Bismuth Sulfosalts from the Nistru Metallogenetic Field, Baia Mare Zone, NW Romania
Abstract
1. Introduction
2. Geological Setting
3. Hydrothermal Mineralization
4. Materials and Methods
4.1. Reflected Light Microscopy
4.2. Electron Probe Microanalysis
4.3. Fluid Inclusion Microthermometry
5. Results
5.1. Mineral Assemblages
5.2. Bismuth Minerals
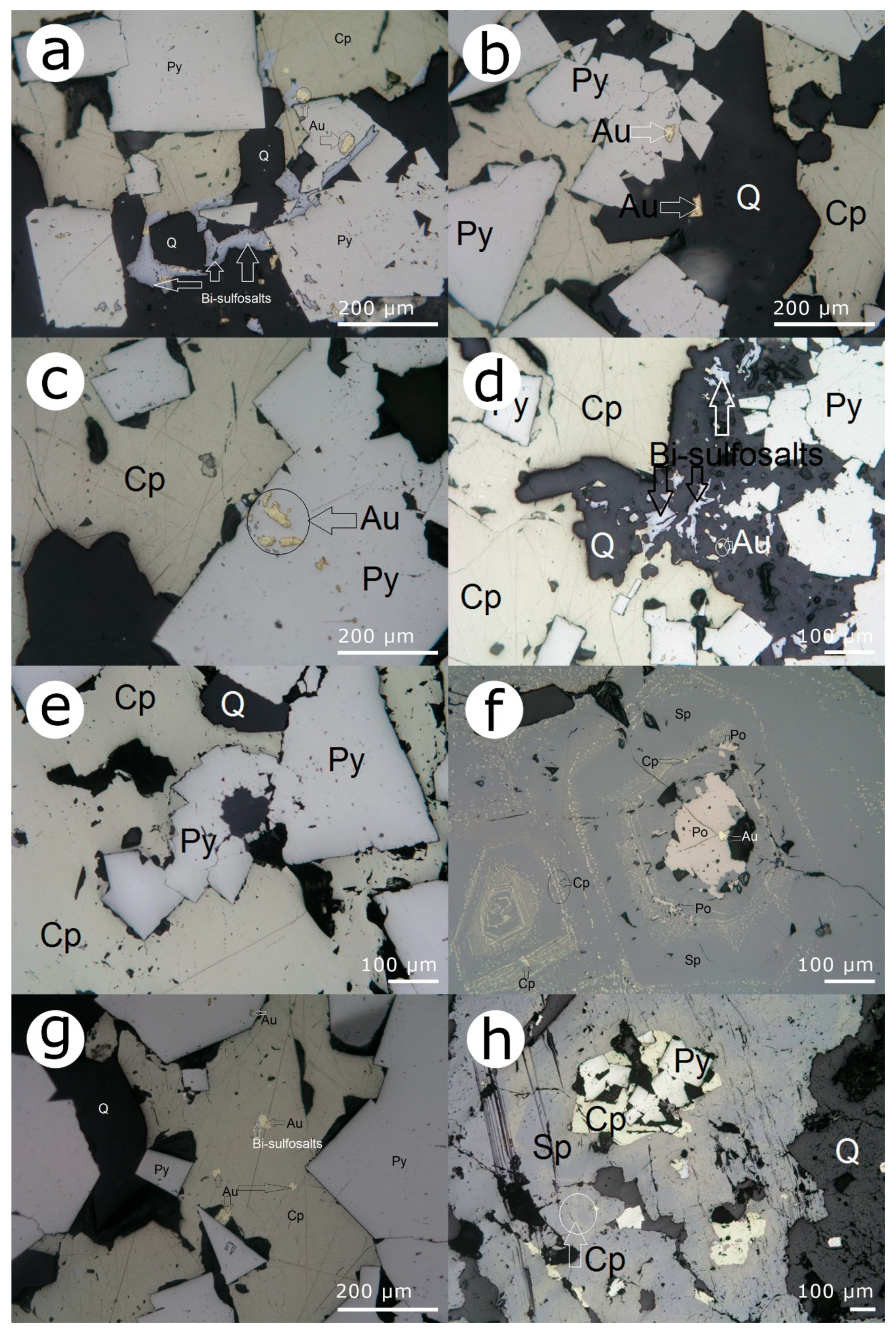
- (i)
- As inclusions in coarse chalcopyrite that cements grains of corroded pyrite (Figure 3a).
- (ii)
- Along the marginal areas of coarse chalcopyrite at the margin adjoining quartz from the vein matrix, associated with isometric native gold (Figure 3b,e).
- (iii)
- As anhedral crystals, associated with the gold and chalcopyrite that are included in the quartz matrix (Figure 3c).
- (iv)
- Commonly, as grains dispersed in the quartz vein matrix.
- (v)
- As aggregates in which bismuthinite derivatives and lillianite homologs occur either alone or intergrown with one another (Figure 3d,f).
- (vi)
- Lillianite-gustavite crystals with matildite inclusions are associated with bismuthine lamellae (Figure 4b,c).
- (vii)
- Lamellar to granular crystals of lillianite-gustavite included in chalcopyrite are rimmed by a thin film of wittichenite (Figure 3c).
- (viii)
- Occurs sporadically as inclusions in chalcopyrite (Figure 3f).

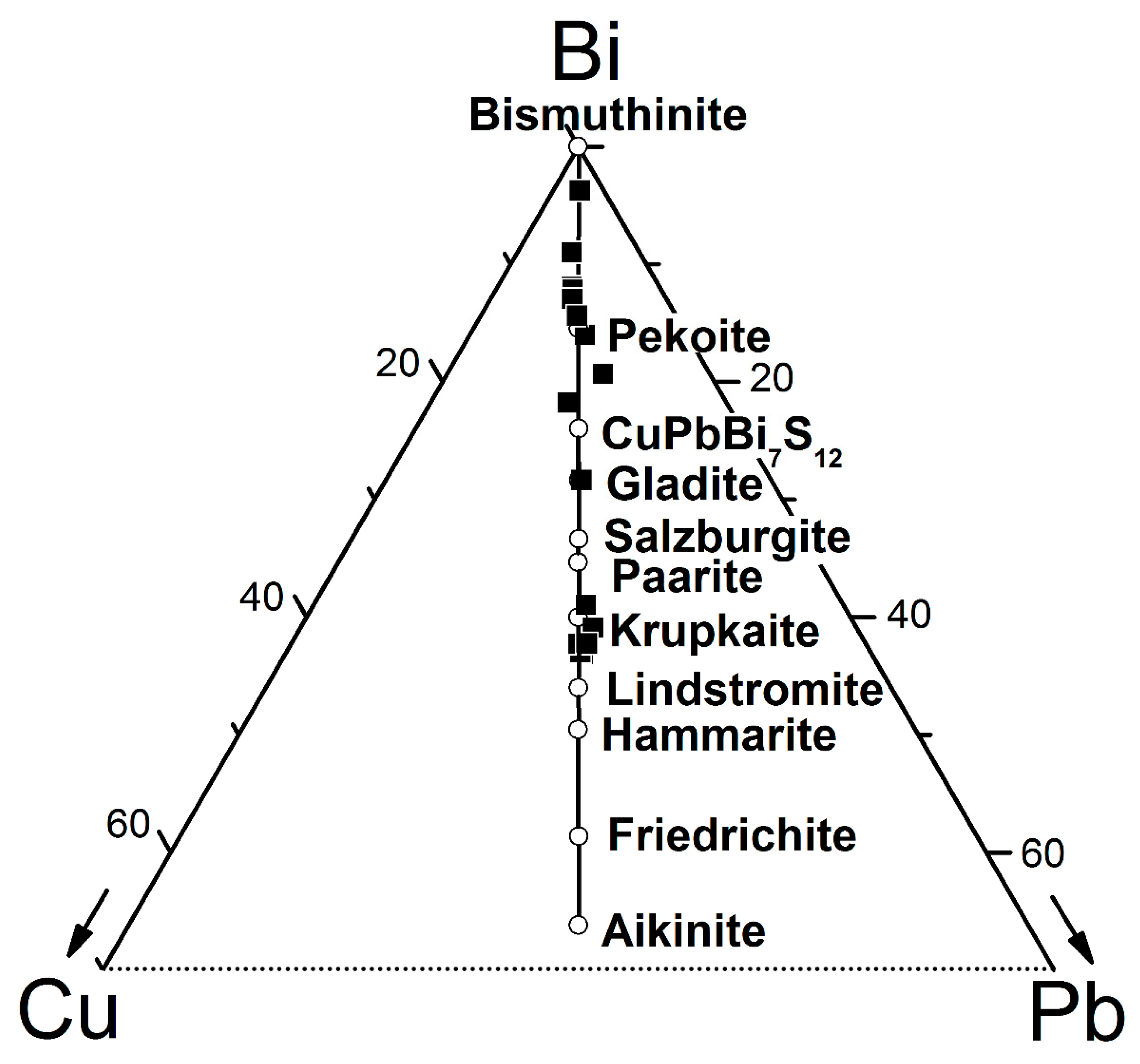
5.2.1. Bismuthinite-Aikinite Series

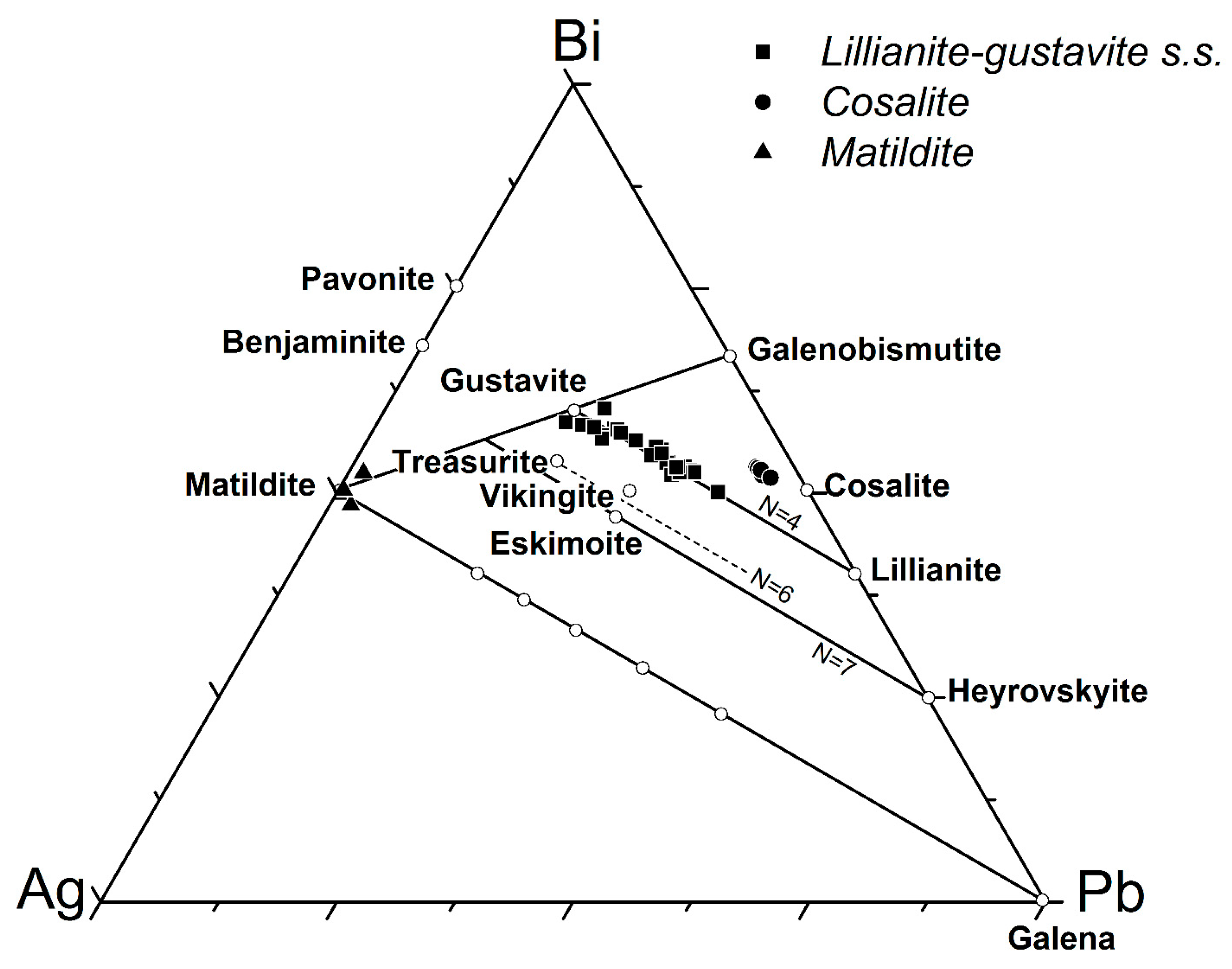
5.2.2. Lillianite-Gustavite Homologous Series
5.2.3. Matildite
5.2.4. Cosalite
5.2.5. Wittichenite
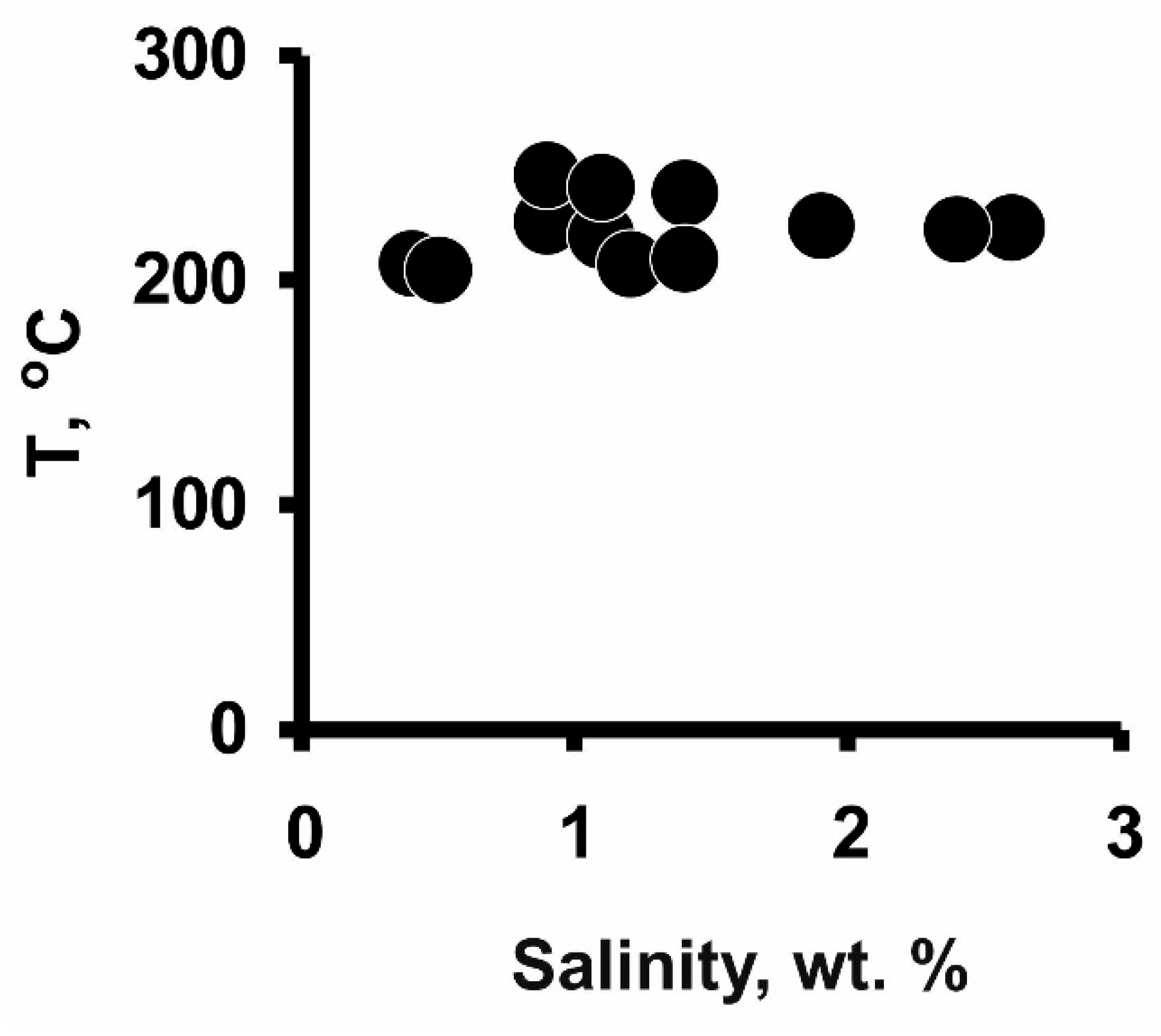
5.3. Fluid Inclusion Data
6. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borodaev, Y.S.; Garavelli, A.; Garbarino, C.; Grillo, S.M.; Mozgova, N.N.; Paar, W.H.; Topa, D.; Vurro, F. Rare Sulfosalts from Vulcano, Aeolian Islands, Italy. V. Selenian Heyrovskyite. Can. Mineral. 2003, 41, 429–440. [Google Scholar] [CrossRef]
- Marquez-Zavalia, M.F.; Galliski, M.A.; Cerny, P.; Chapman, R. An Assemblage of Bismuth-Rich, Tellurium-Bearing Minerals in the El Quemado Granitic Pegmatite, Nevados De Palermo, Salta, Argentina. Can. Mineral. 2013, 50, 1489–1498. [Google Scholar] [CrossRef]
- Meng, L.; Huang, F.; Gao, W.; Gao, R.; Zhao, F.; Zhou, Y.; Li, Y. Multi-Step Gold Refinement and Collection Using Bi-Minerals in the Laozuoshan Gold Deposit, NE China. Minerals 2022, 12, 1137. [Google Scholar] [CrossRef]
- Pažout, R. Lillianite homologues from Kutná Hora ore district, Czech Republic: A case of large-scale Sb for Bi substitution. J. Geosci. 2017, 62, 37–57. [Google Scholar] [CrossRef]
- Pažout, R.; Sejkora, J.; Šrein, V. Bismuth and bismuth-antimony sulphosalts from Kutná Hora vein Ag-Pb-Zn ore district, Czech Republic. J. Geosci. 2017, 62, 59–76. [Google Scholar] [CrossRef]
- Števko, M.; Sejkora, J. Bismuth, lead-bismuth and lead-antimony sulfosalts from the granite-hosted hydrothermal quartz veins at the Elisabeth mine, Gemerská Poloma, Spišsko-gemerské rudohorie Mts., Slovakia. J. Geosci. 2021, 66, 157–173. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, X.; Wu, Z.; Yang, T.; Li, D.; Ren, Y.; Liu, Q.; Zhu, K.; Yu, H. Mineralogy of Bi-sulfosalts and tellurides from the Yaoan gold deposit, southwest China: Metallogenic implications. Ore. Geol. Rev. 2018, 98, 126–140. [Google Scholar] [CrossRef]
- Marcoux, E.; Milesi, J.P.; Sohearto, S.; Rinawan, R. Noteworthy mineralogy of the Au-Ag-Sn-W(Bi) epithermal ore deposit of Cirotan, West Java, Indonesia. Can. Mineral. 1993, 31, 727–744. [Google Scholar]
- Voudouris, P.C.; Melfos, V.; Spry, P.G.; Moritz, R.C.; Papavassiliou, C.; Falalakis, G. Mineralogy and geochemical environment of formation of the Perama Hill high-sulfidation epithermal Au-Ag-Te-Se deposit, Petrota Graben, NE Greece. Min. Pet. 2011, 103, 79–100. [Google Scholar] [CrossRef]
- Buzatu, A.; Damian, G.; Dill, H.G.; Buzgar, N.; Apopei, A.I. Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania)—New bismuth minerals occurrence. Ore Geol. Rev. 2015, 65, 132–147. [Google Scholar] [CrossRef]
