Abstract
Iron ore is the main raw material of the iron and steel metallurgy industry, but quartz in iron ore reduces metallurgical efficiency and increases metallurgical costs. Therefore, iron ore desiliconization by flotation plays an important role in the iron and steel metallurgy industry. In this study, 4-tert-butyl-catechol (TBC) was designed as a collector to directly float out hematite from quartz. The micro-flotation tests demonstrated that under pH ~9.0, 1 × 10−5 mol·L−1 TBC recovered 98% hematite from its mixture with quartz, while the recovery of quartz was only about 17%. Zeta potential and contact angle results inferred that the adsorption affinity of TBC toward hematite was greater than that to quartz. The results of FTIR and XPS inferred that TBC adsorbed on the Fe3+ sites of hematite interfaced via the O atom of its two adjacent hydroxyl groups to form a stable five-membered chelating ring at pH 9.0. This study offered new research insight on the development of novel collectors for hematite flotation through bionics technology.
1. Introduction
Steel is one of the most important metal materials and it plays a vital role in human development and social progress [1]. With the development of the economy, the steel demand around the world continues to be high [2]. However, due to the depletion of high-quality iron ore resources, the quartz-associated iron ores have to be processed to enrich iron minerals [3]. In the smelting process, quartz significantly increases the consumption of alkaline fluxing agents (limestone) [4], which reduces the blast furnace efficiency and increases energy consumption, the amount of slag produced, and the costs. Therefore, the removal of quartz from iron concentrates is of great significance for the efficient utilization of iron ore resources [5]. As a crucial enrichment technology, the flotation separation of quartz from iron ores has also become one of the hot research areas.
Currently, there are three main approaches for separation of quartz from iron ores: reverse flotation with anionic collectors [6], reverse flotation with cationic collectors [7], and direct flotation [8]. In the reverse flotation process of hematite, the common anionic collectors are fatty acids. Bai et al.’s study showed that the flotation recovery of hematite by sodium oleate was over 80% at pH 8.2 [9], but sodium oleate exhibited poor solubility and flotation selectivity. The traditional cationic collectors were dominated by amine collectors, and dodecylamine is one of the most representative cationic collectors. Zhang et al.’s research showed that by using starch as a depressant, dodecylamine could float hematite from quartz [10], but dodecylamine also displayed some issues, such as viscous and big foam and weak solubility [11]. The ether amines are more soluble than fatty amines, while their low flotation selectivity and rich foaming property limit their wide application [12]. At present, fatty acids are dominant collectors used in the reverse flotation process of hematite production [13]. To achieve a satisfactory separation efficiency, the flotation process is recommended to be performed above 35 °C, which significantly increases the production costs [14]. Direct flotation is floating out iron oxide minerals from iron ores, thus removing quartz from iron concentrates [15]. The direct flotation collectors for hematite are anionic surfactants [16], such as fatty acids [17] and petroleum sulfonates [18], which have the advantages of a simple reagent scheme and low cost [19]. While using fatty acid collectors, the floatability of hematite is usually weak, and its separation efficiency from quartz is low [20]. In addition, to increase their dispersity in water, it is necessary to heat the flotation pulp [21]. To solve the negative impact of quartz on the steel metallurgy process, flotation desilication with more efficient collectors is a crucial research hotspot.
To address the above problems, researchers have developed some new collectors for flotation separation of hematite and quartz, such as dodecyl hydroxyethyl dimethyl ammonium bromide [22], poly (N-isopropyl acrylamide) [23], (E)-8,11-dihydroperoxyoctadec-9-enoic acid (EDEA) [24], sodium oleate (NaOL)-dodecylamine acetate (DAA) [25], modified sodium oleate (MSO) [26], mixed cationic C12 amine and anionic sulphate/oleate collectors [27], Curdlan [28], and innovative biosurfactants [29]. Luo et al. first synthesized a novel surfactant, 2-((2-(decyloxy)ethyl)amino)lauric acid (LDEA), substituting 3-(decyloxy)propan-1-amine (DPA) on the α-carbon position of lauric acid, and utilized it as a collector for the flotation of hematite mineral [30]. Luo et al. synthesized a new fatty acid collector, α-bromodecanoic acid, by introducing a strongly electronegative Br atom at the α-carbon position, which could complete floatation separation of hematite from quartz at 16 °C and pH 11.5, but it had large potential hazardous property [31]. Guo et al. designed a novel quaternary ammonium collector, N-(2-hydroxyethyl)-N, N-dimethyl-3-[(1-oxododecyl)amino]-1-propanaminium, which exhibited good water solubility and effectively floated hematite from quartz by using starch as the depressant [32]. Liu et al. synthesized a novel collector, N-(2,3-propanediol)-N-dodecylamine (PDDA), by introducing one propylene glycol group into DDA, which had better solubility and hydrophobicity than DDA [33]. Although these new collectors alleviated the shortcomings of traditional collectors, the problems of high costs, the need to add a depressant, and low-temperature performance are still serious. Therefore, it is very important to develop environmentally friendly, economical, efficient, and temperature-adaptive collectors.
