Distribution, Occurrence and Enrichment Causes of Sodium in Middle Jurassic Coal from Zhundong Coalfield, Xinjiang
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Collection and Preparation of Sample
3.2. Analytical Procedures
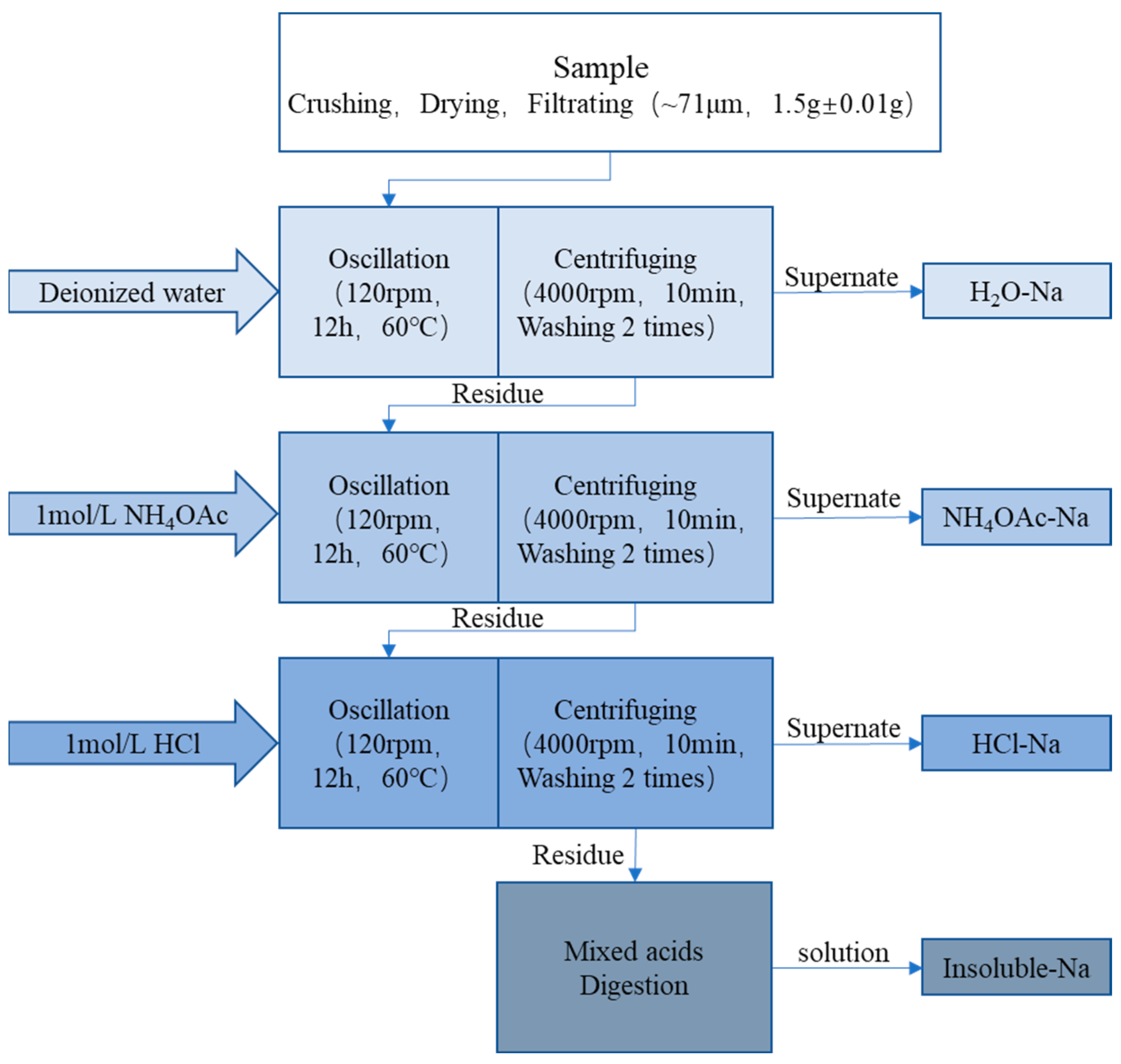
3.3. Sequential Extraction Experiment
4. Results and Analysis
4.1. Proximate and Ultimate Analysis, Total Sulfur
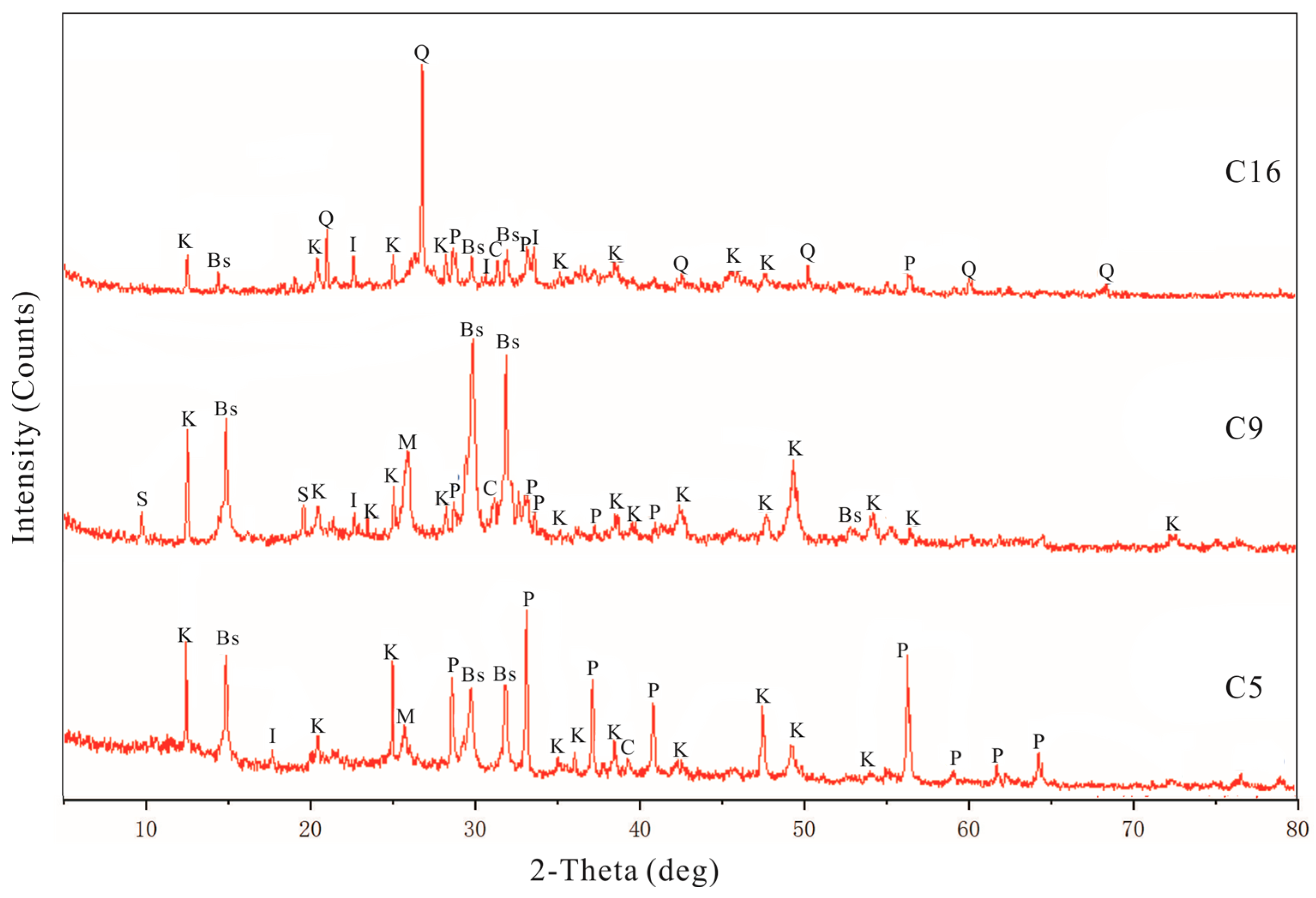
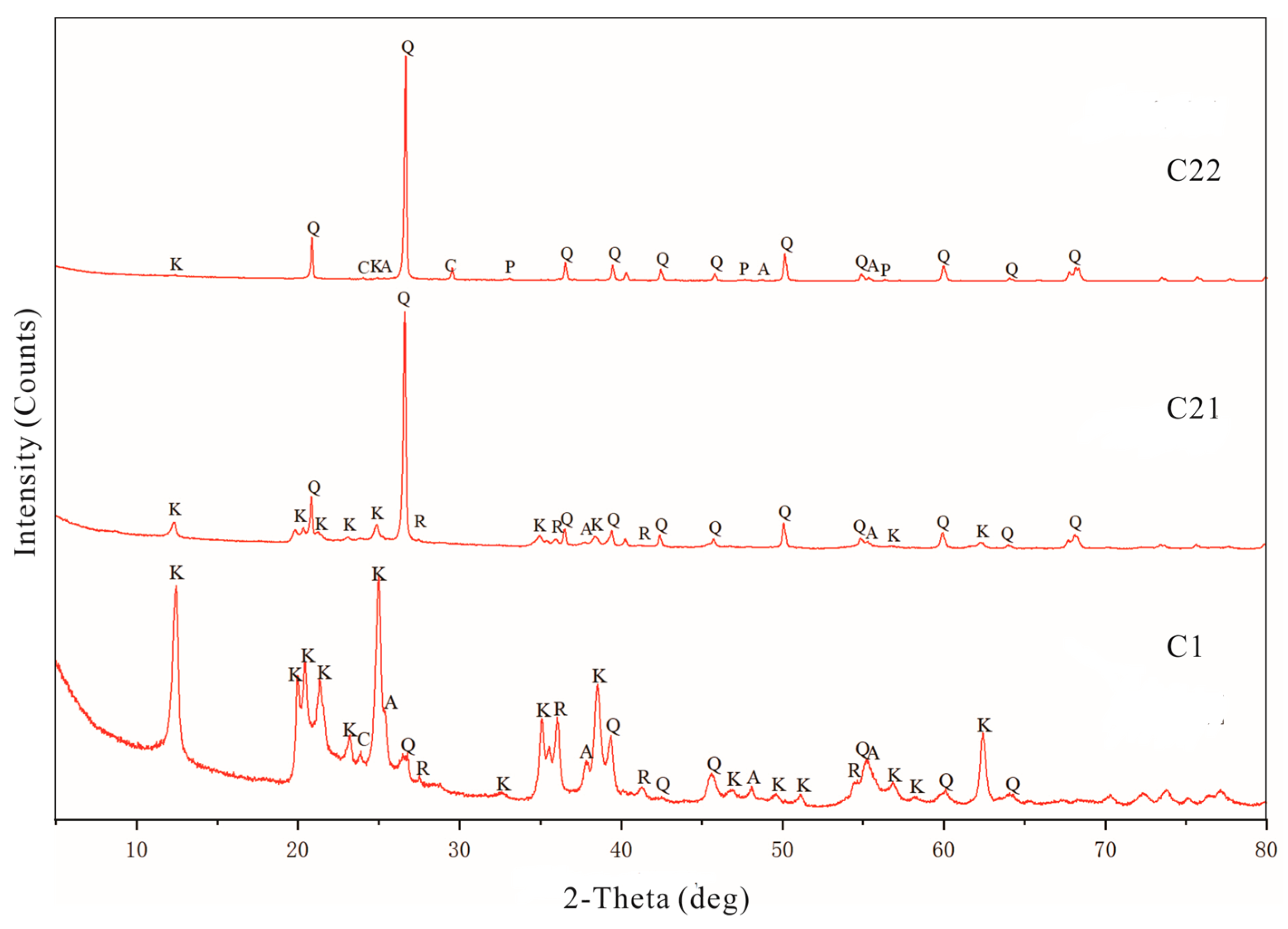
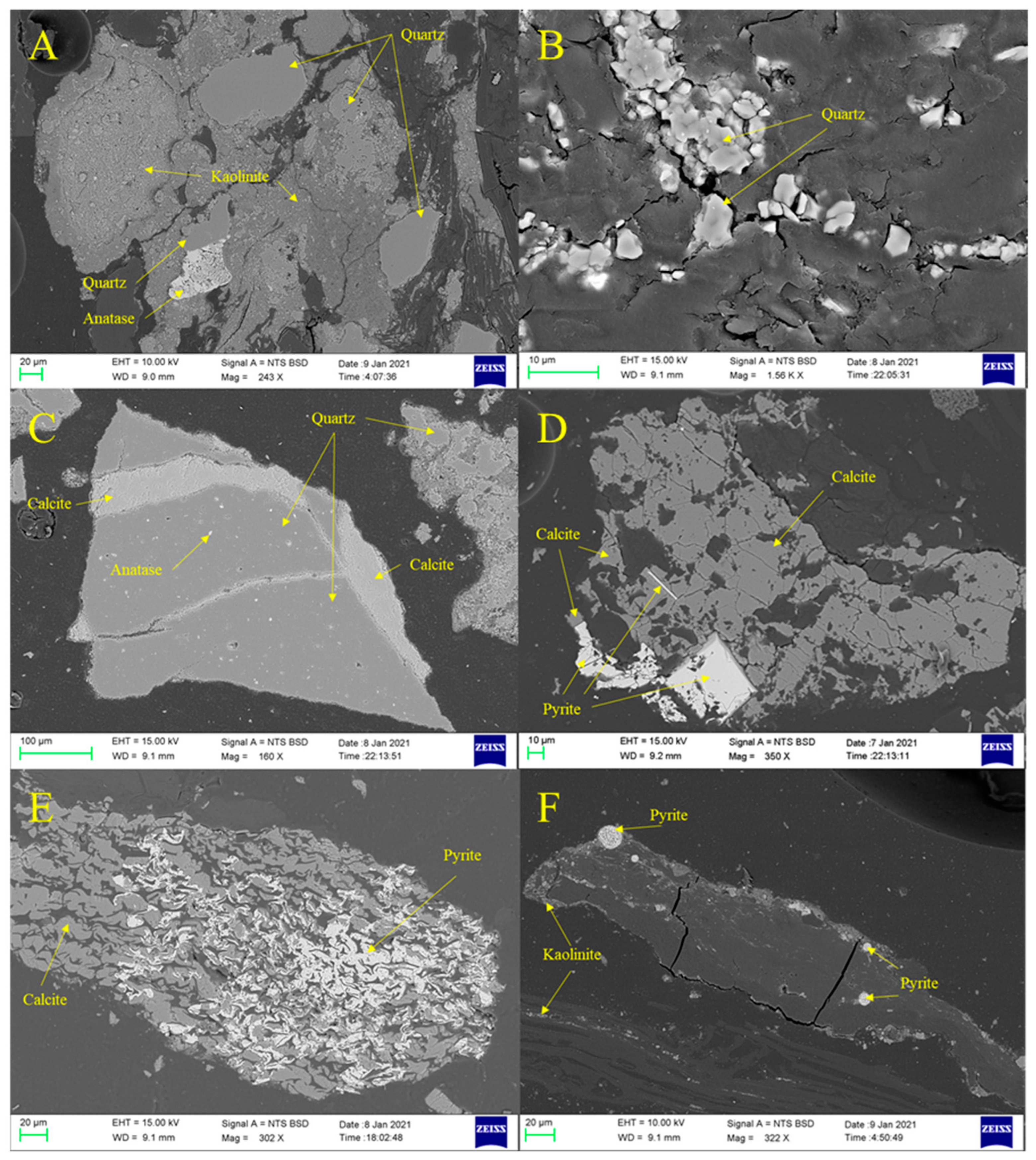
