Mineralogy of Agates with Amethyst from the Tevinskoye Deposit (Northern Kamchatka, Russia)
Abstract
1. Introduction
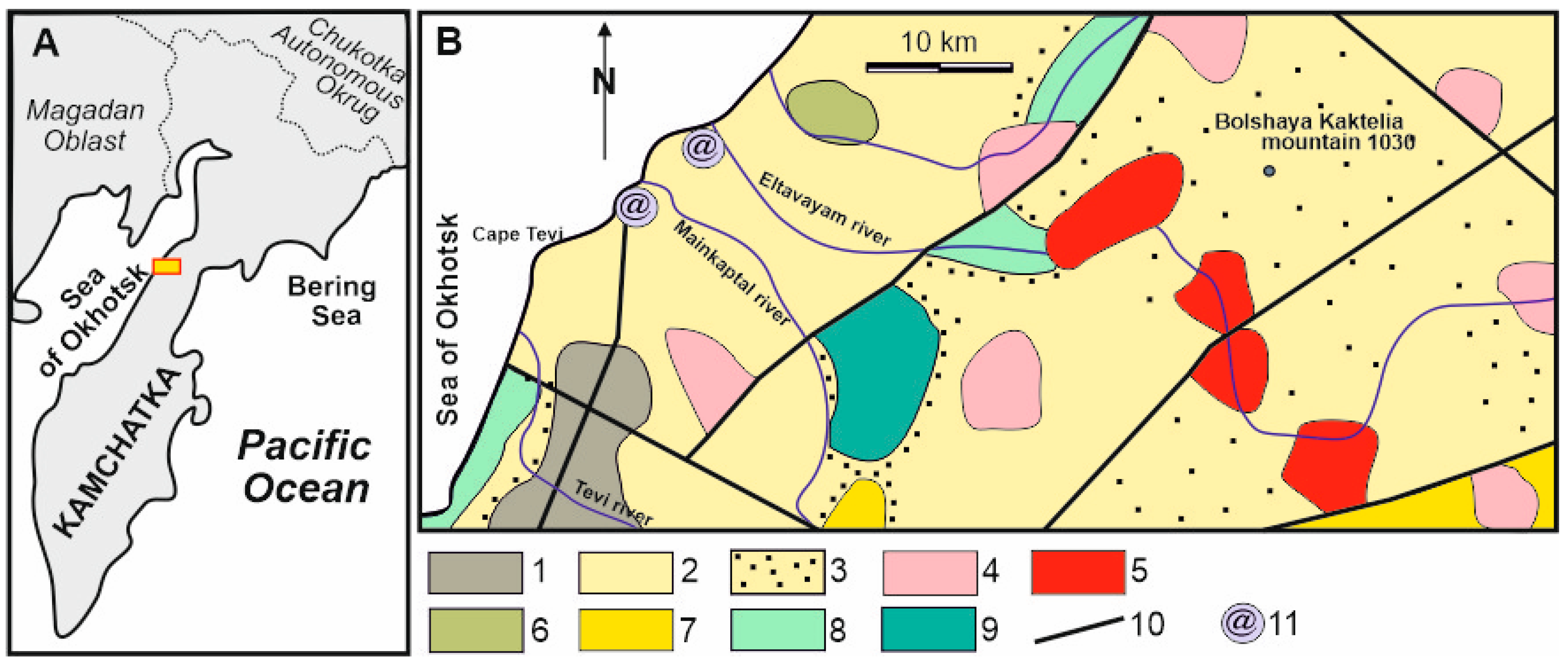
2. Geological Setting
2.1. Host Rocks
2.2. Tevinskoye Deposit Description
3. Materials and Methods
4. Results
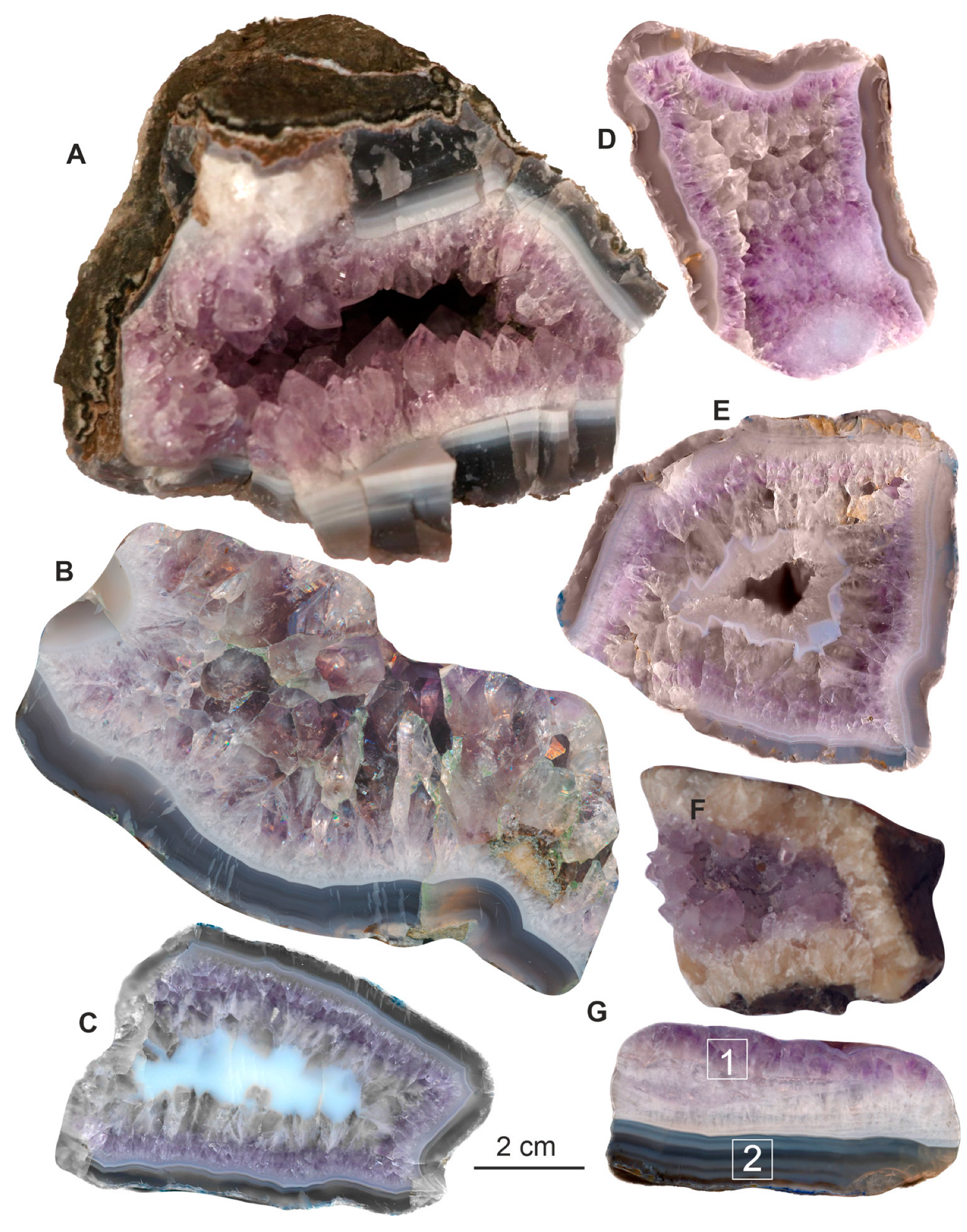
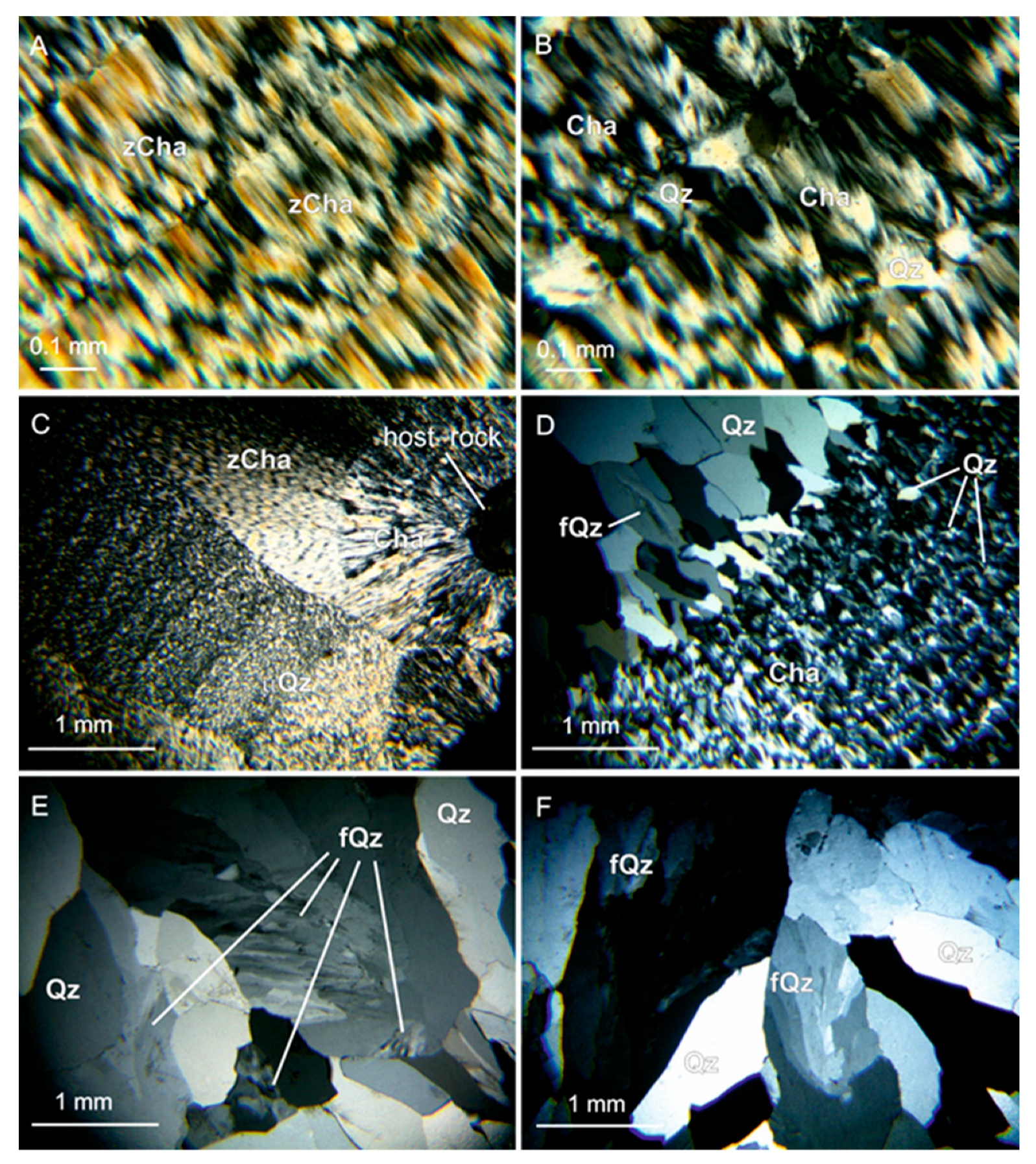
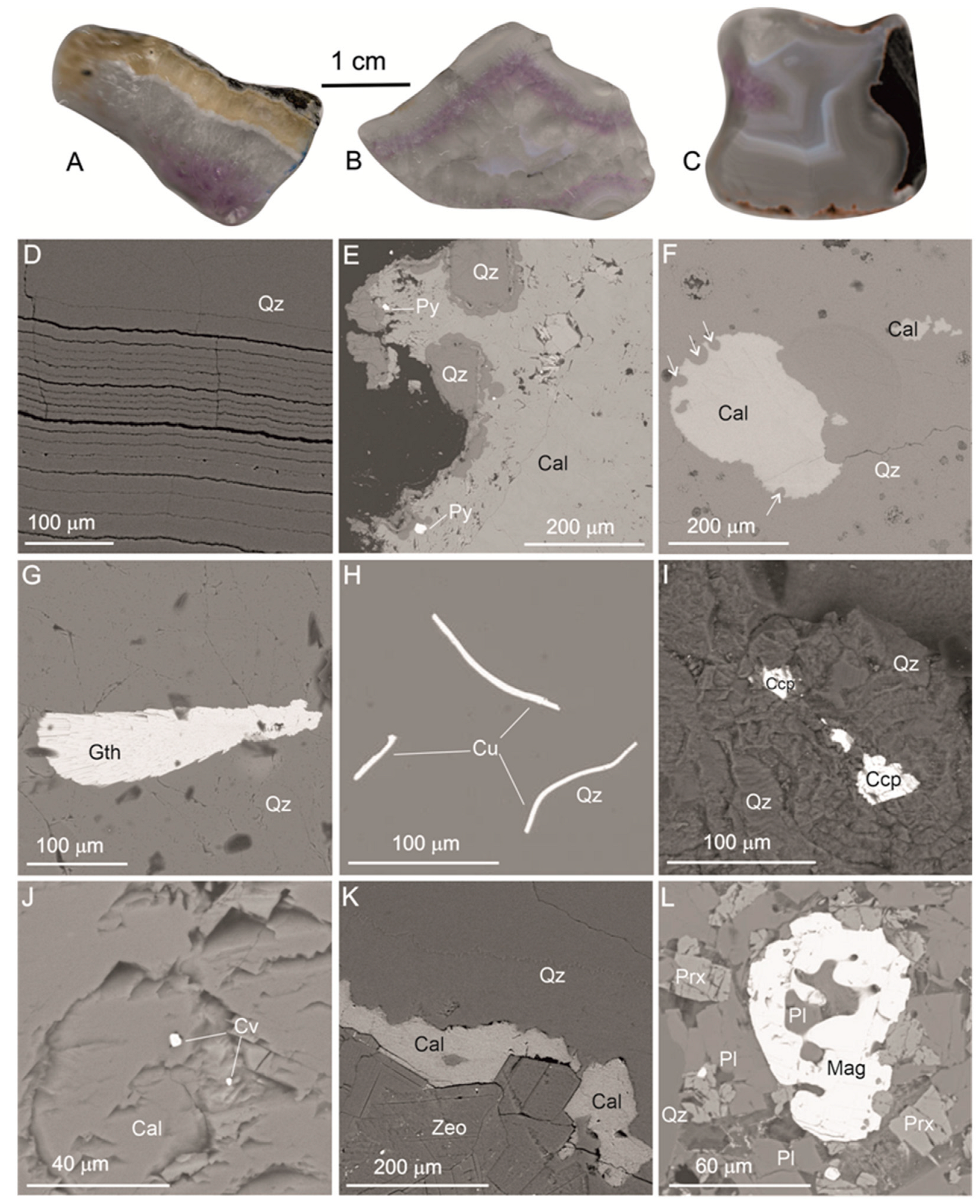
4.1. Macro- and Microscopic Observation
4.2. SEM-EDS Investigation
4.3. X-ray Diffraction
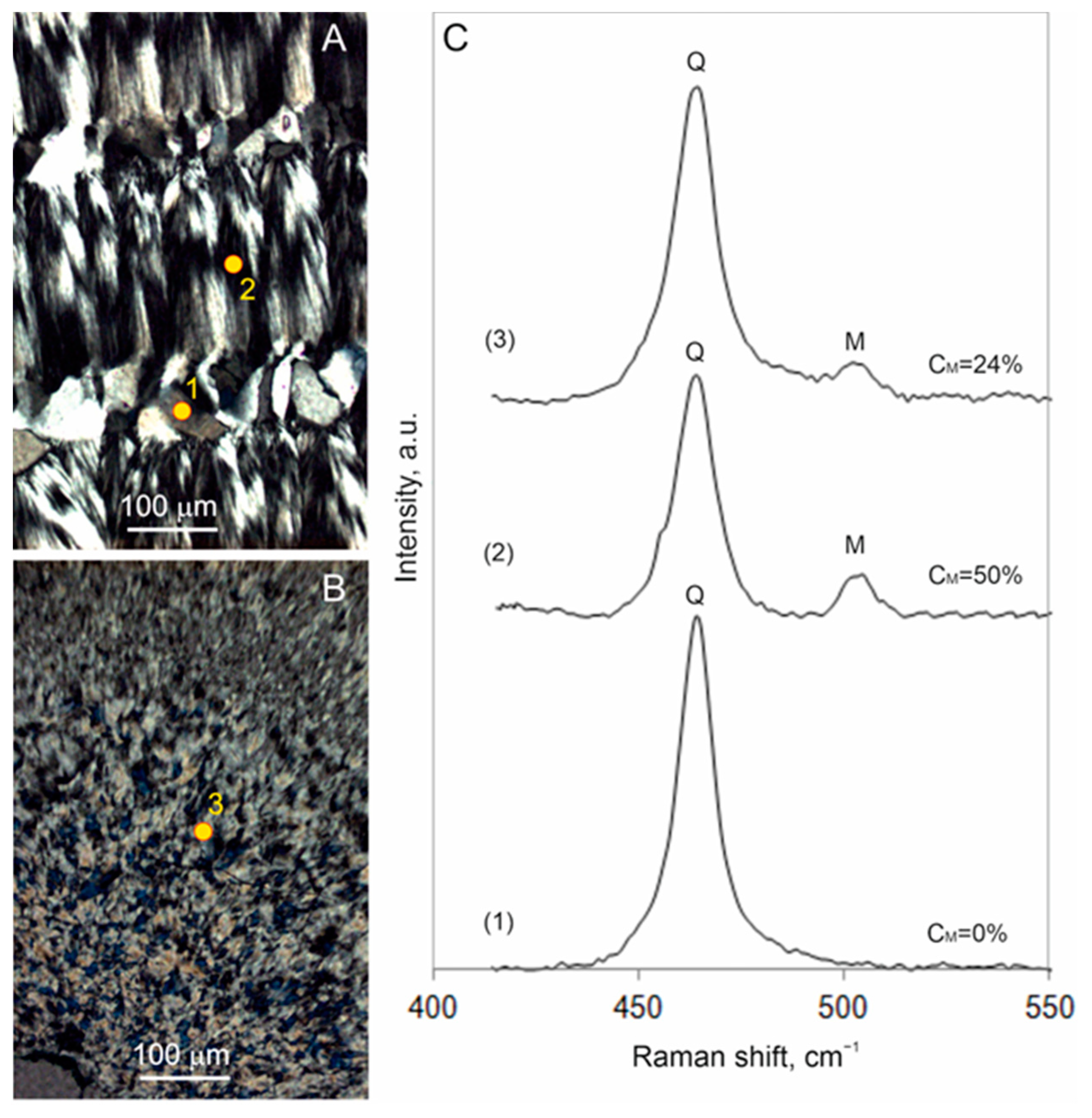
4.4. Raman Spectroscopy
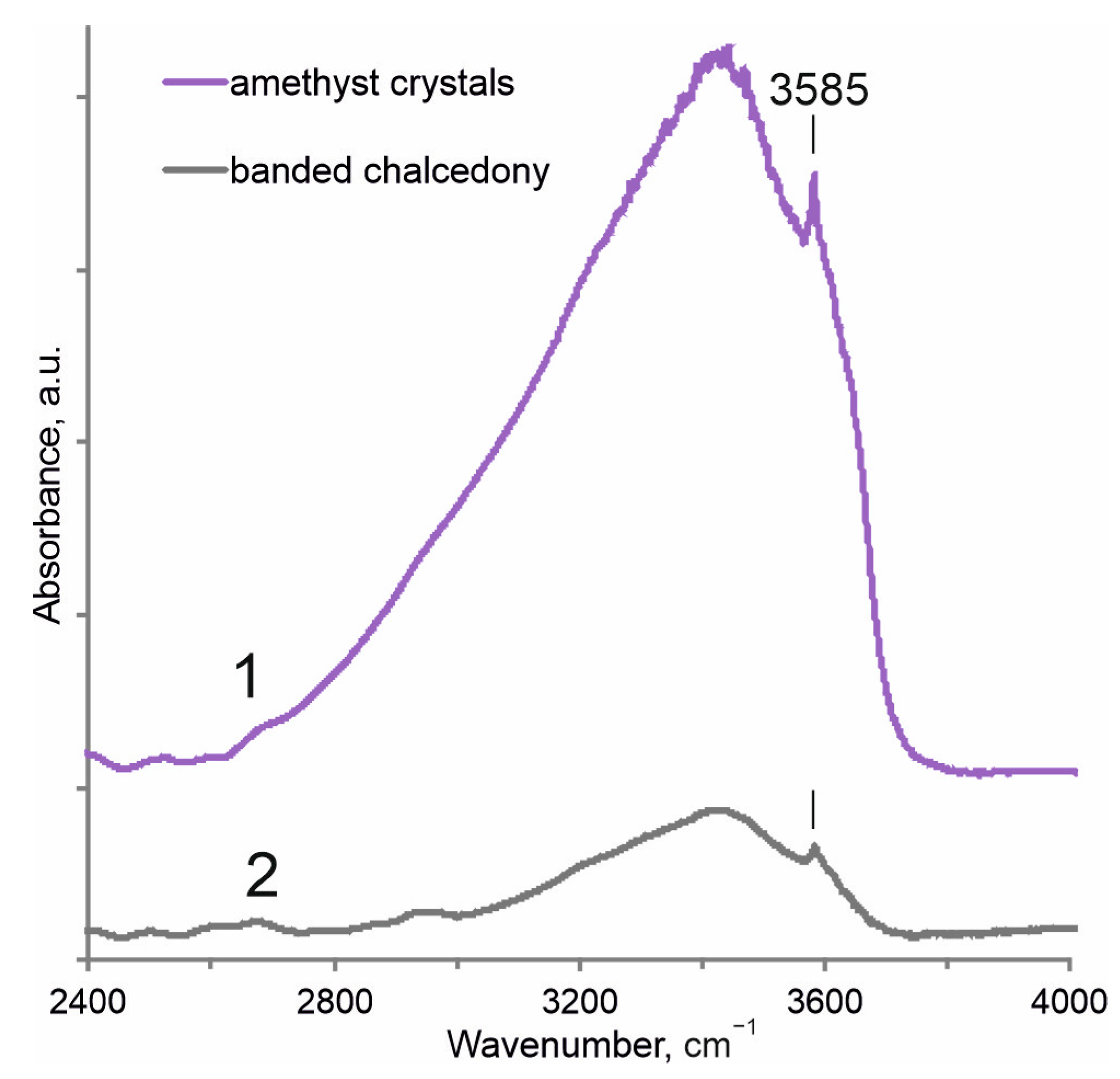
4.5. IR Spectroscopy
4.6. Fluid Inclusions
4.7. Oxygen Isotope Composition
5. Discussion
5.1. Micro-Texture of Agate and Amethyst
5.2. Water in Quartz and Chalcedony
5.3. Mineral Assemblage of Amethyst-Bearing Agate Occurrences
5.4. T,P,X-Conditions of Amethyst Formation in Agate Geodes in Altered Volcanic Rocks
5.5. Genesis of Amethyst in Agates
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Guidelines for the Search and Prospective Assessment of Deposits of Colored Stones (Jewelry, Ornamental, Decorative Facing); v.1. Amethyst; Mingeo USSR: Moscow, Russia, 1974; p. 43. (In Russian)
- Lieber, W. Amethyst: Geschichte, Eigenschaften, Fundorte; Christian Weise Verlag: München, Germany, 1994; 188p. [Google Scholar]