- Chovan, M.; Mikuš, T.; Prcúch, J.; Bača, B. Assemblage of Ag–Pb–Bi±Cu sulfosalts from the Bieber vein, Banská Štiavnica deposit, Slovakia. Acta Geol. Slovaca 2021, 13, 191–198. [Google Scholar]
- Cook, N.J. Bismuth and bismuth-antimony sulphosalts from Neogene vein mineralization, Baia Borşa area, Maramureş, Romania. Mineral. Mag. 1997, 61, 387–409. [Google Scholar] [CrossRef]
- Jelen, S.; Prsek, J.; Kovalenker, V.A.; Topa, D.; Sejkora, J.; Stevko, M.; Ozdin, D. Bismuth Sulfosalts of the Cuprobismutite, Pavonite and Aikinite Series from the Rozalia Mine, Hodrusa-Hamre, Slovakia. Can. Mineral. 2012, 50, 325–340. [Google Scholar] [CrossRef]
- Kouzmanov, K.; Bogdanov, K.; Ramboz, C. Te- and Bi-bearing assemblages in the Elshitsa and Radka epithermal deposits, Central Srednogorie, Bulgaria: Mineralogy and genetical features. Mineral. Petrol. Sofia 2005, 43, 108–112. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J. Intergrowths of bismuth sulphosalts from the Ocna de Fier Fe-skarn deposit, Banat, Southwest Romania. Eur. J. Mineral. 2000, 12, 899–917. [Google Scholar] [CrossRef]
- Cioflica, G.; Jude, R.; Lupulescu, M.; Simon, G.; Damian, G. New data on the Bi-minerals from the mineralizations related to Paleocene magmatites in Romania. Rom. J. Mineral. 1995, 72, 9–23. [Google Scholar]
- Dana, C.D.P.; Agangi, A.; Idrus, A.; Lai, C.-K.; Simbolon, D.R. Bi-Ag-Sulfosalts and Sulfoarsenides in the Ruwai Zn-Pb-Ag Skarn Deposit, Central Borneo, Indonesia. Minerals 2022, 12, 1564. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Pršek, J.; Melfos, V.; Voudouris, P.C.; Maliqi, F.; Kozub-Budzyń, G. Bismuth minerals from the Stan Terg deposit (Trepça, Kosovo). Neues Jahrb. Mineral. Abh. 2015, 192, 317–333. [Google Scholar] [CrossRef]
- Kołodziejczyk, J.; Pršek, J.; Voudouris, P.C.; Melfos, V. Bi-sulphotellurides associated with Pb-Bi-(Sb ± Ag, Cu, Fe) sulphosalts: An example from the Stan Terg deposit in Kosovo. Geol. Carpathica 2017, 68, 366–381. [Google Scholar] [CrossRef]
- Mederski, S.; Pršek, J.; Dimitrova, D.; Hyseni, B. A Combined EPMA and LA-ICP-MS Investigation on Bi-Cu-Au Mineralization from the Kizhnica Ore Field (Vardar Zone, Kosovo). Minerals 2021, 11, 1223. [Google Scholar] [CrossRef]
- Mederski, S.; Pršek, J.; Kołodziejczyk, J.; Kluza, K.; Melfos, V.; Adamek, K.; Dimitrova, D. Mineralogical and geochemical studies of Cu-Bi-Ag±W ores from Janjevo (Kosovo): Insights into the Bi sulfosalt mineralogy and the distribution of bismuth in base metal sulfides. J. Geosci. 2023, 68, 139–162. [Google Scholar] [CrossRef]
- Xiang-Ping, G.; Watanabe, M.; Ohkawa, M.; Hoshino, K.; Shibata, Y.; Desong, C. Felbertalite and Related Bismuth Sulfosalts from the Funiushan Copper Skarn Deposit, Nanjing, China. Can. Mineral. 2001, 39, 1641–1652. [Google Scholar] [CrossRef]
- Bristol, S.K.; Spry, P.G.; Voudouris, P.C.; Melfos, V.; Mathur, R.D.; Fornadel, A.P.; Sakellaris, G.A. Geochemical and geochronological constraints on the formation of shear-zone hosted Cu-Au-Bi-Te mineralization in the Stanos area, Chalkidiki, northern Greece. Ore. Geol. Rev. 2015, 66, 266–282. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Damian, F.; Damian, G. Gold scavenged by bismuth melts: An example from Alpine shear-remobilizates in the Highiş Massif, Romania. Mineral. Petrol. 2006, 87, 351–384. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Spry, P.G.; Mavrogonatos, C.; Sakellaris, G.A.; Bristol, S.K.; Melfos, V.; Fornadel, A.P. Bismuthinite derivatives, lillianite homologues, and bismuth sulfotellurides as indicators of gold mineralization in the Stanos shear-zone related deposit, Chalkidiki, northern Greece. Can. Mineral. 2013, 51, 119–142. [Google Scholar] [CrossRef]
- Makovicky, E. Modular crystal chemistry of sulphosalts ans other complex sulphides. In European Mineralogical Union, Notes in Modular Aspects of Minerals; Merlino, S., Ed.; European Mineralogical Union: Dublin, Ireland, 1997; Volume 1, pp. 237–271. ISBN 9780903056472. [Google Scholar] [CrossRef]
