Insights into the Heterogeneity of the Mercury Isotopic Fingerprint of the Idrija Mine (Slovenia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Preparation
2.3. Analysis
3. Results and Discussion
4. Conclusions
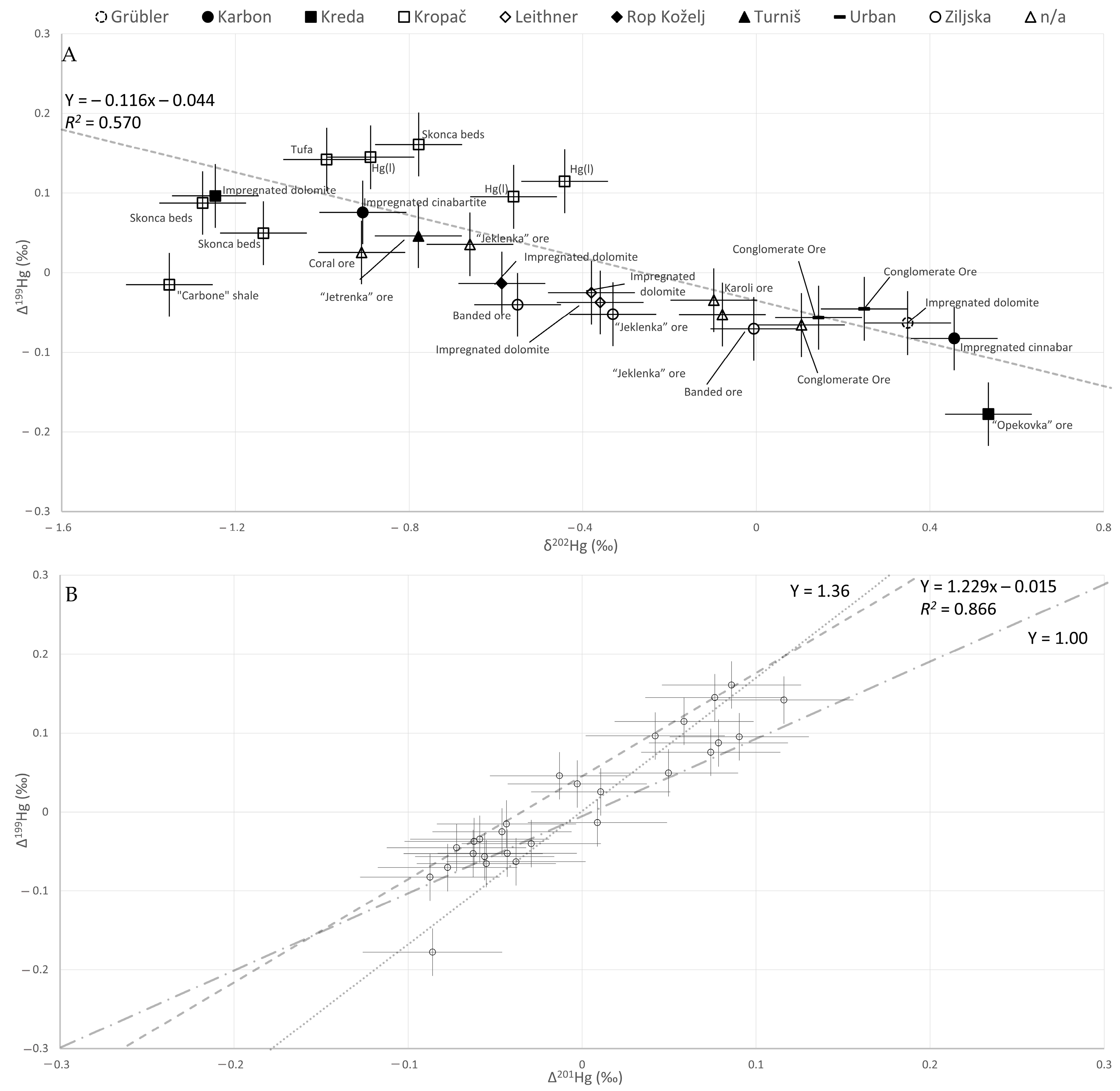
- The isotopic fingerprint of the Idrija mine is generally relatively broad as comparable to most other findings, with the MDF fingerprint (δ202Hg) ranging from −1.35‰ to 0.46‰.