- Klemme, S.; Berndt, J.; Mavrogonatos, C.; Flemetakis, S.; Baziotis, I.; Voudouris, P.; Xydous, S. On the Color and Genesis of Prase (Green Quartz) and Amethyst from the Island of Serifos, Cyclades, Greece. Minerals 2018, 8, 487. [Google Scholar] [CrossRef]
- Commin-Fischer, A.; Berger, G.; Polvé, M.; Dubois, M.; Sardini, P.; Beaufort, D.; Formoso, M.L.L. Petrography and chemistry of SiO2 filling phases in the amethyst geodes from the Serra Geral Formation deposit, Rio Grande do Sul, Brazil. J. S. Am. Earth Sci. 2010, 29, 751–760. [Google Scholar] [CrossRef]
- Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gilg, H.A. The genesis of the amethyst geodes at Artigas (Uruguay) and the paleohydrology of the Guarani aquifer: Structural, geochemical, oxygen, carbon, strontium isotope and fluid inclusion study. Int. J. Earth Sci. 2010, 99, 927–947. [Google Scholar] [CrossRef]
- Hartmann, L.A.; Duarte, L.C.; Massonne, H.J.; Michelin, C.; Rosenstengel, L.M.; Bergmann, M.; Theye, T.; Pertille, J.; Arena, K.R.; Duarte, S.K.; et al. Sequential opening and filling of cavities forming vesicles, amygdales and giant amethyst geodes in lavas from the southern Paraná volcanic province, Brazil and Uruguay. Int. Geol. Rev. 2012, 54, 1–14. [Google Scholar] [CrossRef]
- Duarte, L.C.; Hartmann, L.A.; Vasconcelos, M.A.Z.; Medeiros, J.T.N.; Theye, T. Epigenetic formation of amethyst-bearing geodes from Los Catalanes gemological district, Artigas, Uruguay, southern Paraná Magmatic Province. J. Volcanol. Geotherm. Res. 2009, 184, 427–436. [Google Scholar] [CrossRef]