Bionics is a pivotal field to promote the technological innovation and progress [34]. Researchers have crafted innovative materials, including hydrogels and membranes, drawing inspiration from the adhesive properties of marine mussels [35]. From their research, catechol is a unique structural component [36], which could interact with surfaces through hydrogen bonding, coordination bonds, or hydrophobic interactions [36,37,38,39,40]. Especially, catechol exhibits a strong affinity to iron and its oxides. Additionally, catechol compounds possess good solubility and low-temperature dispersity performance [41]. This shows that catechol has a strong affinity for iron minerals and may have better solubility and low-temperature performance than sodium oleate. Therefore, it is interesting to introduce catechol surfactants as flotation collectors for hematite separation and purification.
This paper investigates the performance of 4-tert-butylcatechol (TBC) in flotation separation of hematite from quartz. Afterwards, the flotation mechanism of TBC to hematite is explored via the contact angle, zeta potential, adsorption capacity, Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS). This work also provides new research insight on the development of novel collectors through bionics technology.
2. Materials and Experimental Methods
2.1. Materials
The high-purity samples of hematite and quartz were purchased from Ye Mineral Company, Guangzhou, China, and their 38–74 μm particles were prepared sequentially by crushing, grinding, and sieving. The results of their X-ray diffraction (XRD) and X-ray fluorescence (XRF) are shown in Figure 1 and Table 1, respectively, indicating a high purity.
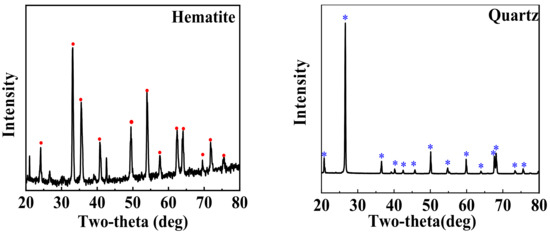
Figure 1.
XRD patterns of hematite and quartz (red dot: the peak of hematite crystal, blue *: the peak of quartz crystal).

Table 1.
The XRF of hematite and quartz.
TBC, hydrochloric acid (HCl), and sodium hydroxide solutions (NaOH) were obtained from Aladdin Reagents Company. Distilled water was used throughout the tests.
2.2. Micro-Flotation Tests
The micro-flotation experiments were performed in a Hallimond tube with 220 mL volume [43], and the operation processes are shown in Figure 2. Each experiment used 2 g samples (the mass ratio of hematite to quartz for their artificial mixture flotation was 1:1), with a particle size ranging from 38 to 74 μm. The pulp pH was regulated using NaOH and HCl solutions [44]. After weighing and analyzing the tailings and concentrates, as listed in Figure 2, the flotation recovery was calculated by Equation (1) (for single mineral) or Equations (2) and (3) (for the mixed minerals) [45]. In Equations (1)–(3), m1 and m2 represent the weight of concentrates and tailings, β1 and β2 are the hematite content in concentrates and tailings, respectively, and r represents the flotation recovery. The reported results were the average recovery of three independent tests:
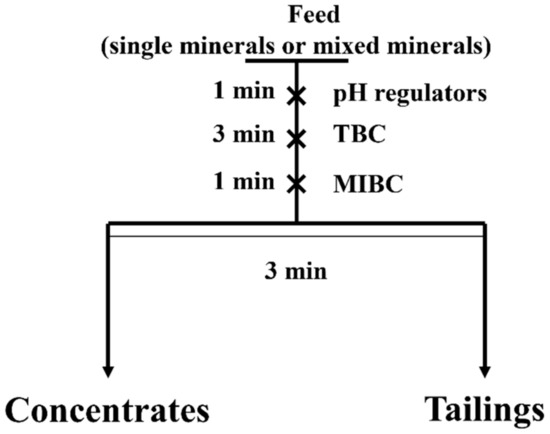
Figure 2.
The operation process of flotation.
2.3. Contact Angle (CA) Measurements
The slices of hematite and quartz were polished by SiC sandpaper [46]. After that, they were submerged in 220 mL of 1.0 × 10−5 mol·L−1 TBC solutions for 10 min. Following that, the slices were removed and washed, and their contact angles were determined using the JC2000C contact-angle device (Zhongchen Digital China, Shanghai, China) [47].
2.4. Zeta Potential Experiments
The −5 μm mineral particles were used to measure their zeta potential on a Brookhaven’s ZetaPlus analyzer (Brookhaven Instruments, New York, NY, USA). After adding 50 mg samples into 50 mL of 1.0 × 10−2 mol·L−1 KCl electrolyte, NaOH or HCl solutions were added to regulate the suspension pH. Then, 1 × 10−5 mol·L−1 TBC solutions were added into the suspension, which was stirred for an extra 10 min before the zeta potential was measured.
2.5. The Adsorption Capacity Measurement
The spectrophotometry approach was used to determine the adsorption capacity of TBC on the hematite surface with a UV-visible spectrophotometer (Evolution 201, Thermo Fisher Scientific, Waltham, MA, USA) [48]. In each experiment, 0.2 g of mineral samples was placed in a conical flask containing distilled water, and the pH was adjusted. Then, the concentration of TBC was adjusted to 1.1 × 10−4 mol·L−1 (18.26 mg·L−1). After shaking the slurry for 12 h, the TBC concentration in the supernatant was calculated by the measured absorbance. Based on the concentration difference of TBC in solutions before and after adsorption, the adsorption capacity of TBC on hematite surfaces was obtained.
