Selective Separation Behavior and Study on the Interaction Mechanism of 2-Hydroxy-3-Naphthylmethyl Hydroxamic Acid and Cassiterite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
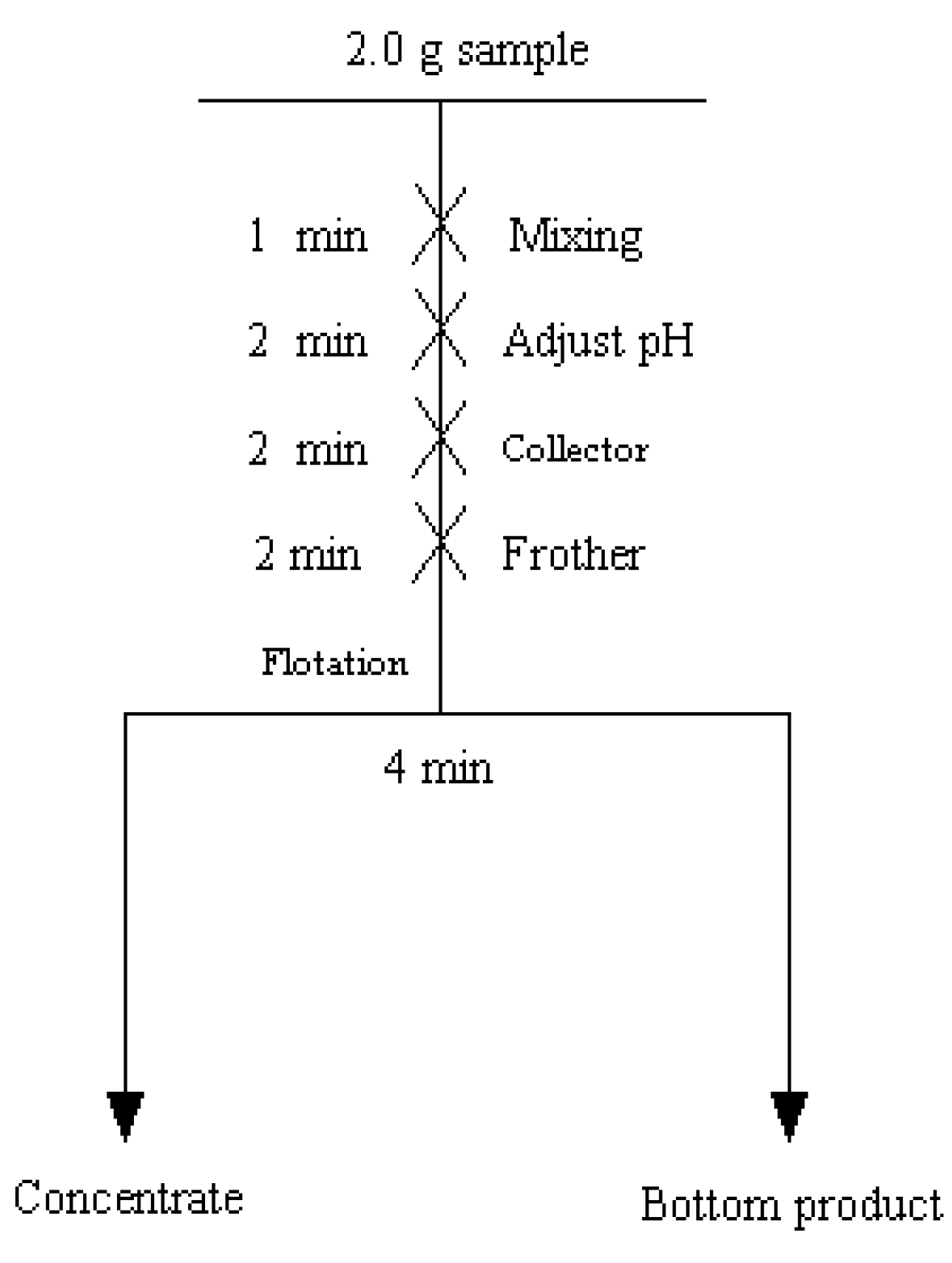
2.2.1. Micro-Flotation Experiments
2.2.2. Zeta Potential Measurements
2.2.3. FT-IR Spectroscopy Analysis
2.2.4. XPS Analysis
3. Results and Discussion
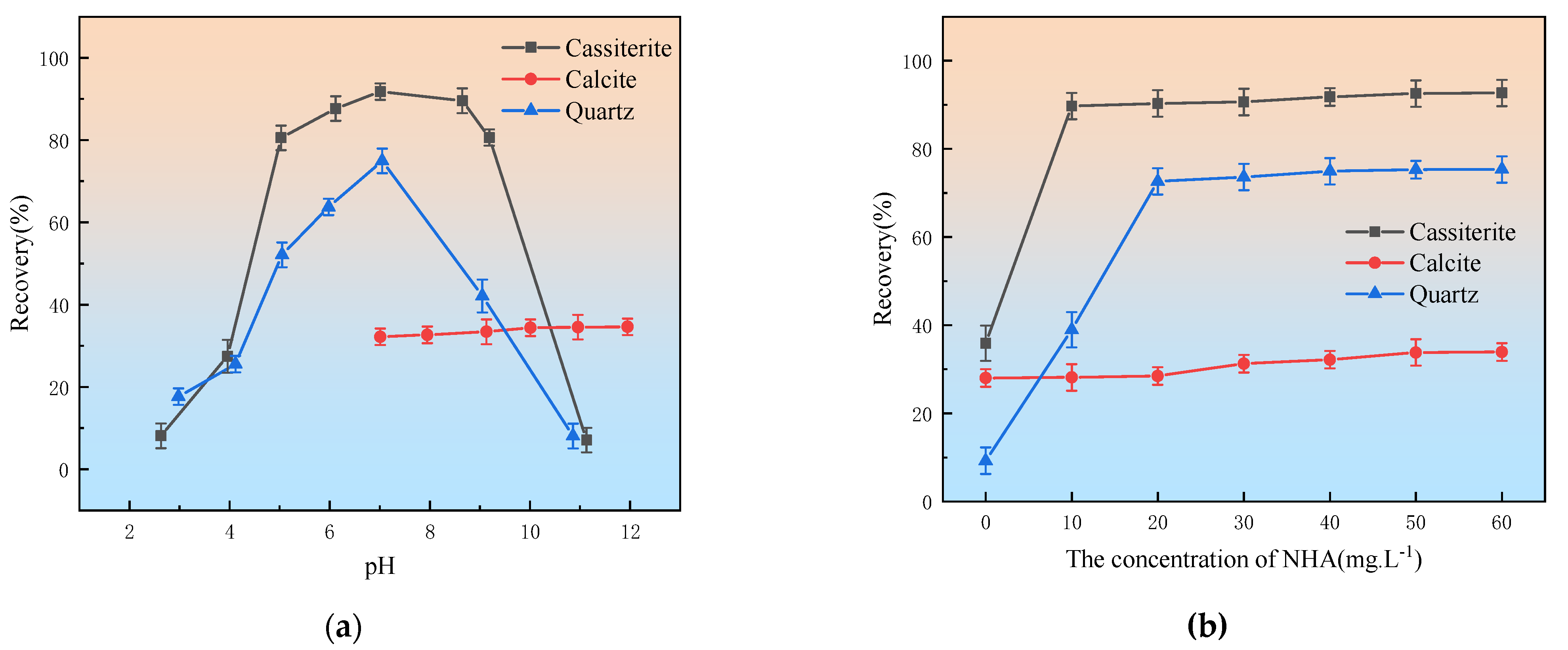
3.1. Flotation Experiments
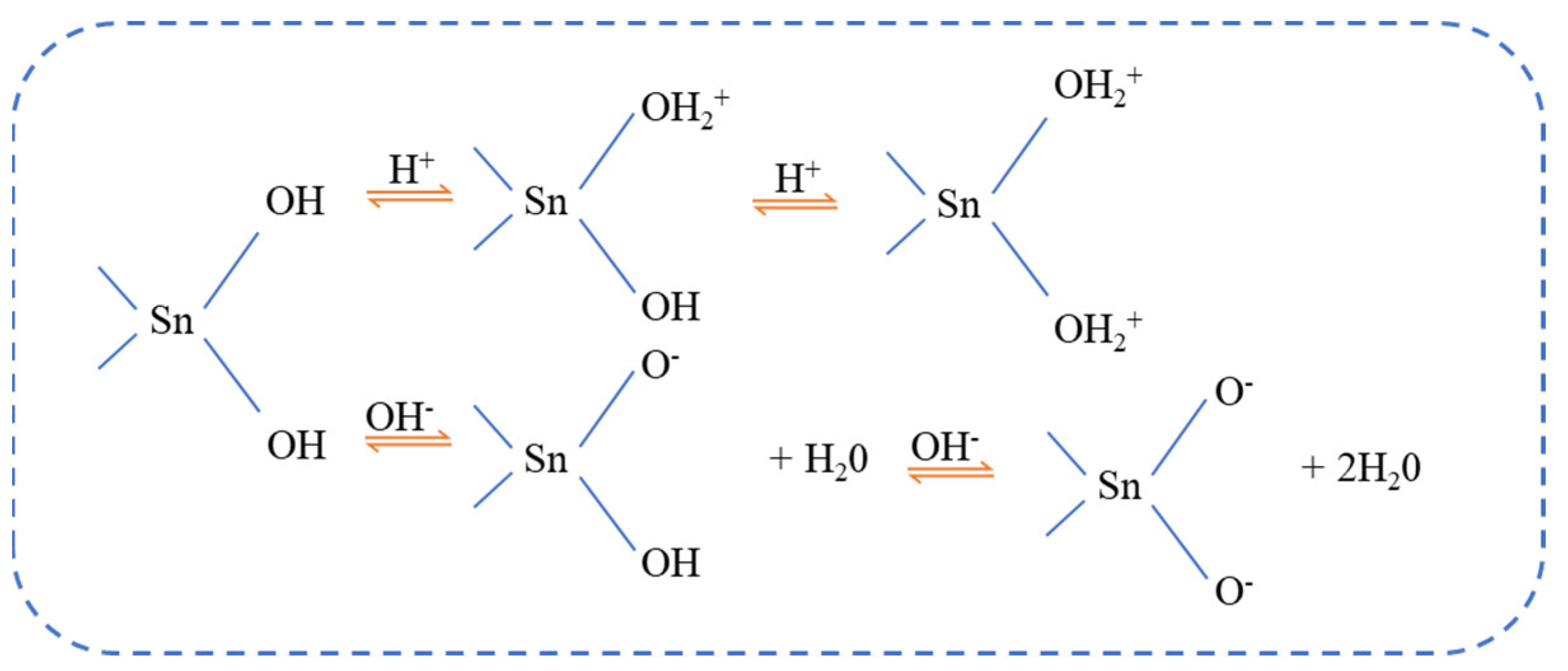
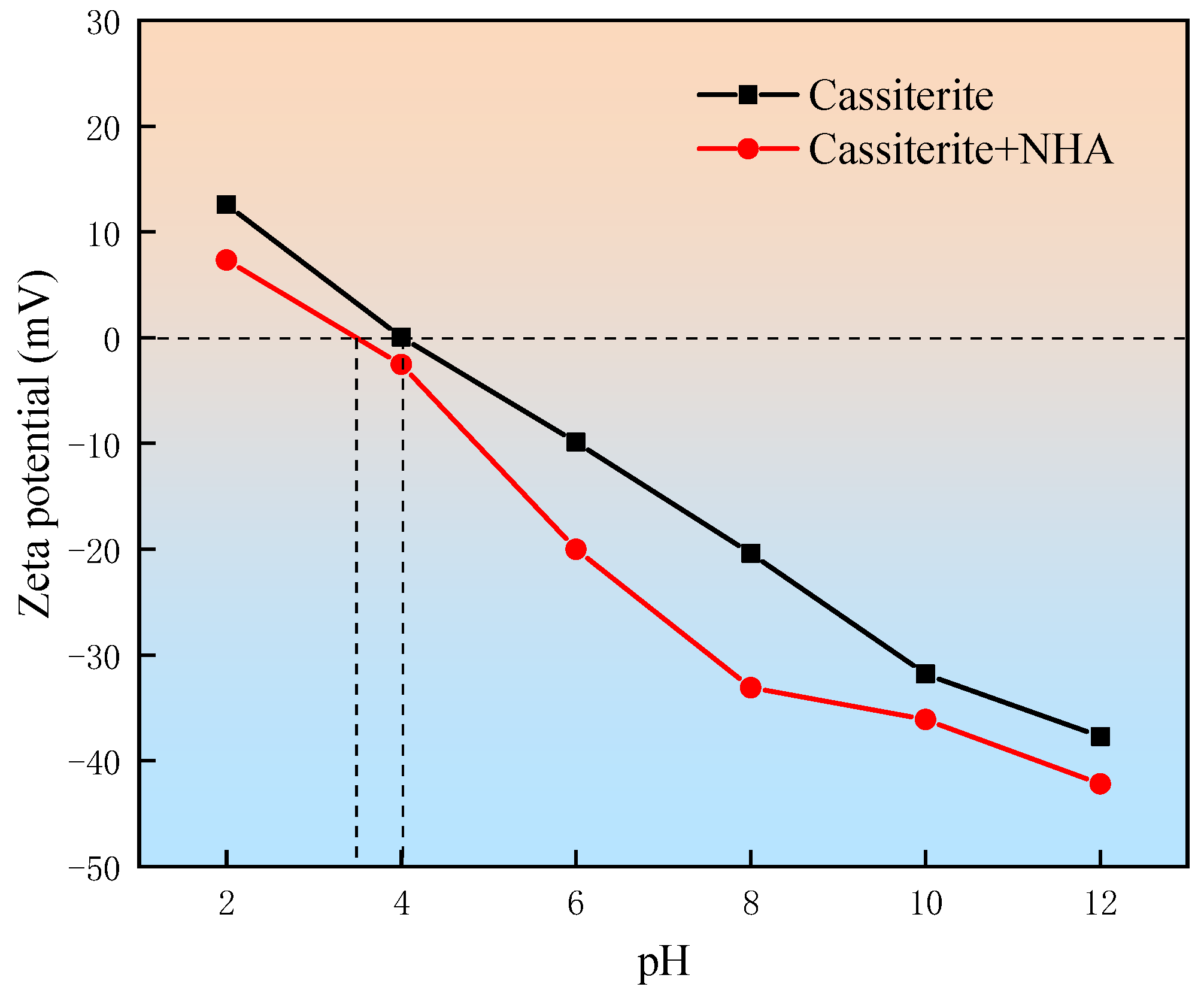
3.2. Zeta Potential Measurements
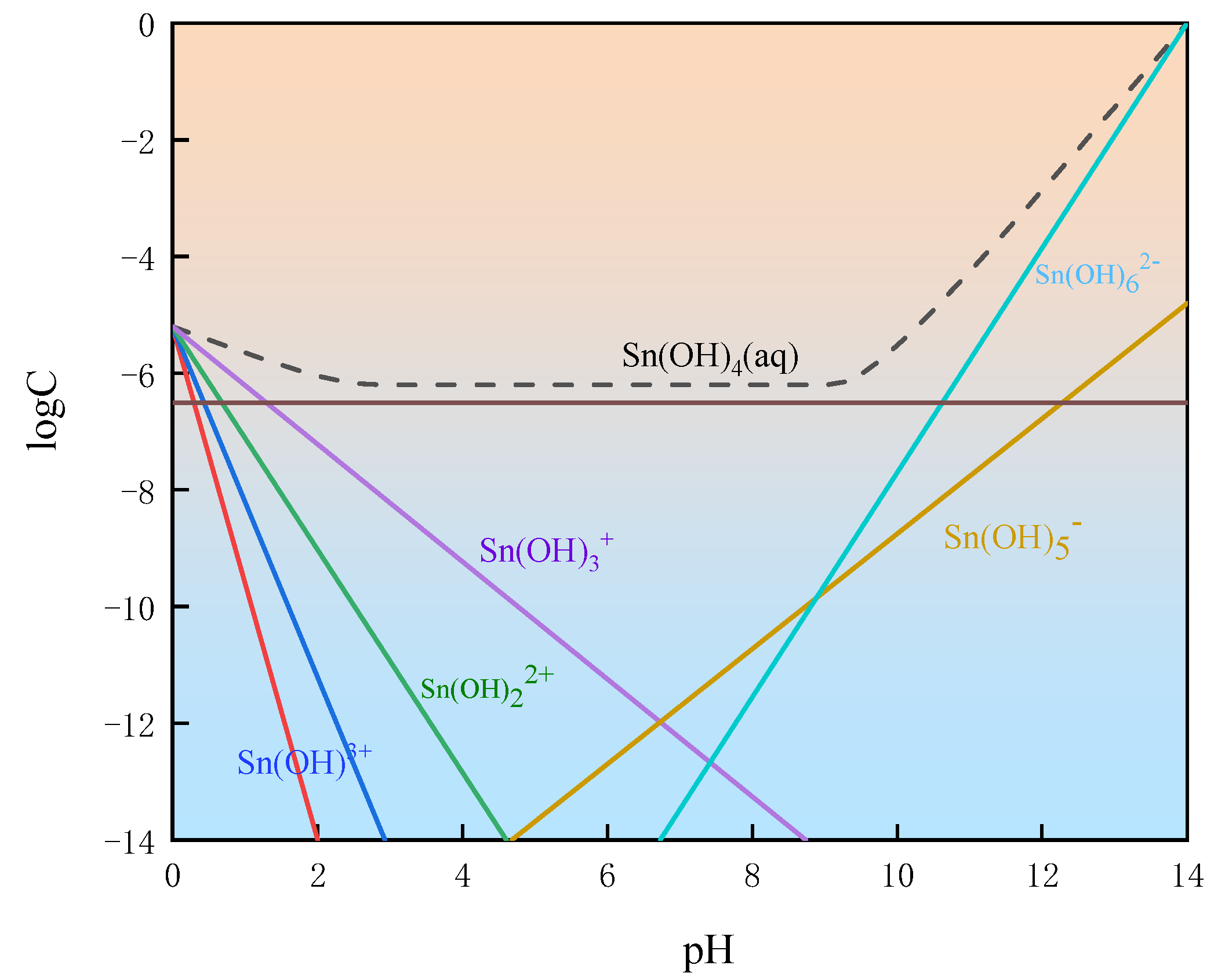
3.3. The Logarithmic Graph Dissolved Components of Cassiterite (LSD)
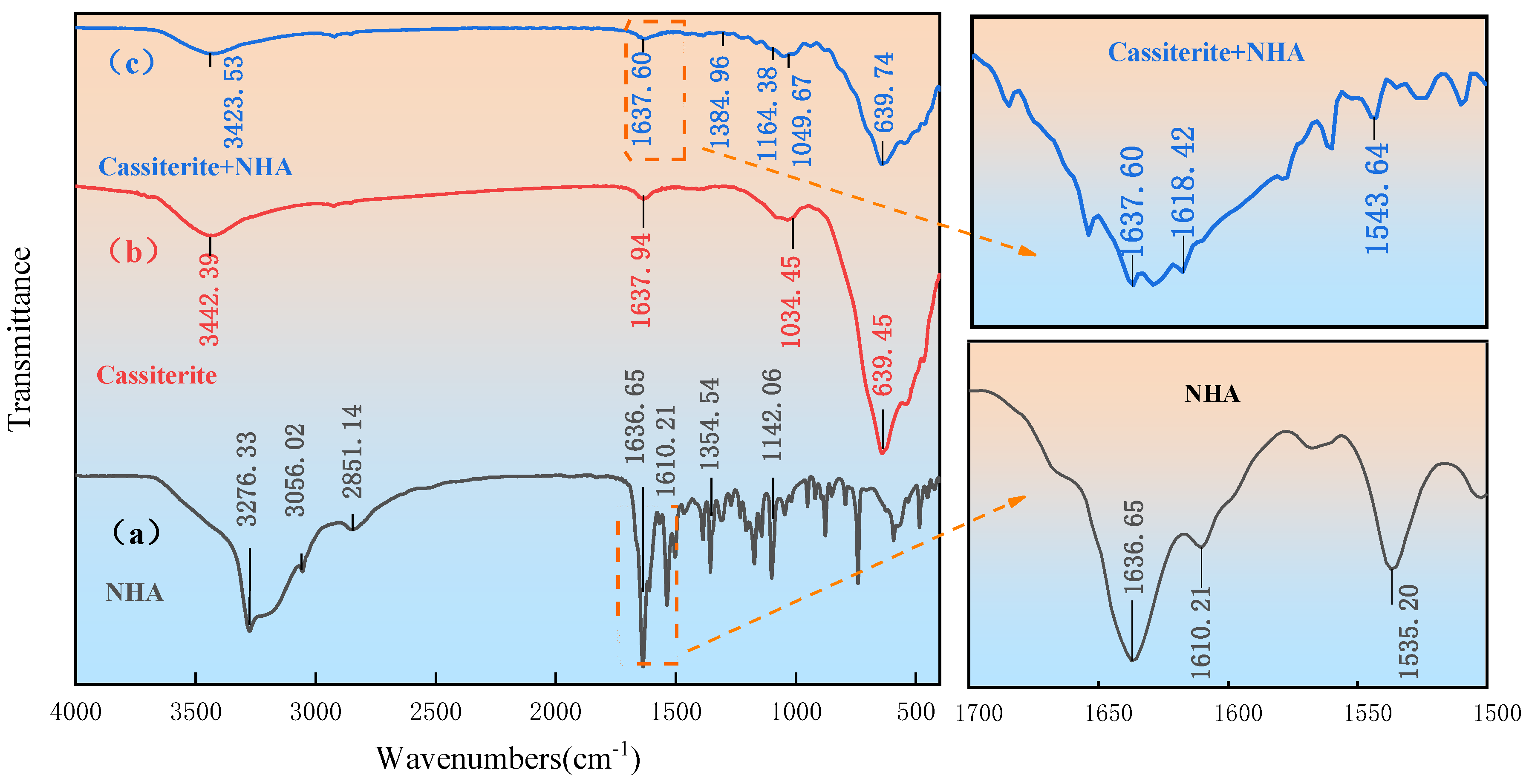
3.4. FT-IR Results
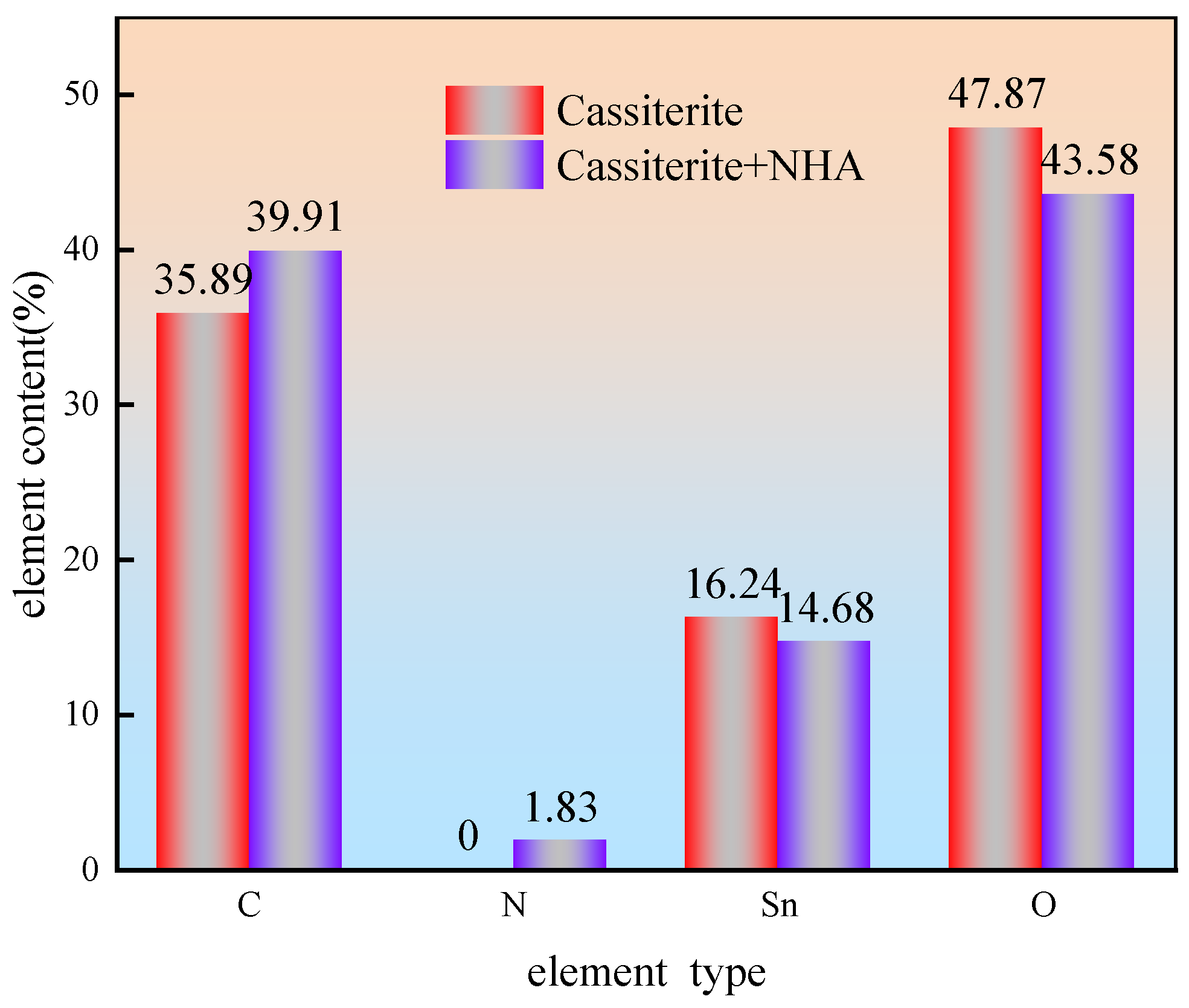
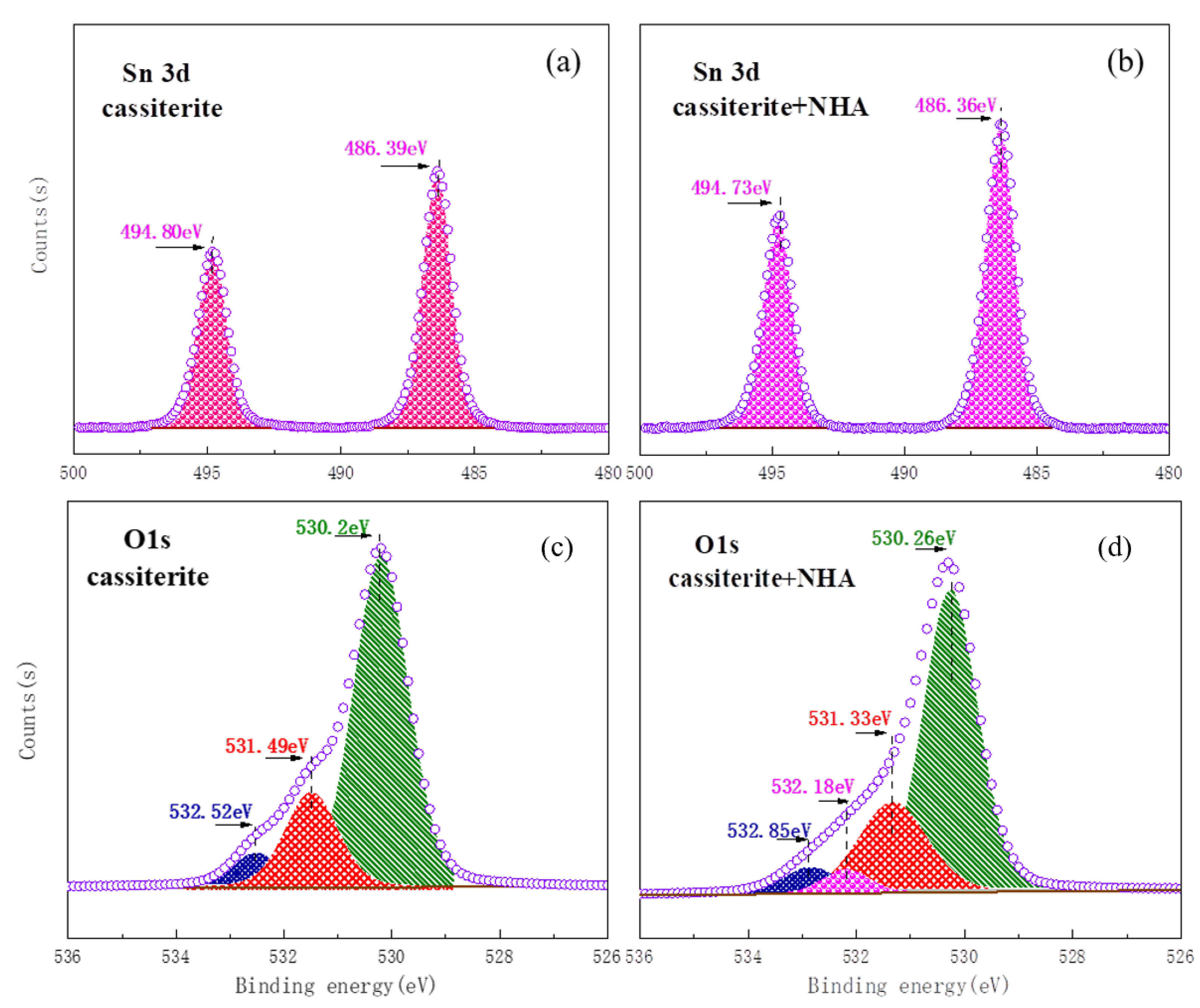
3.5. XPS Analysis
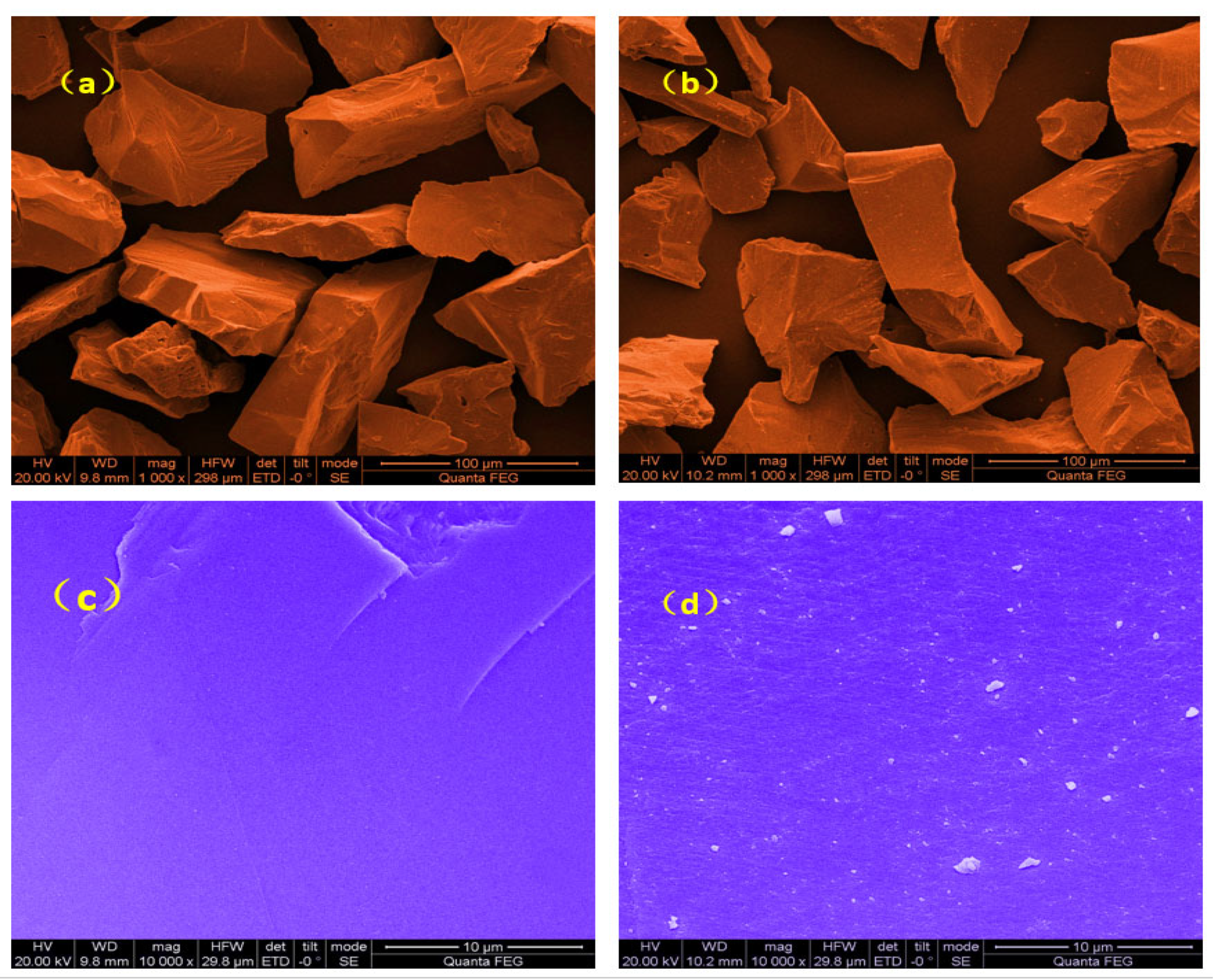
3.6. Scanning Electron Microscopy (SEM) Analysis
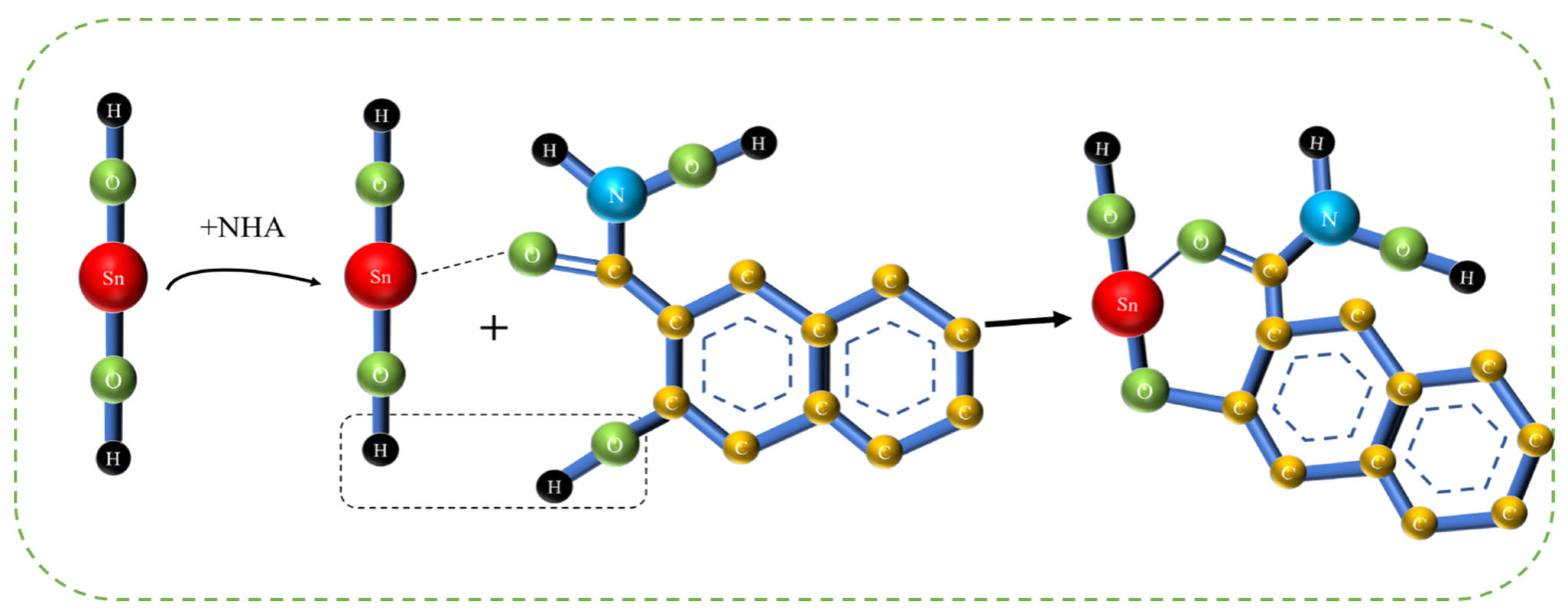