- The MIF ranges are similar to those of other mines, with Δ199Hg ranging from −0.16‰ to 0.18‰.
- Only one example of a relatively homogenous fingerprint was observed at the Kropač excavation field.
- The isotopic fingerprints of cinnabar and Hg(l) from the same excavation site are statistically indistinguishable.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNEP. Guidance on Monitoring of Mercury and Mercury Compounds to Support Evaluation of the Effectiveness of the Minamata Convention; UNEP: Geneva, Switzerland, 2022. [Google Scholar]
- UNEP. Global Mercury Assessment 2018; UNEP: Geneva, Switzerland, 2019. [Google Scholar]
- Kwon, S.Y.; Blum, J.D.; Yin, R.; Tsui, M.T.-K.; Yang, Y.H.; Choi, J.W. Mercury stable isotopes for monitoring the effectiveness of the Minamata Convention on Mercury. Earth Sci. Rev. 2020, 203, 103111. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Minamata Convention on Mercury; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Branfireun, B.; Chételat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S.; Zhang, T.; et al. Science of the Total Environment Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklöf, K.; Meng, B.; Mitchell, C.; Osterwalder, S.; Schuster, P.F.; Webster, J.; et al. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647. [Google Scholar] [CrossRef]
- Berce, B. Geologija živosrebrnega rudišča Idrija. Geol. Trans. Rep. 1958, 4, 1–61. [Google Scholar]
- Placer, L. Rekonstrukcija krovne zgradbe idrijsko Žirovskega ozemlja. Geologija 1973, 16, 317–334. [Google Scholar]
- Placer, L. Tektonski Razvoj Idrijskega Rudišča [Structural History of the Idrija Mercury Deposit]. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 1982. Volume 94. [Google Scholar]
- Drovenik, M.; Dolenec, T.; Režun, B.; Pezdič, J. On the mercury ore from the Grübler orebody, Idrija. Geologija 1990, 33, 397–446. [Google Scholar] [CrossRef]
- Mlakar, I.; Drovenik, M. Strukturne in generske posebnosti idrijskega rudišča. Geologija 1971, 14, 67–126. [Google Scholar]
- Mlakar, I. Geological structure and mineralization of the Idrija ore deposit. Geologija 1972, 15, 47–62. [Google Scholar]
- Placer, L.; Čar, J. Srednjetriadna zgradba idrijskega ozemlja. Geologija 1977, 20, 141–166. [Google Scholar]
- Mlakar, I. Osnovni parametri proizvodnje rudnika Idrija skozi stoletja do danes. Irdijski Razgledi 1974, 21, 1–40. [Google Scholar]
- Kotnik, J.; Horvat, M.; Dizdarevič, T. Current and past mercury distribution in air over the Idrija Hg mine region, Slovenia. Atmos Environ. 2005, 39, 7570–7579. [Google Scholar] [CrossRef]
- Čar, J. Mineralized rocks and ore residues in the Idrija region. In Proceedings of the Meeting of Researchers o Idrija as a Natural and Anthoropogenic Laboratory—Mercury as a Major Pollutant, Idrija, Slovenija, 24–25 May 1996; pp. 10–15. [Google Scholar]
- Kocman, D.; Horvat, M. Non-point source mercury emission from the Idrija Hg-mine region: GIS mercury emission model. J. Environ. Manag. 2011, 92, 2038–2046. [Google Scholar] [CrossRef]
- Božič, D.; Živković, I.; Hudobivnik, M.J.; Kotnik, J.; Amouroux, D.; Štrok, M.; Horvat, M. Fractionation of mercury stable isotopes in lichens. Chemosphere 2022, 309, 136592. [Google Scholar] [CrossRef]
- Čar, J. Ladinian skonca beds of the Idrija Ore Deposit (W Slovenia). Geologija 2013, 56, 151–174. [Google Scholar]
- Tsui, M.T.K.; Blum, J.D.; Kwon, S.Y. Review of stable mercury isotopes in ecology and biogeochemistry. Sci. Total Environ. 2020, 716, 135386. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.D.; Sherman, L.S.; Johnson, M.W. Mercury isotopes in earth and environmental sciences. Annu. Rev. Earth Planet Sci. 2014, 42, 249–269. [Google Scholar] [CrossRef]
