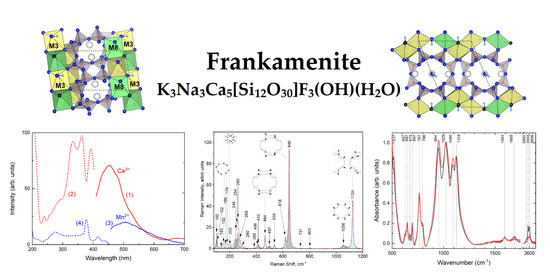
Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties
Abstract
1. Introduction
2. Materials and Methods
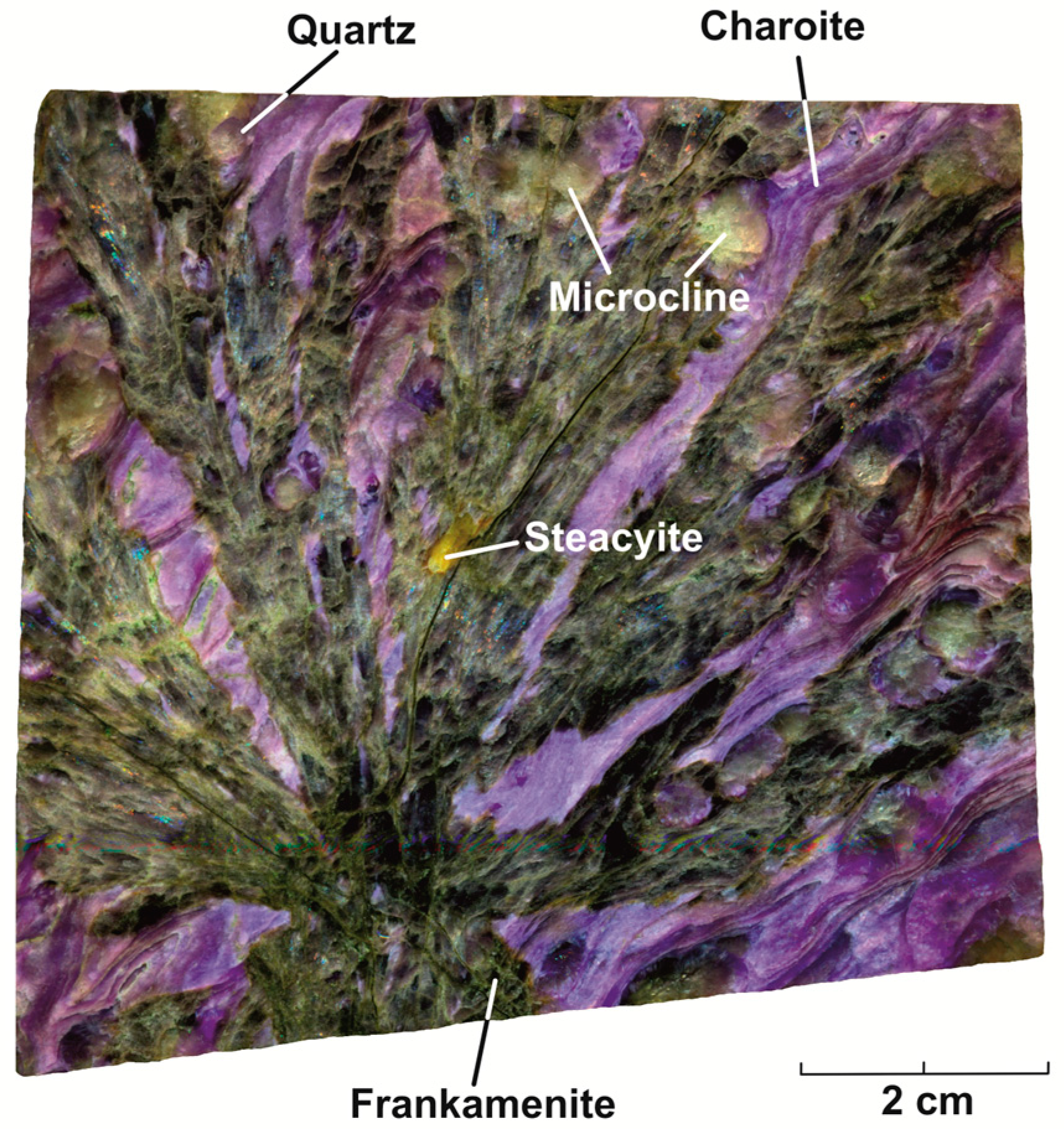
2.1. Sample Description
2.2. Crystal–Chemical Analysis