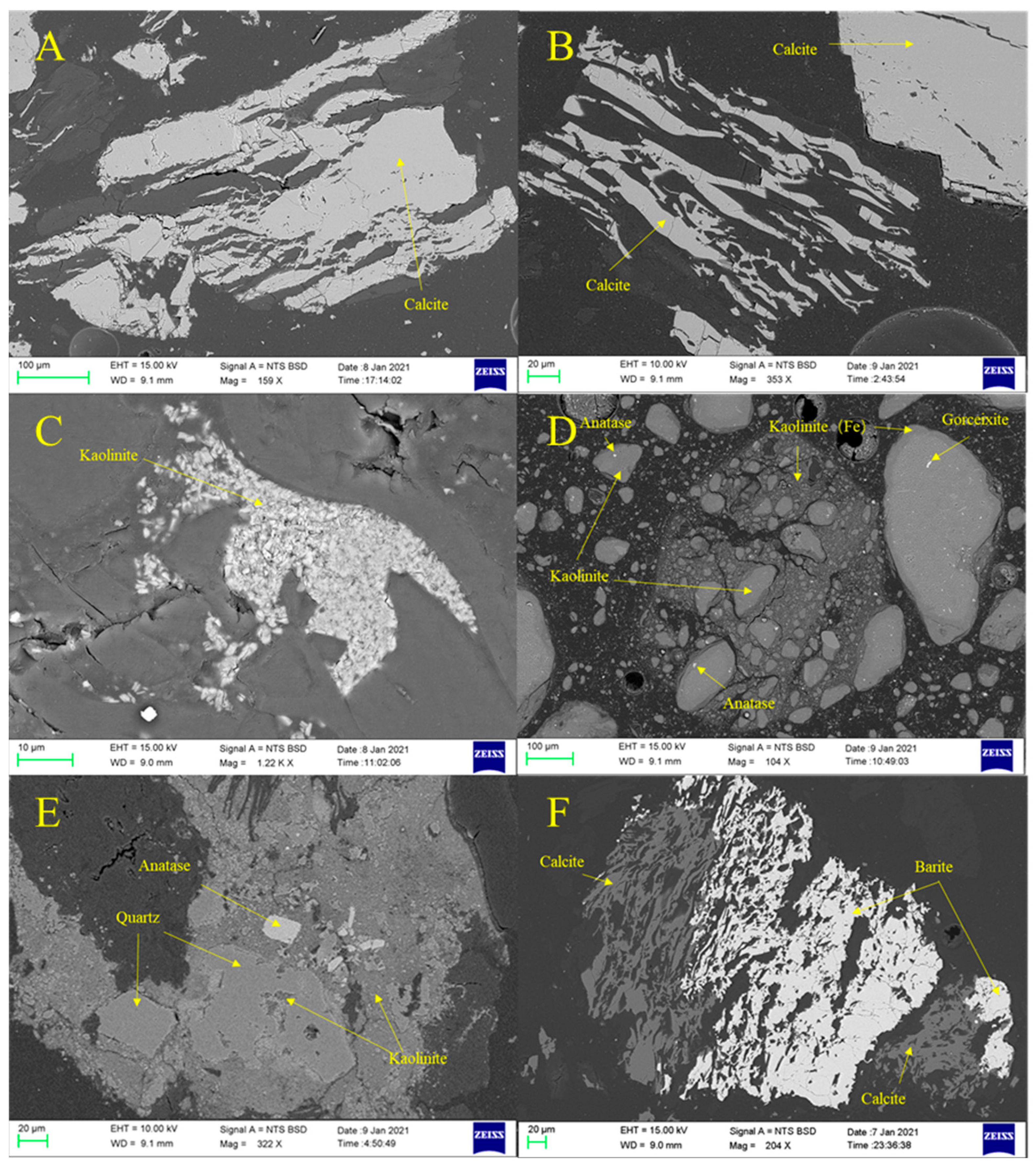
4.2. Mineralogical Characteristics
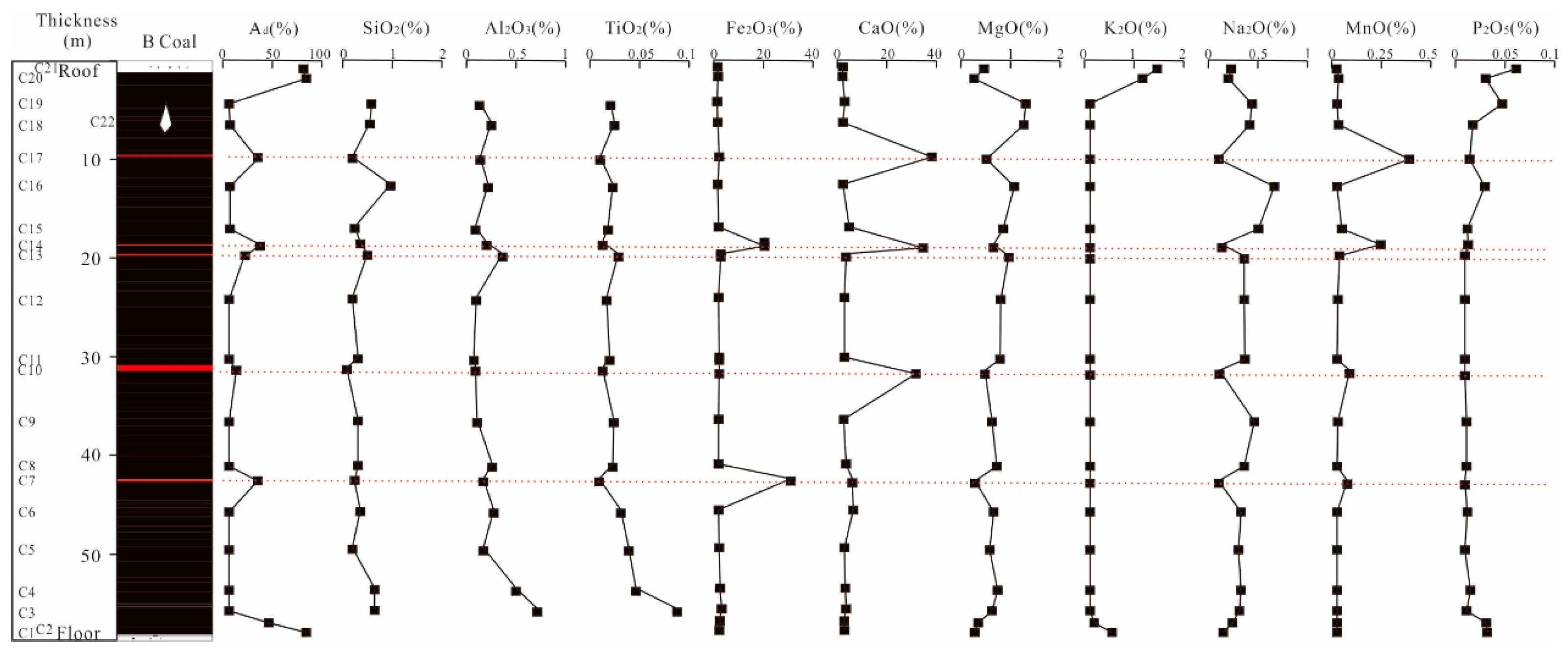
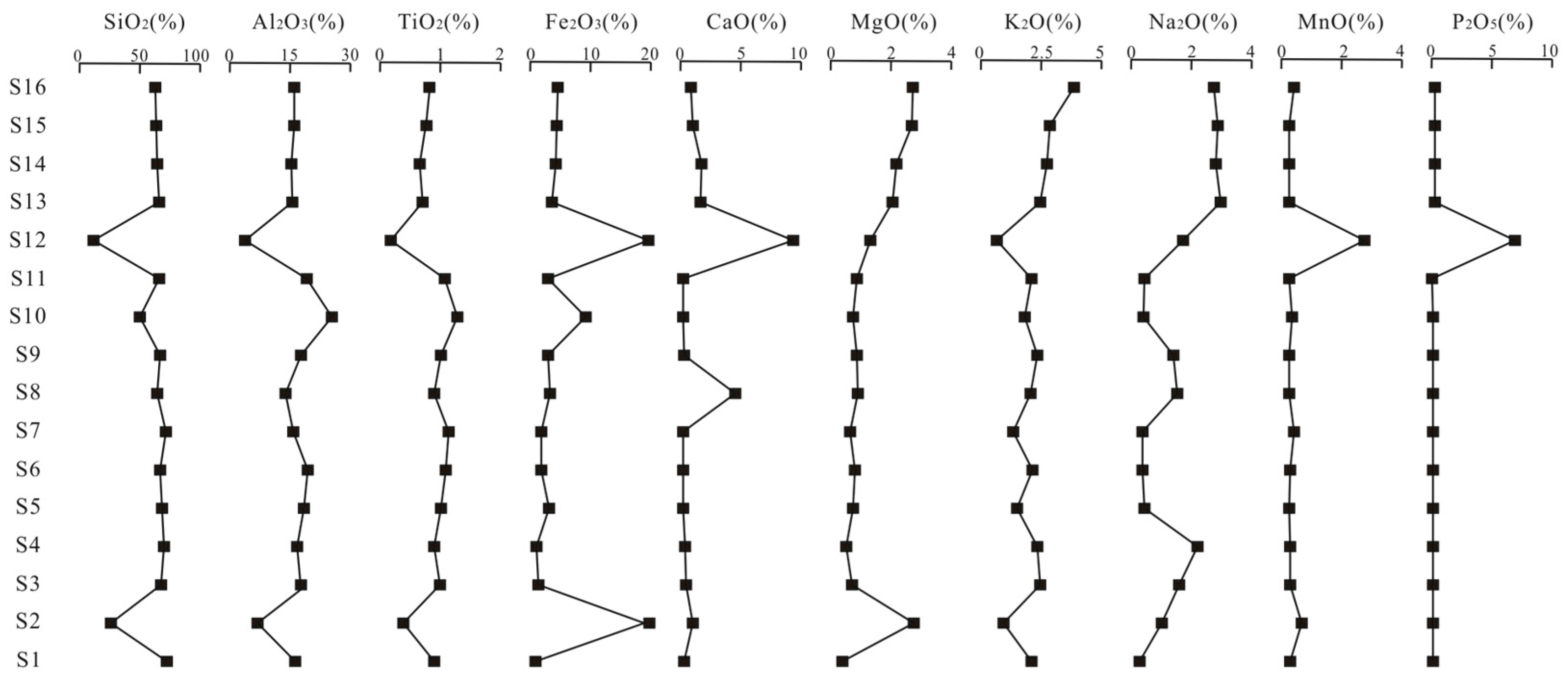
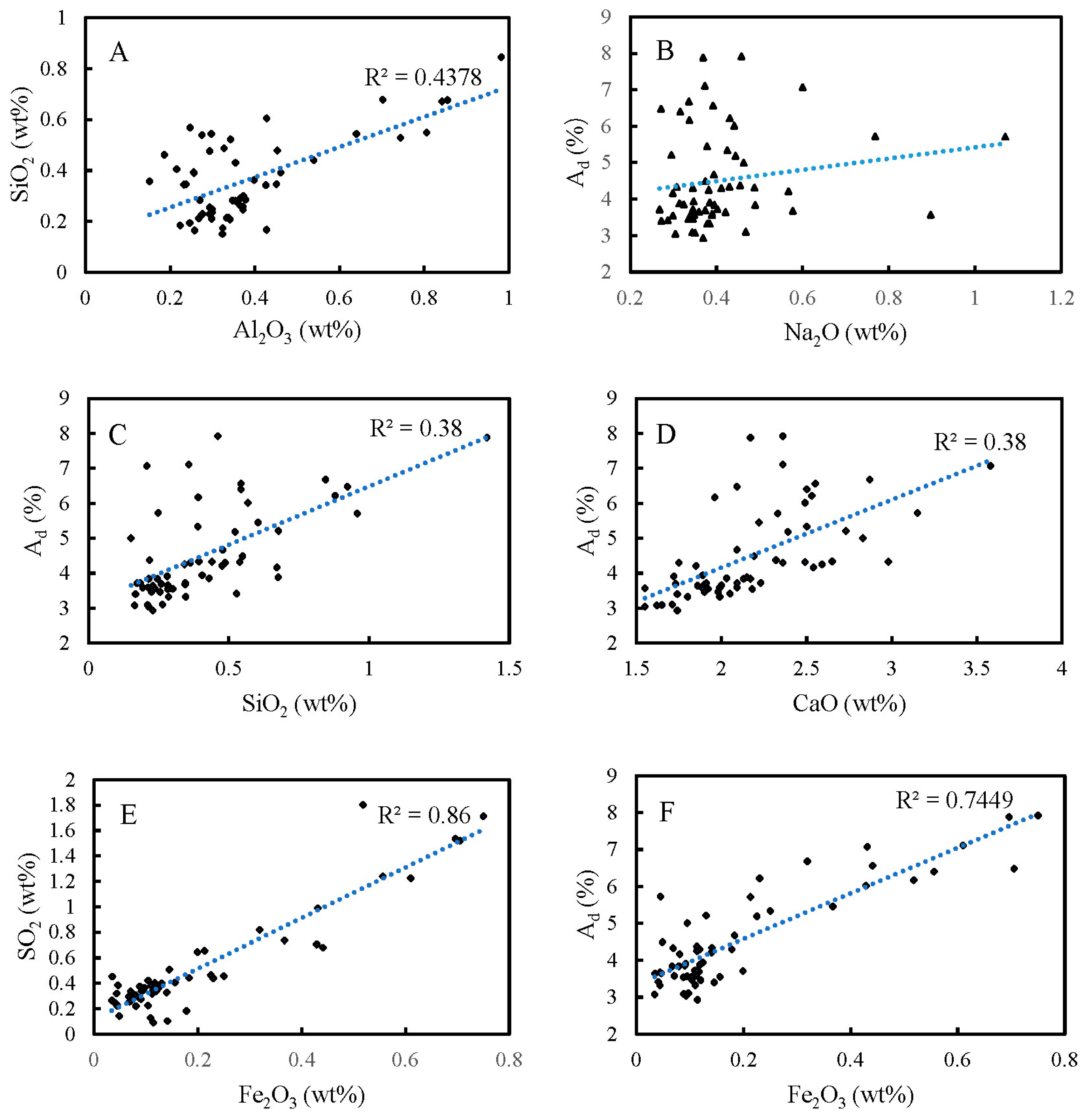
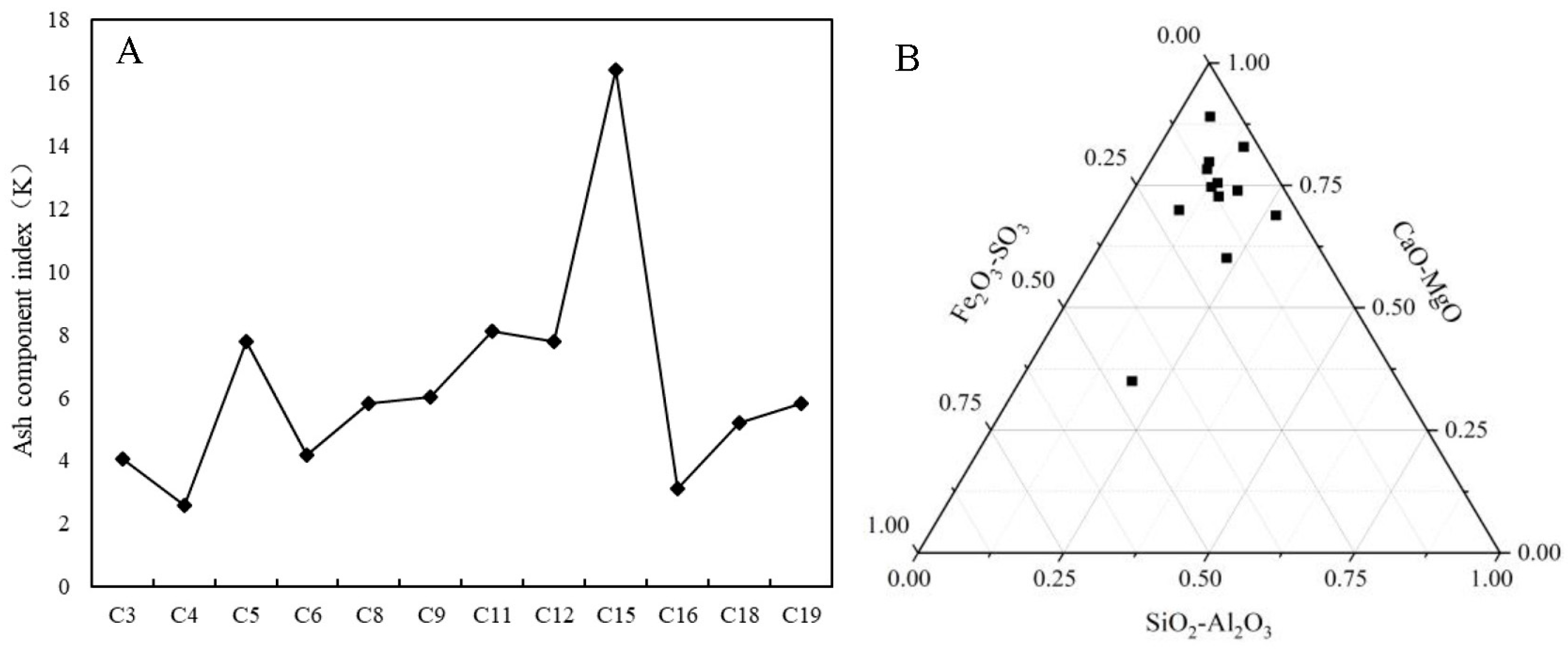
4.3. Analysis of Major Element
4.4. Distribution of Sodium
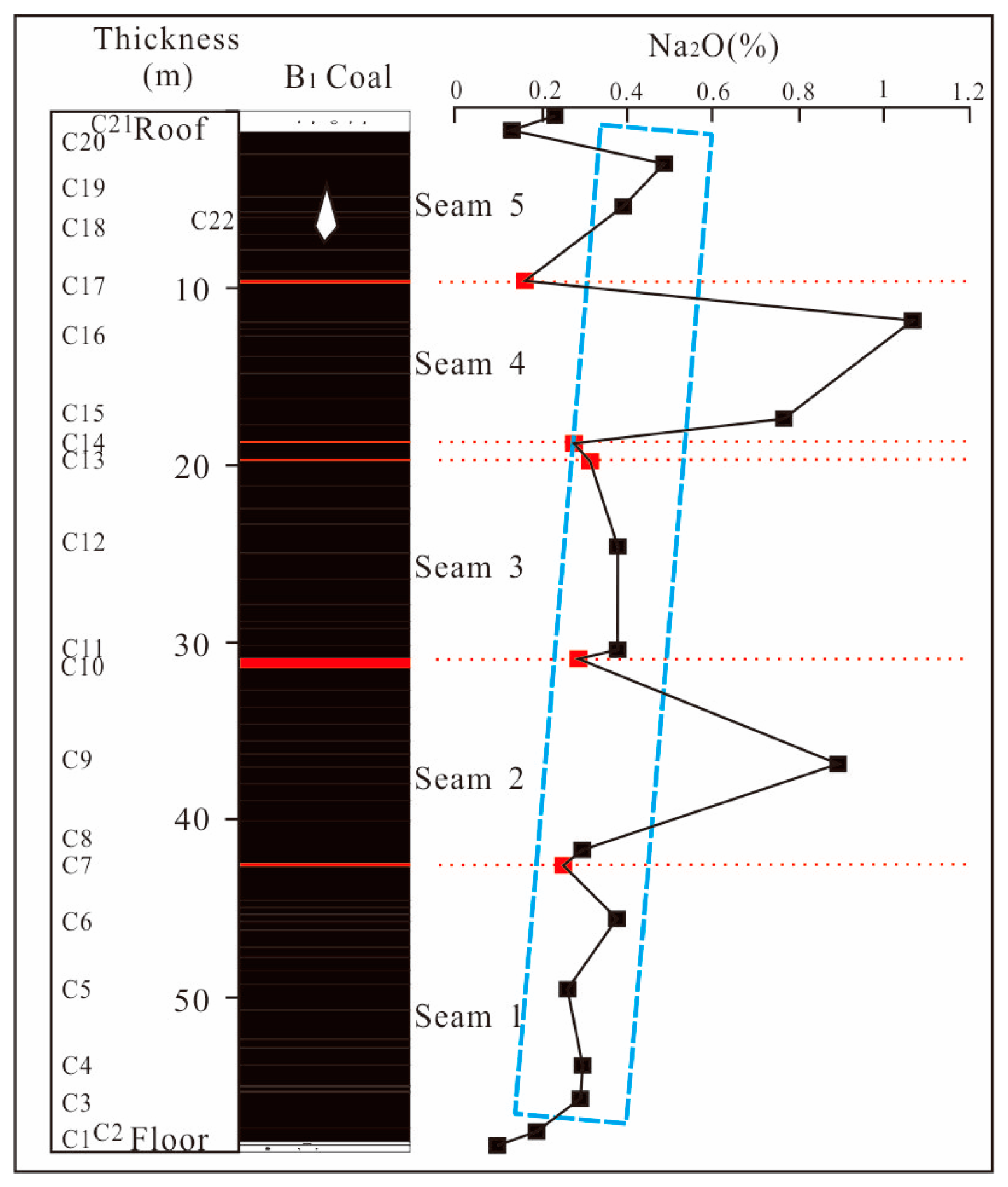
4.4.1. Distribution of Na in Coal Seams
4.4.2. Distributional of Na from Epipedon to Coal Seams
4.5. Occurrence of Sodium
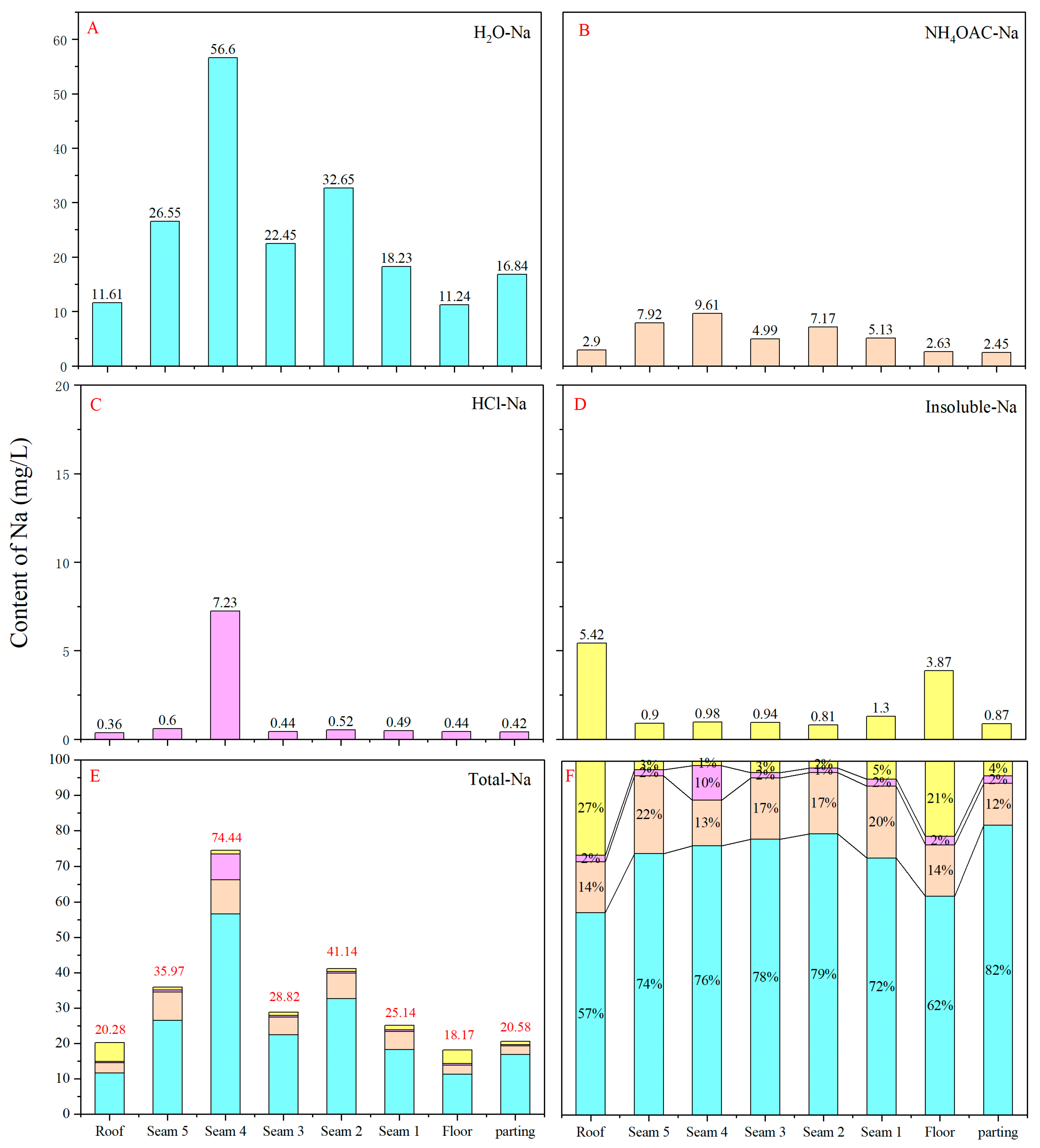
