar] [CrossRef]
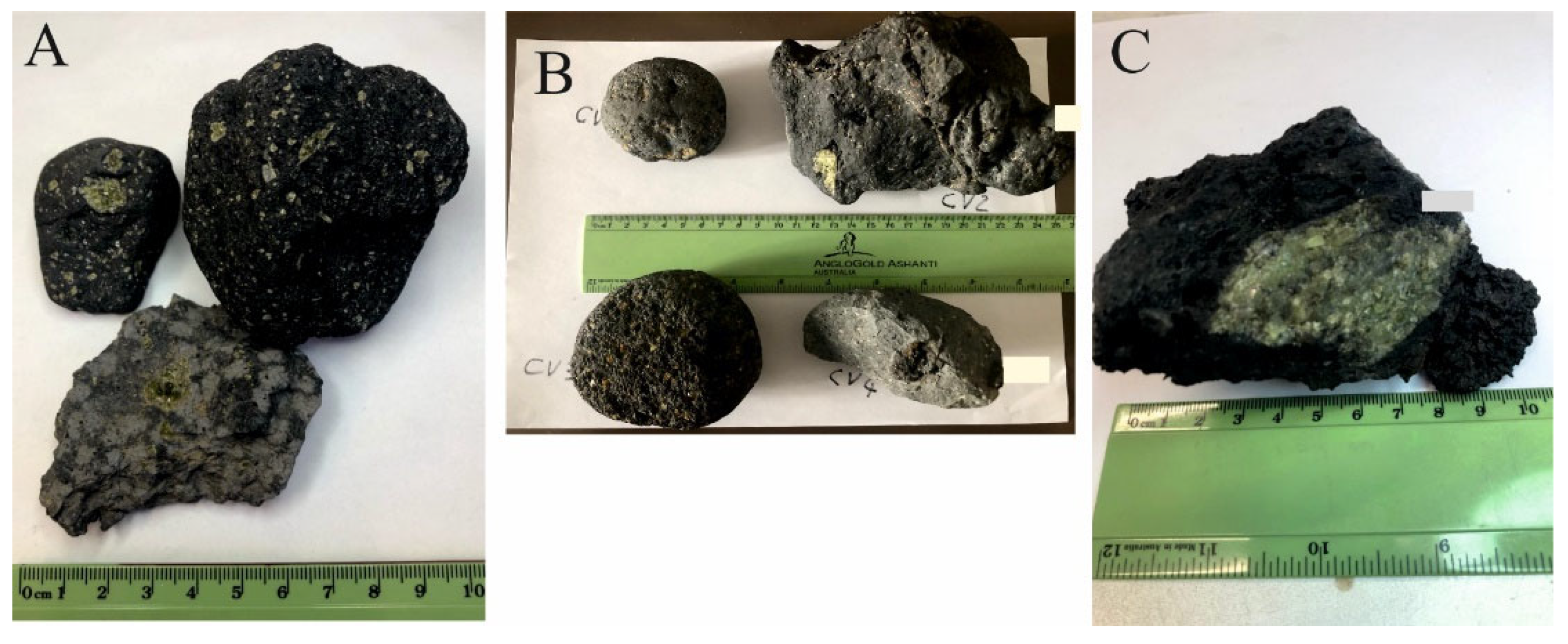

| Island | Location | Type of Rock | Geographic Coordinates |
|---|---|---|---|
| Azores: | |||
| Flores | Lagoa Funda | Basalt tuff | 39°39′82″ N, 31°21′49″ W |
| Terceira | Pico Gordo | Basalt flow | 38°80′02″ N, 27°26′20″ W |
| Sao Miguel | Sete Cidades | Lava flow | 37°53′56″ N, 25°49′17″ W |
| Canary Islands: | |||
| Lanzarote | Playa Malva | Dunite xenolith, basalt | 29°03′50″ N, 13°46′03″ W |
| Puerto Calero | Dunite xenolith, basalt | 28°54′59″ N, 13°42′28″ W | |
| Cape Verde: | |||
| Santo Antao | Tarrafal | Nephelinite–phonolite series | 16°57′17″ N, 25°18′29″ W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccarini, F.; Garuti, G.; Moser, R.; Mavrogonatos, C.; Voudouris, P.; Pimentel, A.; Nazzareni, S. Mineral Chemistry of Olivine, Oxy-Spinel, and Clinopyroxene in Lavas and Xenoliths from the Canary, Azores, and Cape Verde Islands (Macaronesia, North Atlantic Ocean): New Data and Comparisons with the Literature. Minerals 2024, 14, 161. https://doi.org/10.3390/min14020161
Zaccarini F, Garuti G, Moser R, Mavrogonatos C, Voudouris P, Pimentel A, Nazzareni S. Mineral Chemistry of Olivine, Oxy-Spinel, and Clinopyroxene in Lavas and Xenoliths from the Canary, Azores, and Cape Verde Islands (Macaronesia, North Atlantic Ocean): New Data and Comparisons with the Literature. Minerals. 2024; 14(2):161. https://doi.org/10.3390/min14020161
Chicago/Turabian StyleZaccarini, Federica, Giorgio Garuti, Reinhard Moser, Constantinos Mavrogonatos, Panagiotis Voudouris, Adriano Pimentel, and Sabrina Nazzareni. 2024. "Mineral Chemistry of Olivine, Oxy-Spinel, and Clinopyroxene in Lavas and Xenoliths from the Canary, Azores, and Cape Verde Islands (Macaronesia, North Atlantic Ocean): New Data and Comparisons with the Literature" Minerals 14, no. 2: 161. https://doi.org/10.3390/min14020161
APA StyleZaccarini, F., Garuti, G., Moser, R., Mavrogonatos, C., Voudouris, P., Pimentel, A., & Nazzareni, S. (2024). Mineral Chemistry of Olivine, Oxy-Spinel, and Clinopyroxene in Lavas and Xenoliths from the Canary, Azores, and Cape Verde Islands (Macaronesia, North Atlantic Ocean): New Data and Comparisons with the Literature. Minerals, 14(2), 161. https://doi.org/10.3390/min14020161











