Uranium Occurrence State and Its Implication for Sandstone-Type Uranium Mineralization within the Hanbazhai Area of the Longchuanjiang Basin, China
Abstract
1. Introduction
2. Geological Setting
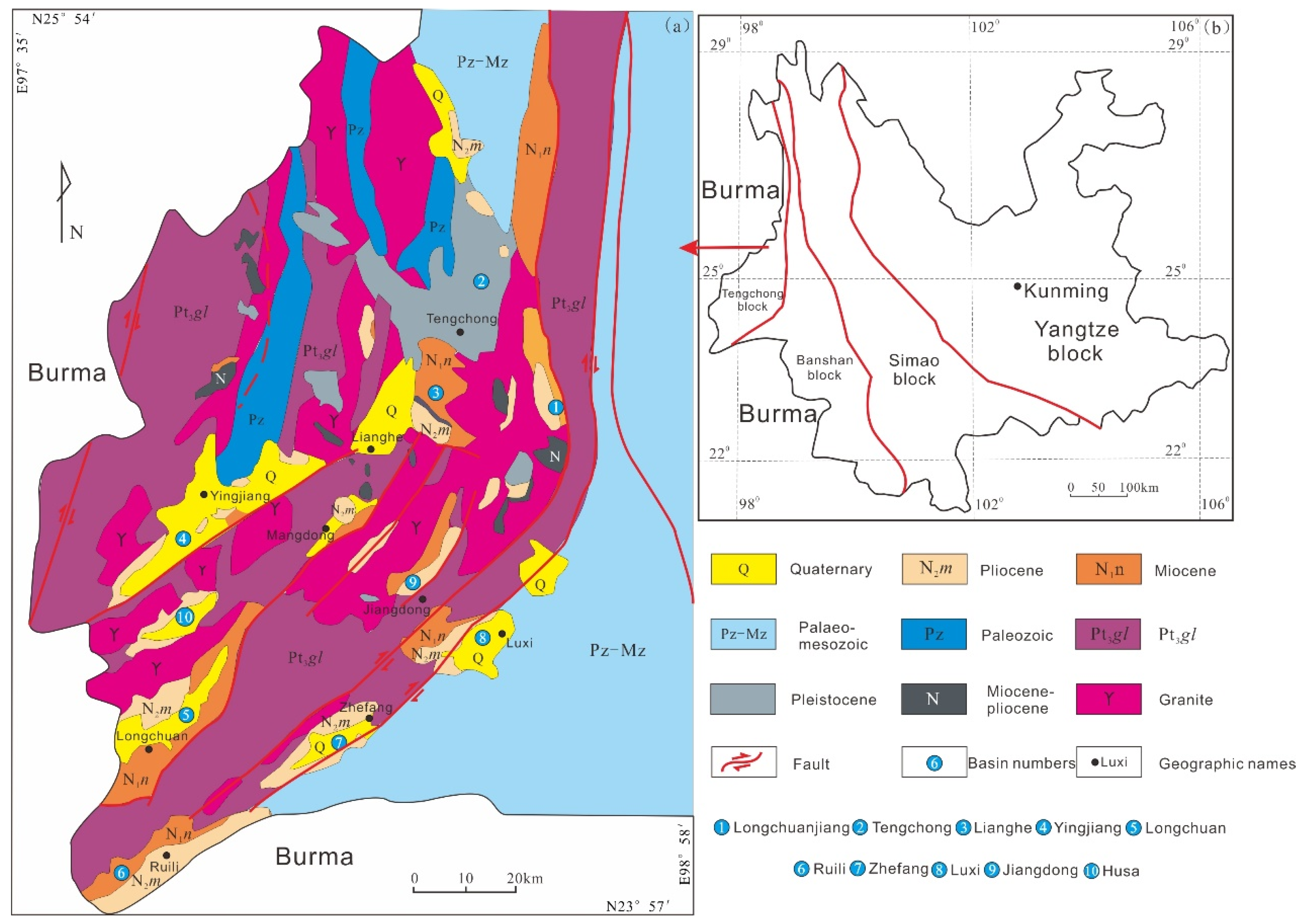
2.1. The Longchuanjiang Basin
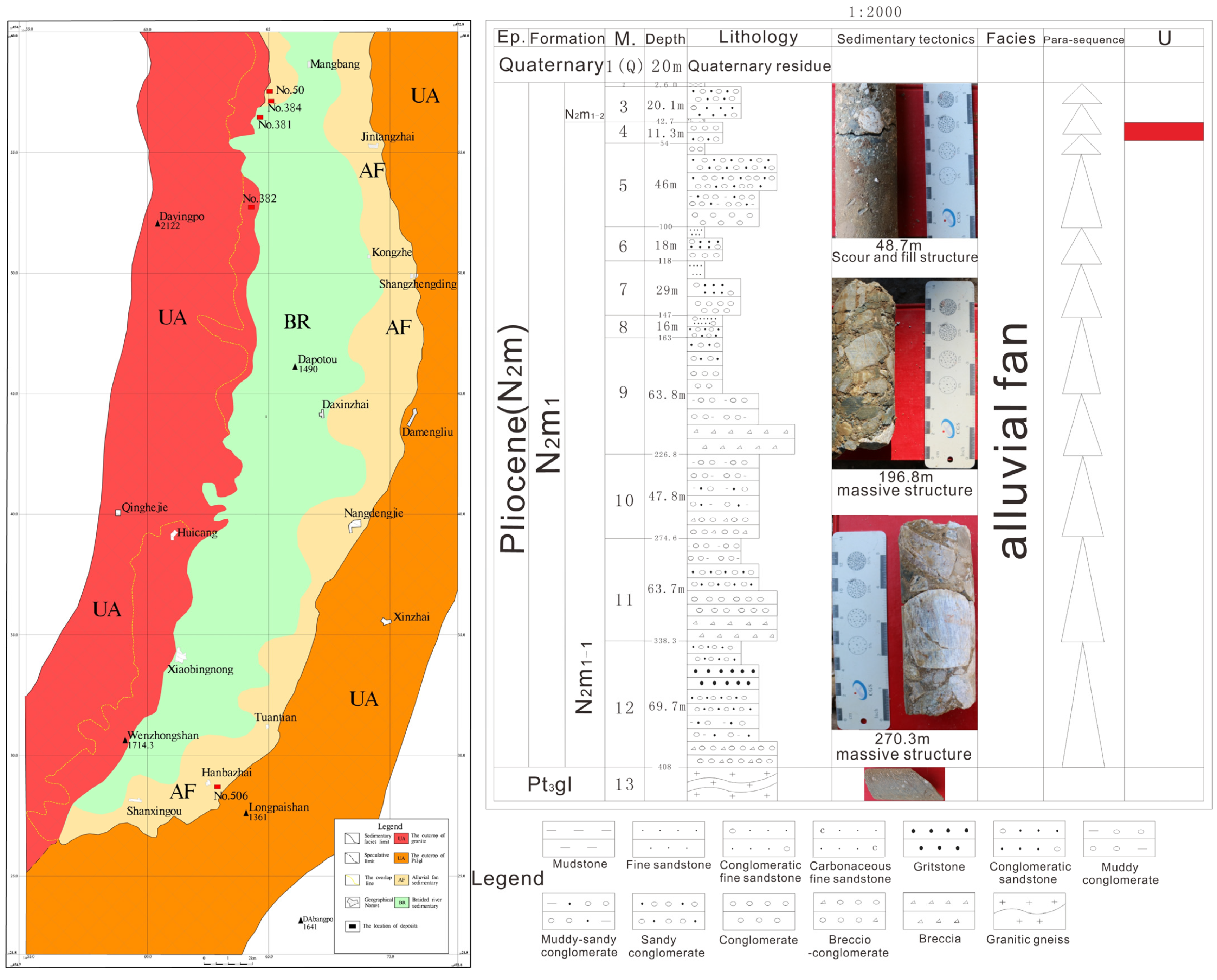
2.2. The Hanbazhai Uranium Deposit
3. Samples and Analytical Methods
3.1. Samples
3.1.1. Sandy Conglomerate
3.1.2. Sandstone
3.2. Analytical Methods
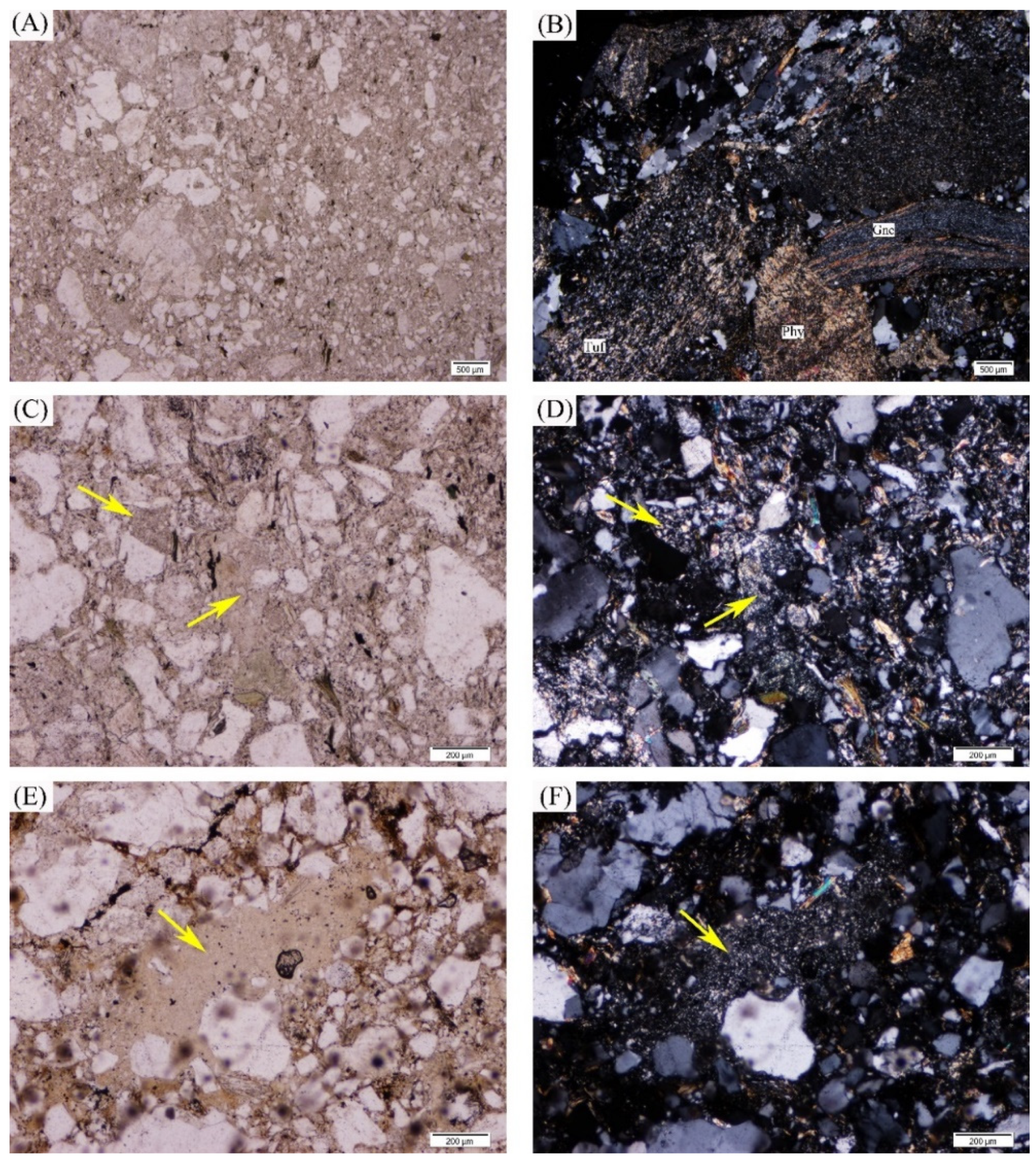
3.2.1. Optical Microscopy
3.2.2. EDS and SEM
3.2.3. EPMA
3.2.4. Chemical Sequential Extraction Experiment
4. Results
