Unlocking Strategic and Critical Raw Materials: Assessment of Zinc and REEs Enrichment in Tailings and Zn-Carbonate in a Historical Mining Area (Montevecchio, SW Sardinia)
Abstract
:1. Introduction
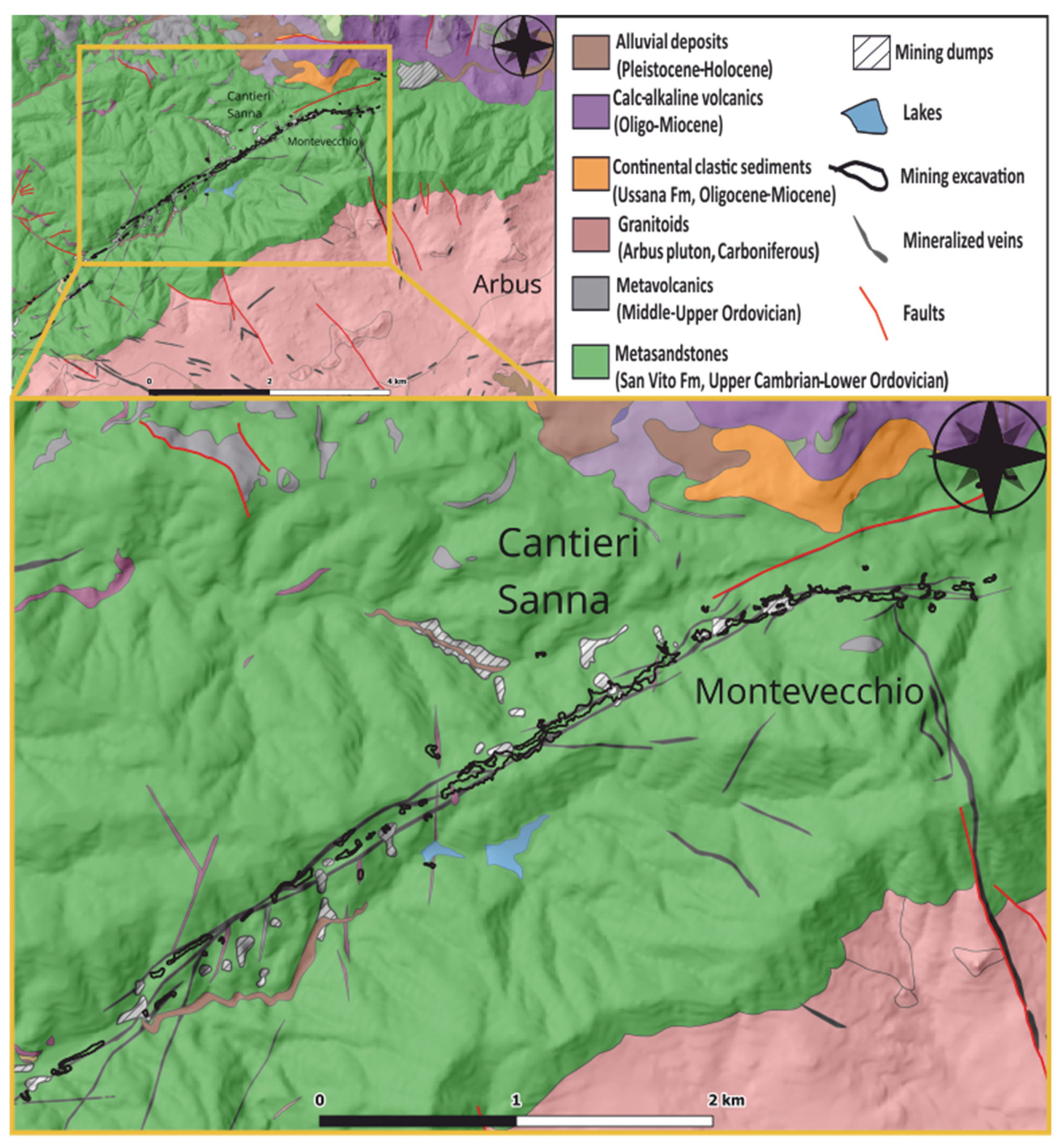
2. Geological Setting
3. The Montevecchio Ore Deposits
The Sanna Vein
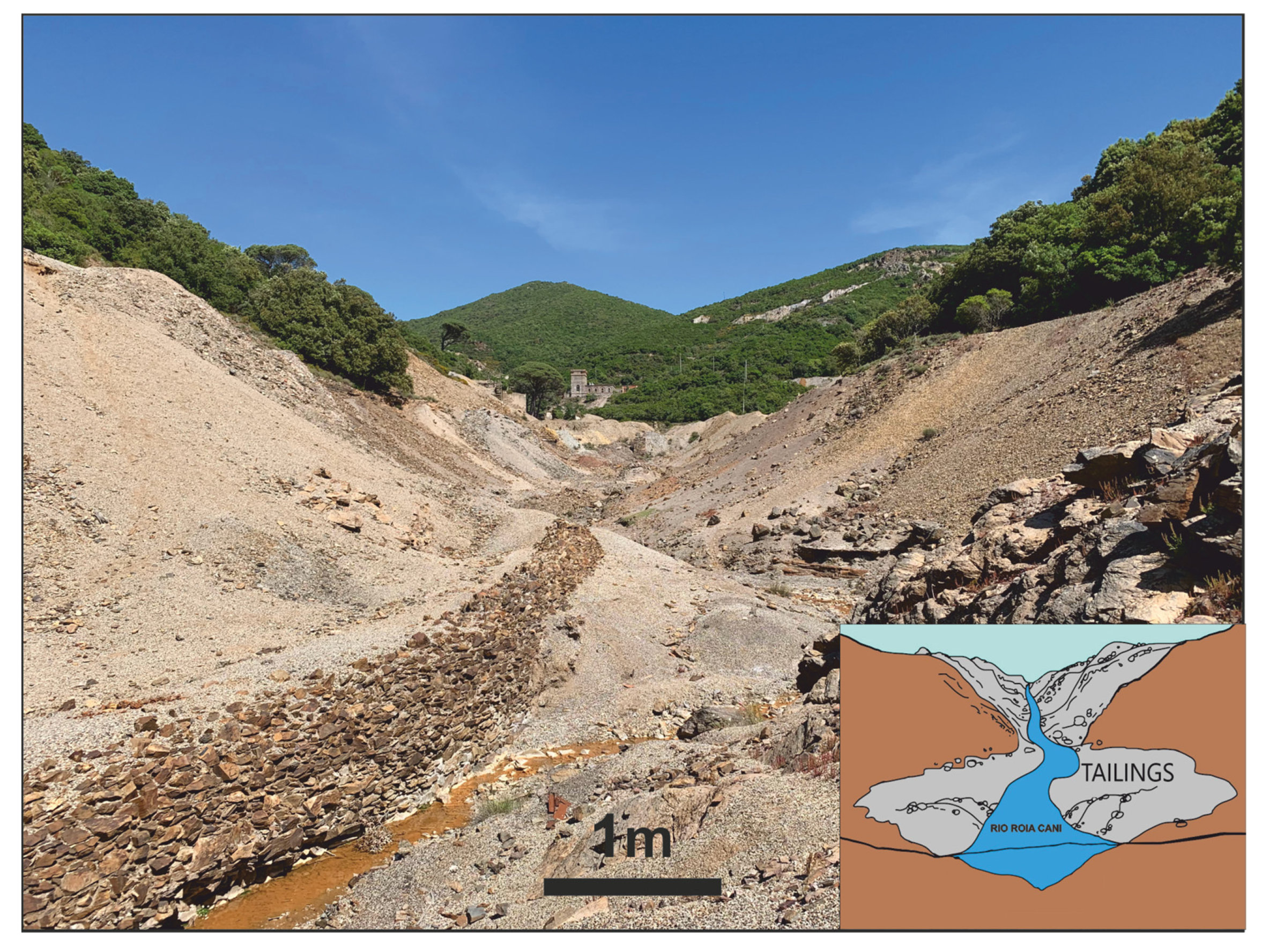
4. The Sanna Plant Area
