Fluid Evolution of Greisens from Krupka Sn-W Ore District, Bohemian Massif (Czech Republic)
Abstract
1. Introduction
2. Geological Settings
2.1. The Krušné Hory/Erzgebirge Crystalline Complex
2.2. Krušné Hory/Erzgebirge Batholith
2.3. Geological Situation of the Krupka Ore District
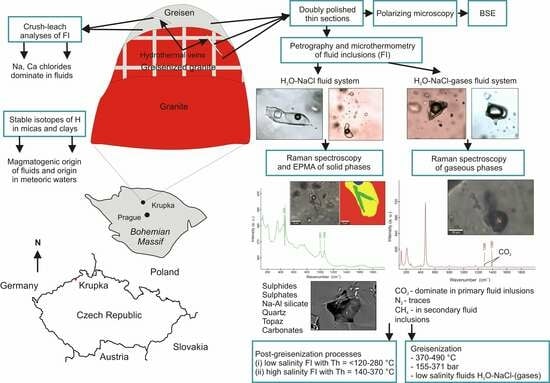
3. Materials and Methods
4. Results
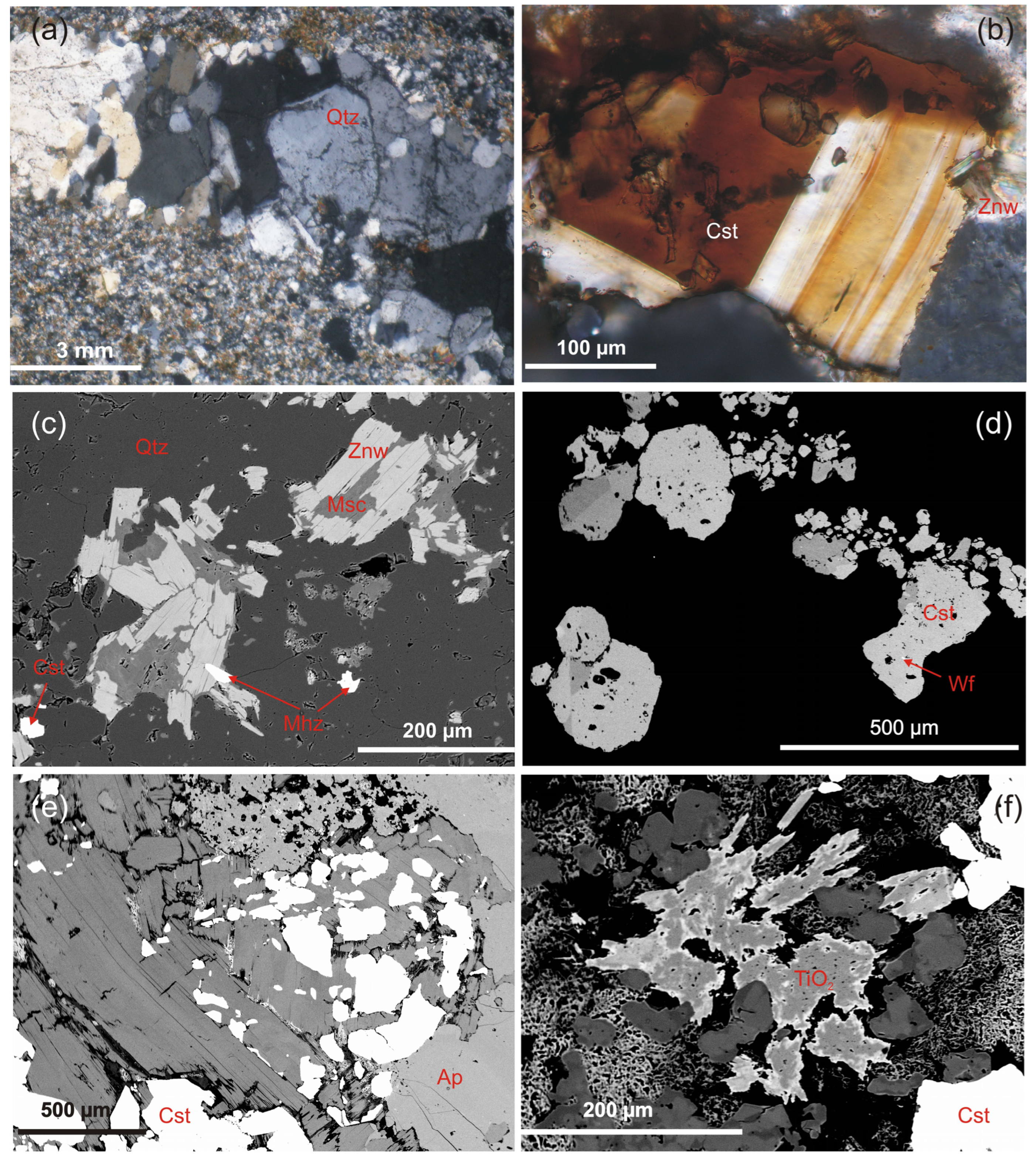
4.1. Sample Types and Mineralogy
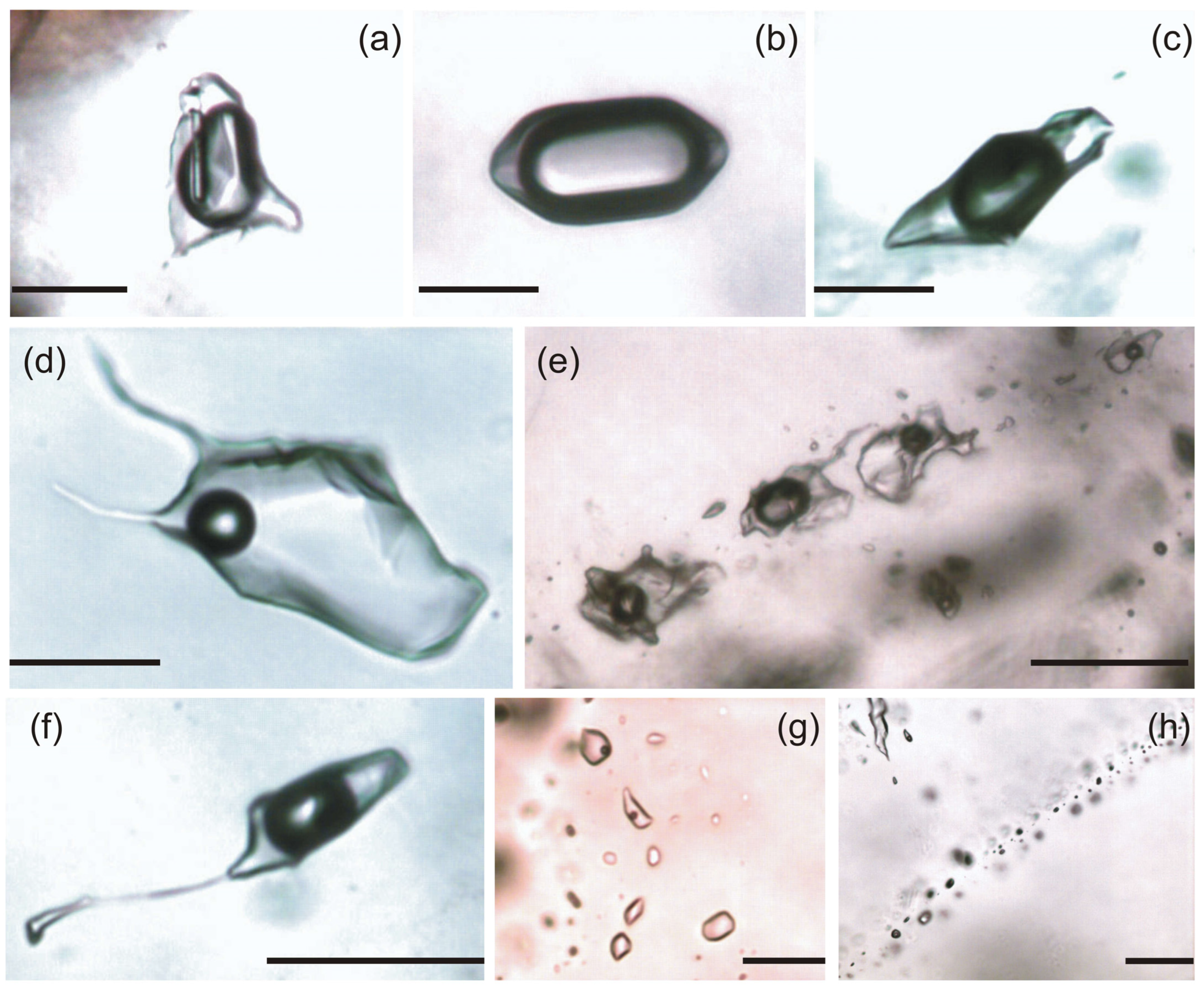
4.2. Petrography of Fluid Inclusions
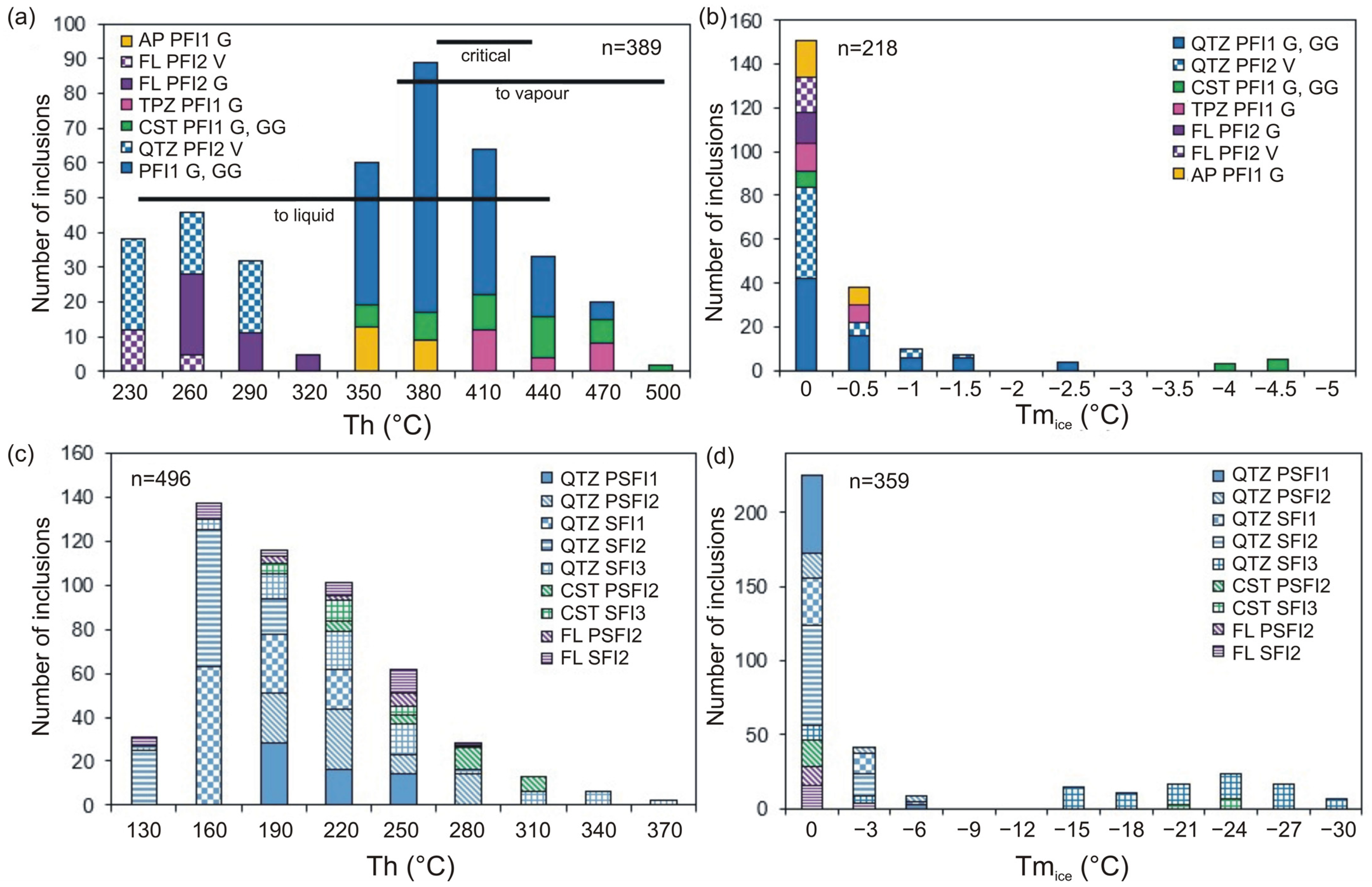
4.3. Microthermometry of Fluid Inclusions
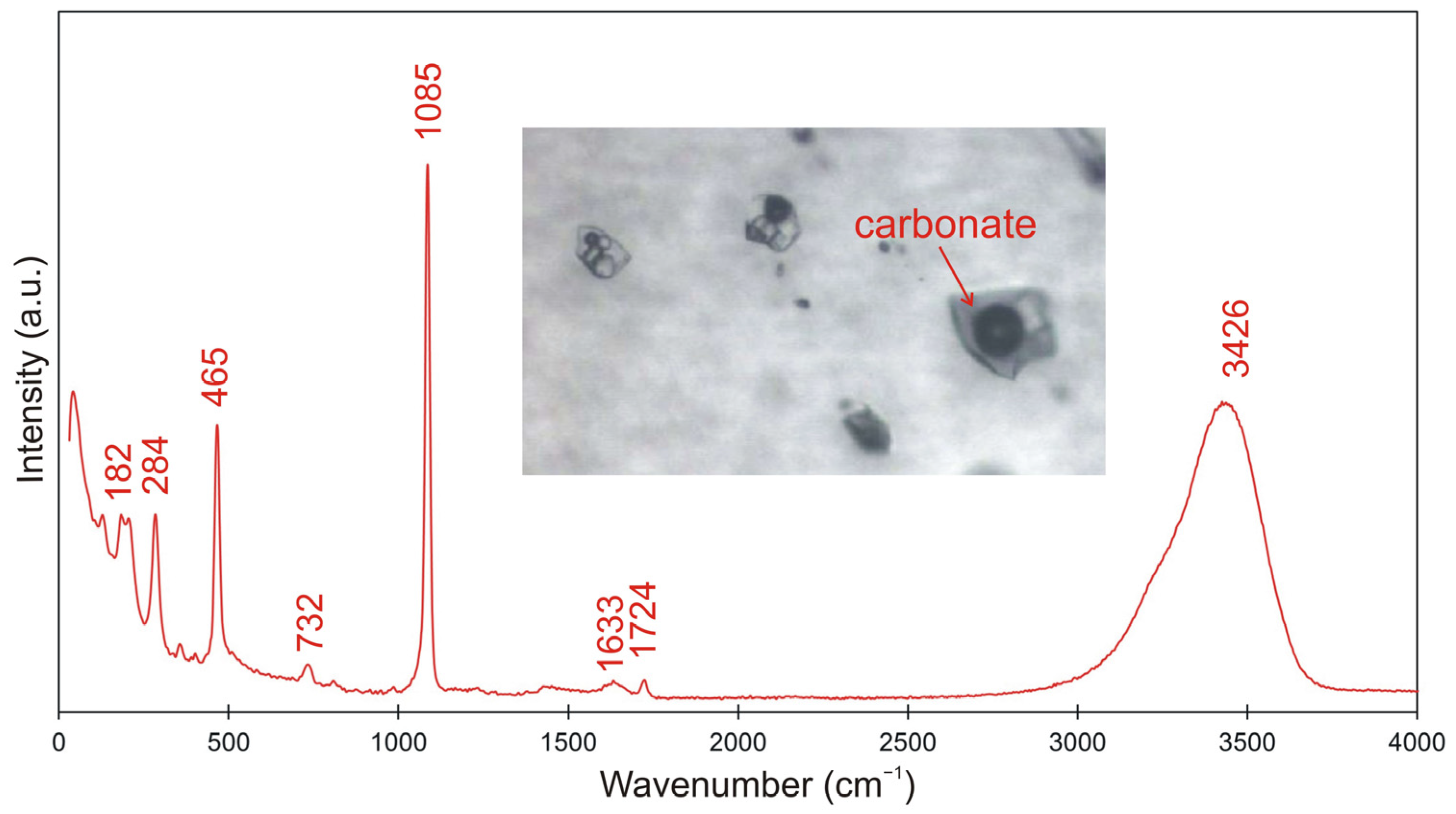
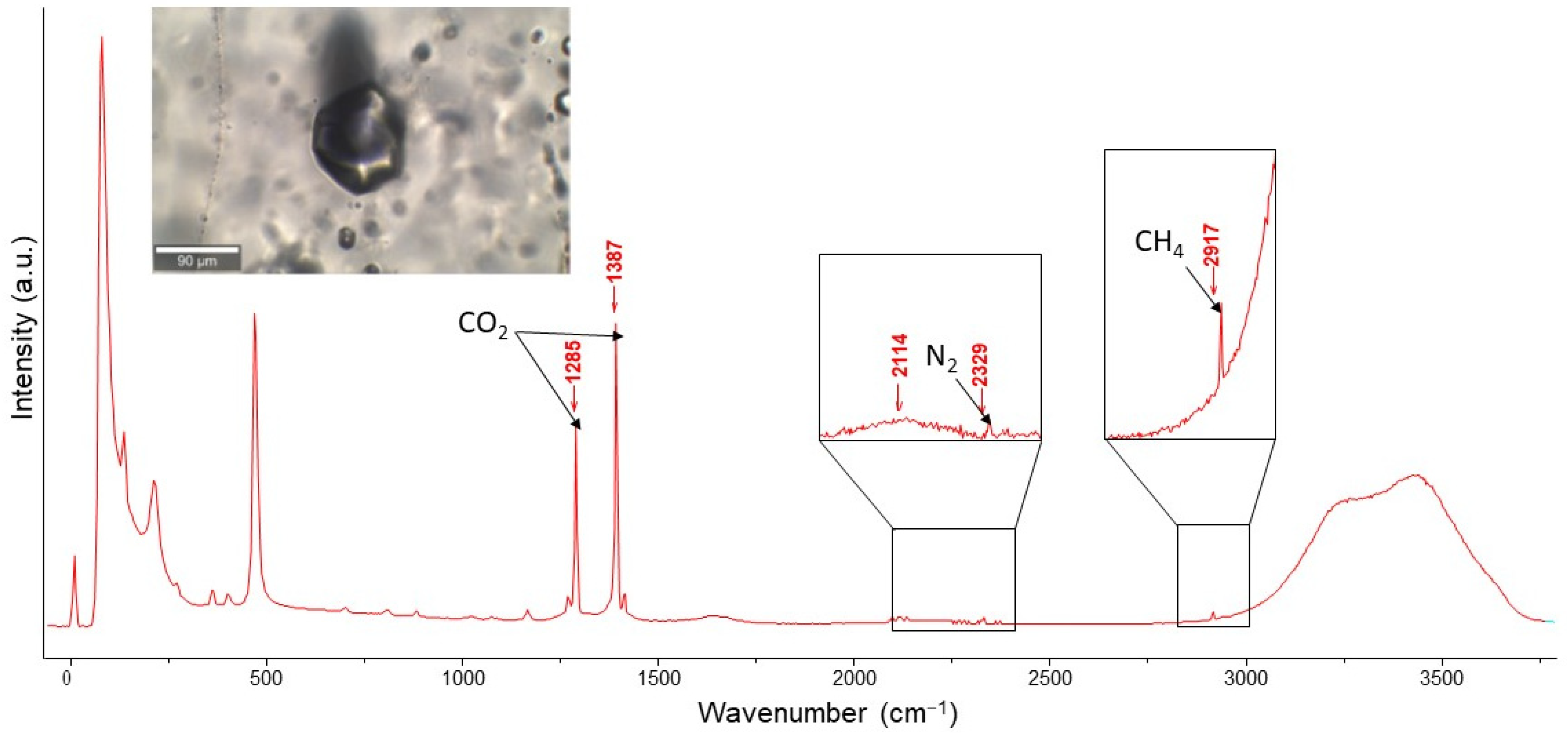
4.4. Composition of Vapor Phase
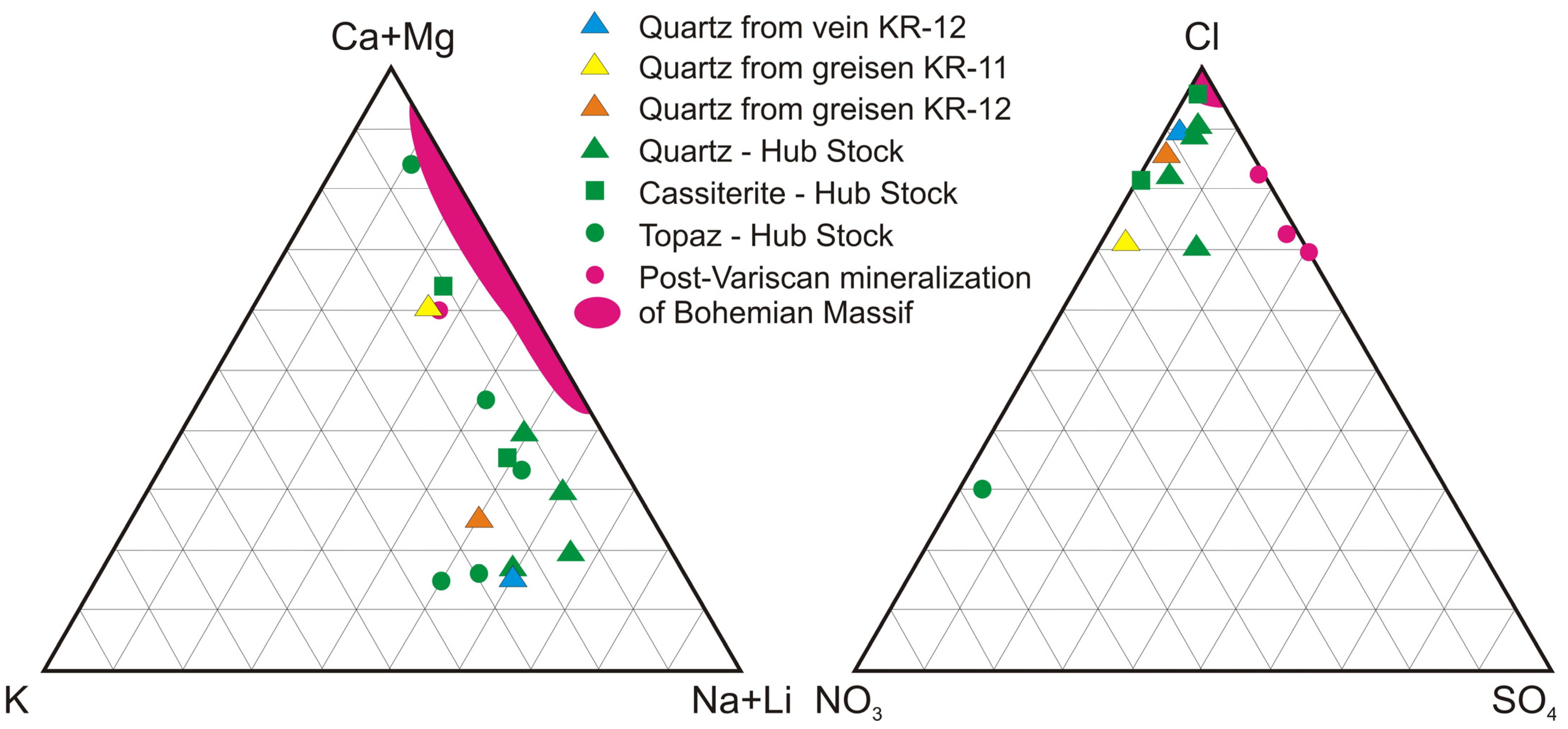
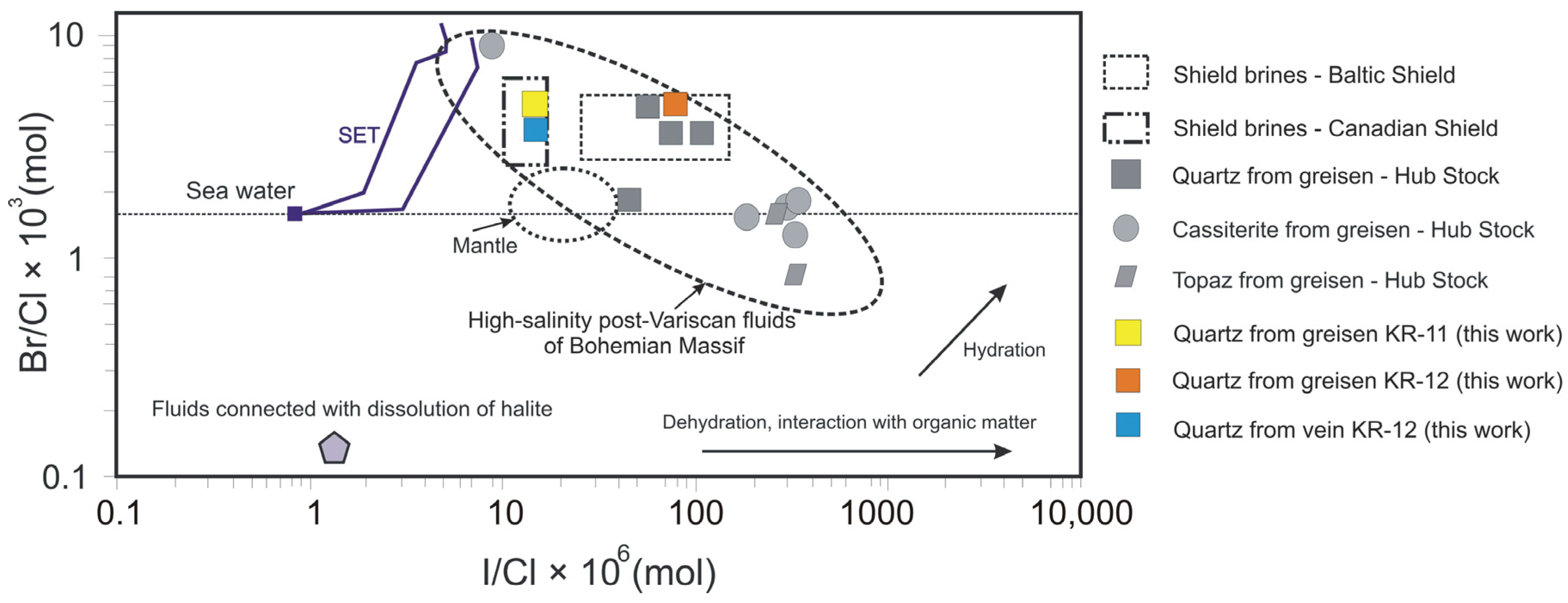
4.5. Crush-Leach Analyses of Fluid Inclusions
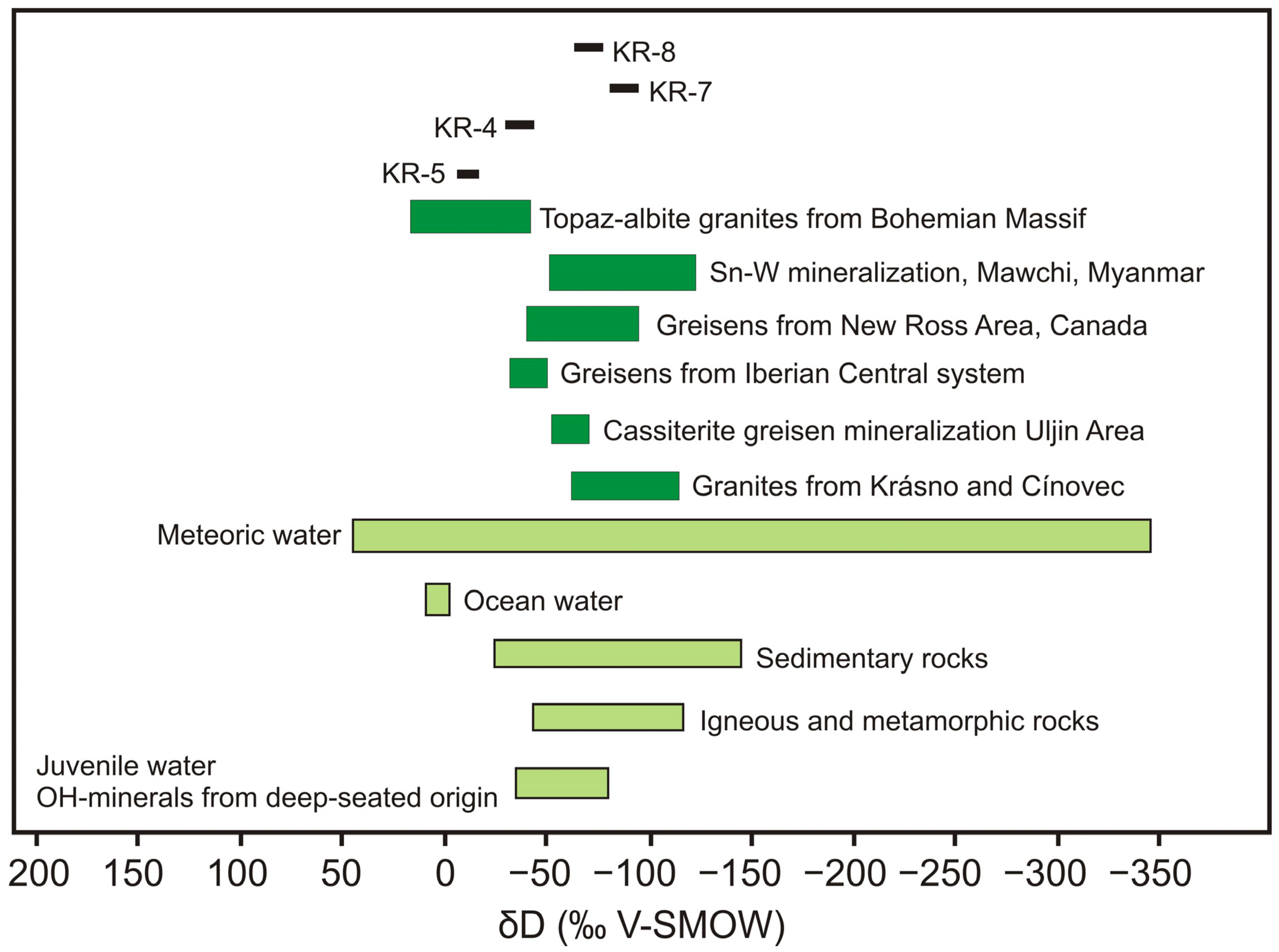
4.6. Hydrogen Isotopes
5. Discussion
5.1. Chemical Composition of Fluids
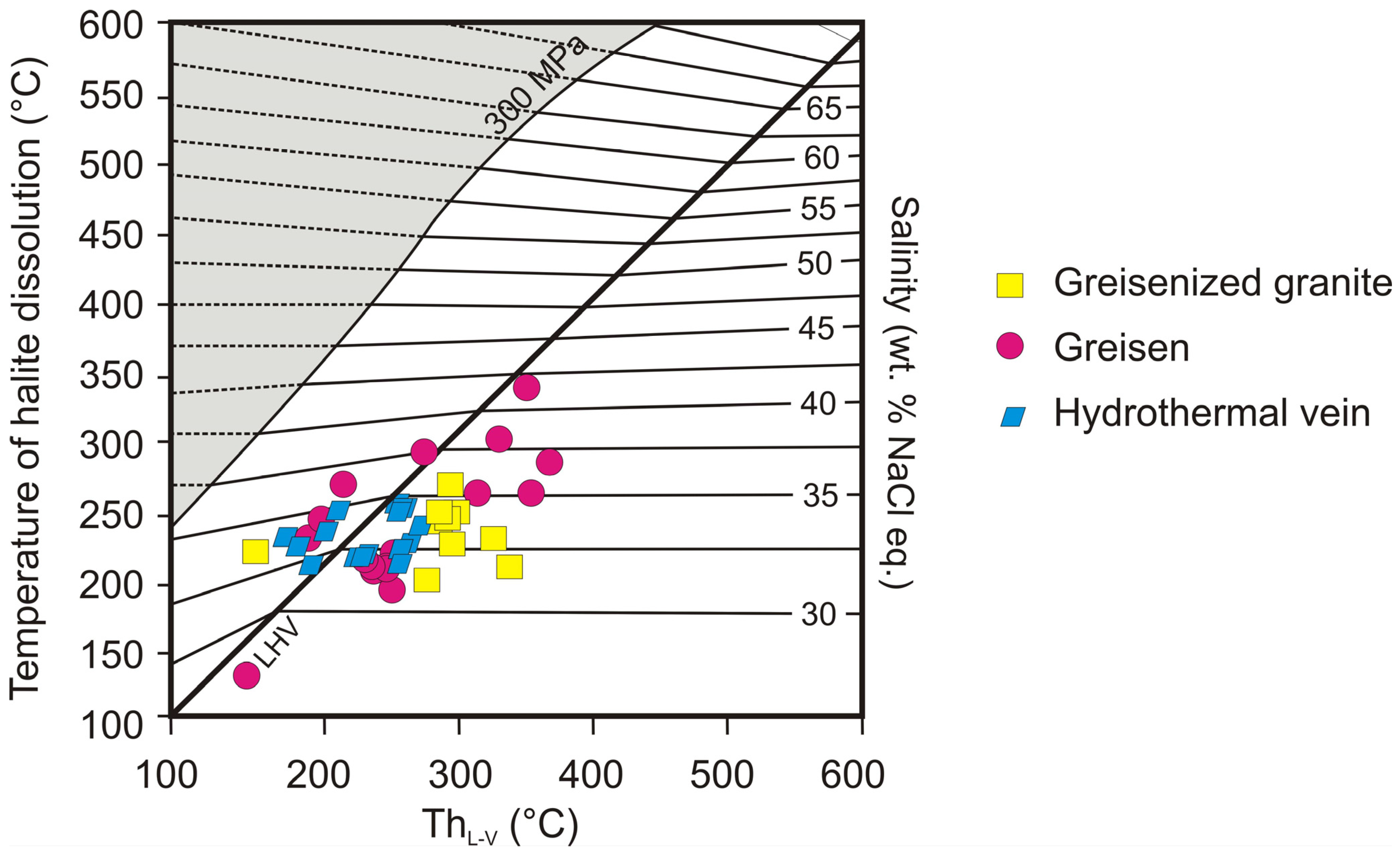
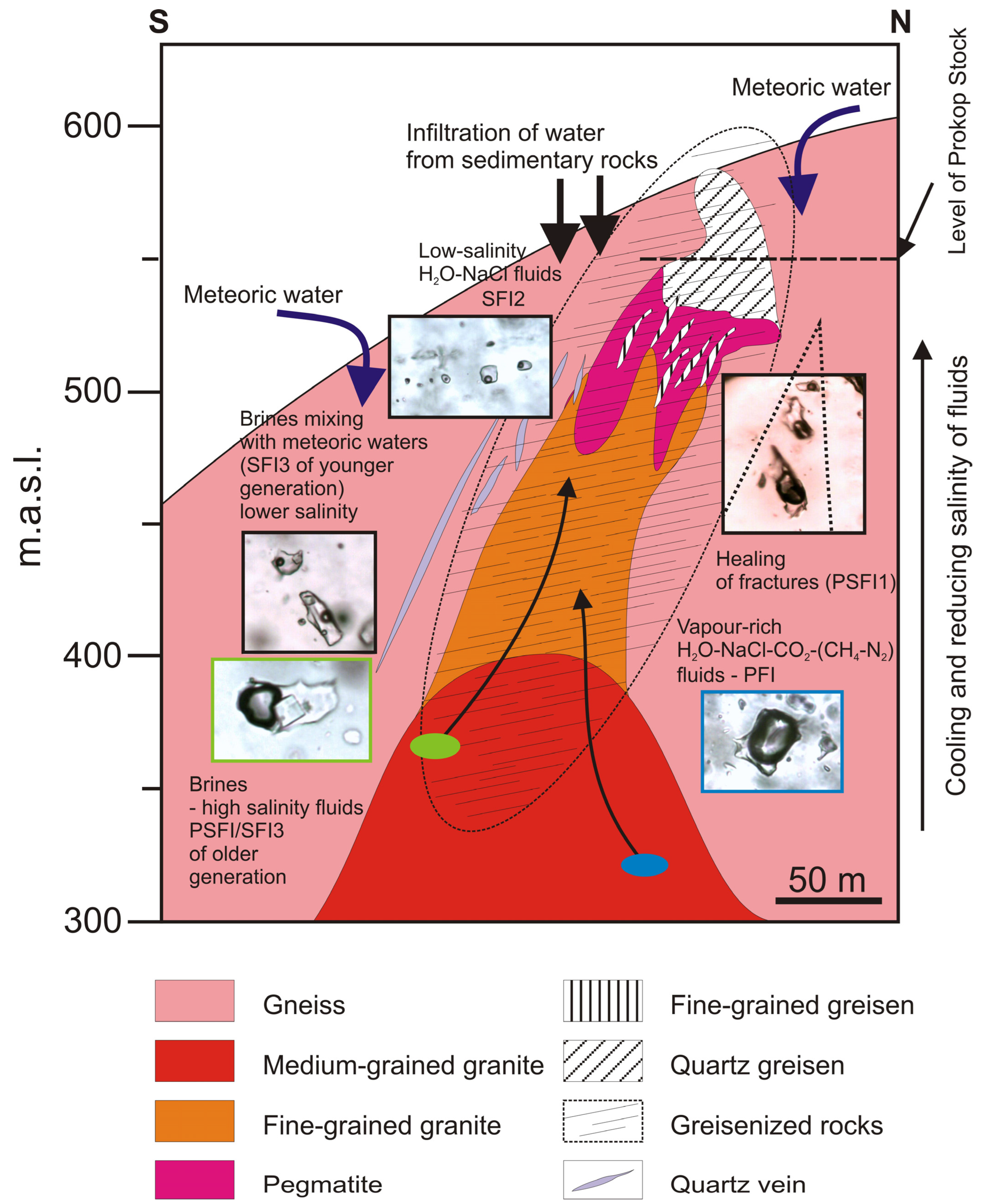
5.2. P-T Conditions of Greisenization and Post-Greisenization Processes
5.3. Origin of Fluids
