Author Contributions
Conceptualisation, Data Curation, Formal Analysis, Investigation, Methodology, Software, Writing—original draft: M.G.R.; Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing—review and editing: J.P.M. Writing—review and editing, Validation, Investigation, Resources: T.M.M. All authors have read and agreed to the published version of the manuscript.
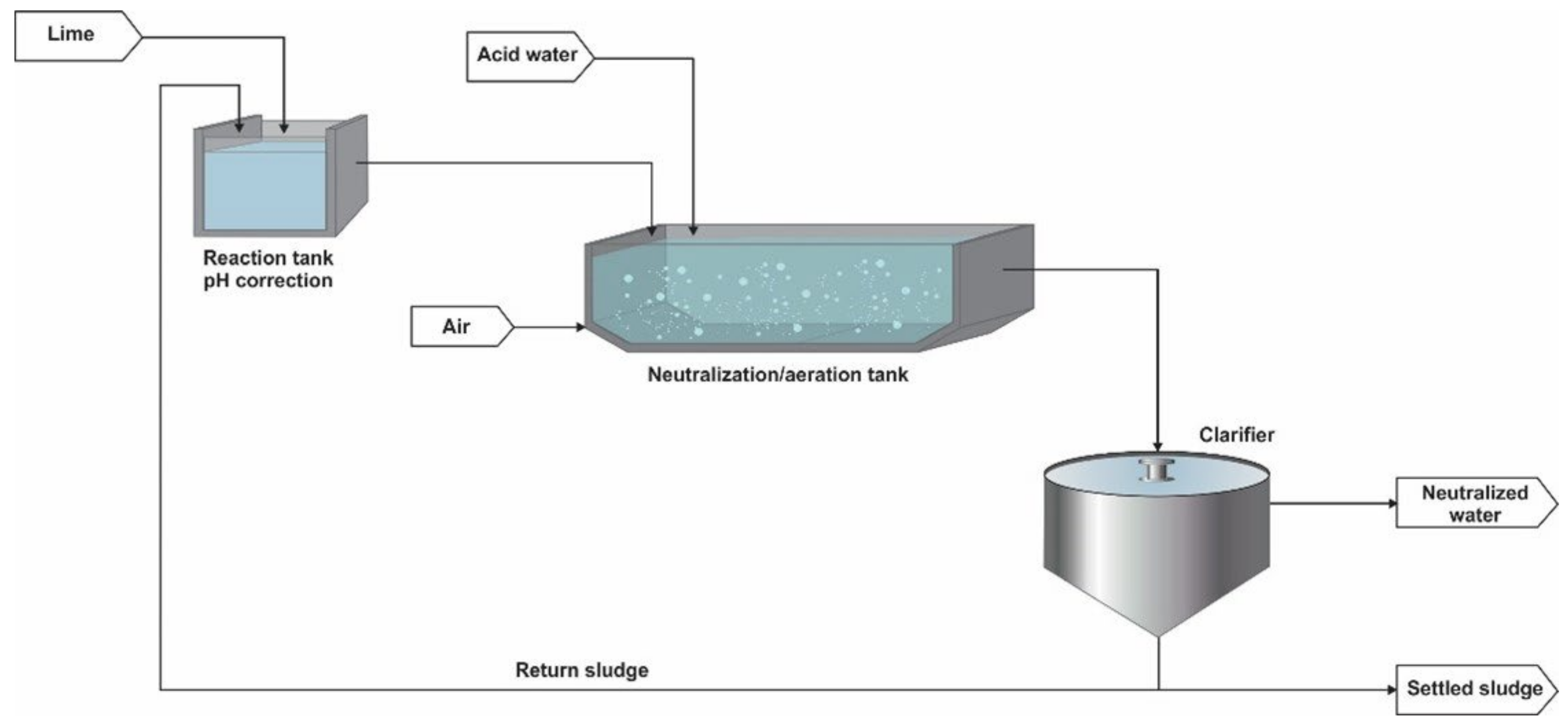
Figure 1.
Neutralisation process of High-Density Sludge for AMD treatment [
3,
12].
Figure 1.
Neutralisation process of High-Density Sludge for AMD treatment [
3,
12].
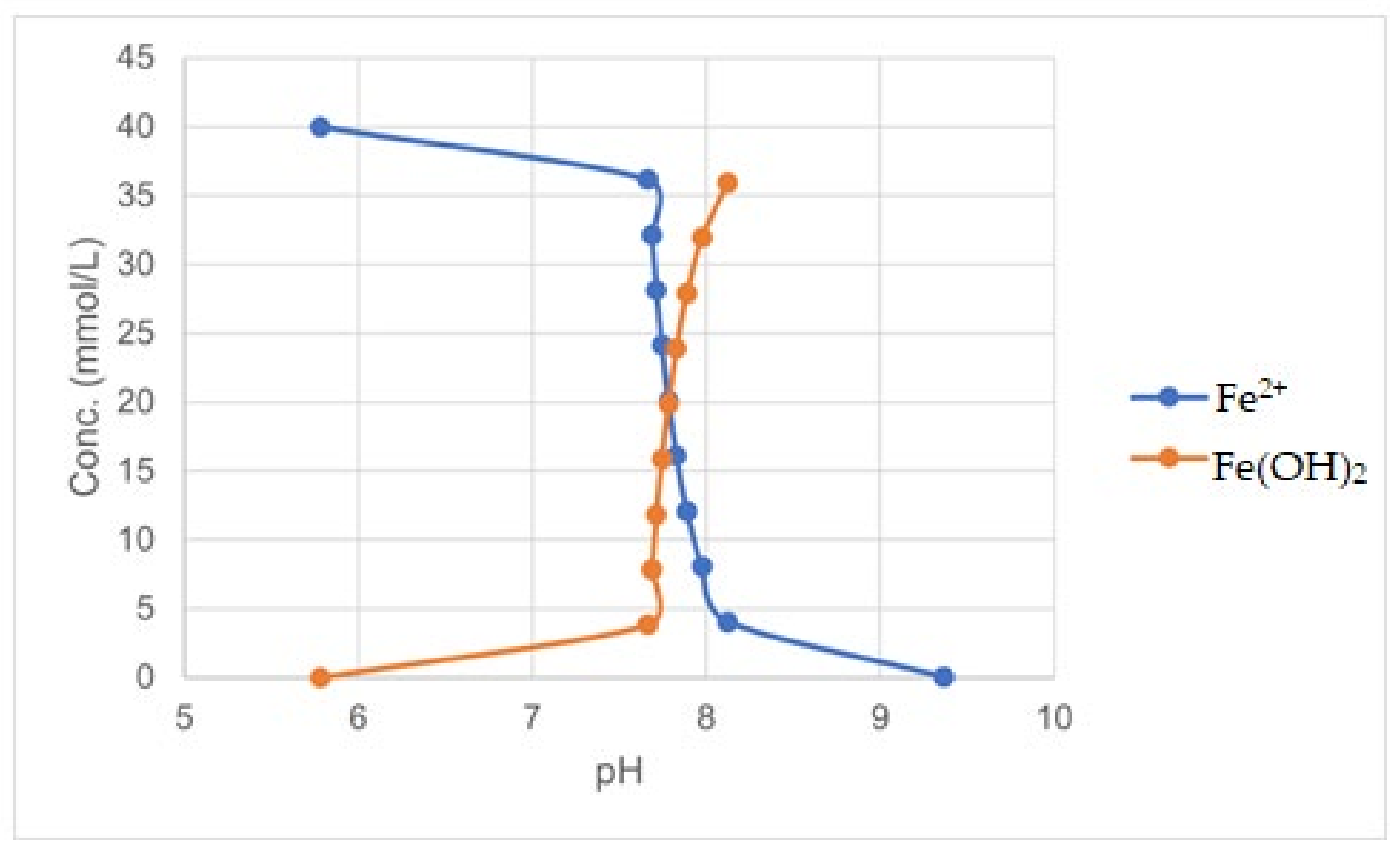
Figure 2.
Effect of pH on Fe(OH)2 precipitation (OLI simulation).
Figure 2.
Effect of pH on Fe(OH)2 precipitation (OLI simulation).
