Geochemical Characteristics of Aluminum-Bearing Iron Ores: A Case Study from the Kolijan Karst-Type Bauxite Deposit, Northwestern Iran
Abstract
1. Introduction
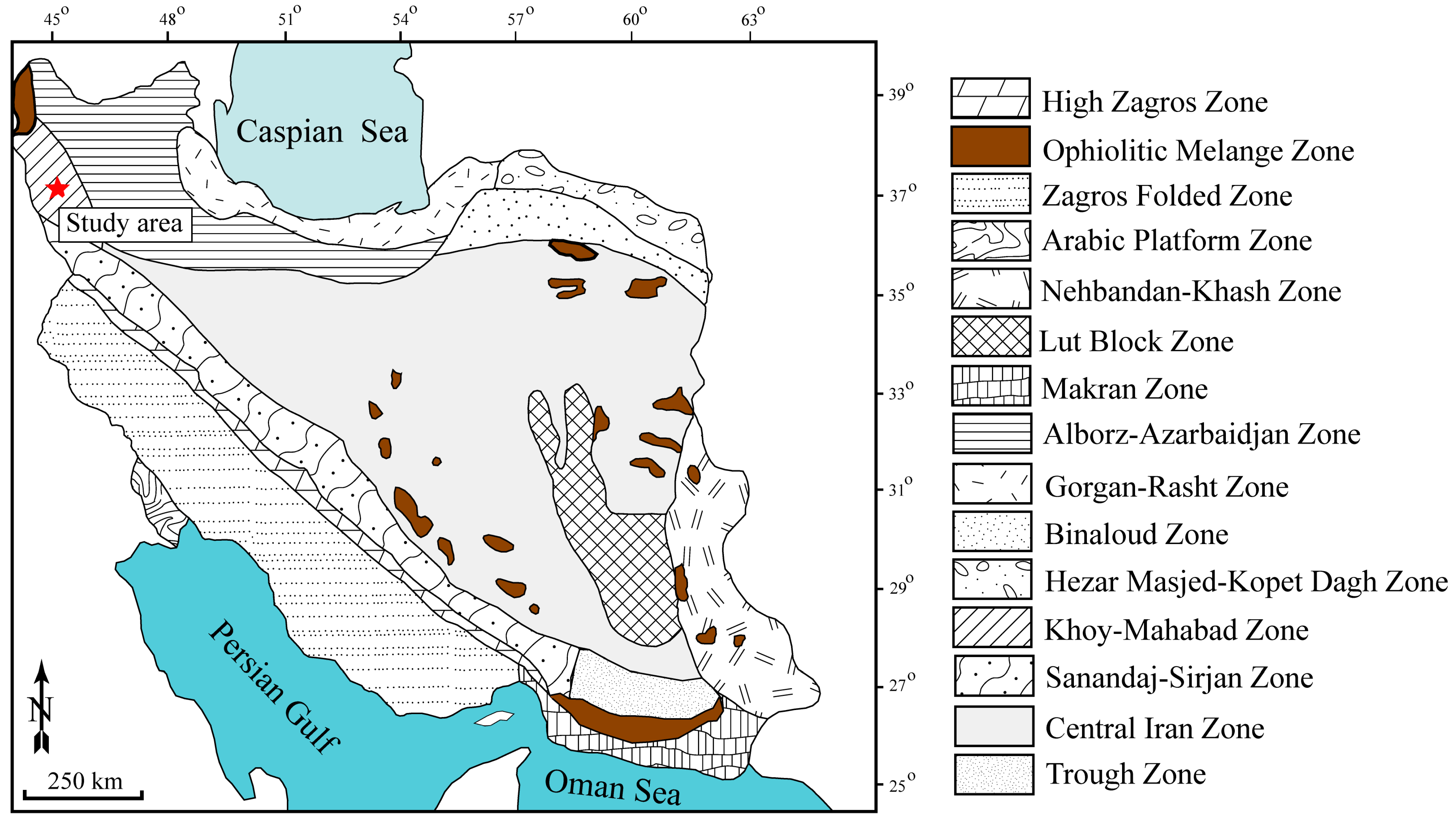
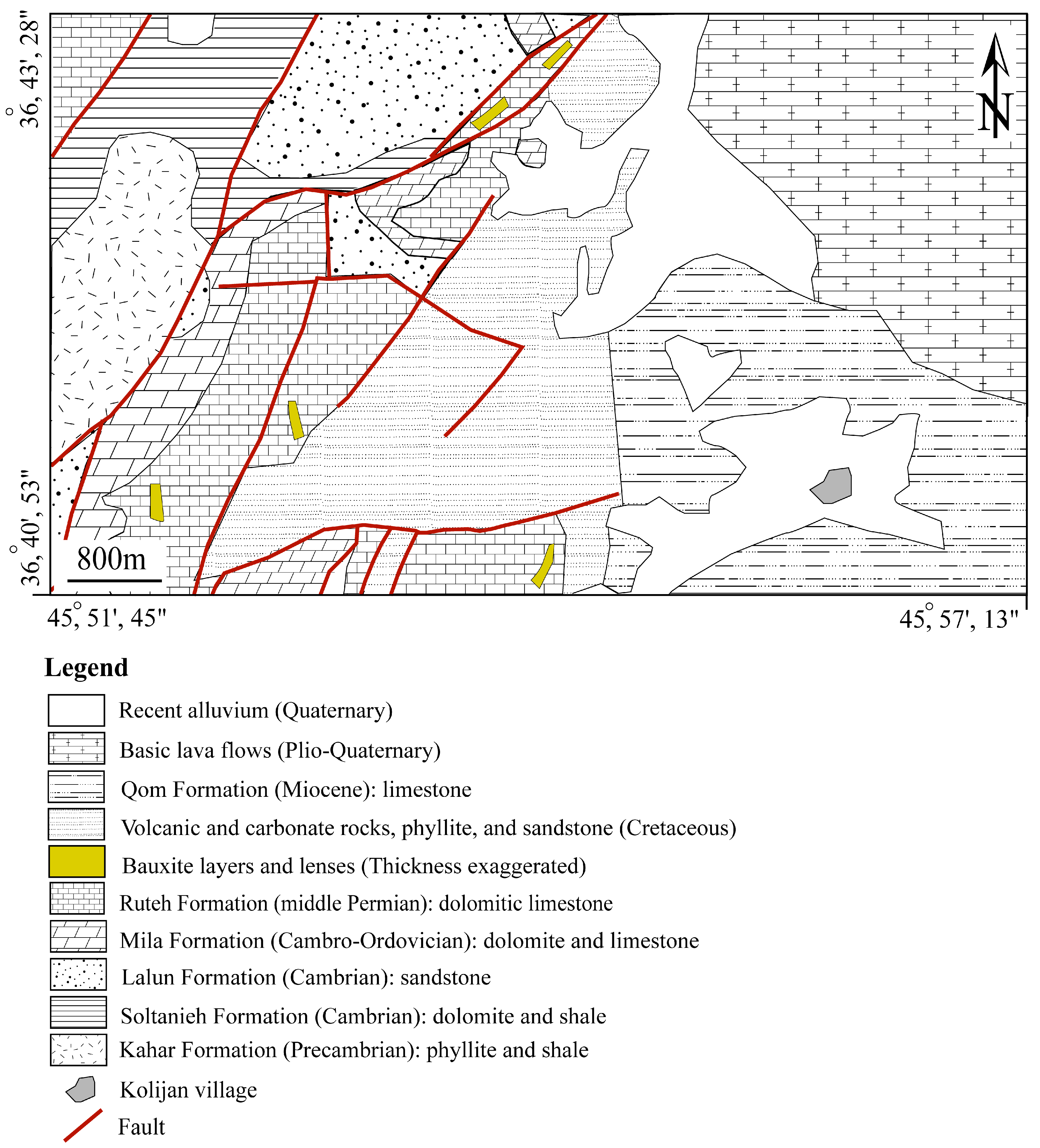
2. Geological Setting of the Deposit
3. Method of Investigation
4. Results and Discussion
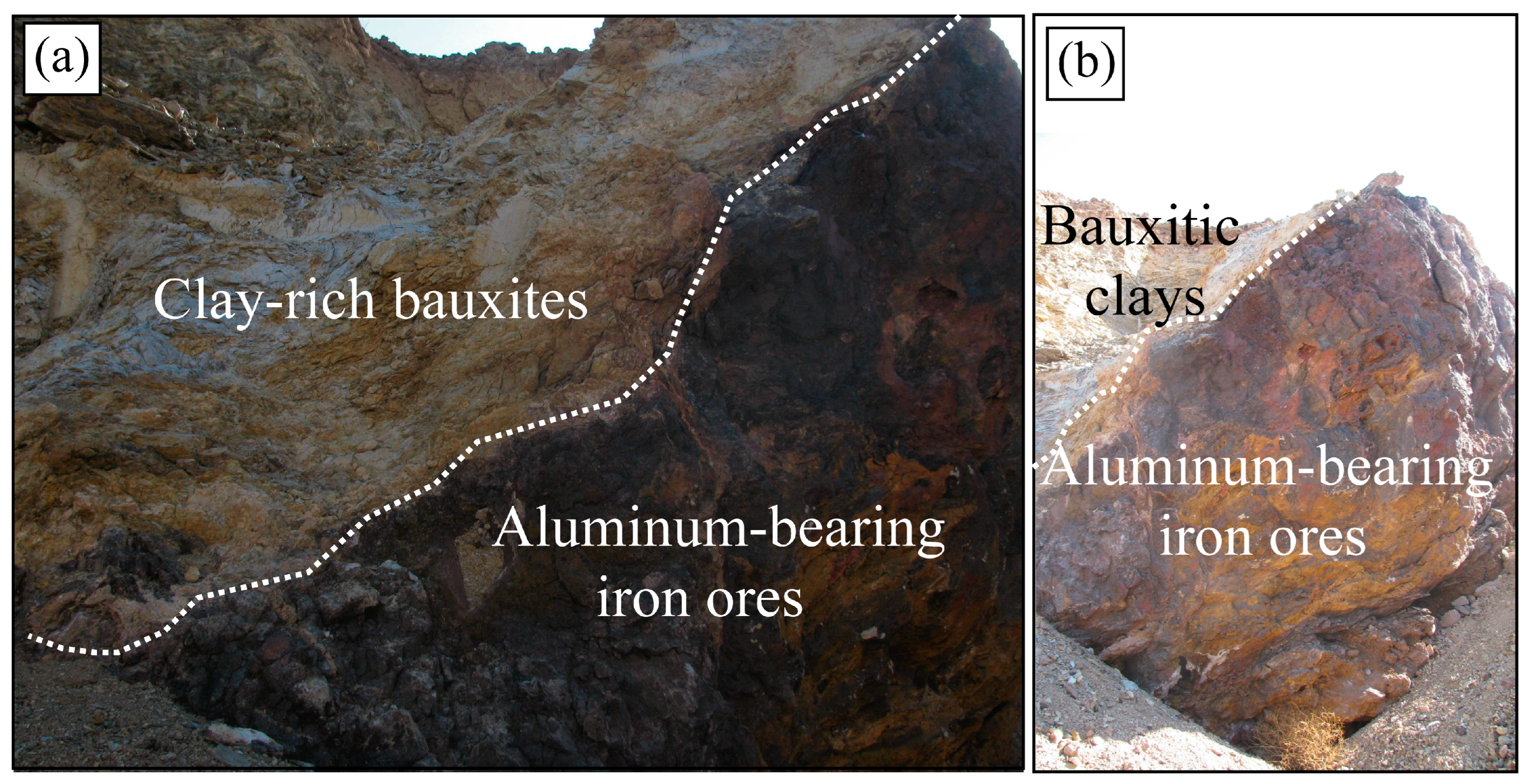
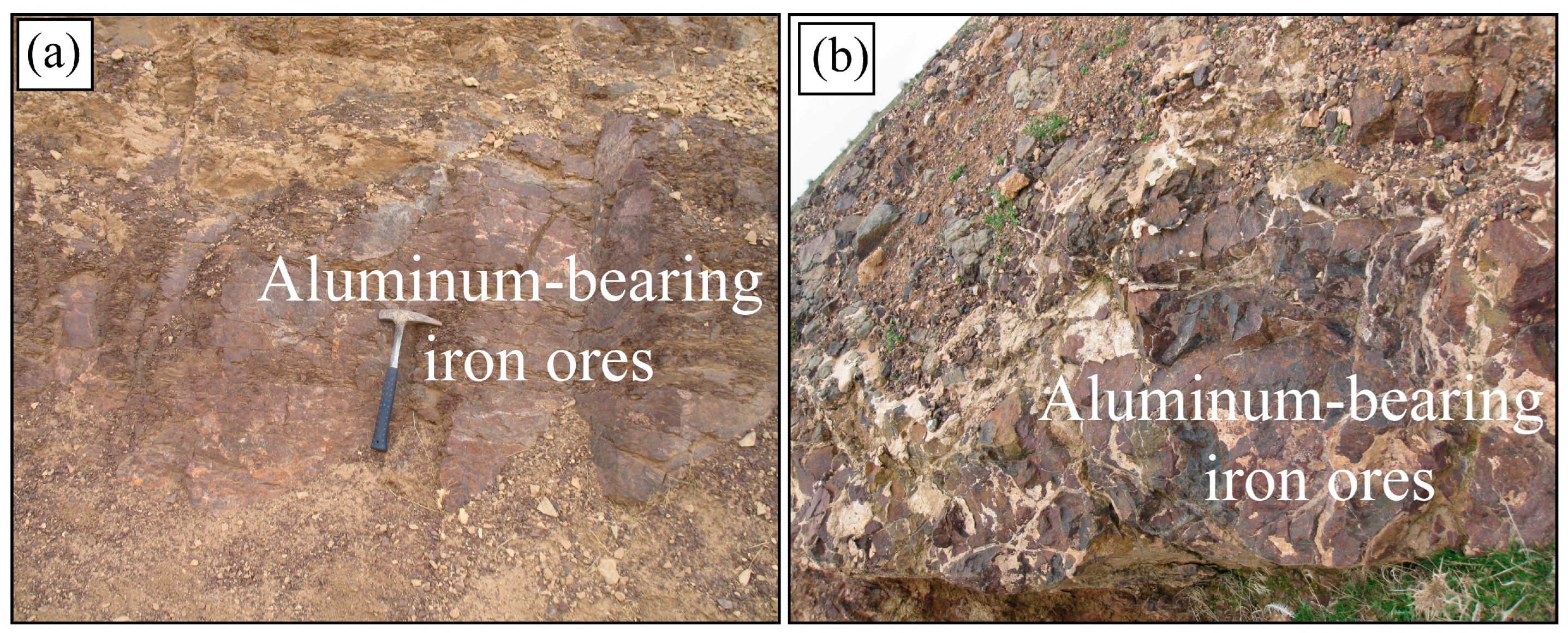
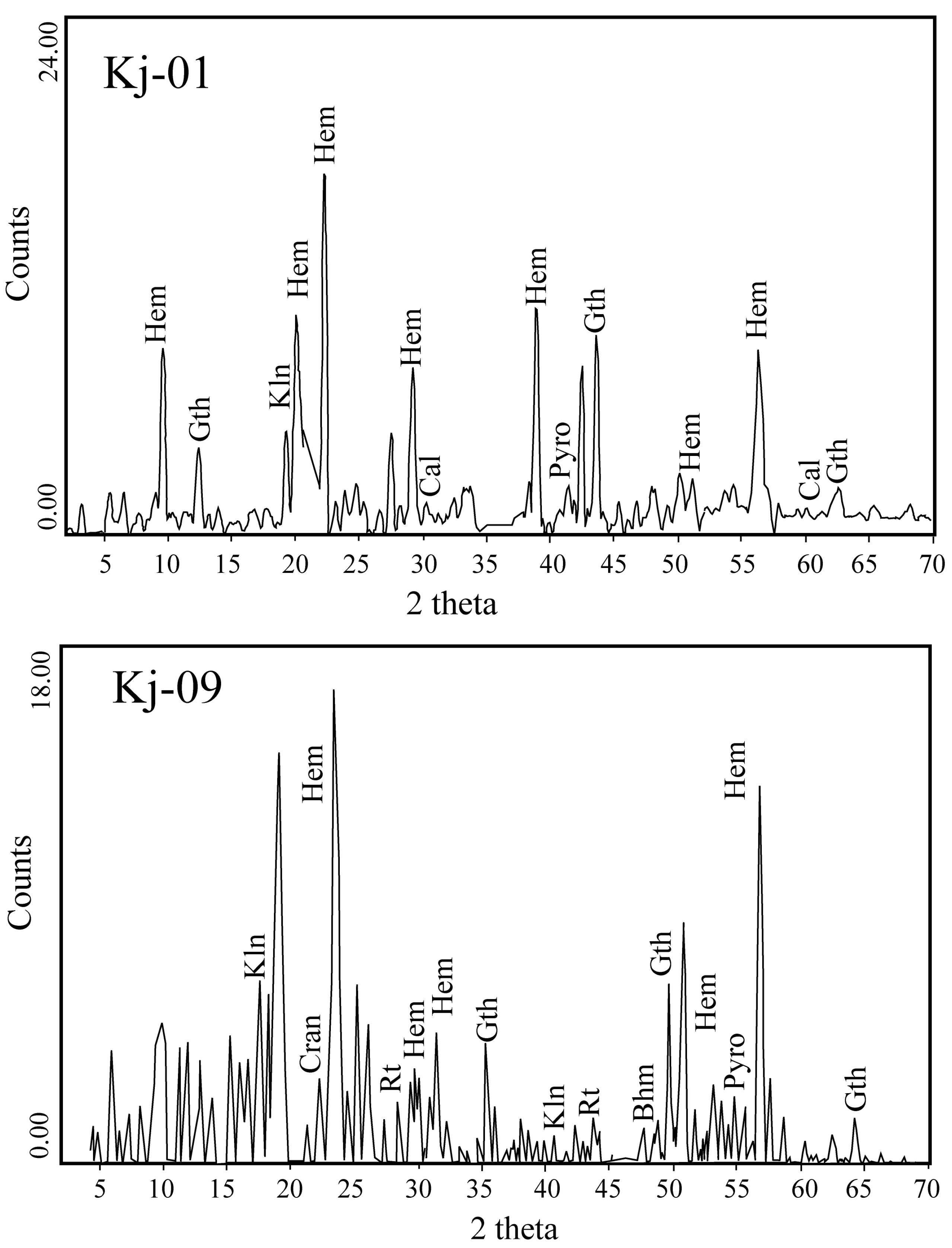
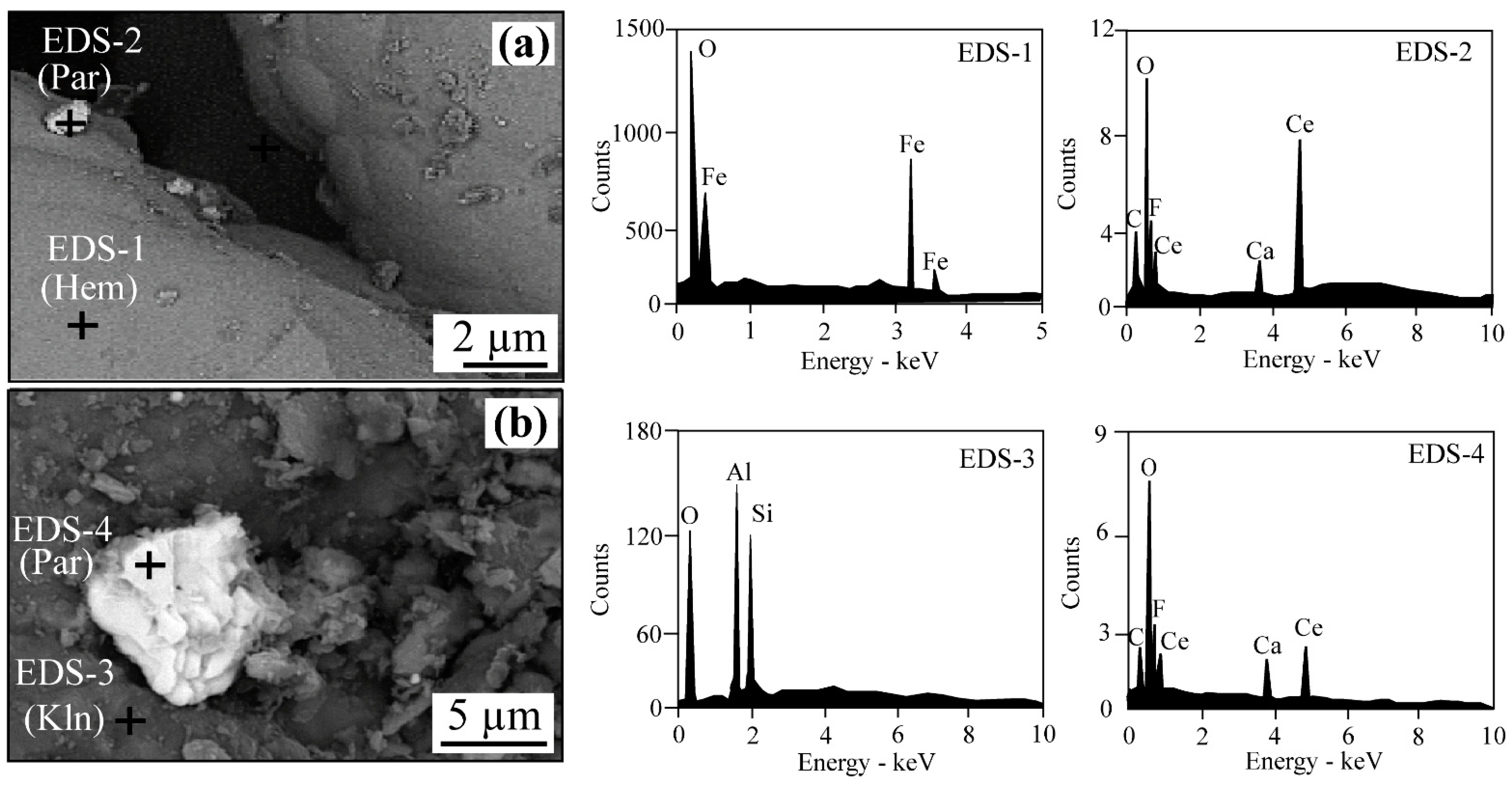
4.1. Texture and Mineralogy of the Al-Bearing Iron Ores

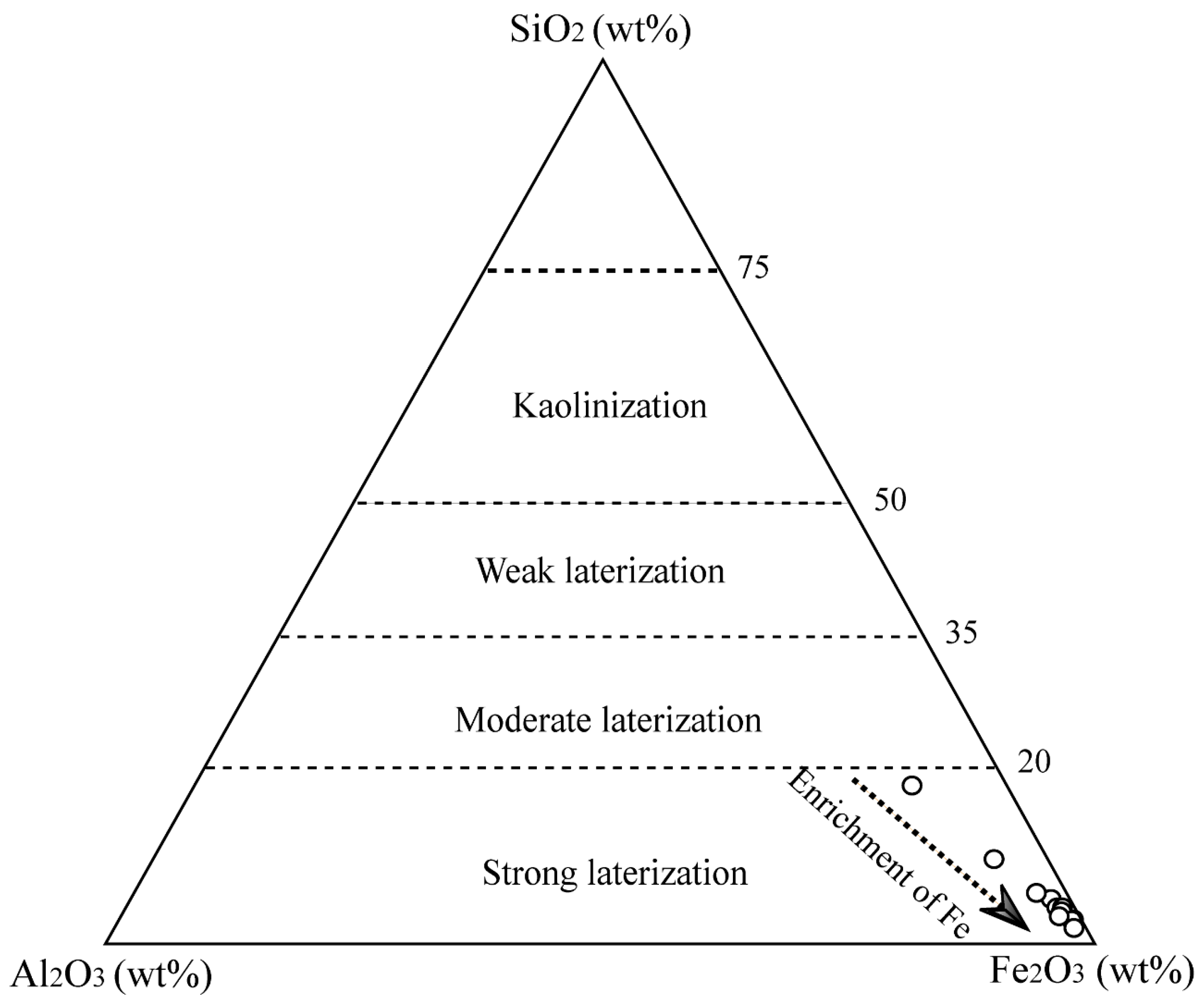
4.2. Distribution of Major Elements in Kolijan Iron Ore
4.3. Distribution of Trace Elements in Kolijan Iron Ore
| Detection Limit | Kj-01 | Kj-02 | Kj-03 | Kj-04 | Kj-05 | Kj-06 | Kj-07 | Kj-08 | Kj-09 | Kj-10 | Kj-11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (wt%) | 0.01 | 3.72 | 1.67 | 1.85 | 2.81 | 1.47 | 1.88 | 2.33 | 4.68 | 8.15 | 14.42 | 1.88 |
| Al2O3 | 0.01 | 1.68 | 0.32 | 0.36 | 1.02 | 0.27 | 0.74 | 0.75 | 2.71 | 4.62 | 8.12 | 1.11 |
| Fe2O3 | 0.01 | 82.99 | 89.93 | 88.74 | 85.87 | 91.12 | 86.81 | 88.14 | 79.45 | 72.89 | 60.9 | 84.87 |
