Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method
Abstract
1. Introduction
2. Methodology for this Review
3. Enhanced Weathering
3.1. Rock Type
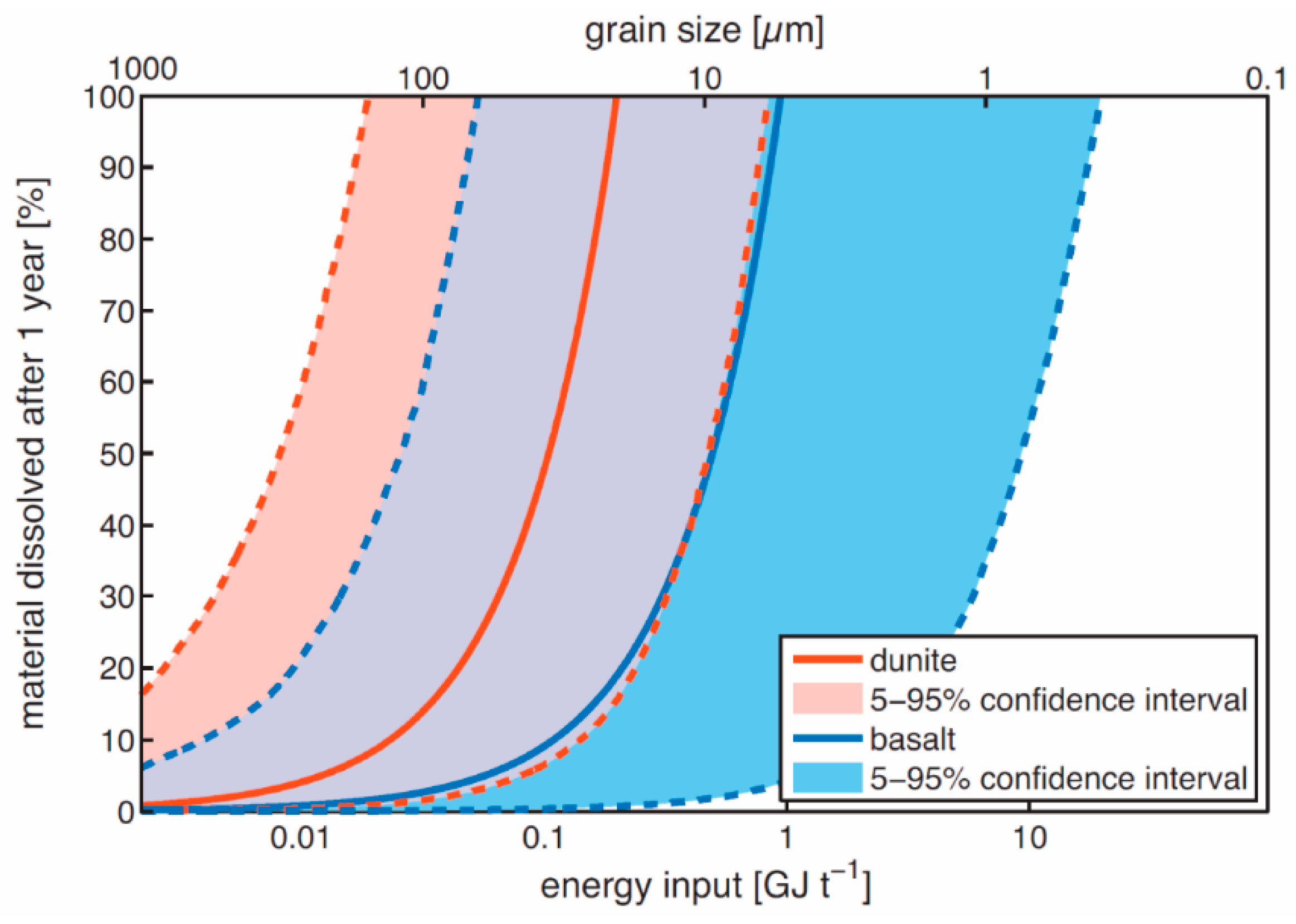
3.2. Particle Size, Shape and Reactive Surface Area
3.3. Spreading Ground Rock
3.4. Mineral Dissolution and Carbon Storage
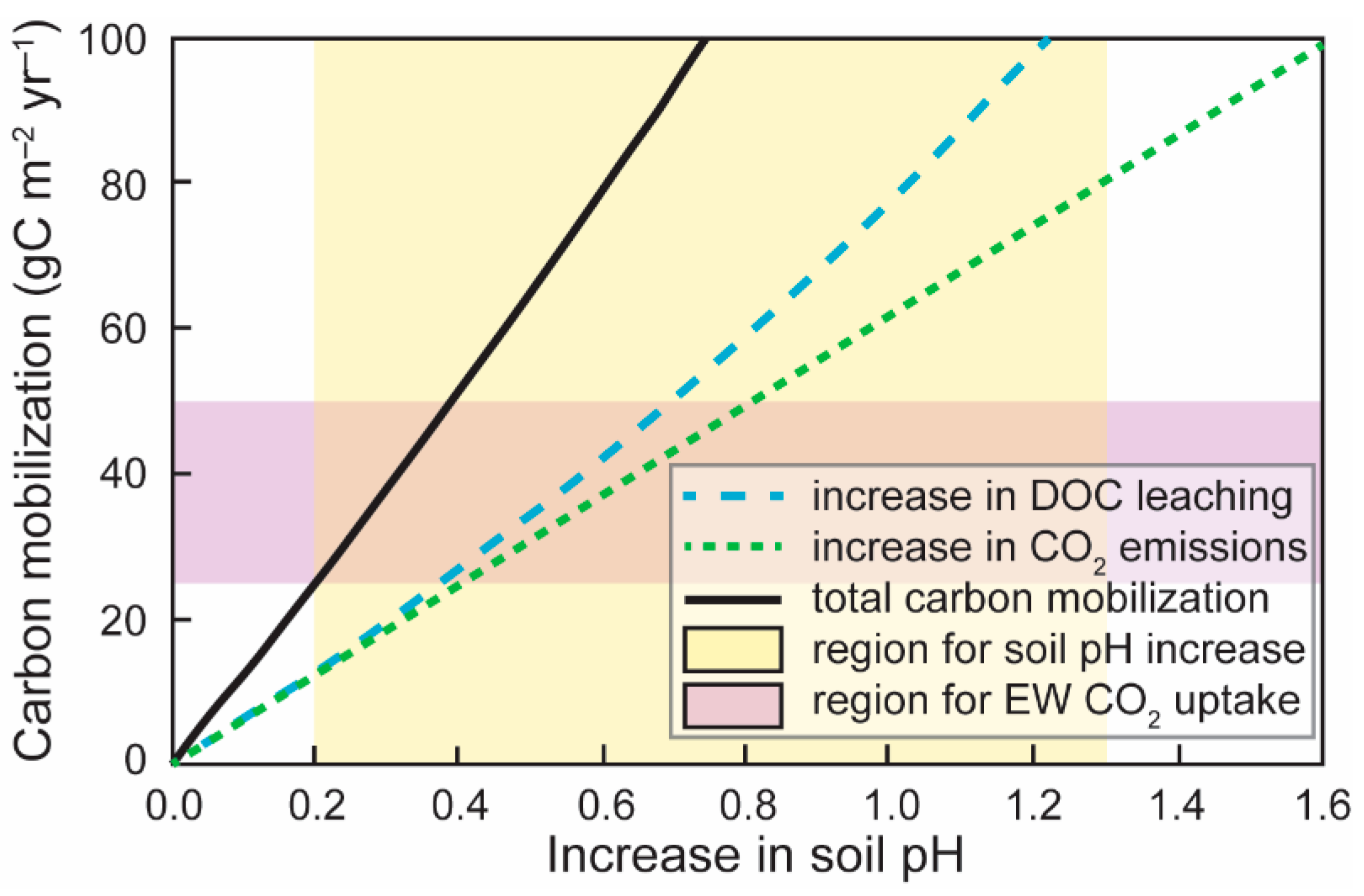
3.5. Acidity
3.6. Feedback Loops
4. Techniques for Implementing Enhanced Weathering
4.1. Implementation Techniques
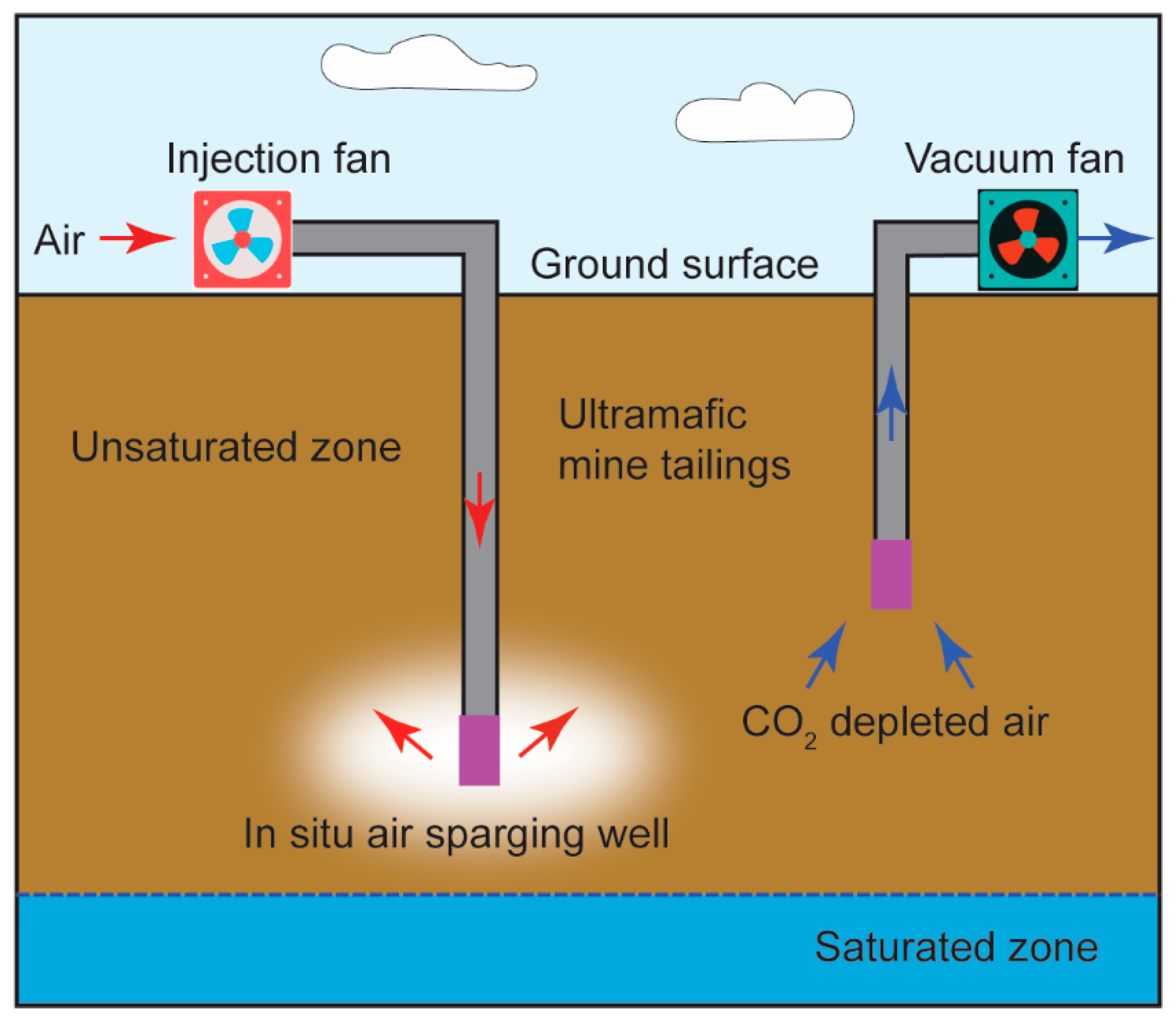
4.2. In Situ and Ex Situ Carbon Dioxide Removal
4.3. Rock Selection for EW Implementation
5. Environmental Benefits of Enhanced Weathering
5.1. Carbon Dioxide Removal
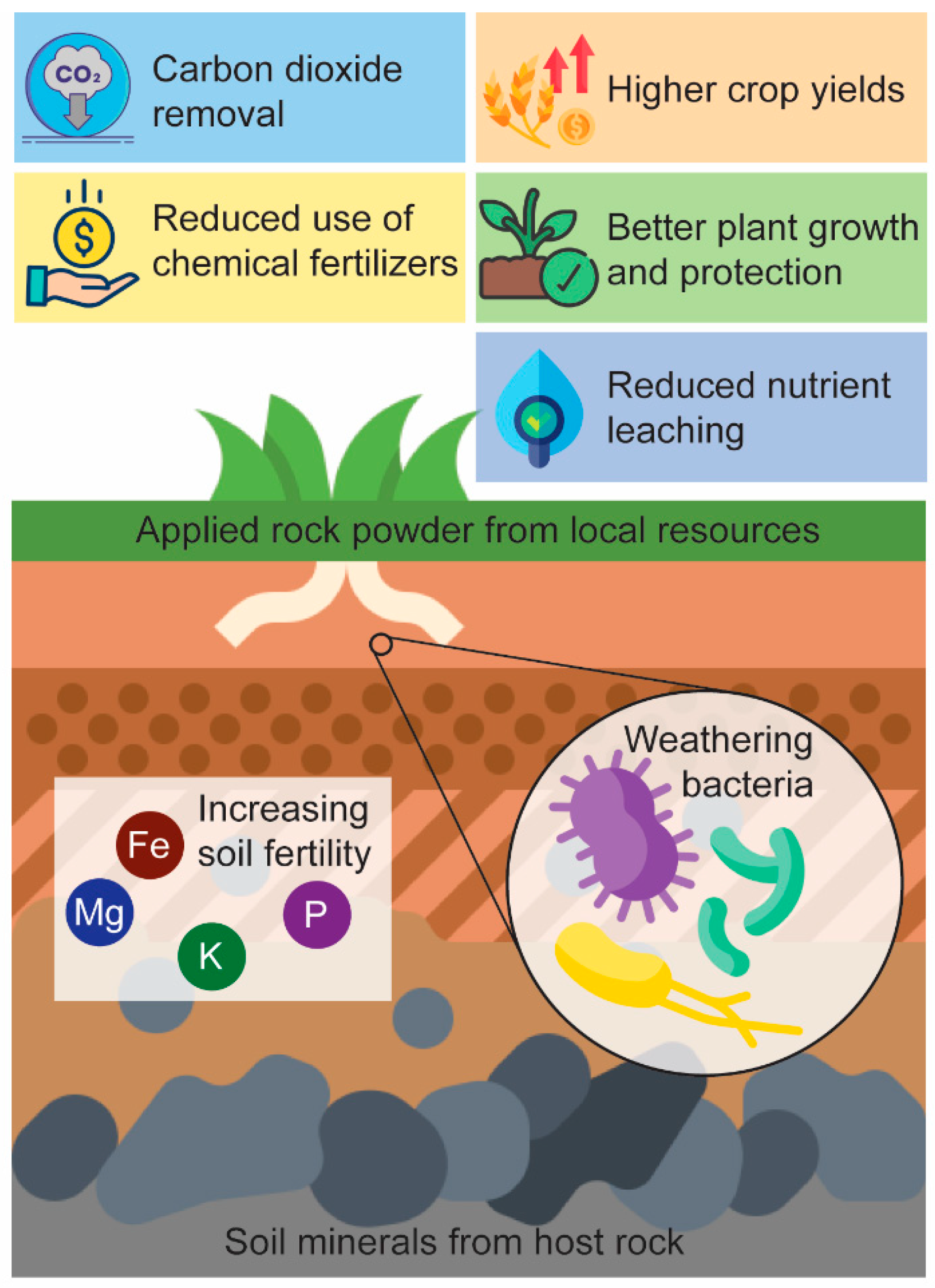
5.2. Soil Fertility
6. Potential Impact on Ecosystems and Biodiversity
6.1. Terrestrial Ecosystems
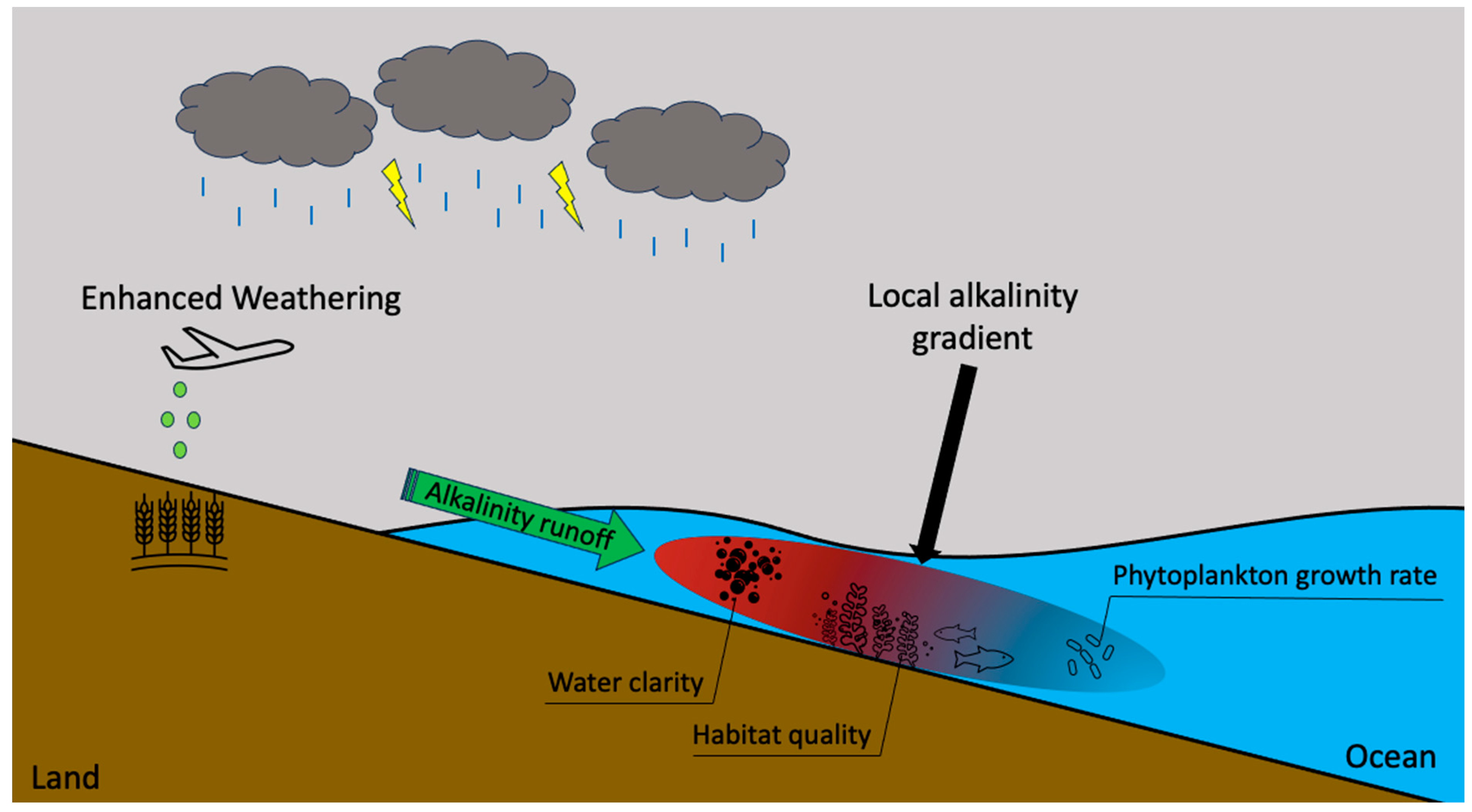
6.2. Marine Ecosystems
7. Water and Air Quality Considerations
7.1. Water and Soil Quality
7.2. Air Quality
8. Other Potential Risks and Challenges
9. Integration with Sustainable Development Goals
10. Conclusions
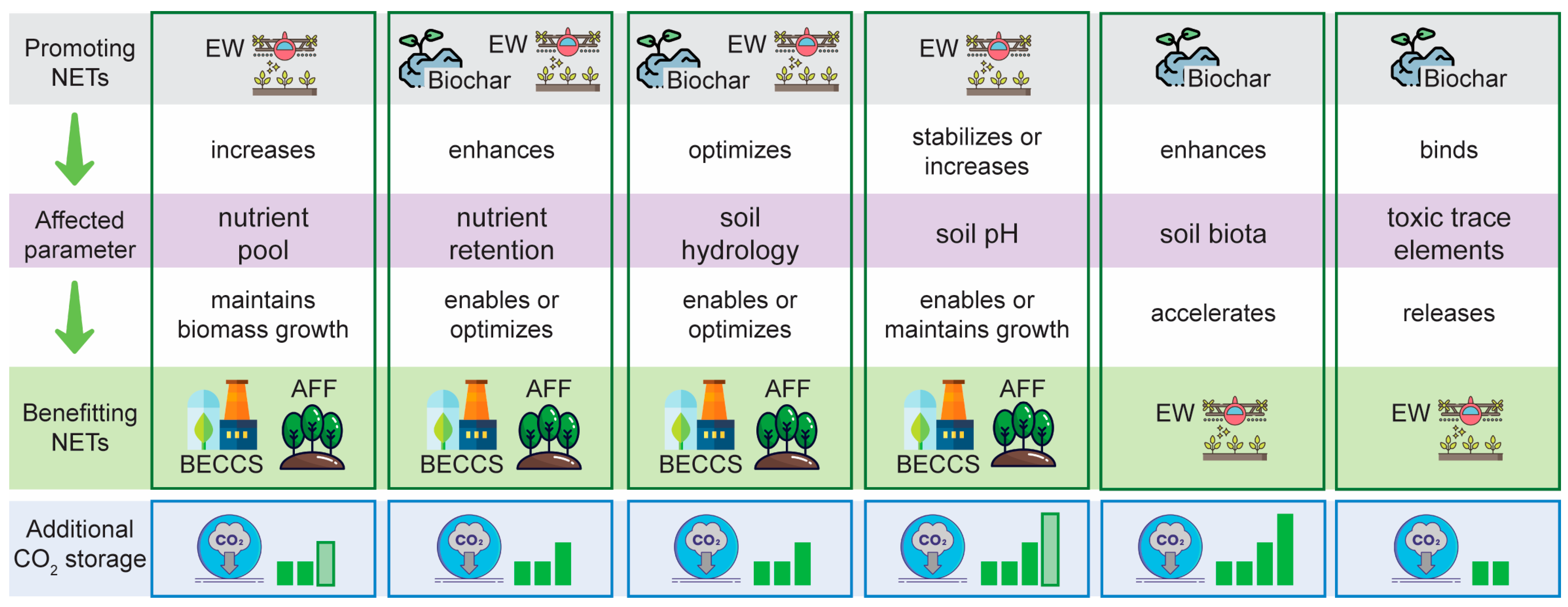
11. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; p. 184. [Google Scholar]
- UNFCCC. The Paris Agreement; UNFCCC: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Cobo, S.; Negri, V.; Valente, A.; Reiner, D.M.; Hamelin, L.; Mac Dowell, N.; Guillen-Gosalbez, G. Sustainable scale-up of negative emissions technologies and practices: Where to focus. Environ. Res. Lett. 2023, 18, 023001. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Krijgsman, P. Enhanced weathering: An effective and cheap tool to sequester CO2. Clim. Chang. 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Köhler, P.; Hartmann, J.; Wolf-Gladrow, D.A. Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. USA 2010, 107, 20228–20233. [Google Scholar] [CrossRef]
- Eufrasio, R.M.; Kantzas, E.P.; Edwards, N.R.; Holden, P.B.; Pollitt, H.; Mercure, J.F.; Koh, S.C.L.; Beerling, D.J. Environmental and health impacts of atmospheric CO2 removal by enhanced rock weathering depend on nations’ energy mix. Commun. Earth Environ. 2022, 3, 106. [Google Scholar] [CrossRef]
- Ng, W.Y.; Low, C.X.; Putra, Z.A.; Aviso, K.B.; Promentilla, M.A.B.; Tan, R.R. Ranking negative emissions technologies under uncertainty. Heliyon 2020, 6, e05730. [Google Scholar] [CrossRef]
- Smith, P.; Davis, S.J.; Creutzig, F.; Fuss, S.; Minx, J.; Gabrielle, B.; Kato, E.; Jackson, R.B.; Cowie, A.; Kriegler, E.; et al. Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Chang. 2016, 6, 42–50. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13, 034010. [Google Scholar] [CrossRef]
- Bullock, L.A.; James, R.H.; Matter, J.; Renforth, P.; Teagle, D.A.H. Global carbon dioxide removal potential of waste materials from metal and diamond mining. Front. Clim. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Renforth, P. The negative emission potential of alkaline materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Rau, G.H.; Willauer, H.D.; Ren, Z.J. The global potential for converting renewable electricity to negative-CO2-emissions hydrogen. Nat. Clim. Chang. 2018, 8, 621–625. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Saharudin, D.M.; Azapagic, A. Environmental sustainability of negative emissions technologies: A review. Sustain. Prod. Consum. 2022, 33, 608–635. [Google Scholar] [CrossRef]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Montserrat, F.; Renforth, P.; Hartmann, J.; Leermakers, M.; Knops, P.; Meysman, F.J.R. Olivine Dissolution in Seawater: Implications for CO2 Sequestration through Enhanced Weathering in Coastal Environments. Environ. Sci. Technol. 2017, 51, 3960–3972. [Google Scholar] [CrossRef]
- Amann, T.; Hartmann, J. Ideas and perspectives: Synergies from co-deployment of negative emission technologies. Biogeosciences 2019, 16, 2949–2960. [Google Scholar] [CrossRef]
- Taylor, L.L.; Quirk, J.; Thorley, R.M.S.; Kharecha, P.A.; Hansen, J.; Ridgwell, A.; Lomas, M.R.; Banwart, S.A.; Beerling, D.J. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Chang. 2016, 6, 402–406. [Google Scholar] [CrossRef]
- Cox, E.; Edwards, N.R. Beyond carbon pricing: Policy levers for negative emissions technologies. Clim. Policy 2019, 19, 1144–1156. [Google Scholar] [CrossRef]
- Zhang, S.; Planavsky, N.J.; Katchinoff, J.; Raymond, P.A.; Kanzaki, Y.; Reershemius, T.; Reinhard, C.T. River chemistry constraints on the carbon capture potential of surficial enhanced rock weathering. Limnol. Oceanogr. 2022, 67, S148–S157. [Google Scholar] [CrossRef]