- Duarte, L.C.; Hartmann, L.A.; Ronchi, L.H.; Berner, Z.; Theye, T.; Massonne, H.J. Stable isotope and mineralogical investigation of the genesis of amethyst geodes in the Los Catalanes gemological district, Uruguay, southernmost Paraná volcanic province. Miner. Depos. 2011, 46, 239–255. [Google Scholar] [CrossRef]
- Pršek, J.; Dumańska-Słowik, M.; Powolny, T.; Natkaniec-Nowak, L.; Toboła, T.; Zych, D.; Skrepnicka, D. Agates from WesternAtlas (Morocco)—Constraints from Mineralogical and Microtextural Characteristics. Minerals 2020, 10, 198. [Google Scholar] [CrossRef]
- Godovikov, A.A.; Ripinen, O.I.; Motorin, S.G. Agates; Nedra: Moscow, Russia, 1987; p. 368. (In Russian) [Google Scholar]
- Goncharov, V.I.; Gorodinsky, M.E.; Pavlov, G.F.; Savva, N.E.; Fadeev, A.P.; Vartanov, V.V.; Gunchenko, E.V. Chalcedony of North.-East. of the USSR; Science: Moscow, Russia, 1987; p. 192. (In Russian) [Google Scholar]
- Rozhkova, V.V. Morphology and properties of the North Timan agates. Proc. Inst. Geol. Komi Phil. Acad. Sci. USSR 1984, 46, 70–77. (In Russian) [Google Scholar]
- Sedov, B.M. Upper Olsky Agates; Okhotnik, Russia, 2019; 244p. [Google Scholar]
