Experimental Investigation on the Combination of Enzyme-Induced Calcium Carbonate Precipitation and Organic Materials for Underground Backfilling Preparation
Abstract
1. Introduction
2. Test Materials and Methods
2.1. Test Materials
2.2. Test Schemes
2.3. Evaluation of Mineralization Effect
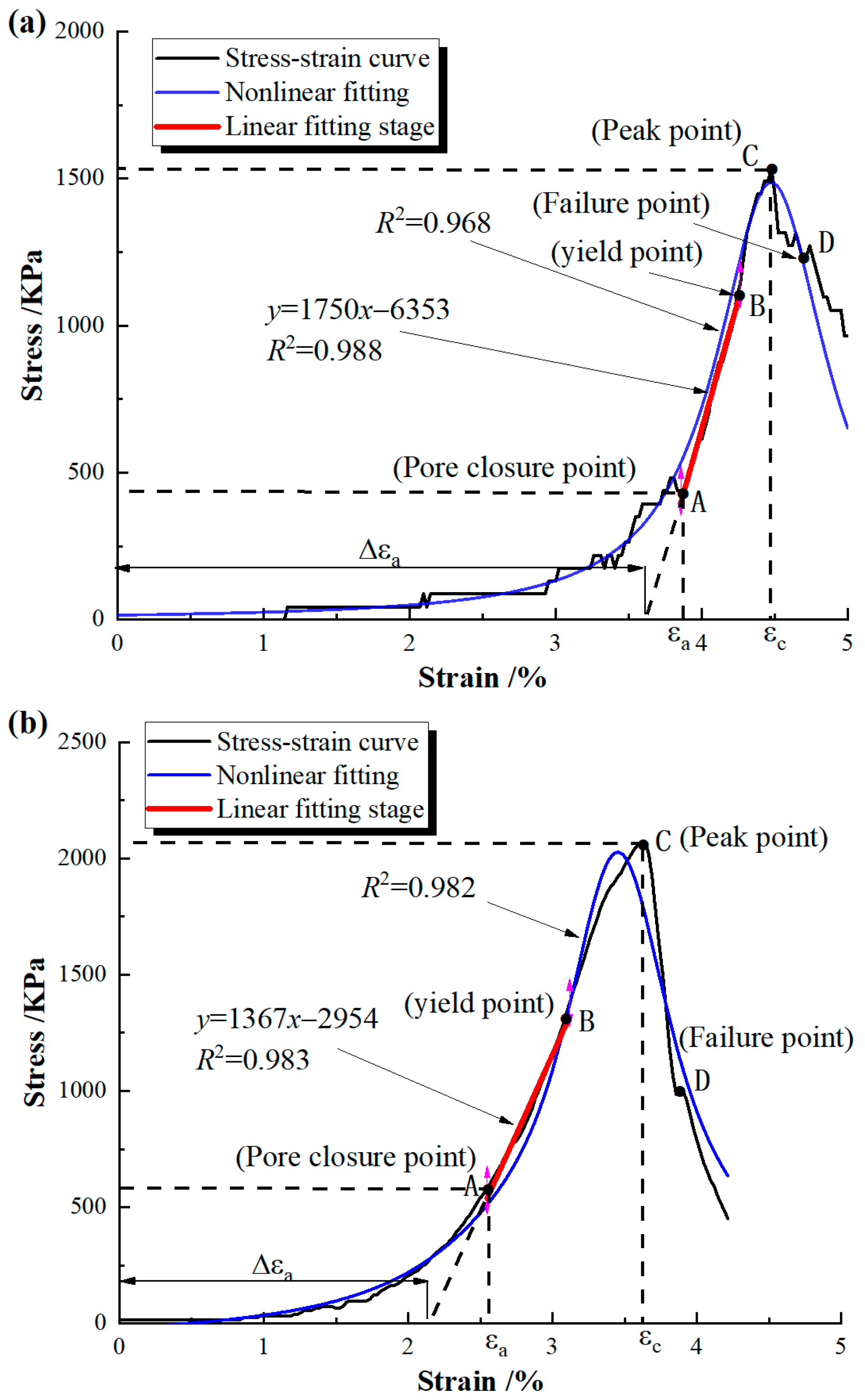
2.3.1. UCS Test
2.3.2. Determination of Calcium Carbonate Production
2.3.3. SEM and XRD Analysis
3. Results
3.1. UCS Test
3.2. Calcium Carbonate Content
3.3. SEM/XRD
4. Discussion
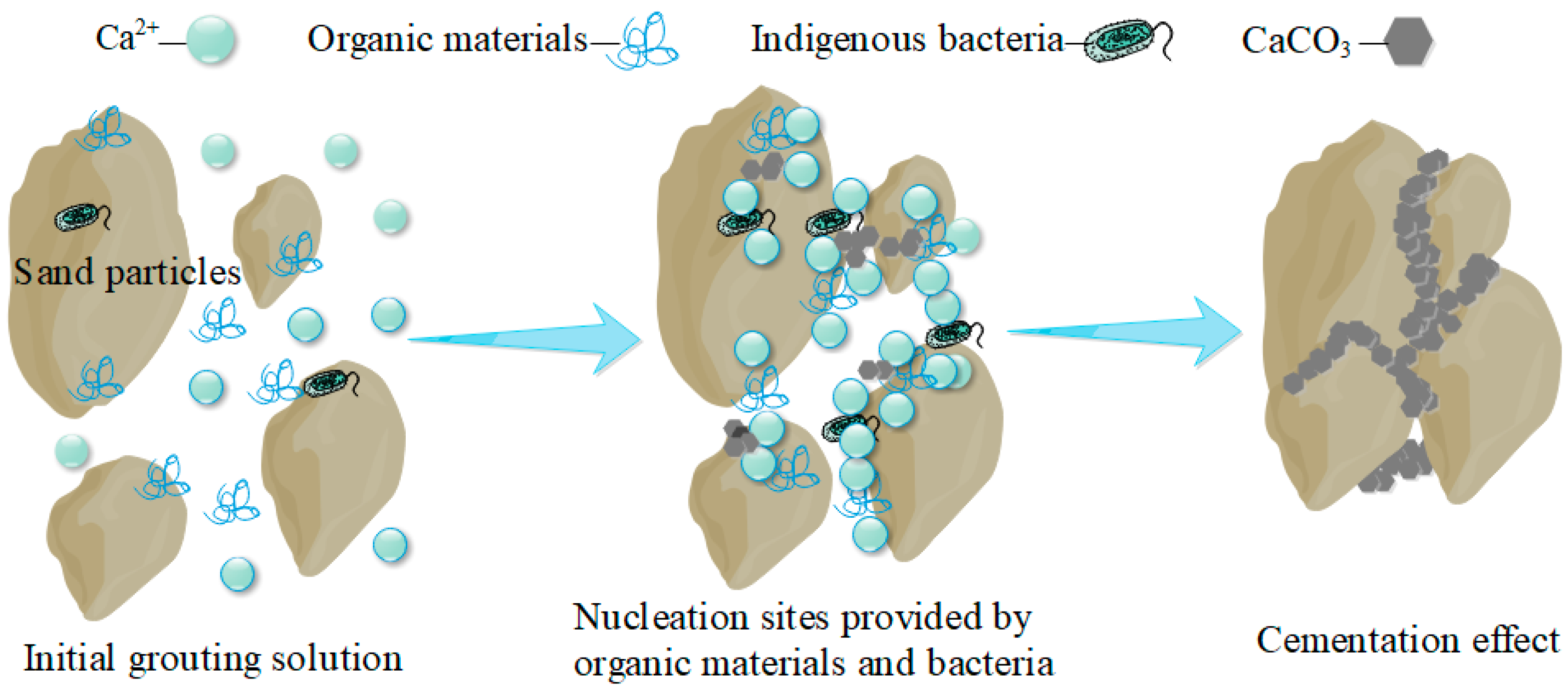
4.1. Mechanism Analysis
4.2. Further Prospects
4.2.1. Research on Biomineralization of Coal-Based Solid Waste
4.2.2. Research on the Self-Healing Function of Biomineralized Materials
5. Conclusions
- (1)
- Peptone, yeast powder, and skimmed milk powder all yielded positive outcomes in relation to the results of EICP. Notably, skimmed milk powder exhibited the most pronounced enhancement effect, followed by peptone, whereas yeast powder displayed a comparatively weaker enhancement effect;
- (2)
- Compared to traditional EICP with a strength of 1.53 MPa, the UCS of EICP combined with peptone, yeast powder, and skimmed milk powder reached 2.03 MPa, 1.60 MPa, and 2.54 MPa, respectively, with growth rates of 32.68%, 4.57%, and 66.01%. Furthermore, in traditional EICP, the proportion of calcium carbonate content is 5.48%. The calcium carbonate content of EICP combined with peptone, yeast powder, and skimmed milk powder reaches 7.44%, 6.02%, and 8.88%, respectively, with growth rates of 35.7%, 9.85%, and 62.04%. In addition, there is also a significant improvement in the uniformity of calcium carbonate, the difference in which between the top and bottom of traditional EICP is 3.1%. The range of EICP combined with peptone, yeast powder, and skimmed milk powder is around 2.0%, 2.5%, and 1.0%, respectively;
- (3)
- Compared to traditional EICP, the crystal morphology of calcite and vaterite varies, with different distributions, when formed using the combination of EICP and skimmed milk powder on the surface and joints of sand particles. These multiple couplings provide evidence that the combination of EICP and organic materials enhances the morphology and structure of calcium carbonate crystals.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Sun, Q.; Qiao, J.; Wang, S. Geological guarantee of coal green mining. J. China Coal Soc. 2020, 45, 8–15. (In Chinese) [Google Scholar]