- Zhuang, W.; Song, X.C.; Liu, M.; Wang, Q.; Song, J.M.; Duan, L.Q.; Li, X.G.; Yuan, H.M. Potential capture and conversion of CO2 from oceanwater through mineral carbonation. Sci. Total Environ. 2023, 867, 161589. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Theodoro, S.H. Enabling food security through use of local rocks and minerals. Extr. Ind. Soc.-Int. J. 2020, 7, 480–487. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. CO2 Sequestration in Chrysotile Mining Residues-Implication of Watering and Passivation under Environmental Conditions. Ind. Eng. Chem. Res. 2012, 51, 8726–8734. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Comparative study of five Quebec ultramafic mining residues for use in direct ambient carbon dioxide mineral sequestration. Chem. Eng. J. 2014, 245, 56–64. [Google Scholar] [CrossRef]
- McCutcheon, J.; Dipple, G.M.; Wilson, S.; Southam, G. Production of magnesium-rich solutions by acid leaching of chrysotile: A precursor to field-scale deployment of microbially enabled carbonate mineral precipitation. Chem. Geol. 2015, 413, 119–131. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Brady, P.V.; Park, A.H.A. Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage. Fuel 2020, 277, 117900. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in situ CO2 Capture and Storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Khudhur, F.W.K.; MacDonald, J.M.; Macente, A.; Daly, L. The utilization of alkaline wastes in passive carbon capture and sequestration: Promises, challenges and environmental aspects. Sci. Total Environ. 2022, 823, 153553. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling; USGS: Reston, VA, USA, 2004; p. 64. [Google Scholar]
- Gras, A.; Beaudoin, G.; Molson, J.; Plante, B. Atmospheric carbon sequestration in ultramafic mining residues and impacts on leachate water chemistry at the Dumont Nickel Project, Quebec, Canada. Chem. Geol. 2020, 546, 119661. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, K.U. Influence of surface passivation and water content on mineral reactions in unsaturated porous media: Implications for brucite carbonation and CO2 sequestration. Geochim. Cosmochim. Acta 2015, 148, 477–495. [Google Scholar] [CrossRef]
- Lechat, K.; Lemieux, J.M.; Molson, J.; Beaudoin, G.; Hebert, R. Field evidence of CO2 sequestration by mineral carbonation in ultramafic milling wastes, Thetford Mines, Canada. Int. J. Greenh. Gas Control 2016, 47, 110–121. [Google Scholar] [CrossRef]
- Dietzen, C.; Rosing, M.T. Quantification of CO2 uptake by enhanced weathering of silicate minerals applied to acidic soils. Int. J. Greenh. Gas Control 2023, 125, 103872. [Google Scholar] [CrossRef]
- Mohammed, S.M.O.; Brandt, K.; Gray, N.D.; White, M.L.; Manning, D.A.C. Comparison of silicate minerals as sources of potassium for plant nutrition in sandy soil. Eur. J. Soil Sci. 2014, 65, 653–662. [Google Scholar] [CrossRef]
- Swoboda, P.; Doring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef] [PubMed]
- Meysman, F.J.R.; Montserrat, F. Negative CO2 emissions via enhanced silicate weathering in coastal environments. Biol. Lett. 2017, 13, 20160905. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, G.; Calabrese, S.; Porporato, A.; Noto, L.V. Effects of precipitation seasonality, irrigation, vegetation cycle and soil type on enhanced weathering—Modeling of cropland case studies across four sites. Biogeosciences 2022, 19, 3877–3896. [Google Scholar] [CrossRef]
- Amann, T.; Hartmann, J.; Struyf, E.; Garcia, W.D.; Fischer, E.K.; Janssens, I.; Meire, P.; Schoelynck, J. EnhancedWeathering and related element fluxes—A cropland mesocosm approach. Biogeosciences 2020, 17, 103–119. [Google Scholar] [CrossRef]
- Blanc-Betes, E.; Kantola, I.B.; Gomez-Casanovas, N.; Hartman, M.D.; Parton, W.J.; Lewis, A.L.; Beerling, D.J.; DeLucia, E.H. In silico assessment of the potential of basalt amendments to reduce N2O emissions from bioenergy crops. Glob. Chang. Biol. Bioenergy 2021, 13, 224–241. [Google Scholar] [CrossRef]
- Buckingham, F.L.; Henderson, G.M.; Holdship, P.; Renforth, P. Soil core study indicates limited CO2 removal by enhanced weathering in dry croplands in the UK. Appl. Geochem. 2022, 147, 105482. [Google Scholar] [CrossRef]
- Dorn, R.I. Assessing biological soil crusts as agents of Ca-Mg silicate dissolution and CO2 sequestration. Phys. Geogr. 2021, 42, 529–541. [Google Scholar] [CrossRef]
- Goll, D.S.; Ciais, P.; Amann, T.; Buermann, W.; Chang, J.F.; Eker, S.; Hartmann, J.; Janssens, I.; Li, W.; Obersteiner, M.; et al. Potential CO2 removal from enhanced weathering by ecosystem responses to powdered rock. Nat. Geosci. 2021, 14, 545–549. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.E.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C-4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Chang. Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
- Lewis, A.L.; Sarkar, B.; Wade, P.; Kemp, S.J.; Hodson, M.E.; Taylor, L.L.; Yeong, K.L.; Davies, K.; Nelson, P.N.; Bird, M.I.; et al. Effects of mineralogy, chemistry and physical properties of basalts on carbon capture potential and plant-nutrient element release via enhanced weathering. Appl. Geochem. 2021, 132, 105023. [Google Scholar] [CrossRef]