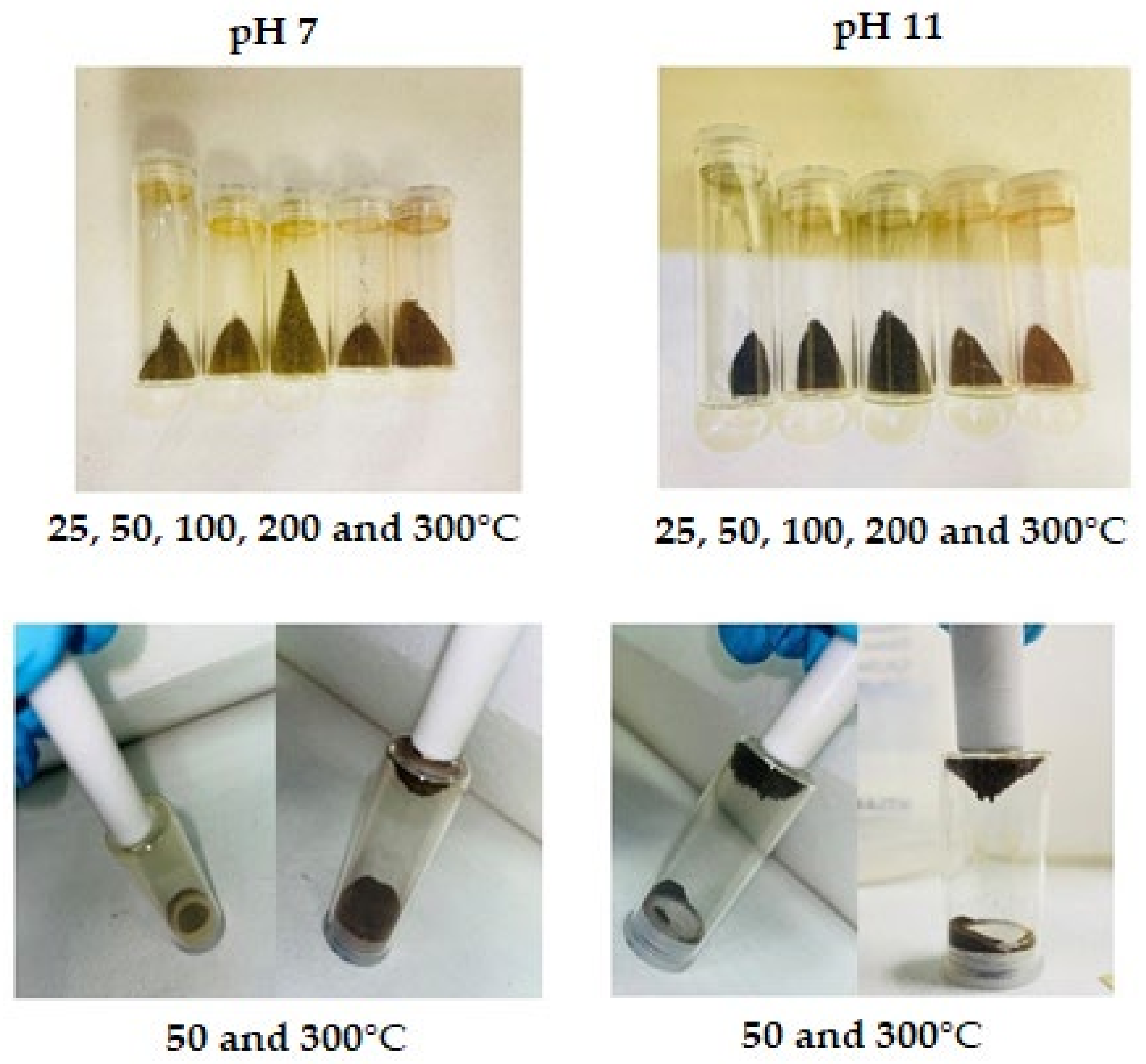
Figure 3.
Magnetite produced with NaOH as alkali.
Figure 3.
Magnetite produced with NaOH as alkali.
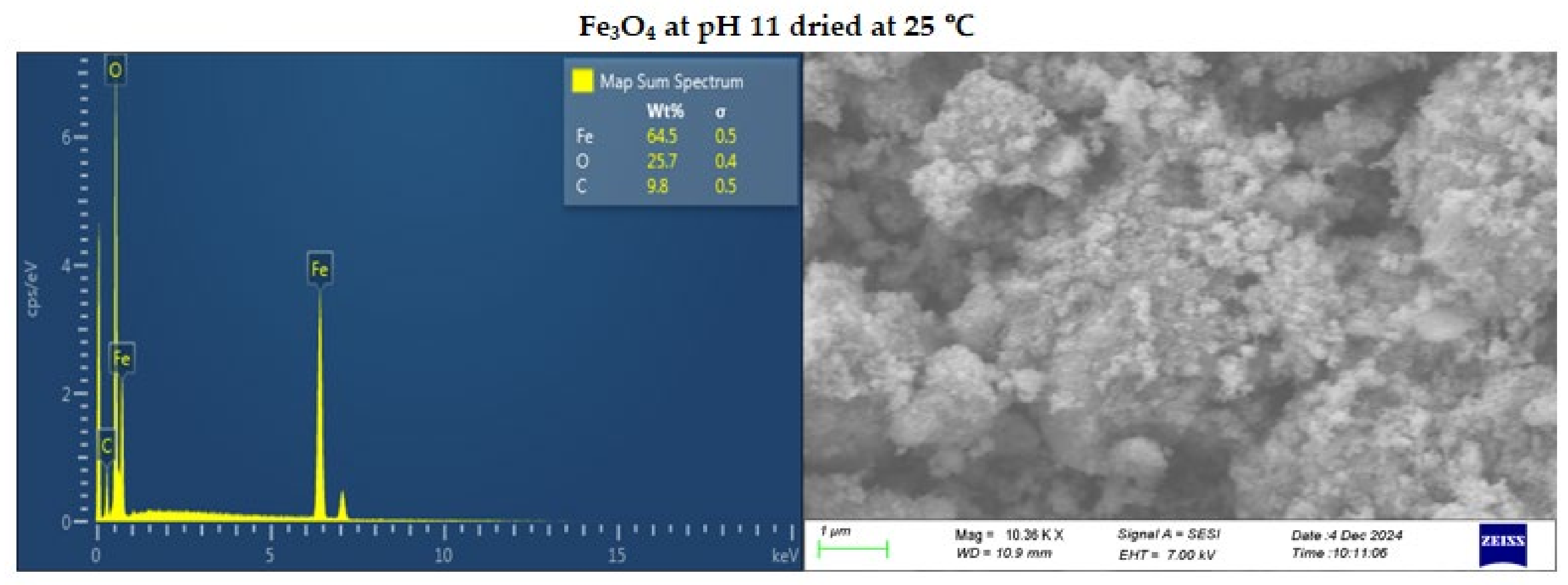
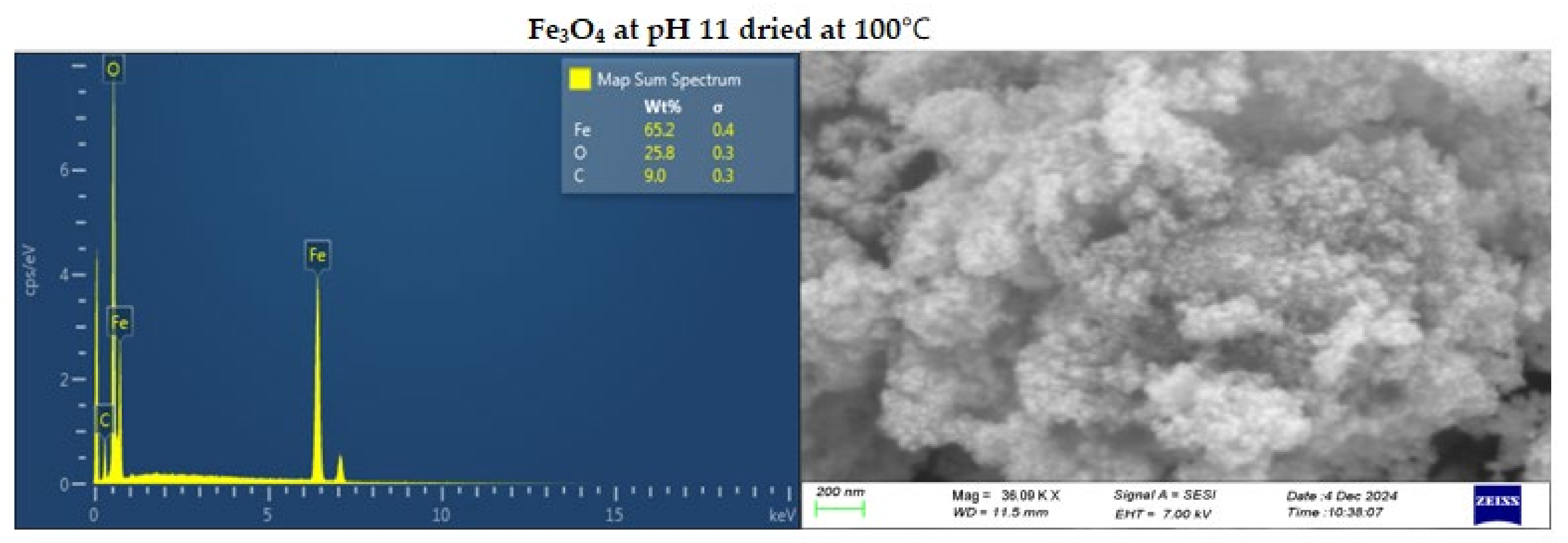
Figure 4.
SEM-EDS image of Fe3O4 at pH 11 dried at 25 (top) and 100℃ (bottom).
Figure 4.
SEM-EDS image of Fe3O4 at pH 11 dried at 25 (top) and 100℃ (bottom).