| CaO | 0.01 | 1.97 | 0.22 | 0.26 | 1.12 | 0.18 | 1.07 | 0.33 | 0.66 | 1.12 | 0.36 | 1.88 |
| Na2O | 0.01 | 0.03 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 |
| MgO | 0.01 | 0.23 | 0.18 | 0.22 | 0.23 | 0.13 | 0.2 | 0.14 | 0.22 | 0.54 | 0.91 | 0.17 |
| K2O | 0.01 | 0.05 | 0.01 | 0.01 | 0.03 | 0.01 | 0.09 | 0.06 | 0.19 | 0.09 | 0.19 | 0.32 |
| TiO2 | 0.01 | 0.64 | 0.08 | 0.08 | 0.41 | 0.08 | 0.25 | 0.08 | 1.61 | 1.57 | 2.65 | 0.41 |
| MnO | 0.01 | 0.71 | 0.52 | 0.62 | 0.67 | 0.41 | 0.77 | 0.59 | 1.02 | 0.96 | 1.09 | 0.91 |
| P2O5 | 0.01 | 0.61 | 0.26 | 0.31 | 0.31 | 0.21 | 0.92 | 0.32 | 1.02 | 1.35 | 1.23 | 1.22 |
| L.O.I | 0.01 | 7.35 | 6.77 | 7.52 | 7.45 | 6.03 | 7.21 | 7.21 | 8.35 | 8.65 | 10.02 | 7.16 |
| Sum | – | 99.98 | 99.97 | 99.98 | 99.94 | 99.92 | 99.95 | 99.96 | 99.93 | 99.96 | 99.91 | 99.94 |
| U (ppm) | 0.05 | 8.24 | 6.69 | 6.11 | 5.67 | 6.28 | 8.75 | 7.05 | 9.6 | 13.82 | 18.26 | 10.39 |
| Th | 0.05 | 0.78 | 0.10 | 0.12 | 0.44 | 0.11 | 0.34 | 0.19 | 1.07 | 2.43 | 4.27 | 0.58 |
| Ba | 0.5 | 214.4 | 79.5 | 96.1 | 155.2 | 63 | 191.1 | 140.6 | 215.3 | 203.2 | 120.3 | 286.1 |
| Hf | 0.2 | 2.1 | 0.5 | 0.8 | 1.4 | 0.2 | 0.8 | 1.2 | 3.1 | 3.7 | 6.6 | 0.8 |
| Cr | 10 | 1010 | 940 | 920 | 980 | 910 | 880 | 960 | 870 | 580 | 470 | 850 |