- Bergquist, B.A.; Blum, J.D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 318, 417–420. [Google Scholar] [CrossRef]
- Stetson, S.J.; Gray, J.E.; Wanty, R.B.; Macalady, D.L. Isotopic variability of mercury in ore, mine-waste calcine, and leachates of mine-waste calcine from areas mined for mercury. Environ. Sci. Technol. 2009, 43, 7331–7336. [Google Scholar] [CrossRef]
- Gray, J.E.; Pribil, M.J.; Higueras, P.L. Mercury isotope fractionation during ore retorting in the Almadén mining district, Spain. Chem. Geol. 2013, 357, 150–157. [Google Scholar] [CrossRef]
- Pribil, M.J.; Rimondi, V.; Costagliola, P.; Lattanzi, P.; Rutherford, D.L. Assessing mercury distribution using isotopic fractionation of mercury processes and sources adjacent and downstream of a legacy mine district in Tuscany, Italy. Appl. Geochem. 2020, 117, 104600. [Google Scholar] [CrossRef]
- Wiederhold, J.G.; Smith, R.S.; Siebner, H.; Jew, A.D.; Brown, G.E.; Bourdon, B.; Kretzschmar, R. Mercury isotope signatures as tracers for Hg cycling at the new idria Hg mine. Environ. Sci. Technol. 2013, 47, 6137–6145. [Google Scholar] [CrossRef]
- Donovan, P.M.; Blum, J.D.; Yee, D.; Gehrke, G.E.; Singer, M.B. An isotopic record of mercury in San Francisco Bay sediment. Chem. Geol. 2013, 349–350, 87–98. [Google Scholar] [CrossRef]
- Gehrke, G.E.; Blum, J.D.; Marvin-DiPasquale, M. Sources of mercury to San Francisco Bay surface sediment as revealed by mercury stable isotopes. Geochim. Cosmochim. Acta 2011, 75, 691–705. [Google Scholar] [CrossRef]
- Smith, R.S.; Wiederhold, J.G.; Jew, A.D.; Brown, G.E.; Bourdon, B.; Kretzschmar, R. Stable Hg isotope signatures in creek sediments impacted by a former Hg mine. Environ. Sci. Technol. 2015, 49, 767–776. [Google Scholar] [CrossRef]
- Smith, C.N.; Kesler, S.E.; Blum, J.D.; Rytuba, J.J. Isotope geochemistry of mercury in source rocks, mineral deposits and spring deposits of the California Coast Ranges, USA. Earth Planet. Sci. Lett. 2008, 269, 399–407. [Google Scholar] [CrossRef]
- Yin, R.; Feng, X.; Meng, B. Stable mercury isotope variation in rice plants (Oryza sativa L.) from the Wanshan mercury Mining District, SW China. Environ. Sci. Technol. 2013, 47, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Feng, X.; Wang, J.; Li, P.; Liu, J.; Zhang, Y.; Chen, J.; Zheng, L.; Hu, T. Mercury speciation and mercury isotope fractionation during ore roasting process and their implication to source identification of downstream sediment in the Wanshan mercury mining area, SW China. Chem. Geol. 2013, 336, 72–79. [Google Scholar] [CrossRef]
- Baptista-Salazar, C.; Hintelmann, H.; Biester, H. Distribution of mercury species and mercury isotope ratios in soils and river suspended matter of a mercury mining area. Environ. Sci. Process. Impacts 2018, 20, 621–631. [Google Scholar] [CrossRef]
- Foucher, D.; Ogrinc, N.; Hintelmann, H. Tracing mercury contamination from the Idrija mining region (Slovenia) to the gulf of Trieste using Hg isotope ratio measurements. Environ. Sci. Technol. 2009, 43, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Peel, K.; Weiss, D.; Chapman, J.; Arnold, T.; Coles, B. A simple combined sample-standard bracketing and inter-element correction procedure for accurate mass bias correction and precise Zn and Cu isotope ratio measurements. J. Anal. At. Spectrom. 2008, 23, 103–110. [Google Scholar] [CrossRef]
- National Institute of Standards & Technology. Certificate of Analysis Standard Reference Material 3133 Mercury (Hg) Standard Solution; Department of Commerce, National Institute of Standards & Technology: Gaithersburg, MD, USA, 2016.