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, H.; Tischendorf, G.; Palchen, W.; Klemm, I.; Ossenkopf, W. Zur Petrographie und Geochemie der Granite des Erzgebirges. Geologie 1972, 21, 457–494. [Google Scholar]
- Teuscher, E.O. Primäre Bildungen des granitischen Magmas und seine Restlösungen im Massif von Eibenstock-Neudeck. Miner. Petr. Mitt. 1936, 19, 211–266. [Google Scholar]
- Beus, A.A.; Zalashkova, N.E. High-temperature postmagmatic metasomatic processes in grantioids. Izv. Akad. Nauk. SSSR Ser. Geol. 1962, 4, 13–31. [Google Scholar]
- Štemprok, M. Petrology and the vertical extent of mineralization in the Cínovec (Zinnwald) granite cupola. Sbor. Geol. Věd Lož. Geol. Miner. 1965, 5, 7–106. [Google Scholar]
- Tischendorf, G.; Förster, H.J. Hercynian granite magmatism and related metallogenesis in the Erzgebirge. Monogr. Ser. Miner. Depos. 1994, 31, 5–23. [Google Scholar]
- Kovalenko, V. Petrology and Geochemistry of Rare Metal Granites; Nauka: Novosibirsk, Russia, 1977; p. 206. [Google Scholar]
- Černý, P.; Blevin, P.L.; Cuney, M.; London, D. Granite-Related Ore Deposits. Econ. Geol. 2006, 107, 337–370. [Google Scholar] [CrossRef]
- Jarchovský, T.; Pavlů, D. Albite-topaz microgranite from Horní Slavkov (Slavkovský les Mts.) NW Bohemia. Věst. Ústř. Úst. Geol. 1991, 66, 13–22. [Google Scholar] [CrossRef][Green Version]