2.6. FTIR Tests
The FTIR spectra of TBC, hematite, and TBC-treated hematite were measured on a Nicolet FTIR-740 spectrometer (Thermo Nicolet Corporation, Madison, WI, USA) [49]. The suspension, which contained 0.5 g of minerals (−5 μm) and 50 mL of 1.0 × 10−4 mol·L−1 TBC solutions (pH ~9.0), was stirred at 25 °C for 6 h. The TBC-treated hematite was then obtained by filtrating the suspension and washing the filter cake three times with water. After drying in a vacuum oven, the FTIR spectra were measured through the KBr disk approach.
2.7. XPS Tests
Thermo Fisher Scientific’s ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used for the XPS experiments. The X-ray source was an Al Kα ray with 1486.7 eV photoelectron energy. The vacuum degree of the test chamber was 10−9–10−8 Torr, and the pass energy was set as 20.0 eV. After being corrected with 284.8 eV C 1s binding energy, the XPS curves were fitted and analyzed using Thermo Avantage 5.9 software [50].
3. Results and Discussion
3.1. Micro-Flotation
3.1.1. Single Mineral
Figure 3 shows the effect of pH and the initial TBC concentration on the flotation recovery of hematite and quartz. Figure 3A indicates that the recovery of hematite increased with rising pH and reached 88.47% at pH ~9.0. Then, hematite recovery decreased with the increase in pH. Compared with hematite, the flotation recovery of quartz was below 25% at pH 6.0–11.0. Thus, the flotation separation of hematite from quartz by TBC might be favorable at pH ~9.0. Figure 3B shows that as the initial concentration of TBC increased from 3 × 10−6 to 15 × 10−6 mol·L−1, the recovery of hematite increased significantly from 6.09% to 93.78%, while the recovery of quartz only slightly increased from 11.34% to 29.74%.
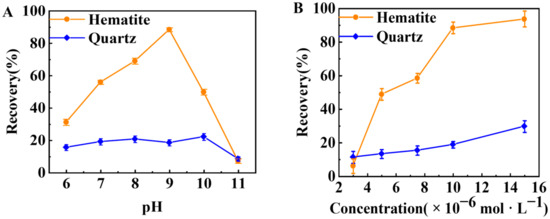
Figure 3.
Effect of (A) pH (1 × 10−5 mol·L−1 TBC) and (B) the initial TBC concentration (pH ~9.0) on the flotation recovery of hematite and quartz.
3.1.2. Artificially Mixed Minerals
The flotation performances of TBC were further investigated by artificially mixed minerals. Figure 4 shows the flotation recovery of hematite and quartz from their artificially mixed minerals as a function of pH and the initial TBC concentration. Figure 4A indicates that with rising pH, the flotation recovery of hematite increased and reached 96.31% at pH 9.0, and then decreased. Meanwhile, the recovery of quartz remained below 22% at pH 6.0–11.0. Figure 4B shows that as the initial TBC concentration increased from 5 × 10−6 to 1 × 10−5 mol·L−1, the recovery of hematite increased from 61.52% to 98.31%, while the recovery of quartz only increased from 10.23% to 17.61%. Therefore, TBC was an efficient collector for the flotation separation of hematite from quartz.
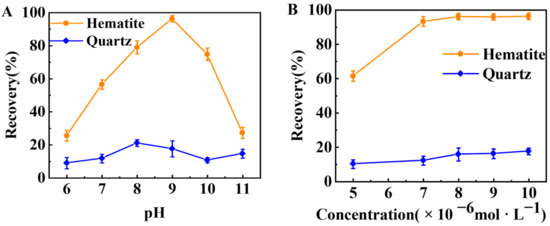
Figure 4.
Flotation recovery of hematite and quartz in the artificially mixed minerals as a function of (A) pH (1 × 10−5 mol·L−1 TBC) and (B) the initial TBC concentration (pH ~9.0).
3.1.3. The Effect of Pulp Temperature
The effect of temperature on the flotation recovery of hematite and quartz from their mixture by TBC was further investigated. Figure 5 shows that as the temperature increased from 5 to 30 °C, the recovery of hematite rose from 42.54% to 96.92%, while the recovery of quartz only increased from 9.78% to 24.74%. At 20 °C, the recoveries of hematite and quartz were 84.31% and 16.22%, respectively. This meant that the favorable temperature for TBC flotation separation of hematite and quartz was above 20 °C. The flotation temperature of sodium oleate is generally higher than 35 °C [14], so TBC has stronger temperature applicability than sodium oleate.
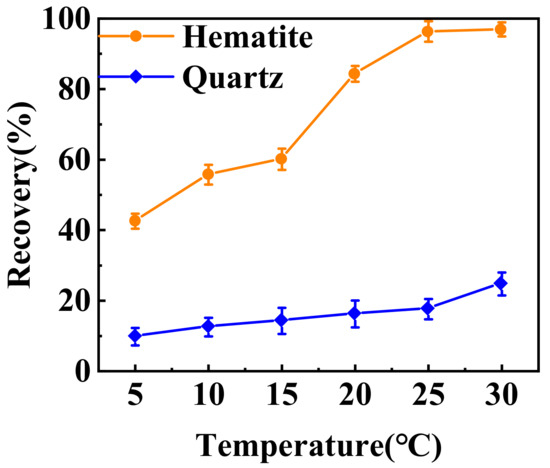
Figure 5.
