Abstract
The combined pollution of organics and heavy metals represents a significant environ-mental problem that has attracted widespread attention. This explores the treatment of methylene blue (MB) and Cu(II), which are common pollutants in dye wastewater, and the recycling of Cu. A magnetized vaterite (V-M) was synthesized using Bacillus velezensis, and its structure and magnetic performance were investigated. The effects and mechanisms of removing MB-Cu(II) composite pollution using V-M and H2O2 in combination were estimated. The results indicated that V-M is a combination of organic and inorganic substances, with 21.5 wt% organic matter and multiple organic functional groups, including O-H, -SH, and others. The combination of V-M and H2O2 can achieve a maximum removal percentage of 90% for MB-Cu(II) pollution. The analysis showed that MB was oxidized by the ·OH generated from the H2O2-based Fenton-like reaction, and was catalyzed by the Fe3O4 in V-M. The immobilization of Cu(II) by V-M was mostly realized through the binding of the organic substances on the surface of the V-M, multilayer adsorption, and a replacement reaction with Ca(II). Magnetic separation and the addition of diluted HCl were used for the recycling of the Cu(II) enriched by V-M, with a recycling percentage reaching 85%. This study introduced a novel approach to the remediation of MB-Cu(II) composite pollution, and the recycling of Cu(II).
1. Introduction
With the increasing impact of human activities and the acceleration of global industrialization, the diversity and quantity of pollutants continue to rise, making the combined pollution of organics and heavy metals a common phenomenon [1]. The heavy metals present in water and soil, which are mainly derived from industrial sources, can not only be absorbed or accumulated in living organisms, damaging the ecosystem and human health, but also interact with other organo-pollutants, resulting in a more harmful form of combined pollution [2]. It is thus a key focus among researchers to reduce such combined contamination of organics and heavy metals.
Dyes are among the most common organo-pollutants. Among all dyes, methylene blue (MB) is the most challenging organo-pollutant in wastewater treatment. It accounts for approximately 50% of all dye production [3,4]. MB has a complex structure with high chroma, which can reduce the light transmittance of the solution [5], and is difficult to biodegrade [6]. MB exerts a long-term detrimental influence on the growth of animals and plants, as well as on human health. Copper salts, a major component in the production of dyes, were employed as a moderant to optimize the performance of printing and dyeing products, resulting in the generation of considerable quantities of Cu(II) in dye wastewater. An excess of Cu(II) can damage the structure and functionality of proteins, enzymes, and DNA. This can lead to adverse effects on organ function and even result in metabolic disorders and, in extreme cases, death [7]. Furthermore, the presence of Cu(II) in polluted dye water can interact with organic substances, thereby amplifying the toxicity and enhancing the damaging effect on cells [8]. Therefore, common heavy metals and organic substances can be represented by Cu(II) and MB, respectively, to explore a novel approach to the treatment of organic substance–heavy-metal-contaminated water.
Calcium carbonate is one of the most economical porous materials. Its carbonate group has been shown to enhance the replacement reaction between metal ions, making it a widely used material for the immobilization of heavy metal ions [9,10,11,12]; however, a multitude of adsorbents, including calcium carbonate, can prove challenging to recycle and may lead to secondary pollution. Moreover, the adsorption method may not achieve the chroma standard for wastewater containing a significant quantity of organic dyes and exhibiting a high chroma. A Fenton-like reaction has been assessed as effective in the degradation of organic dyes. It has attracted considerable interest due to its broad pH range and ease of application, and it is widely applied in the treatment of organic pollutants in water [13,14,15]. Fe3O4 is an environmentally friendly, cost-effective material that can be easily separated. It has been shown to be able to catalyze the production of ·OH from H2O2, and the ·OH can decompose MB. While this method has been demonstrated to facilitate the eventual oxidation of MB to CO2 and H2O, the underlying degradation process remains to be elucidated [16,17]. Some studies combined Fe3O4 and calcium carbonate for the removal of heavy metals and organic pollutants. Islam et al. [11] synthesized a carbonate-based mesoporous magnetic adsorbent (IO@CaCO3), which comprised acicular iron oxide and calcite, and exhibited remarkable capabilities for the removal of As(V), Cr(VI), and Pb(II). Wang et al. [18] synthesized magnetic mesoporous calcium carbonate-based nanocomposites that could immobilize Pb(II) and Cd(II), and demonstrated an ion-exchange-based heavy metal adsorbent process. In addition to the use of chemically synthesized CaCO3, biogenic CaCO3, synthesized by microorganisms, presents a unique organic–inorganic composite structure and often exhibits superior heavy metal adsorption capabilities [19,20], making it a popular material for the remediation of such pollution. Paul et al. [21] combined Fe3O4 with biomineralized vaterite and calcite, respectively, to assess their capability to degrade the MB and Rhodamine B. Their findings implied that the presence of CaCO3 can improve the catalytic activity and that vaterite-supported Fe3O4 shows a superior result. Despite the fact that the aforementioned studies broadened the scope of application of calcium carbonate, it remains costly and requires intricate processing. Furthermore, the studies did not investigate removal methods of composite pollution involving both organics and heavy metals.
Bacillus velezensis (B. velezensis) is widely distributed in the soil and has a clear genetic background and excellent biosafety record [22]. It is a powerful microbe that can induce biogenetic CaCO3 precipitation [23], as elaborated in our previous work [24]. In this study, a magnetized vaterite (V-M) is synthesized under the induction of bacteria, and its magnetic performance and mineral structure are characterized. The mechanism of using V-M as an adsorbent and a catalyst for a Fenton-like reaction to remove composite pollution (MB-Cu) is expounded. Subsequently, the magnetic V-M with immobilized Cu could be recycled by magnetic separation, and the copper could then be mobilized by the addition of diluted HCl. This study presents a novel approach to the removal of composite contamination from MB and Cu(II).
2. Materials and Methods
2.1. Materials
The strain used in the experiment was isolated from biofertilizer, identified as Bacillus velezensis (Gene Bank No. CP037417.1), and was kept in our laboratory. The medium used in this study was lysogeny broth (LB) liquid medium (100 mL of ultra-pure water, 1 g of tryptone, 0.5 g of yeast extract, and 1 g of NaCl, 6.5 ≤ pH ≤ 7.5). The Fe3O4, CaCl2, CuSO4·5H2O, NaOH, MB, 30% H2O2 solution, 36% hydrochloric acid (HCl), and tertiary butanol (TB) were of analytical grade and sourced from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, and Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Ultra-pure water was used to prepare all the solutions. Then, 0.1 mol/L NaOH and 0.1 mol/L HCl were used to adjust the pH values.
2.2. Experimental Methods
2.2.1. Preparation of V-M and Structure Analysis
Here, 0.05% (m/v) Fe3O4, 2% B. velezensis inoculum, and 0.8% CaCl2 were added into a 100 mL sterilized LB liquid medium, and then cultured at 30 °C, 180 rpm for seven days. V-M was obtained from the centrifuged medium (5804R, Eppendorf, Hamburg, Germany, 9000 rpm, 8 min) and was dried at 65 °C and ground. Meanwhile, the biogenetic vaterite without Fe3O4 was synthesized by using the same method for structural comparison.