- Blum, J.D.; Johnson, M.W. Recent developments in mercury stable isotope analysis. Rev. Miner. Geochem. 2017, 82, 733–757. [Google Scholar] [CrossRef]
- National Institute of Standards & Technology. Report of Investigation Reference Material 8610 Isotopes in UM-Almaden Mono-Elemental Secondary Standard This Mercury; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2017.
- Estrade, N.; Carignan, J.; Sonke, J.E.; Donard, O.F. Measuring hg isotopes in bio-geo-environmental reference materials. Geostand Geoanal Res. 2009, 34, 79–93. [Google Scholar] [CrossRef]
- Sherman, L.S.; Blum, J.D.; Nordstrom, D.K.; McCleskey, R.; Barkay, T.; Vetriani, C. Mercury isotopic composition of hydrothermal systems in the Yellowstone Plateau volcanic field and Guaymas Basin sea-floor rift. Earth Planet Sci. Lett. 2009, 279, 86–96. [Google Scholar] [CrossRef]
- Demers, J.D.; Blum, J.D.; Brooks, S.C.; Donovan, P.M.; Riscassi, A.L.; Miller, C.L.; Zheng, W.; Gu, B. Hg isotopes reveal in-stream processing and legacy inputs in East Fork Poplar Creek, Oak Ridge, Tennessee, USA. Environ. Sci. Process. Impacts 2018, 20, 686–707. [Google Scholar] [CrossRef] [PubMed]
| Type of Sample | Description |
|---|---|
| Carbone shale | Carbone shales are the oldest rocks of the Idrija ore deposit. They are composed of mud, clays, silt, and silicate sand with occasional inclusions of conglomerates, which are interchanged rapidly. Afterwards, the tectonic processes shape the shales into various bodies with the common name of Carbone shales. These rocks are gray to dark gray in color and very soft, which makes them difficult to excavate. Carbone shales include Hg(l) and cinnabar, with the ratio between the two commonly being 1:1. The Hg content reached up to 10% of Hg, but in most cases, it was around 0.3%. |
| Impregnated cinnabar | Impregnated cinnabar is represented by pyrite (FeS2) and cinnabar (HgS) concretions in mudstone that were impregnated with Hg(l). The concretion is composed of pyrite, with mostly idiomorphic cryosections substituted with HgS. A detailed description is given by Mlakar and Drovenik [11]. |
