Abstract
Europium (Eu) in coal and sedimentary rocks has important mineral resource potential as well as being a crucial parameter in geochemical studies that can represent changes in the depositional environment during coal deposition and identify the depositional source region. Therefore, it is essential to realize the precise measurement of Eu in coal as this could be a useful parameter for paleoenvironmental reconstruction studies and the exploration of mineral resources. During inductively coupled plasma mass spectrometry (ICP-MS) analysis, polyatomic ions of Ba may interfere with Eu, causing the observed value to be higher than the actual value. This paper develops a new approach for Eu determination by using a high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS). The mass spectral interference and correction of Eu in the coal and sedimentary rock samples at low, medium, and high resolutions were investigated. The results showed that in the high-resolution mode (resolution = 10,000 amu), the interference of polyatomic ions of Ba could be distinguished from Eu; hence, Eu was determined under this circumstance. Under the optimal experimental circumstances, the detection limit was 0.006 μg/mL, the relative standard deviation was 0.80%–1.22%, and the linear correlation coefficient of the standard curve was over 0.9999. The recoveries of the 103Rh internal standard solution ranged from 94.41% to 100.10%. This method was verified using standard reference materials and selected samples, which demonstrated its high sensitivity, accuracy, and reliability, and a low detection limit, making it appropriate for detecting Eu in samples of coal and sedimentary rocks.
1. Introduction
Coal and coal-bearing strata have attracted much attention in recent decades [1,2,3,4] as promising alternative raw sources for rare earth elements (REEs), not only because the REE concentrations in many coals, coal-bearing strata, or coal ashes are equal to or higher than those found in conventional types of REE ores [5,6] but also because the worldwide demand for REEs in recent years has been greater than supply. Among REEs, europium (Eu) is classified as one of the most important critical rare earth elements [7,8,9] that has been widely industrially used.
Europium could also be a useful geochemical parameter for geological researches. As one of only two rare earth elements (Ce and Eu) sensitive to redox environments, Eu exists primarily as Eu3+ and is only reduced to Eu2+ under extreme environmental conditions like unusually strong reducing environments or the influence of high-temperature hydrothermal fluids above 250 °C [10,11,12], leading to anomalies of concentration. Additionally, as the temperature rises, the degree of Eu3+ reduction to Eu2+ increases [13]. Eu anomalies in coal and sedimentary rocks are typically inherited from the eroded source rocks of the sediments rather than from the weathering, denudation, transportation, and deposition of the materials derived from eroded source rocks [14,15,16,17,18,19]. Therefore, Eu anomalies have been extensively studied to indicate sedimentary environments, the supply of erosional source areas, and regional geological history evolution [9,18,19,20,21,22]. Therefore, accurate Eu determination of concentrations in coal and sedimentary rock samples is crucial.
Due to its high sensitivity, low detection limit, and broad linear range, inductively coupled plasma mass spectrometry (ICP-MS) has become one of the most competitive and promising methods for trace and ultratrace REE analysis in geological materials [7,23,24,25] and is widely used in trace element testing compared to other analytical techniques like neutron activation analysis [26,27,28,29,30], X-ray fluorescence spectrometry [31,32,33], laser-induced breakdown spectrometry [34,35,36,37], atomic absorption spectroscopy [38,39,40,41], and inductively coupled plasma atomic emission spectrometry [42,43,44,45]. There have been many reports of concentrations of trace elements in coals that were successfully determined by ICP-MS [46,47,48,49,50,51,52]. However, the determination of Eu by ICP-MS is often interfered with due to the overlapping of M+, MO+, and MOH+ ions [53,54,55], especially the polyatomic ions BaO and BaOH formed by Ba [20,22,56,57]. In addition to studying the effects of different conditions on the formation of polyatomic particle interferences [54,58,59,60,61,62,63], Jarvis et al. [64] used the higher yields of doubly charged ions to avoid the effects of oxides and hydroxides of Ba by determining doubly charged Eu ions, but the decrease in sensitivity was very significant when using this method to determine the content of ultratrace Eu. Yan et al. [65] used the AG50W-x8 cation exchange resin to separate Ba in the dissolving solution and then combined it with ICP-MS to achieve an accurate determination of Eu.
High-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) employs an electrostatic analyzer to compensate for the diffusion of the ion beam generated by the ICP source, delivering an ion beam with a much narrower ion energy range to the magnetic sector and thus distinguishing the weak mass number difference between the interfering and target elements to resolve most polyatomic ion interference problems [66]. Rodushkin et al. [67] used HR-ICP-MS for the determination of multi-elements in coal; nevertheless, the mass spectral interference of Eu in coal samples at low, medium, and high resolution has not been systematically reported; therefore, there is a potential underestimation of Eu determination in coal or sedimentary materials by HR-ICP-MS. In this study, a method for HR-ICP-MS-based Eu determination is presented. At low, medium, and high resolutions, the mass spectrum interferences and calibration of the target element were investigated. Calibration curves, detection limits, precision, accuracy, and internal standard solution recoveries were used to validate the method. The precise determination of Eu in samples of coal and sedimentary rock was accomplished using the established methodology.