4.1. Characteristics of the Uranium Contents in Each Geochemical Phase
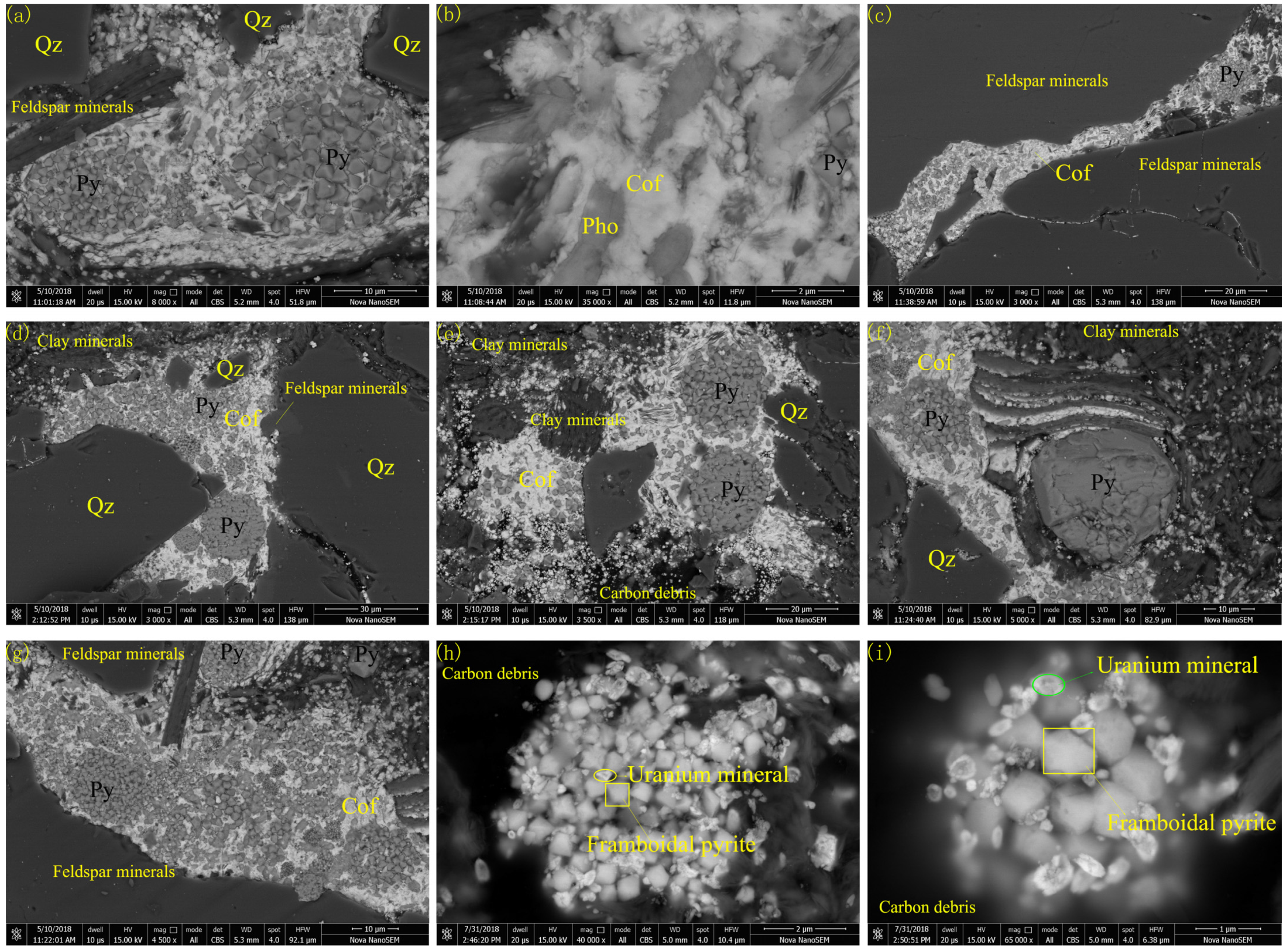
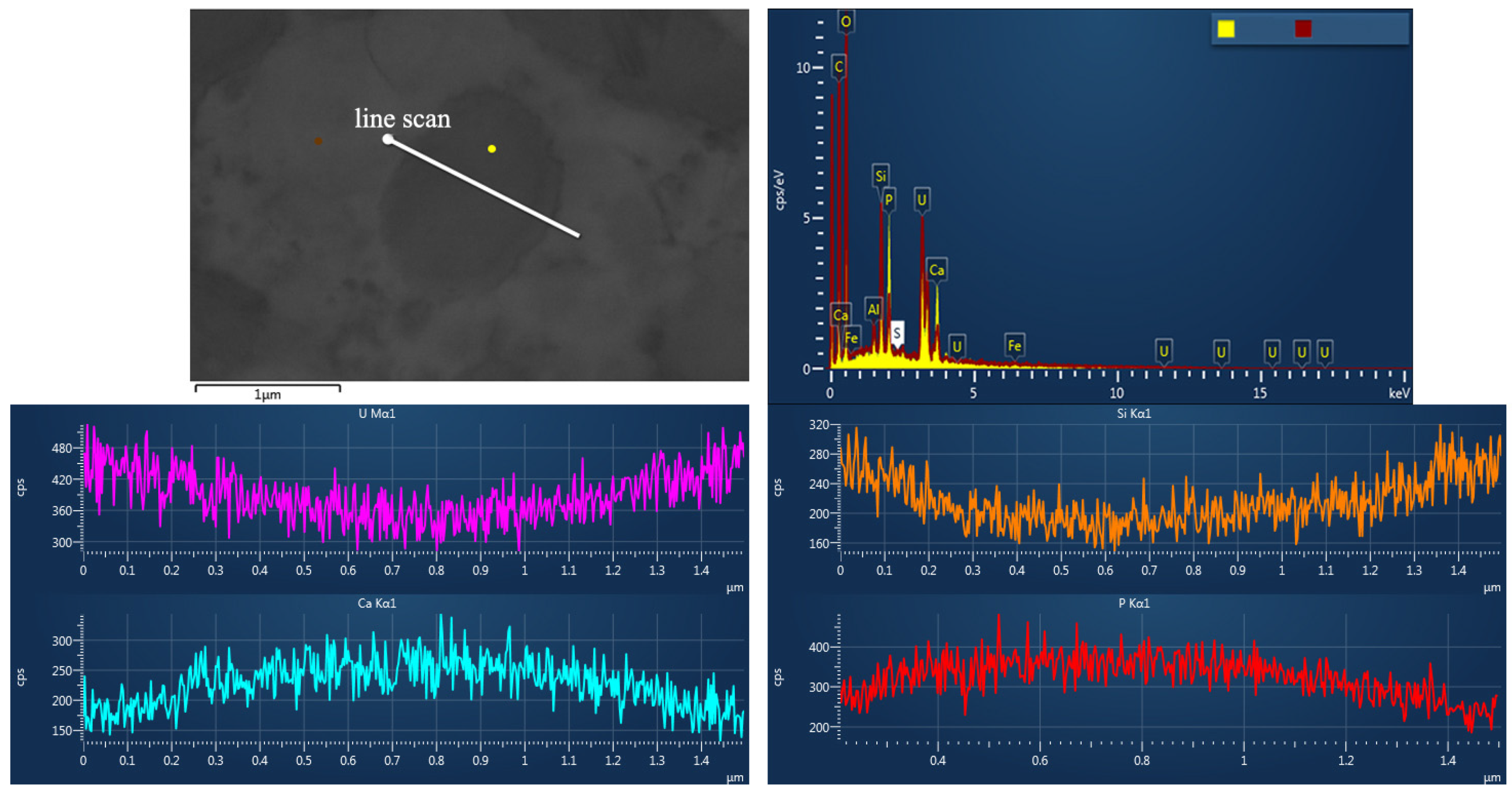
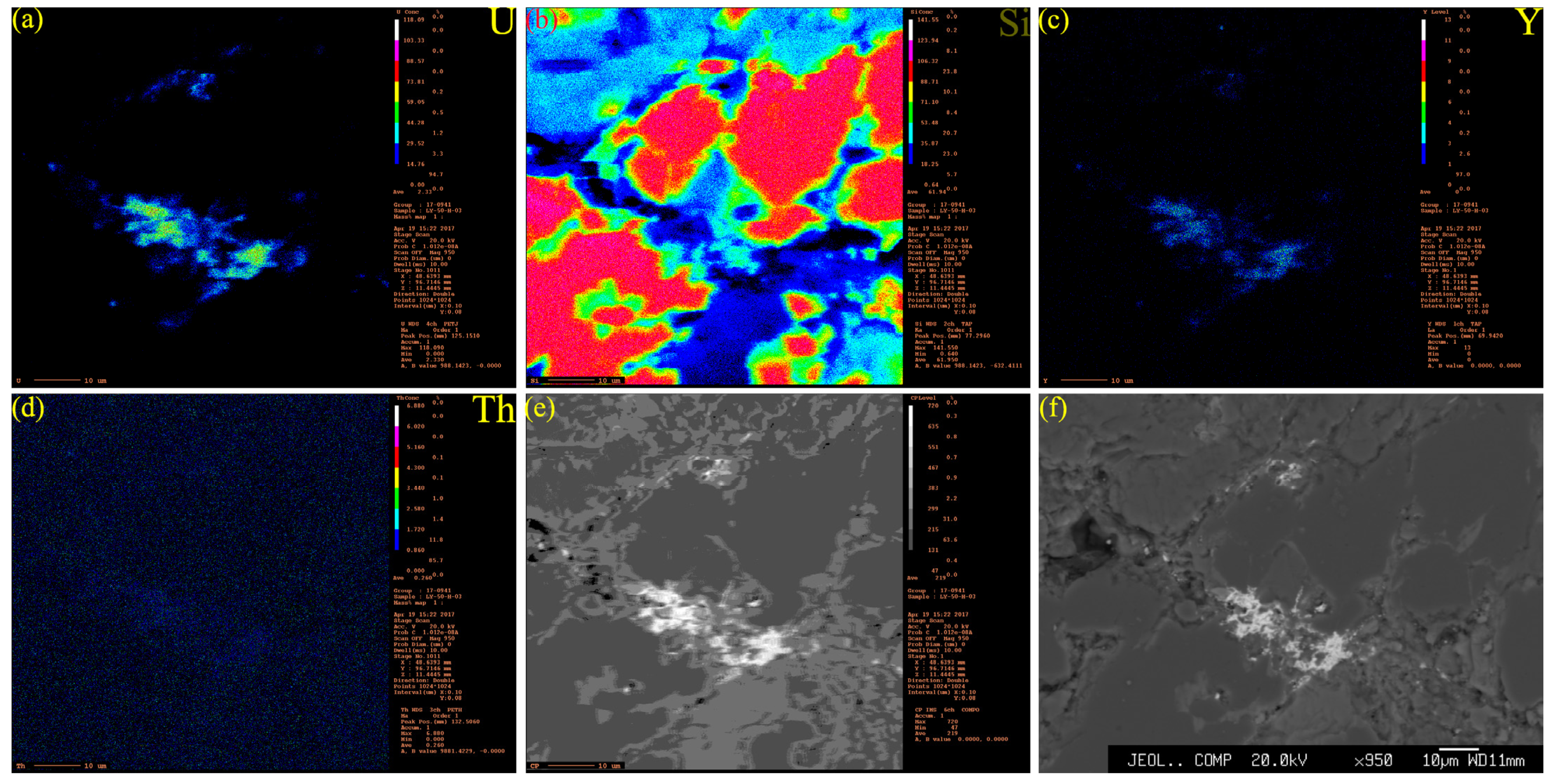
4.2. Types of Uranium Minerals and Their Compositional Analysis
- (1)
- As a common mineral, coffinite has shown a close relationship with sandstone-type uranium deposits but rarely appears in the analysis. The composition of coffinite was approximately 50.1%–60.59% UO2, 14.32%–17.78% SiO2, approximately 4.67%–6.92% P2O5, 1.92%–6.47% CaO, 0.1%–3.85% Al2O3, 0.32%–3.6% FeO and 1.02%–3.7% Y2O3, together with small amounts of K2O, Na2O, MgO, TiO2, SO3, ThO2 and rare earth elements (REEs). The average UO2 content was 55.40%, and SiO2 on average was 15.96%. The data and backscattering image results demonstrated that the contents of UO2 and SiO2 in coffinite were low and that impure elements varied widely, probably because coffinite particles were mostly at the submicron–micron level and coffinite is a metamict mineral that easily forms extremely dispersed uraninite and amorphous SiO2 [35], so the chemical composition of coffinite is susceptible to the effects of symbiotic minerals (quartz, pyrite, feldspar, and clay minerals).