5. Materials and Methods
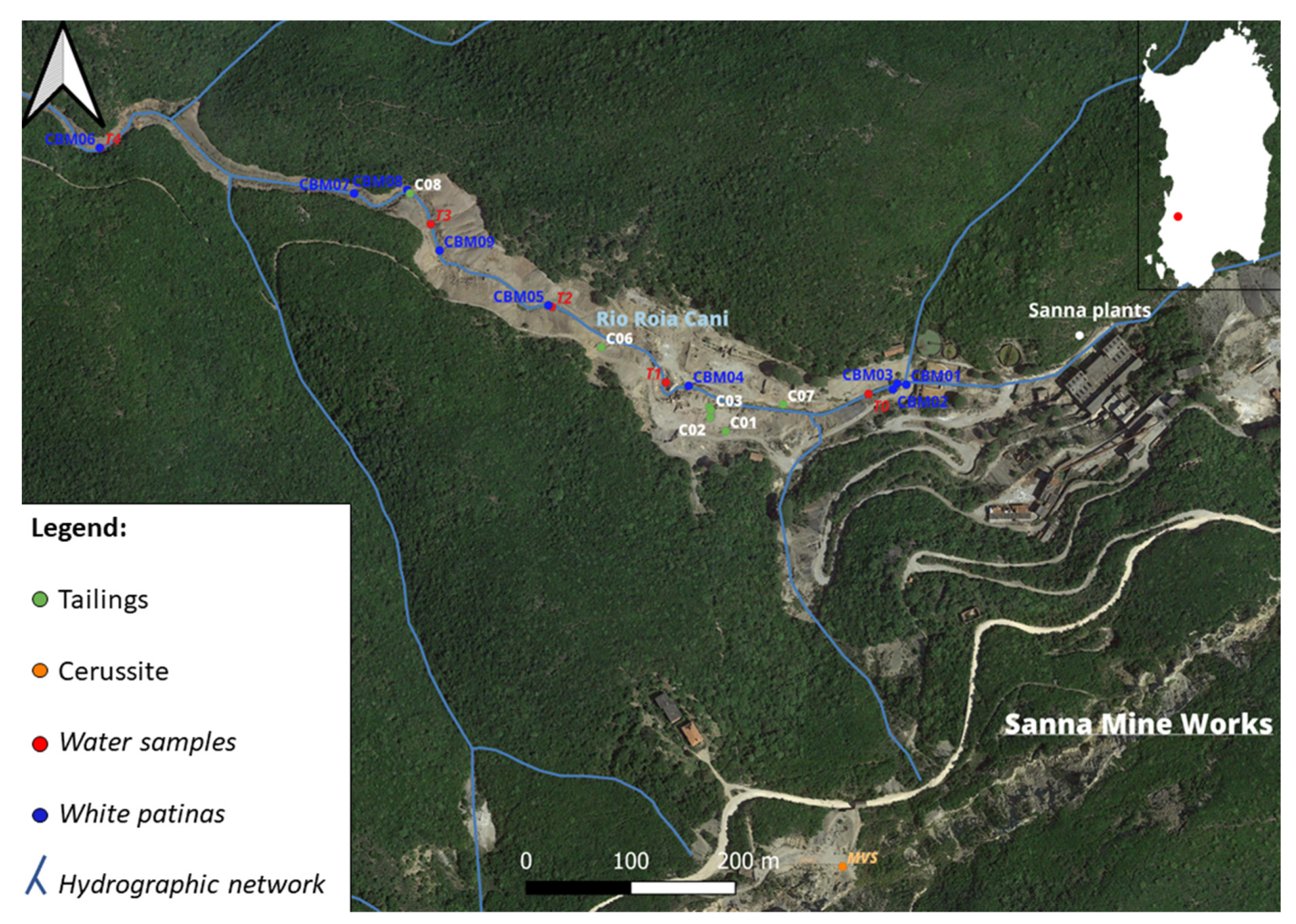
5.1. Sampling
5.2. X-ray Diffraction Analyses
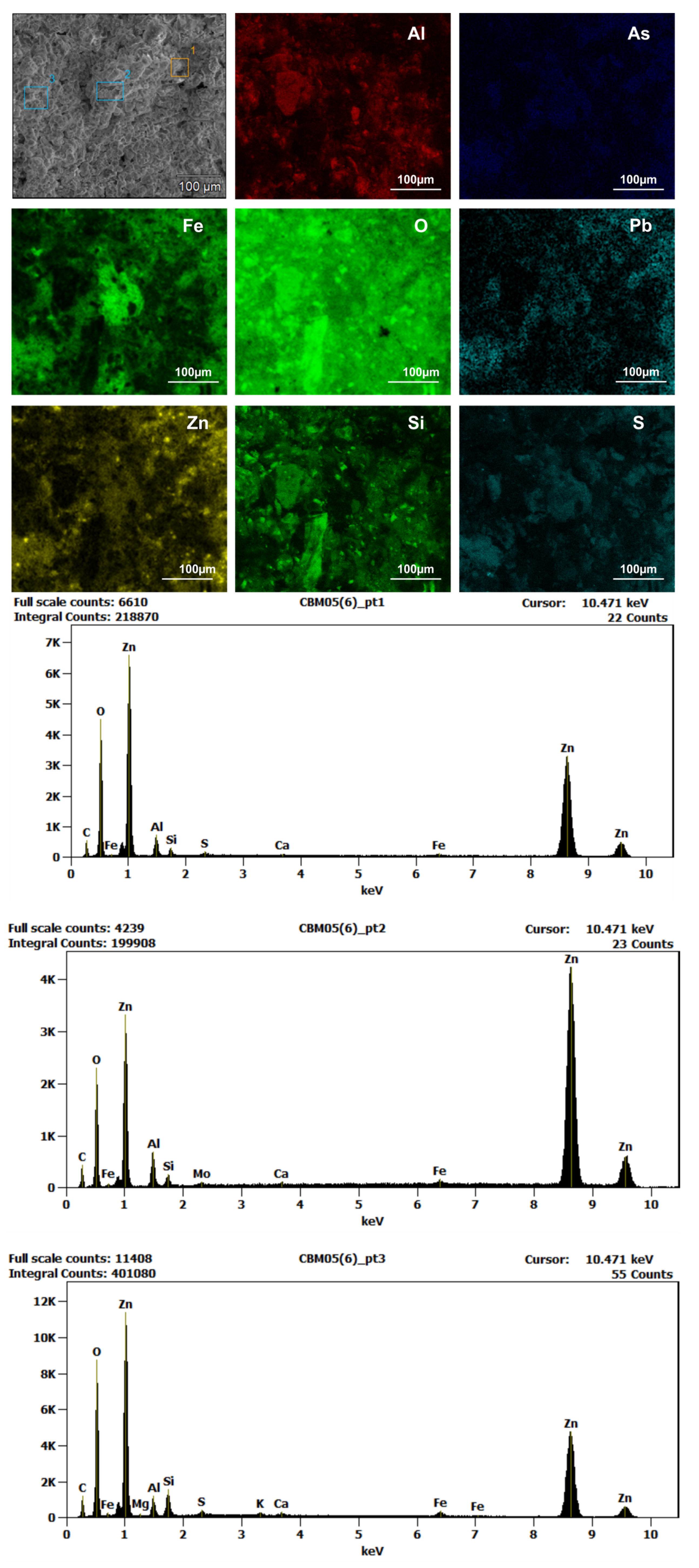
5.3. Scanning Elecron Microscope Analyses
5.4. Inductively Coupled Plasma Spectrometry
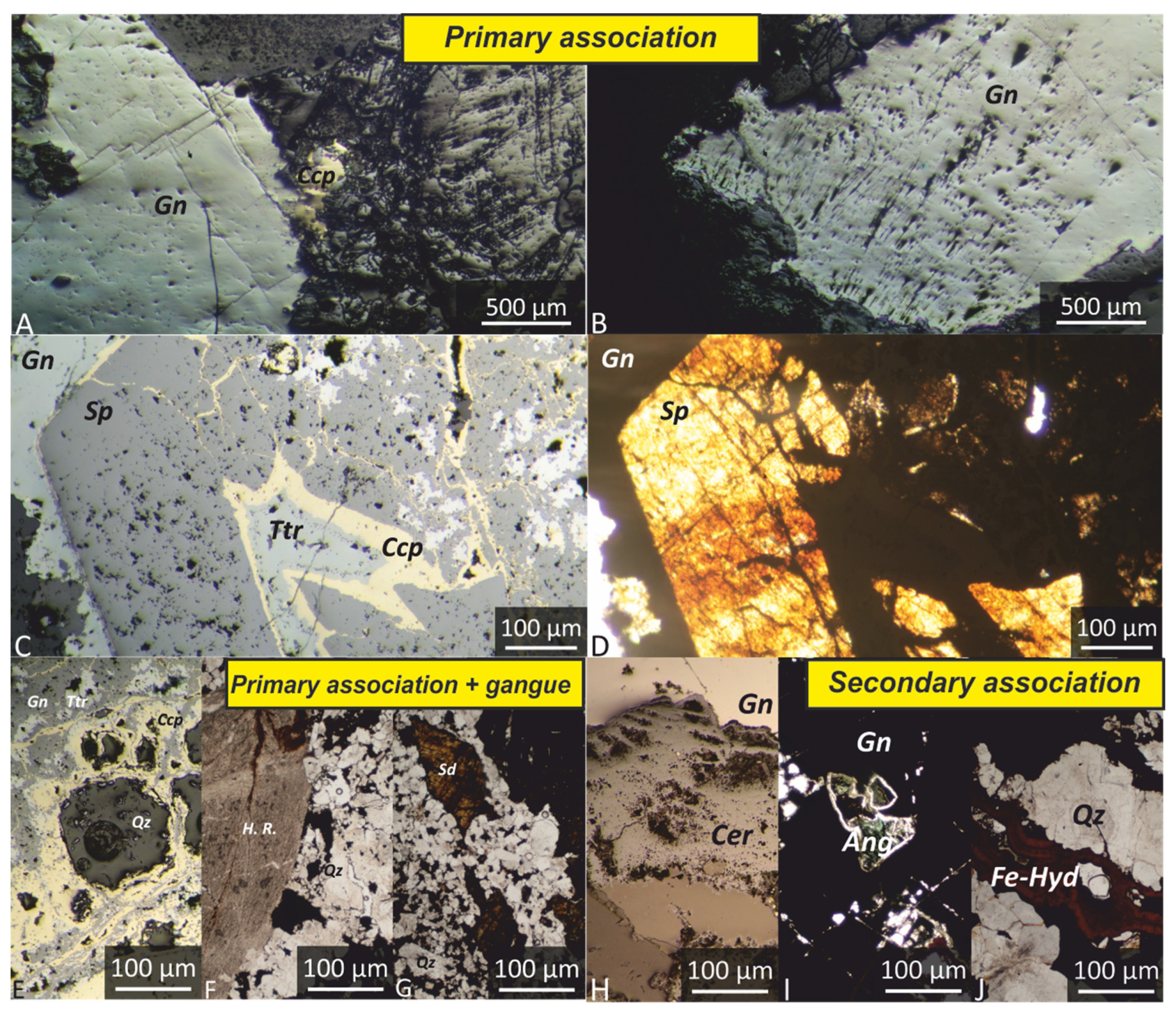
6. Analytical Results
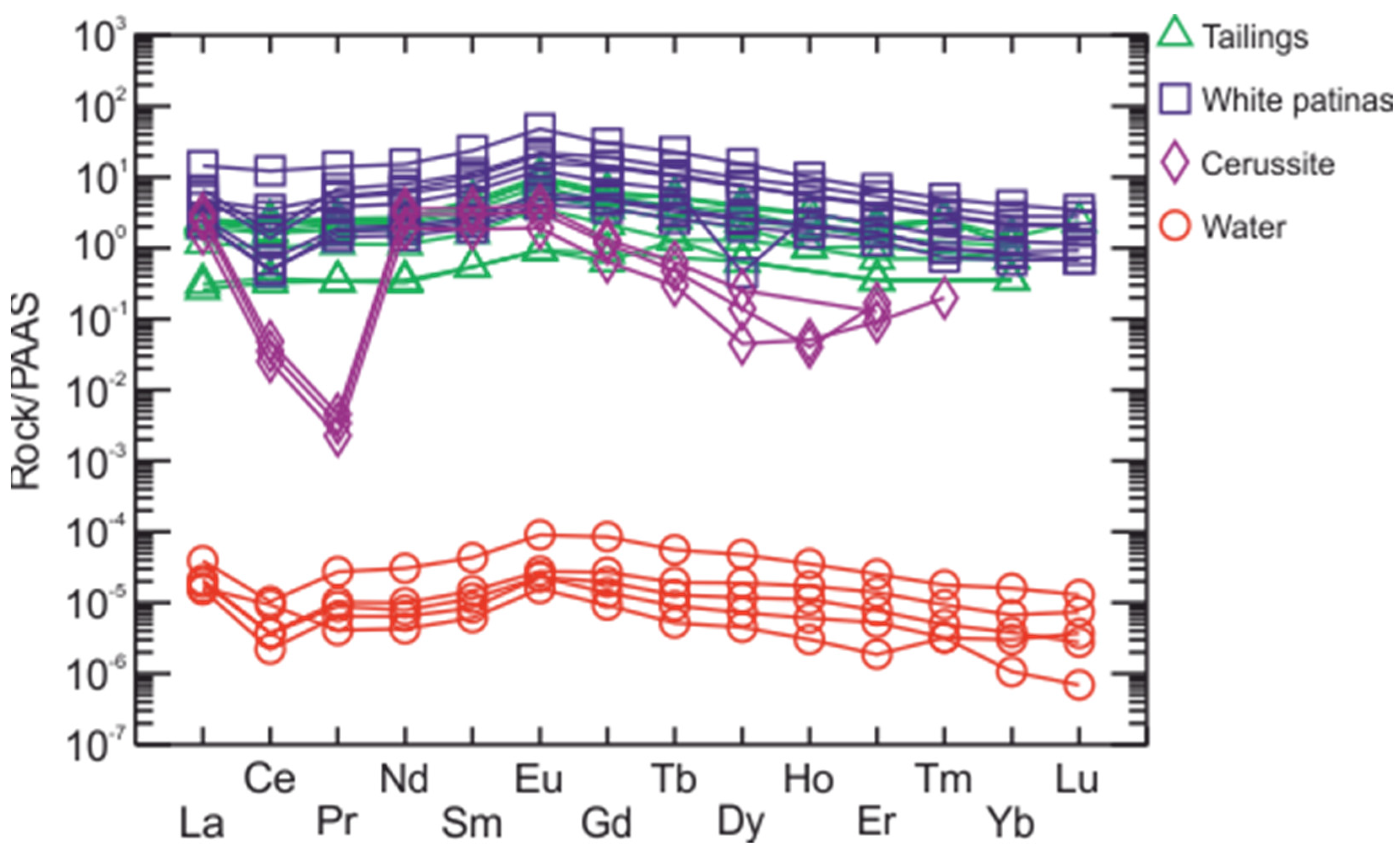
6.1. Tailings
6.2. The White Patinas
6.3. Cerussite
6.4. Water Analyses
7. Discussion
7.1. Environmental Issues and Resilience Factors
7.2. Raw Materials Potential
7.3. Insights for Metals Recovery
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Study on the Critical Raw Materials for the EU. Fifth List. Final Report 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 20 October 2023).