2. Experimental Method
2.1. Instruments
For concentration determination of Eu in coal and sedimentary rock samples, an Attom ES (Nu Instruments, Wrexham, UK) high-resolution inductively coupled plasma mass spectrometer (HR-ICP-MS) was utilized. A Milestone UltraClave (Sorisole, Italy) microwave high-pressure reactor, which can fully digest the samples with a rapid speed under high-temperature and -pressure conditions, was used to digest the samples. The ultrapure water required for the experiment was made using a Milli-Q IQ 7010 Ultrapure water system (18.2 MΩ·cm, Merck Millipore, Molsheim, France). For further purification of the guaranteed reagents (GR) HNO3 (65%) and HF (40%), a DuoPUR acid purification system (Milestone, Milan, Italy) and an SD-2000 acid purification system (Labtech, Beijing, China) were utilized, respectively.
2.2. Reagents and Gases
The reagents needed in the experiment include a series of Eu standard solutions, an internal standard solution, and a tuning solution. The intermediate solution was diluted from the 100 μg/mL standard reference solution (CCS1, Inorganic Ventures, Christiansburg, VA, USA) to 1 μg/mL. The intermediate solution was aspirated into six polyfluoroalkoxy (PFA) volumetric flasks of 0 mL, 0.1 mL, 1 mL, 3 mL, 5 mL, and 10 mL to establish the calibration curve of six concentration levels (0, 1, 10, 30, 50, and 100 μg/L). Next, 2 mL of purified HNO3 was added to each flask and diluted to the tick mark with ultrapure water.
An internal standard stock solution of 103Rh (10 μg/mL) and a tuning solution containing multi-elements (1 μg/L) were diluted from 1000 μg/mL Rh standard solution (GSB 04-1746-2004, National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials, Beijing, China) and 1000 μg/mL multi-elements standard solution (GNM-M241186-2013, National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials, Beijing, China), respectively. The GR HNO3 and GR HF after purification were used for standard solution preparation and sample digestion. Ultrapure argon was used as the cooling, auxiliary, and nebulizer gas.
2.3. Investigated Samples
Four standard reference materials, including two coal samples (SRM2682b and SRM2685b, National Institute of Standards and Technology, Gaithersburg, MD, USA (NIST)) and two sedimentary rocks (GSR-6 and GSR-20, National Institute of Metrology, Beijing, China (NIM)), were chosen as references in order to evaluate the stability and accuracy of the HR-ICP-MS for Eu concentration determination. In addition, to cover the range of Ba/Eu ratios more comprehensively, twelve samples covering multiple lithologies, including six coal samples and six sedimentary rock samples, were selected from 120 samples from coal deposits in China for analysis in this study. Table 1 provides a thorough description of these samples.

Table 1.
Description of all investigated samples.
2.4. Sample Pre-Treatment
Fifty milligrams of coal samples crushed to 200 mesh were weighed precisely into the digestion tube, and 5 mL of purified HNO3 and 2 mL of purified HF were added. On the other hand, 2 mL of purified HNO3 and 5 mL of purified HF were added to the sedimentary rock samples. Two blank samples were inserted in each batch to correct for the influences of external factors on the test results, such as the presence of the element to be measured in the used reagent and contamination during sample pre-treatment.
The digestion tank of the UltraClave reactor was loaded with 5 mL of HNO3 and 150 mL of ultrapure water. The microwave digestion program was configured as stated in Table 2, and Figure 1 depicts the actual running curve.

Table 2.
Microwave program for sample digestion.
Figure 1.
The actual running curve for the sample digestion program.
Since the REEs would react with HF during the digestion process and form insoluble complexes [70], causing the results of REE determination to be lower than the certified values, after digestion, the samples were transferred to PFA digestion cups and heated to 180 °C on an electric hot plate for acid-driving to minimize the effect of HF. To thoroughly dissolve the REEs from the complexes, 5 mL of HNO3 solution (v:v = 1:1) was added to the solution after the acid-driving. This solution was then covered and heated on the electric hot plate for 4 h. The samples were moved to PFA volumetric flasks and diluted with ultrapure water to 100 mL after reaching room temperature in preparation for further analysis.
2.5. Determination Procedure
The optimization procedure may start when the plasma has been ignited for 20 to 30 min and the set spray chamber temperature has been reached. The tuning solution was first aspirated, and then the torch position, nebulizer pressure, and ion optics were optimized to maximize the ion signal. The HR-ICP-MS optimization settings are displayed in Table 3. A magnet calibration program was then performed to ensure its stability before the equipment was prepared for sample testing.

Table 3.
Optimized instrumental parameters for HR-ICP-MS.