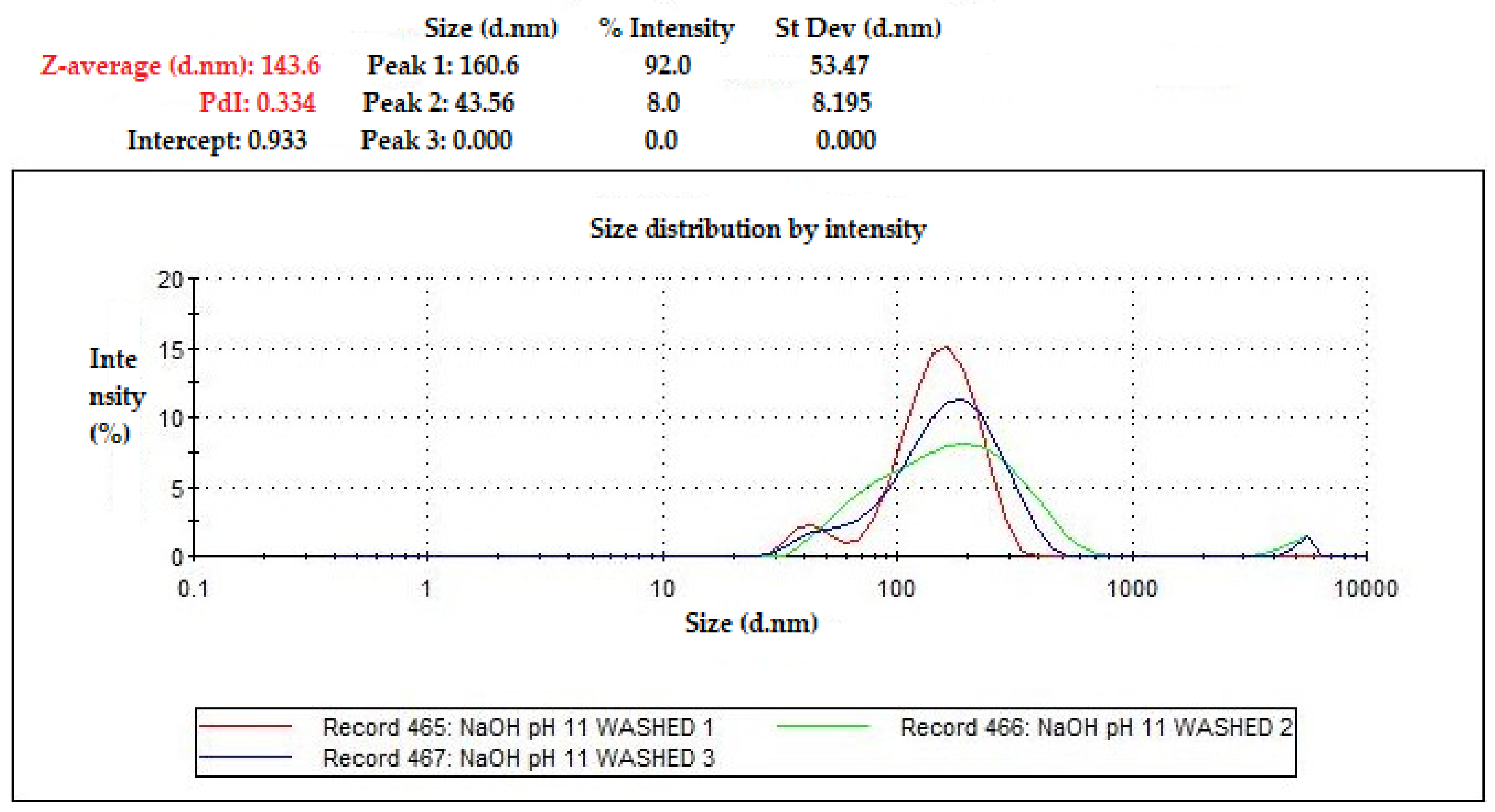
Figure 5.
Particle size of Fe3O4 (pH 11) measured using Zetasizer.
Figure 5.
Particle size of Fe3O4 (pH 11) measured using Zetasizer.
Figure 6.
Goethite formation from Fe(OH)3 when only CaCO3 was used for treatment.
Figure 6.
Goethite formation from Fe(OH)3 when only CaCO3 was used for treatment.
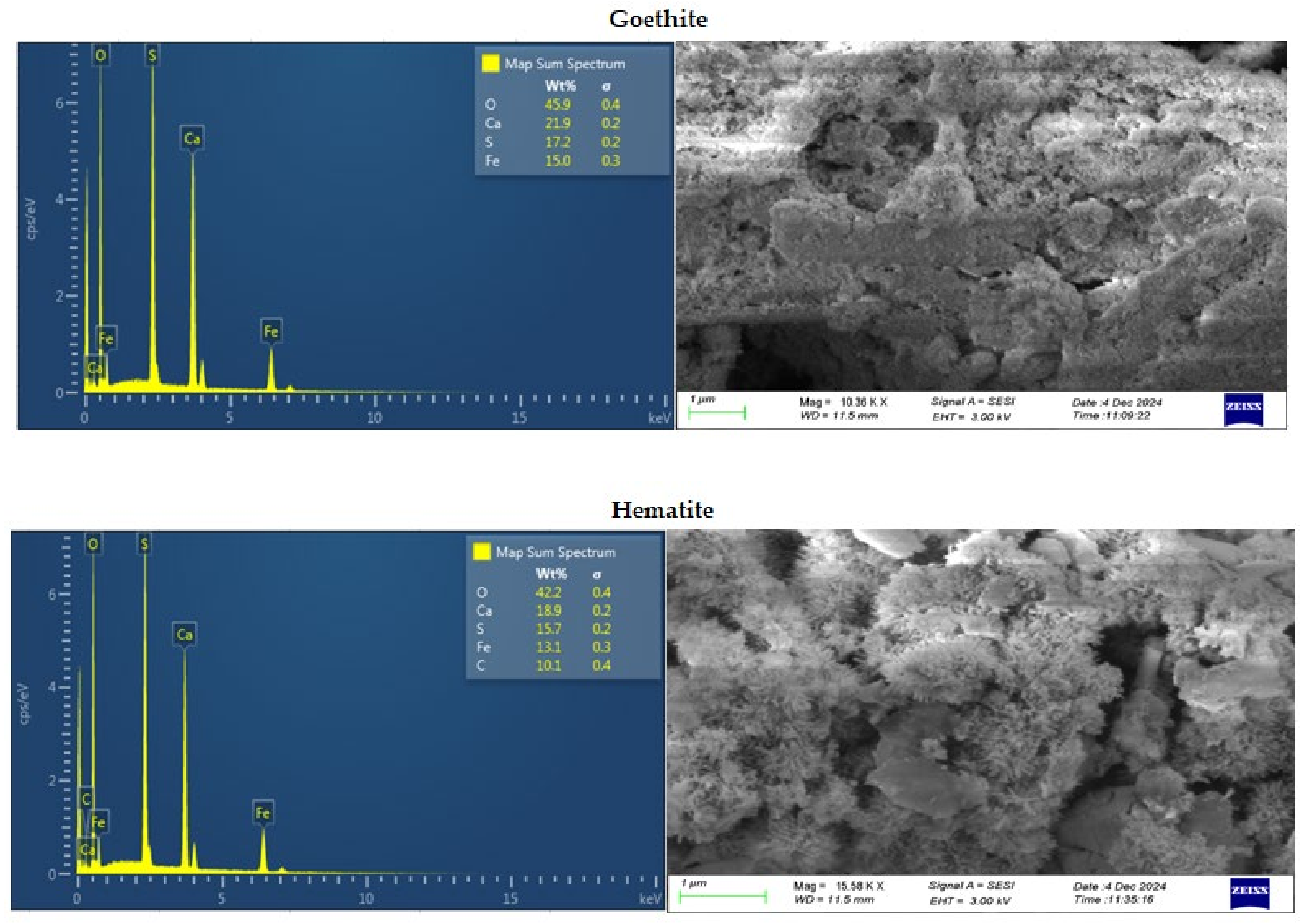
Figure 7.
SEM/EDS images of goethite (top) and hematite (bottom).
Figure 7.
SEM/EDS images of goethite (top) and hematite (bottom).
Figure 8.
XRD spectra of goethite.
Figure 8.
XRD spectra of goethite.
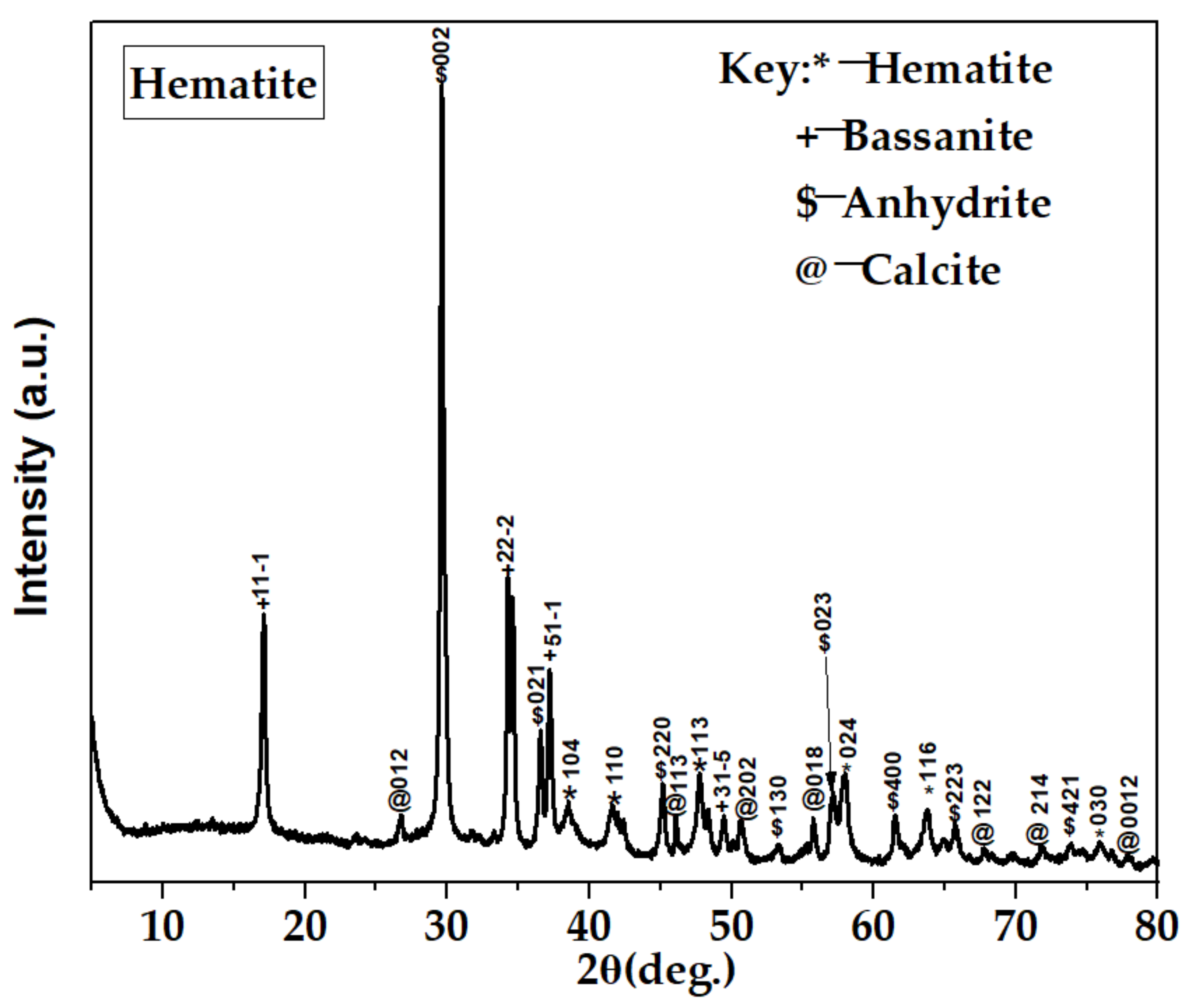
Figure 9.