Flotation recovery of hematite and quartz in the artificially mixed minerals function of pulp temperature (pH ~9.0 and 1 × 10−5 mol·L−1 TBC).
3.2. Contact Angle
Figure 6 shows the effect of pH on the contact angle of TBC-treated hematite and TBC-treated quartz. The contact angles of fresh hematite and quartz were 18° and 13°, respectively. As represented in Figure 6A, after being treated with 1 × 10−5 mol·L−1 TBC, the contact angle of hematite increased from 32° to 48° as the pH increased from 6.0 to 9.0, and then decreased rapidly when the pH increased to 11.0. These results were consistent with the effect of pH on the flotation recovery of hematite. Figure 6B shows that after TBC treatment, the contact angle of the hematite surface was significantly greater than that of the quartz surface at pH 7.0–10.0. Therefore, the contact angle results deduced that the hydrophobic effect of TBC on the hematite surface was stronger than that on the quartz surface.
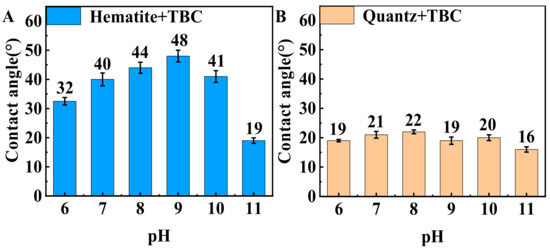
Figure 6.
The contact angle of the (A) TBC-treated hematite and (B) TBC-treated quartz as a function of pH (1.0 × 10−5 mol·L−1 TBC).
3.3. Zeta Potential
Figure 7 shows the effect of pH on the zeta potential of quartz and hematite with or without TBC. As shown in Figure 7A, the isoelectric point (IEP) of hematite was observed near pH ~5.0, which was consistent with the reported value [51]. In the presence of TBC, the zeta potential of hematite particles decreased, especially under pH > 6.0, inferring an adsorption of TBC on the surface of hematite.
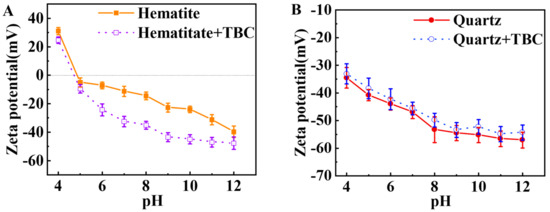
Figure 7.
Effect of pH on the zeta potential of (A) quartz and (B) hematite before and after 1 × 10−5 mol·L−1 TBC treatment.
As shown in Figure 7B, the zeta potential of quartz was negative at pH 4.0–12.0. In the presence of TBC, the zeta potential of quartz particles slightly shifted positively, implying a weak adsorption of TBC on the quartz surface. The zeta potential results deduced the superior adsorption affinity of TBC to hematite rather than quartz under pH > 6.0.
3.4. The Adsorption Capacity of TBC
The adsorption capacity of TBC on the surface of hematite under different pH values is shown in Figure 8, which indicates that as the pH increased from 6.0 to 9.0, the adsorption capacity of TBC on hematite increased from 6.33 to 15.24 mg·g−1; subsequently, with pH increasing to 10.0 and 11.0, the adsorption capacity of TBC decreased to 12.44 and 1.93 mg·g−1, respectively. The results revealed that the preferable pH value for TBC adsorption onto the hematite surface appeared at 8.0–10.0, being in agreement with the flotation and contact angle findings.
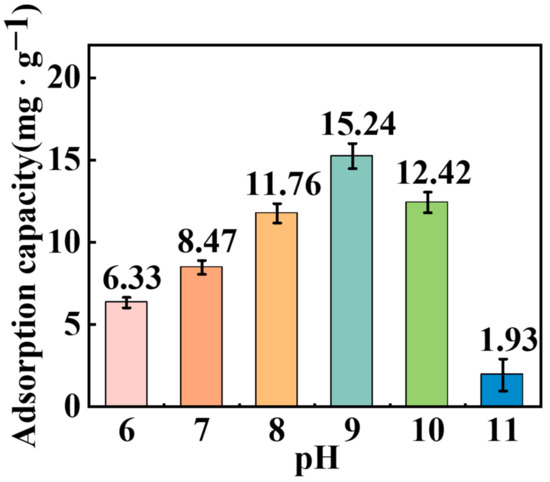
Figure 8.
Effect of pH on the adsorption of TBC on the surface of hematite (1.1 × 10−3 mol·L−1 TBC initial concentration).