- Eisenreich, M.; Breiter, K. Krupka Sn-W-Mo deposits in Eastern Krušné hory Mts. Věst. Čes. Geol. Úst. 1993, 63, 3. [Google Scholar]
- Breiter, K. From explosive breccia to unidirectional solidification textures: Magmatic evolution of a phosphorus- and fluorine-rich granite system (Podlesí, Krušné hory Mts., Czech Republic). Bull. Geosci. 2002, 77, 67–92. [Google Scholar]
- René, M.; Škoda, R. Nb-Ta-Ti oxides fractionation in rare-metal granites: Krásno-Horní Slavkov ore district, Czech Republic. Miner. Petrol. 2011, 103, 37–48. [Google Scholar] [CrossRef]
- Thomas, R. Estimation of the viscosity and the water content of silicate melts from melt inclusion data. Eur. J. Miner. 1994, 6, 511–535. [Google Scholar] [CrossRef]
- Jarchovský, T. The nature and genesis of greisen stock at Krásno, Slavkovský les area—Western Bohemia, Czech Republic. J. Czech Geol. Soc. 2006, 51, 201–216. [Google Scholar] [CrossRef][Green Version]
- Dolejš, D.; Štemprok, M. Magmatic and hydrothermal evolution of Li-F granites: Cínovec and Krásno intrusions, Krušné hory batholith, Czech Republic. Bull. Geosci. 2001, 76, 77–79. [Google Scholar]