The X-ray diffraction (XRD) data were collected on an BTX-III diffractometer (Olympus, Tokyo, Japan) equipped with CoKα radiation. The Fourier transform infrared spectroscopy (FTIR) was performed with a Nicolet Nexus 670 FTIR Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The FTIR spectra were recorded with 32 scans per spectrum at a resolution of 4 cm−1. The thermal gravimetric–derivative thermal gravimetric (TG-DTG) curve was obtained from a STA7300 thermal analysis system (PerkinElmer, Waltham, MA, USA). The purge gas was N2, with a flow rate of 100 mL/min and a heating rate of 10 °C/min. The scanning electron microscope and energy-dispersive X-ray spectroscopy (SEM-EDS) data were collected on a Supra 55 (Carl Zeiss, Oberkochen, Germany). The magnetic performance of V-M was obtained using a 7404 vibrating sample magnetometer (Lake Shore Cryotronics, Westerville, OH, USA).
2.2.2. Factors Influencing the Removal of MB-Cu Composite Pollution
A 50 mL centrifuge tube was used for the study, and the reaction system was controlled to a volume of 20 mL. Unless otherwise stated, 0.05 g of V-M was added, and the concentrations of H2O2, Cu(II), and MB were maintained at 50 mmol/L, 200 mg/L [25], and 200 mg/L [26], respectively. Considering that wastewater containing Cu(II) is typically acidic [27,28], and in light of relevant research findings [26,29], the pH was adjusted to 3. The solution was kept at 25 °C at 100 rpm for 24 h. Thereafter, it was subjected to centrifugation at 9000 rpm for 8 min. Subsequently, the supernatant was collected for determining the concentrations of Cu(II) and MB using a flame atomic absorption spectrophotometer (AAS, AA-6300C, Shimadzu, Kyoto, Japan) and spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA, λ = 665 nm), respectively. Three replicates were set for each experiment.
The following methods were used to assess the related influencing factors:
- A.
- Effects of the initial quantity of V-M and concentration of H2O2
The quantity of V-M was set to 0.005, 0.01, 0.03, 0.05, and 0.1 g. Following the experiment, the concentrations of Cu(II) and MB were measured. The removal percentages of both Cu(II) and MB were then calculated.
The concentration of H2O2 was set to 5, 10, 20, 40, 50, 100, 150, and 200 mmol/L. Following the experiment, the concentrations of Cu(II) and MB were measured. The removal percentages of both Cu(II) and MB were then calculated.
Equations (1) and (2) illustrate the methodology for calculating the removal percentages of Cu(II) and MB:
where Ae and De represent the removal percentage of Cu(II) (%) and degradation percentage of MB (%), respectively, while Ci and Ce represent the initial concentration and equilibrium concentration of Cu(II) or MB (mg/L), respectively.
- B.
- Effect of the initial pH
The initial pH was set to 2.0, 3.0, 4.0, 5.0, and 6.0 using HCl (12 mol/L) or NaOH (12.5 mol/L). Following the experiment, the equilibrium pH (pHe) was measured, and the removal percentages of Cu(II) and MB were calculated using Equations (1) and (2).
- C.
- Effect of the initial concentrations of pollutants
The concentration of Cu(II) was kept at 200 mg/L and the concentration of MB was set at 5, 10, 20, 50, 100, 150, and 200 to estimate the effect of the concentration of MB. Similarly, the concentration of MB was kept at 200 mg/L and the concentration of Cu(II) was set at 5, 10, 20, 50, 100, 150, and 200 mg/L to assess the effect of the concentration of Cu(II). The removal percentages of MB and Cu(II) were calculated using Equations (1) and (2), and the unit adsorption capacity (Qe, mg/g) of Cu(II) by V-M was calculated using Equation (3):
where Ci and Ce represent the initial concentration and equilibrium concentration of Cu(II) (mg/L), respectively, V represents the volume of the adsorbent solution (L), and M represents the weight of V-M (g).
- D.
- The recycling condition of the Cu(II) immobilized by V-M
After the removal reaction was finished, the V-M was separated by a magnet. Different concentrations of HCl (0.001, 0.01, 0.05, and 0.1 mol/L) were used to dissolve V-M. The supernatant was collected after 30 min, the concentrations of Cu(II) and MB in the supernatant were tested, and the recycling percentage (Re, %) was calculated using Equation (4):
where Ci and CR represent the initial concentration and recycled concentration (mg/L), respectively.
2.2.3. Analysis of the Pollutant Removal Mechanism
- A.
- The role of OH
Different concentrations of tertiary butanol (0, 20, and 40 mol/L) were added to the reaction system, with the concentrations of H2O2, Cu(II), and MB maintained at 50 mmol/L, 200 mg/L, and 200 mg/L, respectively. The samples were collected at different times (10, 30, 60, and 150 min), and the removal percentages of Cu(II) and MB were calculated.
- B.
- Adsorption isotherm and adsorption kinetic analysis
Based on the data collected (Section 2.2.2, C), a Langmuir isotherm equation (Equation (5)) and Freundlich isotherm equation (Equation (6)) were applied to fit the data [30,31]:
where Ce and Qe represent the equilibrium concentration (mg/L) and equilibrium adsorption capacity (mg/g) of Cu(II), respectively, KL represents the adsorption coefficient of the Langmuir isotherm equation, KF represents the relative adsorption capacity of the adsorbent, and n is a constant representing the adsorption strength.
The experiment was conducted in accordance with the methodology outlined in Section 2.2.2. The samples were collected at different times (5 to 1440 min). The adsorption capacity (Qt, mg/g) was calculated using Equation (7):
where Ci and Ct represent the initial concentration and concentration at time t of Cu(II), V represents the volume of the adsorbent solution (L), and M represents the weight of V-M (g).
The linear equations of pseudo-first-order dynamics (Equation (8)) and pseudo-second-order dynamics (Equation (9)) were used to fit the data [32]:
where Qt represents the unit adsorption capacity at time t of Cu(II) by V-M (mg/g), Qe represents the unit adsorption capacity at equilibrium (mg/g), while K1 and K2 are the pseudo-first-order kinetic model constant (1/min) and pseudo-second-order kinetic model constant (g/mg·min), respectively.
3. Results and Discussion
3.1. Characterization of V-M
3.1.1. Structural Analysis and Characterization
The structural analysis and characterization results are shown in Figure 1.