4.5.1. Sequential Extraction Results
4.5.2. Correlation Analysis
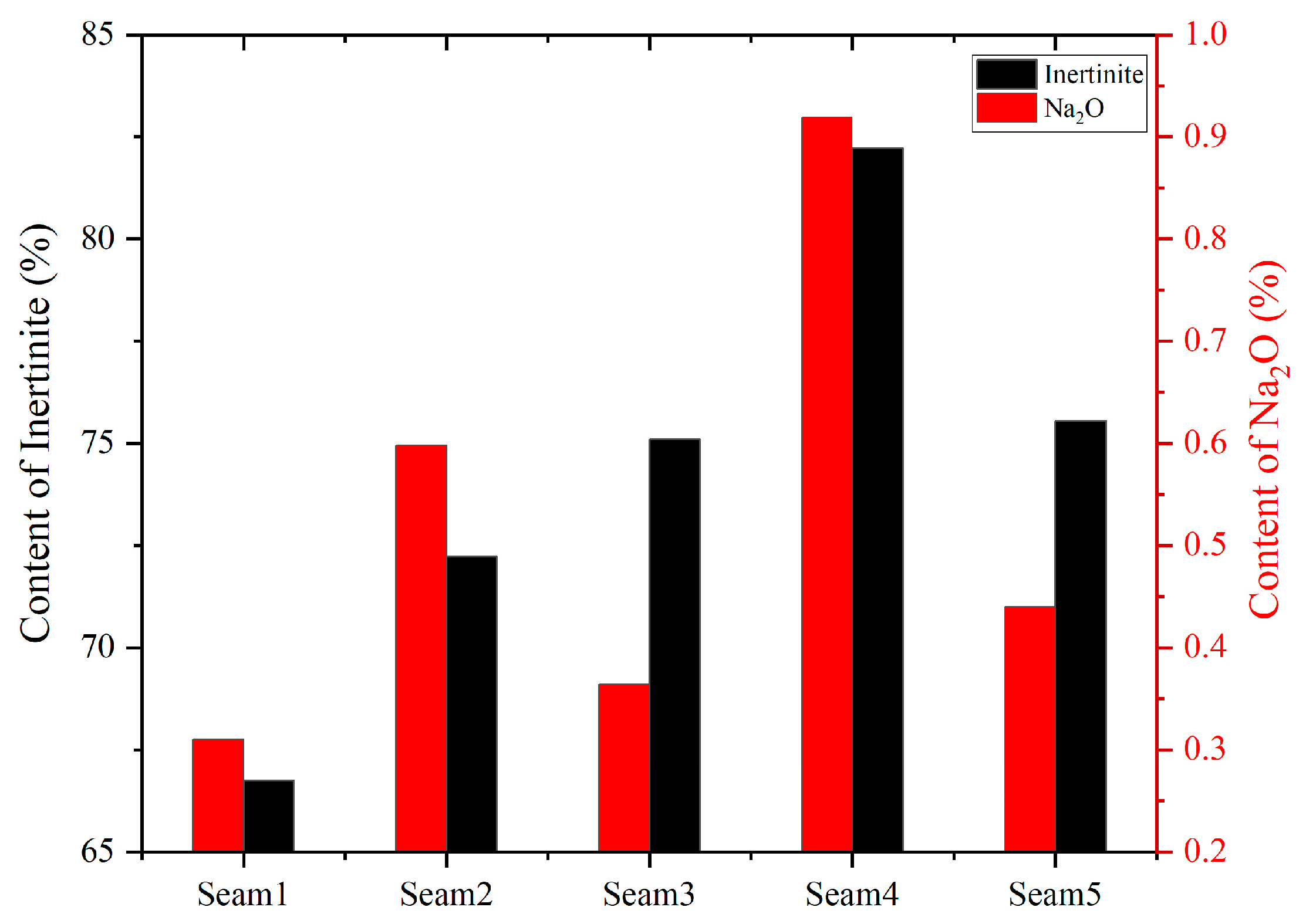
4.5.3. Relationship between Sodium and the Inertinite
5. Discussion
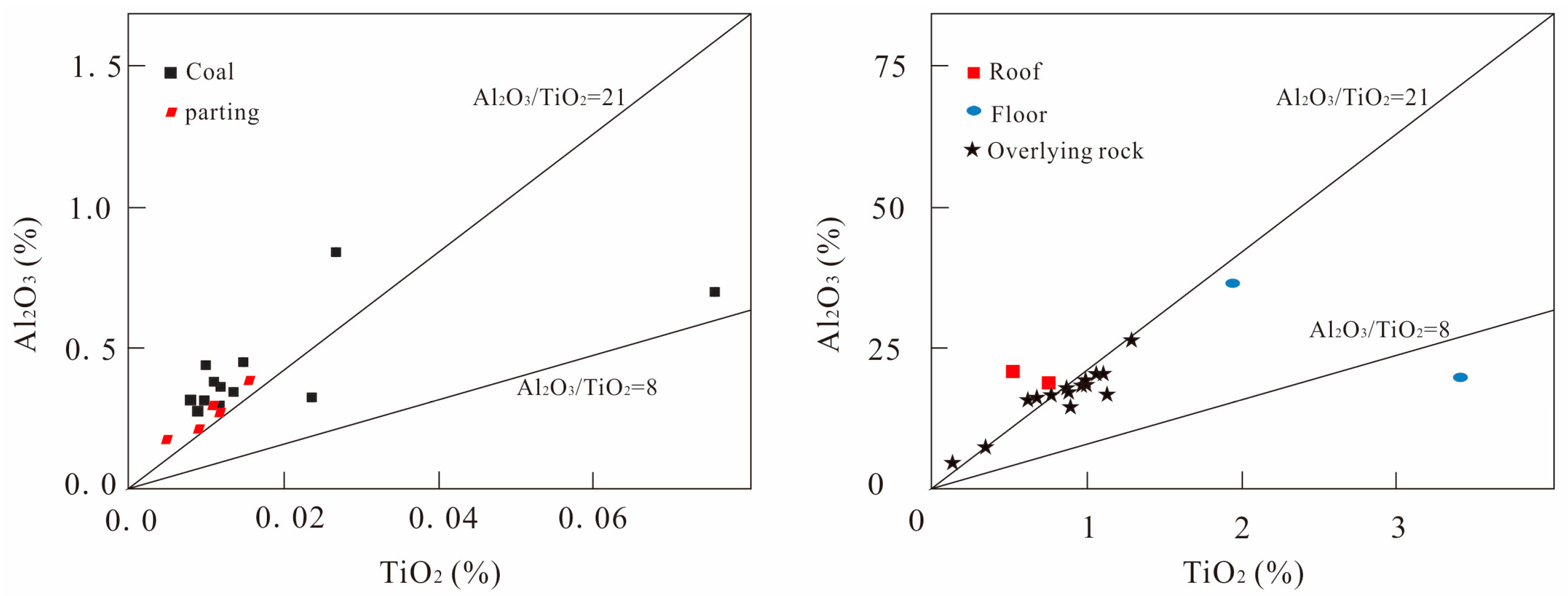
5.1. Sources of Sediments
5.2. Environment of Sedimentation
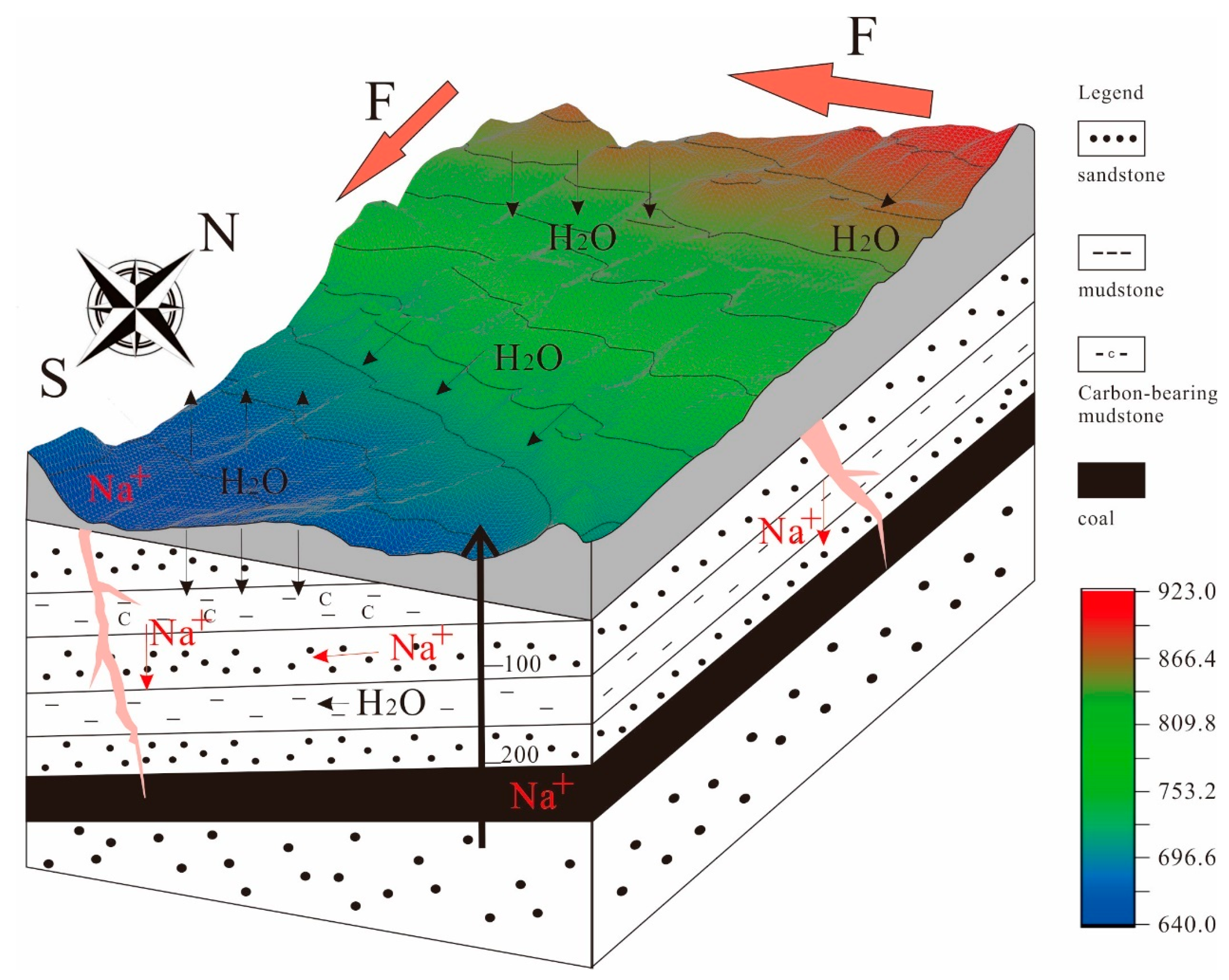
5.3. Hydrological Conditions
5.4. Patterns of Sodium Transport and Enrichment
6. Conclusions
- (1)