2.3. Ab Initio Calculations
2.4. Infrared Spectroscopy
2.5. Raman Spectroscopy
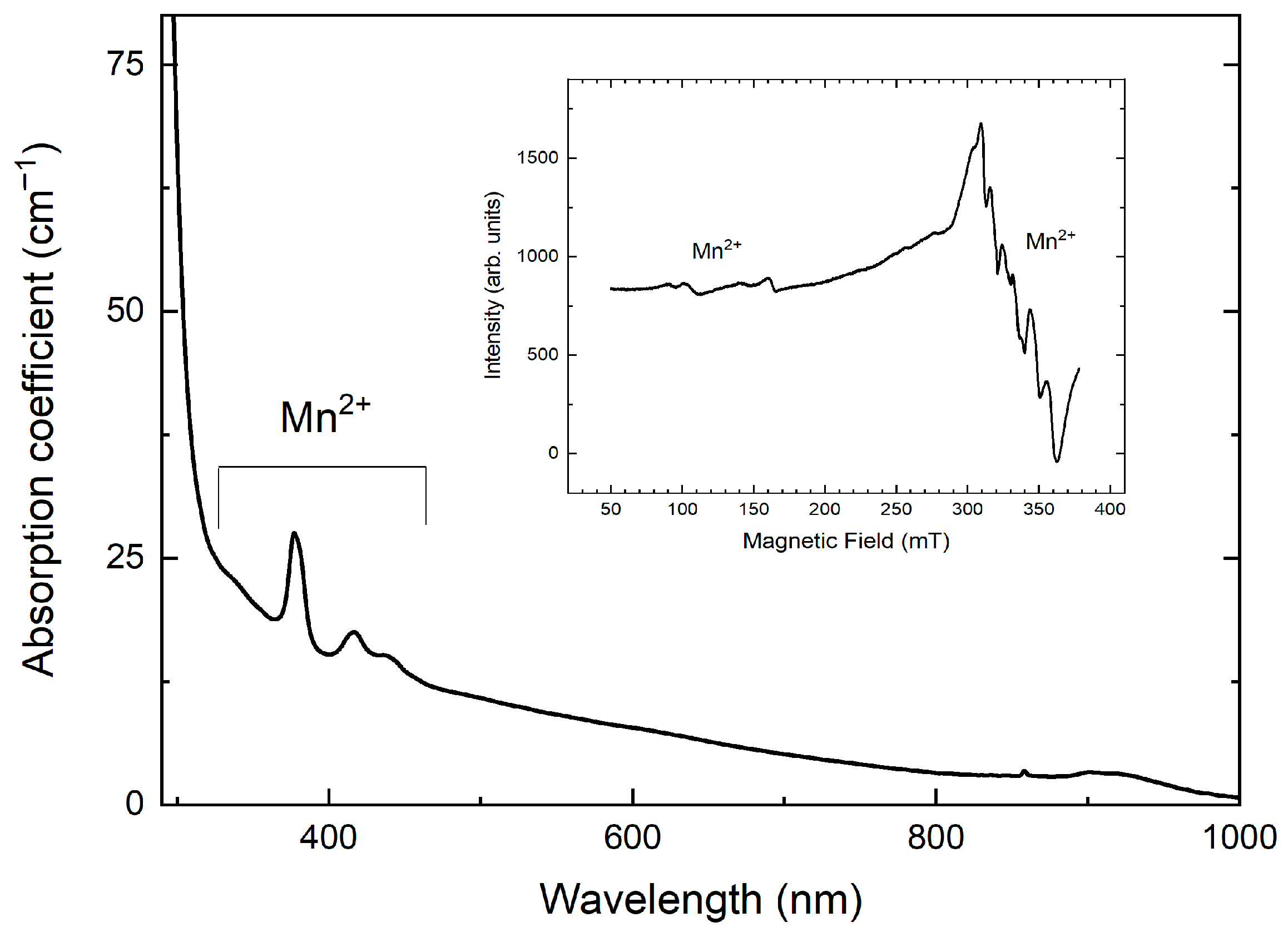