| Co | 0.5 | 26.3 | 29.4 | 28.6 | 27.3 | 30.3 | 31.6 | 32.1 | 27.1 | 24.5 | 14.6 | 34.5 |
| Nb | 0.2 | 1.6 | 0.7 | 0.7 | 1.1 | 0.7 | 0.9 | 0.7 | 3.9 | 3.6 | 6.2 | 1.1 |
| Cs | 0.01 | 0.29 | 0.13 | 0.05 | 0.17 | 0.05 | 0.18 | 0.16 | 0.68 | 0.51 | 0.51 | 0.82 |
| Rb | 0.2 | 1.3 | 0.3 | 0.2 | 1.0 | 0.6 | 0.9 | 0.6 | 2.4 | 1.6 | 2.6 | 3.6 |
| V | 5 | 149 | 99 | 105 | 127 | 93 | 80 | 102 | 138 | 183 | 312 | 84 |
| Ga | 0.1 | 5.0 | 3.2 | 3.3 | 4.1 | 3.2 | 3.2 | 2.6 | 3.4 | 5.2 | 7.5 | 3.0 |
| Sr | 0.1 | 227.9 | 102.1 | 124.8 | 176.4 | 79.3 | 195.1 | 133.1 | 173.8 | 170.8 | 76.3 | 265.4 |
| Y | 0.5 | 1.6 | 0.6 | 0.7 | 1.1 | 0.6 | 2.1 | 2.3 | 1.2 | 3.8 | 9.7 | 1.8 |
| Ta | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.6 | 0.1 |
| Zr | 2 | 11 | 4 | 4 | 8 | 4 | 7 | 7 | 24 | 41 | 71 | 10 |
| Pb | 5 | 71 | 74 | 74 | 70 | 46 | 109 | 65 | 124 | 116 | 152 | 110 |
| Ni | 5 | 71 | 102 | 95 | 78 | 109 | 92 | 83 | 79 | 70 | 42 | 88 |
| La (ppm) | 0.5 | 6.1 | 1.7 | 2.6 | 4.4 | 0.9 | 4.9 | 2.3 | 12.5 | 10.9 | 14.6 | 7.3 |
| Ce | 0.5 | 42.3 | 16.5 | 19.6 | 32.5 | 7.1 | 49.8 | 21.3 | 94.3 | 94.2 | 99.6 | 74.2 |
| Pr | 0.03 | 1.25 | 0.33 | 0.49 | 0.87 | 0.17 | 1.61 | 0.84 | 2.47 | 2.75 | 2.78 | 2.72 |