3. Results and Discussion
3.1. Mass Spectral Interferences and Correction
The determination of Eu by the ICP-MS method inevitably involves polyatomic ion interference, among which the most prominent are the oxides and hydroxides formed by natural isotopes of Ba [20]. Especially when the Ba/Eu is 1000 or higher, the interference of polyatomic ions of Ba to Eu is significantly enhanced [57,65,71]. Table 4 shows the interference of oxides and hydroxides formed by Ba to Eu. By selecting an appropriate resolution, the mass spectral peaks of Eu and the interfering ions can be distinguished for the purpose of accurate quantification. The mass spectral peaks of Eu at different resolutions are displayed in Figure 2.

Table 4.
Interference table of oxides and hydroxides formed by Ba to 153Eu.
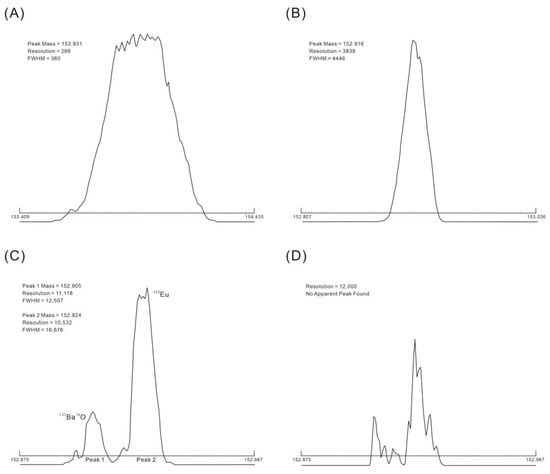
Figure 2.
Peak shape of Eu at different resolutions. (A–D) represent the peaks of the Eu at resolutions of 300, 4000, 10,000, and 12,000 amu, respectively.
The observed values of Eu are significantly higher than the certified values because, in low and medium-resolution modes, the mass spectral peaks of the interfering ions, such as polyatomic ions of Ba (137Ba16O, 136Ba16OH), overlap with the 153Eu mass spectral peak (Figure 2A,B). The mass spectral peak of the interfering ions may be entirely separated from 153Eu in the high-resolution mode (Figure 2C), and the measured Eu values are more in line with the anticipated concentrations. When the resolution increases, the capacity of the device to distinguish the elements to be measured from the examined spectra increases, while analytical sensitivity diminishes as ion transport efficiency declines. Even though the mass spectral peak of the interfering ions may be completely isolated from Eu when the resolution reaches 12,000 amu, the signal is severely attenuated and the instrument is unable to make an accurate diagnosis (Figure 2D). Thus, the high-resolution mode (Resolution = 10,000 amu) should be selected for the measurement.
3.2. Calibration Curves and Method Detection Limit
The lowest concentration of each element that the instrument may identify is represented by the method detection limit (MDL), which is an essential parameter for analytical testing. The MDL was determined by three times the standard deviation of the 11 results for 11 independent blank samples. Table 5 displays the calibration cures, the linear correlation coefficients, and the MDLs of Eu at different resolutions. The correlation coefficients of the standard curve were above 0.9999 at all three resolutions, indicating good linearity and accurate results. The MDLs ranged from 0.003 to 0.008 μg/L.

Table 5.
Calibration curves and method detection limit (MDL) of Eu under different resolutions.
3.3. Precision, Accuracy, and Recovery
In order to evaluate the precision and accuracy of the method for the determination of coal and sedimentary rock samples, the four standard reference materials were prepared according to the experimental method, and the average of six measurements was used as the result to calculate the relative error (RE), the standard deviation (SD), and the relative standard deviation (RSD).
The certified and observed values of Eu, RE, SD, and RSD of the four standard reference materials at different resolutions as well as the recovery of the internal standard solution of 10 μg/mL 103Rh are listed in Table 6.

Table 6.
Observed (Obs) and certified (Cer) values of Eu (μg/g) in standard reference coal and sedimentary rock samples, as well as RE (%), SD (%), RSD (%), and internal solution recovery (Rec, %) of the HR-ICP-MS analysis.
The REs of the standard reference materials were less than 10% for all conditions, except for NIST 2682b at low and medium resolutions. This is due to the fact that Ba/Eu in NIST 2682b was higher than 1000 and the polyatomic ions of Ba interfered with the determination of Eu at low and medium resolutions. The RSDs ranged from 0.33% to 1.46%, demonstrating the high precision of the method. The recoveries of the internal standard solutions ranged from 93.94% to 112.11%, which suggests the stability of the instrument and the analysis.
3.4. Analytical Results
The concentration of the REEs for the samples in Table 1 are listed in Table 7. In addition to the four standard reference materials, the REEs in the other Chinese samples were normalized by the upper continental crust (UCC; UCC data are from Taylor and McLennan [72]), and the distribution patterns of the REEs in the samples were plotted in Figure 3.

Table 7.
Concentrations of REEs (μg/g) for selected samples in low resolution (LR), medium resolution (MR), and high resolution (HR) mode.