- Chen, T.T.; Kirchner, E.; Paar, W. Friedrichite Cu5Pb5Bi7S18 a new member of the aikinite-bismuthinite series. Can. Mineral. 1978, 16, 1127–1130. [Google Scholar]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series, Part I: General properties and definitions. Neues Jahrb. Mineral. Abh. 1977, 130, 264–287. [Google Scholar]
- Makovicky, E.; Karup-Møller, S. Chemistry and crystallography of the lillianite homologous series, Part II: Definition of new minerals: Eskimoite, vikingite, ourayite and treasurite. Redefinition of schirmerite and new data on the lillianite-gustavite solid-solution series. Neues Jahrb. Mineral. Abh. 1977, 131, 56–82. [Google Scholar]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Mineral. 2008, 20, 7–62. [Google Scholar] [CrossRef]
- Zajzon, N.; Szentpéteri, K.; Fehér, B.; Szakáll, S.; Kristály, F. Krupkaite from Avram Iancu mine, Băiţa (Bihor) metallogenic district, Romania. In Proceedings of the Joint 5th Mineral Sciences in the Carpathians Conference and 3rd Central-European Mineralogical Conference, Miskolc, Hungary, 20–21 April 2012; University of Miskolc: Miskolc, Hungary, 2012. [Google Scholar]
- Ilinca, G. Rare sulphosalt minerals in Romania. Acta Mineral.-Petrogr 2006, 5, 42–46. [Google Scholar]
- Ciobanu, C.L.; Pring, A.; Cook, N.J. Micron-to nano-scale intergrowths among members of the cuprobismutite series and paděraite: HRTEM and microanalytical evidence. Mineral. Mag. 2004, 68, 279–300. [Google Scholar] [CrossRef]
- Cook, N.J. Bismuth sulphosalts from hydrothermal vein deposits of Neogene Age, N.W. Romania. Mitt. Osterr. Miner. Ges. 1998, 143, 19–39. [Google Scholar]
- Costin, D.; Vlad, S. Ore formation at Varatec-Baiut (Baia Mare region, East Carpathians Romania. Geochem. Mineral. Petrol. 2005, 43, 64–68. [Google Scholar]
- Damian, G.; Ciobanu, C.L.; Cook, N.J.; Damian, F. Bismuth sulphosalts from the galena-matildite series in the Cremenea vein, Şuior, Baia Mare district, Romania. Neues Jahrb. Mineral. Abh. 2008, 185, 199–213. [Google Scholar] [CrossRef]
- Damian, F. Bismuth minerals-native gold association in the copper mineralisation from Nistru-Baia Mare zone. Stud. Univ. Babeş-Bolyai Cluj-Napoca Geol. 1999, XLIV, 151–158. [Google Scholar]
- Plotinskaya, O.Y.; Damian, F.; Yu, P.V.; Kovalenker, V.A.; Damian, G. Tellurides occurrences in the Baia Mare region, Romania. Carpathian J. Earth Environ. Sci. 2009, 4, 89–100. [Google Scholar]
- Biruk, S.V.; Skakun, L.Z. Bismuth minerals of the Beregovo ore field: Mineral assemblages and spatial zonation (Transcarpathian, Ukraine). Geol. Q. 2000, 44, 39–46. [Google Scholar]
- Tooth, B.; Brugger, J.; Ciobanu, C.; Liu, W. Modeling of gold scavenging by bismuth melts coexisting with hydrothermal fluids. Geology 2008, 36, 815–818. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Pring, A.; Brugger, J.; Danyushevsky, L.V.; Shimizu, M. ‘Invisible gold’ in bismuth chalcogenides. Geochim. Cosmochim. Acta 2009, 73, 1970–1999. [Google Scholar] [CrossRef]
- Seghedi, I.; Downes, H.; Szakács, A.; Mason, P.R.D.; Thirlwall, M.F.; Roșu, E.; Pécskay, Z.; Márton, E.; Panaiotu, C. Neogene-Quaternary magmatism and geodynamics in the Carpathian-Pannonian region: A synthesis. Lithos 2004, 72, 117–146. [Google Scholar] [CrossRef]
- Edelstein, O.; Bernad, A.; Kovacs, M.; Crihan, M.; Pécskay, Z. Preliminary data regarding the K-Ar ages of some eruptive rocks from Baia Mare Neogene volcanic zone. Rev. Roum. Geol 1992, 36, 45–60. [Google Scholar]
- Pécskay, Z.; Edelstein, O.; Kovacs, M.; Bernád, A.; Crihan, M. K/Ar age determination of Neogene volcanic rocks from the Gutâi Mts. (Eastern Carpathians, Romania). Geol. Carpathica 1994, 54, 357–363. [Google Scholar]
- Damian, F.; Damian, G.; Constantina, C. The subvolcanic magmatic rocks from the Nistru zone (Gutii Mountains). Carpathian J. Earth Environ. Sci. 2009, 4, 101–122. [Google Scholar]