3.5. FTIR Analyses
The FTIR spectra of TBC, hematite, and TBC-treated hematite are listed in Figure 9. For TBC, the peaks at 3060 and 2961 cm−1 corresponded to the C-H stretching vibrations of the benzene ring and methyl groups [52], respectively. The C=C vibrations of the benzene ring appeared at 1600 and 1521 cm−1, the hydroxyl (-OH) peaks emerged at 3344, 1367, and 1119 cm−1 [53], and the C-C vibrations of the tert-butyl group appeared at 1276 cm−1. After the interaction of TBC with hematite, the new peaks at 1478 and 2961 cm−1 appeared on the surface of hematite, which were assigned to the C=C vibrations of the benzene ring and the C-H vibrations of the methyl group, respectively. The FTIR spectroscopy results suggested the adsorption of TBC on the hematite surface.
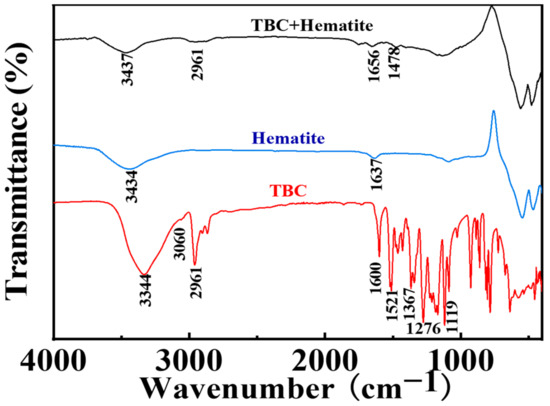
Figure 9.
FTIR spectra of TBC and hematite before and after the reaction with TBC.
3.6. XPS Results
The adsorption mechanism of TBC on hematite at pH ~9 was further explored by XPS. Figure 10A,B show the survey XPS spectra and interface element contents of hematite before and after TBC treatment, which demonstrated that after TBC adsorption, the atomic proportion of C in the interface of hematite increased significantly, while that of O and Fe decreased, inferring the cover of TBC on the hematite surface.
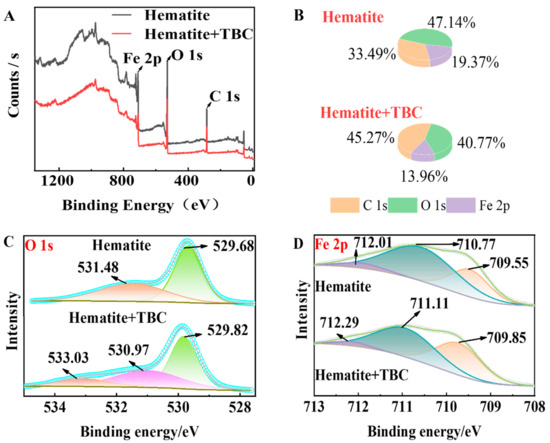
Figure 10.
XPS spectrum (A) and atomic concentration (B) of hematite before and after TBC treatment, and XPS high-resolution (C) O1s and (D) Fe 2p3/2 spectra of hematite before and after TBC treatment.
The Fe 2p3/2 and O1s high-resolution XPS spectra of hematite before and after TBC treatment are shown in Figure 10C,D. Figure 10C indicates that the O1s XPS of the pure hematite were composed of two peaks at 529.68 and 531.48 eV, which were attributed to the lattice and interface O atoms of hematite, respectively [54,55]. After TBC treatment, a new peak at 533.03 eV appeared on the hematite surface, which might be due to the O atoms of TBC chemisorbed on hematite. The XPS peaks of the lattice and interface O atoms for the TBC-modified hematite appeared at 529.82 and 530.97 eV, respectively. The lowered binding energy of interface O atoms implied that some OH species might be removed from the hematite surface.
Figure 10D demonstrates that the Fe 2p3/2 XPS spectra of pure hematite were divided into three peaks at ~712.01, 710.77, and 709.55 eV, which were assigned to Fe(III), Fe(III), and Fe(II) adsorption bands, respectively [56,57,58]. After TBC treatment, the three Fe 2p3/2 XPS peaks all shifted to the higher binding energies of ~712.29, 711.11, and 709.85 eV [59], and the proportion of adsorption bands at 709.85 eV increased significantly. These results implied that TBC chemisorbed on the hematite surface, accompanied by the reduction of some Fe(III) into Fe(II).
As a whole, the fine XPS of Fe 2p3/2 and O1s suggested that the TBC–Fe complexes were formed on the hematite surface where the O atoms of TBC bonded with the surface Fe atom(s) of hematite.
3.7. Discussion
The binding model of the catechol group of Dopa to the metal material surface involved at least three different forms: molecular adsorption (through H-bond), partially dissociated monodentate adsorption, and fully dissociated bidentate adsorption [60]. Dopa could strongly interact with Fe(III) or Fe(II) via its catechol group to form the stable complexes [61].
The zeta potential results deduced a special affinity of hematite toward TBC, and the adsorption capacity experiments revealed that the preferable pH for hematite adsorption of TBC appeared at 8.0–10.0, being in agreement with the flotation and contact angle findings. In addition, the FTIR spectra suggested the adsorption of TBC on the hematite surface, while the XPS suggested that the TBC–Fe complexes were formed on the hematite surface where TBC’s two O atoms bonded with the surface Fe atom(s) of hematite.