3.7. Adsorption Model and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Y. Comprehensive Utilization of Stannary Polymetallic Oxide. Mod. Min. 2009, 25, 20–22. [Google Scholar]
- Liu, J.; Han, Y.; Zhu, Y.; Chen, W. Research Status and Prospective on Separation Technology of Fine Cassiterite. Met. Mine. 2014, 10, 76–81. [Google Scholar]
- Senior, G.D. The Role of Dissolved Metal Ionic Species in the Phosphonic Acid Flotation of Cassiterite; University of British Columbia: Kelowna, Canada, 1987; p. 1. [Google Scholar]
- Zhang, H. Study on Mechanisms and Application of Combined Collectors in Fine Cassiterite Flotation; Central South University: Changsha, China, 2011. (In Chinese) [Google Scholar]
- Zhang, W. Study on the Mechanism and Application of Metal Ions in Cassiterite Flotation System; Central South University: Changsha, China, 2014. (In Chinese) [Google Scholar]
- He, M. Experimental study on high-silica fine-grained cassiterite. Nonferrous Met. (Miner. Process. Sect.) 2018, 1, 43–47. [Google Scholar]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Xu, J. Synthesis of 1-Hydroxy-2-naphthylhydroximic acids and application to collecting rare earth minerals. Nonferrous Met. 2002, 54, 72–73. [Google Scholar]
- Zhao, R. Experimental study on recovering rare-earth from the tailling. J. Inn. Mong. Univ. Sci. Technol. 2012, 1, 9–13. [Google Scholar]
- Xiong, W.; Wang, M.; Xiao, J.; Chen, D. Selective Adsorption of 2-Hydroxy-3-Naphthalene Hydroxamic Acid on the Surface of Bastnaesite and Calcite. Minerals 2022, 12, 1341. [Google Scholar] [CrossRef]
- Guo, Z.; Khoso, S.A.; Wang, J. Interaction mechanism of 2-hydroxy-3-naphthyl hydroxamic acid and l-hydroxy-2-naphthyl hydroxamic acid in the flotation separation of bastnaesite/fluorite: Experiments and first-principles calculations. Sep. Purif. Technol. 2021, 285, 120307. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Q. Flotation fine wolframite slime using naphthalene hydroxyl hydroxamic acid—A study. Min. Metall. Eng. 1998, 4, 35–37. [Google Scholar]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Qiu, H. Flotation and Its Mechanism of Fine Cassiterite; Wuhan University of Technology: Wuhan, China, 2018. (In Chinese) [Google Scholar]