XRD spectra of hematite.
Figure 9.
XRD spectra of hematite.
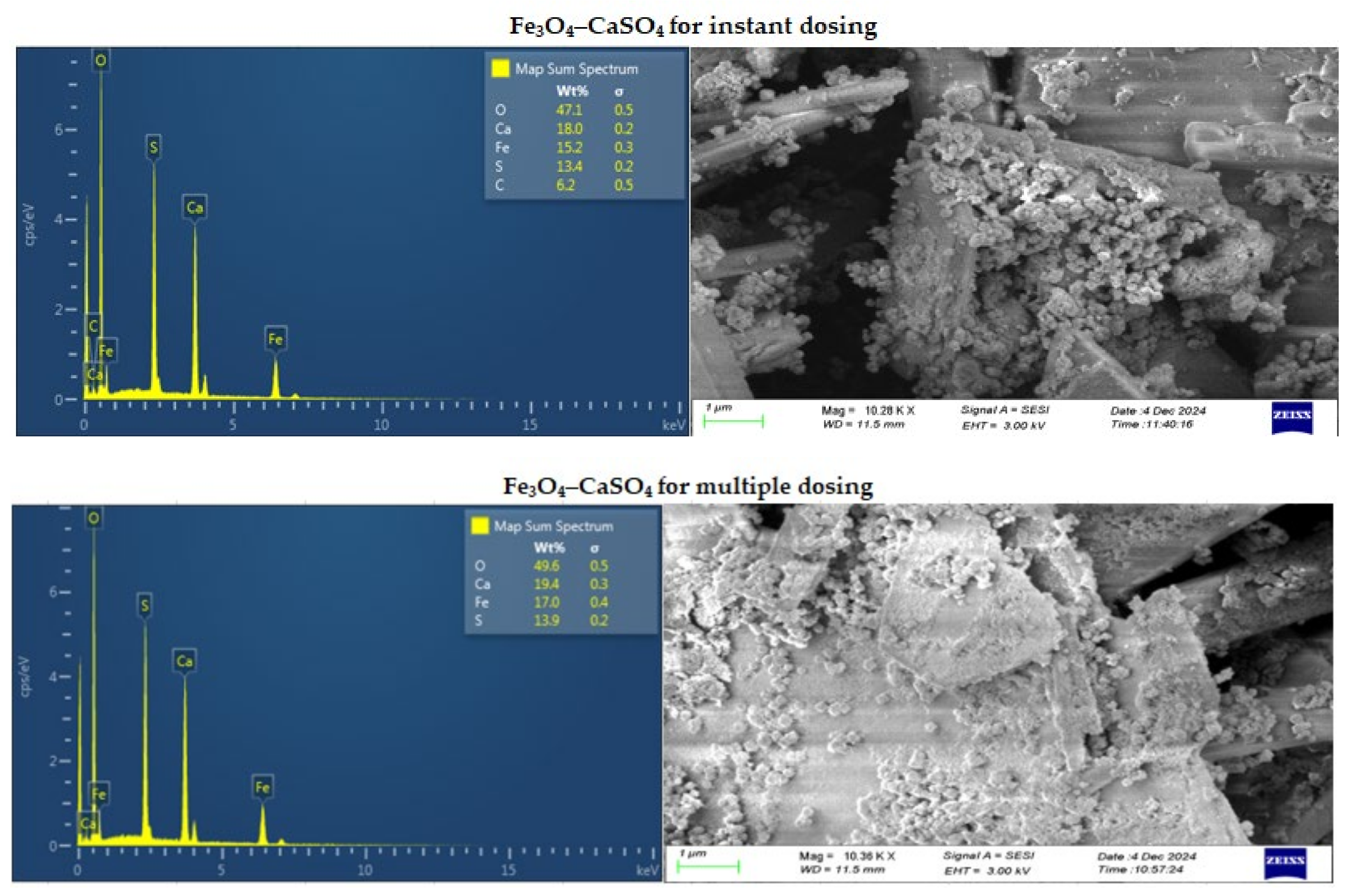
Figure 10.
SEM/EDS images of Fe3O4–CaSO4 for instant (top) and multiple (bottom) dosing.
Figure 10.
SEM/EDS images of Fe3O4–CaSO4 for instant (top) and multiple (bottom) dosing.
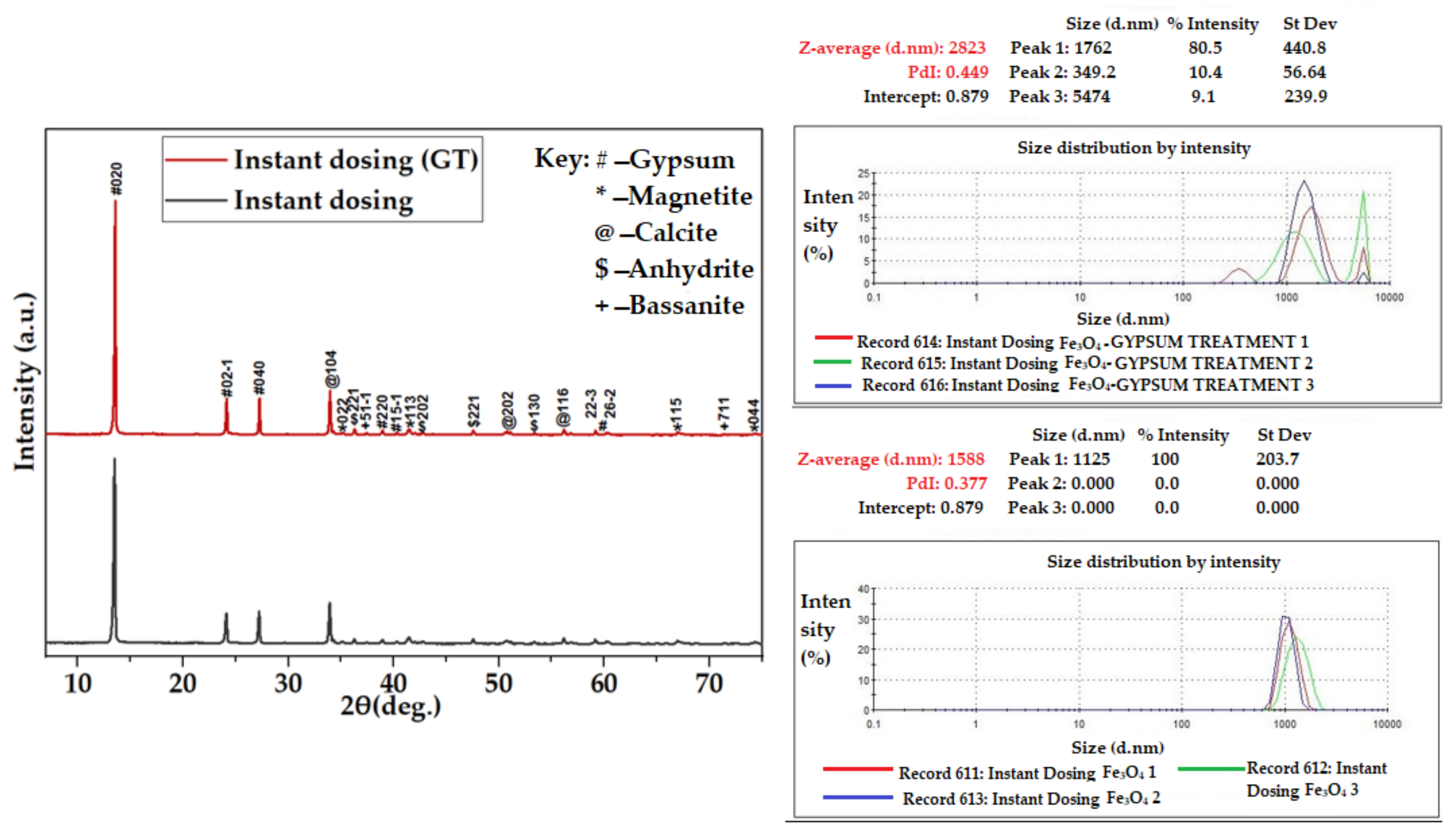
Figure 11.