- Borcoş, M.; Lang, B.; Peltz, S.; Stan, N. Neogene volcanism evolution in the west part of the Gutâi Mountains (Negreşti-Seini-Băiţa). Stud. Teh. Econ. Ser. I 1972, 6, 7–36. [Google Scholar]
- Sandulescu, M. Geotectonica Romaniei, 01. 10. 1984 ed; Editura Tehnica: Bucharest, Romania, 1984; p. 336. [Google Scholar]
- Lang, B.; Edelstein, O.; Steinitz, G.; Kovacs, M.; Halga, S. Ar-Ar dating of adularia-a tool in understanding genetic relations between volcanism and mineralization: Baia Mare Area (Gutîi Mountains), Northwestern Romania. Econ. Geol. 1994, 87, 174–180. [Google Scholar] [CrossRef]
- Borisenko, A.S. Study of the salt composition of Fluid Inclusions in Minerals with Cryometric Methods. Geol. Geofiz. 1977, 18, 16–27. [Google Scholar]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O–NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; De Vivo, B., Frezzotti, M.L., Eds.; Pontignano: Siena, Italy, 1994; pp. 117–130. [Google Scholar]
- Kalyuzhnyi, V.A. Principles of knowledge about mineral forming fluids. Fluid Incl. Res. Proc. COFFI 1985, 15, 289–333. [Google Scholar]
- Brown, P.E. A microcomputer program for the reduction and investigation of fluid inclusion data. Am. Mineral. 1989, 74, 1390–1393. [Google Scholar]
- Kryazhev, S.G.; Prokof’ev, V.Y.; Vasyuta, Y.V. Use of ICP MS Method for Analysis of Composition of Ore-Forming Fluids. Vestn. Mosk. Univ. 2006, 4, 30–36. [Google Scholar]
- Plotinskaya, O.Y.; Damian, G.; Damian, F. Sphalerite assemblages and composition in the Baia Mare region, Eastern Carpathians, Romania (preliminary data). Rom. J. Miner. Depos. 2014, 87, 87–90. [Google Scholar]
- Makovicky, E.; Makovicky, M. Representation of compositions in the bismuthinite-aikinite series. Can. Mineral. 1978, 16, 405–409. [Google Scholar]
- Topa, D.; Makovicky, E.; Balic-Zunic, T. Mineralogical Data on Salzburgite and Paarite, Two New Members of the Bismuthinite Aikinite Series. Can. Mineral. 2005, 43, 909–917. [Google Scholar] [CrossRef]
- Pring, A.; Hyde, B.G. Structural disorder in Lindströmite:A Bismuthinite-Aikinite derivative. Can. Mineral. 1987, 25, 393–399. [Google Scholar]
- Topa, D.; Makovicky, E.; Balic-Zunic, T. The structural role of excess Cu and Pb in gladite and krupkaite based on new refinements of their structure. Can. Mineral. 2002, 40, 1147–1159. [Google Scholar] [CrossRef][Green Version]
- Topa, D.; Makovicky, E.; Paar, W.H. Composition Ranges and Exsolution Pairs for the Members of the Bismuthinite-Aikinite Series from Felbertal, Austria. Can. Mineral. 2002, 40, 849–869. [Google Scholar] [CrossRef]
- Foord, E.E.; Shawe, D.R.; Conklin, N.M. Coexisting galena, PbSss and sulfosalts: Evidence for multiple episodes of mineralization in the Round Mountain and Manhattan gold district, Nevada. Can. Mineral. 1988, 26, 355–376. [Google Scholar]
- Harris, D.C.; Thorpe, R.I. New observations on matildite. Can. Mineral. 1969, 9, 655–662. [Google Scholar]
- Karup-Møller, S. Mineralogy of some Ag-(Cu)-Pb-Bi sulphide associations. Bull. Geol. Soc. Den. 1977, 26, 41–68. [Google Scholar]
- Moëlo, Y.; Marcoux, E.; Makovicky, E.; Karup-Møller, S.; Legendre, O. Homologues de la lillianite (gustavite, vikingite, heyrovskyite riche en Ag et Bi) de l’indice à W-As-(Pb, Bi, Ag) de la Roche-Balue (Loire-Atlantique, France). Bull. Minéral. 1987, 110, 43–64. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E. The Crystal Chemistry of Cosalite Based on New Electron-Microprobe Data and Single-Crystal Determinations of the Structure. Can. Mineral. 2010, 48, 1081–1107. [Google Scholar] [CrossRef][Green Version]
- Roedder, E. Fluid Inclusions. Rev. Mineral. 1984, 12, 644. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Damian, F.; Cook, N.J. Bi-sulphosalts from the cupriferous mineralisations from Nistru, Baia Mare area. Rom. J. Mineral. 1999, 79, 27. [Google Scholar]