- Wu, Q.; Tu, K.; Zeng, Y.; Liu, S. Discussion on the main problems and countermeasures for building an upgrade version of main energy (coal) industry in China. J. China Coal Soc. 2019, 44, 1625–1636. (In Chinese) [Google Scholar]
- Zhang, D.; Fan, G.; Liu, Y.; Ma, L. Field trials of aquifer protection in longwall mining of shallow coal seams in China. Int. J. Rock Mech. Min. Sci. 2010, 47, 908–914. [Google Scholar] [CrossRef]
- Hill, J.G.; Price, D.R. The impact of deep mining on an overlying aquifer in western Pennsylvania. Groundw. Monit. Remediat. 1983, 3, 138–143. [Google Scholar] [CrossRef]
- Karaman, A.; Akhiev, S.S.; Carpenter, P.J. A new method of analysis of water-level response to a moving boundary of a longwall mine. Water Resour. Res. 1999, 35, 1001–1010. [Google Scholar] [CrossRef]
- Howladar, M.F. Coal mining impacts on water environs around the Barapukuria coal mining area, Dinajpur, Bangladesh. Environ. Earth Sci. 2012, 70, 215–226. [Google Scholar] [CrossRef]
- Robertson, J. Challenges in sustainably managing groundwater in the Australian Great Artesian Basin: Lessons from current and historic legislative regimes. Hydrogeol. J. 2019, 28, 343–360. [Google Scholar] [CrossRef]
- Wu, M.; Gao, Y.; He, J.; Liu, Y. Laboratory study on use of soybean urease-induced calcium carbonate precipitation with xanthan gum for stabilization of desert sand against wind erosion. Chin. J. Geotech. Eng. 2020, 42, 1914–1921. (In Chinese) [Google Scholar]
- Wu, L.; Miao, L.; Sun, X.; Chen, R.; Wang, C. Experimental study on solidifying sand using plant-derived urease induced calcium carbonate precipitation. Chin. J. Geotech. Eng. 2020, 349, 120–126. (In Chinese) [Google Scholar]
- Jia, Y.; Chen, J. Laboratory Study on the Use of Urease-Induced Calcium Carbonate Precipitation for Stabilization of Coal Fly Ash. Minerals 2023, 13, 185. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; Tang, X.; Chu, J. Calcium carbonate precipitation catalyzed by soybean urease as an improvement method for fine-grained soil. Soils Found. 2019, 59, 1631–1637. [Google Scholar] [CrossRef]
- Dakhane, A.; Das, S.; Hansen, H.; O’Donnell, S.; Hanoon, F.; Rushton, A.; Peria, C.; Neithalath, N. Crack healing in cementitious mortars using Enzyme-Induced carbonate precipitation: Quantification based on fracture response. J. Mater. Civ. Eng. 2018, 30, 04018035. [Google Scholar] [CrossRef]