- Ďurišová, J.; Charoy, B.; Weisbrod, A. Fluid inclusion studies in minerals from tin and tungsten deposits in the Krušné Hory Mountains (Czechoslovakia). Bull. Minéral. 1979, 102, 665–675. [Google Scholar] [CrossRef]
- Ďurišová, J. Formation conditions of greisen parageneses of the western Erzgebirge Mountains. Věst. Ústř. Úst. Geol. 1984, 59, 141–152. (In Czech) [Google Scholar]
- Dolníček, Z.; René, M.; Prochaska, W.; Kovář, M. Fluid evolution of the Hub Stock, Horní Slavkov–Krásno Sn–W ore district, Bohemian Massif, Czech Republic. Miner. Depos. 2012, 47, 821–833. [Google Scholar] [CrossRef]
- Štemprok, M.; Pivec, E.; Langrová, A. The petrogenesis of a wolframite-bearing greisen in the Vykmanov granite stock, Western Krušné hory pluton (Czech Republic). Bull. Geosci. 2005, 80, 165–184. [Google Scholar]
- Matte, P.; Maluski, H.; Rajlich, P.; Franke, W. Terrane boundaries in the Bohemian Massif: Result of large-scale Variscan shearing. Tectonophysics 1990, 177, 151–170. [Google Scholar] [CrossRef]
- Klomínský, J.; Jarchovský, T.; Rajpot, G.S. Atlas of Plutonic Rocks and Orthogneisses in the Bohemian Massif, Saxothuringicum; Czech Geological Survey: Prague, Czech Republic, 2010; p. 94.