- Baksheev, I.A.; Damian, F.; Damian, G.; Prokofiev, V.Y.; Bryzgalov, I.A.; Marushchenko, L.I. Chemical Composition of Phlogopite, Tourmaline and Illite from Hydrothermal Alterations of the Nistru Deposit, Baia Mare, Romania. Carpath J. Earth Env. 2016, 11, 547–564. [Google Scholar]
- Xu, X.-W.; Zhang, B.-L.; Liang, G.-H.; Qin, K.-Z. Zoning of mineralization in hypogene porphyry copper deposits: Insight from comb microfractures within quartz–chalcopyrite veins in the Hongshan porphyry Cu deposit, western Yunnan, SW China. J. Asian Earth Sci. 2012, 56, 218–228. [Google Scholar] [CrossRef]
- Arehart, G.B.; Chryssoulis, S.L.; Kesler, S.E. Gold and Arsenic in Iron Sulfides from Sediment-Hosted Disseminated Gold Deposits-Implications for Depositional Processes. Econ. Geol. Bull. Soc. 1993, 88, 171–185. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Marcoux, E.; Molo, Y.; Leistel, J.M. Bismuth and cobalt minerals as indicators of stringer zones to massive sulphide deposits, Iberian Pyrite Belt. Miner. Depos. 1996, 31, 1–26. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Naumov, V.B.; Prokof’ev, V.Y.; Jelen, S.; Gaber, M. Compositions of Magmatic Melts and Evolution of Mineral-Forming Fluids in the Banska Stiavnica Epithermal Au–Ag–Pb–Zn Deposit, Slovakia: A Study of Inclusions in Minerals. Geochem. Int. 2006, 44, 118–136. [Google Scholar] [CrossRef]
- Beane, R.E.; Bodnar, R.J. Hydrothermal Fluids and Hydrothermal Alteration in Porphyry Copper Deposits. Porphyry Copp. Depos. Am. Cordill. 1995, 83–93. [Google Scholar]
- Prokofiev, V.Y.; Kovalenker, V.A.; Jeleň, S.; Borisenko, A.S.; Borovikov, A.A. Determination of Metal Concentrations in Fluid Inclusions Formed during the Magmatic and Hydrothermal Stages of Formation of the Epithermal Au–Ag Polymetallic Deposit of Banska Stiavnica (Western Carpathians) with the LA–ICP–MS Method. Dokl. Earth Sci. 2011, 440, 1249–1252. [Google Scholar] [CrossRef]
- Yue, Q.; Zhai, D.; Zhao, G.; Zhao, Q.; Liu, J. The Occurrence and Chemical Composition of Bismuth-Bearing Minerals in the Niuxingba-Liumukeng Ag-Pb-Zn Deposit, Jiangxi Province, South China. Minerals 2024, 14, 53. [Google Scholar] [CrossRef]
- Shin, D.; Park, H.I.; Lee, I.; Lee, K.S.; Hwang, J. Hydrothermal As–Bi mineralization in the Nakdong deposits, South Korea: Insight from fluid inclusions and stable isotopes. Can. Mineral. 2004, 42, 1465–1481. [Google Scholar] [CrossRef]
- Yunsheng, R.; Liandeng, L.; Huihuang, Z. Gold deposits rich in bismuth minerals: An important type of gold deposits. In Proceedings of the Mineral Deposit Research: Meeting the Global Challenge: Proceedings of the Eighth Biennial SGA Meeting Beijing, Beijing, China, 18–21 August 2005; pp. 581–583. [Google Scholar]
- Cioflica:, G.; Jude, R.; Berbeleac, I.; Udubasa, S. Neogene gold mineralization types in Romania. In Proceedings of the XVII Congress of Carpathian-Balkan Geological Association, Bratislava, Romania, 1–4 September 2002. [Google Scholar]



| Number | Weight% | Mineral | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | Total | ||
| NV8 Bi-1 | 75.68 | 3.91 | 0.02 | 0.05 | 0.01 | 1.68 | 0.18 | 0.09 | 17.94 | 99.57 | close to bismuthinite |
| NV8 Bi-2 | 76.64 | 2.78 | 0.01 | 0.04 | 0.03 | 1.27 | 0.12 | 0.09 | 18.84 | 99.81 | bismuthinite |
| NV8 Bi-3 | 69.81 | 6.53 | 0.04 | 0.04 | 0.00 | 3.18 | 0.65 | 0.15 | 18.63 | 99.01 | close to CuPbBi7S12 |
| Nistru 1D | 71.19 | 8.95 | 0.00 | 0.00 | 0.00 | 2.11 | 0.29 | 0.13 | 18.57 | 101.24 | pekoite |
| Nistru 2D | 74.65 | 4.17 | 0.00 | 0.00 | 0.00 | 1.69 | 0.16 | 0.15 | 18.77 | 99.59 | close to bismuthinite |
| Nistru 3D | 77.51 | 2.93 | 0.09 | 0.00 | 0.00 | 1.88 | 0.64 | 0.26 | 18.71 | 102.02 | close to bismutine |
| Nistru 4D | 74.89 | 4.09 | 0.00 | 0.00 | 0.00 | 2.04 | 0.92 | 0.34 | 18.10 | 100.38 | pekoite |
| Nistru 5D | 66.64 | 12.02 | 0.00 | 0.09 | 0.00 | 3.95 | 0.35 | 0.12 | 17.77 | 100.94 | gladite |
| Nistru 6D | 55.09 | 19.13 | 0.10 | 0.10 | 0.00 | 5.51 | 0.22 | 0.17 | 17.17 | 97.49 | krupkaite |
| Nistru 7D | 55.85 | 18.88 | 0.00 | 0.11 | 0.00 | 6.37 | 0.68 | 0.00 | 17.76 | 99.65 | krupkaite |