XRD spectra for Fe3O4-CaSO4 in instant dosing.
Figure 11.
XRD spectra for Fe3O4-CaSO4 in instant dosing.
Figure 12.
XRD spectra for Fe3O4–CaSO4 in multiple dosing.
Figure 12.
XRD spectra for Fe3O4–CaSO4 in multiple dosing.
Figure 13.
Particle size of Fe3O4–CaSO4 (instant and multiple dosing) measured using Zetasizer.
Figure 13.
Particle size of Fe3O4–CaSO4 (instant and multiple dosing) measured using Zetasizer.
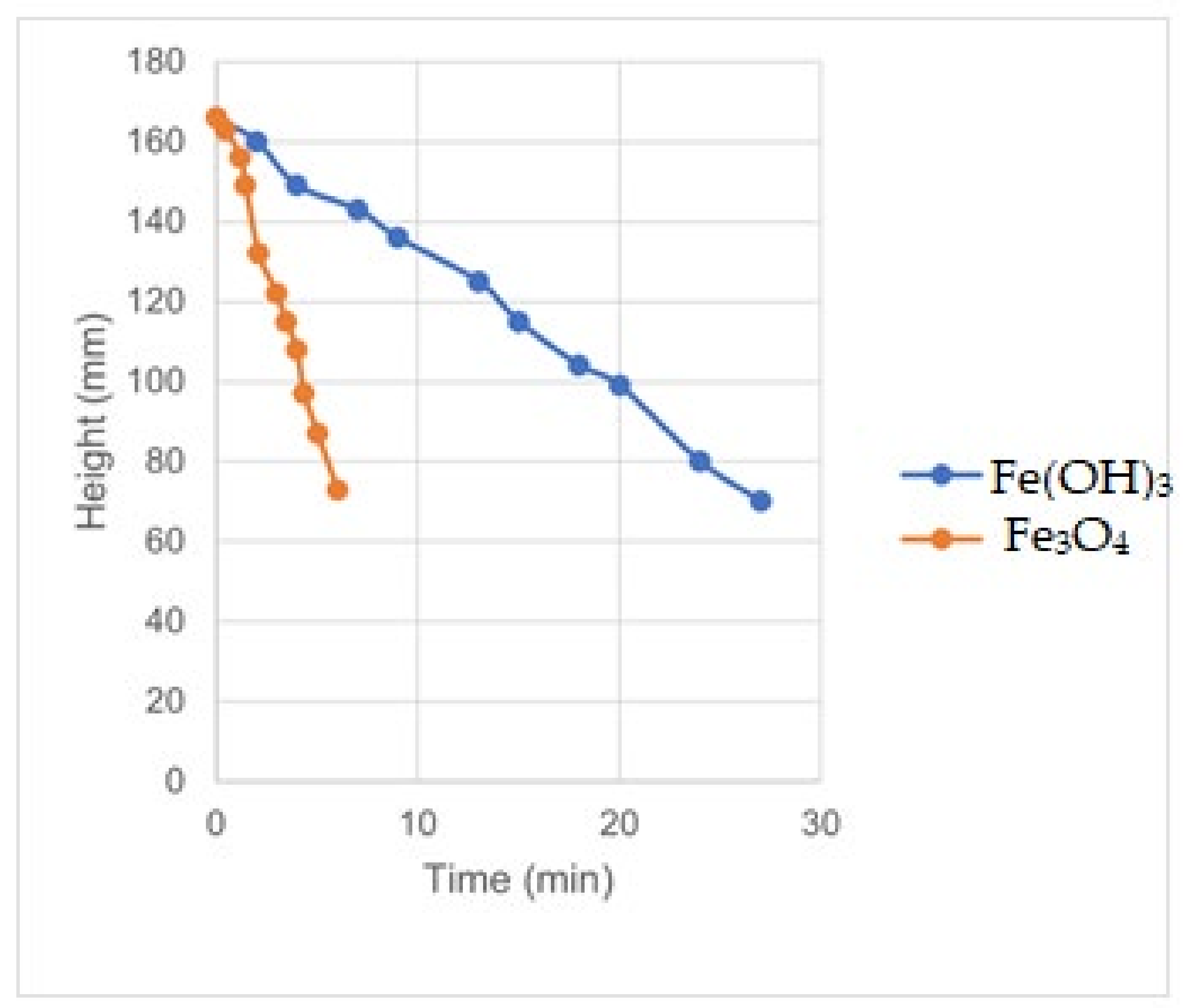
Figure 14.
Settling of Fe(OH)3 and Fe3O4-rich sludge.
Figure 14.
Settling of Fe(OH)3 and Fe3O4-rich sludge.
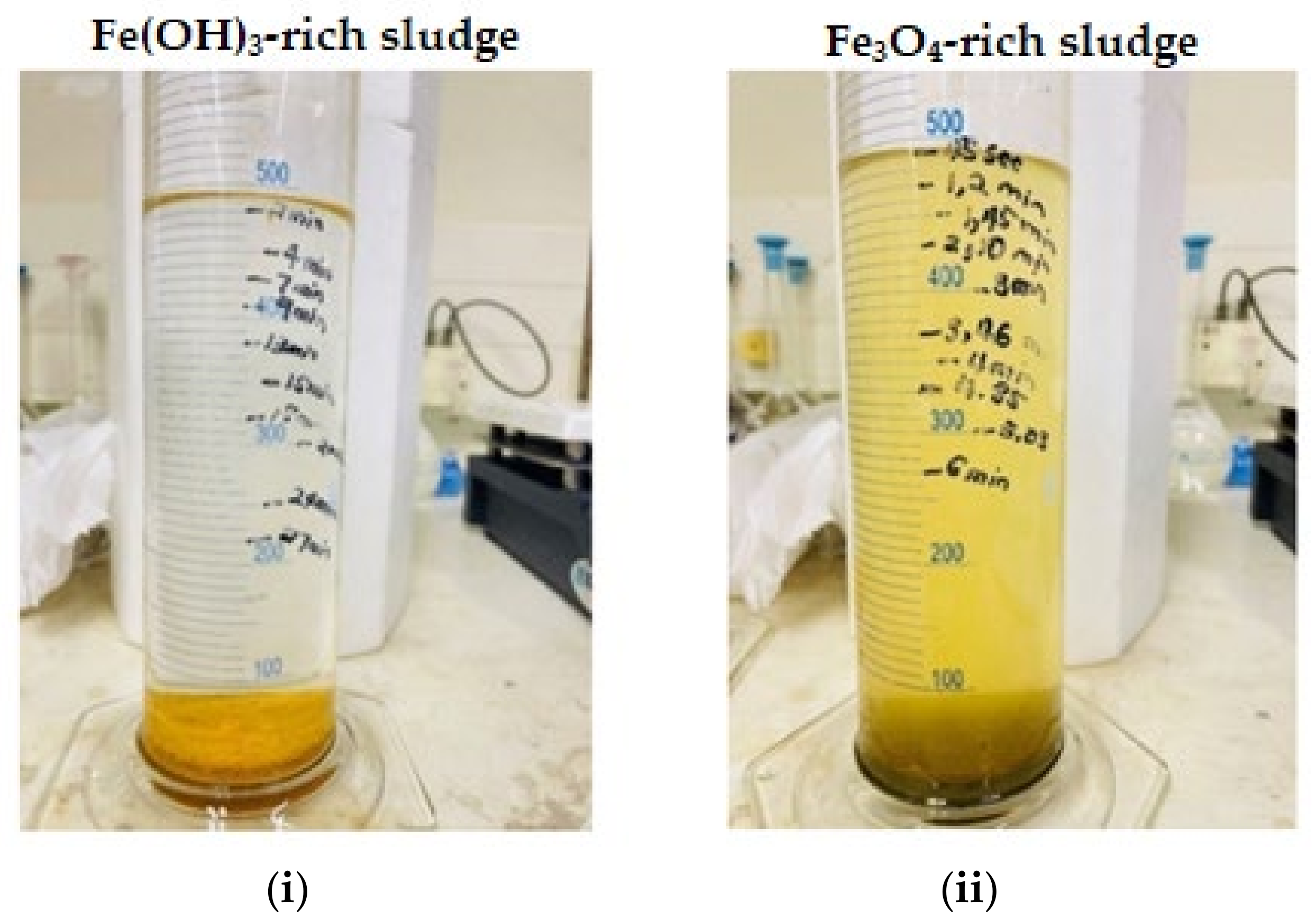
Figure 15.
Sludge settling for (i) Fe(OH)3-rich sludge and (ii) Fe3O4-rich sludge.
Figure 15.
Sludge settling for (i) Fe(OH)3-rich sludge and (ii) Fe3O4-rich sludge.
Table 1.
Acid required to keep Fe2(SO4)3 in solution (OLI simulation).
Table 1.
Acid required to keep Fe2(SO4)3 in solution (OLI simulation).
| Fe2(SO4)3 [mmol] | H2SO4 [mmol] | pH | Fe(OH)3 (Bernalite)—Sol [mmol] | Fe3+ Liq1 [mmol] | SO42− Liq1 [mmol] |
|---|
| 40.00 | 0.00 | 1.79 | 22.72 | 57.28 | 120.00 |
| 40.00 | 4.00 | 1.79 | 20.47 | 59.53 | 124.00 |
| 40.00 | 8.00 | 1.79 | 18.20 | 61.80 | 128.00 |
| 40.00 | 12.00 | 1.79 | 15.93 | 64.07 | 132.00 |
| 40.00 | 16.00 | 1.79 | 13.65 | 66.35 | 136.00 |
| 40.00 | 20.00 | 1.79 | 11.36 | 68.64 | 140.00 |
| 40.00 | 24.00 | 1.79 | 9.07 | 70.93 | 144.00 |
| 40.00 | 28.00 | 1.79 | 6.76 | 73.24 | 148.00 |
| 40.00 | 32.00 | 1.79 | 4.45 | 75.55 | 152.00 |
| 40.00 | 36.00 | 1.79 | 2.13 | 77.87 | 156.00 |
| 40.00 | 40.00 | 1.79 | 0.00 | 80.00 | 160.00 |
Table 2.