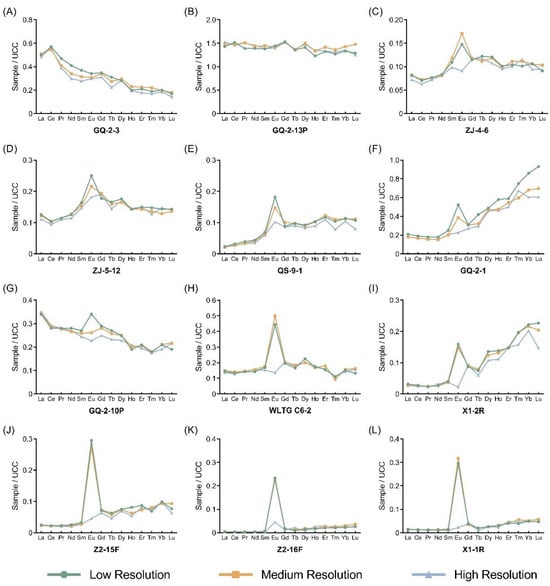
Figure 3.
UCC-normalized REE distribution patterns for samples at different resolutions. (A–L), Correspond to the selected 12 Chinese samples.
For the samples in which Ba/Eu is less than 1000 (GQ-2-3 and GQ-2-13P), the concentrations of Eu at different resolutions are fairly close, suggesting that the influence of Ba can be ignored (Figure 3A,B). As for samples in which Ba/Eu is higher than 1000, the Eu concentration in the samples is overestimated in low and medium-resolution modes due to the influence of the polyatomic ions of Ba. In contrast, the polyatomic ions of Ba are separated in the high-resolution mode and the actual value of Eu is obtained. Among them, the Ba/Eu ratios in ZJ-4-6, ZJ-5-12, QS-9-1, GQ-2-1, and GQ-2-10P are larger than 1000 but less than 10,000, and typically, the interference of Ba on Eu determination does not exceed 100% of the actual value of Eu. The samples that ought to have shown no Eu anomalies, weak positive Eu anomalies, or even weak negative Eu anomalies show positive Eu anomalies at low and medium resolutions (Figure 3C–G). The interference from Ba can reach up to several times (WLTG C6-2, X1-2R, Z2-15F, and Z2-16F) or even thirteen times (X1-1R) that of Eu, and the samples exhibit significant positive Eu anomalies at low and medium resolutions (Figure 3H–L), which indicates that the effect of Ba polyatomic ions on Eu is extremely significant when Ba/Eu is greater than 10,000. Thus, this demonstrates the reliability and effectiveness of this method to separate the interference of Ba using the high-resolution mode of HR-ICP-MS.
4. Conclusions
A new reliable method for the determination of Eu in coal and sedimentary rocks was presented in this paper. The accurate determination of Eu was achieved by using an HR-ICP-MS combined with microwave digestion. By optimizing the instrument parameters and selecting the best resolution, the analytical accuracy of Eu was improved. In the high-resolution mode, the mass spectral peaks of Eu and the polyatomic ions of Ba can be separated by using the slight difference in mass numbers between them, thus effectively separating Eu from the interfering ions and eliminating the mass spectral interference. Compared to Yan et al. [65], this method omits the step of Ba separation, which greatly simplifies the operation process while ensuring the accuracy of the results. This method has been successfully applied to coal and sedimentary rock samples and has the advantages of a low detection limit and high precision and accuracy.