The pKa1 and pKa2 of TBC were 9.5 and 14.0 [62], respectively. Under pH < 8.0, TBC existed in water solutions mainly as its molecular species, at pH 8.0–10.0, TBC existed as its deprotonated form TBC−, and under pH > 14.5, the completely deprotonated species TBC2− dominated in solutions. The molecular species of TBC might interact with hematite through weak interactions, such as the H-bond, which renders TBC with a weak collecting ability to hematite. The deprotonated form TBC− possessed a strong electron-donating ability, which could bond iron atoms on the hematite surface. As a result, TBC− improved the hydrophobicity and floatability of hematite under pH 8.0–10.0. Although the completely deprotonated species TBC2− had a stronger electron-donating ability, under pH > 10.0, TBC was easy to auto-oxidize to quinone configuration, which dramatically weakened its affinity to iron atoms. Therefore, TBC did not return good results of flotation, contact angle, adsorption capacity, and zeta potential under pH > 10.0.
Based on the above experimental results and analyses, a possible adsorption model of TBC on the hematite surface at pH ~9.0 was suggested and shown in Figure 11. It demonstrated that TBC’s two O atoms bonded with the surface Fe atom(s) of hematite to form the five-membered chelating ring, while the H atom of TBC’s hydroxyl formed an H-bond with the O atom of the hematite surface.
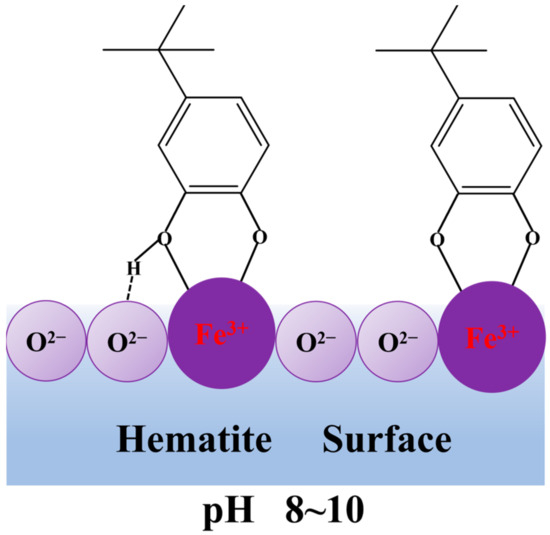
Figure 11.
Possible adsorption model of TBC on the hematite surface.
4. Conclusions
Using TBC as a collector, the flotation separation of hematite from quartz was carried out. Based on the experimental findings and discussion, the main conclusions were drawn, as follows:
- (1)
- At pH 9.0, 1 × 10−5 mol·L−1 TBC recovered over 96% hematite and only 17% quartz from the artificial mixture. TBC exhibited an excellent flotation selectivity for separation of hematite from quartz.
- (2)
- TBC significantly enhanced the hydrophobicity of the hematite surface, while it had little effect on that of quartz, which increased their surface hydrophobicity difference, being a key factor to realize their selective flotation separation.
- (3)
- At pH 9.0, TBC adsorbed on the Fe-active site on the hematite interface mainly via its deprotonated species TBC− to form a stable five-membered chelating ring.
Author Contributions
Conceptualization, C.D. and G.L.; methodology, C.D. and G.L.; validation, C.D.; formal analysis, C.D. and J.Y.; resources, G.L.; data curation, C.D.; writing—original draft preparation, C.D. and J.Y.; writing—review and editing, C.D., J.Y. and G.L.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (51925406).
Data Availability Statement
The data that support the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bulayani, M.M.; Raghupatruni, P.; Mamvura, T.; Danha, G. Exploring Low-Grade Iron Ore Beneficiation Techniques: A Comprehensive Review. Minerals 2024, 14, 796. [Google Scholar] [CrossRef]
- Yuan, S.; Xiao, H.; Wang, R.; Li, Y.; Gao, P. Improved iron recovery from low-grade iron ore by efficient suspension magnetization roasting and magnetic separation. Miner. Eng. 2022, 186, 107761. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.-Z.; Yao, J.; Zhu, Z.-L.; Sun, H.-R.; Chen, K.-Q.; Wang, L.-Y. Differential adsorption of a high-performance collector at solid–liquid interface for the selective flotation of hematite from quartz. J. Mol. Liq. 2021, 339, 116828. [Google Scholar] [CrossRef]
- Moon, S.; Kim, E.; Noh, S.; Triwigati, P.T.; Choi, S.; Park, Y. Carbon mineralization of steel and iron-making slag: Paving the way for a sustainable and carbon-neutral future. J. Environ. Chem. Eng. 2024, 12, 112448. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Xie, X.; Tong, X.; Zhu, Y.; Xie, R. New Low-Temperature Collector for Flotation Separation of Quartz and Hematite after Reduction Roasting and Its Mechanism. Langmuir 2024, 40, 23986–23993. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Guo, Z.; Sun, W.; Zhang, C. Selective separation mechanism of hematite from quartz by anionic reverse flotation: Implications from surface hydroxylation. Appl. Surf. Sci. 2023, 614, 156056. [Google Scholar] [CrossRef]
- Sahoo, H.; Rath, S.S.; Rao, D.S.; Mishra, B.K.; Das, B. Role of silica and alumina content in the flotation of iron ores. Int. J. Miner. Process. 2016, 148, 83–91. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Bai, S.; Li, J.; Bi, Y.; Yuan, J.; Wen, S.; Ding, Z. Adsorption of sodium oleate at the microfine hematite/aqueous solution interface and its consequences for flotation. Int. J. Min. Sci. Technol. 2023, 33, 105–113. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Sun, W.; Chen, D.; Li, S.; Han, M.; Yu, H.; Zhang, C. Selective adsorption mechanism of dodecylamine on the hydrated surface of hematite and quartz. Sep. Purif. Technol. 2021, 275, 119137. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite—Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Li, W.; Song, Y.; Xu, W.; Li, K.; Zhang, Y. Mechanism of Modified Ether Amine Agents in Petalite and Quartz Flotation Systems under Weak Alkaline Conditions. Minerals 2023, 13, 825. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, L.; Chen, W. Probing the role of hydrophobic groups on the performance of fatty acid surfactants for hematite flotation. J. Mol. Liq. 2024, 411, 125674. [Google Scholar] [CrossRef]
