Release Behavior of Pb(II) Ions on the Galena Surface: Dissolution Experiment, DFT Calculation, and MD Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Dissolution Experiments
2.2. Computational Details
3. Results
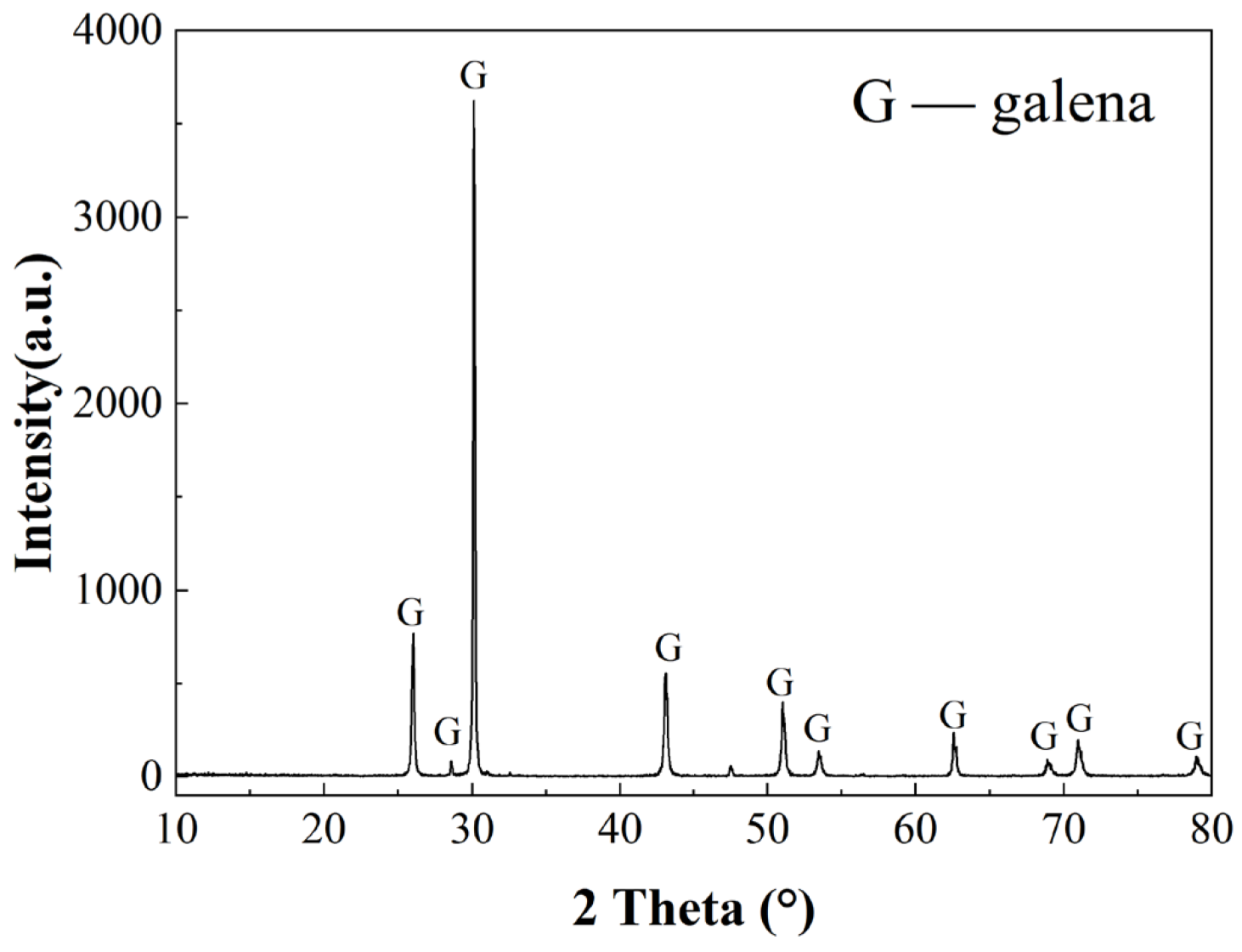
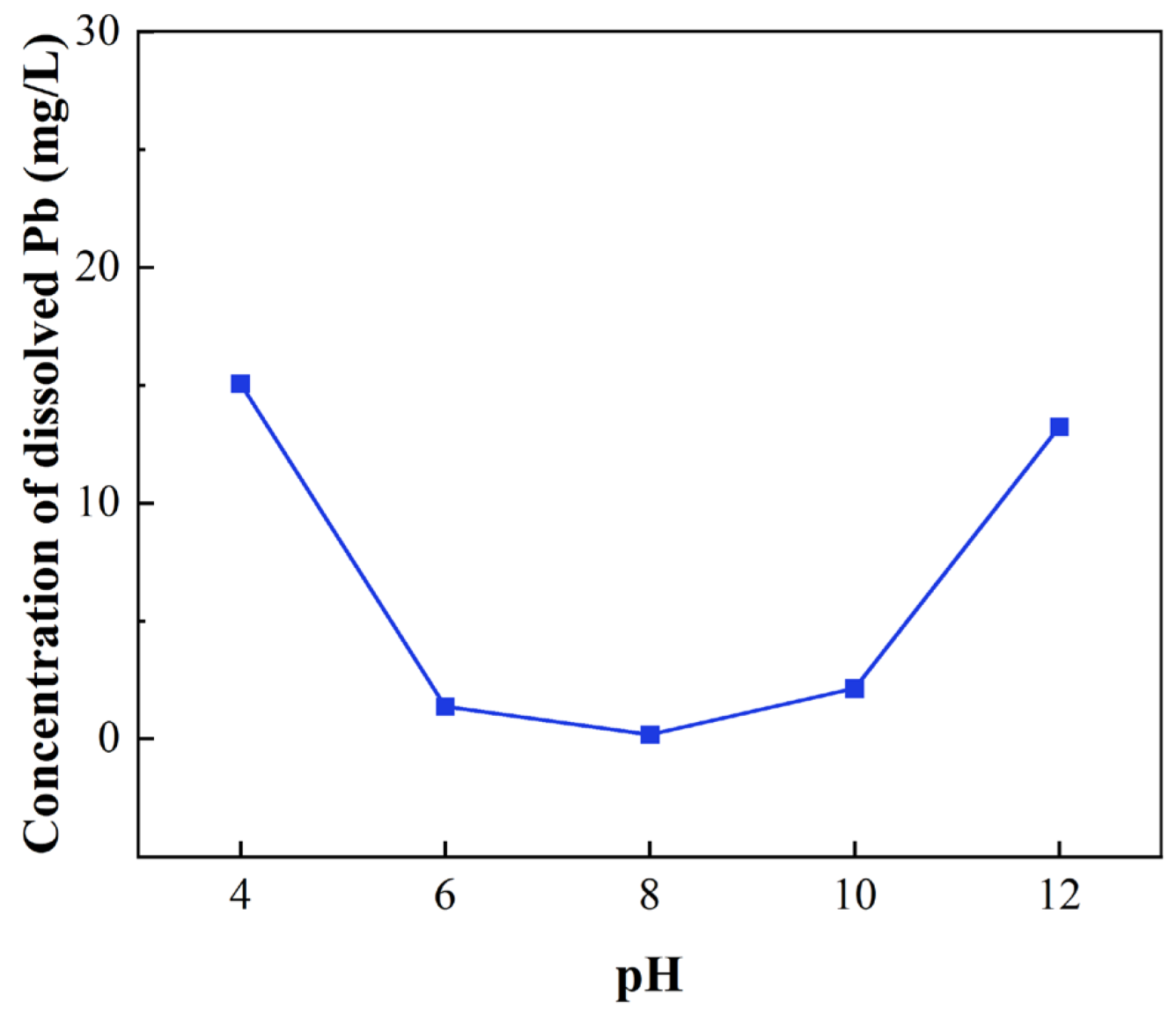
3.1. Pure Mineral Dissolution Experiments
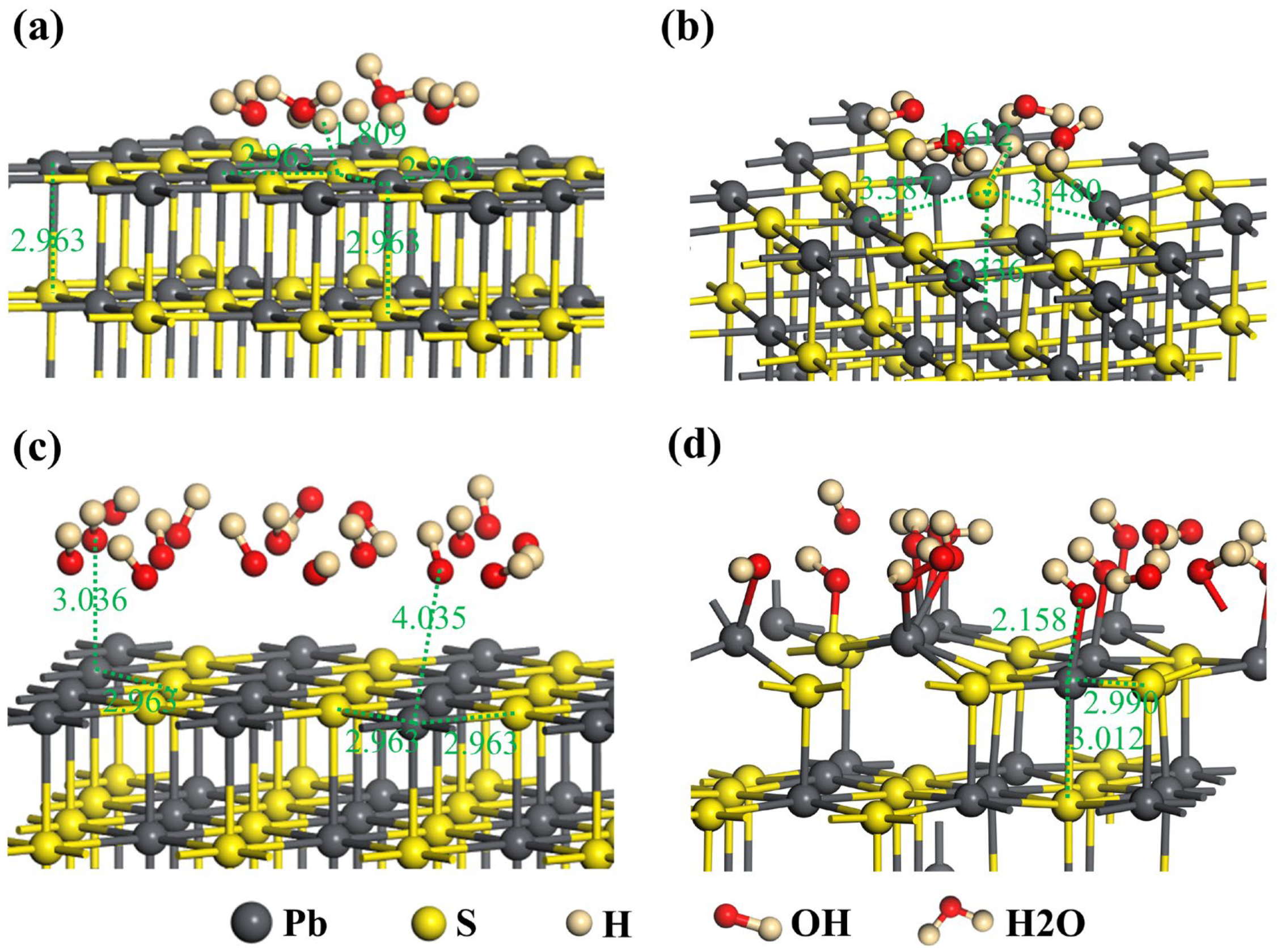
3.2. DFT Calculation
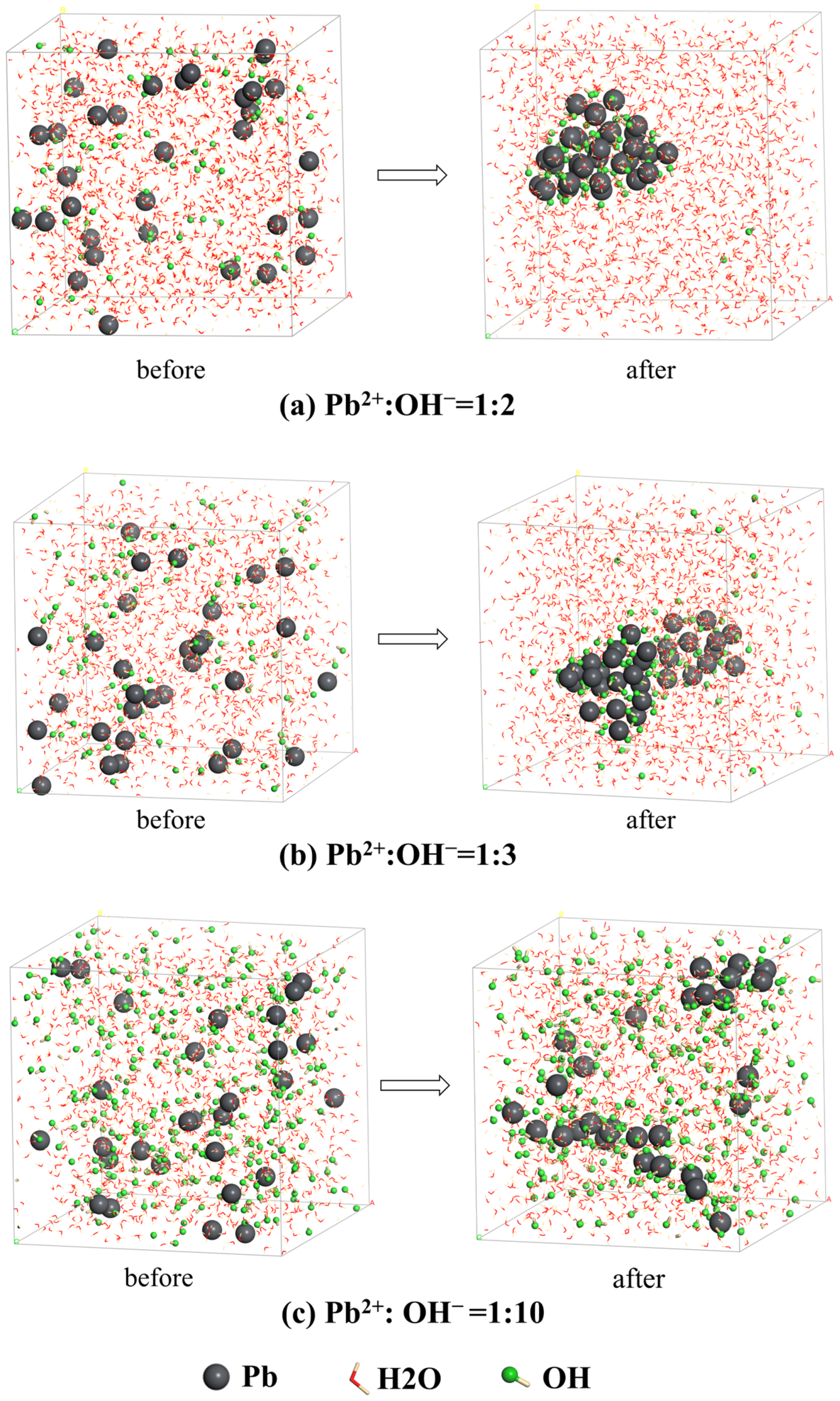
3.3. MD Simulation
3.4. SEM Analysis of Minerals
4. Conclusions
- (1)
- Dissolution experiments showed that the release of Pb2+ from the galena surface is closely related to the pH of the extract solutions, and acidic or highly alkaline environments are conducive to the dissolution of Pb(II) ions.
- (2)
- The results of DFT calculations indicated that Pb atoms on the surface of galena have a tendency to release into the liquid phase under both acidic or alkaline conditions but are more easily released in an acidic solution than the alkaline solution.
- (3)
- The MD simulations suggested that Pb ions form hydroxide aggregates with a certain concentration of OH− under alkaline conditions, while excessive OH− could lead to the dispersion and dissolution of hydroxide aggregates. That is to say, the formation of lead hydroxyl complexes does not always result in the formation of precipitates. Only when hydroxyl complex clusters are formed, and the particle size of the clusters reaches a certain value can precipitation be formed. If the content of hydroxide ions in the solution is too high, it may lead to the re-dispersion of lead hydroxide complex clusters, resulting in the precipitation and dissolution of hydroxides. The ratio of cations to anions in clusters is not a constant value but varies within a range, which is significantly different from the ratio described by stoichiometry in traditional solution chemistry.