- Taylor, L.L.; Driscoll, C.T.; Groffman, P.M.; Rau, G.H.; Blum, J.D.; Beerling, D.J. Increased carbon capture by a silicate-treated forested watershed affected by acid deposition. Biogeosciences 2021, 18, 169–188. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.; Turvey, C.C.; Morgan, B.; Tait, A.W.; McCutcheon, J.; Fallon, S.J.; Southam, G. Carbon accounting of mined landscapes, and deployment of a geochemical treatment system for enhanced weathering at Woodsreef Chrysotile Mine, NSW, Australia. J. Geochem. Explor. 2021, 220, 106655. [Google Scholar] [CrossRef]
- Boschi, C.; Dini, A.; Baneschi, I.; Bedini, F.; Perchiazzi, N.; Cavallo, A. Brucite-driven CO2 uptake in serpentinized dunites (Ligurian Ophiolites, Montecastelli, Tuscany). Lithos 2017, 288, 264–281. [Google Scholar] [CrossRef]
- Blackmore, S.; Vriens, B.; Sorensen, M.; Power, I.M.; Smith, L.; Hallam, S.J.; Mayer, K.U.; Beckie, R.D. Microbial and geochemical controls on waste rock weathering and drainage quality. Sci. Total Environ. 2018, 640, 1004–1014. [Google Scholar] [CrossRef]
- Manning, D.A.C. Mineral stabilities in soils: How minerals can feed the world and mitigate climate change. Clay Miner. 2022, 57, 31–40. [Google Scholar] [CrossRef]
- Ribeiro, I.D.A.; Volpiano, C.G.; Vargas, L.K.; Granada, C.E.; Lisboa, B.B.; Passaglia, L.M.P. Use of Mineral Weathering Bacteria to Enhance Nutrient Availability in Crops: A Review. Front. Plant Sci. 2020, 11, 590774. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of Ultramafic Tailings by Acidithiobacillus spp. for CO2 Sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef]
- Power, I.M.; McCutcheon, J.; Harrison, A.L.; Wilson, S.A.; Dipple, G.M.; Kelly, S.; Southam, C.; Southam, G. Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals 2014, 4, 399–436. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating Mineral Carbonation Using Carbonic Anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef]
- Renforth, P.; Campbell, J.S. The role of soils in the regulation of ocean acidification. Philos. Trans. R. Soc. B-Biol. Sci. 2021, 376, 20200174. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.; Power, I.M.; Shuster, J.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon Sequestration in Biogenic Magnesite and Other Magnesium Carbonate Minerals. Environ. Sci. Technol. 2019, 53, 3225–3237. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.; Thom, J.M.; Dipple, G.M.; Gabites, J.E.; Southam, G. The hydromagnesite playas of Atlin, British Columbia, Canada: A biogeochemical model for CO2 sequestration. Chem. Geol. 2009, 260, 286–300. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Thom, J.M.; Dipple, G.M.; Southam, G. Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada. Geochem. Trans. 2007, 8, 13. [Google Scholar] [CrossRef]
- Harrington, K.J.; Hilton, R.G.; Henderson, G.M. Implications of the Riverine Response to Enhanced Weathering for CO2 removal in the UK. Appl. Geochem. 2023, 152, 105643. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem. Eng. J. 2014, 240, 394–403. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Tooley, C.; Mulders, J.J.P.A.; Renforth, P. The dissolution of olivine added to soil at 4 °C: Implications for enhanced weathering in cold regions. Front. Clim. 2022, 4, 827698. [Google Scholar] [CrossRef]
- Nowamooz, A.; Dupuis, J.C.; Beaudoin, G.; Molson, J.; Lemieux, J.M.; Horswill, M.; Fortier, R.; Larachi, F.; Maldague, X.; Constantin, M.; et al. Atmospheric Carbon Mineralization in an Industrial-Scale Chrysotile Mining Waste Pile. Environ. Sci. Technol. 2018, 52, 8050–8057. [Google Scholar] [CrossRef] [PubMed]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon Mineralization: From Natural Analogues to Engineered Systems. In Geochemistry of Geologic CO2 Sequestration; DePaolo, D.J., Cole, D.R., Navrotsky, A., Bourg, I.C., Eds.; Reviews in Mineralogy & Geochemistry; De Gruyter: Berlin, Germany, 2013; Volume 77, pp. 305–360. [Google Scholar]
- Pronost, J.; Beaudoin, G.; Lemieux, J.M.; Hebert, R.; Constantin, M.; Marcouiller, S.; Klein, M.; Duchesne, J.; Molson, J.W.; Larachi, F.; et al. CO2-depleted warm air venting from chrysotile milling waste (Thetford Mines, Canada): Evidence for in-situ carbon capture from the atmosphere. Geology 2012, 40, 275–278. [Google Scholar] [CrossRef]
- Zarandi, A.E.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Nesquehonite as a carbon sink in ambient mineral carbonation of ultramafic mining wastes. Chem. Eng. J. 2017, 314, 160–168. [Google Scholar] [CrossRef]
- Wilson, S.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Mayer, K.U.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, 25, 121–140. [Google Scholar] [CrossRef]
- Hartmann, J.; West, A.J.; Renforth, P.; Kohler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Durr, H.H.; Scheffran, J. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227, 97–106. [Google Scholar] [CrossRef]