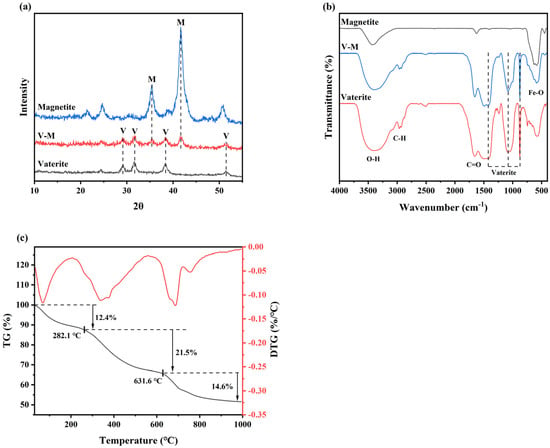
Figure 1.
Structural analysis and characterization of V-M: (a) XRD pattern (Vaterite, V: ICDD PDF No. 72-0506; Magnetite, M: ICDD PDF No. 88-0315), (b) FTIR pattern, and (c) TG/DTG curve.
The XRD results showed that in the absence of Fe3O4 in the medium, the precipitate synthesized by B. velezensis was vaterite; however, upon addition of Fe3O4, the precipitate exhibited diffraction peaks characteristic of both vaterite and Fe3O4 (Figure 1a), indicating that the mineral synthesized by B. velezensis was an Fe3O4-containing vaterite, designated V-M. Except for those vibration peaks of vaterite (1426, 1087, 879, and 743 cm−1) [33] and Fe3O4 (585 cm−1) [34], the FTIR pattern (Figure 1b) suggested that V-M possesses certain organic groups, including O-H (3407 cm−1), C-H (2964 and 2933 cm−1), C-O (1688 cm−1), and -SH (569 cm−1) [35], implying that V-M also contains many organic groups other than vaterite and Fe3O4. Previous research conducted in our laboratory identified these organic compounds as the primary metabolites of B. velezensis [36], which is consistent with the findings of other studies exploring the mechanism of action and factors influencing microbial participation in the precipitation of CaCO3 [37,38]. These metabolites serve as nucleation sites and are incorporated into the lattice of vaterite, forming a distinctive organic-inorganic complex structure that enhances the stability of the vaterite. Additionally, organic compounds play a role in the adsorption of pollutants [39,40], contributing to the favorable Cu(II) fixation properties of V-M (see below). The TG/DTG patterns are shown in Figure 1c. There were three mass-loss steps with increasing temperature: the mass loss between 27.6 and 282.1 °C was attributed to the evaporation of water (12.4 wt%), the mass loss between 282.1 and 631.6 °C was caused by the degradation of organic matter (21.5 wt%), and CaCO3 was degraded at 631.6–992.5 °C, with a 14.6% loss of mass [38,41].
SEM was used to characterize the B. velezensis-synthesized vaterite, Fe3O4, and V-M, and the results are shown in Figure 2.
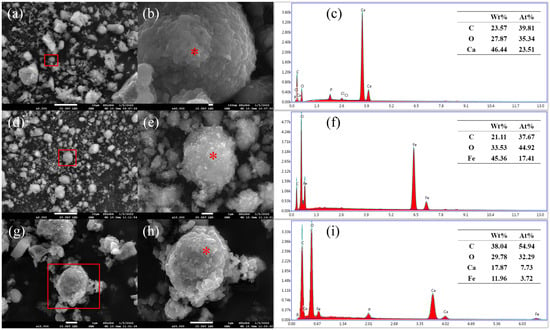
Figure 2.
SEM and EDS data of samples: (a–c) SEM and EDS data of vaterite, (d–f) SEM and EDS data of Fe3O4, and (g–i) SEM and EDS data of V-M. A zoom of the samples is encircled in red.
The mineral synthesized by B. velezensis was vaterite without the presence of Fe3O4. According to previous research in our laboratory [42], it has a porous surface with a diameter of approximately 4.88 µm. Upon the introduction of Fe3O4 into the synthesis system, B. velezensis produced a spherical or irregular porous structure with a diameter of approximately 1 µm (Figure 2g). EDS indicated that there are C, O, Ca, Fe, and other elements in V-M (Figure 2i).
Based on the aforementioned findings, it can be posited that V-M is an Fe3O4-containing vaterite.
3.1.2. Magnetic Performance of V-M
The hysteresis loop can reflect the magnetization properties of ferromagnetic materials, which is the relationship curve of magnetization with the magnetic field strength. The hysteresis loops of Fe3O4 and V-M are shown in Figure 3. The saturation magnetization of Fe3O4 (Figure 3a) and V-M (Figure 3b) was 20.174 and 2.986 emu/g. The coercivity of V-M was 0.047 kOe, which made it almost free of hysteresis, and it could be re-dispersed after being separated by an external magnetic field, exhibiting paramagnetism [43]. In comparison to the coercivity of Fe3O4, the coercivity of V-M remained largely unaltered, suggesting that the synthesis of V-M by B. velezensis in this study had no discernible effect on the magnetic properties of Fe3O4.
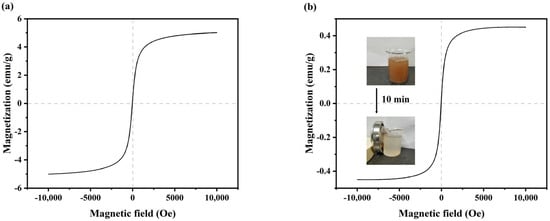
Figure 3.
Magnetic performance of samples: (a) the hysteresis loop of Fe3O4 and (b) the hysteresis loop of V-M.
V-M was fully mixed in water to create a suspension. When an external magnetic field was applied, V-M could be completely sucked to one side of the beaker within 10 min (Figure 3b). The absence of solid particles in the water indicated that V-M could be collected through the application of a magnetic field, exhibiting effective magnetic separation performance and a notable magnetic field response. However, the liquid after the magnet separation was not clear (Figure 3b). This was due to the presence of residual vaterite and bacteria within the solid precipitate, which remained uncombined with Fe3O4 and was, therefore, unable to be attracted by a magnet. Nevertheless, the majority of V-M could be recycled through the use of supplementary magnets.
3.2. Factors Influencing the Removal of MB-Cu Composite Contamination
3.2.1. Effects of the Initial Quantity of V-M and Concentration of H2O2
The Fenton-like reaction has a broad range of potential applications for the degradation of organic pollutants. The addition of H2O2 and adsorbents has a direct impact on the removal of pollutants, with a substantial body of research examining the effects of these additions on the removal of pollutants [26,44,45]. To identify the optimal quantity and ratio of H2O2 and adsorbent materials, the removal effects of varying quantities of V-M, acting as both a Fenton-like reaction catalyst and an adsorbent, and of different concentrations of H2O2 on the combined pollution of organics and heavy metals were analyzed, as illustrated in Figure 4.
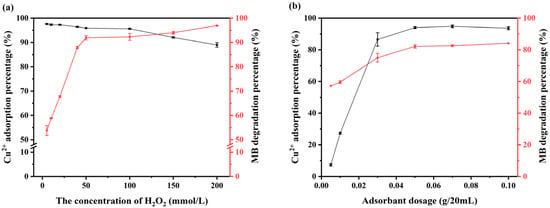
Figure 4.