- (4)
- The surface morphological observation by SEM can well support the calculation and simulation results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortada, U.; Hidalgo, M.C.; Martínez, J.; Rey, J. Impact in soils caused by metal(loid)s in lead metallurgy. The case of La Cruz Smelter (Southern Spain). J. Geochem. Explor. 2018, 190, 302–313. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Meng, X.; Ou, P.; Lv, X.; Zhang, L.; Liu, L.; Chen, F.; Qiu, G. Mineralogical phase transformation of Fe containing sphalerite at acidic environments in the presence of Cu2+. J. Hazard. Mater. 2021, 403, 124058. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Long, X.; Li, Y. DFT study of coadsorption of water and oxygen on galena (PbS) surface: An insight into the oxidation mechanism of galena. Appl. Surf. Sci. 2017, 420, 714–719. [Google Scholar] [CrossRef]
- Zárate-Gutiérrez, R.; Lapidus, G.T.; Morales, R.D. Aqueous oxidation of galena and pyrite with nitric acid at moderate temperatures. Hydrometallurgy 2012, 115–116, 57–63. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Gong, B.; Wu, P.; Huang, Z.; Li, Y.; Yang, S.; Dang, Z.; Ruan, B.; Kang, C. Efficient inhibition of heavy metal release from mine tailings against acid rain exposure by triethylenetetramine intercalated montmorillonite (TETA-Mt). J. Hazard. Mater. 2016, 318, 396–406. [Google Scholar] [CrossRef]
- Jin, G.; Wang, L.; Zheng, K.; Li, H.; Liu, Q. Influence of pH, Pb2+, and temperature on the electrochemical dissolution of galena: Environmental implications. Ionics 2015, 22, 975–984. [Google Scholar] [CrossRef]
- Rebello, S.; Anoopkumar, A.N.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Kim, S.H.; Pandey, A. Hazardous minerals mining: Challenges and solutions. J. Hazard. Mater. 2021, 402, 123474. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Huang, C.P. The dissolution of PbS(s) in dilute aqueous solutions. J. Colloid Interface Sci. 1989, 131, 537–549. [Google Scholar] [CrossRef]
- Bao, Z.; Al, T.; Couillard, M.; Poirier, G.; Bain, J.; Shrimpton, H.K.; Finfrock, Y.Z.; Lanzirotti, A.; Paktunc, D.; Saurette, E.; et al. A cross scale investigation of galena oxidation and controls on mobilization of lead in mine waste rock. J. Hazard. Mater. 2021, 412, 125130. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Liu, J.; Aruguete, D.M.; Jinschek, J.R.; Donald Rimstidt, J.; Hochella, M.F. The non-oxidative dissolution of galena nanocrystals: Insights into mineral dissolution rates as a function of grain size, shape, and aggregation state. Geochim. Cosmochim. Acta 2008, 72, 5984–5996. [Google Scholar] [CrossRef]
- Baba, A.A.; Adekola, F.A. A study of dissolution kinetics of a Nigerian galena ore in hydrochloric acid. J. Saudi Chem. Soc. 2012, 16, 377–386. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Jin, G.; Zheng, K.; Wang, L. Assessing the influence of humic acids on the weathering of galena and its environmental implications. Ecotoxicol. Environ. Saf. 2018, 158, 230–238. [Google Scholar] [CrossRef]
- Giudici, G.D.; Ricci, P.; Lattanzi, P.; Anedda, A. Dissolution of the (001) surface of galena: An in situ assessment of surface speciation by fluid-cell micro-Raman spectroscopy. Am. Mineral. 2007, 92, 518–524. [Google Scholar] [CrossRef]