- Sejkora, J.; Breiter, K. Historical Krupka ore district, Erzgebirge Mountains. Bull. Miner. Petrol. Odd. Nár. Muz. 1999, 7, 29. (In Czech) [Google Scholar]
- Janečka, J.; Malásek, F.; Štemprok, M.; Tischendorf, G.; Zoubek, V. Metallogeny of Tin and Tungsten in the Krušné Hory—Erzgebirge; International Geological Correlation Programme—Excursion Guide; Ústřední Ústav Geologický: Prague, Czech Republic, 1974. [Google Scholar]
- Schumacher, F. Die Erzgebirgishe Metallprovinz und ihre Genesis. Erzmetall 1933, 30, 161–166. [Google Scholar]
- Watznauer, A. Die erzgebirgischen Granitintrusionen. Geologie 1954, 6–7, 688–706. [Google Scholar]
- Breiter, K.; Förster, H.-J.; Seltmann, R. Variscan silicic magmatism and related tin-tungsten mineralization in the Erzgebirge-Slavkovský les metallogenic province. Miner. Depos. 1999, 34, 505–521. [Google Scholar] [CrossRef]
- Forster, H.-J.; Tischendorf, G.; Trumbull, R.B.; Gottesmann, B. Late-Collisional Granites in the Variscan Erzgebirge, Germany. J. Pet. 1999, 40, 1613–1645. [Google Scholar] [CrossRef]
- Škvor, V. Geology of the Czech Part of the Erzgebirge Mountains and Smrčiny; Ústřední Ústav Geologický: Prague, Czech Republic, 1975; p. 48. (In Czech) [Google Scholar]
- Škvor, V. The Krušné Hory Mountains pluton and its interpretation. Věst. Ústř. Úst. Geol. 1986, 61, 65–71. (In Czech) [Google Scholar]
- Siebel, W.; Trzebski, R.; Stettner, G.; Hecht, L.; Casten, U.; Höhndorf, A.; Műller, P. Granitoid magmatism of the NW Bohemian massif revealed: Gravity data, composition, age relations and phase concept. Geol. Rund. 1997, 86, 545–563. [Google Scholar] [CrossRef]
- Romer, R.L.; Thomas, R.; Stein, H.J.; Rhede, D. Dating multiply overprinted Sn-mineralized granites—Examples from the Erzgebirge, Germany. Miner. Depos. 2007, 42, 337–359. [Google Scholar] [CrossRef]
- Tichomirova, M.; Leonhardt, D. New age determination (Pb/Pb zircon evaporation, Rb/Sr) on the granites from Aus-Schwarzenberg and Eibenstock, Western Erzgebirge, Germany. Z. Geol. Wiss. 2010, 38, 99–123. [Google Scholar]
- Štemprok, M.; Blecha, V. Variscan Sn–W–Mo metallogeny in the gravity picture of the Krušné hory/Erzgebirge granite batholith (Central Europe). Ore Geol. Rev. 2015, 69, 285–300. [Google Scholar] [CrossRef]
- Richter, P.; Stettner, G. Geochemische und petrographishe Untersuchungen der Fichtelgebirgsgranite. Geologica Bavar. 1979, 78, 144. [Google Scholar]
- Štemprok, M.; Šulcek, Z. Geochemical profile through an ore-bearing lithium granite. Econ. Geol. 1969, 64, 392–404. [Google Scholar] [CrossRef]
- Peterková, T.; Dolejš, D. Magmatic-hydrothermal evolution of highly evolved granite stock Knöttel near Krupka in Erzgebirge. Zpr. Geol. Výzk. 2017, 50, 189–194. [Google Scholar] [CrossRef]
- Peterková, T.; Dolejš, D. Magmatic-hydrothermal transition of Mo-W-mineralized granite-pegmatite-greisen system recorded by trace elements in quartz: Krupka district, Eastern Krušné hory/Erzgebirge. Chem. Geol. 2019, 523, 179–202. [Google Scholar] [CrossRef]
- Žák, L. Origin of the molybdenite and feldspar deposit of Krupka. II. Paragenetic relations. Acta Univ. Carol. Geol. 1966, 22, 167–195. [Google Scholar]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Weare, J.H. An equation of state for the CH4-CO2-H2O system: I. Pure systems from 0 to 1000 °C and 0 to 8000 bar. Geochim. Cosmochim. Acta 1992, 56, 2605–2617. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Weare, J.H. An equation of state for the CH4-CO2-H2O system: II. Mixtures from 50 to 1000 °C and 0 to 1000 bar. Geochim. Cosmochim. Acta 1992, 56, 2619–2631. [Google Scholar] [CrossRef]
- Bowers, T.S.; Helgeson, H.C. Calculation of the thermodynamic and geochemical consequences of nonideal mixing in the system H2O-CO2-NaCl fluids at high pressures and temperatures. Geochim. Cosmochim. Acta 1983, 47, 1247–1275. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Frantz, J.D. Determination of the homogenization temperatures and densities of supercritical fluids in the system NaCl–KCl–CaCl2–H2O using synthetic fluid inclusions. Chem. Geol. 1987, 64, 335–345. [Google Scholar] [CrossRef]
- Bakker, R.J. Adaptation of the Bowers and Helgeson (1983) equation of state to the H2O–CO2–CH4–N2–NaCl system. Chem. Geol. 1999, 154, 225–236. [Google Scholar] [CrossRef]
- Available online: https://fluids.unileoben.ac.at/Computer.html (accessed on 17 December 2023).
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; De Vivo, B., Frezzotii, M.L., Eds.; Short Course IMA: Pontignano-Siena, Italy, 1994; pp. 117–130. [Google Scholar]
- Knight, C.; Bodnar, R. Synthetic fluid inclusions: IX. Critical PVTX properties of NaCl-H2O solutions. Geochim. Cosmochim. Acta 1989, 53, 3–8. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Burke, E.A.J. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Hurai, V.; Huraiová, M.; Slobodník, M.; Thomas, R. Geofluids Development in Microthermometry, Spectroscopy, Thermodynamics, and Stable Isotopes, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Vennemann, T.V.; O´Neil, J.R. A simple and inexpensive method of hydrogen isotope and water analyses of minerals and rocks based on zinc reagent. Chem. Geol. 1993, 103, 227–234. [Google Scholar] [CrossRef]
- Suzuoki, T.; Epstein, S. Hydrogen isotope fractionation between OH-bearing minerals and water. Geochim. Cosmochim. Acta 1976, 40, 1229–1240. [Google Scholar] [CrossRef]
- Gilg, H.A.; Sheppard, S.M. Hydrogen isotope fractionation between kaolinite and water revisited. Geochim. Cosmochim. Acta 1996, 60, 529–533. [Google Scholar] [CrossRef]
- Lecumberri-Sanchez, P.; Steele-MacInnis, M.; Bodnar, R.J. A numerical model to estimate trapping conditions of fluid inclusions that homogenize by halite disappearance. Geochim. Cosmochim. Acta 2012, 92, 14–22. [Google Scholar] [CrossRef]
- Webster, J.; Thomas, R.; Förster, H.J.; Seltman, R.; Tappen, C. Geochemical evolution of halogen-enriched granite magmas and mineralizing fluids of the Zinnwald tin-tungsten mining district, Erzgebirge, Germany. Miner. Depos. 2004, 39, 452–472. [Google Scholar] [CrossRef]