- Svetova, E.; Svetov, S. Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia). Minerals 2020, 10, 1106. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Tarantola, A.; Götze, J.; Alfieris, D.; Maneta, V.; Psimis, I. Amethyst Occurrences in Tertiary Volcanic Rocks of Greece: Mineralogical, Fluid Inclusion and Oxygen Isotope Constraints on Their Genesis. Minerals 2018, 8, 324. [Google Scholar] [CrossRef]
- Kigai, I.N. The genesis of agates and amethyst geodes. Can. Miner. 2019, 57, 867–883. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, Geochemistry and Genesis of Agate—A Review. Minerals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Moxon, T.; Palyanova, G. Agate Genesis: A Continuing Enigma. Minerals 2020, 10, 953. [Google Scholar] [CrossRef]
- Palyanova, G. Editorial for Special Issue “Agates: Types, Mineralogy, Deposits, Host Rocks, Ages and Genesis”. Minerals 2021, 11, 1035. [Google Scholar] [CrossRef]
- Slyadnev, B.I.; Borovtsov, A.K.; Burmakov, Y.A.; Sidorenko, V.I.; Sapozhnikova, L.P.; Tararin, I.A.; Badredinov, Z.G.; Surikov, S.N.; Sidorov, M.D.; Sidorov, E.G.; et al. State Geological Map of the Russian Federation. Scale 1: 1,000,000 (Third Generation). Series Koryaksko-Kurilskaya. Sheet O-57—Palana. Explanatory Letter. St. Petersburg: VSEGEI Cartographic Factory, 2013. 296p. (In Russian). Available online: https://www.vsegei.ru/ru/info/pub_ggk1000-3/Koryaksko-Kurilskaya/o-57.php (accessed on 10 January 2023).