- Gao, Y.; Qiu, X.; Wang, L. Study on the collecting performance and mechanism on wolframite using hydroxyoxime acid. Min. Metall. Eng. 2014, 34, 84–87. [Google Scholar]
- Liu, C.; Zhang, W.; Song, S.; Li, H. Study on the activation mechanism of lead ions in wolframite flotation using benzyl hydroxamic acid as the collector. Miner. Eng. 2019, 141, 105859. [Google Scholar] [CrossRef]
- Sałdyka, M.; Mielke, Z. Photochemistry of acetohydroxamic acid in solid argon, F.T.I.R.; theoretical studies. J. Phys. Chem. A 2018, 122, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Ke, L. Study on the Mechanism of Auxiliary Collector BYSN of Cassiterite; Central South University: Changsha, China, 2012; p. 425. (In Chinese) [Google Scholar]
- Chen, W. Research on the Surface Properties and Floatability of Fine Cassiterite; Northeastern University: Boston, MA, USA, 2014. (In Chinese) [Google Scholar]
- Zhu, Y. Research on the collection performance of fine-grained cassiterite by the new chelating collector, D.X.S. Mod. Min. 2016, 32, 67–69. [Google Scholar]
- Thomas, B.; Skariah, B. Spray deposited Mg-doped SnO2 thin film LPG sensor: X.P.S.; EDX analysis in relation to deposition temperature doping. J. Alloys Compd. 2015, 625, 231–240. [Google Scholar] [CrossRef]
- Shipochka, M.; Eliyas, A.; Stambolova, I.; Blaskov, V.; Vassilev, S.; Simeonova, S.; Balashev, K. Synthesis of TiO2 on SnO2 bicomponent system and investigation of its structure and photocatalytic activity. Mater. Chem. Phys. 2018, 220, 249–259. [Google Scholar] [CrossRef]
- Tian, M.; Sultan Ahmed, K.; Wang, L.; Sun, W.; Zhang, C.; Hu, Y. Selective separation behavior and its molecular mechanism of cassiterite from quartz using cupferron as a novel flotation collector with a lower dosage of Pb2+ ions. Appl. Surf. Sci. 2019, 486, 228–238. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Ou, L. Study on the depression mechanism of zinc sulfate on talc in chalcopyrite flotation. Colloids Surf. A 2021, 619, 126474. [Google Scholar] [CrossRef]