Effects of the initial quantity of V-M and concentration of H2O2: (a) effect of the initial concentration of H2O2 and (b) effect of the initial quantity of V-M.
As illustrated in Figure 4, the degradation percentage of MB gradually increased with an increase in the H2O2 concentration in the system upon addition of 0.05 g of V-M. Conversely, the removal percentage of Cu(II) displayed an inverse trend. At a concentration of 50 mmol/L, the degradation percentage of MB was observed to reach a high level, without a notable decline in the removal percentage of Cu(II); however, at concentrations exceeding 100 mmol/L, the removal percentage of Cu(II) showed a considerable reduction (Figure 4a). This phenomenon may be attributed to the elevated generation of ·OH when the H2O2 content was excessive, which accelerated the degradation of MB while disrupting the organic–inorganic composite structure of the V-M. At a concentration of 50 mmol/L, the removal percentages of MB and Cu(II) initially increased with the addition of V-M, subsequently reaching equilibrium. This indicates that the addition of V-M had a beneficial effect on the removal of MB-Cu composite pollution. Upon reaching a V-M addition of 0.05 g, the removal percentages of MB and Cu(II) exhibited a near-equilibrium state (Figure 4b). This can be attributed to the insufficient availability of adsorption sites and iron donors provided by the low V-M addition, which consequently resulted in a diminished production of ·OH radicals during the Fenton-like reaction process. This ultimately led to a reduced percentage of removal. In light of the economic cost and pollution control considerations, the subsequent experiments were conducted with a quantity of V-M and a concentration of H2O2 of 0.05 g and 50 mmol/L, respectively. However, further investigation is required to assess the effects of the addition sequence of the two on the removal percentage.
3.2.2. Effect of the Initial pH
The pH of wastewater can affect the stability of V-M and the formation of ·OH, which exerts a significant influence on the efficacy of the treatment process. Figure 5 illustrates the effect of varying initial pH values on the treatment of MB-Cu composite pollution.
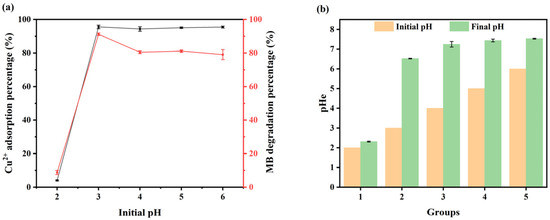
Figure 5.
(a) Effect of the initial pH, and (b) pH changes before and after treatment. The designation “groups” is used to indicate a linearly ascending initial pH.
Figure 5 shows that the removal percentages of Cu(II) and MB were markedly influenced by alterations in the initial pH value of the solution. As the pH increased, the removal percentages initially exhibited an upward trend, subsequently reaching a point of equilibrium (Figure 5a). At a pH of 2, the removal percentages of Cu(II) and MB were low due to the fact that V-M was unable to exist in a stable state under conditions of strong acidity. At a pH of 3, the removal percentages of Cu(II) and MB reached 91.2% and 95.5%, respectively. Thereafter, an increase in the pH value had a negligible effect on the removal of Cu(II). The percentage of degradation of MB slightly declined with an increase in pH, yet remained at approximately 80% (Figure 5a), suggesting that acidic conditions were more conducive to the degradation of MB. This is due to the superior performance of Fe3O4 in catalyzing the formation of ·OH under acidic conditions [46]. As the pH increased, the capacity to generate ·OH decreased, thereby reducing the percentage of degradation of MB. Furthermore, the combination of V-M with H2O2 treatment resulted in a notable elevation in the pH value following the reaction (p < 0.01; Figure 5b). This suggests that V-M, as a Fenton-like catalyst, not only showed a broad pH range of applicability but also demonstrated a robust capacity to regulate pH, addressing the issue of system acidity following the conventional Fenton-like reaction and the necessity for alkaline solution supplementation.
3.2.3. Effect of the Initial Concentrations of Pollutants
An investigation into the effects of the initial pollutant concentration on the efficacy of the treatment process can ascertain the applicable concentration range of pollutants under specific conditions and reveal the influence of the pollutant concentration on the reaction process. Figure 6 illustrates the effects of the initial concentration of pollutants on the combined V-M and H2O2 treatment of MB-Cu composite pollution.
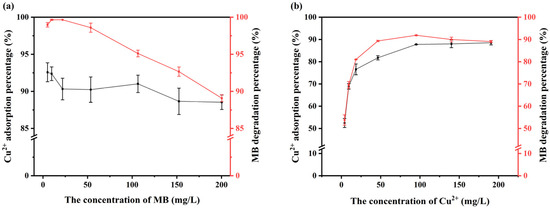
Figure 6.
Effects of the initial concentrations of pollutants: (a) effect of the initial concentration of MB and (b) effect of the initial concentration of Cu(II).
As the concentration of MB in the system increased, the rates of removal of Cu(II) and MB slightly decreased, yet they remained at a relatively high level (Figure 6a). When the initial concentration of MB was less than 50 mg/L, the percentage of degradation was approximately 100%; however, as the initial concentration of MB increased, the percentage of degradation subsequently declined. This is attributable to the restricted concentration of H2O2 and the concomitant reduction in the production of ·OH, which were insufficient to facilitate the complete degradation of excess MB. On the other hand, the elevated Cu(II) concentration in the mixed system not only increased the removal percentage of Cu(II) but also facilitated the degradation of MB (Figure 6b). This is due to the fact that both Cu(II) and Fe(II) are capable of promoting the generation of H2O2 to produce OH. The specific principle is illustrated in Equations (10) and (11) [47,48]. Therefore, it can be reasonably deduced that the presence of an appropriate Cu(II) can improve the efficiency of production of ·OH, which in turn will increase the percentage of degradation of MB.
In conclusion, the percentage of degradation of MB can be affected by the concentration of Cu(II), while the removal percentage of Cu(II) was essentially uninfluenced by the initial concentration of MB when V-M was employed to treat MB-Cu composite pollution. In a mixed system where the concentration of MB is less than 50 mg/L and the concentration of Cu(II) is greater than 100 mg/L, the percentage of removal of both can exceed 90%.
3.2.4. The Recycling Condition of the Cu(II) Immobilized by V-M
The recycling of metal ions from magnetically separated adsorbed samples represents a significant economic opportunity. The dissolution of V-M by HCl releases those heavy metals that have been adsorbed. Figure 7 illustrates the effects of varying HCl concentrations on the recycling of Cu(II). The magnetically separated samples showed robust retention of Cu(II) at a 0.001 mol/L HCl concentration (pH = 3.0), indicating that V-M exhibited notable acid resistance. The recycling percentage of Cu(II) increased notably (p < 0.01) when the concentration of HCl was set to 0.01 mol/L, with the highest recycling (in percentage terms) of Cu(II) observed at an HCl concentration of 0.05 mol/L, reaching 85.82%. The capacity of V-M to recycle Cu(II) is mediocre in comparison to other Cu(II) recycling methods (Table 1). The findings indicated that the V-M synthesized in this study exhibited a notable adsorption capacity for Cu(II). The utilization of V-M facilitated the separation and recycling of heavy metal ions in composite pollution.