- Cao, S.; Yin, W.; Yang, B.; Zhu, Z.; Sun, H.; Sheng, Q.; Chen, K. Insights into the influence of temperature on the adsorption behavior of sodium oleate and its response to flotation of quartz. Int. J. Min. Sci. Technol. 2022, 32, 399–409. [Google Scholar] [CrossRef]
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- Ma, M. Froth Flotation of Iron Ores. Int. J. Min. Eng. Miner. Process. 2012, 1, 56–61. [Google Scholar] [CrossRef]
- Hou, Y.; Sobhy, A. New Insights on Sodium Oleate Adsorption on Quartz for Iron Direct Flotation under Weak-Acidic Condition. Tenside Surfactants Deterg. 2021, 58, 237–242. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Han, Y.; Parra-Álvarez, N.; Claremboux, V.; Kawatra, S.K. Flotation of Iron Ores: A Review. Miner. Process. Extr. Metall. Rev. 2021, 42, 184–212. [Google Scholar] [CrossRef]
- Houot, R. Beneficiation of iron ore by flotation—Review of industrial and potential applications. Int. J. Miner. Process. 1983, 10, 183–204. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Niu, F.; Gao, S. Molecular dynamics study on selective flotation of hematite with sodium oleate collector and starch-acrylamide flocculant. Appl. Surf. Sci. 2022, 592, 153208. [Google Scholar] [CrossRef]
- Luo, A.; Chen, J. Low-temperature collector for smithsonite flotation: Experiments and DFTB+ study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133651. [Google Scholar] [CrossRef]
- Liu, W.; Tong, K.; Ding, R.; Liu, W.; Zhao, P.; Sun, W.; Zhao, Q.; Zhao, S. Synthesis of a novel hydroxyl quaternary ammonium collector and its selective flotation separation of quartz from hematite. Miner. Eng. 2023, 200, 108109. [Google Scholar] [CrossRef]
- Ng, W.S.; Sonsie, R.; Forbes, E.; Franks, G.V. Flocculation/flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector. Miner. Eng. 2015, 77, 64–71. [Google Scholar] [CrossRef]
- Fang, J.; Ge, Y.; Liu, S.; Yu, J.; Liu, C. Investigations on a novel collector for anionic reverse flotation separation of quartz from iron ores. Physicochem. Probl. Miner. Process. 2021, 57, 136–155. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Yang, Y.; Liu, J.; Zeng, X.; Deng, W. Selective flotation separation of ilmenite from titanaugite using mixed anionic/cationic collectors. Int. J. Miner. Process. 2017, 166, 102–107. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Y.; Cao, Y. Reverse Flotation of Quartz from Magnetite Ore with Modified Sodium Oleate. Miner. Process. Extr. Metall. Rev. 2013, 34, 320–330. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Kumari, N.; Bhagat, R.P. Adsorption mechanism of mixed collector systems on hematite flotation. Miner. Eng. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Han, W.; Zhu, Y.; Ge, W.; Liu, J.; Li, Y. Curdlan as a new depressant of hematite for quartz-hematite reverse flotation separation. Miner. Eng. 2022, 185, 107708. [Google Scholar] [CrossRef]
- Pereira, A.R.M.; Hacha, R.R.; Torem, M.L.; Merma, A.G.; Silvas, F.P.C.; Abhilash, A. Direct hematite flotation from an iron ore tailing using an innovative biosurfactant. Sep. Sci. Technol. 2021, 56, 2978–2988. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Y.; Sun, C.; Li, Y.; Han, Y. The flotation behavior and adsorption mechanisms of 2-((2-(decyloxy)ethyl)amino)lauric acid on quartz surface. Miner. Eng. 2018, 117, 121–126. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Y.; Sun, C.; Li, Y.; Han, Y. Flotation and adsorption of a new collector α-Bromodecanoic acid on quartz surface. Miner. Eng. 2015, 77, 86–92. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Liu, W.; Zhao, P.; Chen, X. Efficient separation of quartz from hematite for a novel quaternary ammonium collector: Separation performance, comparative study and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134564. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Tong, K.; Shen, Y.; Zhao, Q.; Zhao, S.; Sun, W. Novel polyhydroxy cationic collector N-(2,3-propanediol)-N-dodecylamine: Synthesis and flotation performance to hematite and quartz. Int. J. Min. Sci. Technol. 2023, 33, 115–122. [Google Scholar] [CrossRef]
- Dou, W.; Zeng, X.; Zhu, S.; Zhu, Y.; Liu, H.; Li, S. Mussel-Inspired Injectable Adhesive Hydrogels for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 9100. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Liu, J.; Zeng, H. Mussel-Inspired Cation−π Interactions: Wet Adhesion and Biomimetic Materials. Langmuir 2023, 39, 17600–17610. [Google Scholar] [CrossRef]