Author Contributions
Formal analysis, S.Z.; investigation, R.J., J.F., K.T., Q.H. and N.S.; methodology, S.Z.; supervision, J.L.; writing—original draft, S.Z.; writing—review and editing, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (No. 2021YFC2902003) and the National Natural Science Foundation of China (No. 42272194).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The editors and three reviewers are acknowledged sincerely for their very detailed comments on this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Chitlango, F.Z.; Wagner, N.J.; Moroeng, O.M. Characterization and Pre-Concentration of Rare Earth Elements in Density Fractionated Samples from the Waterberg Coalfield, South Africa. Int. J. Coal Geol. 2023, 275, 104299. [Google Scholar] [CrossRef]
- Di, S.; Dai, S.; Nechaev, V.P.; French, D.; Graham, I.T.; Zhao, L.; Finkelman, R.B.; Wang, H.; Zhang, S.; Hou, Y. Mineralogy and Enrichment of Critical Elements (Li and Nb-Ta-Zr-Hf-Ga) in the Pennsylvanian Coals from the Antaibao Surface Mine, Shanxi Province, China: Derivation of Pyroclastics and Sediment-Source Regions. Int. J. Coal Geol. 2023, 273, 104262. [Google Scholar] [CrossRef]
- Hou, Y.; Dai, S.; Nechaev, V.P.; Finkelman, R.B.; Wang, H.; Zhang, S.; Di, S. Mineral Matter in the Pennsylvanian Coal from the Yangquan Mining District, Northeastern Qinshui Basin, China: Enrichment of Critical Elements and a Se-Mo-Pb-Hg Assemblage. Int. J. Coal Geol. 2023, 266, 104178. [Google Scholar] [CrossRef]
- Nechaev, V.P.; Dai, S.; Chekryzhov, I.Y.; Tarasenko, I.A.; Zin’kov, A.V.; Moore, T.A. Origin of the Tuff Parting and Associated Enrichments of Zr, REY, Redox-Sensitive and Other Elements in the Early Miocene Coal of the Siniy Utyes Basin, Southwestern Primorye, Russia. Int. J. Coal Geol. 2022, 250, 103913. [Google Scholar] [CrossRef]
- Strzałkowska, E. Rare Earth Elements and Other Critical Elements in the Magnetic Fraction of Fly Ash from Several Polish Power Plants. Int. J. Coal Geol. 2022, 258, 104015. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, S.; French, D.; Spiro, B.; Graham, I.; Liu, J. Hydrothermally-Altered Coal from the Daqingshan Coalfield, Inner Mongolia, Northern China: Evidence from Stable Isotopes of C within Organic Matter and C-O-Sr in Associated Carbonates. Int. J. Coal Geol. 2023, 276, 104330. [Google Scholar] [CrossRef]
- Dai, S.; Xie, P.; Ward, C.R.; Yan, X.; Guo, W.; French, D.; Graham, I.T. Anomalies of Rare Metals in Lopingian Super-High-Organic-Sulfur Coals from the Yishan Coalfield, Guangxi, China. Ore Geol. Rev. 2017, 88, 235–250. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in Coals Preserved within Marine Carbonate Successions: Geochemical and Mineralogical Data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2014, 50, 159–186. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal Deposits as Potential Alternative Sources for Lanthanides and Yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium Redox Equilibria in Aqueous Solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Bau, M. Rare-Earth Element Mobility during Hydrothermal and Metamorphic Fluid-Rock Interaction and the Significance of the Oxidation State of Europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Zhou, Y.; Chou, C.-L.; Wang, X.; Zhao, L.; Zhu, X. Mineralogy and Geochemistry of a Superhigh-Organic-Sulfur Coal, Yanshan Coalfield, Yunnan, China: Evidence for a Volcanic Ash Component and Influence by Submarine Exhalation. Chem. Geol. 2008, 255, 182–194. [Google Scholar] [CrossRef]
- Uysal, I.T.; Golding, S.D. Rare Earth Element Fractionation in Authigenic Illite–Smectite from Late Permian Clastic Rocks, Bowen Basin, Australia: Implications for Physico-Chemical Environments of Fluids during Illitization. Chem. Geol. 2003, 193, 167–179. [Google Scholar] [CrossRef]
- Eskenazy, G.M. Rare Earth Elements in a Sampled Coal from the Pirin Deposit, Bulgaria. Int. J. Coal Geol. 1987, 7, 301–314. [Google Scholar] [CrossRef]
- Qi, H.; Hu, R.; Zhang, Q. REE Geochemistry of the Cretaceous Lignite from Wulantuga Germanium Deposit, Inner Mongolia, Northeastern China. Int. J. Coal Geol. 2007, 71, 329–344. [Google Scholar] [CrossRef]
- Yossifova, M.G.; Eskenazy, G.M.; Valčeva, S.P. Petrology, Mineralogy, and Geochemistry of Submarine Coals and Petrified Forest in the Sozopol Bay, Bulgaria. Int. J. Coal Geol. 2011, 87, 212–225. [Google Scholar] [CrossRef]