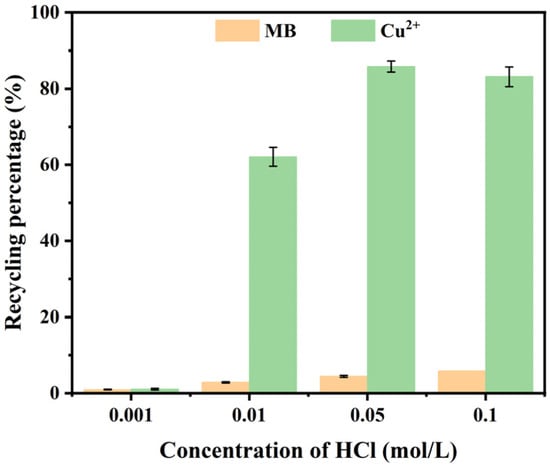
Figure 7.
Recycling percentage of Cu(II) and release percentage of MB at different concentrations of HCl.

Table 1.
The comparison of recycling percentages of Cu(II).
It is noteworthy that the release of MB also occurred during the recycling of Cu(II), but only up to 5.83%. This finding suggests that the process of MB removal by V-M in combination with H2O2 was dominated by the oxidative decomposition of MB by ·OH produced by the Fenton-like reaction, and that a small amount of MB was also adsorbed by V-M.
3.3. Analysis of the Removal Mechanism
3.3.1. The Role of ·OH
The ability of ·OH to degrade organic matter efficiently is well documented. Tertiary butanol (TB), a widely used ·OH scavenger, has been found to trap ·OH and prevent its oxidation of organic matter with great efficiency [48]. Figure 8 illustrates the effects of varying TB concentrations on the removal efficiency of MB and Cu(II).
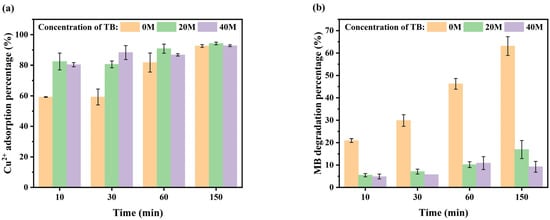
Figure 8.
Effects of TB on the treatment effect of MB-Cu composite pollution: (a) effect of TB on the adsorption percentage of Cu(II) and (b) effect of TB on the degradation percentage of MB.
The findings indicated that, when a high concentration of ·OH inhibitor was present in the system, the degradation percentage of MB decreased significantly with an increase in the TB concentration (p < 0.01; Figure 8b). This phenomenon can be attributed to the fact that the TB can trap the OH produced by H2O2 and prevent its oxidation of MB, as illustrated by Equations (12)–(15). In these equations, the ≡ symbol denotes the iron surface site [54,55,56,57]:
Equations (12) and (13) reveal the mechanism by which Fe(II) and Fe(III), at the surface site of Fe3O4, react with H2O2 to form ·OH. Equations (14) and (15) illustrate the degradation of MB by ·OH and the capture of ·OH by TB to form alkyl radicals, respectively.
Furthermore, the adsorption efficiency of Cu(II) increased with the TB concentration. This phenomenon can be attributed to the ability of TB to capture the OH generated by H2O2, thereby impeding its oxidation of organic matter in V-M. This, in turn, facilitates the adsorption of Cu(II). In conclusion, the degradation of MB was primarily attributed to the action of OH radicals, which were predominantly generated through the reaction of H2O2 with Fe3O4 in V-M.
3.3.2. Analysis of Pollutant Removal Mechanism
To investigate the distribution of Cu(II) in the solid–liquid phase when V-M was combined with H2O2 to treat MB-Cu composite pollution, the Langmuir and Freundlich adsorption isotherms were employed to fit the experimental data. Figure 9 illustrates the adsorption isotherm of Cu(II) and the resulting change in the concentration of Ca(II) in solution. The findings indicated that the variation in the Ca(II) concentration in the system was largely consistent with that of the adsorption isotherm, exhibiting an increase alongside the equilibrium concentration of Cu(II). This suggested the possibility of a displacement phenomenon occurring between Cu(II) and Ca(II) in V-M during the adsorption of Cu(II). As the initial equilibrium concentration of Cu(II) increased, so too did the displacement of Ca(II). The results of the isotherm adsorption fitting (Figure S1) demonstrated that the determination coefficient of the Freundlich adsorption isotherm equation was higher (R2 = 0.98627). This suggested that the Freundlich model was more appropriate for describing the adsorption process of Cu(II) by V-M. In other words, the adsorption of Cu(II) by V-M can be defined as a multimolecular layer adsorption process occurring on a heterogeneous surface [58,59].
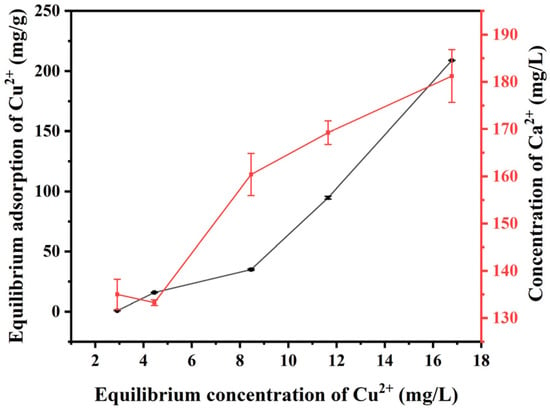
Figure 9.
Effects of changes in the equilibrium concentration of Cu(II) on the equilibrium adsorption of Cu(II) and the concentration of Ca(II) in the system during the treatment of MB-Cu composite pollution by V-M combined with H2O2.
Figure 10 illustrates the adsorption kinetics of Cu(II) by V-M. The findings indicated that the unit adsorption capacity of Cu(II) increased with the prolongation of the adsorption period. Additionally, the concentration of Ca(II) within the system exhibited a parallel trend to that of Cu(II), which was in accordance with the results of isothermal adsorption.
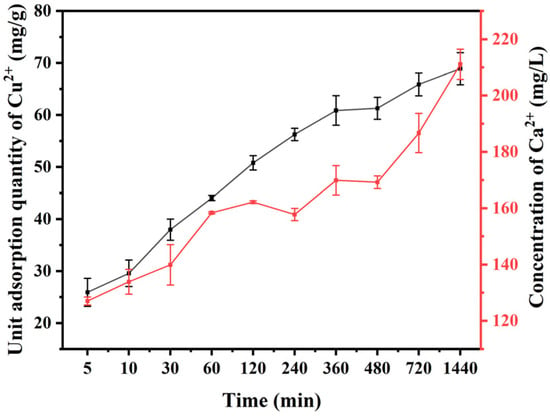
Figure 10.