- Monasterio-Guillot, L.; Fernandez-Martinez, A.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Carbonation of calcium-magnesium pyroxenes: Physical-chemical controls and effects of reaction-driven fracturing. Geochim. Cosmochim. Acta 2021, 304, 258–280. [Google Scholar] [CrossRef]
- Baumann, M.; Dittrich, S.; Korner, M.; von Oheimb, G. Liming in spruce stands: What effect does the number of lime applications have on the herb layer? Eur. J. For. Res. 2019, 138, 723–735. [Google Scholar] [CrossRef]
- White, A.F.; Brantley, S.L. The effect of time on the weathering of silicate minerals: Why do weathering rates differ in the laboratory and field? Chem. Geol. 2003, 202, 479–506. [Google Scholar] [CrossRef]
- Ramezanian, A.; Dahlin, A.S.; Campbell, C.D.; Hillier, S.; Oeborn, I. Assessing biogas digestate, pot ale, wood ash and rockdust as soil amendments: Effects on soil chemistry and microbial community composition. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2015, 65, 383–399. [Google Scholar] [CrossRef]
- Romheld, V.; Marschner, H. Genotypical differences among gramineous species in release of phytosiderophores and uptake of iron phytosiderophores. Plant Soil. 1990, 123, 147–153. [Google Scholar] [CrossRef]
- Wild, B.; Gerrits, R.; Bonneville, S. The contribution of living organisms to rock weathering in the critical zone. Npj Mater. Degrad. 2022, 6, 1–16. [Google Scholar] [CrossRef]
- Wood, C.; Harrison, A.L.; Power, I.M. Impacts of dissolved phosphorus and soil-mineral-fluid interactions on CO2 removal through enhanced weathering of wollastonite in soils. Appl. Geochem. 2023, 148, 105511. [Google Scholar] [CrossRef]
- Washbourne, C.L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid Removal of Atmospheric CO2 by Urban Soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.A.C.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Control 2013, 17, 309–317. [Google Scholar] [CrossRef]
- ten Berge, H.F.M.; van der Meer, H.G.; Steenhuizen, J.W.; Goedhart, P.W.; Knops, P.; Verhagen, J. Olivine Weathering in Soil, and Its Effects on Growth and Nutrient Uptake in Ryegrass (Lolium perenne L.): A Pot Experiment. PLoS ONE 2012, 7, e42098. [Google Scholar] [CrossRef]
- Dietzen, C.; Harrison, R.; Michelsen-Correa, S. Effectiveness of enhanced mineral weathering as a carbon sequestration tool and alternative to agricultural lime: An incubation experiment. Int. J. Greenh. Gas Control 2018, 74, 251–258. [Google Scholar] [CrossRef]
- Haque, F.; Chiang, Y.W.; Santos, R.M. Alkaline Mineral Soil Amendment: A Climate Change “Stabilization Wedge”? Energies 2019, 12, 2299. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- Gentile, E.; Tarantola, F.; Lockley, A.; Vivian, C.; Caserini, S. Use of aircraft in ocean alkalinity enhancement. Sci. Total Environ. 2022, 822, 153484. [Google Scholar] [CrossRef]
- Foteinis, S.; Campbell, J.S.; Renforth, P. Life Cycle Assessment of Coastal Enhanced Weathering for Carbon Dioxide Removal from Air. Environ. Sci. Technol. 2023, 57, 6169–6178. [Google Scholar] [CrossRef]
- Fakhraee, M.; Planavsky, N.J.; Reinhard, C.T. Ocean alkalinity enhancement through restoration of blue carbon ecosystems. Nat. Sustain. 2023, 6, 1087–1094. [Google Scholar] [CrossRef]
- Ren, H.W.; Hu, Y.B.; Liu, J.H.; Zhang, Z.; Mou, L.; Pan, Y.N.; Zheng, Q.; Li, G.; Jiao, N.Z. Response of a Coastal Microbial Community to Olivine Addition in the Muping Marine Ranch, Yantai. Front. Microbiol. 2022, 12, 805361. [Google Scholar] [CrossRef]
- Bullock, L.A.; Yang, A.D.; Darton, R.C. Kinetics-informed global assessment of mine tailings for CO2 removal. Sci. Total Environ. 2022, 808, 152111. [Google Scholar] [CrossRef] [PubMed]
- Paulo, C.; Power, I.M.; Stubbs, A.R.; Wang, B.L.; Zeyen, N.; Wilson, S.A. Evaluating feedstocks for carbon dioxide removal by enhanced rock weathering and CO2 mineralization. Appl. Geochem. 2021, 129, 104955. [Google Scholar] [CrossRef]
- Khalidy, R.; Santos, R.M. The fate of atmospheric carbon sequestrated through weathering in mine tailings. Miner. Eng. 2021, 163, 106767. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Bradshaw, P.M.D.; Harrison, A.L. Prospects for CO2 mineralization and enhanced weathering of ultramafic mine tailings from the Baptiste nickel deposit in British Columbia, Canada. Int. J. Greenh. Gas Control 2020, 94, 102895. [Google Scholar] [CrossRef]
- Paulo, C.; Power, I.M.; Zeyen, N.; Wang, B.L.; Wilson, S. Geochemical modeling of CO2 sequestration in ultramafic mine wastes from Australia, Canada, and South Africa: Implications for carbon accounting and monitoring. Appl. Geochem. 2023, 152, 105630. [Google Scholar] [CrossRef]
- Tan, R.R.; Aviso, K.B.; Bandyopadhyay, S.; Foo, D.C.Y.; Klemes, J.J. Circular economy meets the drawdown economy: Enhanced weathering of industrial solid waste as a win-win solution. Resour. Conserv. Recycl. 2022, 178, 106029. [Google Scholar] [CrossRef]