| Nd | 0.1 | 4.1 | 1.3 | 1.5 | 2.8 | 1.1 | 7.3 | 5.6 | 10.7 | 12.5 | 11.8 | 13.1 |
| Sm | 0.03 | 1.18 | 0.24 | 0.33 | 0.76 | 0.15 | 2.54 | 1.81 | 1.86 | 3.37 | 1.98 | 4.75 |
| Eu | 0.03 | 1.62 | 0.45 | 1.28 | 1.95 | 0.26 | 7.48 | 2.75 | 2.02 | 7.67 | 3.00 | 7.77 |
| Gd | 0.05 | 1.57 | 0.45 | 0.60 | 1.09 | 0.29 | 4.81 | 2.37 | 1.14 | 5.96 | 2.9 | 9.01 |
| Tb | 0.01 | 0.20 | 0.05 | 0.07 | 0.14 | 0.04 | 0.73 | 0.32 | 0.29 | 0.93 | 0.47 | 1.39 |
| Dy | 0.05 | 1.32 | 0.47 | 0.57 | 0.95 | 0.36 | 4.20 | 1.98 | 1.56 | 5.65 | 3.45 | 7.84 |
| Ho | 0.01 | 0.20 | 0.11 | 0.15 | 0.17 | 0.07 | 0.80 | 0.31 | 0.34 | 1.02 | 0.58 | 1.46 |
| Er | 0.03 | 0.44 | 0.24 | 0.35 | 0.39 | 0.13 | 1.51 | 0.99 | 0.90 | 2.23 | 1.79 | 2.67 |
| Tm | 0.01 | 0.09 | 0.02 | 0.02 | 0.05 | 0.03 | 0.16 | 0.10 | 0.14 | 0.28 | 0.24 | 0.31 |
| Yb | 0.03 | 0.43 | 0.19 | 0.24 | 0.33 | 0.15 | 0.84 | 0.57 | 0.73 | 1.39 | 1.34 | 1.45 |
| Lu | 0.01 | 0.11 | 0.02 | 0.02 | 0.06 | 0.02 | 0.12 | 0.12 | 0.12 | 0.24 | 0.26 | 0.22 |
| ∑LREE (La–Eu) (ppm) | – | 56.55 | 20.52 | 25.80 | 43.28 | 9.68 | 73.63 | 34.6 | 123.85 | 131.39 | 133.76 | 109.84 |