| Opekovka ore | Opekovka, or brick, ore was named by the miners. Opekovka ore is relatively common in the mine but is usually found in rather small deposits. |
| Jeklenka ore | Jeklenka, or steel, ore is a gaelic cinnabar type of ore composed of a cinnabar rich in organic matter. It has a fluidic structure and a metallic gray hue. |
| Impregnated dolomite | Dolomite CaMg(CO3)2 impregnated with Hg, mostly as cinnabar, which fills in the pores in dolomite and, in some cases, substitutes for the original rock. |
| Skonca beds | A geological formation containing both cinnabar and Hg(l). A detailed description is offered by Čar [19] |
| Hg(l) | Elemental Hg0 in liquid form. |
| Tufa | Tufa is composed of particles of silicate (SiO2), claystone, and plankton radiolites and some other particles. The matrix is mostly chalcedony (SiO2, trigonal) and pyrite. In the polished thin sections, a gradual granularity can be observed. The color ranges from green, in Tufa that is poorer in Hg, to the red, in Tufa that is richer in Hg. |
| Banded ore | Banded ore presents an interchanging sequence of layers of mudstone with cinnabar-impregnated chalcedony. Many infield fractures with cinnabar are observed. The chalcedony impregnated with cinnabar has the distinct color of Opekovka ore. |
| Jetrenka ore | Jetrenka, or liver, ore is syngenetically formed Hg in bituminous shale or, in some cases, a bituminous radiolarite. Jetrenka ores are found next to slip-faults where the organic matter mixed with cinnabar got smeared over the fault and produced a distinct liver-like color. |
| Conglomerate ore | Conglomerate ore consists of unsorted, differently sized rounded clasts in a sand-sized dolomite matrix. It contains veins and impregnations of cinnabar, which primarily substitutes for the matrix. |
| Coral ore | Coral ore is a syngenetic ore represented by black bituminous silicate sandstone that is rich in brachiopod shales. Miners mistook the brachiopod shells for corals, giving this ore its name. It is a part of the Skonca beds. |