- Aviso, K.B.; Lee, J.Y.; Ubando, A.T.; Tan, R.R. Fuzzy optimization model for enhanced weathering networks using industrial waste. Clean Technol. Environ. Policy 2022, 24, 21–37. [Google Scholar] [CrossRef]
- Jia, X.P.; Zhang, Z.T.; Wang, F.; Li, Z.W.; Wang, Y.T.; Aviso, K.B.; Foo, D.Y.C.; Nair, P.; Tan, R.R.; Wang, F. Regional carbon drawdown with enhanced weathering of non-hazardous industrial wastes. Resour. Conserv. Recycl. 2022, 176, 105910. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, Z.W.; Aviso, K.B.; Tan, R.R.; Wang, F.; Jia, X.P. Multi-period optimization for CO2 sequestration potential of enhanced weathering using non-hazardous industrial wastes. Resour. Conserv. Recycl. 2023, 189, 106766. [Google Scholar] [CrossRef]
- Bullock, L.A.; Alcalde, J.; Tornos, F.; Fernandez-Turiel, J.L. Geochemical carbon dioxide removal potential of Spain. Sci. Total Environ. 2023, 867, 161287. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.R.; Belmonte, B.A.; Benjamin, M.F.D.; Andiappan, V.; Aviso, K.B. Optimization of enhanced weathering networks with alternative transportation modes. Carbon Resour. Convers. 2022, 5, 167–176. [Google Scholar] [CrossRef]
- Snaebjornsdottir, S.O.; Sigfusson, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Rinder, T.; von Hagke, C. The influence of particle size on the potential of enhanced basalt weathering for carbon dioxide removal -Insights from a regional assessment. J. Clean. Prod. 2021, 315, 128178. [Google Scholar] [CrossRef]
- Miller, Q.R.S.; Schaef, H.T.; Kaszuba, J.P.; Gadikota, G.; McGrail, B.P.; Rosso, K.M. Quantitative Review of Olivine Carbonation Kinetics: Reactivity Trends, Mechanistic Insights, and Research Frontiers. Environ. Sci. Technol. Lett. 2019, 6, 431–442. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Declercq, J.; Saldi, G.D.; Gislason, S.R.; Schott, J. Olivine dissolution rates: A critical review. Chem. Geol. 2018, 500, 1–19. [Google Scholar] [CrossRef]
- Renforth, P. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 2012, 10, 229–243. [Google Scholar] [CrossRef]
- Li, J.J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a high-speed stirred mill for mineral carbonation. Int. J. Miner. Metall. Mater. 2015, 22, 1005–1016. [Google Scholar] [CrossRef]
- Li, J.J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a planetary mill for mineral carbonation. Int. J. Miner. Process. 2017, 158, 18–26. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Ioannou, I.; Delimitis, A.; Efstathiou, A.M.; Kyratsi, T. Ball Milling Effect on the CO2 Uptake of Mafic and Ultramafic Rocks: A Review. Geosciences 2018, 8, 406. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Vasiliades, M.A.; Petallidou, K.C.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. A method to enhance the CO2 storage capacity of pyroxenitic rocks. Greenh. Gases-Sci. Technol. 2015, 5, 577–591. [Google Scholar] [CrossRef]
- Stillings, M.; Shipton, Z.K.; Lunn, R.J. Mechanochemical processing of silicate rocks to trap CO2. Nat. Sustain. 2023, 6, 780–788. [Google Scholar] [CrossRef]
- Kantzas, E.P.; Martin, M.V.; Lomas, M.R.; Eufrasio, R.M.; Renforth, P.; Lewis, A.L.; Taylor, L.L.; Mecure, J.F.; Pollitt, H.; Vercoulen, P.V.; et al. Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nat. Geosci. 2022, 15, 382–389. [Google Scholar] [CrossRef]
- Lefebvre, D.; Goglio, P.; Williams, A.; Manning, D.A.C.; de Azevedo, A.C.; Bergmann, M.; Meersmans, J.; Smith, P. Assessing the potential of soil carbonation and enhanced weathering through Life Cycle Assessment: A case study for Sao Paulo State, Brazil. J. Clean. Prod. 2019, 233, 468–481. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Kroeger, J.; Planavsky, N.; Yao, Y. Techno-Economic and Life Cycle Assessment of Enhanced Rock Weathering: A Case Study from the Midwestern United States. Environ. Sci. Technol. 2023, 57, 13828–13837. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Gomari, S.R.; Hughes, D.; Sidiq, A.; Shifa, F. Process-based life cycle assessment of waste clay for mineral carbonation and enhanced weathering: A case study for northeast England, UK. J. Clean. Prod. 2023, 424, 138914. [Google Scholar] [CrossRef]
- Vakilifard, N.; Kantzas, E.P.; Edwards, N.R.; Holden, P.B.; Beerling, D.J. The role of enhanced rock weathering deployment with agriculture in limiting future warming and protecting coral reefs. Environ. Res. Lett. 2021, 16, 094005. [Google Scholar] [CrossRef]
- Gillman, G.P.; Burkett, D.C.; Coventry, R.J. Amending highly weathered soils with finely ground basalt rock. Appl. Geochem. 2002, 17, 987–1001. [Google Scholar] [CrossRef]
- Dong, H.L.; Huang, L.Q.; Zhao, L.D.; Zeng, Q.; Liu, X.L.; Sheng, Y.Z.; Shi, L.; Wu, G.; Jiang, H.C.; Li, F.R.; et al. A critical review of mineral-microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, nwac128. [Google Scholar] [CrossRef] [PubMed]