- McLennan, S.M.; Taylor, S.R. Sedimentary Rocks and Crustal Evolution: Tectonic Setting and Secular Trends. J. Geol. 1991, 99, 1–21. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Yerin, Ü.O.; Oskay, R.G.; Bulut, Y.; Córdoba, P. Enrichment and Distribution of Elements in the Middle Miocene Coal Seams in the Orhaneli Coalfield (NW Turkey). Int. J. Coal Geol. 2021, 247, 103854. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare Earth Element-Bearing Coals from the Russian Far East Deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A Review of Anomalous Rare Earth Elements and Yttrium in Coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of Yttrium and Rare-Earth Elements in the Penge and Kuruman Iron-Formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Duan, P.; Wang, W.; Liu, X.; Sang, S.; Ma, M.; Zhang, W. Differentiation of Rare Earth Elements and Yttrium in Different Size and Density Fractions of the Reshuihe Coal, Yunnan Province, China. Int. J. Coal Geol. 2019, 207, 1–11. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; French, D.; Jia, S.; Hood, M.M.; Garrison, T.M. Mineralogical and Geochemical Compositions of Late Permian Coals and Host Rocks from the Guxu Coalfield, Sichuan Province, China, with Emphasis on Enrichment of Rare Metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Wei, C.; Ye, L.; Hu, Y.; Huang, Z.; Danyushevsky, L.; Wang, H. LA-ICP-MS Analyses of Trace Elements in Base Metal Sulfides from Carbonate-Hosted Zn-Pb Deposits, South China: A Case Study of the Maoping Deposit. Ore Geol. Rev. 2021, 130, 103945. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, R.; Gao, J.; Leng, C.; Gao, W.; Gong, H. Trace and Minor Elements in Sulfides from the Lengshuikeng Ag–Pb–Zn Deposit, South China: A LA–ICP–MS Study. Ore Geol. Rev. 2022, 141, 104663. [Google Scholar] [CrossRef]
- Chatt, A.; Kulathilake, A.I.; Nyarku, S.K. Determination of Trace Elements in Particulate and Soluble Fractions of Seawater by Neutron Activation Analysis. Mar. Chem. 1983, 12, 223. [Google Scholar] [CrossRef]
- Ohde, S. Determination of Rare Earth Elements in Carbonatites from the Kangankunde Mine, Malawi by Instrumental Neutron Activation Analysis. J. Radioanal. Nucl. Chem. 2003, 257, 433–435. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Ding, W.; Liu, P.; Yao, W. Using Rare Earth Element Tracers and Neutron Activation Analysis to Study Rill Erosion Process. Appl. Radiat. Isot. 2006, 64, 402–408. [Google Scholar] [CrossRef]
- Kumar, K.; Saion, E.; Halimah, M.K.; Ck, Y.; Hamzah, M.S. Rare Earth Element (REE) in Surface Mangrove Sediment by Instrumental Neutron Activation Analysis. J. Radioanal. Nucl. Chem. 2014, 301, 667–676. [Google Scholar] [CrossRef]
- Chand, M.; Ashok Kumar, G.V.S.; Senthilvadivu, R.; Usha Lakshmi, K.; Serajuddin, M.; Ramadevi, G.; Kumar, R. Analysis of Coal Ash Samples from Thermal Power Plants of India for Their Gallium Content Using NAA and EDXRF Techniques. Appl. Radiat. Isot. 2022, 187, 110336. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; O’Rielly, D.; Wilson, R.; Das, K.; Wade, B. Mineral Chemistry of Rare Earth Element (REE) Mineralization, Browns Ranges, Western Australia. Lithos 2013, 172–173, 192–213. [Google Scholar] [CrossRef]
- Smoliński, A.; Stempin, M.; Howaniec, N. Determination of Rare Earth Elements in Combustion Ashes from Selected Polish Coal Mines by Wavelength Dispersive X-Ray Fluorescence Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2016, 116, 63–74. [Google Scholar] [CrossRef]
- Turner, A.; Chan, C.C.; Brown, M.T. Application of Field-Portable-XRF for the Determination of Trace Elements in Deciduous Leaves from a Mine-Impacted Region. Chemosphere 2018, 209, 928–934. [Google Scholar] [CrossRef]
- Gaft, M.; Dvir, E.; Modiano, H.; Schone, U. Laser Induced Breakdown Spectroscopy Machine for Online Ash Analyses in Coal. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1177–1182. [Google Scholar] [CrossRef]
- Stankova, A.; Gilon, N.; Dutruch, L.; Kanicky, V. A Simple LIBS Method for Fast Quantitative Analysis of Fly Ashes. Fuel 2010, 89, 3468–3474. [Google Scholar] [CrossRef]
- Decrée, S.; Savolainen, M.; Mercadier, J.; Debaille, V.; Höhn, S.; Frimmel, H.; Baele, J.-M. Geochemical and Spectroscopic Investigation of Apatite in the SIILINJÄRVI Carbonatite Complex: Keys to Understanding Apatite Forming Processes and Assessing Potential for Rare Earth Elements. Appl. Geochem. 2020, 123, 104778. [Google Scholar] [CrossRef]