- Fedorov, P.I.; Kovalenko, D.V.; Bayanova, T.B.; Serov, P.A. Early Cenozoic Magmatism in the Continental Margin of Kamchatka. Petrology 2008, 16, 261–278. [Google Scholar] [CrossRef]
- Fedorov, P.I.; Kovalenko, D.V.; Ageeva, O.A. Western Kamchatka–Koryak Continental-Margin Volcanogenic Belt: Age, Composition, and Sources. Geochem. Int. 2011, 49, 768–792. [Google Scholar] [CrossRef]
- Fedorov, P.I.; Kovalenko, D.V.; Perepelov, A.B.; Dril, S.I. Composition of sources of the Kinkil complex of western Kamchatka according to isotope-geochemical data. Bull. KRAUNC Earth Sci. 2019, 1, 54–72. [Google Scholar] [CrossRef]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Borisenko, A.S. Analysis of the Salt Composition of solutions of gas-liquid inclusions in minerals by cryometry. In The Use of Methods of Thermobarogeochemistry in the Search and Study of Ore Deposits; Laverov, N.P., Ed.; Nedra: Moscow, Russia, 1982; pp. 37–47. (In Russian) [Google Scholar]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 79–108. [Google Scholar]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for NaCl–H2O fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; De Vivo, B., Frezzotti, M.L., Eds.; Virginia Polytechnic Inst State Univ: Blacksburg, VA, USA, 1994; pp. 117–131. [Google Scholar]
- Sharp, Z.D. A laser-based microanalytical method for the in-situ determination of oxygen isotope ratios of silicates and oxides. Geochim. Cosmochim. Acta 1990, 54, 1353–1357. [Google Scholar] [CrossRef]
- Dong, G.; Morrison, G.; Jaireth, S. Quartz textures in epithermal veins, Queensland—Classification, origin, and implication. Econ. Geol. 1995, 90, 1841–1856. [Google Scholar] [CrossRef]
- Murata, J.; Norman, M.B. An index of crystallinity for quartz. Am. J. Sci. 1976, 276, 1120–1130. [Google Scholar] [CrossRef]
- Kuznetsov, S.K.; Svetova, E.N.; Shanina, S.N.; Filippov, V.N. Minor Elements in Quartz from Hydrothermal-Metamorphic Veins in the Nether Polar Ural Province. Geochemistry 2012, 50, 911–925. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Huang, E.; Li, Y.-H.; Hung, H.-T.; Jiang, J.-H.; Liu, T.-C.; Chen, H.-F. Raman Spectroscopic Characteristics of Zeolite Group Minerals. Minerals 2021, 11, 167. [Google Scholar] [CrossRef]
- White, S.N. Laser Raman spectroscopy as a technique for identification of seafloor hydrothermal and cold seep minerals. Chem. Geol. 2009, 259, 240–252. [Google Scholar] [CrossRef]
- Kats, A. Hydrogen in Alpha-quartz. Philips Res. Rep. 1962, 17, 201–279. [Google Scholar]
- Aines, R.D.; Rossman, G.R. Water in minerals? A peak in the infrared. Geophys. Res. 1984, 89, 4059–4071. [Google Scholar] [CrossRef]
- Gilg, H.A.; Krüger, Y.; Taubald, H.; van den Kerkhof, A.M.; Frenz, M.; Morteani, M. Mineralisation of amethyst-bearing geodes in Ametista do Sul (Brazil) from low-temperature sedimentary brines: Evidence from monophase liquid inclusions and stable isotopes. Miner. Depos. 2014, 49, 861–877. [Google Scholar] [CrossRef]
- Matsuhisa, Y.; Goldsmith, J.R.; Clayton, R.N. Oxygen isotopic fractionation in the system quarz-albite–anorthite-water. Geochim. Cosmochim. Acta 1979, 43, 1131–1140. [Google Scholar] [CrossRef]
- Sander, M.V.; Black, J.E. Crystallization and recrystallization of growth-zoned vein quartz crystals from epithermal systems; implications for fluid inclusion studies. Econ. Geol. 1988, 83, 1052–1060. [Google Scholar] [CrossRef]
- Marinova, I.; Ganev, V.; Titorenkova, R. Colloidal origin of colloform-banded textures in the Paleogene low-sulfidation Khan Krum gold deposit, SE Bulgaria. Miner. Depos. 2014, 49, 49–74. [Google Scholar] [CrossRef]
- Yilmaz, T.I.; Duschl, F.; Di Genova, D. Feathery and network-like filamentous textures as indicators for the re-crystallization of quartz from a metastable silica precursor at the Rusey Fault Zone, Cornwall, UK. Solid Earth 2016, 7, 1509–1519. [Google Scholar] [CrossRef]
- Moxon, T.; Rios, S. Moganite and water content as a function of age in agate: An XRD and thermogravimetric study. Eur. J. Mineral. 2004, 4, 693–706. [Google Scholar] [CrossRef]