| Karoli ore | Karoli ores are among the most unique ores of the deposit, as they are only found in the deepest part of the mine of the same name. The genesis of this type of ore has not been sufficiently studied. It is, however, known that it is an epigenetic ore with strong pyrite mineralization, which was later crushed and substituted with cinnabar. |
| Parameter | Setting |
|---|---|
| Sampler cone | Ni, FB9 |
| Skimmer cone | Ni, HS1-7 |
| RF power | 1300 W |
| Ar cooling gas | 13.0 L/min |
| Ar auxiliary gas | 0.8 L/min |
| Ar sweep gas flow | 20 mL/min |
| Ar mix gas flow | 70 mL/min |
| SnCl2 and sample uptake rate | 0.9–1.1 mL/min |
| Mass separation | 1 |
| Blocks | 1 |
| Measurements per block | 30 |
| Magnet delay time [s] | 2 |
| Transfer time [s] | 50 |
| Wash time—5% HNO3 | 10 s |
| Analytical concentration | 1–1.5 ng/mL |
| Sensitivity | 1–5 V |
| Total analysis time | 11 min |
| Sample | δ199Hg | δ200Hg | δ201Hg | δ202Hg | δ204Hg | Δ199Hg | Δ200Hg | Δ201Hg | Δ204Hg | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (‰) | (‰) | (‰) | (‰) | (‰) | (‰) | (‰) | (‰) | (‰) | ||||
| NIST 8610 | This study | N = 16 | Avg. | −0.15 | −0.27 | −0.45 | −0.54 | −0.82 | −0.01 | 0.01 | −0.04 | −0.01 |
| 2SD | (0.04) | (0.05) | (0.08) | (0.10) | (0.17) | (0.04) | (0.03) | (0.03) | (0.04) | |||
| [38] | n.a. | Avg. | −0.17 | −0.27 | −0.46 | −0.56 | −0.82 | −0.03 | −0,00 | −0.04 | 0.00 | |
| 2SD | (0.01) | (0.01) | (0.02) | (0.03) | (0.07) | (0.02) | (0.01) | (0.01) | (0.02) | |||
| NIST 2711a | This study | N = 6 | Avg. | −0.20 | −0.07 | −0.22 | −0.14 | −0.19 | −0.16 | 0.00 | −0.11 | 0.02 |
| 2SD | (0.04) | (0.07) | (0.09) | (0.12) | (0.17) | (0.03) | (0.02) | (0.02) | (0.05) | |||
| [39] | N = 6 | Avg. | −0.26 | −0.11 | −0.34 | −0.24 | n.a. | −0.20 | 0.01 | −0.16 | n.a. | |
| 2SD | (0.22) | (0.24) | (0.27) | (0.37) | n.a. | (0.17) | (0.15) | (0.10) | n.a. | |||
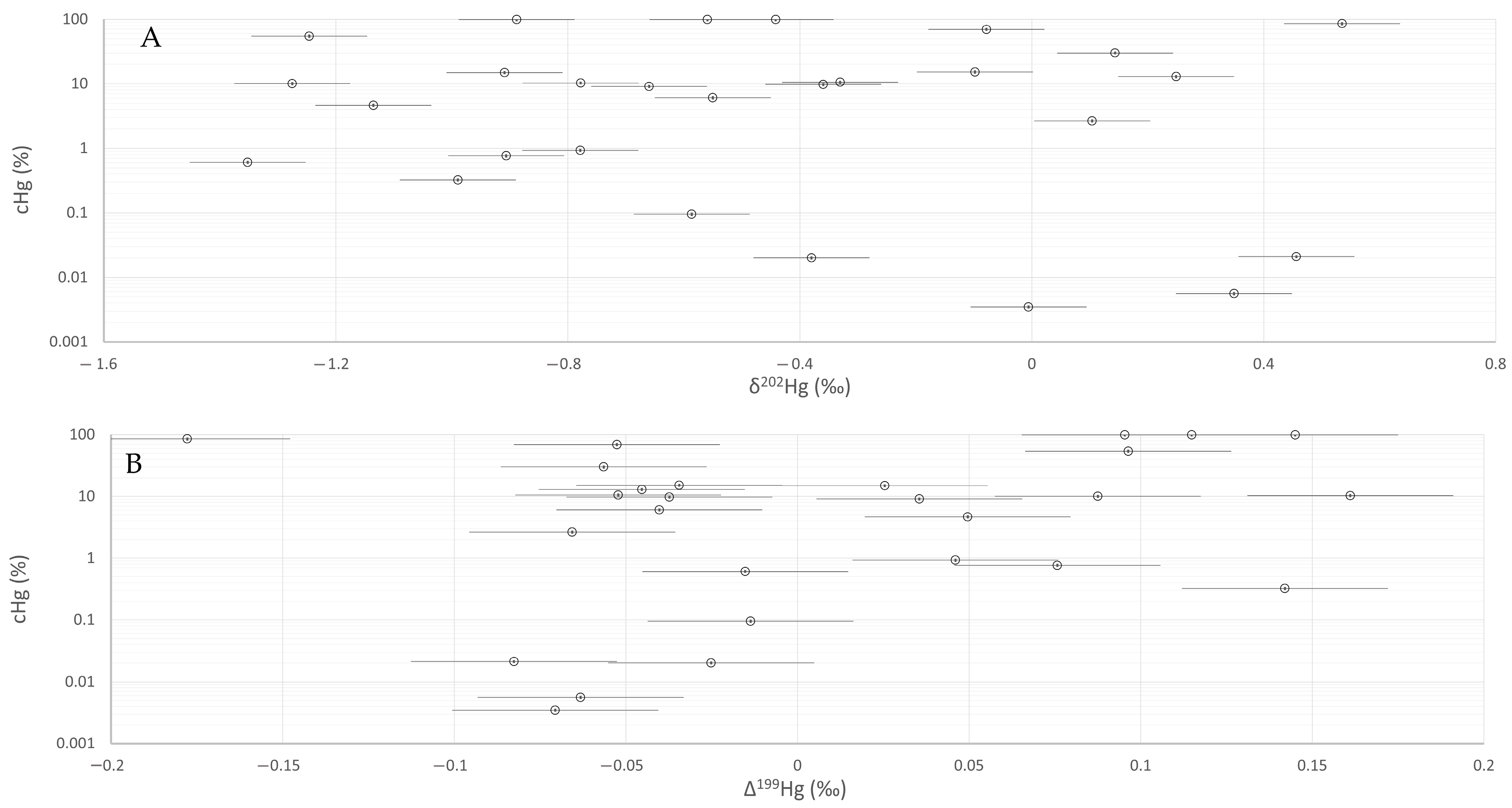
| Type of Sample and Museum Identification | Geologic Period [Ma] | Excavation Field and Level | δ199Hg (‰) | δ200Hg (‰) | δ201Hg (‰) | δ202Hg (‰) | δ204Hg (‰) | Δ199Hg (‰) | Δ200Hg (‰) | Δ201Hg (‰) | Δ204Hg (‰) | cHg (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbone shale | Carboniferous (358.9–298.9) | Kropač (I/20) | −0.36 | −0.67 | −1.06 | −1.35 | −2.03 | −0.02 | 0.01 | −0.04 | −0.01 | 0.608 |