| ∑HREE (Gd–Lu) (ppm) | – | 4.36 | 1.55 | 2.02 | 3.18 | 1.09 | 13.17 | 6.76 | 5.22 | 17.70 | 11.03 | 24.35 |
| ∑REE (La–Lu) (ppm) | – | 60.91 | 22.07 | 27.82 | 46.46 | 10.77 | 86.8 | 41.36 | 129.07 | 149.09 | 144.79 | 134.19 |
| (La/Yb)N | – | 1.05 | 0.66 | 0.80 | 0.99 | 0.44 | 0.43 | 0.30 | 1.27 | 0.58 | 0.81 | 0.37 |
| (∑LREE/∑HREE)N | – | 7.29 | 7.45 | 7.18 | 7.65 | 4.99 | 3.14 | 2.88 | 13.34 | 4.18 | 6.82 | 2.54 |
| Eu/Eu* | – | 3.64 | 4.25 | 8.87 | 6.55 | 3.82 | 6.53 | 4.06 | 4.24 | 5.23 | 3.83 | 3.63 |
| Ce/Ce* | – | 0.93 | 3.49 | 5.02 | 3.96 | 3.79 | 4.14 | 4.04 | 3.49 | 3.87 | 3.92 | 3.57 |
| La/Y | – | 3.81 | 2.83 | 3.71 | 4.00 | 1.50 | 2.33 | 1.00 | 10.42 | 2.87 | 1.51 | 4.06 |
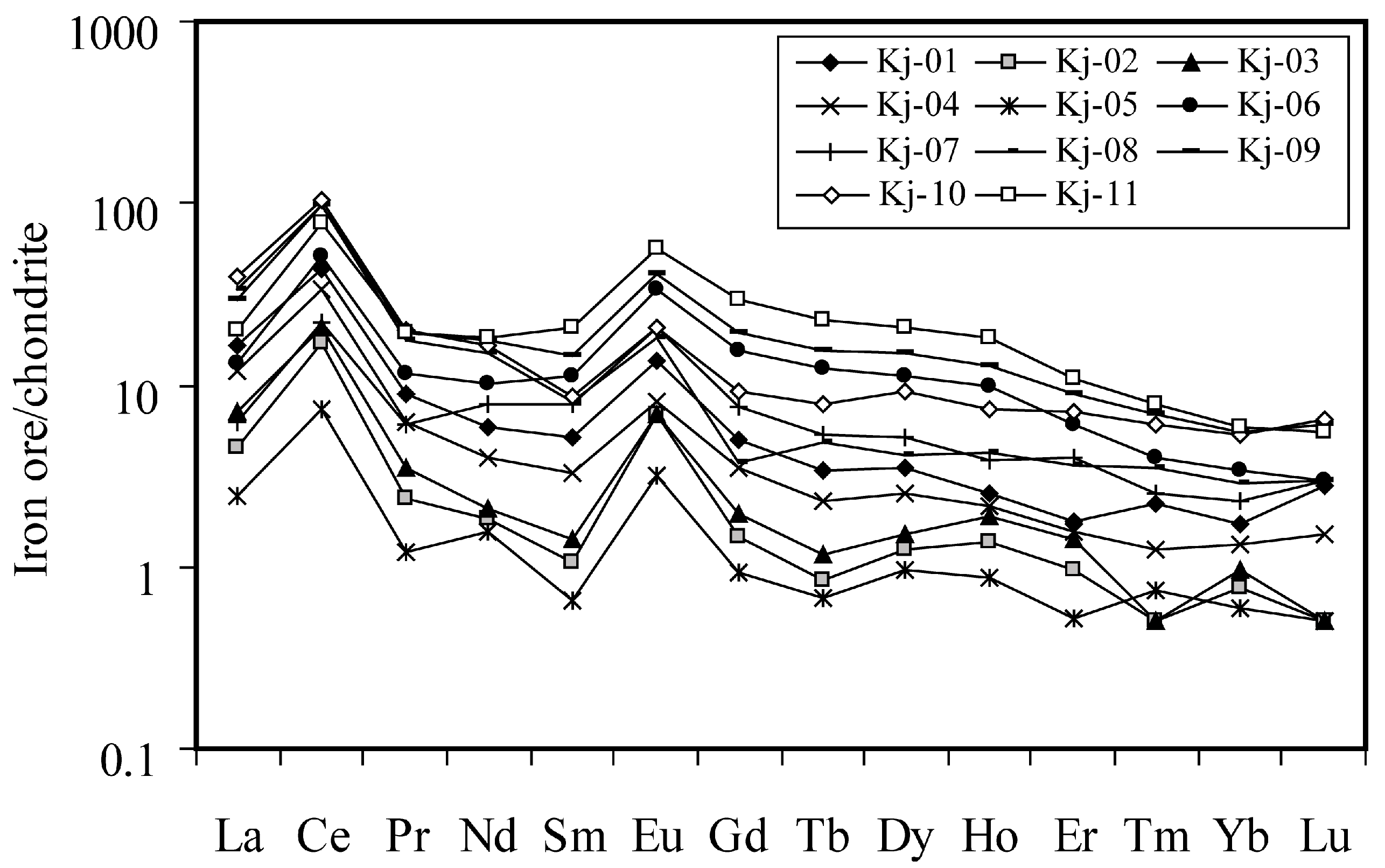
4.4. Distribution Patterns of REE in Kolijan Al-Bearing Iron Ores
4.5. Mineralogical Controls on REE Distribution in the Kolijan Al-Bearing Iron Ores
4.6. Ce and Eu Anomalies in the Kolijan Al-Bearing Iron Ores
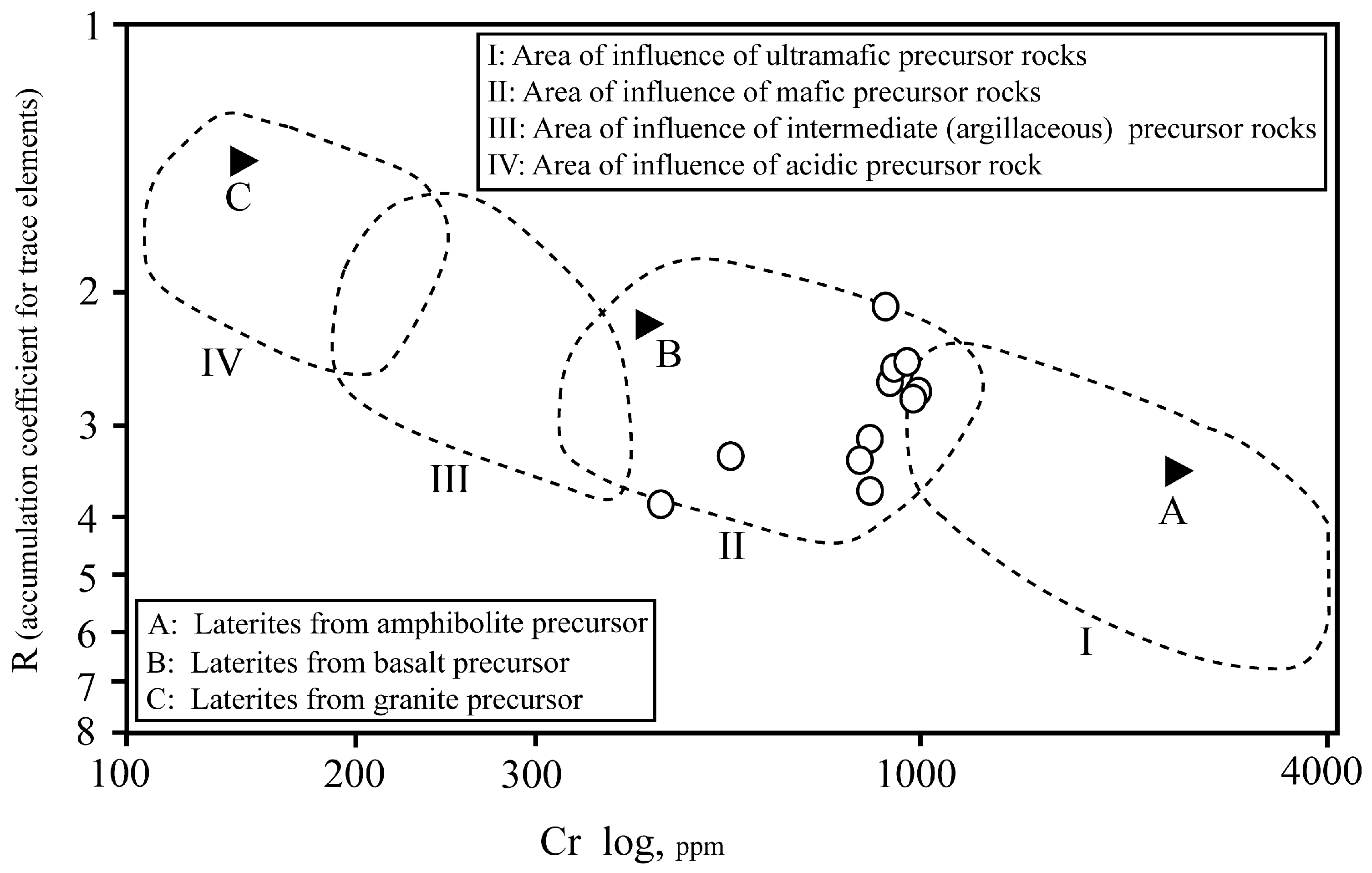
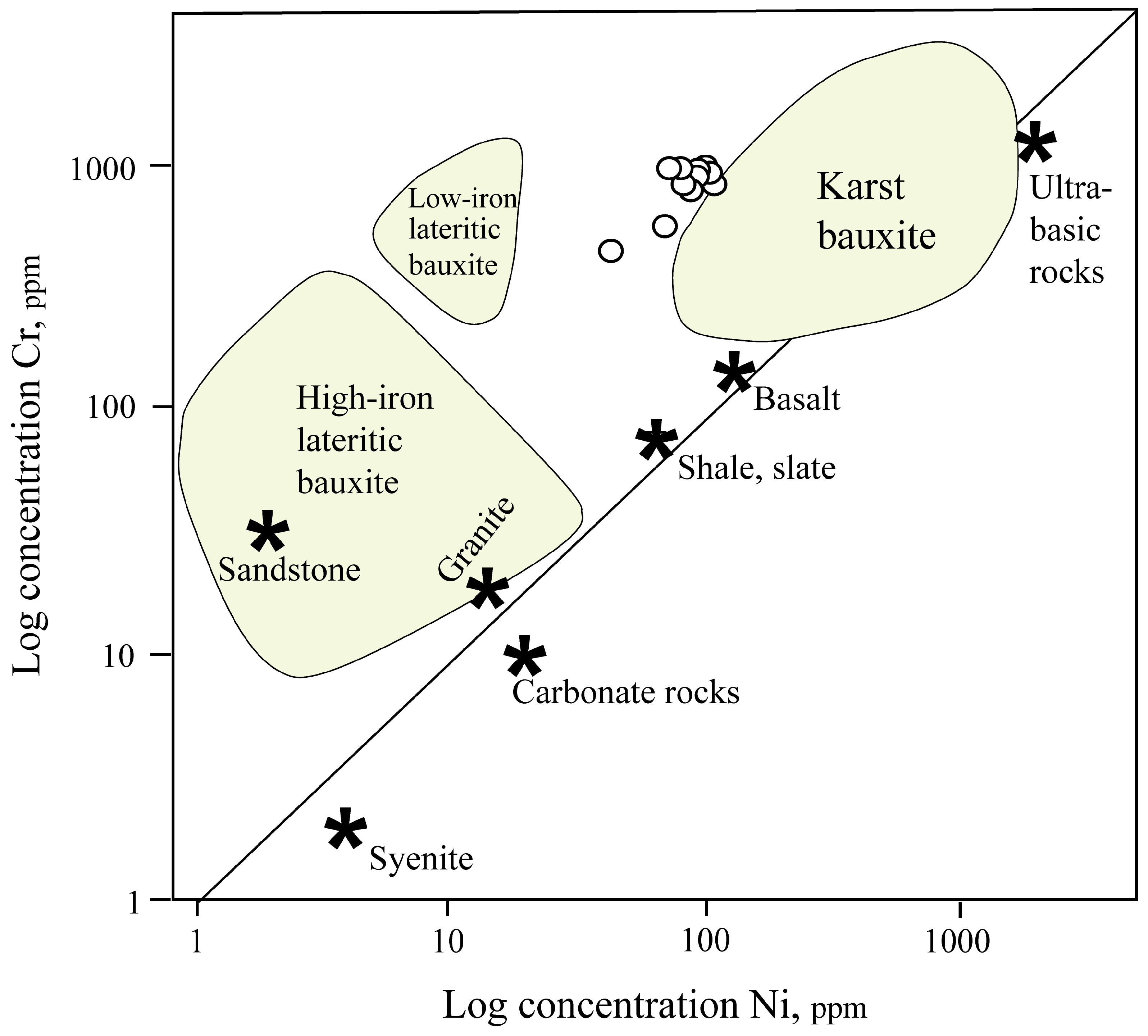
4.7. Protolith of the Kolijan Al-Bearing Iron Ores
5. Conclusions
- Microscopic observations show that the ores developed in multiple stages, and diagenetic and epigenetic processes were important during their formation.