- Moxon, T.; Reed, S.J.B.; Zhang, M. Metamorphic effects on agate found near the Shap granite, Cumbria: As demonstrated by petrography, X-ray diffraction spectroscopic methods. Miner. Mag. 2007, 71, 461–476. [Google Scholar] [CrossRef]
- Moxon, T.; Carpenter, M.A. Crystallite growth kinetics in nanocrystalline quartz (agate and chalcedony). Miner. Mag. 2009, 73, 551–568. [Google Scholar] [CrossRef]
- Moxon, T. Agates: A study of ageing. Eur. J. Mineral. 2002, 14, 1109–1118. [Google Scholar] [CrossRef]
- Svetova, E.N.; Svetov, S.A. Agates from Mesoproterozoic Volcanics (Pasha–Ladoga Basin, NW Russia): Characteristics and Proposed Origin. Minerals 2023, 13, 62. [Google Scholar] [CrossRef]
- Moxon, T.; Nelson, D.R.; Zhang, M. Agate recrystallization: Evidence from samples found in Archaean and Proterozoic host rocks, Western Australia. Aust. J. Earth Sci. 2006, 53, 235–248. [Google Scholar] [CrossRef]
- Conte, A.; Della Ventura, G.; Rondeau, B.; Romani, M.; Guidi, M.C.; La, C.; Napoleoni, C.; Lucci, F. Hydrothermal genesis and growth of the banded agates from the Allumiere-Tolfa volcanic district (Latium, Italy). Phys. Chem. Miner. 2022, 49, 39. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Liu, X.; Li, G.; Yu, X.; Wang, Y.; Li, H.; Liu, H.; Shan, G.; Li, T.; et al. Thermal Process of Rock Crystal: Cause of Infrared Absorption Band at 3585 cm−1. Crystals 2021, 11, 1083. [Google Scholar] [CrossRef]
- Aines, R.D.; Kirby, S.H.; Rossman, G.R. Hydrogen speciation in synthetic quartz. Phys. Chem. Miner. 1984, 11, 204–212. [Google Scholar] [CrossRef]
- Stalder, R.; Konzett, J. OH-defects in quartz in the system quartz–albite–water and granite–water between 5 and 25 kbar. Phys. Chem. Miner. 2012, 39, 817–827. [Google Scholar] [CrossRef]
- Fukuda, J. Water in Rocks and Minerals—Species, Distributions, and Temperature Dependences. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Tohoku University: Sendai, Japan, 2012. [Google Scholar] [CrossRef]
- Stünitz, H.; Thust, A.; Heilbronner, R.; Behrens, H.; Kilian, R.; Tarantola, A.; Fitz Gerald, J.D. Water redistribution in experimentally deformed natural milky quartz single crystals-Implications for H2O-weakening processes. J. Geophys. Res. Solid Earth 2017, 122, 866–894. [Google Scholar] [CrossRef]
- Palyanova, G.; Sidorov, E.; Borovikov, A.; Seryotkin, Y. Copper-Containing Agates of the Avacha Bay (Eastern Kamchatka, Russia). Minerals 2020, 10, 1124. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Kutyev, F.T.; Anikin, P.P. Native Copper Agates of the Kuril-Kamchatka Province. In Native Metals in Postmagmatic Formations; Yakutsk Publishing House: Yakutsk, Russia, 1985; pp. 72–73. (In Russian) [Google Scholar]
- Saveliev, D.P. A scattering of agates at Cape Vertikalny, Eastern Kamchatka. Bull. Kamchatka Reg. Assoc. Educ. Sci. Cent. Ser. Earth Sci. 2020, 47, 3. [Google Scholar] [CrossRef]
- McArthur, J.R.; Jennings, E.A.; Kissin, S.A.; Sherlock, R.L. Stable-isotope, fluid-inclusion, and mineralogical studies relating to the genesis of amethyst, Thunder Bay Amethyst Mine, Ontario. Can. J. Earth Sci. 2011, 30, 1955–1969. [Google Scholar] [CrossRef]
- Khakimov, A.K. Some features of the mineralogy and genesis of agate bodies in the Ijevan region of Armenia. News High. Educ. Inst. Geol. Explor. 1965, 7, 45–56. (In Russian) [Google Scholar]
- Troilo, F.; Harfi, A.; El Bittarello, E.; Costa, E. Amethyst from Boudi, Morocco. Gems Gemmol. 2015, 51, 32–40. [Google Scholar] [CrossRef]
- Gilg, H.A.; Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gatter, I.; Strieder, A.J. Genesis of amethyst geodes in basaltic rocks of the Serra Geral Formation (Ametista do Sul, Rio Grande do Sul, Brazil): A fluid inclusion, REE, oxygen, carbon, and Sr isotope study on basalt, quartz, and calcite. Miner. Depos. 2003, 38, 1009–1025. [Google Scholar] [CrossRef]
- Kile, D.E. Mineralogy of the Amethyst Mines in the Thunder Bay Area, Thunder Bay, Ontario, Canada. Rocks Miner. 2019, 94, 306–343. [Google Scholar] [CrossRef]