- Zwart, E.W.; Touret, J.L.R. Melting behavior and composition of aqueous fluid inclusions on fluorite and calcite: Application within system H2O-CaCl2-NaCl. Eur. J. Miner. 1994, 6, 773–786. [Google Scholar] [CrossRef]
- Ulmanová, J.; Dolníček, Z. New occurrence of vein W-mineralization in Tanvald granite from Jablonec nad Nisou—Mineralogy, chemical compositions of minerals and fluid inclusions. Bull. Min. Petrolog. 2019, 27, 193–204. (In Czech) [Google Scholar]
- Nasdala, L.; Smith, D.C.; Kaindl, R.; Ziemann, M.A. Raman spectroscopy: Analytical perspectives in mineralogical research. EMU Notes Miner. 2004, 6, 20. [Google Scholar] [CrossRef]
- Tsuruoka, S. The Evolution of Hydrothermal Fluids from the Deep Porphyry Environment to the Shallow Epithermal Environment. Ph.D. Thesis, Colorado School of Mines, Faculty and the Board of Trustees, Golden, CO, USA, 2017. Volume 197. p. 17. [Google Scholar]
- Borisenko, A.S. Izučenije solevogo sostava rastvorov gazovožidkich vklučenij v mineralach metodom kriometrii. Geolog. Geofiz. 1977, 8, 16–27. [Google Scholar]
- Klemm, W. Chemical evolution of hydrothermal solutions during Variscan and post-Variscan mineralization in Erzgebirge, Germany. In Metallogeny of Collisional Orogens: Focused on the Erzgebirge and Comparable Metallogenic Settings; Seltmann, R., Kämpf, H., Möller, P., Eds.; Czech Geological Survey: Prague, Czech Republic, 1994; pp. 150–158. [Google Scholar]
- Charoy, B. Ploemeur kaolin deposit (Brittany), an example of hydrothermal alteration. Pétrologie 1975, 1, 253–266. [Google Scholar]
- Charoy, B. Definition of Importance des Phenomenes Deuteriques et des Fluids Associes dans les Granites. Consequences Metallogeniques; Sciences de la Terre: Paris, France, 1979; p. 364. [Google Scholar]
- Dolníček, Z.; Fojt, B.; Prochaska, W.; Kučera, J.; Sulovský, P. Origin of the Zálesí U–Ni–Co–As–Ag/Bi deposit, Bohemian Massif, Czech Republic: Fluid inclusion and stable isotope constraints. Miner. Depos. 2009, 44, 81–97. [Google Scholar] [CrossRef]
- Kotlánová, M.; Dolníček, Z. Origin and chemical composition of fluids of post-Variscan hydrothermal mineralization at locality Zlatý důl near Hlubočky (Lower Carboniferous of the Nízký Jeseník Upland). Geol. Výzk. Mor. Slez. 2016, 23, 74–80. (In Czech) [Google Scholar] [CrossRef][Green Version]
- Edmonds, M.; Woods, A.W. Exsolved volatiles in magma reservoirs. J. Volcanol. Geotherm. Res. 2018, 368, 13–30. [Google Scholar] [CrossRef]
- Thomas, R. Fluid evolution in relation to the emplacement of the Variscan granites in the Erzgebirge region: A review of the melt and fluid inclusion evidence. In Metallogeny of Collisional Orogens; Seltmann, R., Kämpf, H., Möller, P., Eds.; Czech Geological Survey: Prague, Czech Republic, 1994; pp. 70–81. [Google Scholar]
- Thomas, R.; Förster, H.J.; Appel, K.; Webster, J. Formation of extremely F-rich hydrous melt fractions and hydrothermal fluids during differentiation of highly evolved tin-granite magmas: A melt/fluid-inclusion study. Contrib. Miner. Pet. 2005, 148, 582–601. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Morishita, Y.; Nishio, Y. Ore Genesis of the Takatori Tungsten–Quartz Vein Deposit, Japan: Chemical and Isotopic Evidence. Minerals 2021, 11, 765. [Google Scholar] [CrossRef]
- Tornos, F.; Delgado, A.; Casquet, C.; Galindo, C. 300 Million years of episodic hydrothermal activity: Stable isotope evidence from hydrothermal rocks of the Eastern Iberian Central System. Miner. Depos. 2000, 35, 551–569. [Google Scholar] [CrossRef]
- Moon, S.H.; Park, H.-I.; Ripley, E.M.; Lee, I. Mineralogic and stable isotope studies of cassiterite greisen mineralization in the Uljin area, Korea. Econ. Geol. 1996, 91, 916–933. [Google Scholar] [CrossRef]
- Carruzzo, S.; Kontak, D.J.; Clarke, D.B.; Kyser, T.K. An integrated fluid-mineral stable-isotope study of the granite-hosted mineral deposits of the New Ross area, South Mountain Batholith, Nova Scotia, Canada: Evidence for multiple reservois. Canad. Miner. 2004, 42, 1425–1441. [Google Scholar] [CrossRef]
- Myint, A.Z.; Yonezu, K.; Boyce, A.J.; Selby, D.; Scherstén, A.; Tindell, T.; Watanabe, K.; Swe, Y.M. Stable isotope and geochronological study of the Mawchi Sn-W deposit, Myanmar: Implications for timing of mineralization and ore genesis. Ore Geol. Rev. 2018, 95, 663–679. [Google Scholar] [CrossRef]


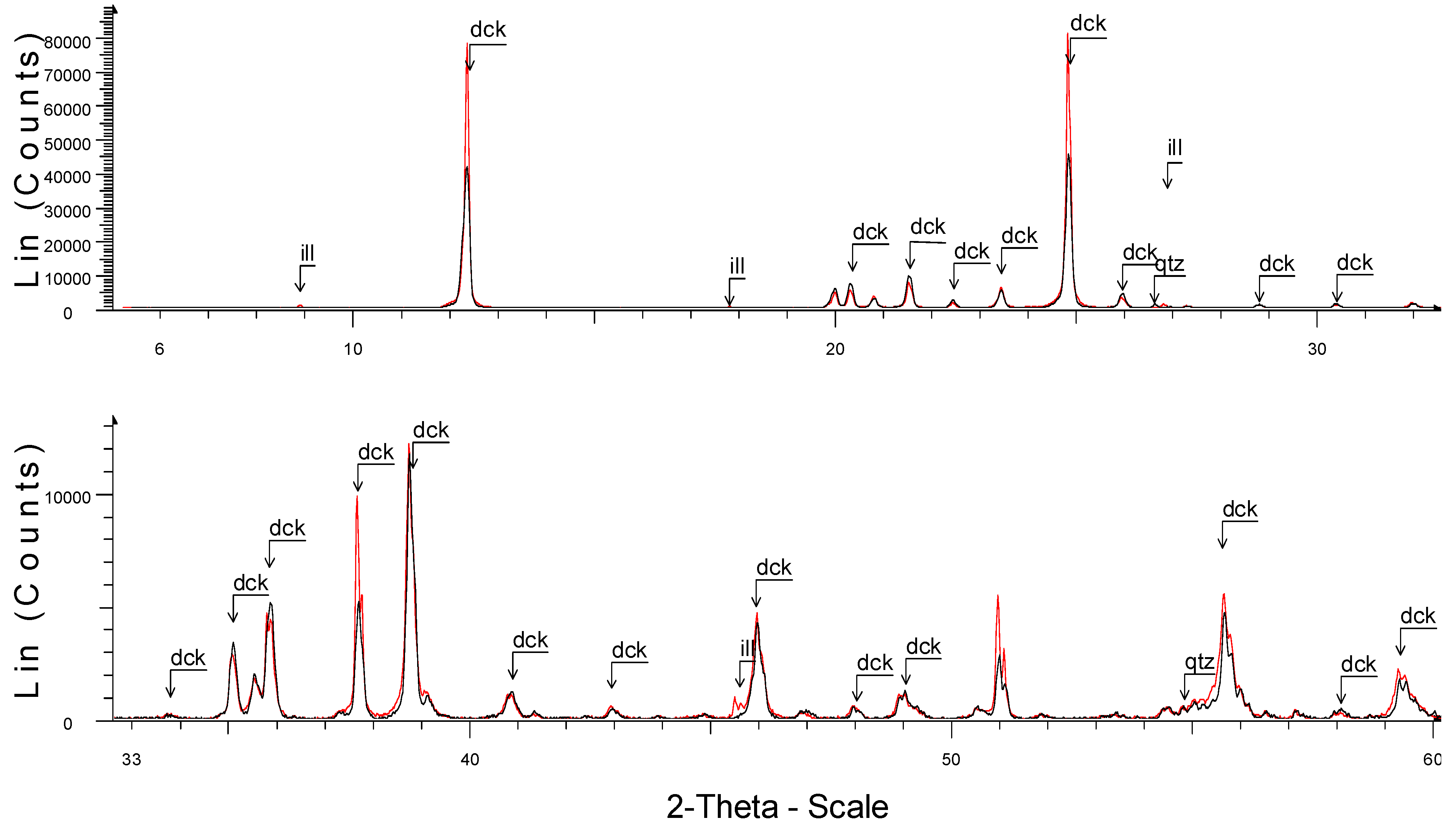





| Sample | Locality | Type of Sample | Mineral Assemblages |
|---|---|---|---|
| KNM-1 | Knötel, Prokop Stock | greisen | quartz, Li-mica, wolframite, molybdenite |
| KNM-2 | Knötel | greisen | quartz, Li-mica, apatite, cassiterite, TiO2 phase, wolframite |
| KNM-3 | Steinknochen, Martin adit | hydrothermal vein | quartz, oxi-hydroxides of Fe |
| KNM-4 | Knötel | greisen | quartz, Li-mica, wolframite |
| KR-17 | Knötel | greisenized granite | quartz, micas, K-feldspar, plagioclase, cassiterite, |
| KR-3 | Knötel, adit Sedmi spáčů | greisen | quartz, Li-mica, cassiterite, wolframite, chalcopyrite, pyrite, galena, arsenopyrite |
| KR-4 | Knötel | greisen | quartz, Li-mica, wolframite, dickite, illite, fluorite |
| KR-5 | Knötel | greisen | quartz, protolithionite/zinnwaldite, cassiterite |
| KR-7 | Knötel | greisen | quartz, phengite/Li-phengite, molybdenite, fluorite, woframite |
| KR-8 | Knötel, Prokop Stock | greisen | quartz, molybdenite, wolframite, fluorite, dickite |
| KR-10 | Knötel, Prokop Stock | greisen | quartz, Li-mica, cassiterite, wolframite |
| KR-11 | Knötel, Prokop Stock | greisen | quartz, mica, molybdenite, Bi-minerals |
| KR-12 | Knötel, Prokop Stock | greisen | quartz, wolframite, fluorite, clay minerals |
| KR-12 | Knötel, Prokop Stock | hydrothermal vein | quartz, fluorite, clay mineral |
| PRK-1 | Preisselberg | greisenized granite | quartz, plagioclase, K-feldspar, Li-mica, cassiterite, zircon |
| PRK-1 | Preisselberg | greisen | quartz, Li-mica, zinnwaldite, muscovite, cassiterite, topaz |
| PRK-1 | Preisselberg | hydrothermal vein | quartz |