| Nistru 8D | 55.82 | 18.39 | 0.00 | 0.00 | 0.00 | 6.35 | 0.70 | 0.17 | 17.74 | 99.17 | krupkaite |
| Nistru 9D | 57.18 | 17.43 | 0.00 | 0.11 | 0.00 | 5.41 | 0.33 | 0.11 | 17.69 | 98.26 | krupkaite |
| 196/2 | 76.29 | 2.18 | 0.06 | 0.00 | 0.24 | 1.97 | 0.85 | 0.09 | 19.13 | 100.82 | pekoite |
| N-1/3 | 78.53 | 1.11 | 0.06 | 0.13 | 0.09 | 0.43 | 0.03 | 0.16 | 18.90 | 99.45 | bismuthinite |
| NV-9/2 | 56.82 | 18.67 | 0.00 | 0.08 | 0.01 | 6.33 | 0.54 | 0.05 | 17.89 | 100.39 | krupkaite |
| NV-9/3 | 56.47 | 18.12 | 0.00 | 0.09 | 0.00 | 6.10 | 0.81 | 0.00 | 18.25 | 99.84 | krupkaite |
| Chemical Formula on the Basis of 8 Cations. | |||||||||||
| Number | Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | naikinite | |
| NV8 Bi-1 | 7.45 | 0.39 | 0.00 | 0.01 | 0.00 | 0.54 | 0.07 | 0.02 | 11.51 | 12.63 | close to bismuthinite |
| NV8 Bi-2 | 7.40 | 0.27 | 0.00 | 0.01 | 0.01 | 0.40 | 0.04 | 0.02 | 11.85 | 9.40 | bismuthinite |
| NV8 Bi-3 | 6.61 | 0.62 | 0.01 | 0.01 | 0.00 | 0.99 | 0.23 | 0.04 | 11.50 | 24.42 | close to CuPbBi7S12 |
| Nistru 1D | 6.79 | 0.86 | 0.00 | 0.00 | 0.00 | 0.66 | 0.10 | 0.03 | 11.55 | 21.38 | pekoite |
| Nistru 2D | 7.19 | 0.41 | 0.00 | 0.00 | 0.00 | 0.54 | 0.06 | 0.04 | 11.78 | 12.98 | close to bismuthinite |
| Nistru 3D | 7.32 | 0.28 | 0.01 | 0.00 | 0.00 | 0.58 | 0.23 | 0.07 | 11.51 | 13.82 | close to bismutine |
| Nistru 4D | 7.20 | 0.40 | 0.00 | 0.00 | 0.00 | 0.65 | 0.33 | 0.09 | 11.34 | 17.40 | pekoite |
| Nistru 5D | 6.37 | 1.16 | 0.00 | 0.02 | 0.00 | 1.24 | 0.13 | 0.03 | 11.06 | 33.02 | gladite |
| Nistru 6D | 5.35 | 1.87 | 0.02 | 0.02 | 0.00 | 1.76 | 0.08 | 0.04 | 10.86 | 51.29 | krupkaite |
| Nistru 7D | 5.21 | 1.78 | 0.00 | 0.02 | 0.00 | 1.95 | 0.24 | 0.00 | 10.80 | 55.02 | krupkaite |
| Nistru 8D | 5.22 | 1.73 | 0.00 | 0.00 | 0.00 | 1.95 | 0.25 | 0.04 | 10.81 | 54.72 | krupkaite |
| Nistru 9D | 5.46 | 1.68 | 0.00 | 0.02 | 0.00 | 1.70 | 0.12 | 0.03 | 11.01 | 48.37 | krupkaite |
| 196/2 | 7.14 | 0.21 | 0.01 | 0.00 | 0.04 | 0.61 | 0.30 | 0.02 | 11.67 | 15.47 | pekoite |
| N-1/3 | 7.65 | 0.11 | 0.01 | 0.02 | 0.02 | 0.14 | 0.01 | 0.04 | 12.00 | 3.77 | bismuthinite |
| NV-9/2 | 5.28 | 1.75 | 0.00 | 0.01 | 0.00 | 1.93 | 0.19 | 0.01 | 10.83 | 53.58 | krupkaite |
| NV-9/3 | 5.21 | 1.69 | 0.00 | 0.01 | 0.00 | 1.85 | 0.28 | 0.00 | 10.97 | 53.52 | krupkaite |
| Number | Weight % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | Total | |||
| 1P Bi-1 | 45.64 | 29.46 | 0.17 | 0.01 | 5.49 | 1.03 | 0.55 | 0.15 | 16.61 | 99.11 | ||
| 1P Bi-2 | 46.79 | 30.70 | 0.11 | 0.04 | 5.25 | 0.99 | 0.42 | 0.11 | 16.45 | 100.86 | ||
| 1P Bi-3 | 45.34 | 30.46 | 0.24 | 0.11 | 5.43 | 0.64 | 0.26 | 0.13 | 16.40 | 99.01 | ||
| 196 Bi-1 | 50.89 | 22.17 | 0.19 | 0.12 | 7.59 | 1.69 | 0.68 | 0.23 | 17.09 | 100.65 | ||
| 196 Bi-2 | 51.26 | 22.70 | 0.21 | 0.10 | 7.52 | 0.63 | 0.15 | 0.28 | 16.87 | 99.72 | ||
| 196 Bi-3 | 51.64 | 19.16 | 0.15 | 0.18 | 9.07 | 0.59 | 0.05 | 0.30 | 16.90 | 98.04 | ||
| NV8 Bi-3 | 54.01 | 20.54 | 0.00 | 0.04 | 7.62 | 0.92 | 0.18 | 0.06 | 17.20 | 100.57 | ||
| NV9 Bi-1 | 47.78 | 27.20 | 0.11 | 0.07 | 5.77 | 0.88 | 0.25 | 0.21 | 16.59 | 98.86 | ||
| NV9 Bi-2 | 48.15 | 26.59 | 0.10 | 0.10 | 5.98 | 0.80 | 0.25 | 0.16 | 16.63 | 98.76 | ||
| 1P Bi-4 | 45.13 | 29.01 | 0.09 | 0.16 | 5.45 | 0.38 | 0.39 | 0.18 | 16.71 | 97.50 | ||
| 1P Bi-5 | 47.67 | 26.83 | 0.13 | 0.28 | 6.49 | 1.16 | 0.22 | 0.17 | 16.57 | 99.52 | ||
| 10D | 42.95 | 34.24 | 0.00 | 0.00 | 4.24 | 0.40 | 0.14 | 0.24 | 16.26 | 98.47 | ||
| 11D | 46.52 | 28.54 | 0.15 | 0.14 | 5.82 | 1.89 | 0.98 | 0.15 | 16.66 | 100.85 | ||
| 12D | 47.7 | 29.11 | 0.00 | 0.19 | 6.07 | 1.20 | 0.41 | 0.14 | 15.91 | 100.73 | ||
| 13D | 47.32 | 27.33 | 0.00 | 0.06 | 5.86 | 1.51 | 0.56 | 0.20 | 16.42 | 99.26 | ||
| 14D | 46.60 | 30.70 | 0.10 | 0.13 | 5.66 | 1.23 | 0.42 | 0.00 | 16.78 | 101.62 | ||
| 15D | 46.06 | 31.58 | 0.13 | 0.31 | 5.11 | 1.53 | 0.70 | 0.15 | 16.36 | 101.93 | ||