- Müller, S.; Meima, J.A.; Rammlmair, D. Detecting REE-Rich Areas in Heterogeneous Drill Cores from Storkwitz Using LIBS and a Combination of k-Means Clustering and Spatial Raster Analysis. J. Geochem. Explor. 2021, 221, 106697. [Google Scholar] [CrossRef]
- Agterdenbos, J.; van Elteren, J.T.; Bax, D.; Ter Heege, J.P. The Determination of Selenium with Hydride Generation AAS-IV. Spectrochim. Acta Part B At. Spectrosc. 1986, 41, 303–316. [Google Scholar] [CrossRef]
- Macalalad, E.; Bayoran, R.; Ebarvia, B.; Rubeška, I. A Concise Analytical Scheme for 16 Trace Elements in Geochemical Exploration Samples Using Exclusively AAS. J. Geochem. Explor. 1988, 30, 167–177. [Google Scholar] [CrossRef]
- Carrone, G.; Morzan, E.; Candal, R.; Tudino, M. Optimization of Sample Injection in TS-Ff-AAS for Determination of Trace Elements. Microchem. J. 2021, 160, 105608. [Google Scholar] [CrossRef]
- Abd Manan, T.S.; Beddu, S.; Mohamad, D.; Mohd Kamal, N.L.; Wan Mohtar, W.H.; Khan, T.; Jusoh, H.; Sarwono, A.; Ali, M.M.; Che Muda, Z.; et al. Physicochemical and Leaching Properties of Coal Ashes from Malaysian Coal Power Plant. Chem. Phys. Lett. 2021, 769, 138420. [Google Scholar] [CrossRef]
- Iwashita, A.; Nakajima, T.; Takanashi, H.; Ohki, A.; Fujita, Y.; Yamashita, T. Effect of Pretreatment Conditions on the Determination of Major and Trace Elements in Coal Fly Ash Using ICP-AES. Fuel 2006, 85, 257–263. [Google Scholar] [CrossRef]
- Iwashita, A.; Nakajima, T.; Takanashi, H.; Ohki, A.; Fujita, Y.; Yamashita, T. Determination of Trace Elements in Coal and Coal Fly Ash by Joint-Use of ICP-AES and Atomic Absorption Spectrometry. Talanta 2007, 71, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, N.S.; Shaverina, A.V.; Tsygankova, A.R.; Saprykin, A.I. Analysis of High-Purity Germanium Dioxide by ETV-ICP-AES with Preliminary Concentration of Trace Elements. Talanta 2016, 155, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xiong, Y.; Wang, N.; Song, Z.; Yang, J.; Qiu, X.; Zhu, L. Determination of Trace Elements in High-Purity Quartz Samples by ICP-OES and ICP-MS: A Normal-Pressure Digestion Pretreatment Method for Eliminating Unfavorable Substrate Si. Anal. Chim. Acta 2020, 1110, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Arbuzov, S.I.; Chekryzhov, I.Y.u.; Verkhoturov, A.A.; Spears, D.A.; Melkiy, V.A.; Zarubina, N.V.; Blokhin, M.G. Geochemistry and Rare-Metal Potential of Coals of the Sakhalin Coal Basin, Sakhalin Island, Russia. Int. J. Coal Geol. 2023, 268, 104197. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of Trace Elements in Chinese Coals: A Review of Abundances, Genetic Types, Impacts on Human Health, and Industrial Utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, Mineralogy, and Geochemistry of the Ge-Rich Coal from the Wulantuga Ge Ore Deposit, Inner Mongolia, China: New Data and Genetic Implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Palozzi, J.; Bailey, J.G.; Tran, Q.A.; Stanger, R. A Characterization of Rare Earth Elements in Coal Ash Generated during the Utilization of Australian Coals. Int. J. Coal Prep. Util. 2023, 43, 2106–2135. [Google Scholar] [CrossRef]
- Shen, M.; Dai, S.; French, D.; Graham, I.T.; Spiro, B.F.; Wang, N.; Tian, X. Geochemical and Mineralogical Evidence for the Formation of Siderite in Late Permian Coal-Bearing Strata from Western Guizhou, SW China. Chem. Geol. 2023, 637, 121675. [Google Scholar] [CrossRef]
- Wang, N.; Dai, S.; Wang, X.; Nechaev, V.P.; French, D.; Graham, I.T.; Zhao, L.; Song, X. New Insights into the Origin of Middle to Late Permian Volcaniclastics (Nb-Zr-REY-Ga-Rich Horizons) from Eastern Yunnan, SW China. Lithos 2022, 420–421, 106702. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, S.; Wang, X.; Zhao, L.; Nechaev, V.P.; French, D.; Graham, I.T.; Zheng, J.; Wang, Y.; Dong, M. Critical Element (NB-Ta-Zr-Hf-REE-Ga-Th-u) Mineralization in Late Triassic Coals from the Gaosheng Mine, Sichuan Basin, Southwestern China: Coupled Effects of Products of Sediment-Source-Region Erosion and Acidic Water Infiltration. Int. J. Coal Geol. 2022, 262, 104101. [Google Scholar] [CrossRef]
- Raut, N.M.; Huang, L.-S.; Aggarwal, S.K.; Lin, K.-C. Determination of Lanthanides in Rock Samples by Inductively Coupled Plasma Mass Spectrometry Using Thorium as Oxide and Hydroxide Correction Standard. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 809–822. [Google Scholar] [CrossRef]