- Blengini, G.A.; Mathieux, F.; Mancini, L.; Nyberg, M.; Cavaco Viegas, H.; Salminen, J.; Garbarino, E.; Orveillion, G.; Saveyn, H. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine Tailings Dams: Characteristics, Failure, Environmental Impacts, and Remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Ali, M.A.H.; Mewafy, F.M.; Qian, W.; Alshehri, F.; Almadani, S.; Aldawsri, M.; Aloufi, M.; Saleem, H.A. Mapping Leachate Pathways in Aging Mining Tailings Pond Using Electrical Resistivity Tomography. Minerals 2023, 13, 1437. [Google Scholar] [CrossRef]
- Miler, M.; Bavec, Š.; Gosar, M. The Environmental Impact of Historical Pb-Zn Mining Waste Deposits in Slovenia. J. Environ. Manag. 2022, 308, 114580. [Google Scholar] [CrossRef]
- Moroni, M.; Naitza, S.; Ruggieri, G.; Aquino, A.; Costagliola, P.; De Giudici, G.; Caruso, S.; Ferrari, E.; Fiorentini, M.L.; Lattanzi, P. The Pb-Zn-Ag Vein System at Montevecchio-Ingurtosu, Southwestern Sardinia, Italy: A Summary of Previous Knowledge and New Mineralogical, Fluid Inclusion, and Isotopic Data. Ore Geol. Rev. 2019, 115, 103194. [Google Scholar] [CrossRef]
- Ministero Dell’ambiente e Della Tutela del Territorio. Perimetrazione del Sito di Interesse Nazionale del Sulcis-Iglesiente-Guspinese. GU Serie Generale n. 121 del 27-05-2003. Suppl. Ordinario n. 83. 2003. Available online: https://www.gazzettaufficiale.it/eli/gu/2003/05/27/121/so/83/sg/pdf (accessed on 20 October 2023).
- Progemisa SpA. Interventi di Bonifica e Ripristino Ambientale Dell’area Mineraria Dismessa di Montevecchio Ponente. Scheda di Intervento di Emergenza E 3.1—Piano di Caratterizzazione; Public report; Progemisa SpA: Cagliari, Italy, 2003. [Google Scholar]
- Sedda, L. Analisi Ambientale Dell’area di Montevecchio Ponente: Stato Dell’arte E Tecnologie. Master’s Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2021. [Google Scholar]
- Piano Regionale di Gestione dei Rifiuti. Piano di Bonifica Siti Inquinati. Schede dei Siti Minerari. Allegato 5; Regione Autonoma Sardegna: Cagliari, Italy, 2003. [Google Scholar]
- Studio per il Piano di Recupero dell’area Mineraria Dimessa di Montevecchio—Ingurtosu; Progemisa SpA: Cagliari, Italy, 2001; pp. 190–203.
- Cidu, R.; Fanfani, L. Overview of the environmental geochemistry of mining districts in southwestern Sardinia, Italy. Geochem. Explor. Environ. Anal. 2002, 2, 243–251. [Google Scholar] [CrossRef]
- Cidu, R.; Frau, F. Impact of the Casargiu Mine Drainage (SW Sardinia, Italy) on the Mediterranean Sea. In Proceedings of the International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009; pp. 926–931. [Google Scholar]
- Cidu, R.; Frau, F.; Da Pelo, S. Drainage at Abandoned Mine Sites: Natural Attenuation of Contaminants in Different Seasons. Mine Water Environ. 2011, 30, 113–126. [Google Scholar] [CrossRef]
- Frau, F.; Medas, D.; Da Pelo, S.; Wanty, R.B.; Cidu, R. Environmental Effects on the Aquatic System and Metal Discharge to the Mediterranean Sea from a Near-Neutral Zinc-Ferrous Sulfate Mine Drainage. Water Air Soil Pollut. 2015, 226, 55. [Google Scholar] [CrossRef]
- De Giudici, G.; Medas, D.; Cidu, R.; Lattanzi, P.; Podda, F.; Frau, F.; Rigonat, N.; Pusceddu, C.; Da Pelo, S.; Onnis, P.; et al. Application of hydrologic-tracer techniques to the Casargiu adit and Rio Irvi (SW-Sardinia, Italy): Using enhanced natural attenuation to reduce extreme metal loads. Appl. Geochem. 2018, 96, 42–54. [Google Scholar] [CrossRef]
- De Giudici, G.; Medas, D.; Cidu, R.; Lattanzi, P.; Rigonat, N.; Frau, I.; Podda, F.; Marras, P.A.; Dore, E.; Frau, F.; et al. Assessment of origin and fate of contaminants along mining-affected Rio Montevecchio (SW Sardinia, Italy): A hydrologic-tracer and environmental mineralogy study. Appl. Geochem. 2019, 109, 104420. [Google Scholar] [CrossRef]
- Medas, D.; Lattanzi, P.; Podda, F.; Meneghini, C.; Trapananti, A.; Sprocati, A.; Casu, M.A.; Musu, E.; De Giudici, G. The amorphous Zn biomineralization at Naracauli stream, Sardinia: Electron microscopy and X-ray absorption spectroscopy. Environ. Sci. Pollut. Res. 2014, 21, 6775–6782. [Google Scholar] [CrossRef]