| 16D | 45.4 | 30.74 | 0.00 | 0.00 | 4.97 | 1.44 | 0.99 | 0.00 | 16.53 | 100.07 | ||
| 18D | 45.61 | 31.45 | 0.10 | 0.05 | 4.87 | 1.08 | 0.78 | 0.15 | 16.52 | 100.61 | ||
| 19D | 46.21 | 30.15 | 0.10 | 0.01 | 6.15 | 0.20 | 0.10 | 0.00 | 16.43 | 99.35 | ||
| 20D | 46.71 | 30.94 | 0.00 | 0.05 | 5.72 | 0.32 | 0.00 | 0.00 | 16.56 | 100.30 | ||
| 1P/1 | 46.48 | 29.81 | 0.14 | 0.11 | 5.54 | 1.17 | 0.67 | 0.05 | 16.50 | 100.47 | ||
| 1P/2 | 46.86 | 30.06 | 0.12 | 0.12 | 5.70 | 0.29 | 0.33 | 0.07 | 16.49 | 100.04 | ||
| 196/1 | 50.82 | 21.62 | 0.19 | 0.21 | 8.46 | 1.26 | 0.33 | 0.22 | 16.48 | 99.59 | ||
| N1/2 | 51.39 | 22.23 | 0.22 | 0.15 | 8.71 | 0.93 | 0.18 | 0.17 | 16.96 | 100.94 | ||
| N1/4 | 52.30 | 17.61 | 0.00 | 0.13 | 9.90 | 1.51 | 0.06 | 0.22 | 17.25 | 98.98 | ||
| N1/5 | 52.53 | 20.8 | 0.27 | 0.23 | 8.75 | 0.68 | 0.07 | 0.2 | 16.85 | 100.38 | ||
| N1/7 | 50.01 | 22.79 | 0.19 | 0.21 | 7.32 | 1.03 | 0.28 | 0.13 | 16.61 | 98.57 | ||
| NV9/1 | 48.45 | 24.23 | 0.08 | 0.07 | 6.71 | 0.84 | 0.39 | 0.05 | 16.89 | 97.71 | ||
| Chemical Formula on the Basis of 11 Atoms | ||||||||||||
| Number | Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | N | L | x |
| 1P Bi-1 | 2.51 | 1.63 | 0.02 | 0.00 | 0.58 | 0.19 | 0.11 | 0.02 | 5.94 | 3.91 | 63.52 | 0.61 |
| 1P Bi-2 | 2.57 | 1.70 | 0.01 | 0.00 | 0.56 | 0.18 | 0.09 | 0.02 | 5.88 | 3.80 | 61.64 | 0.56 |
| 1P Bi-3 | 2.53 | 1.71 | 0.02 | 0.01 | 0.59 | 0.12 | 0.05 | 0.02 | 5.95 | 3.97 | 61.27 | 0.60 |
| 196 Bi-1 | 2.68 | 1.18 | 0.02 | 0.01 | 0.78 | 0.29 | 0.13 | 0.03 | 5.88 | 3.86 | 87.34 | 0.81 |
| 196 Bi-2 | 2.78 | 1.24 | 0.02 | 0.01 | 0.79 | 0.11 | 0.03 | 0.04 | 5.97 | 3.84 | 86.50 | 0.79 |
| 196 Bi-3 | 2.81 | 1.05 | 0.01 | 0.02 | 0.96 | 0.11 | 0.01 | 0.04 | 5.99 | 4.20 | 93.88 | 1.03 |
| NV8 Bi-3 | 2.89 | 1.11 | 0.00 | 0.00 | 0.79 | 0.16 | 0.04 | 0.01 | 6.00 | 3.56 | 96.42 | 0.75 |
| NV9 Bi-1 | 2.64 | 1.52 | 0.01 | 0.01 | 0.62 | 0.16 | 0.05 | 0.03 | 5.97 | 3.72 | 70.99 | 0.61 |
| NV9 Bi-2 | 2.66 | 1.48 | 0.01 | 0.01 | 0.64 | 0.15 | 0.05 | 0.02 | 5.98 | 3.73 | 73.09 | 0.63 |
| 1P Bi-4 | 2.51 | 1.63 | 0.01 | 0.02 | 0.59 | 0.07 | 0.08 | 0.03 | 6.07 | 3.91 | 63.82 | 0.61 |
| 1P Bi-5 | 2.61 | 1.48 | 0.01 | 0.03 | 0.69 | 0.21 | 0.04 | 0.02 | 5.91 | 3.97 | 72.56 | 0.72 |
| 10D | 2.43 | 1.96 | 0.00 | 0.00 | 0.47 | 0.07 | 0.03 | 0.04 | 6.00 | 3.93 | 48.90 | 0.47 |
| 11D | 2.48 | 1.54 | 0.01 | 0.01 | 0.60 | 0.33 | 0.20 | 0.02 | 5.80 | 3.91 | 66.89 | 0.64 |
| 12D | 2.64 | 1.63 | 0.00 | 0.02 | 0.65 | 0.22 | 0.08 | 0.02 | 5.74 | 3.94 | 67.37 | 0.65 |
| 13D | 2.59 | 1.51 | 0.00 | 0.01 | 0.62 | 0.27 | 0.11 | 0.03 | 5.86 | 3.80 | 70.32 | 0.63 |
| 14D | 2.51 | 1.67 | 0.01 | 0.01 | 0.59 | 0.22 | 0.08 | 0.00 | 5.90 | 3.97 | 62.52 | 0.62 |
| 15D | 2.49 | 1.72 | 0.01 | 0.03 | 0.54 | 0.27 | 0.14 | 0.02 | 5.77 | 3.86 | 58.98 | 0.55 |
| 16D | 2.47 | 1.69 | 0.00 | 0.00 | 0.52 | 0.26 | 0.20 | 0.00 | 5.86 | 3.81 | 59.48 | 0.54 |
| 18D | 2.49 | 1.73 | 0.01 | 0.00 | 0.51 | 0.19 | 0.16 | 0.02 | 5.88 | 3.80 | 58.06 | 0.52 |
| 19D | 2.58 | 1.70 | 0.01 | 0.00 | 0.67 | 0.04 | 0.02 | 0 | 5.98 | 4.16 | 64.25 | 0.69 |
| 20D | 2.59 | 1.73 | 0.00 | 0.00 | 0.62 | 0.06 | 0.00 | 0.00 | 5.99 | 4.00 | 62.34 | 0.62 |
| 1P/1 | 2.53 | 1.64 | 0.01 | 0.01 | 0.59 | 0.21 | 0.14 | 0.01 | 5.86 | 3.88 | 63.79 | 0.60 |
| 1P/2 | 2.60 | 1.68 | 0.01 | 0.01 | 0.61 | 0.05 | 0.07 | 0.01 | 5.96 | 3.93 | 64.04 | 0.62 |
| 196/1 | 2.75 | 1.18 | 0.02 | 0.02 | 0.89 | 0.22 | 0.07 | 0.03 | 5.82 | 4.17 | 87.72 | 0.95 |
| N1/2 | 2.74 | 1.20 | 0.02 | 0.01 | 0.90 | 0.16 | 0.04 | 0.02 | 5.90 | 4.26 | 86.73 | 0.98 |
| N1/4 | 2.77 | 0.94 | 0.00 | 0.01 | 1.02 | 0.26 | 0.01 | 0.03 | 5.95 | 4.39 | 96.97 | 1.16 |
| N1/5 | 2.83 | 1.13 | 0.02 | 0.02 | 0.91 | 0.12 | 0.01 | 0.03 | 5.92 | 4.09 | 91.39 | 0.95 |
| N1/7 | 2.74 | 1.26 | 0.02 | 0.02 | 0.78 | 0.19 | 0.06 | 0.02 | 5.93 | 3.87 | 84.83 | 0.79 |
| NV9/1 | 2.66 | 1.34 | 0.01 | 0.01 | 0.71 | 0.15 | 0.08 | 0.01 | 6.04 | 3.84 | 79.49 | 0.73 |
| Number | Weight % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | Total | |