- Lu, Y. Effect of particle size on the oxidation and flotation behavior of galena particles. Physicochem. Probl. Miner. Process. 2019, 55, 208–216. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Duan, H.; Wang, B.; Han, C.; Wei, D. The adsorption mechanism of calcium ion on quartz (101) surface: A DFT study. Powder Technol. 2018, 329, 158–166. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Chen, X.; Bai, S.; Liu, D.; Cao, Q. DFT computation of Cu adsorption on the S atoms of sphalerite (110) surface. Miner. Eng. 2013, 46–47, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Xia, S.; Yu, L. Adsorption of Pb(II) on the kaolinite(001) surface in aqueous system: A DFT approach. Appl. Surf. Sci. 2015, 339, 28–35. [Google Scholar] [CrossRef]
- Chen, J.; Ke, B.; Lan, L.; Li, Y. DFT and experimental studies of oxygen adsorption on galena surface bearing Ag, Mn, Bi and Cu impurities. Miner. Eng. 2015, 71, 170–179. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, T.; Ren, S.; Qiu, X. Research on flotation mechanism of wolframite activated by Pb(II) in neutral solution. Appl. Surf. Sci. 2020, 530, 147036. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, K.; Liu, Q.; Wang, L.; Feng, X.; Li, H. Galena weathering in simulated alkaline soil: Lead transformation and environmental implications. Sci. Total Environ. 2021, 755 Pt 2, 142708. [Google Scholar] [CrossRef]
- Güler, T. Redox behavior of galena in alkaline condition. Ionics 2017, 24, 221–227. [Google Scholar] [CrossRef]
- Zha, L.; Li, H.; Wang, N. Electrochemical Study of Galena Weathering in NaCl Solution: Kinetics and Environmental Implications. Minerals 2020, 10, 416. [Google Scholar] [CrossRef]
- Gabr, A.A.; Ali, M.A.; Orabi, A.H.; Osman, H.M.; Elyan, S.S. A novel method has been developed to efficiently recover valuable lead, zinc, and rare earth elements from hazardous waste generated by glass polishing. Arab. J. Basic Appl. Sci. 2023, 30, 513–525. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, D.; Li, F.; Ralston, J. Oxidation of Galena: II. Electrokinetic Study. J. Colloid Interface Sci. 1994, 164, 345–354. [Google Scholar] [CrossRef]


| Geometric Optimization Parameters | |
| Module | CASTEP |
| Functional | GGA-PW91 |
| Energy Cutoff | 350 eV |
| Pseudopotentials K-point set | Ultrasoft 3 × 3 × 1 |
| Convergence tolerance | |
| Energy | 1.0 × 10−5 eV/atom |
| Max. force | 0.03 eV/Å |
| Max. displacement | 0.001 Å |
| Max. stress | 0.05 GPa |
| Dynamics | System Models |
|---|---|
| Module | Forcite |
| Forcefield | COMPASS |
| Ensemble | NPT |
| Thermostat | Nose |
| Barostat | Berendsen |
| Temperature | 298 K |
| Pressure | 1.0 × 10−5 Gpa |
| Dynamics | System models |
| Module | Forcite |
| Time step | 1.0 Fs |
| Number of steps | 3.0 × 106 |
| Total time | 3000 ps |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Xu, S.; Huang, H.; Ren, S. Release Behavior of Pb(II) Ions on the Galena Surface: Dissolution Experiment, DFT Calculation, and MD Simulation. Minerals 2024, 14, 1075. https://doi.org/10.3390/min14111075
Xiao M, Xu S, Huang H, Ren S. Release Behavior of Pb(II) Ions on the Galena Surface: Dissolution Experiment, DFT Calculation, and MD Simulation. Minerals. 2024; 14(11):1075. https://doi.org/10.3390/min14111075
Chicago/Turabian StyleXiao, Minsi, Shitong Xu, Haiwei Huang, and Sili Ren. 2024. "Release Behavior of Pb(II) Ions on the Galena Surface: Dissolution Experiment, DFT Calculation, and MD Simulation" Minerals 14, no. 11: 1075. https://doi.org/10.3390/min14111075
APA StyleXiao, M., Xu, S., Huang, H., & Ren, S. (2024). Release Behavior of Pb(II) Ions on the Galena Surface: Dissolution Experiment, DFT Calculation, and MD Simulation. Minerals, 14(11), 1075. https://doi.org/10.3390/min14111075