- Podda, F.; Medas, D.; De Giudici, G.; Ryszka, P.; Wolowski, K.; Turnau, K. Zn biomineralization processes and microbial biofilm in a metal-rich stream (Naracauli, Sardinia). Environ. Sci. Pollut. Res. 2014, 21, 6793–6808. [Google Scholar] [CrossRef]
- Cuccuru, S.; Naitza, S.; Secchi, F.; Puccini, A.; Casini, L.; Pavanetto, P.; Linnemann, U.; Hofmann, M.; Oggiano, G. Structural and metallogenic map of late Variscan Arbus Pluton (SW Sardinia, Italy). J. Maps 2016, 12, 860–865. [Google Scholar] [CrossRef]
- Carmignani, L.; Oggiano, G.; Barca, S.; Conti, P.; Salvadori, I.; Eltrudis, A.; Funedda, A.; Pasci, S. Geologia Della Sardegna: Note Illustrative Della Carta Geologica Della Sardegna in Scala 1:200.000, Memorie Descrittive Della Carta Geologica d’Italia (Vol. 60); Servizio Geologico d’Italia: Rome, Italy, 2001; p. 283. [Google Scholar]
- Annino, E.; Barca, S.; Costamagna, L.G.; Annino, E.; Barca, S.; Costamagna, L.G. Lineamenti stratigrafico-strutturali dell’Arburese (Sardegna sud-occidentale). Rend. Semin. Fac. Sci. Univ. Cagliari. 2016, 70, 403–426. [Google Scholar]
- Assorgia, A.; Brotzu, P.; Morbidelli, L.; Nicoletti, M.; Traversa, G. Successione e cronologia (K-Ar) degli eventi vulcanici del complesso calco-alcalino oligo-miocenico dell’Arcuentu (Sardegna centro-occidentale). Period. di Mineral. 1984, 53, 89–102. [Google Scholar]
- Salvadori, I. Studio geo-minerario della zona di Salaponi (Sardegna Sud-occidentale). Boll. Soc. Geol. Ital. 1958, 77, 91–126. [Google Scholar]
- Salvadori, I.; Zuffardi, P. Guida per l’escursione a Montevecchio e all’Arcuentu. Itinerari geologici, mineralogici e giacimentologici in Sardegna. Ente Min. Sardo 1973, 1, 29–44. [Google Scholar]
- Moroni, M.; Rossetti, P.; Naitza, S.; Magnani, L.; Ruggieri, G.; Aquino, A.; Tartarotti, P.; Franklin, A.; Ferrari, E.; Castelli, D.; et al. Factors controlling hydrothermal nickel and cobalt mineralization—Some suggestions from historical ore deposits in Italy. Minerals 2015, 9, 429. [Google Scholar] [CrossRef]
- Zuffardi, P. Fenomeni di ricircolazione nel giacimento di Montevecchio e l’evoluzione in profondità della sua mineralizzazione. Res. Ass. Min. Sarda 1962, 1–2, 17–73. [Google Scholar]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Mondillo, N.; Boni, M.; Balassone, G.; Spoleto, S.; Stellato, F.; Marino, A.; Santoro, L.; Spratt, J. Rare earth elements (REE)—Minerals in the Silius fluorite vein system (Sardinia, Italy). Ore Geol. Rev. 2016, 74, 211–224. [Google Scholar] [CrossRef]
- Corda, V. Studi Sull’impatto Ambientale Delle Discariche Minerarie a Montevecchio Ponente. Master’s Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2022. [Google Scholar]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical characterization of mine waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Mulenshi, J.; Gilbricht, S.; Chelgani, S.C.; Rosenkranz, J. Systematic characterization of historical tailings for possible remediation and recovery of critical metals and minerals—The Yxsjöberg case. J. Geochem. Explor. 2021, 226, 106777. [Google Scholar] [CrossRef]
- Wang, C.; Harbottle, D.; Liu, Q.; Xu, Z. Current state of fine mineral tailings treatment: A critical review on theory and practice. Miner. Eng. 2014, 58, 113–131. [Google Scholar] [CrossRef]
- Cidu, R.; Caboi, R.; Fanfani, L.; Frau, F. Acid drainage from sulfides hosting gold mineralization (Furtei, Sardinia). Environ. Geol. 1997, 30, 231–237. [Google Scholar] [CrossRef]
- Jurjovec, J.; Ptacek, C.J.; Blowes, D.W. Acid neutralization mechanisms and metal release in mine tailings: A laboratory column experiment. Geochim. Cosmochim. Acta 2002, 66, 1511–1523. [Google Scholar] [CrossRef]
- Frau, F.; Ardau, C. Geochemical controls on arsenic distribution in the Baccu Locci stream catchment (Sardinia, Italy) affected by past mining. Appl. Geochem. 2003, 18, 1373–1386. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L. Release, transport and attenuation of metals from an old tailings impoundment. Appl. Geochem. 2005, 20, 639–659. [Google Scholar] [CrossRef]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef]
- Da Pelo, S.; Musu, E.; Cidu, R.; Frau, F.; Lattanzi, P. Release of toxic elements from rocks and mine wastes at the Furtei gold mine (Sardinia, Italy). J. Geochem. Explor. 2009, 100, 142–152. [Google Scholar] [CrossRef]