- Busato, J.G.; dos Santos, L.F.; de Paula, A.M.; Sodre, F.F.; de Oliveira, A.L.; Dobbss, L.B.; Martins, E.D.; Jindo, K. Can co-application of silicate rock powder and humic-like acids increase nutrient uptake and plant growth in weathered tropical soil? Acta Agric. Scand. Sect. B-Soil Plant Sci. 2022, 72, 761–774. [Google Scholar] [CrossRef]
- Luchese, A.V.; Leite, I.J.; Giaretta, A.P.; Alves, M.L.; Missio, R.F. Use of quarry waste basalt rock powder as a soil remineralizer to grow soybean and maize. Heliyon 2023, 9, e14050. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, L.T.; Silva, G.N.; Holsback, H.M.S.; Oliveira, C.D.; Marcante, N.C.; Martins, E.D.; Santos, F.L.D.; Santos, E.F. Potential of basalt dust to improve soil fertility and crop nutrition. J. Agric. Food Res. 2022, 10, 100443. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Oliveira, M.L.S.; Oliveira, L.F.S.; Schneider, I.A.H.; Kautzmann, R.M. Application of andesite rock as a clean source of fertilizer for eucalyptus crop: Evidence of sustainability. J. Clean. Prod. 2020, 256, 120432. [Google Scholar] [CrossRef]
- Jariwala, H.; Haque, F.; Vanderburgt, S.; Santos, R.M.; Chiang, Y.W. Mineral-Soil-Plant-Nutrient Synergisms of Enhanced Weathering for Agriculture: Short-Term Investigations Using Fast-Weathering Wollastonite Skarn. Front. Plant Sci. 2022, 13, 929457. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Maity, A.; Chari, M.S.; Banerjee, A.; Biswas, D.R. Low-grade silicate minerals as value-added natural potash fertilizer in deeply weathered tropical soil. Geoderma 2023, 433, 116433. [Google Scholar] [CrossRef]
- Guo, F.X.; Wang, Y.P.; Zhu, H.Y.; Zhang, C.Y.; Sun, H.W.; Fang, Z.L.; Yang, J.; Zhang, L.S.; Mu, Y.; Man, Y.B.; et al. Crop productivity and soil inorganic carbon change mediated by enhanced rock weathering in farmland: A comparative field analysis of multi-agroclimatic regions in central China. Agric. Syst. 2023, 210, 103691. [Google Scholar] [CrossRef]
- Van Straaten, P. Farming with rocks and minerals: Challenges and opportunities. An. Acad. Bras. Cienc. 2006, 78, 731–747. [Google Scholar] [CrossRef]
- Daniell, A.; van Tonder, D.M. Opportunity for Increasing the Soil Quality of Non-arable and Depleted Soils in South Africa: A Review. J. Soil. Sci. Plant Nutr. 2023, 23, 2476–2487. [Google Scholar] [CrossRef]
- Santos, R.M.; Araujo, F.; Jariwala, H.; Khalidy, R.; Haque, F.; Chiang, Y.W. Pathways, roundabouts, roadblocks, and shortcuts to safe and sustainable deployment of enhanced rock weathering in agriculture. Front. Earth Sci. 2023, 11, 1215930. [Google Scholar] [CrossRef]
- Nunes, J.M.G.; Kautzmann, R.M.; Oliveira, C. Evaluation of the natural fertilizing potential of basalt dust wastes from the mining district of Nova Prata (Brazil). J. Clean. Prod. 2014, 84, 649–656. [Google Scholar] [CrossRef]
- Anda, M.; Shamshuddin, J.; Fauziah, C.I. Improving chemical properties of a highly weathered soil using finely ground basalt rocks. Catena 2015, 124, 147–161. [Google Scholar] [CrossRef]
- Gillman, G.P.; Burkett, D.C.; Coventry, R.J. A laboratory study of application of basalt dust to highly weathered soils: Effect on soil cation chemistry. Aust. J. Soil Res. 2001, 39, 799–811. [Google Scholar] [CrossRef]
- Farhadi-Machekposhti, M.; Valdes-Abellan, J.; Pla, C.; Benavente, D.; Pachepsky, Y. Impact of marble powder amendment on hydraulic properties of a sandy soil. Int. Agrophysics 2020, 34, 223–232. [Google Scholar] [CrossRef]
- Garcia, W.D.; Amann, T.; Hartmann, J.; Karstens, K.; Popp, A.; Boysen, L.R.; Smith, P.; Goll, D. Impacts of enhanced weathering on biomass production for negative emission technologies and soil hydrology. Biogeosciences 2020, 17, 2107–2133. [Google Scholar] [CrossRef]
- Horn, R.; Taubner, H.; Wuttke, M.; Baumgartl, T. Soil physical-properties related to soil-structure. Soil Tillage Res. 1994, 30, 187–216. [Google Scholar] [CrossRef]
- Colombi, T.; Braun, S.; Keller, T.; Walter, A. Artificial macropores attract crop roots and enhance plant productivity on compacted soils. Sci. Total Environ. 2017, 574, 1283–1293. [Google Scholar] [CrossRef]
- Moosdorf, N.; Renforth, P.; Hartmann, J. Carbon Dioxide Efficiency of Terrestrial Enhanced Weathering. Environ. Sci. Technol. 2014, 48, 4809–4816. [Google Scholar] [CrossRef]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H.; et al. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef]
- Futa, B.; Kraska, P.; Andruszczak, S.; Gierasimiuk, P.; Jaroszuk-Sierocinska, M. Impact of Subsurface Application of Compound Mineral Fertilizer on Soil Enzymatic Activity under Reduced Tillage. Agronomy 2021, 11, 2213. [Google Scholar] [CrossRef]
- Romero, F.; Hilfiker, S.; Edlinger, A.; Held, A.; Hartman, K.; Labouyrie, M.; van der Heijden, M.G.A. Soil microbial biodiversity promotes crop productivity and agro-ecosystem functioning in experimental microcosms. Sci. Total Environ. 2023, 885, 163683. [Google Scholar] [CrossRef] [PubMed]