| Sample | Mineral | No. of FI | Genesis | Phase Composition | LVR | ThL (°C) | ThV (°C) | ThC (°C) | Tmice (°C) | Te (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| Greisenized granite | ||||||||||
| PRK-1 | qtz | 17 | PFI1 | L+V | 0.05–0.6 | 351–441 | 392–456 | - | −2.5 to 0 | −22.8 to −23.0 |
| PRK-1 | qtz | 15 | PSFI1 | L+V | 0.4–0.7 | 236–252 | 247–265 | - | −1.5 to 0 | - |
| PRK-1 | qtz | 14 | PSFI2 | L+V | 0.7–0.95 | 178–295 | - | - | −5.2 to 0 | - |
| PRK-1 | qtz | 13 | SFI1 | L+V | 0.4–0.95 | 156–234 | - | - | −3.5 to 0 | - |
| PRK-1 | qtz | 14 | SFI2 | L+V | 0.8–0.95 | 115–205 | - | - | −0.6 to 0 | - |
| PRK-1 | cst | 14 | PFI1 | L+V | 0.1–0.5 | 362–423 | 396–498 | - | −0.2 to 0 | - |
| PRK-1 | cst | 9 | PSFI2 | L+V | 0.7–0.95 | 208–311 | - | - | −1.5 to 0 | - |
| PRK-1 | cst | - | SFI1/PSFI1 | V (?) | - | - | - | - | - | |
| KR-17 | qtz | 13 | PFI1 | L+V | 0.05–0.5 | 342–416 | 382–421 | - | −2.5 to 0 | −22.8 |
| KR-17 | qtz | 11 | PSFI2 | L+V | 0.7–0.95 | 182–235 | - | - | −0.8 to 0 | −23.0 to −23.1 |
| KR-17 | qtz | 8 | SFI1 | L+V | 0.4–0.95 | 165–228 | - | - | −3.3 to 0 | - |
| KR-17 | qtz | 12 | SFI2 | L+V | 0.7–0.95 | 117–192 | - | - | −0.3 to 0 | - |
| KR-17 | cst | 9 | PFI1 | L+V | 0.2–0.5 | 378–435 | 363–465 | - | −4.6 to −0.1 | |
| Greisen | ||||||||||
| PRK-1 | qtz | 24 | PFI1 | L+V | 0.1–0.6 | 377–401 | 392–425 | 389–412 | −2.3 to −0.1 | - |
| PRK-1 | qtz | 13 | PSFI1 | L+V | 0.3–0.6 | 205–249 | - | - | −1.3 to 0 | - |
| PRK-1 | qtz | 8 | PSFI2 | L+V | 0.7–0.95 | 231–282 | - | - | −3.6 to 0 | - |
| PRK-1 | qtz | 16 | SFI1 | L+V | 0.5–0.9 | 149–227 | - | - | −4.0 to 0 | - |
| PRK-1 | qtz | 15 | SFI2 | L, L+V | 0.7–1.0 | 121–199 | - | - | −2.6 to −0.1 | −23 |
| PRK-1 | cst | 16 | PFI1 | L+V | 0.1–0.5 | 362–425 | 383–406 | - | −0.2 to 0 | - |
| PRK-1 | cst | 8 | SFI2 | L+V | 0.7–0.95 | 145–201 | - | - | ||
| PRK-1 | tpz | 16 | PFI1 | L+V | 0.05–0.6 | 393–423 | 408–456 | 401–416 | −0.7 to 0 | - |
| KR-3 | qtz | 16 | PFI1 | L+V | 0.2–0.5 | 367–402 | 398–431 | 406, 413 | −2.1 to 0 | |
| KR-4 | qtz | 14 | PFI1 | L+V | 0.1–0.5 | 345–387 | 376–469 | −0.8 to −0.1 | ||
| KR-4 | fl | 9 | PFI2 | L+V | 0.7–0.9 | 224–289 | - | - | −0.2 to 0 | - |
| KR-5 | qtz | 10 | PFI1 | L+V | 0.2–0.5 | 354–421 | 382–439 | −2.0 to 0 | ||
| KR-7 | qtz | 10 | PFI1 | L+V | 0.2–0.6 | 372–418 | 390–447 | −1.5 to 0 | ||
| KR-8 | qtz | 12 | PFI1 | L+V | 0.1–0.5 | 345–421 | 364–473 | −2.5 to −0.2 | ||
| KR-8 | fl | 11 | PFI2 | L+V | 0.7–0.95 | 254–296 | - | - | −0.1 to 0 | - |
| KR-11 | qtz | 31 | PFI1 | L+V | 0.1–0.5 | 357–406 | 370–428 | 397–409 | −0.5 to 0 | |
| KR-12 | qtz | 27 | PFI1 | L+V | 0.1–0.5 | 348–411 | 381–489 | 397–421 | −2.1 to −0.2 | −22.8 |
| KNM-1 | qtz | 18 | PFI1 | L+V | 0.1–0.5 | 383–396 | 356–402 | −2.3 to 0 | ||
| KNM-4 | qtz | 15 | PFI1 | L+V | 0.2–0.5 | 361–380 | 377–421 | −1.7 to −0.1 | ||
| KR-11 | qtz | 8 | PSFI1 | L+V | 0.5–0.7 | 208–232 | - | - | −1.0 to 0 | - |
| KR-12 | qtz | 12 | PSFI1 | L+V | 0.5–0.7 | 203–212 | - | - | −0.5 to 0 | - |
| KNM-1 | qtz | 6 | PSFI1 | L+V | 0.5–0.7 | 216–262 | - | - | −0.3 to 0 | - |
| KR-12 | qtz | 11 | PSFI2 | L+V | 0.8–0.95 | 201–232 | - | - | −3.1 to 0 | - |
| KR-11 | qtz | 21 | SFI1 | L+V | 0.6–0.8 | 190–213 | - | - | −2.5 to 0 | - |
| KR-12 | qtz | 28 | SFI1 | L+V | 0.5–0.8 | 175–209 | - | - | −3.2 to 0 | - |
| KNM-1 | qtz | 7 | SFI1 | L+V | 0.6–0.7 | 169–193 | - | - | −4.0 to −0.2 | - |
| KR-5 | qtz | 13 | SFI2 | L+V | 0.8–0.95 | 116–186 | - | - | −4.2 to 0 | −37.7 |
| KR-11 | qtz | 21 | SFI2 | L, L+V | 0.8–1.0 | 145–174 | - | - | −2.5 to −0.3 | −38.4 |
| KR-12 | qtz | 28 | SFI2 | L, L+V | 0.8–1.0 | 121–206 | - | - | −5.8 to 0 | −37.3, −37.5 |
| KNM-1 | qtz | 9 | SFI2 | L, L+V | 0.8–1.0 | 139–152 | - | - | −0.4 to 0 | |
| KNM-4 | qtz | 11 | SFI2 | L, L+V | 0.8–1.0 | 119–128 | - | - | −0.2 to 0 | |
| KR-5 | cst | 10 | PFI1 | L+V | 0.1–0.5 | 352–440 | 376–462 | - | −0.2 to 0 | - |
| KR-10 | cst | 14 | PFI1 | L+V | 0.2–0.6 | 336–412 | 399–403 | - | −0.1 to 0 | - |
| KR-5 | cst | 7 | PSFI2 | L+V | 0.8–0.95 | 246–289 | 316–322 | - | −2.8 to 0 | - |
| KR-10 | cst | - | SFI1 | L+V, V (?) | 0–0.05 (?) | - | - | - | - | |
| KNM-2 | tpz | 8 | PFI1 | L+V | 0.2–0.5 | 398–431 | 405–416 | 412–423 | −0.4 to 0 | - |
| KNM-2 | ap | 21 | PFI1 | L+V | 0.1–0.5 | 342–390 | - | - | −0.5 to 0 | −22.8 to −22.9 |
| KR-11 | fl | 12 | PFI2 | L+V | 0.6–0.9 | 216–301 | - | - | −0.2 to 0 | - |
| KR-11 | fl | 6 | PSFI2 | L+V | 0.8–0.95 | 176–241 | - | - | −1.3 to 0 | - |
| KR-11 | fl | 13 | SFI2 | L+V | 0.7–0.95 | 121–247 | - | - | −2.1 to 0 | - |
| Hydrothermal vein | ||||||||||
| KNM-3 | qtz | 23 | PFI2 | L+V | 0.5–0.9 | 218–291 | - | - | −1.1 to 0 | |
| KNM-3 | qtz | 4 | PSFI1 | L+V | 0.5–0.7 | 213–266 | - | - | −1.2 to 0 | - |
| KNM-3 | qtz | 12 | PSFI2 | L+V | 0.8–0.95 | 177–242 | - | - | −6.5 to 0 | - |
| KNM-3 | qtz | 13 | SFI1 | L+V | 0.4–0.9 | 165–230 | - | - | −3.5 to 0 | - |
| KNM-3 | qtz | 21 | SFI2 | L+V | 0.7–0.95 | <50–201 | - | - | −2.6 to 0 | - |
| KR-12 | qtz | 42 | PFI2 | L+V | 0.4–0.7 | 256–302 | - | - | −0.4 to 0 | −22.9 |
| KR-12 | qtz | 17 | PSFI2 | L+V | 0.8–0.95 | 199–245 | - | - | −1.5 to 0 | - |
| KR-12 | qtz | 21 | SFI1 | L+V | 0.7–0.9 | 160–202 | - | - | −3.5 to 0 | - |
| KR-12 | qtz | 32 | SFI2 | L, L+V | 0.8–0.95 | 137–182 | - | - | −4.5 to 0 | −36.3 to −37.9 |
| KR-12 | fl | 14 | PFI2 | L+V | 0.7–0.95 | 247–276 | - | - | −0.2 to 0 | - |
| KR-12 | fl | 16 | SFI2 | L+V | 0.95 | 115–204 | - | - | −3.8 to 0 | - |
| KR-8 | fl | 6 | PFI2/PSFI1 | L+V | 0.7–0.95 | 191–213 | −0.3 to 0 | |||
| PRK-1 | qtz | 26 | PFI2 | L+V | 0.5–0.8 | 259–279 | - | - | −1.2 to 0 | - |
| PRK-1 | qtz | 8 | SFI1 | L+V | 0.7–0.9 | 148–223 | - | - | −2.8 to 0 | - |
| PRK-1 | qtz | 14 | SFI2 | L, L+V | 0.9–1.0 | <50–162 | - | - | −1.3 to 0 | - |
| Sample | Mineral | Genesis | No. of FI | Phase Composition | LVR | ThL (°C) | Tmice (°C) | Tdsh (°C) |
|---|---|---|---|---|---|---|---|---|
| Greisenized granite | ||||||||
| PRK-1 | qtz | PSFI/SFI3 | 6 | L+V+S | 0.6–0.95 | 151–303 | −31.0 to −14.1 | 162–202 |