- Dascalu, I.; Somacescu, S.; Hornoiu, C.; Calderon-Moreno, J.M.; Stanica, N.; Stroescu, H.; Anastasescu, M.; Gartner, M. Solgel Zn, Fe modified SnO2 powders for CO sensors and magnetic applications. Process Saf. Environ. Prot. 2018, 117, 722–729. [Google Scholar] [CrossRef]
- Kwoka, M.; Waczynska, N.; Kościelniak, P.; Sitarz, M.; Szuber, J. X-ray photoelectron spectroscopy and thermal desorption spectroscopy comparative studies of L-CVD SnO2 ultra thin films. Thin Solid Film. 2011, 520, 913–917. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; He, J.; Hu, Y.; Sun, W.; Yuan, D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]
- Supothina, S.; Guire, M.R.D. Characterization of SnO2 thin films grown from aqueous solutions. Thin Solid Films 2000, 371, 1–9. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Khoso, S.A.; Sun, W.; Hu, Y. Understanding the Activation Mechanism of Pb2+ ion in Benzohydroxamic Acid Flotation of Spodumene: Experimental Findings and DFT Simulations. News Sci. Miner. Eng. 2019, 143, 106006. [Google Scholar] [CrossRef]
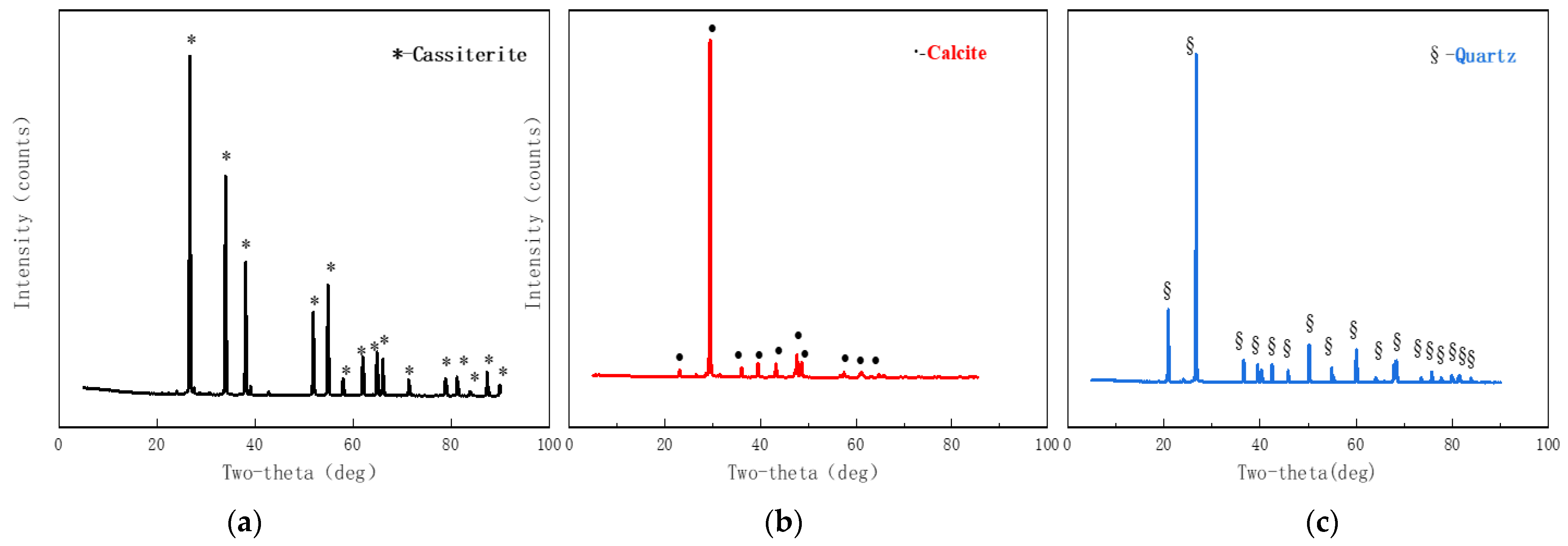
| Agent Name | Molecular Formula | Purity | Manufacturer |
|---|---|---|---|
| Deionized (DI) | H2O | Analytically pure. | BGRIMM Technology Group, Beijing, China |
| Waterhydrochloric acid | HCI | Analytically pure. | Sinopharm Chemical Reagent Co. Ltd., Shanghai, China |
| Sodium hydroxide | NaOH | Analytically pure. | Sinopharm Chemical Reagent Co. Ltd. |
| 2-hydroxy-3-naphthylmethyl hydroxamic acid | C11H8O3 | Analytically pure. | BGRIMM Technology Group |
| Pure terpenic oil | C10H18O2 | Analytically pure. | Sinopharm Chemical Reagent Co. Ltd. |
| Element (%) | Sn | CaCO3 | SiO2 | MgO | Fe | K2O | Cu |
|---|---|---|---|---|---|---|---|
| Cassiterite | 76.72 | – | – | 0.010 | 0.010 | 0.011 | <0.005 |
| Calcite | – | 99.42 | 0.22 | 0.052 | – | – | <0.005 |