- Muynck, W.; Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in micro-bial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, G.; Liu, K.; Zheng, W.; Zhao, Z. Experimental study of EICP combined with organic materials for silt improvement in the Yellow River Flood Area. Appl. Sci. 2020, 10, 7678. [Google Scholar] [CrossRef]
- Khodadadi, T.; Kavazanjian, E.; Paassen, L.; DeJong, J. Bio-Grout materials: A Review. Grouting 2017, GSP 288, 1–12. [Google Scholar]
- Wei, Y.; Xu, H.; Xu, S.; Su, H.; Sun, R.; Huang, D.; Zhao, L.; Hu, Y.; Wang, K.; Lian, X. Synthesis and characterization of calcium carbonate on three kinds of microbial cells templates. J. Cryst. Growth 2020, 547, 125755. [Google Scholar] [CrossRef]
- Knorr, B. Enzyme-Induced Carbonate Precipitation for the Mitigation of Fugitive Dust. Master’s Thesis, Arizona State University, Tempe, AZ, USA, 2014. [Google Scholar]
- Ossai, R.; Rivera, L.; Bandini, P. Experimental study to determine an EICP application method feasible for field treatment for soil erosion control. In Geo-Congress 2020; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 205–213. [Google Scholar]
- Kavazanjian, E.; Hamdan, N. Enzyme induced carbonate precipitation (EICP) columns for ground improvement. In Proceedings of the IFCEE 2015, San Antonio, TX, USA, 17–21 March 2015; pp. 2252–2261. [Google Scholar]
- Kavazanjian, E.; Almajed, A.; Hamdan, N. Bio-inspired soil improvement using EICP soil columns and soil nails. Grouting 2017, GSP 288, 13–22. [Google Scholar]
- Cuccurullo, A.; Gallipoli, D.; Bruno, A.W.; Augarde, C.; Blorderie, C.L. Soil Stabilization Against Water Erosion via Calcite Precipitation by Plant-Derived Urease. In Geotechnical Research for Land Protection and Development; Springer Nature: Cham, Switzerland, 2020; pp. 753–762. [Google Scholar]
- Guo, S.; Zhang, J.; Li, M.; Zhou, N.; Song, W.; Wang, Z.; Qi, S. A preliminary study of solid-waste coal gangue based biomineralization as eco-friendly underground backfill material: Material preparation and macro-micro analyses. Sci. Total Environ. 2021, 770, 145241. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, D.; Wang, S.; Xie, Y.; Yu, Y. Water—Preserved mining with the method named “backfilling while mining”. J. China Coal Soc. 2018, 43, 62–69. (In Chinese) [Google Scholar]