- Hematite and goethite are the main constituents of the Kolijan iron ores, accompanied by lesser amounts of kaolinite, illite, amesite, boehmite, rutile, anatase, calcite, pyrolusite, crandallite, and parisite-(Ce).
- The distribution of trace elements in the Al-bearing iron ores developed by scavenging, co-precipitation, isomorphic substitution, adsorption, and fixation in new mineral phases, such as parisite-(Ce).
- Preferential adsorption of LREE by kaolinite, illite, boehmite, rutile, and anatase resulted in their separation from HREE in the ores; pyrolusite and crandallite host both LREE and HREE in the ores.
- Geochemical ratios, such as La/Y, indicate that fluctuations in the level of the water- table, and the function of carbonate bedrocks as a geochemical barrier caused fractionation and the concentration of REE during formation of the iron ores.
- Strong positive Ce anomalies in the Kolijan iron ores result from alkalinity produced by carbonate bedrocks resulting in precipitation of parisite-(Ce).
- Strong positive Eu anomalies in the ores are closely dependent on alkaline pH.
- Based on geochemical data (Ni and Cr contents and accumulation coefficients of elements), basaltic rocks are the probable protolith of the Kolijan iron ores.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bárdossy, G. Karst Bauxites: Bauxite Deposits on Carbonat e Rocks; Elsevier Scientific Publication: Amsterdam, The Netherlands, 1982; p. 441. [Google Scholar]
- Bárdossy, G.; Aleva, G.J.J. Lateritic Bauxites. Developments in Economic Geology; Elsevier Scientific Publication: Amsterdam, The Netherlands, 1990; Volume 27, p. 624. [Google Scholar]
- Mameli, P.; Mongelli, G.; Oggiano, G.; Dinelli, E. Geological, geochemical and mineralogical features of some bauxite deposits from Nurra (Western Sardinia, Italy): Insights on conditions of formation and parental affinity. Int. J. Earth Sci. 2007, 96, 887–902. [Google Scholar] [CrossRef]
- Abedini, A.; Mongelli, G.; Khosravi, M.; Sinisi, R. Geochemistry and secular trends in the middle–late Permian karst bauxite deposits, northwestern Iran. Ore Geol. Rev. 2020, 124, 103660. [Google Scholar] [CrossRef]
- Abedini, A.; Khosravi, M.; Mongelli, G. The middle Permian pyrophyllite-rich ferruginous bauxite, northwestern Iran, Irano-Himalayan karst belt: Constraints on elemental fractionation and provenance. J. Geochem. Explor. 2022, 233, 106905. [Google Scholar] [CrossRef]
- Ling, K.Y.; Zhu, X.Q.; Tang, H.S.; Li, S.X. Importance of hydrogeological conditions during formation of the karstic bauxite deposits, Central Guizhou Province, Southwest China: A case study at Lindai deposit. Ore Geol. Rev. 2017, 82, 198–216. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhang, Q.; Yang, S.; Liang, Y.; Zhang, Y. Genesis of the Permian karstic Pingguo bauxite deposit, western Guangxi, China. Miner. Depos. 2017, 52, 1031–1048. [Google Scholar] [CrossRef]
- Yu, W.; Algeo, T.J.; Yan, J.; Yang, J.; Du, Y.; Huang, X.; Weng, S. Climatic and hydrologic controls on upper Paleozoic bauxite deposits in South China. Earth-Sci. Rev. 2019, 189, 159–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Li, Y.; Wu, C.; Zheng, C. The “coal-bauxite-iron” structure in the ore-bearing rock series as a prospecting indicator for southeastern Guizhou bauxite mines. Ore Geol. Rev. 2013, 53, 145–158. [Google Scholar] [CrossRef]
- Ling, K.; Wen, H.; Fan, H.; Zhu, X.; Li, Z.; Zhang, Z.; Grasby, S.E. Iron loss during continental weathering in the early Carboniferous period recorded by karst bauxites. JGR Earth Surf. 2023, 128, e2022JF006906. [Google Scholar] [CrossRef]
- Mondillo, N.; Di Nuzzo, M.; Kalaitzidis, S.; Boni, M.; Santoro, L.; Balassone, G. Petrographic and geochemical features of the B3 bauxite horizon (Cenomanian–Turonian) in the Parnassos-Ghiona area: A contribution towards the genesis of the Greek karst bauxites. Ore Geol. Rev. 2022, 143, 104759. [Google Scholar] [CrossRef]
- Öztürk, H.; Hanilçi, N.; Cansu, Z.; Kasapci, C. Formation of Ti-rich bauxite from alkali basalt in continental margin carbonates, Payas region, SE Turkey: Implications for sea level change in the upper cretaceous. Turk. J. Earth Sci. 2021, 30, 116–141. [Google Scholar] [CrossRef]
- Yang, S.; Qingfei, W.; Xuefei, L.; Kan, Z.; Santosh, M.; Deng, J. Global spatio-temporal variations and metallogenic diversity of karst bauxites and their tectonic, paleogeographic and paleoclimatic relationship with the Tethyan realm evolution. Earth Sci. Rev. 2022, 233, 104184. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, Q.F.; Deng, J.; Wang, Y.Z.; Kang, W.; Liu, X.F.; Li, Z.M. Genesis of karst bauxite-bearing sequences in Baofeng, Henan (China), and the distribution of critical metals. Ore Geol. Rev. 2019, 115, 103–161. [Google Scholar] [CrossRef]
- Sinisi, R. Mineralogical and geochemical features of Cretaceous bauxite from San Giovanni Rotondo (Apulia, Southern Italy): A provenance tool. Minerals 2018, 8, 567. [Google Scholar] [CrossRef]
- Reinhardt, N.; Proenza, J.; Villanova-de-Benavent, C.; Aiglsperger, T.; Bover-Arnal, T.; Torró, L. Geochemistry and mineralogy of rare earth elements (REE) in bauxitic ores of the Catalan Coastal Range, NE Spain. Minerals 2018, 8, 562. [Google Scholar] [CrossRef]
- Torró, L.; Proenza, J.A.; Aiglsperger, T.; Bover-Arnal, T.; Villanova-de-Benavent, C.; Rodrigez, D.; Ramírez, A.; Rodrigez, J.; Mosquea, L.A.; Salas, R. Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114–131. [Google Scholar] [CrossRef]
- Radusinović, S.; Jelenković, R.; Pačevski, A.; Simić, V.; Božović, D.; Holclajtner-Antunović, I.; Životić, D. Content and mode of occurrences of rare earth elements in the Zagrad karstic bauxite deposit (Nikšić area, Montenegro). Ore Geol. Rev. 2017, 80, 406–428. [Google Scholar] [CrossRef]
- Luo, C.; Yang, R.; Chen, J.; Gao, L.; Zu, H.; Ni, X. Genesis of the Carboniferous karstic bauxites in Qingzhen region, Central Guizhou, Southwest China. J. Geochem. Explor. 2022, 235, 106955. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhao, L.; Peng, Y.; Ma, Y.; Zhou, Z. Metallogeny of the large-scale Carboniferous karstic bauxite in the Sanmenxia area, southern part of the North China Craton China. Chem. Geol. 2020, 556, 119851. [Google Scholar] [CrossRef]
- Mordberg, L.E. Geochemical evolution of a Devonian diaspore-crandallite-svanbergite-bearing weathering profile in the Middle Timan, Russia. J. Geochem. Explor. 1999, 66, 35–361. [Google Scholar] [CrossRef]
- Yuste, A.; Bauluz, B.; Mayayo, M.J. Genesis and mineral transformations in lower cretaceous karst bauxites (NE Spain): Climatic influence and superimposed processes. Geol. J. 2015, 50, 839–857. [Google Scholar] [CrossRef]
- Yuste, A.; Bauluz, B.; Mayayo, M.J. Origin and geochemical evolution from ferrallitized clays to karst bauxite: An example from the Lower Cretaceous of NE Spain. Ore Geol. Rev. 2017, 84, 67–79. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The rare earth elements potential of Greek bauxite active mines in the light of a sustainable REE demand. J. Sustain. Metall. 2019, 5, 20–47. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Kasama, T.; Church, N.S.; Douvalis, A.P.; Göttlicher, J.; Steininger, R.; Boubnov, A.; Pontikes, Y.; Tzamos, E.; et al. Nano-mineralogy and -geochemistry of high-grade diasporic karst-type bauxite from Parnassos-Ghiona mines Greece. Ore Geol. Rev. 2017, 84, 228–244. [Google Scholar] [CrossRef]