Variation in the Cu(II) unit adsorption and Ca(II) concentration with time in MB-Cu composite pollution treated with V-M combined with H2O2.
The experimental data were fitted using pseudo-first-order and pseudo-second-order kinetic models. The results indicated that the pseudo-second-order kinetic model exhibited a higher degree of correlation and a superior fitting coefficient (R2 = 0.9773; Figure S2; Table 2). The findings suggested that ion exchange occurred during the adsorption of Cu(II) by V-M, with the percentage of adsorption dependent on the concentration of the activation site on the surface of V-M [59].

Table 2.
Pseudo-first-order and pseudo-second-order adsorption kinetic model parameters.
Our previous research found that, in comparison to chemically synthesized minerals, the organic matter present in biominerals not only contributed to the formation of mineral crystals but also enhanced their stability. Furthermore, this organic matter played an essential role in the fixation and adsorption of pollutants [39,40]. Further investigation is required to elucidate the adsorption and fixation of pollutants by organic matter in V-M.
For comparison, the maximum unit adsorption capacity (Qmax) of V-M for Cu(II) in an adsorption system devoid of MB and the Qmax of other materials can be found in Table 3. Among the numerous materials that can adsorb Cu(II), the adsorption performance of V-M was intermediate, and the Qmax of V-M was lower than that of bioderived vaterite, as synthesized in our laboratory [42]. This suggested that the incorporation of Fe3O4 can exert a certain influence on the Cu(II) retention properties of biogenic vaterite. Nevertheless, the magnetic separation characteristics of V-M and its capacity to catalyze the oxidation of MB by H2O2, as a Fenton-like catalyst, were not exhibited by other materials. Consequently, V-M continues to demonstrate advantages in the treatment of complex polluted water bodies.

Table 3.
The comparison of Qmax (mg/g) values for Cu(II).
In conclusion, V-M is a type of porous organic–inorganic complex comprising Fe3O4, and the mechanism of its action in the removal of MB-Cu composite pollution in the presence of H2O2 is illustrated in Figure 11. The main elements are as follows: (1) Magnetic vaterite could be synthesized through the induction of B. velezensis. (2) The Fe3O4 component of V-M was capable of reacting with H2O2 to form ·OH and degrade MB to CO2 and H2O. The presence of Cu(II) was observed to facilitate this reaction. (3) In addition to providing adsorption sites for Cu(II) and MB, the organic functional groups in V-M also stabilized the structure of V-M, thereby extending the pH range of its applicability. (4) Cu(II) was primarily removed through V-M adsorption, which occurred through multilayer adsorption on heterogeneous surfaces. In addition, displacement with Ca(II) played a role in the removal process. (5) Following the adsorption of Cu(II), the V-M could be efficiently collected using an applied magnetic field. (6) The immobilized Cu(II) in the V-M could be efficiently recycled through a diluted HCl treatment.
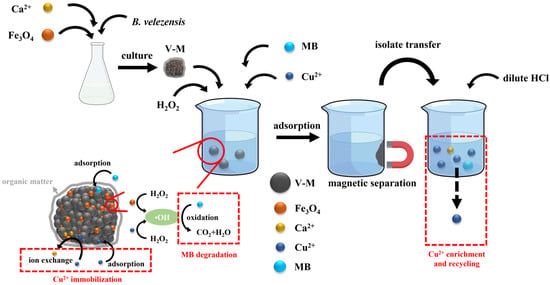
Figure 11.
Mechanism of V-M combined with H2O2 in removing MB-Cu composite pollution.
4. Conclusions
The V-M was synthesized through the use of B. velezensis. V-M can catalyze the production of ·OH from H2O2 for the purpose of degrading MB. Additionally, it is capable of fixing Cu(II) through chemisorption, and Cu(II) can also participate in Fenton-like reactions to facilitate the degradation of MB. Following the treatment of the composite contamination, V-M was separated by magnetic separation. The Cu(II) fixed by V-M could then be recycled by diluted HCl. In conclusion, the combination of V-M and H2O2 was shown to be an effective method for the removal of MB-Cu pollution in water, as well as for the recycling of Cu(II). This provides a novel approach for addressing the combined pollution of organic substances and heavy metals in the environment and the recycling of heavy metals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min14111142/s1. Figure S1: Isothermal adsorption model-fitting curves for Cu(II) by V-M: (a) fitting curve of Langmuir model and (b) fitting curve of Freundlich model. Figure S2: Kinetic curves of adsorption of Cu(II) by V-M: (a) fitting curve of pseudo-first-order dynamics and (b) fitting curve of pseudo-second-order dynamics.
Author Contributions
X.H., investigation, data curation, writing—original draft; M.H., data curation, writing—original draft, writing—review and editing; Y.C. and X.W., data curation, writing—original draft; B.L., conceptualization, supervision, project administration, writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Major Scientific and Technological Achievement Transformation Project of Guizhou Province (Grant No. 2022-Major-010), and the National Natural Science Foundation of China (Grant No. 92351302).