- GB/T 50123–2019; Standard for Geotechnical Test Methods. Ministry of Water Resources of the People’s Republic of China. China Planning Press: Beijing, China, 2019. (In Chinese)
- Lee, S.; Kim, J. An experimental study on enzymatic-induced carbonate precipitation using yellow soybeans for soil stabilization. KSCE J. Civ. Eng. 2020, 24, 2026–2037. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Wang, Y.; Han, X.; Jiang, N.; Wang, J.; Feng, J. The effect of enrichment media on the stimulation of native ureolytic bacteria in calcareous sand. Int. J. Environ. Sci. Technol. 2020, 17, 1795–1808. [Google Scholar] [CrossRef]
- Dhami, N.; Alsubhi, W.; Elizabeth, W.; Abhijit, M. Bacterial community dynamics and biocement formation during stimulation and augmentation: Implications for soil consolidation. Front. Microbiol. 2017, 8, 1267. [Google Scholar] [CrossRef]
- Martin, K.; Hamed, K.; Kavazanjian, E. Enzyme-Induced carbonate precipitation: Scale-up of bio-cemented soil columns. In Geo-Congress 2020; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 96–103. [Google Scholar]
- Woolley, M.; Paassen, L.; Kavazanjian, E. Impact on surface hydraulic conductivity of EICP treatment for fugitive dust mitigation. In Geo-Congress 2020; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 132–140. [Google Scholar]
- Liu, S.; Yu, J.; Zeng, W.; Peng, X.; Cai, Y.; Tu, B. Repair effect of tabia cracks with microbially induced carbonate precipitation. Chin. J. Rock Mech. Eng. 2020, 39, 191–204. (In Chinese) [Google Scholar]
- Pasillas, J.; Khodadadi, H.; Martin, K.; Bandini, P.; Newtson, C.; Kavazanjian, E. Viscosity-Enhanced EICP treatment of soil. In Proceedings of the IFCEE 2018, Orlando, FL, USA, 5–10 March 2018; Volume GSP 296, pp. 145–154. [Google Scholar]
- Kou, H.; Wu, C.; Jang, B.; Wang, D. Spatial distribution of CaCO3 in biocemented sandy slope using surface percolation. J. Mater. Civ. Eng. 2021, 33, 06021004. [Google Scholar] [CrossRef]
- Yang, F. Enzyme-Induced Calcite Precipitation for the Reinforcement of Earthen Sites. Master’s Thesis, Lanzhou University, Lanzhou, China, 2019. (In Chinese). [Google Scholar]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Almajed, A.; Tirkolaei, H.; Kavazanjian, E.; Hamdan, N. Enzyme induced biocementated sand with high strength at low carbonate content. Sci. Rep. 2019, 9, 1135. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Zhang, J. Statistical Damage Model for Whole Deformation and Failure Process of Rock Considering Initial Void Closure. J. Southwest Jiaotong Univ. 2022, 57, 314–321. (In Chinese) [Google Scholar]