- Karadağ, M.M.; Küpeli, S.; Arýk, F.; Ayhan, A.; Zedef, V.; Döyen, A. Rare earth element (REE) geochemistry and genetic implications of the Mortas bauxite deposit (Seydi¸sehir/Konya-Southern Turkey). Chem. Erde Geochem. 2009, 69, 143–159. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Du, Y.; Ling, W.; Wu, L.; Cui, T.; Zhou, Q.; Jin, Z.; Lei, Z.; Weng, S. REE mobility and Ce anomaly in bauxite deposit of WZD area, Northern Guizhou, China. J. Geochem. Explor. 2013, 133, 103–117. [Google Scholar] [CrossRef]
- Hanilçi, N. Geological and geochemical evolution of the Bolkardagi bauxite deposits, Karaman, Turkey: Transformation from shale to bauxite. J. Geochem. Explor. 2013, 133, 118–137. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhang, Q.; Zhang, Y.; Li, Y. Genesis of REE minerals in the karstic bauxite in western Guangxi, China, and its constraints on the deposit formation conditions. Ore Geol. Rev. 2016, 75, 100–115. [Google Scholar] [CrossRef]
- Mongelli, G. Ce-anomalies in the textural components of Upper Cretaceous karst bauxites from the Apulian carbonate platform (southern Italy). Chem. Geol. 1997, 140, 69–79. [Google Scholar] [CrossRef]
- Gu, J.; Huang, Z.; Fan, H.; Jin, Z.; Yan, Z.; Zhang, J. Mineralogy, geochemistry, and genesis of lateritic bauxite deposits in the Wuchuan–Zheng’an–Daozhen area, Northern Guizhou Province, China. J. Geochem. Explor. 2013, 130, 44–59. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Su, H.M.; Jiang, S.Y. Mineralogical control and characteristics of rare earth elements occurrence in Carboniferous bauxites from western Henan Province, north China: A XRD, SEM-EDS and LA-ICP-MS analysis. Ore Geol. Rev. 2019, 114, 103144. [Google Scholar] [CrossRef]
- Mongelli, G.; Boni, M.; Buccione, R.; Sinisi, R. Geochemistry of the Apulian karst bauxites (southern Italy): Chemical fractionation and parental affinities. Ore Geol. Rev. 2014, 63, 9–21. [Google Scholar] [CrossRef]
- Mongelli, G.; Buccione, R.; Gueguen, R.; Langone, A.; Sinisi, R. Geochemistry of the Apulian allochthonous karst bauxite, Southern Italy: Distribution of critical elements and constraints on Late Cretaceous Peri-Tethyan palaeogeography. Ore Geol. Rev. 2016, 77, 246–259. [Google Scholar] [CrossRef]
- Putzolu, F.; Piccolo Papa, A.; Mondillo, N.; Boni, M.; Balassone, G.; Mormone, A. Geochemical characterization of bauxite deposits from the Abruzzi Mining District (Italy). Minerals 2018, 8, 298. [Google Scholar] [CrossRef]
- Lei, Z.; Ling, W.; Wu, H.; Zhang, Y.; Zhang, Y. Geochemistry and mineralization of the Permian bauxites with contrast bedrocks in northern Guizhou, South China. J. Earth Sci. 2023, 34, 487503. [Google Scholar] [CrossRef]
- Mongelli, G.; Boni, M.; Oggiano, G.; Mameli, P.; Sinisi, R.; Buccione, R.; Mondillo, N. Critical metals distribution in Tethyan karst bauxite: The cretaceous Italian ores. Ore Geol. Rev. 2017, 86, 526–536. [Google Scholar] [CrossRef]
- Mongelli, G.; Mameli, P.; Sinisi, R.; Roberto, B.; Oggiano, G. REEs and other critical raw materials in Cretaceous Mediterranean-type bauxite: The case of the Sardinian ore (Italy). Ore Geol. Rev. 2021, 139, 104559. [Google Scholar] [CrossRef]
- Radusinović, S.; Papadopoulos, A. The potential for REE and associated critical metals in karstic bauxites and bauxite residue of Montenegro. Minerals 2021, 11, 975. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Zamanian, H.; Taghipour, B.; Zarasvandi, A.; Buccione, R.; Ellahi, S.S. Mineralogical and geochemical evolution of the Bidgol bauxite deposit, Zagros Mountain Belt, Iran: Implications for ore genesis, rare earth elements fractionation and parental affinity. Ore Geol. Rev. 2017, 86, 755–783. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Mongelli, G. Geology, geochemistry, and genesis of REY minerals of the late Cretaceous karst bauxite deposits, Zagros Simply Folded Belt, SW Iran: Constraints on the ore-forming process. J. Geochem. Explor. 2022, 240, 107030. [Google Scholar] [CrossRef]
- Abedini, A.; Rezaei Azizi, M.; Dill, H.G. Formation mechanisms of lanthanide tetrad effect in limestones: An example from Arbanos district, NW Iran. Carbonates Evaporites 2020, 35, 1–18. [Google Scholar] [CrossRef]
- Abedini, A.; Khosravi, M. Geochemical constraints on the Triassic–Jurassic Amir-Abad karst-type bauxite deposit, NW Iran. J. Geochem. Explor. 2020, 211, 106489. [Google Scholar] [CrossRef]
- Abedini, A.; Khosravi, M. REE geochemical characteristics of the Huri karst-type bauxite Deposit, Irano–Himalayan Belt, Northwestern Iran. Minerals 2023, 13, 926. [Google Scholar] [CrossRef]
- Abedini, A.; Mongelli, G.; Khosravi, M. Geochemical constraints on the middle Triassic Kani Zarrineh karst bauxite deposit, Irano–Himalayan belt, NW Iran: Implications for elemental fractionation and parental affinity. Ore Geol. Rev. 2021, 133, 104099. [Google Scholar] [CrossRef]
- Abedini, A.; Mongelli, G.; Khosravi, M. Geochemistry of the early Jurassic Soleiman Kandi karst bauxite deposit, Irano–Himalayan belt, NW Iran: Constraints on bauxite genesis and the distribution of critical raw materials. J. Geochem. Explor. 2022, 241, 107056. [Google Scholar] [CrossRef]
- Abedini, A.; Khosravi, M.; Mongelli, G. Critical metals distribution in the late Triassic–early Jurassic Nasr-Abad bauxite deposit, Irano–Himalayan karst bauxite belt, NW Iran. Geochemistry 2024, 84, 126039. [Google Scholar] [CrossRef]
- Khosravi, M.; Abedini, A.; Alipour, S.; Mongelli, G. The Darzi-Vali bauxite deposit, West-Azarbaidjan Province, Iran: Critical metals distribution and parental affinities. J. Afr. Earth Sci. 2017, 129, 960–972. [Google Scholar] [CrossRef]
- Khosravi, M.; Vérard, C.; Abedini, A. Palaeogeographic and geodynamic control on the Iranian karst-type bauxite deposits. Ore Geol. Rev. 2021, 139, 104589. [Google Scholar] [CrossRef]
- Nabavi, M.H. An Introduction to the Geology of Iran; Geological Survey of Tehran, Iran: Tehran, Iran, 1976; 105p. [Google Scholar]
- Mutakyahwa, M.K.D.; Ikingura, J.R.; Mruma, A.H. Geology and geochemistry of bauxite deposits in Lushoto district, Usambara Mountains, Tanzania. J. Afr. Earth Sci. 2003, 36, 357–369. [Google Scholar] [CrossRef]
- Valeton, I. Bauxites; Elsevier Scientific: Amsterdam, The Netherlands, 1972; 226p. [Google Scholar]
- Li, Z.; Din, J.; Xu, J.; Liao, C.; Yin, F.; Lu, T.; Cheng, L.; Li, J. Discovery of the REE minerals in the Wulong–Nanchuan bauxite deposits, Chongqing, China: Insights on conditions of formation and processes. J. Geochem. Explor. 2013, 133, 88–102. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Liu, X.; Zhao, L.; Lin, L.; Zhang, Q.; Feng, Y.; Wang, W. Metallogenic process of Permian Taiping karstic bauxite deposit in Youjiang Basin, China. Ore Geol. Rev. 2022, 152, 105258. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, J.; Liu, X.; Zhang, Q.; Sun, S.; Jiang, C.; Zhou, F. Discovery of the REE minerals and its geological significance in the Quyang bauxite deposit, West Guangxi, China. J. Asian Earth Sci. 2010, 39, 701–712. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Norton, S.A. Laterite and bauxite formation. Econ. Geol. 1973, 68, 353–361. [Google Scholar] [CrossRef]