- (2)
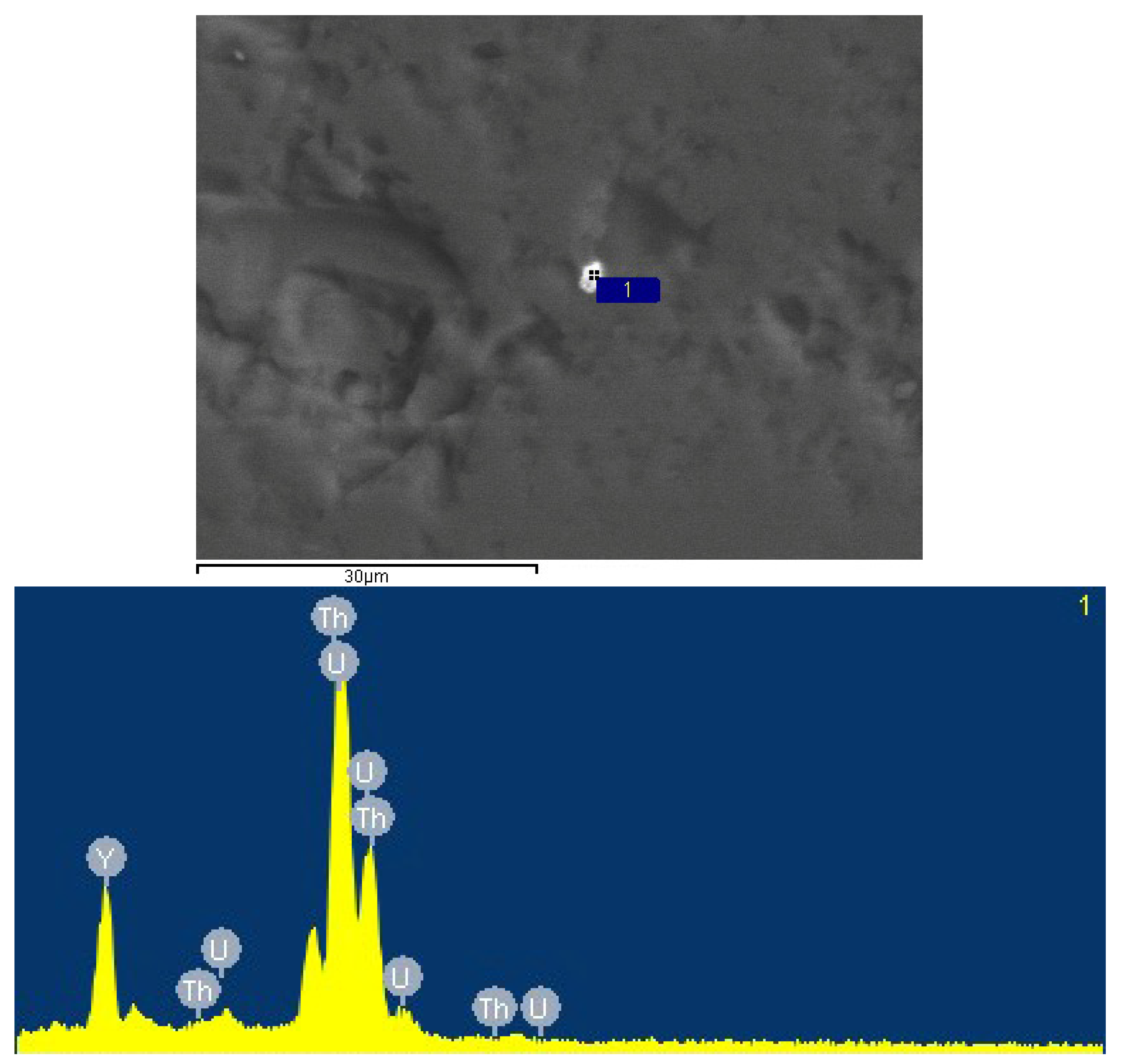
- The composition of thorite was approximately 7.72%–8.79% UO2 and approximately 53.82%–54.32% ThO2, containing small amounts of La and Ce rare earth elements.
- (3)
- According to the electron probe test data, the study sample probably contained uranium phosphosilicate minerals, which were first reported in the Dalmatovo deposit (West Ural, Russia), which is in an infiltrated buried valley [36]. The composition of this mineral was 9.66%–12.40% P2O5, 4.86%–7.18% CaO, 10.36%–12.51% SiO2, and up to 67.81% UO2, including almost no iron, which is frequently found in isomorphic varieties of ningyoite and, less commonly, coffinite.
- (4)
- For monazite, the electron probe results showed that the ThO2 content was more than 4%, and the UO2 content was approximately 0.35%.
4.3. Microscopic Morphology and Symbiotic Relationship of Uranium Minerals
4.3.1. SEM Profiles of Uranium Minerals and Other Minerals
4.3.2. Chemical Information of the Uranium Minerals
5. Discussion
5.1. Discussion on the Formation and Genesis of Uranium Minerals in the Study Area
5.1.1. Enrichment of Primary Uranium Minerals in the Sedimentary–Diagenetic Stage
5.1.2. Geochemical Environment of the Sedimentary–Diagenetic Stage
5.1.3. The Stage of Uranium Enrichment by Later Fluids
5.1.4. The Adsorption of Uranium
6. Conclusions
- (1)
- By using an experiment to sequentially extract uranium, electron probe microanalysis, scanning electron microscopy and energy spectroscopy indicate that the type and mode of occurrence of uranium in the Hanbazhai area of the Longchuanjiang Basin have adsorbed uranium and uranium minerals, which are closely related to framboidal pyrite, feldspar minerals, clay minerals, and organic matter. Uranium minerals include coffinite and uranium phosphosilicates, and U-rich minerals include thorite and xenotime.