- Cao, G.; Liu, S.; Cai, Y.; Yu, J.; Sun, Z. Experimental study on solidification of land reclamation sea sand by EICP combined with targeting activation of microbes producing urease. Rock Soil Mech. 2022, 43, 2241–2252. (In Chinese) [Google Scholar]
- Kiasari, A.; Pakbaz, M.; Ghezelbash, G. Comparison of effects of different nutrients on stimulating indigenous soil bacteria for biocementation. J. Mater. Civ. Eng. 2019, 31, 04019067. [Google Scholar] [CrossRef]
- Bosikov, I.I.; Martyushev, N.V.; Klyuev, R.V.; Tynchenko, V.S.; Kukartsev, V.A.; Eremeeva, S.V.; Karlina, A.I. Complex Assessment of X-ray Diffraction in Crystals with Face-Centered Silicon Carbide lattice. Crystals 2023, 13, 528. [Google Scholar] [CrossRef]
- Elaqra, H.; Godin, N.; Peix, G.; R’Mili, M.; Fantozzi, G. Damage evolution analysis in mortar,during compressive loading using acoustic emission and X-ray tomography: Effects of the sand/cement ratio. Cem. Concr. Res. 2007, 37, 703–713. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Dong, B.; Liu, S.; Yu, J.; Cai, Y.; Tu, B. Evaluation of the effect of natural seawater strengthening calcareous sand based on MICP. Rock Soil Mech. 2021, 42, 1104–1114. (In Chinese) [Google Scholar]
- Liu, S.; Yu, J.; Han, L.; Cai, Y.; Tu, B.; Zhou, J. Experimental study on water resistance of tabia surface with microbially induced carbonate precipitation. Chin. J. Rock Mech. Eng. 2019, 38, 1718–1728. (In Chinese) [Google Scholar]
- Khaleghi, M.; Rowshanzamir, M. Biologic improvement of a sandy soil using single and mixed cultures: A comparison study. Soil Tillage Res. 2019, 186, 112–119. [Google Scholar] [CrossRef]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined co-culture of ureolytic and non-ureolytic bacteria. Biogeosciences 2013, 10, 17249–17273. [Google Scholar]
- Zhang, J.; Zhang, Q.; Zhou, N.; Li, M.; Huang, P.; Li, B. Research progress and prospect of coal based solid waste backfilling mining technology. J. China Coal Soc. 2022, 47, 4167–4181. (In Chinese) [Google Scholar]