| Impregnated cinnabar (499) | Karbon (III/4) | 0.03 | 0.21 | 0.26 | 0.46 | 0.63 | −0.08 | −0.02 | −0.09 | −0.05 | 0.021 | |
| Impregnated cinnabar (508) | Karbon (III/4) | −0.15 | −0.42 | −0.61 | −0.91 | −1.37 | 0.08 | 0.04 | 0.07 | −0.02 | 0.767 | |
| Opekovka ore (513) | Middle Permian (272.9–259.1) | Kreda (VI) | −0.04 | 0.24 | 0.32 | 0.53 | 0.87 | −0.18 | −0.03 | −0.09 | 0.07 | 85.5 |
| Jeklenka ore (525) | n./a. | −0.07 | −0.05 | −0.12 | −0.08 | −0.13 | −0.05 | −0.01 | −0.06 | −0.02 | 69.5 | |
| Impregnated dolomite (542) | Lower Triassic (251.9–247.2) | Grübler (XIII/8) | 0.02 | 0.19 | 0.22 | 0.35 | 0.53 | −0.06 | 0.02 | −0.04 | 0.01 | 0.006 |
| Impregnated dolomite (559) | Kreda (VII) | −0.22 | −0.61 | −0.90 | −1.25 | −1.89 | 0.10 | 0.02 | 0.04 | −0.03 | 54.4 | |
| Impregnated dolomite (964) | Rop Koželj (n.a.) | −0.16 | −0.27 | −0.43 | −0.59 | −0.88 | −0.01 | 0.02 | 0.01 | 0.00 | 0.095 | |
| Impregnated dolomite (806) | Anisian (247.2–242.0) | Leithner (III) | −0.12 | −0.19 | −0.33 | −0.38 | −0.56 | −0.03 | 0.00 | −0.05 | 0.01 | 0.020 |
| Impregnated dolomite (807) | Leithner (III) | −0.13 | −0.18 | −0.33 | −0.36 | −0.54 | −0.04 | 0.00 | −0.06 | 0.00 | 9.75 | |
| Skonca beds | Ladinian (242–237) | Kropač (I/20) | −0.24 | −0.51 | −0.80 | −1.14 | −1.71 | 0.05 | 0.06 | 0.05 | −0.01 | 4.66 |
| Skonca beds | Kropač (I/20) | −0.04 | −0.35 | −0.50 | −0.78 | −1.19 | 0.16 | 0.04 | 0.09 | −0.03 | 10.3 | |
| Skonca beds | Kropač (I/17) | −0.23 | −0.62 | −0.88 | −1.28 | −1.92 | 0.09 | 0.02 | 0.08 | −0.01 | 10.0 | |
| Hg(l) | Kropač (I/20) | −0.05 | −0.24 | −0.33 | −0.56 | −0.88 | 0.10 | 0.04 | 0.09 | −0.04 | 100 | |
| Hg(l) | Kropač (I/20) | −0.08 | −0.41 | −0.59 | −0.89 | −1.33 | 0.14 | 0.04 | 0.08 | −0.01 | 100 | |
| Hg(l) | Kropač (I/20) | 0.00 | −0.16 | −0.27 | −0.44 | −0.64 | 0.11 | 0.06 | 0.06 | 0.02 | 100 | |
| Tufa | Kropač (I/20) | −0.11 | −0.46 | −0.63 | −0.99 | −1.50 | 0.14 | 0.04 | 0.12 | −0.03 | 0.324 | |