- Maier, G.; Bernt, C.; Butler, A. Catechol oxidation: Considerations in the design of wet adhesive materials. Biomater. Sci. 2018, 6, 332–339. [Google Scholar] [CrossRef]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic Enhancement of Dopa-Mediated Adhesion in a Mussel Foot Protein. J. Am. Chem. Soc. 2013, 135, 377–383. [Google Scholar] [CrossRef]
- Yu, J.; Kan, Y.; Rapp, M.; Danner, E.; Wei, W.; Das, S.; Miller, D.R.; Chen, Y.; Waite, J.H.; Israelachvili, J.N. Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proc. Natl. Acad. Sci. USA 2013, 110, 15680–15685. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Xu, H.; Nishida, J.; Ma, W.; Wu, H.; Kobayashi, M.; Otsuka, H.; Takahara, A. Competition between Oxidation and Coordination in Cross-Linking of Polystyrene Copolymer Containing Catechol Groups. ACS Macro Lett. 2012, 1, 457–460. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Liu, W.; Wang, W.; Wang, W. Catechol functionalized hyperbranched polymers as biomedical materials. Prog. Polym. Sci. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Liu, G.; Liu, Y.; Zhong, H. Modulation of the morphology, surface energy and wettability of malachite through a S,O,O-ligand surfactant: Mechanism and hydrophobization. Appl. Surf. Sci. 2020, 505, 144467. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Contact Angle of Bubble with an Immersed-in-Water Particle of Different Materials. Ind. Eng. Chem. Res. 2011, 50, 4207–4211. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, M.; Qiu, Z.; Liu, S.; Yang, L.; Chen, W.; Liu, G. The depressant-free flotation separation of Cu/Zn sulfide minerals with an environmentally friendly triazine-thiol collector. Appl. Surf. Sci. 2024, 678, 161102. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, F.; Qin, W.; Wang, C.; Li, X. Flotation separation of sphalerite from galena using eco-friendly and efficient depressant pullulan. Sep. Purif. Technol. 2022, 295, 121013. [Google Scholar] [CrossRef]
- Qi, J.; Liu, G.; Dong, Y. Probing the hydrophobic mechanism of N-[(3-hydroxyamino)-propoxy]-N-octyl dithiocarbamate toward bastnaesite flotation by in situ AFM, FTIR and XPS. J. Colloid Interface Sci. 2020, 572, 179–189. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, S.; Qi, J.; Yang, L.; Liu, G. Tungsten minerals flotation with 4-alkoxy benzohydroxamic acid: The structure-performance relationship of its C3 derivatives. Miner. Eng. 2023, 203, 108361. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, G.; Liu, S.; Chen, W.; Liu, G. Strengthening flotation enrichment of Pb(Ⅱ)-activated scheelite with N-[(3-hydroxyamino)-propoxy]-N-hexyl dithiocarbamate. J. Ind. Eng. Chem. 2022, 114, 338–346. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Yang, L.; Liu, S.; Chen, W.; Liu, G. The low-carbon flotation separation of chalcopyrite from pyrite with a fire-new alkylamine-triazine-dithiol collector. Appl. Surf. Sci. 2023, 640, 158338. [Google Scholar] [CrossRef]
- Quast, K. The use of zeta potential to investigate the interaction of oleate on hematite. Miner. Eng. 2016, 85, 130–137. [Google Scholar] [CrossRef]
- Roman, C.; Roman, T.; Arsene, C.; Bejan, I.-G.; Olariu, R.-I. Gas-phase IR cross-sections and single crystal structures data for atmospheric relevant nitrocatechols. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120379. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, R.A. (Ed.) Chapter 7-Alcohols and Phenols. In Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: Cambridge, MA, USA, 2001; pp. 125–141. [Google Scholar]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Han, W.; Zhu, Y.; Liu, J.; Li, Y. A novel depressant HPAM of the hematite in reverse cationic flotation of iron ore. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128547. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS and auger spectroscopy studies on mixtures of the oxides SiO2, Al2O3, Fe2O3 and Cr2O3. J. Electron Spectrosc. Relat. Phenom. 1987, 43, 97–112. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Y.V.; Leonhardt, G.; Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 121–124. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Soraya, D.A.D.; Li, C.; Li, H. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127267. [Google Scholar] [CrossRef]
- Terranova, U.; Bowler, D. Adsorption of Catechol on TiO2 Rutile (100): A Density Functional Theory Investigation. J. Phys. Chem. C 2010, 114, 6491–6495. [Google Scholar] [CrossRef]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef]
- Slabbert, N.P. Ionisation of some flavanols and dihydroflavonols. Tetrahedron 1977, 33, 821–824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).