| Matildite | ||||||||||
| 21D | 53.44 | 1.60 | 0.00 | 0.10 | 24.13 | 1.43 | 0.15 | 0.12 | 15.43 | 96.40 |
| 22D | 51.71 | 0.20 | 0.00 | 0.56 | 26.74 | 1.44 | 0.12 | 0.10 | 15.82 | 96.69 |
| N1/10 | 52.43 | 2.39 | 0.04 | 0.11 | 27.46 | 0.55 | 0.03 | 0.04 | 17.42 | 100.47 |
| N1/11 | 53.12 | 0.64 | 0.07 | 0.05 | 26.76 | 0.24 | 0.02 | 0.07 | 17.11 | 98.08 |
| Cosalite | ||||||||||
| 23D | 43.82 | 36.54 | 0.44 | 0.10 | 1.68 | 2.37 | 0.67 | 0.00 | 16.60 | 102.22 |
| 24D | 42.65 | 34.06 | 0.12 | 0.16 | 1.64 | 2.11 | 0.54 | 0.22 | 16.35 | 97.85 |
| 25D | 43.98 | 35.42 | 0.00 | 0.24 | 1.66 | 1.83 | 0.44 | 0.15 | 16.33 | 100.05 |
| 26D | 43.26 | 35.32 | 0.09 | 0.18 | 1.56 | 1.88 | 0.56 | 0.20 | 16.36 | 99.41 |
| NV8 Bi-4 | 42.18 | 36.27 | 0.02 | 0.15 | 1.32 | 2.36 | 0.81 | 0.06 | 16.69 | 99.86 |
| Wittichenite | ||||||||||
| 1P Bi-6 | 40.32 | 0.89 | 0.08 | 0.22 | 3.18 | 32.66 | 1.66 | 0.31 | 19.80 | 99.12 |
| 1P/3 | 41.28 | 1.02 | 0.20 | 0.29 | 2.94 | 32.44 | 0.29 | 0.21 | 19.42 | 98.09 |
| NV6/1 | 39.71 | 0.24 | 0.00 | 0.09 | 3.67 | 34.29 | 0.37 | 0.01 | 20.00 | 98.38 |
| Empirical Chemical Formula | ||||||||||
| (The formulae were calculated based on: 4 atoms (matildite), 36 atoms (cosalite), and 7 atoms (wittichenite) | ||||||||||
| Number | Bi | Pb | Te | Sb | Ag | Cu | Fe | Se | S | |
| Matildite | ||||||||||
| 21D | 1.03 | 0.031 | 0.000 | 0.003 | 0.898 | 0.090 | 0.011 | 0.006 | 1.933 | |
| e22D | 0.970 | 0.004 | 0.000 | 0.018 | 0.972 | 0.089 | 0.008 | 0.005 | 1.934 | |
| N1/10 | 0.937 | 0.043 | 0.001 | 0.003 | 0.951 | 0.032 | 0.002 | 0.002 | 2.029 | |
| N1/11 | 0.973 | 0.012 | 0.002 | 0.002 | 0.949 | 0.015 | 0.002 | 0.004 | 2.042 | |
| Cosalite | ||||||||||
| 23D | 7.759 | 6.526 | 0.128 | 0.030 | 0.576 | 1.380 | 0.444 | 0.000 | 19.157 | |
| 24D | 7.804 | 6.285 | 0.036 | 0.050 | 0.581 | 1.270 | 0.370 | 0.107 | 19.498 | |
| 25D | 8.003 | 6.501 | 0.000 | 0.075 | 0.585 | 1.095 | 0.300 | 0.072 | 19.368 | |
| 26D | 7.874 | 6.484 | 0.027 | 0.056 | 0.550 | 1.125 | 0.381 | 0.096 | 19.407 | |
| NV8 Bi-4 | 7.544 | 6.543 | 0.006 | 0.045 | 0.456 | 1.386 | 0.539 | 0.029 | 19.452 | |
| Wittichenite | ||||||||||
| 1P Bi-6 | 0.969 | 0.021 | 0.003 | 0.009 | 0.148 | 2.580 | 0.149 | 0.019 | 3.101 | |
| 1P/3 | 1.019 | 0.025 | 0.008 | 0.012 | 0.141 | 2.632 | 0.027 | 0.013 | 3.122 | |
| NV6/1 | 0.953 | 0.006 | 0.000 | 0.004 | 0.170 | 2.705 | 0.034 | 0.001 | 3.127 | |
| Index № | n | T hom, °C | T eut, °C | T ice melt, °C | wt. % eq. NaCl | d, g/sm3 |
|---|---|---|---|---|---|---|
| 1 | 3 | 225 | −27 | −1.5 | 2.6 | 0.86 |
| 2 | 7 | 222 | −35 | −1.4 | 2.4 | 0.86 |
| 3 | 3 | 227 | −26 | −0.5 | 0.9 | 0.84 |
| 4 | 3 | 239 | −22 | −0.8 | 1.4 | 0.82 |
| 5 | 2 | 247 | −28 | −0.5 | 0.9 | 0.80 |
| 6 | 3 | 224 | −22 | −1.1 | 1.9 | 0.85 |
| 7 | 2 | 241 | −23 | −0.6 | 1.1 | 0.82 |
| 8 | 2 | 219 | −21 | −0.6 | 1.1 | 0.85 |
| 9 | 3 | 210 | −22 | −0.8 | 1.4 | 0.87 |
| 10 | 3 | 207 | −23 | −0.7 | 1.2 | 0.87 |
| 11 | 2 | 205 | −21 | −0.3 | 0.5 | 0.86 |
| 12 | 3 | 207 | −22 | −0.2 | 0.4 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, F.; Damian, G.; Cook, N.J.; Prokofiev, V.Y.; András, P. Bismuth Sulfosalts from the Nistru Metallogenetic Field, Baia Mare Zone, NW Romania. Minerals 2024, 14, 1182. https://doi.org/10.3390/min14111182
Damian F, Damian G, Cook NJ, Prokofiev VY, András P. Bismuth Sulfosalts from the Nistru Metallogenetic Field, Baia Mare Zone, NW Romania. Minerals. 2024; 14(11):1182. https://doi.org/10.3390/min14111182
Chicago/Turabian StyleDamian, Floarea, Gheorghe Damian, Nigel J. Cook, Vsevolod Yu. Prokofiev, and Peter András. 2024. "Bismuth Sulfosalts from the Nistru Metallogenetic Field, Baia Mare Zone, NW Romania" Minerals 14, no. 11: 1182. https://doi.org/10.3390/min14111182
APA StyleDamian, F., Damian, G., Cook, N. J., Prokofiev, V. Y., & András, P. (2024). Bismuth Sulfosalts from the Nistru Metallogenetic Field, Baia Mare Zone, NW Romania. Minerals, 14(11), 1182. https://doi.org/10.3390/min14111182