- Addison, S.L.; Smaill, S.J.; Garrett, L.G.; Wakelin, S.A. Effects of forest harvest and fertiliser amendment on soil biodiversity and function can persist for decades. Soil Biol. Biochem. 2019, 135, 194–205. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. ECOLOGY Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, W.; Velescu, A.; Leimer, S.; Bigalke, M.; Boy, J.; Valarezo, C. Temporal Trends of Phosphorus Cycling in a Tropical Montane Forest in Ecuador During 14 Years. J. Geophys. Res.-Biogeosci. 2019, 124, 1370–1386. [Google Scholar] [CrossRef]
- Crawford, K.M.; Busch, M.H.; Locke, H.; Luecke, N.C. Native soil microbial amendments generate trade-offs in plant productivity, diversity, and soil stability in coastal dune restorations. Restor. Ecol. 2020, 28, 328–336. [Google Scholar] [CrossRef]
- Gremer, J.R.; Andrews, C.; Norris, J.R.; Thomas, L.P.; Munson, S.M.; Duniway, M.C.; Bradford, J.B. Increasing temperature seasonality may overwhelm shifts in soil moisture to favor shrub over grass dominance in Colorado Plateau drylands. Oecologia 2018, 188, 1195–1207. [Google Scholar] [CrossRef]
- Lange, M.; Habekost, M.; Eisenhauer, N.; Roscher, C.; Bessler, H.; Engels, C.; Oelmann, Y.; Scheu, S.; Wilcke, W.; Schulze, E.D.; et al. Biotic and Abiotic Properties Mediating Plant Diversity Effects on Soil Microbial Communities in an Experimental Grassland. PLoS ONE 2014, 9, e96182. [Google Scholar] [CrossRef]
- Fischer, C.; Tischer, J.; Roscher, C.; Eisenhauer, N.; Ravenek, J.; Gleixner, G.; Attinger, S.; Jensen, B.; de Kroon, H.; Mommer, L.; et al. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil. 2015, 397, 1–16. [Google Scholar] [CrossRef]
- Bach, L.T.; Gill, S.J.; Rickaby, R.E.M.; Gore, S.; Renforth, P. CO2 removal with enhanced weathering and ocean alkalinity enhacement: Potential risks and co-benefits for marine pelagic ecosystems. Front. Clim. 2019, 1, 7. [Google Scholar] [CrossRef]
- Ferderer, A.; Chase, Z.; Kennedy, F.; Schulz, K.G.; Bach, L.T. Assessing the influence of ocean alkalinity enhancement on a coastalphytoplankton community. Biogeosciences 2022, 19, 5375–5399. [Google Scholar] [CrossRef]
- Guo, J.Y.A.; Strzepek, R.; Willis, A.; Ferderer, A.; Bach, L.T. Investigating the effect of nickel concentration on phytoplankton growth to assess potential side-effects of ocean alkalinity enhancement. Biogeosciences 2022, 19, 3683–3697. [Google Scholar] [CrossRef]
- Flipkens, G.; Blust, R.; Town, R.M. Deriving Nickel (Ni(II)) and Chromium (Cr(III)) Based Environmentally Safe Olivine Guidelines for Coastal Enhanced Silicate Weathering. Environ. Sci. Technol. 2021, 55, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Park, H.J.; Cai, Y.J.; Chang, S.X. Environmental Risks in Atmospheric CO2 Removal Using Enhanced Rock Weathering Are Overlooked. Environ. Sci. Technol. 2021, 55, 9627–9629. [Google Scholar] [CrossRef]
- Mustafa, A.; Zulfiqar, U.; Mumtaz, M.Z.; Radziemska, M.; Haider, F.U.; Holatko, J.; Hammershmiedt, T.; Naveed, M.; Ali, H.; Kintl, A.; et al. Nickel (Ni) phytotoxicity and detoxification mechanisms: A review. Chemosphere 2023, 328, 138574. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.; Morgan, B.; Turvey, C.C.; Paterson, D.J.; Jowitt, S.M.; McCutcheon, J.; Southam, G. Fate of transition metals during passive carbonation of ultramafic mine tailings via air capture with potential for metal resource recovery. Int. J. Greenh. Gas Control 2018, 71, 155–167. [Google Scholar] [CrossRef]
- Suhrhoff, T.J. Phytoprevention of Heavy Metal Contamination From Terrestrial Enhanced Weathering: Can Plants Save the Day? Front. Clim. 2022, 3, 820204. [Google Scholar] [CrossRef]
- Dupla, X.; Moller, B.; Baveye, P.C.; Grand, S. Potential accumulation of toxic trace elements in soils during enhanced rock weathering. Eur. J. Soil Sci. 2023, 74, e13343. [Google Scholar] [CrossRef]
- Vienne, A.; Poblador, S.; Portillo-Estrada, M.; Hartmann, J.; Ijiehon, S.; Wade, P.; Vicca, S. Enhanced weathering using basalt rock powder: Carbon sequestration, co-benefits and risks in a mesocosm study with Solanum tuberosum. Front. Clim. 2022, 4, 869456. [Google Scholar] [CrossRef]
- Calabrese, S.; Wild, B.; Bertagni, M.B.; Bourg, I.C.; White, C.; Aburto, F.; Cipolla, G.; Noto, L.V.; Porporato, A. Nano- to Global-Scale Uncertainties in Terrestrial Enhanced Weathering. Environ. Sci. Technol. 2022, 56, 15261–15272. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, R.; Mishra, K.K.; Pandey, J.K.; Udayabhanu, G.N.; Bandopadhyay, A.K. Determination of quartz and its abundance in respirable airborne dust in both coal and metal mines in India. In Proceedings of the 1st International Symposium on Mine Safety Science and Engineering (ISMSSE), Beijing, China, 26–29 October 2011. [Google Scholar]