Fe3O4 with Na2CO3.
Table 2.
Fe3O4 with Na2CO3.
| Reactants | | Solid Products | | Solution |
|---|
| Na2CO3 [mmol] | FeSO4 [mmol] | Fe2(SO4)3 [mmol] | H2SO4 [mmol] | O2 [mmol] | pH | Fe3O4 (Magnetite)—Sol [mmol] | Fe(OH)3 (Bernalite)—Sol [mmol] | CaSO4·2H2O (Gypsum) [mmol] | CaCO3 (Calcite)—Sol [mmol] | CO2—Vap [mmol] | Fe3+ Liq1 [mmol] | Fe2+ Liq1 [mmol] | Ca2+ Liq1 [mmol] | Na+ Liq1 [mmol] | S6+ Liq1 [mmol] | C4+ Liq1 [mmol] | H2O [mmol] |
|---|
| 0 | 40 | 40 | 40 | 0 | 1.83 | 0.00 | 0.00 | 0.0 | 0.0 | 0.00 | 80.00 | 40.00 | 0.00 | 0 | 200 | 0.00 | 55,483 |
| 20 | 40 | 40 | 40 | 0 | 1.85 | 0.00 | 11.99 | 0.0 | 0.0 | 0.00 | 68.01 | 40.00 | 0.00 | 40 | 200 | 20.00 | 55,468 |
| 40 | 40 | 40 | 40 | 0 | 1.87 | 0.00 | 23.79 | 0.0 | 0.0 | 0.00 | 56.21 | 40.00 | 0.00 | 80 | 200 | 40.00 | 55,454 |
| 60 | 40 | 40 | 40 | 0 | 1.90 | 0.00 | 35.36 | 0.0 | 0.0 | 13.63 | 44.64 | 40.00 | 0.00 | 120 | 200 | 46.37 | 55,441 |
| 80 | 40 | 40 | 40 | 0 | 1.94 | 0.00 | 46.66 | 0.0 | 0.0 | 34.14 | 33.34 | 40.00 | 0.00 | 160 | 200 | 45.86 | 55,428 |
| 100 | 40 | 40 | 40 | 0 | 1.99 | 0.00 | 57.56 | 0.0 | 0.0 | 54.66 | 22.44 | 40.00 | 0.00 | 200 | 200 | 45.34 | 55,417 |
| 120 | 40 | 40 | 40 | 0 | 2.08 | 0.00 | 67.78 | 0.0 | 0.0 | 75.18 | 12.22 | 40.00 | 0.00 | 240 | 200 | 44.82 | 55,409 |
| 140 | 40 | 40 | 40 | 0 | 2.25 | 0.00 | 76.42 | 0.0 | 0.0 | 95.72 | 3.58 | 40.00 | 0.00 | 280 | 200 | 44.28 | 55,407 |
| 160 | 40 | 40 | 40 | 0 | 4.21 | 0.00 | 80.00 | 0.0 | 0.0 | 115.98 | 0.00 | 40.00 | 0.00 | 320 | 200 | 44.02 | 55,424 |
| 180 | 40 | 40 | 40 | 0 | 4.62 | 19.60 | 40.80 | 0.0 | 0.0 | 142.54 | 0.00 | 20.40 | 0.00 | 360 | 200 | 37.46 | 55,482 |
| 200 | 40 | 40 | 40 | 0 | 5.16 | 38.34 | 3.33 | 0.0 | 0.0 | 166.79 | 0.00 | 1.66 | 0.00 | 400 | 200 | 33.21 | 55,536 |
Table 3.
Fe3O4 with CaCO3.
Table 3.
Fe3O4 with CaCO3.
| Reactants | | Solid Products | | Solution |
|---|
| CaCO3 [mmol] | FeSO4 [mmol] | Fe2(SO4)3 [mmol] | H2SO4 [mmol] | O2 [mmol] | pH | Fe3O4 (Magnetite)—Sol [mmol] | Fe(OH)3 (Bernalite)—Sol [mmol] | CaSO4·2H2O (Gypsum) [mmol] | CaCO3 (Calcite)—Sol [mmol] | CO2—Vap [mmol] | Fe3+ Liq1 [mmol] | Fe2+ Liq1 [mmol] | Ca2+ Liq1 [mmol] | Na+ Liq1 [mmol] | S6+ Liq1 [mmol] | C4+ Liq1 [mmol] | H2O [mmol] |
|---|
| 0 | 40 | 40 | 40 | 0 | 1.83 | 0.00 | 0.00 | 0.0 | 0.0 | 0.00 | 80.00 | 40.0 | 0.00 | 0.0 | 200.0 | 0.00 | 55,483 |
| 20 | 40 | 40 | 40 | 0 | 1.85 | 0.00 | 11.11 | 4.0 | 0.0 | 0.00 | 68.89 | 40.0 | 16.03 | 0.0 | 196.0 | 20.00 | 55,463 |
| 40 | 40 | 40 | 40 | 0 | 1.86 | 0.00 | 22.71 | 24.6 | 0.0 | 0.00 | 57.29 | 40.0 | 15.38 | 0.0 | 175.4 | 40.00 | 55,408 |
| 60 | 40 | 40 | 40 | 0 | 1.87 | 0.00 | 34.06 | 45.2 | 0.0 | 12.48 | 45.94 | 40.0 | 14.77 | 0.0 | 154.8 | 47.52 | 55,353 |
| 80 | 40 | 40 | 40 | 0 | 1.89 | 0.00 | 45.15 | 65.8 | 0.0 | 32.57 | 34.85 | 40.0 | 14.19 | 0.0 | 134.2 | 47.43 | 55,300 |
| 100 | 40 | 40 | 40 | 0 | 1.92 | 0.00 | 55.90 | 86.4 | 0.0 | 52.66 | 24.10 | 40.0 | 13.60 | 0.0 | 113.6 | 47.34 | 55,247 |
| 120 | 40 | 40 | 40 | 0 | 1.96 | 0.00 | 66.14 | 107.0 | 0.0 | 72.75 | 13.86 | 40.0 | 13.02 | 0.0 | 93.0 | 47.25 | 55,197 |
| 140 | 40 | 40 | 40 | 0 | 2.08 | 0.00 | 75.33 | 127.6 | 0.0 | 92.84 | 4.67 | 40.0 | 12.42 | 0.0 | 72.4 | 47.16 | 55,152 |
| 160 | 40 | 40 | 40 | 0 | 4.10 | 0.00 | 80.00 | 148.2 | 0.0 | 112.70 | 0.00 | 40.0 | 11.79 | 0.0 | 51.8 | 47.32 | 55,128 |
| 180 | 40 | 40 | 40 | 0 | 4.51 | 19.73 | 40.53 | 167.6 | 0.0 | 139.60 | 0.00 | 20.3 | 12.42 | 0.0 | 32.4 | 40.37 | 55,147 |
| 200 | 40 | 40 | 40 | 0 | 5.08 | 38.92 | 2.16 | 184.6 | 0.0 | 170.00 | 0.00 | 1.1 | 15.36 | 0.0 | 15.4 | 34.85 | 55,169 |
Table 4.
Fe(OH)3 with CaCO3 and O2.
Table 4.
Fe(OH)3 with CaCO3 and O2.
| Reactants | | Solid Products | | Solution | | | | | | |
|---|
| CaCO3 [mmol] | FeSO4 [mmol] | Fe2(SO4)3 [mmol] | H2SO4 [mmol] | O2 [mmol] | pH | Fe3O4 (Magnetite)—Sol [mmol] | Fe(OH)3 (Bernalite)—Sol [mmol] | CaSO4·2H2O (Gypsum) [mmol] | CaCO3 (Calcite)—Sol [mmol] | CO2—Vap [mmol] | Fe3+ Liq1 [mmol] | Fe2+ Liq1 [mmol] | Ca2+ Liq1 [mmol] | Na+ Liq1 [mmol] | S6+ Liq1 [mmol] | C4+ Liq1 [mmol] | H2O [mmol] |
|---|
| 0 | 40 | 40 | 40 | 10 | 1.79 | 0.00 | 16.13 | 0.0 | 0.0 | 0.00 | 103.87 | 0.0 | 0.00 | 0.0 | 200.00 | 0.00 | 55,452 |
| 20 | 40 | 40 | 40 | 10 | 1.81 | 0.00 | 27.07 | 2.1 | 0.0 | 0.00 | 92.93 | 0.0 | 17.91 | 0.0 | 197.91 | 20.00 | 55,437 |
| 40 | 40 | 40 | 40 | 10 | 1.81 | 0.00 | 38.88 | 22.7 | 0.0 | 7.44 | 81.12 | 0.0 | 17.26 | 0.0 | 177.26 | 32.56 | 55,380 |
| 60 | 40 | 40 | 40 | 10 | 1.81 | 0.00 | 50.56 | 43.3 | 0.0 | 27.52 | 69.44 | 0.0 | 16.68 | 0.0 | 156.68 | 32.48 | 55,324 |
| 80 | 40 | 40 | 40 | 10 | 1.82 | 0.00 | 62.05 | 63.9 | 0.0 | 47.60 | 57.95 | 0.0 | 16.10 | 0.0 | 136.10 | 32.40 | 55,269 |
| 100 | 40 | 40 | 40 | 10 | 1.82 | 0.00 | 73.33 | 84.5 | 0.0 | 67.68 | 46.67 | 0.0 | 15.55 | 0.0 | 115.55 | 32.32 | 55,214 |
| 120 | 40 | 40 | 40 | 10 | 1.83 | 0.00 | 84.35 | 105.0 | 0.0 | 87.75 | 35.65 | 0.0 | 15.04 | 0.0 | 95.04 | 32.25 | 55,160 |
| 140 | 40 | 40 | 40 | 10 | 1.84 | 0.00 | 95.05 | 125.4 | 0.0 | 107.81 | 24.95 | 0.0 | 14.60 | 0.0 | 74.60 | 32.19 | 55,108 |
| 160 | 40 | 40 | 40 | 10 | 1.87 | 0.00 | 105.30 | 145.7 | 0.0 | 127.87 | 14.70 | 0.0 | 14.29 | 0.0 | 54.29 | 32.13 | 55,058 |
| 180 | 40 | 40 | 40 | 10 | 1.95 | 0.00 | 114.69 | 165.8 | 0.0 | 147.91 | 5.31 | 0.0 | 14.24 | 0.0 | 34.24 | 32.09 | 55,013 |
| 200 | 40 | 40 | 40 | 10 | 3.99 | 0.00 | 120.00 | 185.0 | 0.0 | 167.64 | 0.00 | 0.0 | 15.02 | 0.0 | 15.02 | 32.36 | 54,993 |
Table 5.