- Dumańska-Słowik, M.; Toboła, T.; Jarmołowicz-Szulc, K.; Naglik, B.; Dyląg, J.; Szczerba, J. Inclusion study of hourglass amethyst from Boudi (Morocco) by Raman microspectroscopy and microthermometric measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.H.; Yu, S.H.; Lee, J.D. A fluid inclusion study of an amethyst deposit in the Cretaceous Kyongsang Basin, South Korea. Mineral. Mag. 2001, 65, 477–487. [Google Scholar] [CrossRef]
- Frishman, N.I. Stage and formation conditions of amethyst in “Cape Korabl” deposit. Proc. Fersman Sci. Sess. GI KSC RAS. 2015, 12, 339–341. [Google Scholar]
- Balitsky, V.S.; Machina, I.B.; Mar’in, A.A.; Shigley, J.E.; Rossman, G.R.; Lu, T. Industrial growth, morphology and some properties of bi-colored amethyst-citrine quartz (ametrine). J. Cryst. Growth 2000, 212, 255–260. [Google Scholar] [CrossRef]
- Balitsky, V.S.; Balitskaya, L.V.; Balitsky, D.V.; Semenchenko, S.P.; Humenny, M.V. A Method for Growing Quartz or Amethyst Crystals or Amethyst Druse by the Hydrothermal Method of Temperature Difference in Aqueous Solutions of Ammonium Fluoride. RU 2707771, 29 November 2019. (In Russian). [Google Scholar]
- Zebrev, Y.N.; Chernaya, T.N.; Konovalov, N.I.; Novoselov, V.P.; Oleinik, N.A.; Abdrafikov, S.N.; Popolitov, V.I.; Pisarevsky, Y.V. Method for Obtaining Synthetic Amethyst. RU 2040596 C1, 25 July 1995. (In Russian). [Google Scholar]
- Harris, C. Oxygen-isotope zonation of agates from Karoo volcanics of the Skeleton Coast, Namibia. Am. Mineral. 1989, 74, 476–481. [Google Scholar]
- Götze, J.; Tichomirowa, M.; Fuchs, H.; Pilot, J.; Sharp, Z.D. Geochemistry of agates: A trace element and stable isotope study. Chem. Geol. 2001, 175, 523–541. [Google Scholar] [CrossRef]
- Shen, M.; Lu, Z.; He, X. Mineralogical and Geochemical Characteristics of Banded Agates from Placer Deposits: Implications for Agate Genesis. ACS Omega 2022, 7, 23858–23864. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Ladygin, V.M.; Yanakieva, D.Y.; Frolova, J.V.; Semikolennykh, E.S. Agates in Metavolcanics. Bulletin of the Russian Federal Property Fund. 2014, 71p. (In Russian). Available online: https://www.rfbr.ru/rffi/ru/bulletin/o_1923809#8 (accessed on 10 January 2023).
- Merino, E.; Wang, Y.; Deloule, E. Genesis of agates in flood basalts; twisting of chalcedony fibers and trace-element geochemistry. Am. J. Sci. 1995, 295, 1156–1176. [Google Scholar] [CrossRef]
- Rossman, G.R. Colored varieties of the silica minerals. Rev. Mineral. 1994, 29, 433–467. [Google Scholar]
- Czaja, M.; Kądziołka-Gaweł, M.; Konefał, A.; Sitko, R.; Teper, E.; Mazurak, Z.; Sachanbińskiet, M. The Mossbauer spectra of prasiolite and amethyst crystals from Poland. Phys. Chem. Miner. 2017, 44, 365–375. [Google Scholar] [CrossRef]
- Dedushenko, S.K.; Makhina, I.B.; Mar’in, A.A.; Mukhanov, V.A.; Perfiliev, Y.U.D. What oxidation state of iron determines the amethyst colour? Hyperfine Interact. 2004, 156, 417–422. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Innocenti, M.; Tesi, S.; Romanelli, M.; D’Acapito, F.; Fornaciai, G.; Montegrossi, G.; Pardi, L.A. A Fe K-edge XAS study of amethyst. Phys. Chem. Miner. 2010, 37, 283–289. [Google Scholar] [CrossRef]
- SivaRamaiah, G.; Lin, J.; Pan, Y. Electron paramagnetic resonance spectroscopy of Fe3+ ions in amethyst: Thermodynamic potentials and magnetic susceptibility. Phys. Chem. Miner. 2011, 38, 159–167. [Google Scholar] [CrossRef]
- Fournier, R.O. The behaviour of silica in hydrothermal solutions. Geology and geochemistry of epithermal systems. Rev. Econ. Geol. 1985, 2, 45–61. [Google Scholar]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]


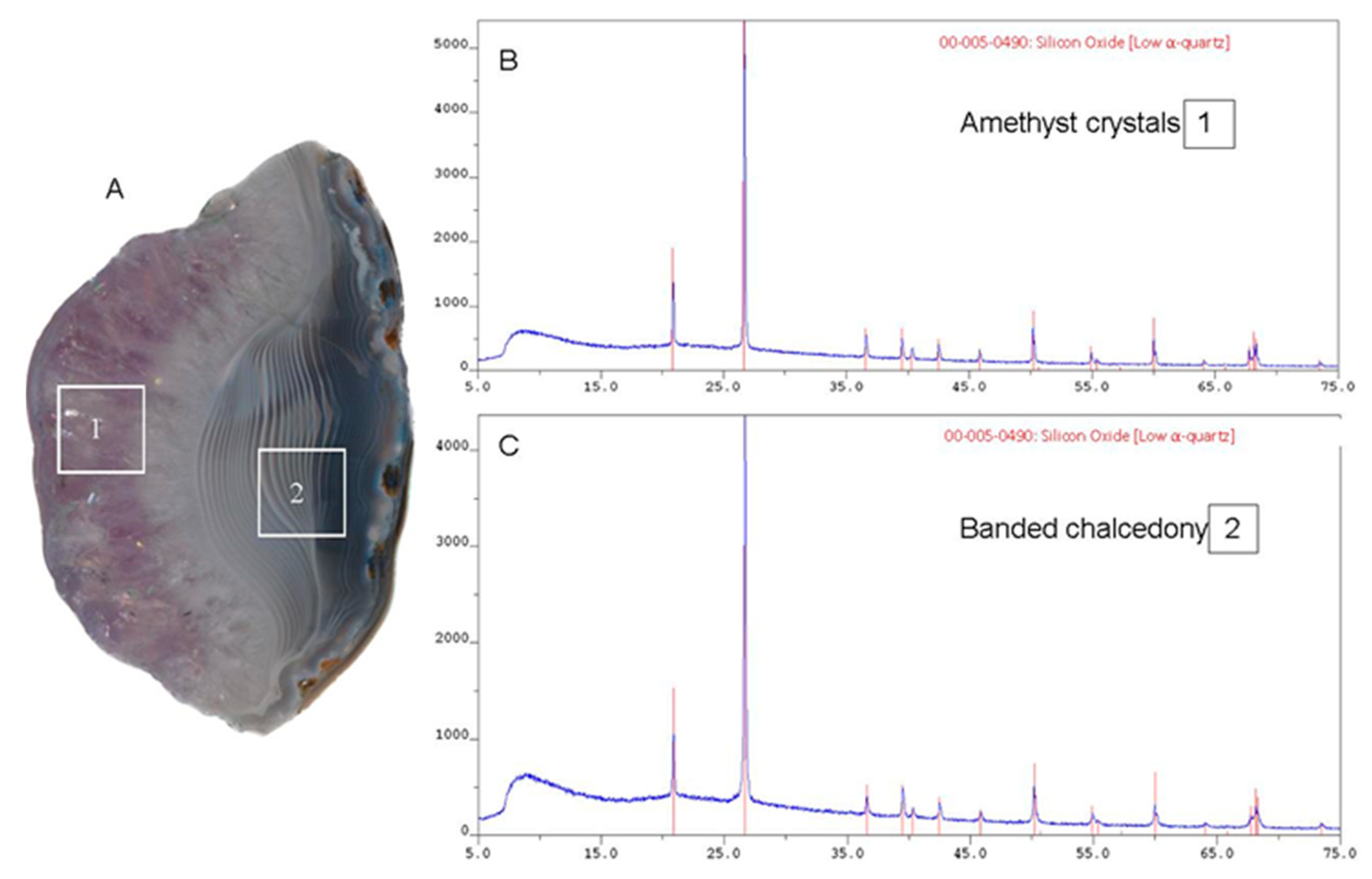
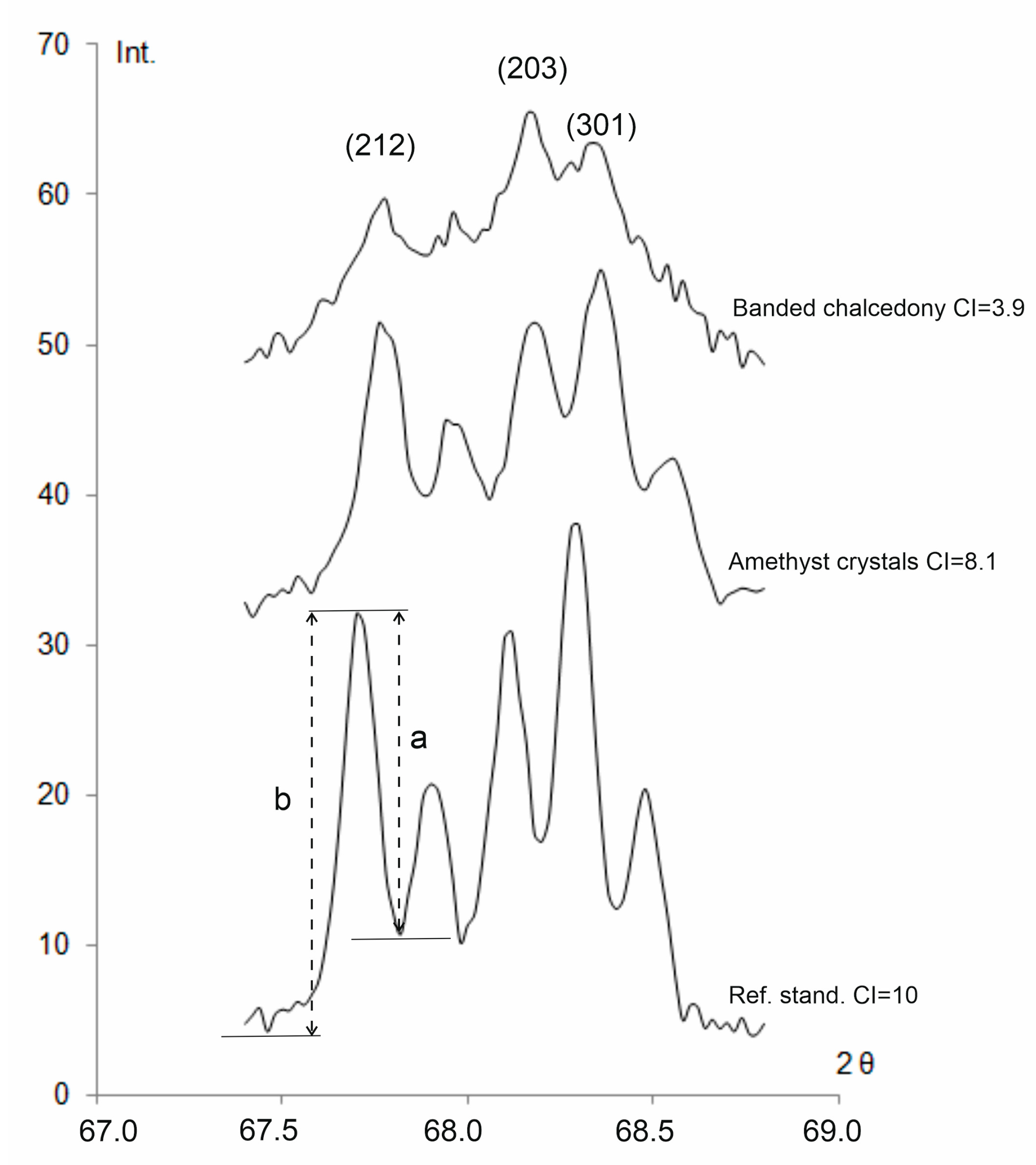



| Sample | a ± Δa, Å | c ± Δc, Å | V, Å3 | Cs, Å | CI |
|---|---|---|---|---|---|
| Amethyst crystals area | 4.9138 ± 0.0001 | 5.4054 ± 0.0002 | 113.03 | 525 | 8.1 |
| Banded chalcedony area | 4.9140 ± 0.0001 | 5.4059 ± 0.0001 | 113.05 | 560 | 3.9 |
| Quartz crystal, ref. std. | 4.9133 ± 0.0001 | 5.4052 ± 0.0001 | 113.00 | 935 | 10 |