- Geng, H.; Wang, F.; Yan, C.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J. Hazard. Mater. 2020, 383, 121136. [Google Scholar] [CrossRef]
- Dore, E.; Fancello, D.; Rigonat, N.; Medas, D.; Cidu, R.; Da Pelo, S.; Frau, F.; Lattanzi, P.; Marras, P.A.; Meneghini, C.; et al. Natural attenuation can lead to environmental resilience in mine environment. Appl. Geochem. 2020, 117, 104597. [Google Scholar] [CrossRef]
- Zuddas, P.; Podda, F. Variations in Physico-Chemical Properties of Water Associated with Bio-Precipitation of Hydrozincite [Zn5(CO3)2(OH)6] in the Waters of Rio Naracauli, Sardinia (Italy). Appl. Geochem. 2005, 20, 507–517. [Google Scholar] [CrossRef]
- Li, M.Y.H.; Zhou, M.F.; Williams-Jones, A.E. The Genesis of Regolith-Hosted Heavy Rare Earth Element Deposits: Insights from the World-Class Zudong Deposit in Jiangxi Province, South China. Econ. Geol. 2019, 114, 541–568. [Google Scholar] [CrossRef]
- Zhukova, I.A.; Stepanov, A.S.; Jiang, S.Y.; Murphy, D.; Mavrogenes, J.; Allen, C.; Chen, W.; Bottrill, R. Complex REE systematics of carbonatites and weathering products from uniquely rich Mount Weld REE deposit, Western Australia. Ore Geol. Rev. 2021, 139 Pt B, 104539. [Google Scholar] [CrossRef]
- Tuduri, J.; Pourret, O.; Chauvet, A.; Gaouzi, A.; Ennaciri, A. Rare earth elements as proxies of supergene alteration processes from the giant Imiter silver deposit. In Proceedings of the 11th Biennal Meeting SGA, Antofagasta, Chile, 2 June 2014. [Google Scholar]
- Li, X.; Liang, X.; He, H.; Li, J.; Ma, L.; Tan, W.; Zhong, Y.; Zhu, J.; Zhou, M.F.; Dong, H. Microorganisms Accelerate REE Mineralization in Supergene Environments. Appl. Environ. Microbiol. 2022, 88, e0063222. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hirata, T.; Shimizu, H.; Ozaki, T.; Fortin, D. A rare earth element signature of bacteria in natural waters? Chem. Geol. 2007, 244, 569–583. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; pp. 1–312. [Google Scholar]
- Kaya, M. Assessment of Secondary Zinc and Lead Resources. In Recycling Technologies for Secondary Zn-Pb Resources; Kaya, M., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Navidi Kashani, A.H.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Mercante, C. Treatment of Mineral-Metallurgical Residues for the Recovery of Useful. Ph.D. Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2021. [Google Scholar]
- Sogos, G. Study of Minerallurgical Processes for the Treatment of Residues from Mining Activities. Ph.D. Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2022. [Google Scholar]
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]
- Golik, V.; Komashchenko, V.; Morkun, V. Innovative technologies of metal extraction from the ore processing mill tailings and their integrated use. Metall. Min. Ind. 2015, 7, 49–52. [Google Scholar]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Cotruvo, J.A. The chemistry of lanthanides in biology: Recent discoveries, emerging principles, and technological applications. ACS Cent. Sci. 2019, 5, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Maleke, M.; Unuofin, J.; Cebekhulu, S. Microbial Recovery of Rare Earth Elements. In Environmental Technologies to Treat Rare Earth Elements Pollution: Principles and Engineering; IWA Publishing: London, UK, 2019; Chapter 8; pp. 179–205. [Google Scholar]
- Johannesson, K.H.; Zhou, X. Geochemistry of the rare earth elements in natural terrestrial waters: A review of what is currently known. Chin. J. Geochem. 1997, 16, 20–42. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L.J. Role of microorganisms in bioleaching of rare earth elements from primary and secondary resources. Appl. Microbiol. Biotechnol. 2019, 103, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Brewer, A.; Reed, D.W.; Lammers, L.N.; Jiao, Y. Recovery of Rare Earth Elements from Low-Grade Feedstock Leachates Using Engineered Bacteria. Environ. Sci. Technol. 2017, 51, 13471–13480. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, D.M.; Gupta, M.; Brewer, A.W.; Ho, L.; Singer, S.L.; Bourcier, W.L.; Woods, S.; Reed, D.W.; Lammers, L.N.; et al. Techno-economic Assessment for Integrating Biosorption into Rare Earth Recovery Process. ACS Sustain. Chem. Eng. 2017, 5, 10148–10155. [Google Scholar] [CrossRef]