Effect of Fe2+ to Fe3+ mole ratio on Fe3O4 formation.
Table 5.
Effect of Fe2+ to Fe3+ mole ratio on Fe3O4 formation.
| NaOH [mmol] | FeSO4 [mmol] | Fe2(SO4)3 [mmol] | H2SO4 [mmol] | O2 [mmol] | pH | Fe3O4 (Magnetite)—Sol [mmol] | Fe(OH)3 (Bernalite)—Sol [mmol] | FeSO4 [mmol] | Fe3+ Liq1 [mmol] | Fe2+ Liq1 [mmol] | S6+ Liq1 [mmol] | Na+ Liq1 [mmol] | H2O [mmol] |
|---|
| 400 | 0 | 40 | 0.00 | 40 | 0 | 12.67 | 0.00 | 80.00 | 0 | 0 | 160 | 400 | 55,588 |
| 400 | 4 | 40 | 0.05 | 40 | 0 | 12.62 | 4.00 | 72.00 | 0 | 0 | 164 | 400 | 55,604 |
| 400 | 8 | 40 | 0.10 | 40 | 0 | 12.57 | 8.00 | 64.00 | 0 | 0 | 168 | 400 | 55,620 |
| 400 | 12 | 40 | 0.15 | 40 | 0 | 12.51 | 12.00 | 56.00 | 0 | 0 | 172 | 400 | 55,636 |
| 400 | 16 | 40 | 0.20 | 40 | 0 | 12.44 | 16.00 | 48.00 | 0 | 0 | 176 | 400 | 55,652 |
| 400 | 20 | 40 | 0.25 | 40 | 0 | 12.36 | 20.00 | 40.00 | 0 | 0 | 180 | 400 | 55,668 |
| 400 | 24 | 40 | 0.30 | 40 | 0 | 12.26 | 24.00 | 32.00 | 0 | 0 | 184 | 400 | 55,684 |
| 400 | 28 | 40 | 0.35 | 40 | 0 | 12.13 | 28.00 | 24.00 | 0 | 0 | 188 | 400 | 55,700 |
| 400 | 32 | 40 | 0.40 | 40 | 0 | 11.96 | 32.00 | 16.00 | 0 | 0 | 192 | 400 | 55,716 |
| 400 | 36 | 40 | 0.45 | 40 | 0 | 11.65 | 36.00 | 8.00 | 0 | 0 | 196 | 400 | 55,732 |
| 400 | 40 | 40 | 0.50 | 40 | 0 | 7.32 | 40.00 | 0.00 | 0 | 0 | 200 | 400 | 55,748 |
Table 6.
Composition of reactants to form magnetite.
Table 6.
Composition of reactants to form magnetite.
| Parameter | | Mol Mass | Fe | Final Sol | Stock Sol | Unit |
|---|
| g/mol | mg/L Fe | mmol/L | mmol/L | g/L | mL/L | Volume/Mass |
|---|
| Total Volume | mL | | | | | | | 1000 | mL |
| Fe2(SO4)3·5.4H2O | mmol | 496.90 | 2234 | 40 | 200 | 99.38 | | 200.0 | mL |
| H2SO4 | mmol | 98.00 | | 40 | 200 | 19.60 | 10.87 | 200.0 | mL |
| H2O | | | | | | | | 400.0 | mL |
| FeSO4·7H2O | mmol | 277.86 | 4468 | 40 | 200 | 55.57 | | 200.0 | mL |
| CaO | mmol | 56.00 | | 200 | 500 | | | 11.20 | g CaO |
| CaCO3 | mmol | 100.00 | | 200 | 500 | | | 20.00 | g CaCO3 |
| Na2CO3 | mmol | 106.00 | | 200 | | | | 21.20 | g Na2CO3 |
Table 7.
Magnetite formation in the absence of gypsum.
Table 7.
Magnetite formation in the absence of gypsum.
| Reactants | Feed | Treated |
|---|
| Conditions | | | | | | |
|---|
| Reagents | Conc. | mol Mass | Conc. | | | | | | |
|---|
| | mmol/L | g | g/L | | | | | | |
| Fe2(SO4)3·5.4H2O | 34.78 | 496.90 | 17.28 | | | | | | |
| H2SO4 | 34.78 | 98.00 | 3.41 | | | | | | |
| FeSO4·7H2O | 34.78 | 277.85 | 9.66 | | | | | | |
| NaOH for Fe3+ and Fe2+ | 304.35 | 40.00 | 12.17 | | | | | | |
| NaOH dosage (g/L) | | | | 0.00 | 12.17 | 12.18 | 12.21 | 12.24 | 12.28 |
| Results | | | | | | | | | |
| pH | | | | 1.80 | 7.00 | 8.20 | 9.08 | 10.12 | 11.08 |
| Fe2+ (mg/L) | | | | 2290 | 502.7 | 167.6 | 111.7 | 111.7 | 55.9 |
| Fe3+ (mg/L) | | | | 4580 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acidity (mg/L CaCO3) | | | | 19,800 | 900 | 200 | 0.00 | 0.00 | 0.00 |
| Temperature | | | | | Magnetic strength after heating (0—none; 10—strong) |
| 25 °C dry | | | | | 0.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| 50 °C dry | | | | | 0.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| 100 °C dry | | | | | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| 200 °C dry | | | | | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| 300 °C dry | | | | | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Temperature | | | | | Colour |
| 25 °C dry | | | | | Brown | Black | Black | Black | Black |
| 50 °C dry | | | | | Brown | Black | Black | Black | Black |
| 100 °C dry | | | | | Brown | Brown | Dark brown | Black | Black |
| 200 °C dry | | | | | Brown | Brown | Brown | Brown | Dark brown |
| 300 °C dry | | | | | Brown | Brown | Brown | Brown | Brown |
Table 8.
Effect of pH on the formation of goethite and magnetite.
Table 8.
Effect of pH on the formation of goethite and magnetite.
| Compound | Formula | pH |
|---|
| | | 8 | 11 |
|---|
| Gypsum | CaSO4·2H2O | 1.9 | 1.5 |
| Magnetite | Fe3O4 | 8.4 | 21.2 |
| Hematite | Fe2O3 | 0.0 | 0.0 |
| Thenardite | Na2SO4 | 53.0 | 57.3 |
| Goethite | HFeO2 | 36.7 | 20.0 |
| Total | | 100.0 | 100.0 |
| Colour | | Black | Black |
Table 9.
Fe(OH)3 and gypsum formation with CaCO3.
Table 9.
Fe(OH)3 and gypsum formation with CaCO3.
| Parameter | Feed | Treated |
|---|
| Water Quality | | | | | | |
|---|
| Time (h) | 0.0 | 0.3 | 1 | 3 | 4 | 5 |
| CaCO3 (g/L) | | 20.00 | | | | |
| pH | 1.3 | 6.1 | 6.2 | 5.4 | 5.9 | 6.1 |
| Fe2+ | 2457 | 2457 | 2457 | 2569 | 2457 | 2457 |
| Fe3+ | 4580 | 0 | 0 | 0 | 0 | 0 |
| Acidity | 19,800.0 | | | | | |
| Sludge colour | | Brown | Brown | Brown | Brown | Brown |
Table 10.
Effect of temperature on the formation of goethite and hematite (XRD Wt%).
Table 10.
Effect of temperature on the formation of goethite and hematite (XRD Wt%).
| Compound | Formula | Temperature (°C) |
|---|
| | | 100 | 300 |
|---|
| Gypsum | CaSO4·2H2O | 2.4 | 0.3 |
| Magnetite | Fe3O4 | 0.0 | 0.5 |
| Calcite | CaCO3 | | 12.1 |
| Bassanite | HCaSO4.5 | 86.3 | 43.4 |
| Anhydrite | CaSO4 | | 37.6 |
| Hematite | Fe2O3 | | 6.1 |
| Thenardite | Na2SO4 | 0.0 | |
| Goethite | HFeO2 | 11.3 | |
| Total | | 100.0 | 100.0 |
| Colour | | Yellow | Red |
Table 11.