- The Yihua coal exhibits relatively high sodium content, with sodium primarily distributed within the coal seams. In descending order, the distribution of sodium within the coal seam samples is as follows: Seam4 > Seam2 > Seam5 > Seam3 > Seam1 > parting > roof > floor. Overall, the sodium content tends to decrease with the increasing depth of the coal seams. Additionally, the coal seam samples exhibit a characteristic where the sodium content is higher in the upper part compared to the lower part of the same coal. In addition, the sodium content in the epipedon, overlying rock stratums and coal seams also showed a decreasing trend with depth.
- (2)
- The sequential extraction experiment revealed that H2O-Na constitutes the primary component of sodium in the Yihua coal, accounting for over 70% of its composition. NH4OAc-Na in the coal seams is significantly higher than in the roof, floor and gangue. Among the different coal seams, Seam4 exhibits higher HCl-Na content, which is influenced by the sodium on organic functional groups in the coal structure. Insoluble Na, on the other hand, is relatively higher in the roof and floor. Although the sodium content in some seams is affected by sodium on organic functional groups in the coal structure or in minerals, the dominant forms of sodium in coal are ionic and hydrated ionic states. The primary mineral content in coal is minimal, mainly consisting of postgenetic minerals such as kaolinite, calcite and gypsum. Therefore, minerals are not the primary carriers of sodium in coal.