- MacLean, W.; Bonavia, F.; Sanna, G. Argillite debris converted to bauxite during karst weathering: Evidence from immobile element geochemistry at the Olmedo Deposit, Sardinia. Miner. Depos. 1997, 32, 607–616. [Google Scholar] [CrossRef]
- Mondillo, N.; Balassone, G.; Boni, M.; Rollinson, G. Karst bauxites in the Campania Apennines (southern Italy): A new approach. Period. Mineral. 2011, 80, 407–432. [Google Scholar]
- Schellmann, W. Geochemical differentiation in laterite and bauxite formation. Catena 1994, 21, 131–143. [Google Scholar] [CrossRef]
- Ajouyed, O.; Hurela, C.; Ammarib, M.; Ben Allalb, L.; Marmier, N. Sorption of Cr(VI) onto natural iron and aluminum (oxy)hydroxides: Effects of pH, ionic strength and initial concentration. J. Hazard. Mater. 2010, 174, 616–622. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.; Cantano, M. Intensive kaolinization during a lateritic weathering event in southwest Spain mineralogical and geochemical inferences from a relict paleosol. Catena 2010, 80, 23–33. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere–speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Jeon, B.; Dempsey, B.A.; Burgos, W.D.; Royer, R.A. Sorption kinetics of Fe(II), Zn (II), Co(II), Ni(II), Cd(II), and Fe(II)/Me(II) onto hematite. Water Res. 2003, 37, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Ramos de Oliveira, L.A.; Rosiere, C.A.; Rios, F.J.; Andrade, S.; de Moraes, R. Chemical fingerprint of iron oxides related to iron enrichment of banded iron formation from the Cauê Formation-Esperança Deposit, Quadrilátero Ferrífero, Brazil: A laser ablation ICP-MS study. Braz. J. Geol. 2015, 45, 193–216. [Google Scholar] [CrossRef]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Boni, M.; Herrington, R. Trace element geochemistry of iron-(oxy)-hydroxides in Ni (Co)-laterites: Review, new data and implications for ore forming processes. Ore Geol. Rev. 2022, 140, 104501. [Google Scholar] [CrossRef]
- Schwertmann, U.; Pfab, G. Structural vanadium in synthetic goethite. Geochim. Cosmochim. Acta 1994, 58, 4349–4352. [Google Scholar] [CrossRef]
- Atun, G.; Bascetin, E. Adsorption of barium on kaolinite, illite and montmorillonite at various ionic strengths. Radiochim. Acta 2003, 91, 223–228. [Google Scholar] [CrossRef]
- Keceli, G. Adsorption kinetics and equilibrium of strontium onto kaolinite. Sep. Sci.Technol. 2015, 50, 72–80. [Google Scholar] [CrossRef]
- Mahoney, J.; Langmuir, D. Adsorption of Sr on kaolinite, illite and montmorillonite at high ionic strengths. Radiochim. Acta 1991, 54, 139–144. [Google Scholar] [CrossRef]
- Taylor, Y.; McLennan, S.M. The Continental Crust: Its Composition and Evolution, 1st ed.; Blackwell: Oxford, UK, 1985. [Google Scholar]
- Braun, J.J.; Pagel, M.; Muller, J.P.; Bilong, P.; Michard, A.; Guillet, B. Cerium anomalies in lateritic profiles. Geochim. Cosmochim. Acta 1990, 54, 781–795. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.F.; Williams-Jones, A.E. Controls on the dynamics of rare earth elements during subtropical hillslope processes and formation of regolith-hosted deposits. Econ. Geol. 2020, 115, 1097–1118. [Google Scholar] [CrossRef]
- Ma, J.; Wei, G.; Xu, Y.; Long, W.; Sun, W. Mobilization and re-distribution of major and trace elements during extreme weathering of basalt in Hainan Island, South China. Geochim. Cosmochim. Acta 2017, 71, 3223–3237. [Google Scholar] [CrossRef]
- Sanematsu, K.; Kon, Y.; Imai, A.; Watanabe, K.; Watanabe, Y. Geochemical and mineralogical characteristics of ion-adsorption type REE mineralization in Phuket, Thailand. Miner. Depos. 2013, 48, 437–451. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Stetzenbach, J.K.; Hodge, V.F. Speciation of the rare earth element neodymium in groundwaters of the Nevada Test Site and Yucca Mountain and implications for actinide solubility. Appl. Geochem. 1995, 10, 565–572. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Stetzenbach, J.K.; Hodge, V.F.; Lyons, W.B. Rare earth element complexation behavior in circumneutral pH groundwaters: Assessing the role of carbonate and phosphate ions. Earth Planet. Sci. Lett. 1996, 139, 305–319. [Google Scholar] [CrossRef]
- Patino, L.C.; Velbel, M.A.; Price, J.R.; Wade, J.A. Trace element mobility during spheroidal weathering of basalts and andesites in Hawaii and Guatemala. Chem. Geol. 2003, 202, 343–364. [Google Scholar] [CrossRef]
- Nesbit, H.W. Mobility and fractionation of rare earth elements during weathering of a granodiorite. Nature 1979, 279, 206–210. [Google Scholar] [CrossRef]
- Crnički, J.; Jurković, I. Rare earth elements in Triassic bauxites of Croatia Yugoslavia. Travaux 1990, 19, 239–248. [Google Scholar]
- Shaw, D.M. Interprétation Géochemique des Éléments en Traces dans les Roches Cristallines; Masson et Cie: Paris, France, 1964. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry, 2nd ed.; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–64. [Google Scholar]
- Özlü, N. Trace element contents of karst bauxites and their parent rocks in the Mediterranean belt. Miner. Depos. 1983, 18, 469–476. [Google Scholar] [CrossRef]
- Schroll, E.; Sauer, D. Beitrag zur Geochemie von Titan, Chrom, Nikel, Cobalt, Vanadium und Molibdan in Bauxitischen gestermen und problem der stofflichen herkunft des Aluminiums. Trav. ICSOBA 1968, 5, 83–96. [Google Scholar]
- Abedini, A.; Calagari, A.A. The mineralogy and geochemistry of Permian lateritic ores in east of Shahindezh, West-Azarbaidjan province. Iran. J. Crystallogr. Mineral. 2012, 20, 59–72. [Google Scholar]




| Kj-01 | Kj-02 | Kj-03 | Kj-04 | Kj-05 | Kj-06 | Kj-07 | Kj-08 | Kj-09 | Kj-10 | Kj-11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematite | ××××× | ××××× | ×××× | ××××× | ××××× | ××××× | ××××× | ×××× | ×××× | ×××× | ×××× |
| Goethite | ×× | ×× | ×× | × | × | × | ×× | ×× | ×× | × | ×× |
| Kaolinite | ac | ac | ac | ac | ac | ac | × | × | × | × | ac |
| Calcite | ac | ac | ac | ac | |||||||
| Pyrolusite | ac | ac | ac | ac | ac | ac | |||||
| Anatase | ac | ac | |||||||||
| Rutile | ac | ac | ac | ac | ac | ||||||
| Crandallite | ac | ac | ac | ac | ac | ||||||
| Illite | ac | ac | |||||||||
| Amesite | ac | ac | ac | ac | |||||||
| Boehmite | ac | ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedini, A.; Khosravi, M. Geochemical Characteristics of Aluminum-Bearing Iron Ores: A Case Study from the Kolijan Karst-Type Bauxite Deposit, Northwestern Iran. Minerals 2024, 14, 151. https://doi.org/10.3390/min14020151
Abedini A, Khosravi M. Geochemical Characteristics of Aluminum-Bearing Iron Ores: A Case Study from the Kolijan Karst-Type Bauxite Deposit, Northwestern Iran. Minerals. 2024; 14(2):151. https://doi.org/10.3390/min14020151
Chicago/Turabian StyleAbedini, Ali, and Maryam Khosravi. 2024. "Geochemical Characteristics of Aluminum-Bearing Iron Ores: A Case Study from the Kolijan Karst-Type Bauxite Deposit, Northwestern Iran" Minerals 14, no. 2: 151. https://doi.org/10.3390/min14020151
APA StyleAbedini, A., & Khosravi, M. (2024). Geochemical Characteristics of Aluminum-Bearing Iron Ores: A Case Study from the Kolijan Karst-Type Bauxite Deposit, Northwestern Iran. Minerals, 14(2), 151. https://doi.org/10.3390/min14020151