Magnetite and gypsum formation with CaCO3 and CaO.
Table 11.
Magnetite and gypsum formation with CaCO3 and CaO.
| Parameter | Unit | Instant Dosing | Multiple Dosing |
|---|
| | | Feed | CaCO3 and CaO | Feed | CaCO3 | CaO |
|---|
| Dosing | | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Time | min | | 0 | 30 | 60 | 120 | 150 | | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 181 |
| CaCO3 | g | | 16.0 | | | | | | 4.0 | 4.0 | 4.0 | 4.0 | | | | |
| CaO | g | | | 3.36 | | | | | | | | | 1.12 | 1.12 | 1.12 | |
| pH | | 1.78 | 1.78 | 5.01 | 6.37 | 6.61 | 7.13 | 1.78 | 1.78 | 2.47 | 2.50 | 5.65 | 6.01 | 6.54 | 6.92 | 7.20 |
| Fe2+ | mg/L | 2402 | 2402 | 2290 | 1173 | 614 | 279 | 2402 | 2402 | 2346 | 2346 | 2346 | 2066 | 1396 | 559 | 223 |
| Fe3+ | mg/L | 4580 | 4580 | 0 | 0 | 0 | 0 | 4580 | 3407 | 614 | 167 | 0 | 0 | 0 | 0 | 0 |
| Acidity | mg/L | 19,800 | 4000 | 3600 | 3100 | 2700 | 800 | 19,800 | 19,400 | 12,000 | 9600 | 2900 | 2500 | 1300 | 800 | 600 |
| Temperature | °C | Magnetic strength after heating (0—none; 10—strong) pH 7.1 | Magnetic strength after heating (0—none; 10—strong) pH 7.2 |
| 50 | | 6.00 | 6.00 |
| 100 | | 8.00 | 8.00 |
| 200 | | 8.00 | 8.00 |
| 300 | | 8.00 | 8.00 |
| Temperature | °C | Colour | Colour |
| 50 | | Brown | Brown |
| 100 | | Black-grey | Black-grey |
| 200 | | Grey | Grey |
| 300 | | Brown | Brown |
Table 12.
Magnetite combined with gypsum.
Table 12.
Magnetite combined with gypsum.
| Compound | Formula | Composition (%) |
|---|
| | | Instant | Multiple |
|---|
| Gypsum | CaSO4·2H2O | 92.2 | 91.0 |
| Magnetite | Fe3O4 | 6.7 | 6.5 |
| Calcite | CaCO3 | 0.8 | 1.3 |
| Bassanite | HCaSO4.5 | 0.0 | 1.0 |
| Anhydrite | CaSO4 | 0.3 | 0.1 |
| Hematite | Fe2O3 | 0.0 | 0.1 |
| Thenardite | Na2SO4 | 0.0 | 0.0 |
| Goethite | HFeO2 | 0.0 | 0.0 |
Table 13.
Single and multiple dosing for sludge production.
Table 13.
Single and multiple dosing for sludge production.
| Parameter | Unit | Instant Dosing | Multiple Dosing |
|---|
| | | Feed | CaCO3 and CaO | Feed | CaCO3 | CaO |
|---|
| Dosing | | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Time | min | | 0 | 30 | 60 | 120 | 150 | | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 181 |
| CaCO3 | g | | 16.0 | | | | | | 4.0 | 4.0 | 4.0 | 4.0 | | | | |
| CaO | g | | | 3.36 | | | | | | | | | 1.12 | 1.12 | 1.12 | |
| pH | | 1.78 | 1.78 | 5.01 | 6.37 | 6.61 | 7.13 | 1.78 | 1.78 | 2.47 | 2.50 | 5.65 | 6.01 | 6.54 | 6.92 | 7.20 |
| Fe2+ | mg/L | 2402 | 2402 | 2290 | 1173 | 614 | 279 | 2402 | 2402 | 2346 | 2346 | 2346 | 2066 | 1396 | 559 | 223 |
| Fe3+ | mg/L | 4580 | 4580 | 0 | 0 | 0 | 0 | 4580 | 3407 | 614 | 167 | 0 | 0 | 0 | 0 | 0 |
| Acidity | mg/L | 19,800 | 4000 | 3600 | 3100 | 2700 | 800 | 19,800 | 19,400 | 12,000 | 9600 | 2900 | 2500 | 1300 | 800 | 600 |
Table 14.
Separation of gypsum and Fe3O4.
Table 14.
Separation of gypsum and Fe3O4.
| Parameter | Instant | Multiple |
|---|
| Time (h) | 24 | 24 |
| Feed | Fe3O4 Gypsum | Fe3O4 Gypsum |
| Total mass in 100 sat gypsum solution (Mg) | 500.00 | 500.00 |
| CaSO4·2H2On (Mg) | 370.00 | 370.00 |
| Fe3O4 (mg) | 130.00 | 130.00 |
| Dry product mass (mg) (100 °C) | 84.90 | 149.50 |
| Magnetite (XRD results) (%) | 6.50 | 11.70 |
| Magnetic | Yes | yes |
Table 15.
XRD results (Wt %).
Table 15.
XRD results (Wt %).
| Compound | Formula | Dosing |
|---|
| Instant (%) | Multiple (%) |
|---|
| Gypsum | CaSO4·2H2O | 91.0 | 82.1 |
| Magnetite | Fe3O4 | 6.5 | 11.7 |
| Calcite | CaCO3 | 1.3 | 5.8 |
| Bassanite | HCaSO4.5 | 1.0 | 0.0 |
| Anhydrite | CaSO4 | 0.1 | 0.1 |
| Hematite | Fe2O3 | 0.1 | 0.3 |
Table 16.
Production of Fe3O4 sludge.
Table 16.
Production of Fe3O4 sludge.
| Parameter | mmol/L | g | L | Mass (g) | Vol (mL) | Stock | g/L | Stock Volume |
|---|
| H2O | | | | | | | | 400 |
| H2SO4 | 4.0 | 98.0 | 1.0 | 0.39 | 0.22 | 5.0 | 1.96 | 200 |
| Fe2(SO4)3·5.4H2O | 4.0 | 496.9 | 1.0 | 1.99 | | 5.0 | 9.94 | 200 |
| FeSO4·7H2O | 4.0 | 277.9 | 1.0 | 1.11 | | 5.0 | 5.56 | 200 |
| CaO for Fe2+ | 5.0 | 56.0 | 1.0 | 0.28 | | | 0.28 | |
| Total Volume (mL) | | | | | | | | 1000 |
| Products | | | | | | | | |
| Fe3O4 | 4.0 | 231.6 | 1.00 | 0.93 | | 5.0 | | 200 |
| Gypsum | 21.0 | 172.0 | 1.00 | 3.61 | | 5.0 | | |
| Total | | 403.6 | 1.00 | 4.54 | | | | |
Table 17.
Production of Fe(OH)3-rich sludge.
Table 17.
Production of Fe(OH)3-rich sludge.
| Parameter | mmol/L | g | L | Mass | Vol | Stock | g/L | Stock Volume |
|---|
| | | | | g | mL | | | |
|---|
| H2O | | | | | | | | 400 |
| H2SO4 | 4.0 | 98.0 | 1.0 | 0.39 | 0.22 | 5.0 | 1.96 | 200 |
| Fe2(SO4)3·5.4H2O | 4.0 | 496.9 | 1.0 | 1.99 | | 5.0 | 9.94 | 200 |
| FeSO4·7H2O | 0.0 | 277.9 | 1.0 | 0.00 | | 5.0 | 0.00 | 200 |
| CaO for Fe2+ | 0.0 | 56.0 | 1.0 | 0.00 | | | 0.00 | |
| Total Volume (mL) | | | | | | | | 1000 |
| Products | | | | | | | | |
| Fe3O4 | 4.0 | 231.6 | 1.00 | 0.93 | | | | |
| Gypsum | 16.0 | 172.0 | 1.00 | 2.75 | | | | |
| Total | | 403.6 | 1.00 | 3.68 | | | | |
Table 18.
Settling rate of Fe3O4/gypsum-rich sludge.
Table 18.
Settling rate of Fe3O4/gypsum-rich sludge.
| Time | Height | Rate |
|---|
| Min | Mm | m/h |
|---|
| 0.0 | 166 | |
| 0.5 | 163 | 0.40 |
| 1.2 | 156 | 0.50 |
| 1.5 | 149 | 0.70 |
| 2.1 | 132 | 0.97 |
| 3.0 | 122 | 0.88 |
| 3.5 | 115 | 0.88 |
| 4.0 | 108 | 0.87 |
| 4.4 | 97 | 0.95 |
| 5.0 | 87 | 0.94 |
| 6.0 | 73 | 0.93 |
| Average | | 0.80 |
Table 19.
Settling rate of Fe(OH)3/gypsum-rich sludge.
Table 19.
Settling rate of Fe(OH)3/gypsum-rich sludge.
| Time | Height | Rate |
|---|
| Min | mm | m/h |
|---|
| 0 | 166 | |
| 2 | 160 | 0.18 |
| 4 | 149 | 0.26 |
| 7 | 143 | 0.20 |
| 9 | 136 | 0.20 |
| 13 | 125 | 0.19 |
| 15 | 115 | 0.20 |
| 18 | 104 | 0.21 |
| 20 | 99 | 0.20 |
| 24 | 80 | 0.22 |
| 27 | 70 | 0.21 |
| Average | | 0.21 |