- (3)
- The coal was deposited in a warm, humid, shallow-lake peat-swamp environment. There was no significant influence from terrestrial or marine sources. The enrichment of sodium is primarily influenced by hydrodynamic conditions. The sodium generated from the weathering of the epipedon and overlying rock strata is dissolved by atmospheric precipitation and surface water, entering groundwater through structural and weathering fissures, as well as rock pores. Sodium-rich groundwater is transported downward and enters the coal seam, where, over the course of lengthy geological processes, sodium accumulates in the form of ions, hydrated ions and organic-matter-bound states within the coal seam pores.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, X.F.; Wang, Y.; Ding, H.; Zhu, C.; Zhang, H.Y. Modes of occurrence of sodium in Zhundong coal. J. China Coal Soc. 2015, 40, 2909–2915. (In Chinese) [Google Scholar] [CrossRef]
- Dahlin, R.S.; Peng, W.; Nelson, M.; Vimalchand, P.; Liu, G. Formation and prevention of agglomerated deposits during the gasification of high-sodium lignite. Energy Fuels 2006, 20, 2465–2470. [Google Scholar] [CrossRef]
- Li, C.Z.; Sathe, C.; Kershaw, J.R.; Pang, Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal. Fuel 2000, 79, 427–438. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, X.; Alastuey, A.; Querol, X.; Li, J. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2010, 82, 51–67. [Google Scholar] [CrossRef]
- Yang, Z.C.; Liu, J.L.; Yao, W. Fouling index of Zhundong coal ash. Clean Coal Technol. 2013, 19, 81–84. (In Chinese) [Google Scholar] [CrossRef]
- Bryers, R.W. Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Wijaya, N.; Choo, T.K.; Zhang, L. Generation of ultra-clean coal from Victorian brown coal—Sequential and single leaching at room temperature to elucidate the elution of individual inorganic elements. Fuel Process. Technol. 2011, 92, 2127–2137. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, X.; Song, S.; Mei, Y.; Zhao, J.; Fang, Y. Optimization of leaching conditions for removing sodium from sodium-rich coals by orthogonal experiments. Fuel 2017, 208, 499–507. [Google Scholar] [CrossRef]
- Li, X.; Bai, Z.Q.; Bai, J.; Han, Y.N.; Kong, L.X.; Li, W. Insight into the Effects of Sodium Species with Different Occurrence Modes on the Structural Features of Residues Derived from Direct Liquefaction of Zhundong Coal by Multiple Techniques. Energy Fuels 2015, 29, 7142–7149. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.L.; Yan, Z.; Liu, K.L.; Xu, Y.Q.; Sheng, C.D.; Pan, W.P. The varying characterization of alkali metals (Na, K) from coal during the initial stage of coal combustion. Energy Fuels 2001, 15, 786–793. [Google Scholar] [CrossRef]
- Sripada, P.P.; Xu, T.; Kibria, M.A.; Bhattacharya, S. Comparison of entrained flow gasification behaviour of Victorian brown coal and biomass. Fuel 2017, 203, 942–953. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Chlorine in coal: A review. Int. J. Coal Geol. 2006, 67, 127–144. [Google Scholar] [CrossRef]
- Spears, D.A.; Zheng, Y. Geochemistry and origin of elements in some UK coals. Int. J. Coal Geol. 1999, 38, 161–179. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Querol, X.; Font, O.; Moreno, N.; Zhou, J.B.; Lei, G.M. High quality of Jurassic Coals in the Southern and Eastern Junggar Coalfields, Xinjiang, NW China: Geochemical and mineralogical characteristics. Int. J. Coal Geol. 2012, 99, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Querol, X.; Font, O.; Moreno, N.; Zhou, J.B. Environmental geochemistry of the feed coals and their combustion by-products from two coal-fired power plants in Xinjiang Province, Northwest China. Fuel 2012, 95, 446–456. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.Y.; Shi, D.Z.; Liu, D.H. Study on sodium removal for Zhundong coal upgrading. Coal Convers. 2013, 36, 14–18. (In Chinese) [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Huang, Q.; Yao, Q. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace. Fuel 2015, 143, 430–437. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, B.; Li, L.; Zhang, H. Experimental Measurement of the Effective Thermal Conductivity of Ash Deposit for High Sodium Coal (Zhun Dong Coal) in a 300 KW Test Furnace. Energy Fuels 2013, 27, 7008–7022. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Wei, B.; Zhang, L.; Tan, H.; Yang, T.; Mikulcic, H.; Duic, N. The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium: A study from ash evaporating to condensing. Appl. Therm. Eng. 2015, 80, 150–159. [Google Scholar] [CrossRef]
- Sathe, C.; Hayashi, J.; Li, C.Z.; Chiba, T. Release of alkali and alkaline earth metallic species during rapid pyrolysis of a Victorian brown coal at elevated pressures. Fuel 2003, 82, 1491–1498. [Google Scholar] [CrossRef]
- Wu, L.; Qiao, Y.; Gui, B.; Wang, C.; Xu, J.; Yao, H.; Xu, M. Effects of Chemical Forms of Alkali and Alkaline Earth Metallic Species on the Char Ignition Temperature of a Loy Yang Coal under O2/N2 Atmosphere. Energy Fuels 2012, 26, 112–117. [Google Scholar] [CrossRef]
- Chadwick, B.L.; Ashman, R.A.; Campisi, A.; Crofts, G.J.; Godfrey, P.D.; Griffin, P.G.; Ottrey, A.L.; Morrison, R.J.S. Development of techniques for monitoring gas-phase sodium species formed during coal combustion and gasification. Int. J. Coal Geol. 1996, 32, 241–253. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhao, L.; Han, T.; Chen, W.; Yan, Y.; Jin, X.; Che, D. Release and Transformation Behaviors of Sodium, Calcium, and Iron during Oxy-fuel Combustion of Zhundong Coals. Energy Fuels 2018, 32, 1242–1254. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.A.; Yan, Y.; Jin, X.; Liu, Y.; Che, D. Release and transformation of sodium during combustion of Zhundong coals. J. Energy Inst. 2016, 89, 48–56. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Yao, Q. Dynamic behavior of sodium release from pulverized coal combustion by phase-selective laser-induced breakdown spectroscopy. Proc. Combust. Inst. 2015, 35, 2339–2346. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.; Shi, D.; Lv, J.; Liu, D.; Guo, X.; Dong, A.; Xiong, S. Research on sodium removal from Zhundong coal. Adv. Mater. Res. 2013, 734–737, 614–618. [Google Scholar] [CrossRef]
- Dai, B.Q.; Wu, X.; De Girolamo, A.; Zhang, L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler. Part 1. Changes on the properties of ash deposits along the furnace. Fuel 2015, 139, 720–732. [Google Scholar] [CrossRef]
- Xu, J.; Yu, D.; Fan, B.; Zeng, X.; Lv, W.; Chen, J. Characterization of Ash Particles from Co-combustion with a Zhundong Coal for Understanding Ash Deposition Behavior. Energy Fuels 2014, 28, 678–684. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Kang, Y.; Miao, Y.; Ren, W.; Wang, T. Safely Burning High Alkali Coal with Kaolin Additive in a Pulverized Fuel Boiler. Energy Fuels 2014, 28, 5640–5648. [Google Scholar] [CrossRef]
- Si, J.; Liu, X.; Xu, M.; Sheng, L.; Zhou, Z.; Wang, C.; Zhang, Y.; Seo, Y.-C. Effect of kaolin additive on PM2.5 reduction during pulverized coal combustion: Importance of sodium and its occurrence in coal. Appl. Energy 2014, 114, 434–444. [Google Scholar] [CrossRef]
- GB/T 482-2008; Sampling of Coal Seams. China Coal Industry Association: Beijing, China, 2008.
- GB/T 212-2008; Proximate Analysis of Coal. China Coal Industry Association: Beijing, China, 2008.
- GB/T 214-2007; Determination of Total Sulfur in Coal. China Coal Industry Association: Beijing, China, 2007.
- GB/T 19227-2008; Determination of Nitrogen in Coal. China Coal Industry Association: Beijing, China, 2008.
- GB/T 476-2008; Determination of Carbon and Hydrogen in Coal. China Coal Industry Association: Beijing, China, 2008.
- GB/T14506.28-2010; Methods for Chemical Analysis of Silicate Rocks—Part 28: Determination of 16 Major and Minor Elements Content. Ministry of Natural Resources: Beijing, China, 2010.
- Grigore, M.; Sakurovs, R. Inorganic matter in Victorian brown coals. Int. J. Coal Geol. 2016, 154, 257–264. [Google Scholar] [CrossRef]
- Li, Z.; Ward, C.R.; Gurba, L.W. Occurrence of non-mineral inorganic elements in macerals of low-rank coals. Int. J. Coal Geol. 2010, 81, 242–250. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, D.H.; Ren, D.Y.; Tang, Y.G.; Shao, L.Y.; Song, H.B. Geochemistry of the late Permian No. 30 coal seam, Zhijin Coalfield of Southwest China: Influence of a siliceous low-temperature hydrothermal fluid. Appl. Geochem. 2004, 19, 1315–1330. [Google Scholar] [CrossRef]
- Benson, S.A.; Holm, P.L. Comparison of inorganic constituents in three low rank coals. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 145–149. [Google Scholar] [CrossRef]
- GB/T15224.1-2018; Classification for Quality of Coal—Part 1: Ash. China Coal Industry Association: Beijing, China, 2018.