- De Giudici, G.; Wanty, R.B.; Podda, F.; Kimball, B.A.; Verplanck, P.L.; Lattanzi, P.; Cidu, R.; Medas, D. Quantifying biomineralization of zinc in the rio naracauli (Sardinia, Italy), using a tracer injection and synoptic sampling. Chem. Geol. 2014, 384, 110–119. [Google Scholar] [CrossRef]
- Buosi, M.; Contini, E.; Enne, R.; Farci, A.; Garbarino, C.; Naitza, S.; Tocco, S. Contributo alla conoscenza dei materiali delle discariche della miniera di Monteponi: I “Fanghi Rossi” dell’elettrolisi, caratterizzazione fisico-geotecnica e chimico-mineralogica, definizione del potenziale inquinante e proposte per possibili interventi. Res. Ass. Min. Sarda 1999, 104, 49–93. [Google Scholar]
- Contini, E.; Naitza, S.; Tocco, S.; Garau, A.; Buosi, M.; Sarritzu, R. Fenomeni di contaminazione da discariche minerarie e metallurgiche nel distretto dell’antimonio del Sarrabus-Gerrei (Sardegna Sud-Orientale): L’area di Su Suergiu-Villasalto. Res. Ass. Min. Sarda 2008, 112, 45–83. [Google Scholar]
- Manca, P.P.; Massacci, G.; Mercante, C. Environmental Management and Metal Recovery: Re-processing of Mining Waste at Montevecchio Site (SW Sardinia). In Proceedings of the 18th Symposium on Environmental Issues and Waste Management in Energy and Mineral Production: SWEMP 2018; Widzyk-Capehart, E., Hekmat, A., Singhal, R., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]



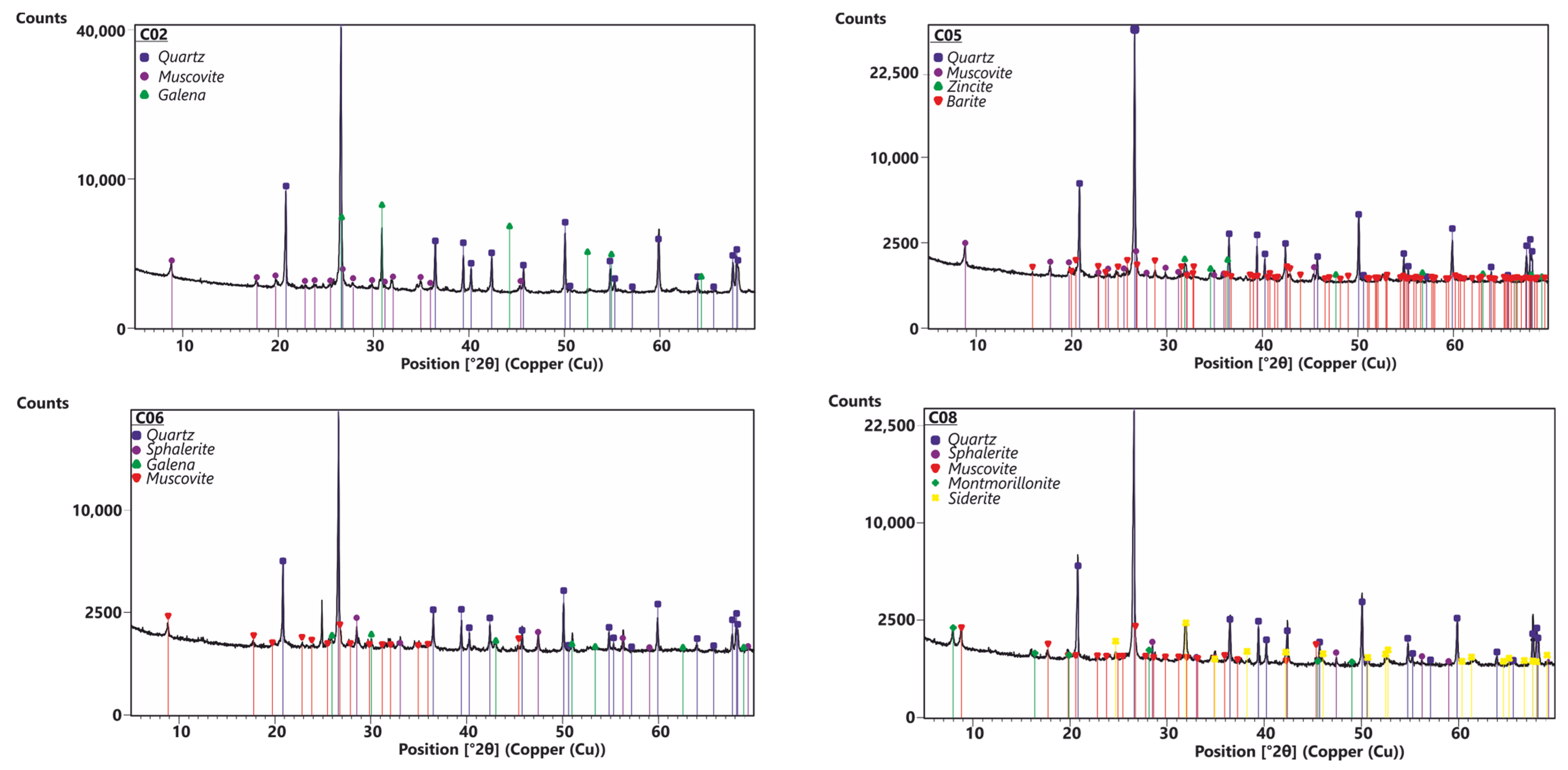
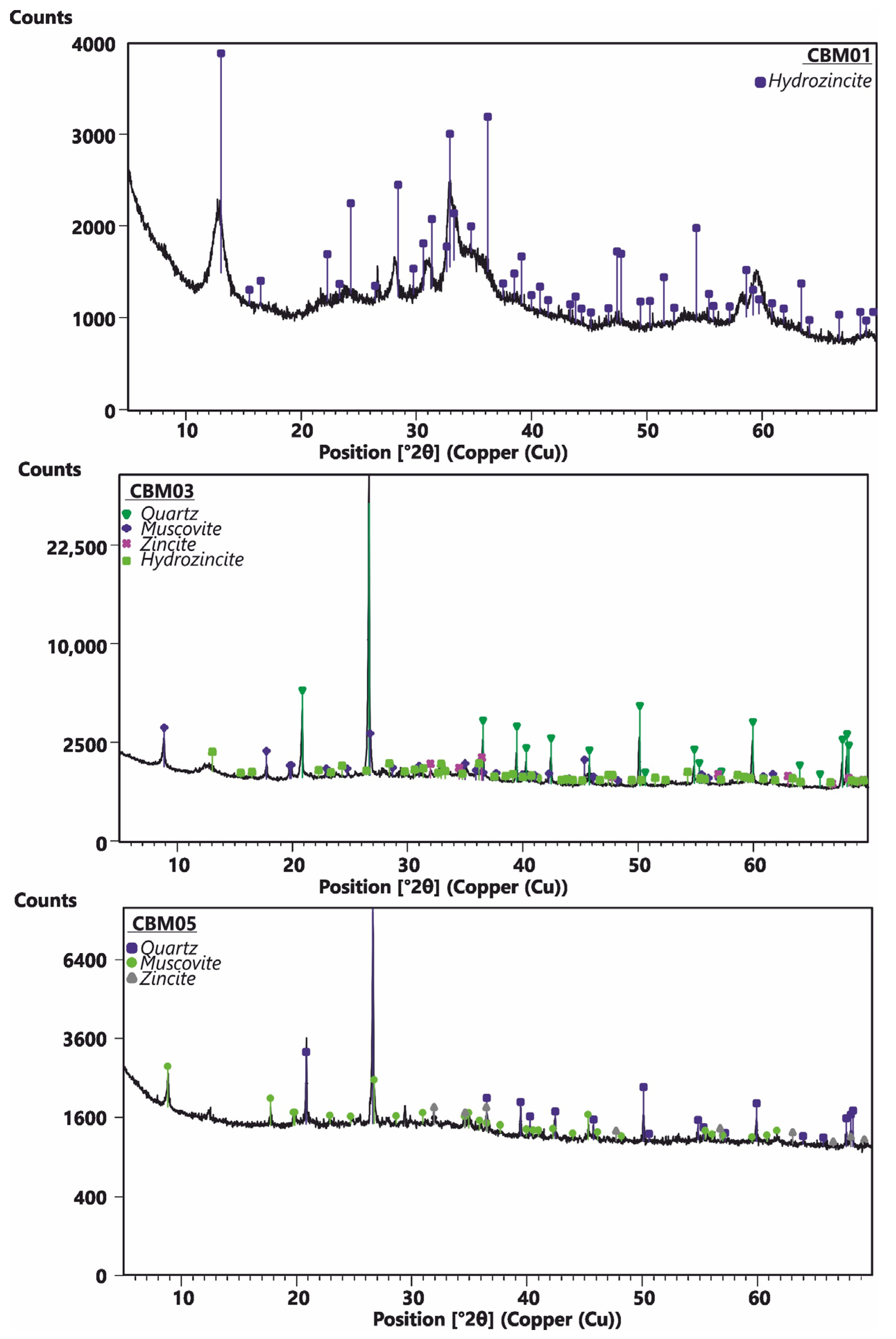
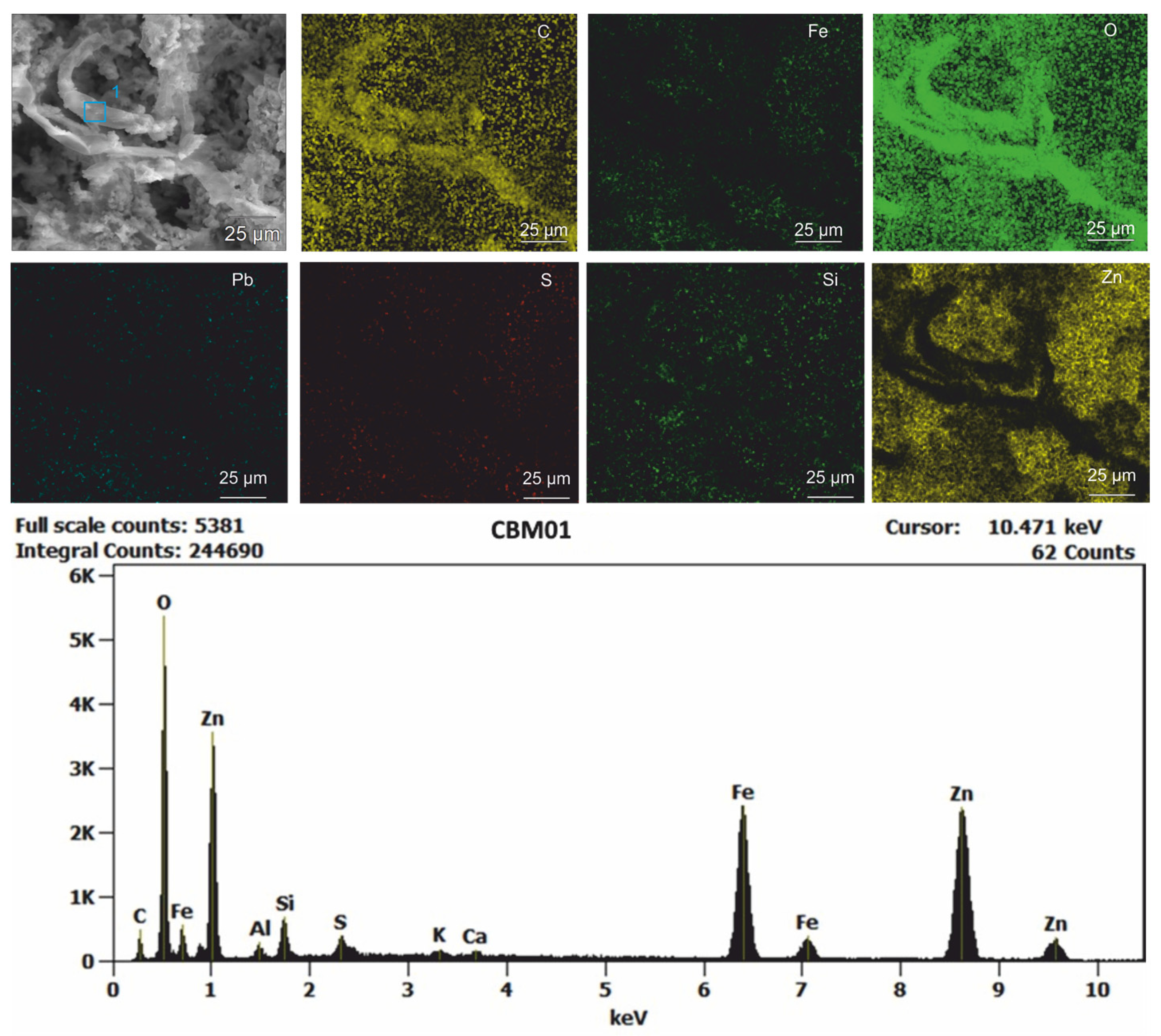

| Layer | Approximately. Thickness | Sample | Granulometry | Mineralogy |
|---|---|---|---|---|
| 1 | 50 cm | C01 | Moderately graded sand with clays | Qz, Ms, Dol |
| 2 | 70 cm | C02 | Poorly graded sand with clays | Qz, Ms, Gn |
| 3 | 40 cm | C03 | Poorly graded sandy silt with clays | Qz, Ms, Brt |
| 4 | 50 cm | C04 | Poorly graded sand with clays | Qz, Ms, Sd, Brt |
| 5 | 20 cm | C05 | Poorly graded sandy silt with clays | Qz, Ms, Znc, Brt |
| 6 | 40 cm | C06 | Poorly graded gravelly sand with clays | Qz, Ms, Sp, Gn |
| 7 | 10 cm | C07 | Moderately well-graded sand with clays | Qz, Ms |
| 8 | 50 cm | C08 | Poorly graded sandy gravel, with clays | Qz, Ms, Sp, Mnt, Sd |
| Sample | Al | As | Ba | Bi | Cd | Co | Cu | Fe | Ga | Ge | In | Mn | Ni | Pb | Sb | Sn | V | W | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C01 | 19,000 | 78 | 1400 | ND | 47 | 15 | 140 | 61,000 | ND | ND | ND | 3100 | 25 | 2400 | 28 | ND | ND | ND | 6800 |
| C02 | 20,100 | 55 | 1200 | ND | 100 | 15 | 110 | 75,400 | 10 | 5 | 2 | 4300 | 35 | 3000 | 20 | 2 | 27 | 2 | 7500 |
| C03 | 19,000 | 57 | 1700 | ND | 110 | 22 | 90 | 80,200 | 12 | 4 | 2 | 4500 | 39 | 2300 | 19 | 3 | 34 | 3 | 8700 |
| C04 | 17,200 | 58 | 2000 | ND | 70 | 26 | 65 | 93,000 | 8 | 4 | 1 | 5900 | 34 | 760 | 14 | 2 | 21 | 2 | 6600 |
| C05 | 22,700 | 60 | 3800 | ND | 140 | 25 | 83 | 88,200 | 13 | 4 | 2 | 5800 | 42 | 1100 | 19 | 3 | 37 | 3 | 11,300 |
| C06 | 18,700 | 430 | 270 | 5 | 180 | 24 | 2070 | 135,000 | 24 | 15 | 13 | 6700 | 23 | 12,300 | 170 | 10 | 25 | 2 | 26,500 |
| C07 | 16,700 | 83 | 5200 | ND | 150 | 36 | 280 | 154,000 | 8 | 5 | 2 | 8000 | 48 | 5200 | 40 | 2 | 17 | 2 | 15,500 |
| C08 | 17,200 | 79 | 2300 | ND | 57 | 15 | 180 | 64,600 | ND | ND | ND | 3300 | 28 | 3800 | 34 | ND | 31 | ND | 8700 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REEs + Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C01 | 9 | 12 | 30 | 3 | 12 | 3 | 1 | 3 | ND | 2 | ND | 1 | ND | 1 | ND | 77 |
| C02 | 27 | 45 | 81 | 10 | 38 | 9 | 4 | 10 | 1 | 6 | 1 | 2 | ND | 2 | ND | 236 |
| C03 | 65 | 83 | 180 | 20 | 83 | 22 | 10 | 26 | 4 | 17 | 3 | 6 | 1 | 3 | ND | 523 |
| C04 | 64 | 97 | 180 | 22 | 86 | 23 | 10 | 25 | 3 | 15 | 2 | 5 | 1 | 3 | ND | 536 |
| C05 | 76 | 91 | 190 | 23 | 92 | 26 | 11 | 29 | 4 | 19 | 3 | 6 | 1 | 4 | 1 | 576 |
| C06 | 35 | 73 | 130 | 16 | 65 | 16 | 6 | 17 | 2 | 9 | 1 | 3 | ND | 2 | ND | 375 |
| C07 | 48 | 88 | 140 | 19 | 74 | 19 | 8 | 20 | 3 | 11 | 2 | 4 | ND | 3 | ND | 439 |
| C08 | 8 | 10 | 26 | 3 | 11 | 3 | 1 | 3 | ND | 2 | ND | 1 | ND | 1 | ND | 69 |