- (2)
- It is considered that uraniferous sandstones have undergone at least two stages of mineralization: the sedimentary–diagenetic stage and the later uranium enrichment by fluid. The geochemical environment of the sedimentary–diagenetic stage is generally a sulfide-reducing environment, and the later fluids are rich in U, Si, P, and Y.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Chen, Y.; Feng, X.; Li, J.-G.; Guo, H.; Miao, P.; Jin, R.; Tang, C.; Zhao, H.; Wang, G.; et al. Uranium Occurrence State in the Tarangaole Area of the Ordos Basin, China: Implications for Enrichment and Mineralization. Ore Geol. Rev. 2019, 115, 103034. [Google Scholar] [CrossRef]
- Burns, P.C.; Finch, R.J. (Eds.) Uranium: Mineralogy, Geochemistry, and the Environment; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2018. [Google Scholar]
- Wu, S.; Wang, D. Uranium concentration by organic matter during diagenesis and mineralization in the continental sandstone-type uranium deposit in North Sichuan. Uranium Geol. 1991, 7, 265–272, (In Chinese with English Abstract). [Google Scholar]
- Alberic, P.; Viollier, E.; Jezequel, D.; Grosbois, C.; Michard, G. Interactions between trace elements and dissolved organic matter in the stagnant anoxic deep layer of a meromictic lake. Limnol. Oceanogr. 2000, 45, 1088–1096. [Google Scholar] [CrossRef]
- Yang, Z. Occurrence and Abundance of V, Cr, Mo, and U in the Late Permian Coals from Yanshan, Yunnan, China. Bull. Mineral. Petrol. Geochem. 2009, 28, 268–271, (In Chinese with English Abstract). [Google Scholar]
- Cuney, M. Evolution of uranium fractionation processes through time: Driving the secular variation of uranium deposit types. Econ. Geol. 2010, 105, 553–569. [Google Scholar] [CrossRef]
- Douglas, G.B.; Butt, C.R.M.; Gray, D.J. Geology, geochemistry and mineralogy of the lignite-hosted Ambassador palaeochannel uranium and multi-element deposit, Gunbarrel Basin, Western Australia. Miner. Depos. 2011, 46, 761–787. [Google Scholar] [CrossRef]
- Hou, H.Q.; Li, Y.R.; Liu, H.J.; Han, S.Y.; Wang, G.; Bai, Y.S.; Wu, B.L. The organic matter characteristics of the Zhiluo Formation and its relationship with uranium mineralization in the North Ordos Basin. Acta Geol. Sin. 2016, 90, 3367–3374, (In Chinese with English Abstract). [Google Scholar]
- Fomina, M.; Charnock, J.M.; Hillier, S.; Alvarez, R.; Gadd, G.M. Fungal transformations of uranium oxides. Environ. Microbiol. 2007, 9, 1696–1710. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Douglas, G.; Grice, K.; Moreau, J.W. Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Sci. Rev. 2016, 159, 160–185. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Etschmann, B.; Brugger, J.; Douglas, G.; Evans, K.; Fisher, L.; Kappen, P.; Moreau, J.W. Characterization of uranium redox state in organic-rich Eocene sediments. Chemosphere 2018, 194, 602–613. [Google Scholar] [CrossRef]
- Rallakis, D.; Michels, R.; Brouand, M.; Parize, O.; Cathelineau, M. The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia. Minerals 2019, 9, 310. [Google Scholar] [CrossRef]
- Whicker, J.J.; Pinder III, J.E.; Ibrahim, S.A.; Stone, J.M.; Breshears, D.D.; Baker, K.N. Uranium partition coefficients (K-d) in forest surface soil reveal long equilibrium times and vary by site and soil size fraction. Health Phys. 2007, 93, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.B.; Tome, F.V.; Lozano, J.C.; Perez-Fernandez, M.A. Influence of soil texture on the distribution and availability of U-238, Th-230, and Ra-226 in soils. J. Environ. Radioact. 2008, 99, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yu, M. The clay mineral characteristics and the relation to uranium mineralization in Turpan-Hami Basin. Contrib. Geol. Miner. Resour. Res. 2005, 20, 188–191, (In Chinese with English Abstract). [Google Scholar]
- Ohnuki, T.; Yoshida, T.; Ozaki, T.; Samadfam, M.; Kozai, N.; Yubuta, K.; Mitsugashira, T.; Kasama, T.; Francis, A.J. Interactions of uranium with bacteria and kaolinite clay. Chem. Geol. 2005, 220, 237–243. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Peng, S.P.; Qin, M.K.; Liu, H.X.; Geng, Y.Y.; Zhang, X.; Ding, B.; Xiu, X.Q. Origin and role of kaolinization in roll-front uranium deposits and its response to ore-forming fluids in the Yili basin, China. Geofluids 2018, 2018, 7847419. [Google Scholar] [CrossRef]
- Wu, D.; Pan, J.; Xia, F.; Huang, G.; Lai, J. The Mineral Chemistry of Chlorites and Its Relationship with Uranium Mineralization from Huangsha Uranium Mining Area in the Middle Nanling Range, SE China. Minerals 2019, 9, 199. [Google Scholar] [CrossRef]
- Duff, M.C.; Coughlin, J.U.; Hunter, D.B. Uranium co-precipitation with iron oxide minerals. Geochim. Cosmochim. Acta 2002, 66, 3533–3547. [Google Scholar] [CrossRef]
- Reeder, R.J.; Nugent, M.; Lamble, G.M.; Tait, C.D.; Morris, D.E. Uranyl incorporation into calcite and aragonite: XAFS and luminescence studies. Environ. Sci. Technol. 2000, 34, 638–644. [Google Scholar] [CrossRef]
- Doinikova, O.A. Uranium deposits with a new phosphate type of blacks. Geol. Ore Depos. 2007, 49, 80–86. [Google Scholar] [CrossRef]
- Bonnetti, C.; Cuney, M.; Michels, R.; Truche, L.; Malartre, F.; Liu, X.; Yang, J. The multiple roles of sulfate-reducing bacteria and Fe-Ti oxides in the genesis of the Bayinwula roll front-type uranium deposit, Erlian Basin, NE China. Econ. Geol. 2015, 110, 1059–1081. [Google Scholar] [CrossRef]
- Chen, Y. Discussion on characteristics, genesis type and genetic model of in-situ leachable sandstone type uranium deppsits in Western Yunnan. Uranium Geol. 1998, 14, 193–199, (In Chinese with English Abstract). [Google Scholar]
- Yao, Y.; Sun, Z. Geophysical characteristics of strata (rocks) and application of geophysical methods in Longchuanjiang Basin. Uranium Geol. 2004, 20, 245–250, (In Chinese with English Abstract). [Google Scholar]
- Sun, Z.; Chen, H.; Zhu, X.; Wu, Y.; Yang, Y.E. Cenozoic coupled basin-mountain and prospecting direction for sandstone-type uranium deposits in Western Yunnan. Uranium Geol. 2007, 23, 289–297, (In Chinese with English Abstract). [Google Scholar]