- GB/T15224.2-2021; Classification for Quality of Coal—Part 2: Sulfur Content. China Coal Industry Association: Beijing, China, 2021.
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O'Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90, 72–99. [Google Scholar] [CrossRef]
- Querol, X.; Chinchon, S.; Lopez, S.A. Iron sulfide precipitation sequence in Albian coals from the Maestrazgo Basin, southeastern Iberian Range, northeastern Spain. Int. J. Coal Geol. 1989, 11, 171–189. [Google Scholar] [CrossRef]
- Liu, D.M.; Yang, Q.; Zhou, C.G.; Tang, D.Z.; Kang, X.D. Occurrence and geological genesis of pyritesin Late Paleozoic coals in North China. Geochimica 1999, 340–350. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, D.D.; Chen, P.; Tang, X.Y.; Hong, A.N. Characteristic Study and Geological Genesis Analysis of Pyrite in No.8 Coal in Huainan Coalfield. Coal Geol. China 2009, 21, 22–26. (In Chinese) [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Ward, C.R.; Spears, D.A.; Booth, C.A. Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int. J. Coal Geol. 1999, 40, 281–308. [Google Scholar] [CrossRef]
- Spears, D.A.; Caswell, S.A. Mineral matter in coals: Cleat minerals and their origin in some coals from the English Midlands. Int. J. Coal Geol. 1986, 6, 107–125. [Google Scholar] [CrossRef]
- Ward, C.R. Minerals in bituminous coals of the Sydney Basin (Australia) and the Illinois Basin (USA). Int. J. Coal Geol. 1989, 13, 455–479. [Google Scholar] [CrossRef]
- Hayashi, K.I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of approximately 1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Et Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xu, Y.-G.; Zhong, Y.-T.; Guan, J.-P. The Guadalupian-Lopingian boundary mudstones at Chaotian (SW China) are clastic rocks rather than acidic tuffs: Implication for a temporal coincidence between the end-Guadalupian mass extinction and the Emeishan volcanism. Lithos 2010, 119, 10–19. [Google Scholar] [CrossRef]
- Dai, S.; Li, T.; Jiang, Y.; Ward, C.R.; Hower, J.C.; Sun, J.; Liu, J.; Song, H.; Wei, J.; Li, Q.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Hailiushu Mine, Daqingshan Coalfield, Inner Mongolia, China: Implications of sediment-source region and acid hydrothermal solutions. Int. J. Coal Geol. 2015, 137, 92–110. [Google Scholar] [CrossRef]
- Chou, C.L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, L. Depositional environment and controlling factors of coal measures source rocks of Yacheng Formation in Yabei depression of Qiongdongnan basin. Acta Petrol. Mineral. 2017, 36, 70–79. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Xia, Y.C.; Ma, L. Study on oil-rich coal occurrence characteristics and sedimentary environment in Yushen Mining Area. Coal Sci. Technol. 2020, 48, 192–197. (In Chinese) [Google Scholar] [CrossRef]
- Wu, B.; Chen, H.S.; Zhu, G.R. Macroelement Characteristics and Sedimentary Environment Analysis of Workable Coal of Mukong Coal Mine in Jinsha of Guizhou. Guizhou Geol. 2013, 30, 266–270. (In Chinese) [Google Scholar] [CrossRef]
- Hao, J.S.; Ge, B.X.; Xie, H.B. The Analysis Method Based on Ash-Composition and Its Application in Coal-Accumulating Environment Reconstruction. Acta Sedimentol. Sin. 2000, 3, 460–464. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, C.; Qu, S.; Zhang, J.; Wang, Y.; Zhang, Y. Distribution, occurrence and leaching dynamic behavior of sodium in Zhundong coal. Fuel 2017, 190, 189–197. [Google Scholar] [CrossRef]
- Yang, H.B.; Chen, L.; Kong, Y.H. A novel classification of structural units in Jungger Basin. Xinjiang Pet. Geol. 2004, 25, 686–688. [Google Scholar] [CrossRef]






| Proximate Analysis | St,d | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | Odaf | Cdaf | Hdaf | Ndaf | ||
| Max | 16.26 | 7.92 | 37.96 | 68.92 | 1.80 | 20.26 | 79.59 | 3.93 | 0.90 |
| Min | 9.28 | 2.93 | 28.54 | 59.61 | 0.09 | 15.29 | 75.80 | 2.76 | 0.56 |
| Average | 12.64 | 4.51 | 31.51 | 65.40 | 0.49 | 17.67 | 77.83 | 3.29 | 0.70 |
| Samples | SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | K2O | Na2O | MnO | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Coal-av | 0.44 | 0.24 | 0.03 | 0.42 | 2.20 | 0.83 | 0.02 | 0.40 | 0.01 | 0.02 |
| parting-av | 0.26 | 0.18 | 0.01 | 10.87 | 23.02 | 0.55 | 0.01 | 0.15 | 0.16 | 0.01 |
| Roof | 64.00 | 14.56 | 0.67 | 0.05 | 0.31 | 0.37 | 1.28 | 0.19 | 0.01 | 0.04 |
| Floor | 34.20 | 23.62 | 2.64 | 0.27 | 0.63 | 0.31 | 0.36 | 0.18 | 0.01 | 0.03 |
| China 1 | 8.47 | 5.98 | 0.33 | 4.85 | 1.23 | 0.22 | 0.19 | 0.16 | 0.02 | 0.09 |
| Loy Yang 2 | 5.41 | 5.15 | 0.31 | 0.79 | 0.18 | 1.10 | 0.08 | 0.89 | n.d | 0.04 |
| Yorks 3 | 10.55 | 4.81 | 0.18 | 2.77 | 0.42 | 0.37 | 0.72 | 0.30 | 0.05 | n.d |
| Samples | Na2O (wt.%) | ||
|---|---|---|---|
| Max | Min | Average | |
| Epipedon | 3.67 | 2.05 | 2.96 |
| Overlying rock | 2.97 | 0.28 | 1.45 |
| Coal | 1.07 | 0.27 | 0.49 |
| Samples | Seam1 | Seam2 | Seam3 | Seam4 | Seam5 |
|---|---|---|---|---|---|
| Na2O (wt.%) | 0.31 | 0.60 | 0.36 | 0.92 | 0.44 |
| Inertinite (%) | 66.74 | 72.23 | 75.10 | 82.23 | 75.55 |
| Vitrinite (%) | 28.69 | 23.60 | 22.50 | 15.52 | 20.95 |
| Exinite (%) | 0.96 | 0.92 | 0.80 | 1.05 | 0.50 |
| Samples | Ca2+ | K+ | Mg2+ | Na+ | Cl− | SO42− |
|---|---|---|---|---|---|---|
| Seam5 | 4.45 | n.d | 4.695 | 26.55 | 6.565 | 42.125 |
| Seam4 | 3.04 | 0.355 | 2.77 | 56.6 | 51.05 | 31.65 |
| Seam3 | 2.24 | n.d | 2.495 | 22.45 | 8.125 | 29.8 |
| Seam2 | 2.64 | n.d | 1.975 | 32.65 | 25.31 | 28 |
| Seam1 | 13.48 | 0.37 | 4.3725 | 18.225 | 6.7625 | 110.925 |
| Zhundong 1 | 4.5 | 0.4 | 1.1 | 66.1 | 44.3 | 22.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, W.; Wang, W.; Duan, P.; He, X.; Lu, Q. Distribution, Occurrence and Enrichment Causes of Sodium in Middle Jurassic Coal from Zhundong Coalfield, Xinjiang. Minerals 2024, 14, 146. https://doi.org/10.3390/min14020146
Wang Y, Wang W, Wang W, Duan P, He X, Lu Q. Distribution, Occurrence and Enrichment Causes of Sodium in Middle Jurassic Coal from Zhundong Coalfield, Xinjiang. Minerals. 2024; 14(2):146. https://doi.org/10.3390/min14020146
Chicago/Turabian StyleWang, Yulong, Wenfeng Wang, Wenlong Wang, Piaopiao Duan, Xin He, and Qingfeng Lu. 2024. "Distribution, Occurrence and Enrichment Causes of Sodium in Middle Jurassic Coal from Zhundong Coalfield, Xinjiang" Minerals 14, no. 2: 146. https://doi.org/10.3390/min14020146
APA StyleWang, Y., Wang, W., Wang, W., Duan, P., He, X., & Lu, Q. (2024). Distribution, Occurrence and Enrichment Causes of Sodium in Middle Jurassic Coal from Zhundong Coalfield, Xinjiang. Minerals, 14(2), 146. https://doi.org/10.3390/min14020146