| Sample | Al | As | Ba | Cd | Co | Cu | Fe | Mn | Ni | Pb | Sb | Sr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBM01 | 1770 | 23 | 31 | 610 | 11 | 850 | 27,700 | 360 | 150 | 14,800 | 8 | 8 | 433,200 |
| CBM02 | 1800 | 41 | 310 | 580 | 18 | 730 | 26,700 | 650 | 130 | 14,600 | 8 | 14 | 415,000 |
| CBM03 | 10,400 | 920 | 16,500 | 550 | 84 | 1430 | 355,200 | 14,400 | 190 | 33,000 | 42 | 200 | 82,400 |
| CBM04 | 550 | 14 | 120 | 640 | 14 | 300 | 4870 | 290 | 170 | 6400 | 5 | 7 | 509,000 |
| CBM05 | 9010 | 270 | 1530 | 400 | 46 | 1690 | 199,800 | 7450 | 90 | 74,500 | 67 | 55 | 90,300 |
| CBM06 | 10,020 | 230 | 11,300 | 680 | 29 | 1490 | 91,400 | 4100 | 92 | 55,600 | 110 | 76 | 274,300 |
| CBM07 | 880 | 23 | 250 | 450 | 10 | 570 | 9350 | 340 | 130 | 20,650 | 13 | 9 | 512,200 |
| CBM08 | 1350 | 29 | 860 | 460 | 9 | 660 | 15,100 | 400 | 120 | 23,370 | 1 | 10 | 489,000 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REEs + Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBM01 | 250 | 250 | 140 | 61 | 270 | 63 | 24 | 89 | 11 | 45 | 7 | 15 | 1 | 8 | 1 | 1235 |
| CBM02 | 200 | 200 | 110 | 45 | 200 | 44 | 17 | 65 | 8 | 34 | 6 | 12 | 1 | 6 | 1 | 949 |
| CBM03 | 250 | 550 | 970 | 120 | 520 | 130 | 52 | 140 | 18 | 72 | 10 | 20 | 2 | 11 | 2 | 2867 |
| CBM04 | 100 | 86 | 38 | 15 | 61 | 13 | 5 | 22 | 3 | 12 | 2 | 5 | ND | 2 | ND | 364 |
| CBM05 | 140 | 180 | 280 | 47 | 220 | 56 | 22 | 68 | 8 | 36 | 5 | 11 | 1 | 6 | 1 | 1081 |
| CBM06 | 91 | 140 | 220 | 33 | 140 | 35 | 14 | 40 | 5 | 2 | 3 | 6 | 1 | 3 | ND | 733 |
| CBM07 | 84 | 86 | 41 | 13 | 51 | 11 | 4 | 17 | 2 | 9 | 2 | 4 | ND | 2 | ND | 326 |
| CBM08 | 100 | 100 | 54 | 17 | 69 | 15 | 6 | 23 | 3 | 13 | 2 | 5 | ND | 2 | ND | 409 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REEs + Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MVS_1 | ND | 110 | 4 | ND | 120 | 20 | 4 | 6 | 1 | 1 | ND | ND | ND | ND | ND | 266 |
| MVS_2 | ND | 96 | 3 | ND | 100 | 17 | 4 | 5 | ND | 1 | ND | 1 | ND | ND | ND | 227 |
| MVS_3 | ND | 61 | 2 | ND | 64 | 10 | 2 | 3 | ND | ND | ND | ND | ND | ND | ND | 142 |
| Sample | T [°C] | pH | Eh [mV] | EC [µs/cm] | TDS [mg/L] | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REEs + Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 16.4 | 6.3 | 490 | 1318 | 989 | 1130 | 730 | 300 | 90 | 340 | 82 | 30 | 130 | 15 | 89 | 17 | 40 | 4 | 19 | 3 | 3019 |
| T1 | 17.5 | 6.1 | 491 | 1301 | 1071 | 190 | 620 | 750 | 36 | 150 | 34 | 17 | 43 | 4 | 21 | 3 | 5 | 1 | 3 | ND | 1877 |
| T2 | 16.6 | 5.7 | 320 | 1390 | 1060 | 1990 | 1490 | 830 | 240 | 1040 | 240 | 97 | 400 | 43 | 220 | 35 | 72 | 7 | 45 | 6 | 6755 |
| T3 | 18.6 | 5.4 | 471 | 1365 | 1030 | 880 | 800 | 290 | 78 | 270 | 64 | 25 | 91 | 10 | 55 | 11 | 22 | 2 | 11 | 1 | 2610 |
| T4 | 20.0 | 4.7 | 414 | 1399 | 1036 | 450 | 580 | 180 | 57 | 220 | 47 | 25 | 64 | 7 | 34 | 6 | 15 | 1 | 8 | 2 | 1696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedda, L.; De Giudici, G.; Fancello, D.; Podda, F.; Naitza, S. Unlocking Strategic and Critical Raw Materials: Assessment of Zinc and REEs Enrichment in Tailings and Zn-Carbonate in a Historical Mining Area (Montevecchio, SW Sardinia). Minerals 2024, 14, 3. https://doi.org/10.3390/min14010003
Sedda L, De Giudici G, Fancello D, Podda F, Naitza S. Unlocking Strategic and Critical Raw Materials: Assessment of Zinc and REEs Enrichment in Tailings and Zn-Carbonate in a Historical Mining Area (Montevecchio, SW Sardinia). Minerals. 2024; 14(1):3. https://doi.org/10.3390/min14010003
Chicago/Turabian StyleSedda, Lorenzo, Giovanni De Giudici, Dario Fancello, Francesca Podda, and Stefano Naitza. 2024. "Unlocking Strategic and Critical Raw Materials: Assessment of Zinc and REEs Enrichment in Tailings and Zn-Carbonate in a Historical Mining Area (Montevecchio, SW Sardinia)" Minerals 14, no. 1: 3. https://doi.org/10.3390/min14010003
APA StyleSedda, L., De Giudici, G., Fancello, D., Podda, F., & Naitza, S. (2024). Unlocking Strategic and Critical Raw Materials: Assessment of Zinc and REEs Enrichment in Tailings and Zn-Carbonate in a Historical Mining Area (Montevecchio, SW Sardinia). Minerals, 14(1), 3. https://doi.org/10.3390/min14010003