- Zhang, C.Y.; Peng, P.A.; Song, J.Z.; Liu, C.S.; Peng, J.; Lu, P.X. Utilization of modified BCR procedure for the chemical speciation of heavy metals in Chinese soil reference material. Ecol. Environ. Sci. 2012, 21, 1881, (In Chinese with English Abstract). [Google Scholar]
- Wan, X.; Wang, W.; Ye, T.M.; Guo, Y.W.; Gao, X.B. A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J. Hazard. Mater. B 2006, 134, 197–201. [Google Scholar] [CrossRef]
- Smeda, A.; Zyrnicki, W. Application of sequential extraction and the ICP-AES method for study of the partitioning of metals in fly ashes. Microchem. J. 2002, 72, 9–16. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, G.; Lei, X.; Xu, H.; Li, L.; Yuan, C.; Wang, Y. Distribution and mode of occurrence of uranium in bottom ash derived from high-germanium coals. J. Environ. Sci. 2016, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, K.; Wu, H.; Chen, X.; Zhang, J.; Kong, R. Uranium mineral occurrence of new sandstone type uranium deposits in Western Yunnan. Sci. Technol. Eng. 2018, 18, 49–55, (In Chinese with English Abstract). [Google Scholar]
- Wei, S. Chinese Uranium Minerals; China Atomic Energy Press: Beijing, China, 1979; pp. 65–73. (In Chinese)
- Min, M.Z.; Zhang, F.S.; Zhao, F.M.; Wang, D.Y. Genesis of Uranium Mineralogy Studies; Atomic Energy Press: Beijing, China, 1992; pp. 95–99. (In Chinese) [Google Scholar]
- Zhang, J. Solution Equilibrium of Uranium Minerals; Atomic Energy Press: Beijing, China, 2008; pp. 156–163. (In Chinese) [Google Scholar]
- Zhang, J.Y.; Wang, A.Z.; Li, X.Y.; Zheng, Z.X.; Li, J.Z. Uranium mineralogy of China; Atomic Energy Press: Beijing, China, 1995; pp. 3–282. (In Chinese) [Google Scholar]
- Li, J.; Chen, Y.; Zhang, C. A Concise Course on Uranium Geology and Exploration; Geology Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Doynikova, O.A.; Sidorenko, G.A.; Sivtsov, A.V. Phosphosilicates of tetravalent uranium. In Doklady Earth Sciences; Pleiades Publishing: New York, NY, USA, 2014; Volume 456, pp. 755–758. [Google Scholar]
- Yu, Z.Q.; Ling, H.F.; Mavrogenes, J.; Chen, P.R.; Chen, W.F.; Fang, Q.C. Metallogeny of the Zoujiashan uranium deposit in the Mesozoic Xiangshan volcanic-intrusive complex, southeast China: Insights from chemical compo-sitions of hydrothermal apatite and metal elements of individual fluid inclusions. Ore Geol. Rev. 2019, 113, 103085. [Google Scholar] [CrossRef]
- Wilkins, M.J.; Livens, F.R.; Vaughan, D.J.; Lloyd, J.R. The impact of Fe(III)-Reducing bacteria on uranium mobility. Biogeochemistry 2006, 78, 125–150. [Google Scholar] [CrossRef]
- Prakash, O.M.; Gihring, T.M.; Dalton, D.D.; Chin, K.J.; Green, S.J.; Akob, D.M.; Wanger, G.; Kostka, J.E. Geobacter daltonii sp. nov., an Fe(Ⅲ)- and uranium(Ⅵ)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination. Int. J. Syst. Evol. Microbiol. 2010, 60, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Latmani, R.; Veeramani, H.; Vecchia, E.D.; Junier, P.; Lezama-Pacheco, J.S.; Suvorova, E.I.; Sharp, G.O.; Wigginton, N.S.; Bargar, J.R. Non-uraninite products of microbial U (VI) reduction. Environ. Sci. Technol. 2010, 44, 9456–9462. [Google Scholar] [CrossRef]
- Latta, D.E.; Kemner, K.M.; Mishra, B.; Boyanov, M.I. Effects of calcium and phosphate on uranium(VI) oxidation: Comparison between nanoparticulate uraninite and amorphous U (VI)- Phosphate. Geochim. Cosmochim. Acta 2016, 174, 122–142. [Google Scholar] [CrossRef]
- Latta, D.E.; Boyanov, M.I.; Kemner, K.M.; O’Loughlin, E.J.; Scherer, M.M. Abiotic reduction of uranium by Fe(Ⅱ) in soil. Appl. Geochem. 2012, 27, 1512–1524. [Google Scholar] [CrossRef]
- Hu, R.Z.; Bi, X.W.; Zhou, M.F.; Peng, J.T.; Su, W.C.; Liu, S.; Qi, H.W. Uranium metallogenesis in South China and its relationship to crustal extension during the Cretaceous to Tertiary. Econ. Geol. 2008, 103, 583–598. [Google Scholar] [CrossRef]
- Dargent, M.; Truche, L.; Dubessy, J.; Bessaque, G.; Marmier, H. Reduction kinetics of aqueous U(Ⅵ) in acidic chloride brines to uraninite by methane, hydrogen or C-graphite under hydrothermal conditions: Implications for the genesis of unconformity-related uranium ore deposits. Geochim. Cosmochim. Acta 2015, 167, 11–26. [Google Scholar] [CrossRef]
- Shamim, A.; Gu, H.; Yang, X. Uranium enrichment in tuff of Upper Triassic in the ordos Basin. Geol. Rev. 2016, 401–402. [Google Scholar]
- Zhang, J.; Xu, G.; Lin, J.; Peng, Y.; Wang, G. The implication of six kinds of new sandstone-type uranium deposits to uranium resources potential in North China. Geol. China 2010, 37, 1434–1449, (In Chinese with English Abstract). [Google Scholar]
- Ma, Y.; Wu, B.; Liu, Y.; Liu, C.; Zhao, Z.; Wang, H.; Song, Z.; Wei, A. Study on uranium occurrence state of sandstone-type uranium deposits in HJQ region, Ordos Basin. Northwestern Geol. 2013, 46, 141–152, (In Chinese with English Abstract). [Google Scholar]
- Raiswell, R.; Berner, R.A. Pyrite formation in euxinic and semi-euxinic sediments. Am. J. Sci. 1985, 285, 710–724. [Google Scholar] [CrossRef]
- Sawlowicz, Z.; Gize, A.P.; Rospondek, M. Organic Matter from Zechstein Copper Deposits (Kupferschiefer) in Poland//Organic Matter and Mineralisation: Thermal Alteration, Hydrocarbon Generation and Role in Metallogenesis; Springer: Dordrecht, The Netherlands, 2000; pp. 220–242. [Google Scholar]
- Wei, H.Y.; Wei, X.M.; Qiu, Z.; Song, H.; Shi, G. Redox conditions across the G-L boundary in South China: Evidence from pyrite morphology and sulfur isotopic compositions. Chem. Geol. 2016, 440, 1–14. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Garciaguinea, J.; Martinezfrias, J.; Gonzalezmartin, R.; Zamora, L. Framboidal pyrites in antique books. Nature 1997, 388, 631. [Google Scholar] [CrossRef]
- Wignall, P.B.; Newton, R. Pyrite framboid diameter as a measure of oxygen deficiency in ancient mudrocks. Am. J. Sci. 1998, 298, 537–552. [Google Scholar] [CrossRef]