| Banded ore (404) | Ziljska (I/15) | −0.18 | −0.27 | −0.44 | −0.55 | −0.87 | −0.04 | 0.01 | −0.03 | −0.05 | 6.07 | |
| Banded ore (414) | Ziljska I/16) | −0.07 | 0.01 | −0.08 | −0.01 | 0.06 | −0.07 | 0.01 | −0.08 | 0.07 | 0.003 | |
| Jeklenka ore (433) | Ziljska (I/14) | −0.14 | −0.16 | −0.29 | −0.33 | −0.50 | −0.05 | 0.00 | −0.04 | −0.01 | 10.57 | |
| Jeklenka ore (435) | n.a. | −0.13 | −0.32 | −0.50 | −0.66 | −1.01 | 0.04 | 0.02 | 0.00 | −0.02 | 9.10 | |
| Conglomerate Ore (580) | Urban (IV) | −0.02 | 0.08 | 0.05 | 0.14 | 0.21 | −0.06 | 0.00 | −0.06 | 0.00 | 30.1 | |
| Conglomerate Ore (581) | Urban (IV) | 0.02 | 0.13 | 0.11 | 0.25 | 0.36 | −0.05 | 0.00 | −0.07 | −0.01 | 13.0 | |
| Jetrenka ore (944) | Turniš (I/4) | −0.15 | −0.38 | −0.60 | −0.78 | −1.22 | 0.05 | 0.01 | −0.01 | −0.06 | 0.929 | |
| Conglomerate Ore | n.a. | n.a. | −0.04 | 0.07 | 0.02 | 0.10 | 0.15 | −0.07 | 0.01 | −0.06 | −0.01 | 2.65 |
| Coral ore (999) | n.a. | −0.20 | −0.45 | −0.67 | −0.91 | −1.39 | 0.03 | 0.01 | 0.01 | −0.03 | 14.9 | |
| Karoli ore | n.a. | −0.06 | −0.04 | −0.13 | −0.10 | −0.16 | −0.03 | 0.01 | −0.06 | −0.01 | 15.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božič, D.; Živković, I.; Dizdarević, T.; Peljhan, M.; Štrok, M.; Horvat, M. Insights into the Heterogeneity of the Mercury Isotopic Fingerprint of the Idrija Mine (Slovenia). Minerals 2023, 13, 1227. https://doi.org/10.3390/min13091227
Božič D, Živković I, Dizdarević T, Peljhan M, Štrok M, Horvat M. Insights into the Heterogeneity of the Mercury Isotopic Fingerprint of the Idrija Mine (Slovenia). Minerals. 2023; 13(9):1227. https://doi.org/10.3390/min13091227
Chicago/Turabian StyleBožič, Dominik, Igor Živković, Tatjana Dizdarević, Martina Peljhan, Marko Štrok, and Milena Horvat. 2023. "Insights into the Heterogeneity of the Mercury Isotopic Fingerprint of the Idrija Mine (Slovenia)" Minerals 13, no. 9: 1227. https://doi.org/10.3390/min13091227
APA StyleBožič, D., Živković, I., Dizdarević, T., Peljhan, M., Štrok, M., & Horvat, M. (2023). Insights into the Heterogeneity of the Mercury Isotopic Fingerprint of the Idrija Mine (Slovenia). Minerals, 13(9), 1227. https://doi.org/10.3390/min13091227