| PRK-1 | cst | PSFI/SFI3 | 8 | L+V+S | 0.5–0.9 | 293–335 | −25.5 to −19.5 | 178–224 |
| Greisen | ||||||||
| PRK-1 | qtz | PSFI/SFI3 | 12 | L+V+S1–3 | 0.7–0.95 | 263–369 | −26.1 to −15.2 | 189–314 |
| KR-3 | cst | PSFI/SFI3 | 6 | L+V+S1–3 | 0.5–0.95 | 141–327 | −23.2 to −21.7 | 145–335 |
| KR-12 | qtz | PSFI/SFI3 | 15 | L+V+S1–6 | 0.5–0.95 | 196–242 | −27.6 to −16.0 | 182–281 |
| KNM-1 | qtz | PSFI/SFI3 | 9 | L+V+S1–6 | 0.6–0.95 | 189–221 | −28.1 to −18.3 | 178–204 |
| KR-11 | qtz | PSFI/SFI3 | 17 | L+V+S1–5 | 0.5–0.95 | 212–248 | −25.8 to −20.4 | 210–270 |
| KR-11 | qtz | SFI3 | 4 | L+V+S | 0.8–0.95 | 182–311 | −1.3 to −0.2 | - |
| KR-10 | cst | PSFI/SFI3 | 5 | L+V+S1–2 | 0.7–0.95 | 192–245 | −24.5 to −19.7 | 214–275 |
| Hydrothermal vein | ||||||||
| KNM-3 | qtz | PSFI/SFI3 | 16 | L+V+S1–5 | 0.6–0.95 | 164–213 | −26.5 to −17.2 | 201–251 |
| KR-12 | qtz | SFI3 | 8 | L+V+S1–2 | 0.7–0.95 | 126–362 | −3.0 to 0 | - |
| KR-12 | qtz | PSFI/SFI3 | 14 | L+V+S1–6 | 0.6–0.95 | 182–265 | −30.8 to −14.3 | 192–231 |
| Sample | Mineral | Genesis | No. of FI | Phase Composition | LVR | ThL (°C) | ThV (°C) | Thc (°C) | Tmice (°C) | Tmcla (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| PRK-1 | cst | PFI1/PSFI1 | 4 | L+V | 0.1–0.4 | - | 399–456 | −0.2 to 0 | 5.6–10.1 | |
| KR-5 | cst | PFI1 | 2 | L+V | 0.1–0.3 | - | 396, 402 | −0.1 to 0 | 4.8, 6.1 | |
| KR-12 | qtz | PFI1 | 2 | L+V | 0.1–0.3 | - | 392 | 408 | −2.1 to 0 | 4.2, 4.7 |
| KNM-1 | qtz | PFI1 | 2 | L+V | 0.2–0.4 | - | 422,438 | −0.8 to 0 | 5.8, 7.3 | |
| KNM-2 | qtz | PFI1 | 5 | L+V | 0.1–0.5 | 389, 401 | 406–419 | −1.4 to −0.1 | 4.2–7.3 | |
| KNM-2 | tpz | PFI1 | 7 | L+V | 0.2–0.5 | 399–413 | 403–408 | 401 | −0.6 to 0 | 4.4–8.4 |
| CH4 | CO2 | N2 | |||
|---|---|---|---|---|---|
| Sample | Mineral | Genesis | mol. % | ||
| KNM-3 | qtz | PFI2/PSFI1 | 28.8 | 71.1 | 0.1 |
| KNM-3 | qtz | PFI2/PSFI1 | 1.3 | 98.7 | 0 |
| KNM-3 | qtz | PFI2/PSFI1 | 100 | 0 | 0 |
| KR-12 | qtz | PFI1 | 0 | 100 | 0 |
| KR-12 | qtz | PFI1 | 2.1 | 96.2 | 1.7 |
| Sample | KR-11 (Greisen) | KR-12 (Greisen) | KR-12 (Vein) |
|---|---|---|---|
| Li+ | 27 | 30 | 32 |
| Na+ | 4496 | 4250 | 4055 |
| K+ | 2582 | 2124 | 1677 |
| Mg2+ | 602 | 173 | 94 |
| Ca2+ | 10,198 | 1878 | 952 |
| F− | 81 | 219 | 250 |
| Cl− | 9866 | 9179 | 8564 |
| Br− | 47 | 38 | 31 |
| I− | 1.4 | 0.6 | 0.1 |
| NO3− | 3829 | 1573 | 845 |
| SO42− | 777 | 186 | 191 |
| Br/Cl × 103 | 4.8 | 4.2 | 3.7 |
| Na/Br | 95 | 111 | 129 |
| I/Cl × 106 | 15 | 66 | 12 |
| Na/K | 1.7 | 2.0 | 2.4 |
| K/Na | 0.6 | 0.5 | 0.4 |
| Cl/SO4 | 13 | 49 | 45 |
| Q+/Q− | 2.3 | 1.2 | 1.0 |
| Mineral | Sample | δDmineral (‰ V-SMOW) | δDfluid (‰ V-SMOW) | Temperature (°C) | Content of Water (%) |
|---|---|---|---|---|---|
| Dickite | KR-4 | −22.2 | −45.7 to −37.9 | 100–250 | 9.79 |
| Dickite | KR-8 | −51.2 | −74.7 to −66.9 | 100–250 | 14.31 |
| Phengite/Li-phengite | KR-7 | −54.6 | −92.4 to −77.8 | 350–400 | 2.34 |
| Protolithionite/zinnwaldite | KR-5 | 33.6 | −20.6 to −6.0 | 350–450 | 0.92 |
| Sample | KNM-2 | KMN-2 | KNM-2 | KNM-1 | KR-5 | KR-12 |
|---|---|---|---|---|---|---|
| Mineral | Tpz | Tpz | Qtz | Qtz | Cst | Qtz |
| Genesis | PFI1 | PFI1 | PFI1 | PFI1 | PFI1 | PFI1 |
| Thv (°C) | 408 | 403 | 412 | 422 | 396 | 392 |
| Tmice (°C) | −0.2 | −0.1 | −0.7 | −0.8 | −0.2 | −2.1 |
| Tmcla (°C) | 4.6 | 6.1 | 5.1 | 5.8 | 5.6 | 4.2 |
| LVR | 0.2 | 0.3 | 0.2 | 0.4 | 0.3 | 0.2 |
| X(H2O) | 0.89 | 0.90 | 0.87 | 0.91 | 0.90 | 0.88 |
| X(CO2) | 0.11 | 0.09 | 0.10 | 0.07 | 0.08 | 0.10 |
| X(NaCl) | 0.00 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Molar volume (cm3/mol) | 83.0 | 56.8 | 79.5 | 42.9 | 58.5 | 82.5 |
| Sample | Mineral | Genesis | Salinity (wt. % NaCl eq.) | Molar Volume (cm3/mol) | mol. % CO2 |
|---|---|---|---|---|---|
| PRK-1 | Cst | PFI1/PSFI1 | 0.0–2.8 | 79.3–83.4 | 12.6–14.6 |
| KR-5 | Cst | PFI1 | 3.1–4.0 | 56.4–55.8 | 8.1–9.4 |
| KR-12 | Qtz | PFI1 | 3.2–3.4 | 81.6–84.9 | 10.7–11.3 |
| KNM-1 | Qtz | PFI1 | 1.3–3.3 | 55.9–81.7 | 2.6–8.9 |
| KNM-2 | Qtz | PFI1 | 1.5–3.9 | 58.6–81.6 | 7.7–14.8 |
| KNM-2 | Tpz | PFI1 | 0.5–4.1 | 56.6–80.2 | 7.6–16.5 |
| Fluid System | H2O-CO2-(NaCl) | H2O-NaCl | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Mineral | KNM-2 tpz | KR-12 qtz | PRK-1 qtz | PRK-1 tpz | KR-3 qtz | KNM-2 tpz | KR-11 qtz | KR-12 qtz |
| Thv (°C) | 403, 408 | 392 | 392–425 | 408–456 | 398–431 | 405–416 | 379–428 | 381–456 |
| Tmice (°C) | −0.1,−0.2 | −2.1 | −2.3 to −0.2 | −0.3 to −0.1 | −2.1 to −0.3 | −0.4 to 0 | −0.5 to 0 | −2.1 to −0.2 |
| Tmcla (°C) | 4.6, 6.1 | 4.2 | ||||||
| LVR | 0.2, 0.3 | 0.2 | 0.2–0.4 | 0.2–0.3 | 0.2–0.3 | 0.2–0.3 | 0.1–0.3 | 0.1–0.4 |
| Mol. % CO2 | 10.7, 8.9 | 9.8 | ||||||
| Bulk molar volume (cm3/mol) | 56.8, 83.0 | 82.4 | 38.3–46.7 | 47.0–95.1 | 39.0–52.9 | 45.2–52.2 | 37.5–60.2 | 33.7–80.54 |
| Pressure (bar) | 352, 371 | 322 | 173–223 | 197–268 | 182–224 | 193–209 | 155–320 | 157–313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krejčí Kotlánová, M.; Dolníček, Z.; René, M.; Prochaska, W.; Ulmanová, J.; Kapusta, J.; Mašek, V.; Kropáč, K. Fluid Evolution of Greisens from Krupka Sn-W Ore District, Bohemian Massif (Czech Republic). Minerals 2024, 14, 86. https://doi.org/10.3390/min14010086
Krejčí Kotlánová M, Dolníček Z, René M, Prochaska W, Ulmanová J, Kapusta J, Mašek V, Kropáč K. Fluid Evolution of Greisens from Krupka Sn-W Ore District, Bohemian Massif (Czech Republic). Minerals. 2024; 14(1):86. https://doi.org/10.3390/min14010086
Chicago/Turabian StyleKrejčí Kotlánová, Michaela, Zdeněk Dolníček, Miloš René, Walter Prochaska, Jana Ulmanová, Jaroslav Kapusta, Vlastimil Mašek, and Kamil Kropáč. 2024. "Fluid Evolution of Greisens from Krupka Sn-W Ore District, Bohemian Massif (Czech Republic)" Minerals 14, no. 1: 86. https://doi.org/10.3390/min14010086
APA StyleKrejčí Kotlánová, M., Dolníček, Z., René, M., Prochaska, W., Ulmanová, J., Kapusta, J., Mašek, V., & Kropáč, K. (2024). Fluid Evolution of Greisens from Krupka Sn-W Ore District, Bohemian Massif (Czech Republic). Minerals, 14(1), 86. https://doi.org/10.3390/min14010086