- Berner, R.A.; Raiswell, R. Burial of organic carbon and pyrite sulfur in sediments over phanerozoic time: A new theory. Geochim. Cosmochim. Acta 1983, 47, 855–862. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Bonnetti, C.; Liu, X.; Cuney, M.; Malartre, F.; Michels, R. The Uranium metallogenic cycle in the intracontinental Erlian Basin, NE China: From source to deposit. Miner. Resour. A Sustain. World 2015, 69, 1–5. [Google Scholar]
- Zhang, C. Geochemical interface for uranium ore fluids. Acta Geol. Sichuan 2005, 25, 86–91, (In Chinese with English Abstract). [Google Scholar]
- Jin, R.S.; Zhang, C.J.; Feng, X.X.; Tao, C.; Zhu, Q.; Li, G.Y. The influence of fluid mixing on the mineralization of sandstone type uranium deposits. Geol. Bull. China 2013, 33, 354–358, (In Chinese with English Abstract). [Google Scholar]
- Xie, H.; Jiao, Y.; Liu, Z.; Li, X.D.; Yi, C.; Rong, H.; Wan, L.L. Occurrence and enrichment mechanism of uranium ore minerals from sandstone-type uranium deposit, Northern Ordos Basin. Earth Sci. 2020, 45, 1531, (In Chinese with English Abstract). [Google Scholar]
- Doinikova, O.A.; Solodov, I.N.; Chertok, M.B. Mineral composition of uranium ore at the Dalmatovo deposit, Russia. Geol. Ore Depos. 2009, 51, 486–495. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, H.; Wang, X.; He, K.; Zhang, S.; Wu, C. Formation conditions and main controlling factors of uranium in marine source rocks. Adv. Earth Sci. 2017, 32, 199–208, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y. The Study of Sediments Adsorption of Uranium in Phosphorus-Enriched Water System; Chengdu University of Technology: Chengdu, China, 2014; pp. 70–87, (In Chinese with English Abstract). [Google Scholar]
- Wang, J. Discussion on geochemical characteristics, mechanism and prospecting model of gley type sandstone uranium mineralization- taking Redwell Uranium deposit as an example. Uranium Geol. 1998, 14, 20–25, (In Chinese with English Abstract). [Google Scholar]
- Ding, W.; Shen, K. Epigenetic alteration of sedimentary rocks at hydrogenic uranium deposit. Uranium Geol. 2001, 17, 83–89, (In Chinese with English Abstract). [Google Scholar]
- Cai, C.F.; Dong, H.L.; Li, H.T.; Xiao, X.; Ou, G.; Zhang, C. Mineralogical and geochemical evidence for coupled bacterial uranium mineralization and hydrocarbon oxidation in the Shashagetai deposit, NW China. Chem. Geol. 2007, 236, 167–179. [Google Scholar] [CrossRef]
- Alessi, D.S.; Lezama-Pacheco, J.S.; Stubbs, J.E.; Janousch, M.; Bargar, J.R.; Persson, P.; Bernier-Latmani, R. The product of microbial uranium reduction includes multiple species with U(IV)—Phosphate coordination. Geochim. Cosmochim. Acta 2014, 131, 115–127. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Welch, S.A.; Taunton, A.E.; Banfield, J.F. Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiol. J. 2002, 19, 343–367. [Google Scholar] [CrossRef]
- Hutchens, E.; Valsami-Jones, E.; Harouiya, N.; Chaïrat, C.; Oelkers, E.H.; McEldoney, S. An experimental investigation of the effect of bacillus megaterium on apatite dissolution. Geomicrobiol. J. 2006, 23, 177–182. [Google Scholar] [CrossRef]
- Toyoda, K.; Nakamura, Y.; Masuda, A. Rare earth elements of Pacific pelagic sediments. Geochim. Cosmochim. Acta 1990, 54, 1093–1103. [Google Scholar] [CrossRef]
- Wei, J.; Tang, C.; Xu, Z.; Chen, L.; Guo, H.; Miao, P.; Du, S.; Zeng, H. Uranium mineral occurrence of the uraniferous rock series in Longhupao region, northern Songliao Basin. Acta Mineral. Sin. 2019, 39, 17, (In Chinese with English Abstract). [Google Scholar]
- Gupta, D.K.; Walther, C. Uranium in Plants and the Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–246. [Google Scholar]
- Catalano, J.G.; Brown, J.R.G.E. Uranyl adsorption onto montmorillonite: Evaluation of binding sites and carbonate complexation. Geochim. Cosmochim. Acta 2005, 69, 2995–3005. [Google Scholar] [CrossRef]

| Sample No. | Depth (m) | Lithology | Measurements |
|---|---|---|---|
| 30ZK30-06 | 123.07 | Grayish-white sandy conglomerate | Chemical compositions (U 70.5 ppm); EDS and SEM; EPMA |
| 30ZK30-08 | 119.63 | Grayish-white sandy conglomerate | Chemical compositions (U 78.7 ppm); EDS and SEM; EPMA |
| 30ZK30-09 | 107 | Light gray sandy conglomerate | Chemical compositions (U 189 ppm); The sequential extraction procedure |
| 30ZK30-10 | 107.6 | Light gray sandy conglomerate | Chemical compositions (U 223 ppm); The sequential extraction procedure |
| 40ZK28-05 | 40.5 | Grayish-white sandy conglomerate | Chemical compositions (U 430 ppm); The sequential extraction procedure; EDS and SEM |
| 40ZK28-06 | 41 | Grayish-white sandy conglomerate | Chemical compositions (U 459 ppm); The sequential extraction procedure; EDS and SEM |
| 50ZK28-03 | 161.83 | Orange pebbly argillaceous sandstone | Chemical compositions (U 71.1 ppm); EDS and SEM; EPMA |
| 50ZK28-31 | 148.3 | Gray-black carbonaceous sandstone | Chemical compositions (U 184 ppm); The sequential extraction procedure |
| 50ZK28-55 | 206 | Orange pebbly argillaceous sandstone | Chemical compositions (U 1159 ppm); The sequential extraction procedure |
| 10ZK24-04-1 | 88.22 | Grayish white pebbly tuffaceous sandstone | Chemical compositions (U 364 ppm); EDS and SEM; EPMA |
| 10ZK24-04-3 | 88.22 | Grayish white pebbly tuffaceous sandstone | Chemical compositions (U 364 ppm); EDS and SEM; EPMA |
| 10ZK24-07 | 89.12 | Grayish white pebbly tuffaceous sandstone | Chemical compositions (U 1976 ppm); EDS and SEM; EPMA |
| 20ZK10-02 | 106.35 | Grayish white pebbly tuffaceous sandstone | EDS and SEM; EPMA |
| 20ZK10-03 | 115.4 | Grayish-white sandy conglomerate | EDS and SEM; EPMA |
| 20ZK46-01 | 149.23 | Grayish-white tuffaceous sandstone | EDS and SEM; EPMA |
| 20ZK46-02 | 158.86 | Grayish-white sandy conglomerate | EDS and SEM; EPMA |
| 30ZK10-01 | 130.39 | Grayish-white sandy conglomerate | EDS and SEM; EPMA |
| 30ZK10-02 | 133.06 | Grayish-white sandy conglomerate | EDS and SEM; EPMA |
| 30ZK10-03 | 159.12 | Gray pebbly fine sandstone | EDS and SEM; EPMA |
| Occurrence State | The Fractionation Procedures |
|---|---|
| water-soluble | Take 1 g sample, dissolve in 20 mL distilled water, oscillate 15 min at room temperature, and centrifuge. |