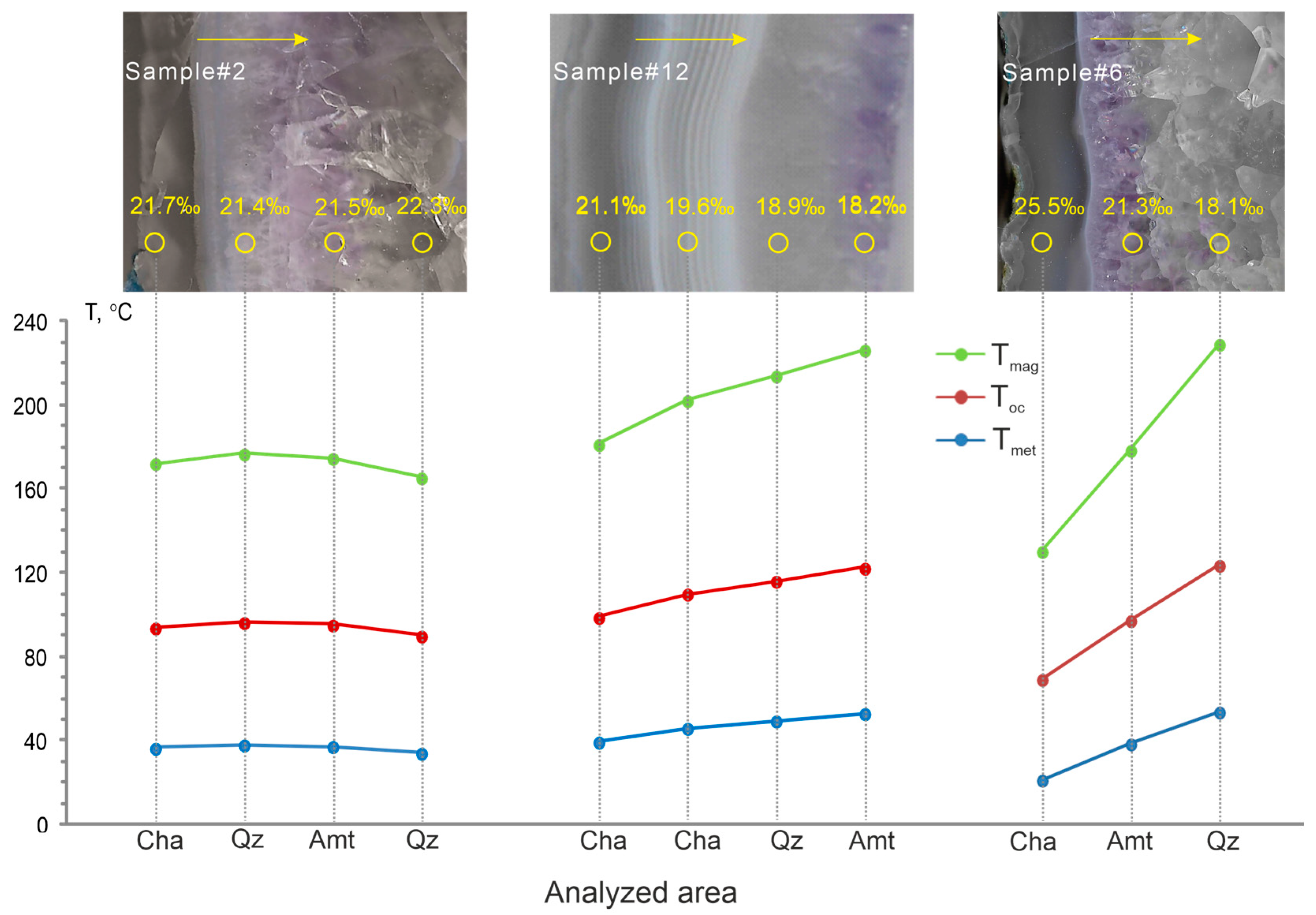
| Analysis Points | Bands Characterization | δ18OSMOW (‰) | Tmet (°C) | Toc (°C) | Tmag (°C) |
|---|---|---|---|---|---|
| #6_1 | fibrous chalcedony (rim) | 25.5 | 21 | 69 | 130 |
| #6_2 | amethyst | 21.3 | 39 | 97 | 179 |
| #6_3 | quartz (core) | 18.1 | 54 | 124 | 229 |
| #2_1 | fibrous chalcedony (rim) | 21.7 | 36 | 94 | 172 |
| #2_2 | quartz | 21.4 | 38 | 96 | 177 |
| #2_3 | amethyst | 21.5 | 37 | 95 | 175 |
| #2_4 | quartz (core) | 22.3 | 34 | 90 | 165 |
| #12_1 | chalcedony (rim) | 21.1 | 39 | 99 | 181 |
| #12_2 | banded chalcedony | 19.6 | 46 | 110 | 202 |
| #12_3 | amethyst | 18.9 | 49 | 116 | 214 |
| #12_4 | quartz (core) | 18.2 | 53 | 122 | 226 |
| Amethyst Occurrences | Host Rocks | Age | Mineral Assemblage | References |
|---|---|---|---|---|
| Tevinskoye (Russia) | Basalts, andesites, dacites | Eocene | Chalcedony, quartz, amethyst, native copper, covellite, chalcopyrite, Ni-pyrite, goethite, calcite, and clinoptilolite. | our data |
| Amethysta do Sul (Brazil) | Basalts, andesites | Early Cretaceous | Chalcedony, quartz, amethyst, celadonite, pyrite, goethite, anhydrite, calcite, gypsum, barite, and fluorite. | [38,61] |
| Arttigas (Uruguay) | - ″ - | - ″ - | Chalcedony, quartz, amethyst, celadonite, goethite, and calcite. | [7] |
| Los Catalanes (Uruguay) | - ″ - | – | Chalcedony, quartz, amethyst celadonite, calcite, fluorite, pyrite–in geodes; zeolites–in amygdales. | [10] |
| Thander Bay and Blue Point (Canada, Ontario) | Silicified mudstone breccia | Proterozoic | Quartz, amethyst, barite, calcite, rutile, native copper, chalcopyrite, galena, marcasite, pyrite, sphalerite, hematite, goethite, kaolinite, and smectite. | [58,62] |
| Boudi (Morocco) | Siltstone, sandstone | Lower Cambrian | Quartz, amethyst, moganite, hematite, calcite-covered amethyst crystals. | [11,60] |
| Ijevan (Armenia) | Tuffs, tuffsandstones | Late Cretaceous | Chalcedony, quartz, amethyst, calcite, goethite, heulandite. Mordenite and chlorite are rare. | [59] |
| Deposits | Amethyst Occurs | T °C | Salinity, wt.% NaCl eq. | P (κbar) | References |
|---|---|---|---|---|---|
| Artigas (Uruguay) | geodes | 50–120 | - ″ - | - ″ - | [7] |
| Serra Geral (Brazil) | geodes | <100 | 0.9–5.6 | - ″ - | [6] |
| Ametista do Sul (Brazil) | geodes | 95–130 | 5.3–0.3 | 0.21–0.29 | [38] |
| Artigas, Uruguay | geodes, breccia | 100–150 | - ″ - | 1.2–5.5 | [9] |
| Boudi (Morocco) | veins | 191–445 | 5.71–13.94 | 0.64–1.31 | [63] |
| Silver Hill (Greece) | - ″ - | 189–205 | 0.9–2.1 | - ″ - | [17] |
| Kassiteres (Greece) | - ″ - | 211–275 | 0.5–3.4 | - ″ - | - ″ - |
| Megala Therma (Greece) | - ″ - | 219–246 | 3.1–4.8 | - ″ - | - ″ - |
| Chondro Vouno (Greece) | - ″ - | 204–221 | 5.3–8.0 | - ″ - | - ″ - |
| Kalogries (Greece) | - ″ - | 139–209 | 3.4–5.6 | - ″ - | - ″ - |
| Eonyang (South Korea) | miarolitic cavities in the aplite | 156–333 | 34–4 | 1–1.5 | [64] |
| Cape Korabl (Russia) | stockworks, veins, breccias | 260–20 | - ″ - | - ″ - | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetova, E.N.; Palyanova, G.A.; Borovikov, A.A.; Posokhov, V.F.; Moroz, T.N. Mineralogy of Agates with Amethyst from the Tevinskoye Deposit (Northern Kamchatka, Russia). Minerals 2023, 13, 1051. https://doi.org/10.3390/min13081051
Svetova EN, Palyanova GA, Borovikov AA, Posokhov VF, Moroz TN. Mineralogy of Agates with Amethyst from the Tevinskoye Deposit (Northern Kamchatka, Russia). Minerals. 2023; 13(8):1051. https://doi.org/10.3390/min13081051
Chicago/Turabian StyleSvetova, Evgeniya N., Galina A. Palyanova, Andrey A. Borovikov, Viktor F. Posokhov, and Tatyana N. Moroz. 2023. "Mineralogy of Agates with Amethyst from the Tevinskoye Deposit (Northern Kamchatka, Russia)" Minerals 13, no. 8: 1051. https://doi.org/10.3390/min13081051
APA StyleSvetova, E. N., Palyanova, G. A., Borovikov, A. A., Posokhov, V. F., & Moroz, T. N. (2023). Mineralogy of Agates with Amethyst from the Tevinskoye Deposit (Northern Kamchatka, Russia). Minerals, 13(8), 1051. https://doi.org/10.3390/min13081051