- Xiang, J.; Qiu, J.; Yuan, L.; Wu, J.; Ma, Z. Characterization and role analysis of bacteria types in self-healing behaviour of cemented paste backfill. J. Build. Eng. 2023, 75, 106964. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Fernandez, C.A.; Haider, M.; Chu, K.; Jian, G.; Nassiri, S.; Zhang, D.; Rousseau, R.; Glezakou, V.A. Toward Self-Healing Concrete Infrastructure: Review of Experiments and Simulations across Scales. Chem. Rev. 2023, 123, 10838–10876. [Google Scholar] [CrossRef]










| Min Dry Density g/cm3 | Max Dry Density g/cm3 | Specific Gravity | Total Organic Carbon g/kg | Total Nitrogen g/kg | Carbon-to-Nitrogen Ratio | Total Soluble Salt g/kg | Water Content % |
|---|---|---|---|---|---|---|---|
| 1.51 | 1.74 | 2.52 | 0.23 | 0.07 | 2.71 | 0.35 | 3.27 |
| Scheme Number | Treatment Method | Organic Material Concentration (g·L−1) | Urease Activity mM/min | Urea mM | CaCl2 mM | pH | Grouting Rounds |
|---|---|---|---|---|---|---|---|
| A | EICP | / | 5.2 | 1000 | 1000 | 9 | 5 |
| B1 | EICP + peptone | 2 | 5.2 | 1000 | 1000 | 9 | 5 |
| B2 | 4 | 5.2 | 1000 | 1000 | 9 | 5 | |
| B3 | 6 | 5.2 | 1000 | 1000 | 9 | 5 | |
| C1 | EICP + yeast powder | 2 | 5.2 | 1000 | 1000 | 9 | 5 |
| C2 | 4 | 5.2 | 1000 | 1000 | 9 | 5 | |
| C3 | 6 | 5.2 | 1000 | 1000 | 9 | 5 | |
| D1 | EICP + skimmed milk powder | 2 | 5.2 | 1000 | 1000 | 9 | 5 |
| D2 | 4 | 5.2 | 1000 | 1000 | 9 | 5 | |
| D3 | 6 | 5.2 | 1000 | 1000 | 9 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.; Ma, L.; Ngo, I.; Osemudiamhen, A.E.; Guo, Z. Experimental Investigation on the Combination of Enzyme-Induced Calcium Carbonate Precipitation and Organic Materials for Underground Backfilling Preparation. Minerals 2024, 14, 153. https://doi.org/10.3390/min14020153
Cao G, Ma L, Ngo I, Osemudiamhen AE, Guo Z. Experimental Investigation on the Combination of Enzyme-Induced Calcium Carbonate Precipitation and Organic Materials for Underground Backfilling Preparation. Minerals. 2024; 14(2):153. https://doi.org/10.3390/min14020153
Chicago/Turabian StyleCao, Guanghui, Liqiang Ma, Ichhuy Ngo, Arienkhe Endurance Osemudiamhen, and Zezhou Guo. 2024. "Experimental Investigation on the Combination of Enzyme-Induced Calcium Carbonate Precipitation and Organic Materials for Underground Backfilling Preparation" Minerals 14, no. 2: 153. https://doi.org/10.3390/min14020153
APA StyleCao, G., Ma, L., Ngo, I., Osemudiamhen, A. E., & Guo, Z. (2024). Experimental Investigation on the Combination of Enzyme-Induced Calcium Carbonate Precipitation and Organic Materials for Underground Backfilling Preparation. Minerals, 14(2), 153. https://doi.org/10.3390/min14020153