- Raut, N.M.; Huang, L.-S.; Lin, K.-C.; Aggarwal, S.K. Uncertainty Propagation through Correction Methodology for the Determination of Rare Earth Elements by Quadrupole Based Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2005, 530, 91–103. [Google Scholar] [CrossRef]
- Zawisza, B.; Pytlakowska, K.; Feist, B.; Polowniak, M.; Kita, A.; Sitko, R. Determination of Rare Earth Elements by Spectroscopic Techniques: A Review. J. Anal. At. Spectrom. 2011, 26, 2373–2390. [Google Scholar] [CrossRef]
- Smirnova, E.V.; Mysovskaya, I.N.; Lozhkin, V.I.; Sandimirova, G.P.; Pakhomova, N.N.; Smagunova, A.A. Spectral interferences from polyatomic Barium ions in inductively plasma mass spectrometry. J. Appl. Spectrosc. 2006, 73, 911–917. [Google Scholar] [CrossRef]
- Loges, A.; Wagner, T.; Barth, M.; Bau, M.; Göb, S.; Markl, G. Negative Ce Anomalies in Mn Oxides: The Role of Ce4+ Mobility during Water–Mineral Interaction. Geochim. Cosmochim. Acta 2012, 86, 296–317. [Google Scholar] [CrossRef]
- Longerich, H.P.; Fryer, B.J.; Strong, D.F.; Kantipuly, C.J. Effects of Operating Conditions on the Determination of the Rare Earth Elements by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Spectrochim. Acta Part B At. Spectrosc. 1987, 42, 75–92. [Google Scholar] [CrossRef]
- Vaughan, M.-A.; Horlick, G.; Tan, S.H. Effect of Operating Parameters on Analyte Signals in Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 1987, 2, 765–772. [Google Scholar] [CrossRef]
- Tan, S.H.; Horlick, G. Background Spectral Features in Inductively Coupled Plasma/Mass Spectrometry. Appl. Spectrosc. 1986, 40, 445–460. [Google Scholar] [CrossRef]
- Tan, S.H.; Horlick, G. Matrix-Effect Observations in Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 1987, 2, 745–763. [Google Scholar] [CrossRef]
- Gray, A.L.; Williams, J.G. System Optimisation and the Effect on Polyatomic, Oxide and Doubly Charged Ion Response of a Commercial Inductively Coupled Plasma Mass Spectrometry Instrument. J. Anal. At. Spectrom. 1987, 2, 599–606. [Google Scholar] [CrossRef]
- Shibata, N.; Fudagawa, N.; Kubota, M. Effects of Hydrogen Mixed with Argon Carrier Gas in Electrothermal Vaporization-Inductively Coupled Plasma-Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1992, 47, 505–516. [Google Scholar] [CrossRef]
- Jarvis, K.E.; Gray, A.L.; McCurdy, E. Avoidance of Spectral Interference on Europium in Inductively Coupled Plasma Mass Spectrometry by Sensitive Measurement of the Doubly Charged Ion. J. Anal. At. Spectrom. 1989, 4, 743–747. [Google Scholar] [CrossRef]
- Yan, X.; Dai, S.; Graham, I.T.; He, X.; Shan, K.; Liu, X. Determination of Eu Concentrations in Coal, Fly Ash and Sedimentary Rocks Using a Cation Exchange Resin and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Int. J. Coal Geol. 2018, 191, 152–156. [Google Scholar] [CrossRef]
- Balcaen, L.; Bolea-Fernandez, E.; Resano, M.; Vanhaecke, F. Inductively Coupled Plasma—Tandem Mass Spectrometry (ICP-MS/Ms): A Powerful and Universal Tool for the Interference-Free Determination of (Ultra)Trace Elements—A Tutorial Review. Anal. Chim. Acta 2015, 894, 7–19. [Google Scholar] [CrossRef]
- Rodushkin, I. Multielement Analysis of Coal by ICP Techniques Using Solution Nebulization and Laser Ablation. Talanta 2000, 51, 743–759. [Google Scholar] [CrossRef]
- Dai, S.; Wang, P.; Ward, C.R.; Tang, Y.; Song, X.; Jiang, J.; Hower, J.C.; Li, T.; Seredin, V.V.; Wagner, N.J.; et al. Elemental and Mineralogical Anomalies in the Coal-Hosted Ge Ore Deposit of Lincang, Yunnan, Southwestern China: Key Role of N2–CO2-Mixed Hydrothermal Solutions. Int. J. Coal Geol. 2015, 152, 19–46. [Google Scholar] [CrossRef]
- Wei, Q.; Rimmer, S.M.; Dai, S. Distribution of Trace Elements in Fractions after Micronization and Density-Gradient Centrifugation of High-Ge Coals from the Wulantuga and Lincang Ge Ore Deposits in China. Energy Fuels 2017, 31, 11818–11837. [Google Scholar] [CrossRef]
- Low, F.; Zhang, L. Microwave Digestion for the Quantification of Inorganic Elements in Coal and Coal Ash Using ICP-OES. Talanta 2012, 101, 346–352. [Google Scholar] [CrossRef]
- Dulski, P. Interferences of Oxide, Hydroxide and Chloride Analyte Species in the Determination of Rare Earth Elements in Geological Samples by Inductively Coupled Plasma-Mass Spectrometry. Fresenius’ J. Anal. Chem. 1994, 350, 194–203. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Oxford Press: Oxford, UK, 1985. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).