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Acknowledgments
The authors would like to thank Jiahong Zhou of Nanjing Normal University for their support in this research project.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sun, Y.; Li, D.; Lu, X.; Sheng, J.; Zheng, X.; Xiao, X. Flocculation of combined contaminants of dye and heavy metal by nano-chitosan flocculants. J. Environ. Manag. 2021, 299, 113589. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Song, H.-W.; Mishra, S.; Zhang, W.; Zhang, Y.-L.; Zhang, Q.-R.; Yu, Z.-G. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef] [PubMed]
- Fajarwati, F.; Anugrahwati, M.; Yanti, I.; Safitri, R.; Yuanita, E. Adsorption study of methylene blue and eriochrome black T dyes on activated carbon and magnetic carbon composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 012025. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Okundaye, B.; Simbi, I.; Ama, O.M.; Darmokoesoemo, H.; Widyaningrum, B.A.; Osibote, O.A. Biosorption of methylene blue using clove leaves waste modified with sodium hydroxide. Results Chem. 2023, 5, 100778. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, G.; Li, P.; Gao, J.; Lv, B.; Li, D. A novel method for photodegradation of high-chroma dye wastewater via electrochemical pre-oxidation. Chemosphere 2010, 80, 410–415. [Google Scholar] [CrossRef]
- Wu, K.; Shi, M.; Pan, X.; Zhang, J.; Zhang, X.; Shen, T.; Tian, Y. Decolourization and biodegradation of methylene blue dye by a ligninolytic enzyme-producing Bacillus thuringiensis: Degradation products and pathway. Enzym. Microb. Technol. 2022, 156, 109999. [Google Scholar] [CrossRef]
- Qi, J.; Li, X.; Zheng, H.; Li, P.; Wang, H. Simultaneous removal of methylene blue and copper(II) ions by photoelectron catalytic oxidation using stannic oxide modified iron(III) oxide composite electrodes. J. Hazard. Mater. 2015, 293, 105–111. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Xiao, D.; Han, D. In vitro effects of brominated flame retardants, selected metals and their mixtures on ethoxyresorufin-O-deethylase activity in Mossambica tilapia liver. Ecotoxicol. Environ. Saf. 2018, 161, 350–355. [Google Scholar] [CrossRef]
- Cai, G.-B.; Zhao, G.-X.; Wang, X.-K.; Yu, S.-H. Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J. Phys. Chem. C 2010, 114, 12948–12954. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Li, P.; Qu, J.; Dou, Z.-F.; Yan, W.-S.; Zhu, J.-F.; Wu, Z.-Y.; Song, W.-G. High adsorption capacity and the key role of carbonate groups for heavy metal ion removal by basic aluminum carbonate porous nanospheres. J. Mater. Chem. 2012, 22, 19898–19903. [Google Scholar] [CrossRef]
- Islam, M.S.; San Choi, W.; Nam, B.; Yoon, C.; Lee, H.-J. Needle-like iron oxide@ CaCO3 adsorbents for ultrafast removal of anionic and cationic heavy metal ions. Chem. Eng. J. 2017, 307, 208–219. [Google Scholar] [CrossRef]
- Wierzba, S.; Makuchowska-Fryc, J.; Kłos, A.; Ziembik, Z.; Ochędzan-Siodłak, W. Role of calcium carbonate in the process of heavy metal biosorption from solutions: Synergy of metal removal mechanisms. Sci. Rep. 2022, 12, 17668. [Google Scholar] [CrossRef]
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, C.; Xing, M.; Zhou, Y. Oxidation of emerging organic contaminants by in-situ H2O2 fenton system. Green Energy Environ. 2023, 9, 417–434. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole degradation by UV and UV/H2O2 advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cheng, S.; Su, T.; Zuo, G.; Zhao, W.; Qi, X.; Wei, W.; Dong, W. Fenton-like catalyst Fe3O4@ polydopamine-MnO2 for enhancing removal of methylene blue in wastewater. Colloids Surf. B Biointerfaces 2019, 181, 226–233. [Google Scholar] [CrossRef]
- Ma, C.; Feng, S.; Zhou, J.; Chen, R.; Wei, Y.; Liu, H.; Wang, S. Enhancement of H2O2 decomposition efficiency by the co-catalytic effect of iron phosphide on the Fenton reaction for the degradation of methylene blue. Appl. Catal. B Environ. 2019, 259, 118015. [Google Scholar] [CrossRef]
- Wang, P.; Shen, T.; Li, X.; Tang, Y.; Li, Y. Magnetic mesoporous calcium carbonate-based nanocomposites for the removal of toxic Pb(II) and Cd(II) ions from water. ACS Appl. Nano Mater. 2020, 3, 1272–1281. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Zhong, Y.; Guan, Y.; Chen, Q.; Du, W.; Liu, G. Biological calcium carbonate enhanced the ability of biochar to passivate antimony and lead in soil. Environ. Sci. Process. Impacts 2023, 25, 1365–1373. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Lei, Y.; Jia, H.; Niu, Q.; Dapaah, M.F.; Gao, Y.; Cheng, L. Green and effective remediation of heavy metals contaminated water using CaCO3 vaterite synthesized through biomineralization. J. Environ. Manag. 2024, 353, 120136. [Google Scholar] [CrossRef]
- Paul, D.; Singhania, A.; Das, G. Catalytic activities of the vaterite and the calcite based solid supported catalysts for spontaneous Fenton-like dye degradation: A comparative study. J. Environ. Chem. Eng. 2022, 10, 107558. [Google Scholar] [CrossRef]
- Cui, L.; Yang, C.; Wei, L.; Li, T.; Chen, X. Isolation and identification of an endophytic bacteria Bacillus velezensis 8-4 exhibiting biocontrol activity against potato scab. Biol. Control 2020, 141, 104156. [Google Scholar] [CrossRef]
- Dikshit, R.; Jain, A.; Dey, A.; Kumar, A. Microbially induced calcite precipitation using Bacillus velezensis with guar gum. PLoS ONE 2020, 15, e0236745. [Google Scholar] [CrossRef]
- Huang, S.; Liu, R.; Sun, M.; Li, X.; Guan, Y.; Lian, B. Transcriptome expression analysis of the gene regulation mechanism of bacterial mineralization tolerance to high concentrations of Cd2+. Sci. Total Environ. 2022, 806, 150911. [Google Scholar] [CrossRef]
- He, G.; Wang, C.; Cao, J.; Fan, L.; Zhao, S.; Chai, Y. Carboxymethyl chitosan-kaolinite composite hydrogel for efficient copper ions trapping. J. Environ. Chem. Eng. 2019, 7, 102953. [Google Scholar] [CrossRef]
- Almeida, C.; Debacher, N.; Downs, A.; Cottet, L.; Mello, C. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloïd Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Badsha, M.A.; Lo, I.M. An innovative pH-independent magnetically separable hydrogel for the removal of Cu(II) and Ni(II) ions from electroplating wastewater. J. Hazard. Mater. 2020, 381, 121000. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Bharti, A.K.; Sadegh, H. Adsorption mechanism of functionalized multi-walled carbon nanotubes for advanced Cu (II) removal. J. Mol. Liq. 2017, 230, 667–673. [Google Scholar] [CrossRef]
- Atar, N.; Olgun, A.; Wang, S. Adsorption of cadmium (II) and zinc (II) on boron enrichment process waste in aqueous solutions: Batch and fixed-bed system studies. Chem. Eng. J. 2012, 192, 1–7. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C. Analyzing concurrent multi-stage adsorption process of activated carbon with a favorable parameter of Langmuir equation. J. Taiwan Inst. Chem. Eng. 2009, 40, 197–204. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-J.; Ma, F.; Li, F.-C.; Zhang, C.-H.; Chen, J.-N. Vaterite induced by Lysinibacillus sp. GW-2 strain and its stability. J. Struct. Biol. 2017, 200, 97–105. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S. A new approach to modification of natural adsorbent for heavy metal adsorption. Bioresour. Technol. 2008, 99, 2516–2527. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Nie, W.; Sun, M.; Li, X.; Lian, B. Facilitated mechanism of biological vaterite stability mediated by Bacillus velezensis and its secretions. ACS Earth Space Chem. 2023, 7, 2019–2030. [Google Scholar] [CrossRef]