| weak acid-extractable | Take another 1 g sample, dissolve it in 40 mL acetic acid solution (1 mol/L), oscillated 16 h at room temperature, and centrifuge. |
| reducible | After washing the solid residue from the previous step, dissolve in 40 mL hydroxylamine hydrochloride solution, oscillated 16 h at room temperature, and centrifuge. |
| oxidable | After washing the solid residue from the previous step, slowly add in 10 mL H202 (30 mL) 3 times, oscillate 1 h at 85 °C, cool to room temperature, add 50 mL NH4Ac solution, oscillated 16 h at room temperature, and centrifuge. |
| residual state | After washing the solid residue from the previous step, add 60 °C distilled water in a water bath and evaporate to dryness; after a constant weight is maintained, remove it and perform acid digestions. |
| Sample No. | ω(U)/μg•g−1 | ||||||
|---|---|---|---|---|---|---|---|
| Water-Soluble | Weak Acid-Extractable | Reducible | Oxidable | Residual | Total Contents (Sequential Extraction) | Total Contents (One Step) | |
| 30ZK30-09 | 0.65 (0.34%) | 22.00 (11.70%) | 6.05 (3.22%) | 57.30 (30.48%) | 102.00 (54.26%) | 188.00 | 189.00 |
| 30ZK30-10 | 1.01 (0.47%) | 21.50 (9.63%) | 1.58 (0.68%) | 140.00 (62.64%) | 59.40 (26.58%) | 223.49 | 223.00 |
| 40ZK28-05 | 3.74 (0.87%) | 181.00 (42.07%) | 27.60 (6.42%) | 85.90 (19.96%) | 132.00 (30.68%) | 430.24 | 430.00 |
| 40ZK28-06 | 3.87 (0.90%) | 158.00 (34.47%) | 33.40 (7.28%) | 112.00 (24.40%) | 151.00 (32.95%) | 458.27 | 459.00 |
| 50ZK28-31 | 1.10 (0.60%) | 31.30 (17.11%) | 0.99 (0.54%) | 77.70 (42.49%) | 71.80 (39.26%) | 182.90 | 184.00 |
| 50ZK28-55 | 11.30 (0.97%) | 145.00 (12.49%) | 17.30 (1.49%) | 24.60 (2.12%) | 963.00 (82.93%) | 1161.20 | 1159.00 |
| Average value | 3.61 (0.82%) | 93.13 (21.05%) | 14.49 (3.77%) | 82.92 (18.67%) | 246.53 (55.59%) | 442.35 | 440.67 |
| Sample No. | 50ZK28-03 | 10ZK24-04-1 | 10ZK24-04-3 | 10ZK24-07 | 20ZK10-02 | 20ZK46-01 | 30ZK10-02 | 30ZK30-06 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Point | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| SiO2 | 16.45 | 17.78 | 14.32 | 14.88 | 11.25 | 10.74 | 10.82 | 10.36 | 10.93 | 11.77 | 12.51 | 11.78 | 10.78 | 10.77 | 16.41 | 20.92 | / |
| TiO2 | 1.2 | 0.68 | 0.53 | 0.77 | / | 0.57 | 0.33 | 1.06 | 0.74 | 0.28 | 0.45 | 0.21 | 0.40 | 0.50 | 0.35 | / | / |
| Al2O3 | 2.01 | 3.85 | 2.61 | 1.8 | 0.14 | 0.29 | 0.17 | 0.08 | 0.08 | 0.29 | 0.17 | 0.13 | 0.31 | 1.07 | 0.10 | / | / |
| FeO | 2.34 | 2.43 | 3.6 | 1.35 | 0.47 | 0.49 | 0.28 | 0.35 | 0.35 | 0.98 | 0.40 | 2.13 | 0.61 | 1.22 | 0.32 | 0.16 | 1.09 |
| MgO | 0.4 | 0.46 | 0.81 | 0.27 | 0.07 | 0.12 | 0.09 | 0.06 | 0.11 | 0.15 | 0.04 | 0.04 | 0.12 | 0.22 | 0.15 | / | / |
| CaO | 2.24 | 1.98 | 2.32 | 2.41 | 4.42 | 6.32 | 7.18 | 5.09 | 4.86 | 6.73 | 6.05 | 5.83 | 7.72 | 7.44 | 6.47 | 0.04 | 0.55 |
| K2O | 0.29 | 1.64 | 0.14 | 0.44 | 0.11 | 0.21 | 0.16 | 0.19 | 0.17 | 0.22 | 0.21 | 0.18 | 0.24 | 0.32 | 0.61 | 0.06 | 0.03 |
| Na2O | 0.15 | 0.23 | 0.19 | 0.15 | 0.13 | 0.11 | 0.06 | / | / | / | / | / | / | / | / | / | |
| P2O5 | 4.99 | 4.67 | 5.35 | 5.49 | 9.46 | 11.96 | 12.40 | 11.24 | 11.35 | 11.92 | 9.66 | 10.61 | 14.03 | 15.70 | 6.92 | 0.59 | 28.58 |
| SO3 | 0.24 | 0.14 | 0.24 | 0.17 | 0.24 | 0.10 | 0.18 | / | 0.05 | 0.12 | 0.13 | 4.83 | 0.55 | 2.54 | 0.06 | / | / |
| UO2 | 57.88 | 50.1 | 57.16 | 60.59 | 8.79 | 65.48 | 63.81 | 65.61 | 67.81 | 63.59 | 65.17 | 60.84 | 61.81 | 58.11 | 51.29 | 7.72 | 0.35 |
| PbO | / | / | 0.09 | 0.29 | 0.18 | 0.08 | 0.1 | / | / | / | / | / | / | / | / | / | / |
| MnO | / | / | / | / | / | 0.32 | / | 0.28 | 0.35 | 0.25 | 0.15 | 0.18 | 0.28 | 0.11 | / | / | / |
| V2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.09 | 0.12 | |
| ThO2 | 1.2 | 0.68 | 0.53 | 0.77 | 54.32 | / | / | / | / | / | / | / | / | / | 3.85 | 53.82 | 4.07 |
| ZrO2 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 1.14 | |
| La2O3 | / | 0.23 | 0.24 | / | / | / | / | / | / | / | / | / | / | / | / | / | 12.3 |
| Y2O3 | 3.15 | 3.04 | 3.7 | 3.4 | 2.57 | 0.58 | 0.64 | 0.57 | 0.69 | 0.54 | 0.45 | 0.43 | 0.67 | 0.69 | 1.02 | 1.01 | 0.93 |
| Ce2O3 | 0.41 | 0.51 | 0.43 | / | 0.44 | 0.39 | 0.27 | / | 0.21 | 0.40 | 0.25 | / | 0.69 | 0.22 | 0.26 | 0.21 | 29.43 |
| AS2O5 | / | 0.04 | / | / | 0.18 | 0.14 | / | 0.05 | 0.12 | 0.04 | / | 0.09 | / | 0.07 | / | / | / |
| Nd2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.60 | 0.44 | / | 9.87 |
| Pr2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.12 | / | / | 2.97 |
| Sm2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 2.37 |
| Gd2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 1.65 |
| Eu2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.19 |
| Dy2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.46 |
| Er2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.34 |
| Tm2O3 | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.14 |
| Total | 91.88 | 88.14 | 91.83 | 92.01 | 92.88 | 97.9 | 96.49 | 94.94 | 97.82 | 97.28 | 95.64 | 97.28 | 98.21 | 99.70 | 88.34 | 85.79 | 95.32 |
| Type of uranium mineral | coffinite | thorite | uranium phosphosilicates | coffinite | thorite | monazite | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Mou, C.; Wu, H. Uranium Occurrence State and Its Implication for Sandstone-Type Uranium Mineralization within the Hanbazhai Area of the Longchuanjiang Basin, China. Minerals 2023, 13, 1037. https://doi.org/10.3390/min13081037
Xia Y, Mou C, Wu H. Uranium Occurrence State and Its Implication for Sandstone-Type Uranium Mineralization within the Hanbazhai Area of the Longchuanjiang Basin, China. Minerals. 2023; 13(8):1037. https://doi.org/10.3390/min13081037
Chicago/Turabian StyleXia, Yu, Chuanlong Mou, and Hao Wu. 2023. "Uranium Occurrence State and Its Implication for Sandstone-Type Uranium Mineralization within the Hanbazhai Area of the Longchuanjiang Basin, China" Minerals 13, no. 8: 1037. https://doi.org/10.3390/min13081037
APA StyleXia, Y., Mou, C., & Wu, H. (2023). Uranium Occurrence State and Its Implication for Sandstone-Type Uranium Mineralization within the Hanbazhai Area of the Longchuanjiang Basin, China. Minerals, 13(8), 1037. https://doi.org/10.3390/min13081037