| Quartz | – | – | 99.21 | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, Z.; Liu, F.; Zhao, C.; Zhu, Y. Selective Separation Behavior and Study on the Interaction Mechanism of 2-Hydroxy-3-Naphthylmethyl Hydroxamic Acid and Cassiterite. Minerals 2024, 14, 29. https://doi.org/10.3390/min14010029
Wu S, Wang Z, Liu F, Zhao C, Zhu Y. Selective Separation Behavior and Study on the Interaction Mechanism of 2-Hydroxy-3-Naphthylmethyl Hydroxamic Acid and Cassiterite. Minerals. 2024; 14(1):29. https://doi.org/10.3390/min14010029
Chicago/Turabian StyleWu, Shipeng, Zhongming Wang, Fang Liu, Chen Zhao, and Yangge Zhu. 2024. "Selective Separation Behavior and Study on the Interaction Mechanism of 2-Hydroxy-3-Naphthylmethyl Hydroxamic Acid and Cassiterite" Minerals 14, no. 1: 29. https://doi.org/10.3390/min14010029
APA StyleWu, S., Wang, Z., Liu, F., Zhao, C., & Zhu, Y. (2024). Selective Separation Behavior and Study on the Interaction Mechanism of 2-Hydroxy-3-Naphthylmethyl Hydroxamic Acid and Cassiterite. Minerals, 14(1), 29. https://doi.org/10.3390/min14010029