- Mwaanga, P.; Silondwa, M.; Kasali, G.; Banda, P.M. Preliminary review of mine air pollution in Zambia. Heliyon 2019, 5, e02485. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Paluchamy, B.; Mishra, D.P.; Panigrahi, D.C.; Klemes, J.J. Airborne respirable dust in fully mechanised underground metalliferous mines e Generation, health impacts and control measures for cleaner production. J. Clean. Prod. 2021, 296, 126524. [Google Scholar] [CrossRef]
- Larkin, C.S.; Andrews, M.G.; Pearce, C.R.; Yeong, K.L.; Beerling, D.J.; Bellamy, J.; Benedick, S.; Freckleton, R.P.; Goring-Harford, H.; Sadekar, S.; et al. Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia. Front. Clim. 2022, 4, 959229. [Google Scholar] [CrossRef]
- Klemme, A.; Rixen, T.; Muller, M.; Notholt, J.; Warneke, T. Destabilization of carbon in tropical peatlands by enhanced weathering. Commun. Earth Environ. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Rickels, W.; Proelss, A.; Geden, O.; Burhenne, J.; Fridahl, M. Integrating Carbon Dioxide Removal Into European Emissions Trading. Front. Clim. 2021, 3, 690023. [Google Scholar] [CrossRef]
- Spence, E.; Cox, E.; Pidgeon, N. Exploring cross-national public support for the use of enhanced weathering as a land-based carbon dioxide removal strategy. Clim. Chang. 2021, 165, 1–18. [Google Scholar] [CrossRef]
- Pidgeon, N.F.; Spence, E. Perceptions of enhanced weathering as a biological negative emissions option. Biol. Lett. 2017, 13, 20170024. [Google Scholar] [CrossRef]
- Cox, E.; Spence, E.; Pidgeon, N. Deliberating enhanced weathering: Public frames, iconic ecosystems and the governance of carbon removal at scale. Public Underst. Sci. 2022, 31, 960–977. [Google Scholar] [CrossRef]
- Smith, P.; Adams, J.; Beerling, D.J.; Beringer, T.; Calvin, K.V.; Fuss, S.; Griscom, B.; Hagemann, N.; Kammann, C.; Kraxner, F.; et al. Land-Management Options for Greenhouse Gas Removal and Their Impacts on Ecosystem Services and the Sustainable Development Goals. Annu. Rev. Environ. Resour. 2019, 44, 255–286. [Google Scholar] [CrossRef]
- West, T.O.; McBride, A.C. The contribution of agricultural lime to carbon dioxide emissions in the United States: Dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 2005, 108, 145–154. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]




| Mineral Family | Mineral | Formula | Release | Typical Content (wt%) | Dissolution Rate Constant |
|---|---|---|---|---|---|
| Feldspatoid | Nepheline | (Na,K)AlSiO4 | K | 4.2 | –2.73 |
| Plagioclase | Anorthite | CaAl2Si2O8 | Ca | 13.6 | –3.50 |
| Mica | Glauconite | K(Fe3+,Al,Mg)2(Si,Al)4O10(OH)2 | K, Mg | 7.5, 3.0 | –4.80 |
| Pyroxene | Wollastonite | CaSiO3 | Ca | 33.6 | –5.37 |
| Feldspatoid | Leucite | KAlSi2O6 | K | 17.4 | –6.00 |
| Pyroxene | Diopside | CaMgSi2O6 | Ca, Mg | 18.6 | –6.36 |
| Tourmaline | Dravite | NaMg3Al6B3Si6O30(OH) | Mg, B | 7.8, 3.5 | –6.50 |
| Olivine | Forsterite | Mg2SiO4 | Mg | 33.6 | –6.85 |
| Amphibole | Hornblende | Ca2(Mg,Fe)4Al[Si2AlO22](OH)2 | Ca, Mg | 8.6, 7.8 | –7.00 |
| Pyroxene | Enstatite | Mg2Si2O6 | Mg | 35.0 | –9.02 |
| Mica | Biotite | K(Fe,Mg)3AlSi3O10(OH)2 | K, Mg | 7.5, 3.6 | –9.84 |
| K-feldspar | Orthoclase | KAlSi3O8 | K | 14.1 | –10.06 |
| Plagioclase | Albite | NaAlSi3O8 | Na | 8.7 | –10.16 |
| Mica | Muscovite | KAl3Si3O10(OH)2 | K | 9.1 | –11.85 |
| Material | Scale | Plant Presence | Dosage (t/ha) | CO2 Capture Metric | Ton CO2/ha/yr | Reference |
|---|---|---|---|---|---|---|
| Basalt | Mesocosm | Yes | 100 | Mg balance | 3.01 | [46] |
| Concrete | Field | No | Not stated | SIC | 85 | [78] |
| Dolerite | Field | Yes | Not stated | SIC | 17.6 | [79] |
| Olivine | Pot | Yes | 204 | Mg balance | 2.69 | [80] |
| Olivine | Mesocosm | Yes | 220 | Mg balance | 0.05 | [41] |
| Olivine | Column | No | 50 | Mg balance | 4.16 | [81] |
| Olivine | Column | No | 127 | Mg balance | 0.30 | [63] |
| Wollastonite | Pot | Yes | 221 | SIC | 39.3 | [82] |
| Wollastonite | Field | Yes | 1.25–5.0 | SIC | 0.28–2.4 | [83] |
| Wollastonite | Watershed | Yes | 3.44 | Ca balance | 0.77 | [48] |
| Wollastonite | Column | No | 221 | Si, Ca, HCO3− | 24.5–52.9 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandeginste, V.; Lim, C.; Ji, Y. Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method. Minerals 2024, 14, 75. https://doi.org/10.3390/min14010075
Vandeginste V, Lim C, Ji Y. Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method. Minerals. 2024; 14(1):75. https://doi.org/10.3390/min14010075
Chicago/Turabian StyleVandeginste, Veerle, Carl Lim, and Yukun Ji. 2024. "Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method" Minerals 14, no. 1: 75. https://doi.org/10.3390/min14010075
APA StyleVandeginste, V., Lim, C., & Ji, Y. (2024). Exploratory Review on Environmental Aspects of Enhanced Weathering as a Carbon Dioxide Removal Method. Minerals, 14(1), 75. https://doi.org/10.3390/min14010075