- Khanjani, M.; Westenberg, D.J.; Kumar, A.; Ma, H. Tuning polymorphs and morphology of microbially induced calcium carbonate: Controlling factors and underlying mechanisms. ACS Omega 2021, 6, 11988–12003. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Hu, M.; Gao, L.; Lian, B. The competitive and selective adsorption of heavy metals by struvite in the Pb(II)-Cd(II)-Zn(II) composite system and its environmental significance. Water Res. 2024, 250, 121087. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Zhang, L.; Yu, C.; Lian, B. Synthesis of biogenic high-magnesium calcite and its experimental immobilization effect on Cd2+. Geomicrobiol. J. 2021, 38, 482–493. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.-Z.; Wang, F.-P.; Huang, Y.-R.; Fu, S.-Q.; Zhou, G.-T. Insights into the formation mechanism of vaterite mediated by a deep-sea bacterium Shewanella piezotolerans WP3. Geochim. Cosmochim. Acta 2019, 256, 35–48. [Google Scholar] [CrossRef]
- Liu, R.; Lian, B. Non-competitive and competitive adsorption of Cd2+, Ni2+, and Cu2+ by biogenic vaterite. Sci. Total Environ. 2019, 659, 122–130. [Google Scholar] [CrossRef]
- Noginova, N.; Chen, F.; Weaver, T.; Giannelis, E.; Bourlinos, A.; Atsarkin, V. Magnetic resonance in nanoparticles: Between ferro-and paramagnetism. J. Phys. Condens. Matter 2007, 19, 246208. [Google Scholar] [CrossRef]
- Akköz, Y.; Coşkun, R.; Delibaş, A. Preparation and characterization of sulphonated bio-adsorbent from waste hawthorn kernel for dye (MB) removal. J. Mol. Liq. 2019, 287, 110988. [Google Scholar] [CrossRef]
- Kannamba, B.; Reddy, K.L.; AppaRao, B. Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lim, W.T.; Park, J.Y.; Kim, Y.H. Effect of pH on Fenton and Fenton-like oxidation. Environ. Technol. 2009, 30, 183–190. [Google Scholar] [CrossRef]
- Eberhardt, M.K.; Ramirez, G.; Ayala, E. Does the reaction of copper(I) with hydrogen peroxide give hydroxyl radicals? A study of aromatic hydroxylation. J. Org. Chem. 1989, 54, 5922–5926. [Google Scholar] [CrossRef]
- Lv, K.; Xu, Y. Effects of polyoxometalate and fluoride on adsorption and photocatalytic degradation of organic dye X3B on TiO2: The difference in the production of reactive species. J. Phys. Chem. B 2006, 110, 6204–6212. [Google Scholar] [CrossRef] [PubMed]
- Zan, F.; Huo, S.; Xi, B.; Zhao, X. Biosorption of Cd2+ and Cu2+ on immobilized Saccharomyces cerevisiae. Front. Environ. Sci. Eng. 2012, 6, 51–58. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, K.; Liu, Q.; Guo, F.; Xiong, Z.; Li, Y.; Wang, Q. A bifunctional adsorbent of silica gel-immobilized Schiff base derivative for simultaneous and selective adsorption of Cu(II) and SO42−. Sep. Purif. Technol. 2018, 191, 61–74. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, R.-Y.; Yu, J.-X.; Wang, H.-S.; Guo, Q.-Y.; Liu, K.-Q.; Chen, H.-D.; Chi, R.-A. Sequential recovery of Cu(II), Cr(III), and Zn(II) from electroplating sludge leaching solution by an on-line biosorption method with dosage controlling. J. Clean. Prod. 2022, 337, 130427. [Google Scholar] [CrossRef]
- Wong, P.; Lam, K.; So, C. Removal and recovery of Cu(II) from industrial effluent by immobilized cells of Pseudomonas putida II-11. Appl. Microbiol. Biotechnol. 1993, 39, 127–131. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Chen, Y.; Qian, H. Extraction and recycling utilization of metal ions (Cu2+, Co2+ and Ni2+) with magnetic polymer beads. Chem. Eng. J. 2012, 200, 104–112. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Xue, X.; Hanna, K.; Abdelmoula, M.; Deng, N. Adsorption and oxidation of PCP on the surface of magnetite: Kinetic experiments and spectroscopic investigations. Appl. Catal. B Environ. 2009, 89, 432–440. [Google Scholar] [CrossRef]
- Hadi, P.; To, M.-H.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef]
- Flyunt, R.; Leitzke, A.; Mark, G.; Mvula, E.; Reisz, E.; Schick, R.; von Sonntag, C. Determination of •OH, O2•−, and hydroperoxide yields in ozone reactions in aqueous solution. J. Phys. Chem. B 2003, 107, 7242–7253. [Google Scholar] [CrossRef]
- Horváth, K.; Vajda, P.; Felinger, A. Multilayer adsorption in liquid chromatography–The surface heterogeneity below an adsorbed multilayer. J. Chromatogr. A 2017, 1505, 50–55. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Heiba, H.F.; Taha, A.A.; Mostafa, A.R.; Mohamed, L.A.; Fahmy, M.A. Preparation and characterization of novel mesoporous chitin blended MoO3-montmorillonite nanocomposite for Cu(II) and Pb(II) immobilization. Int. J. Biol. Macromol. 2020, 152, 554–566. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Heavy metal adsorption using biogenic iron compounds. Hydrometallurgy 2018, 179, 44–51. [Google Scholar] [CrossRef]
- Verma, A.; Singh, A.; Bishnoi, N.R.; Gupta, A. Biosorption of Cu (II) using free and immobilized biomass of Penicillium citrinum. Ecol. Eng. 2013, 61, 486–490. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Erratum to: Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono-and multi-metal systems. Environ. Earth Sci. 2015, 73, 5445. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Chitosan/CaCO3-silane nanocomposites: Synthesis, characterization, in vitro bioactivity and Cu(II) adsorption properties. Int. J. Biol. Macromol. 2018, 114, 149–160. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.-H.; Kim, J.-O.; Cho, D.-W.; Kumar, R.; Baek, S.H.; Kurade, M.B.; Jeon, B.-H. Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 2016, 160, 126–133. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Y.; Liu, X.; Guan, Y.; Chen, L.; Lian, B. Adsorption of Ni2+ and Cu2+ using bio-mineral: Adsorption isotherms and mechanisms. Geomicrobiol. J. 2018, 35, 742–748. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Fang, Y. Using graphene oxide as a superior adsorbent for the highly efficient immobilization of Cu(II) from aqueous solution. J. Mol. Liq. 2014, 199, 237–243. [Google Scholar] [CrossRef]
- Dev, V.V.; Baburaj, G.; Antony, S.; Arun, V.; Krishnan, K.A. Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J. Clean. Prod. 2020, 255, 120309. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, Y.; Zeng, G.; Xu, W.; Yang, C.; Zhang, J. Biosorption of copper(II) by immobilizing Saccharomyces cerevisiae on the surface of chitosan-coated magnetic nanoparticles from aqueous solution. J. Hazard. Mater. 2010, 177, 676–682. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.; Tang, M.; He, Q.; Long, A.; Luo, S.; Sun, S.; Wan, J.; Gao, Y.; Zhou, L. Physically-crosslinked activated CaCO3/polyaniline-polypyrrole-modified GO/alginate hydrogel sorbent with highly efficient removal of copper(II) from aqueous solution. Chem. Eng. J. 2022, 431, 133375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).