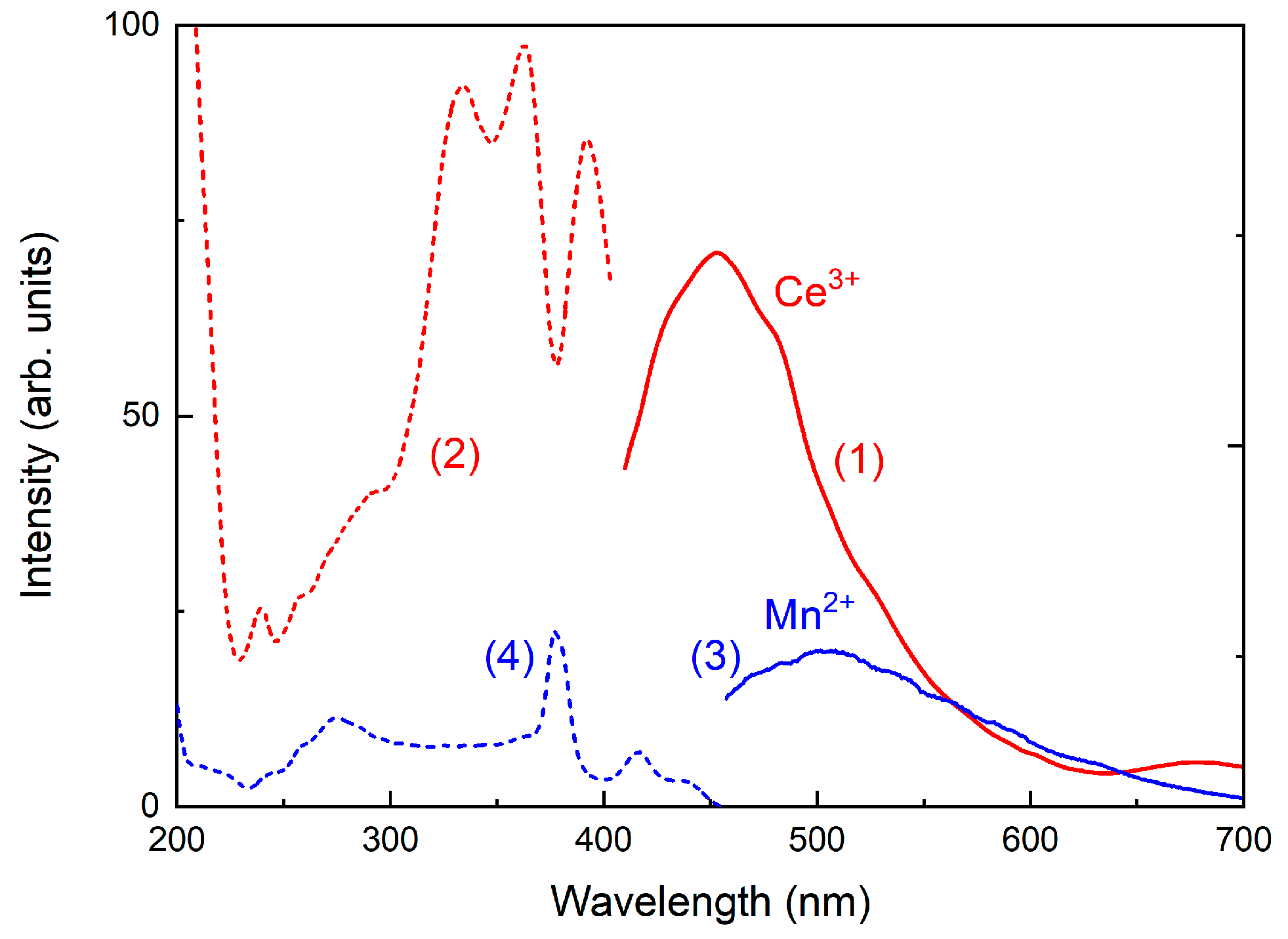
2.6. Optical Spectroscopy and ESR
3. Results
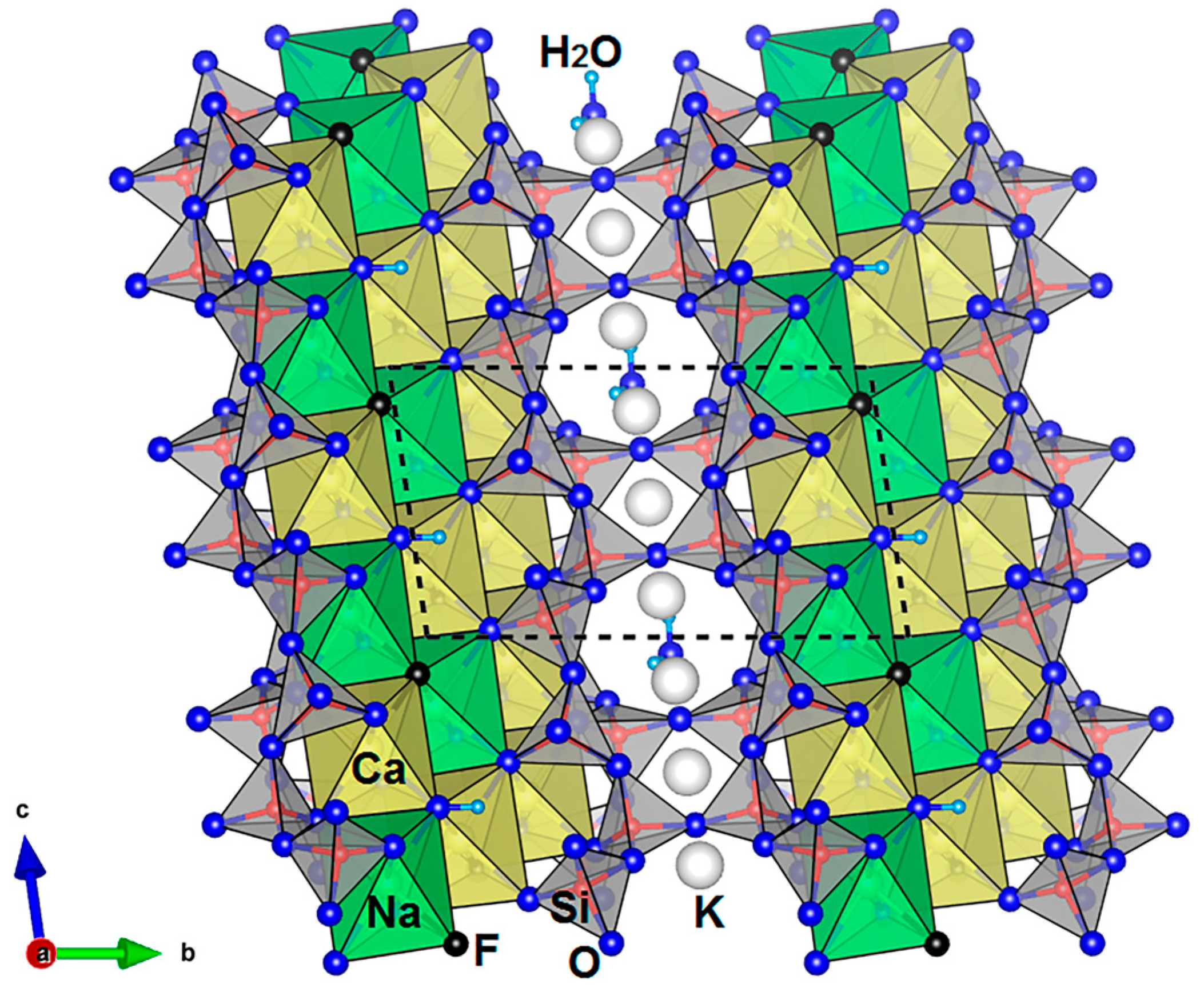
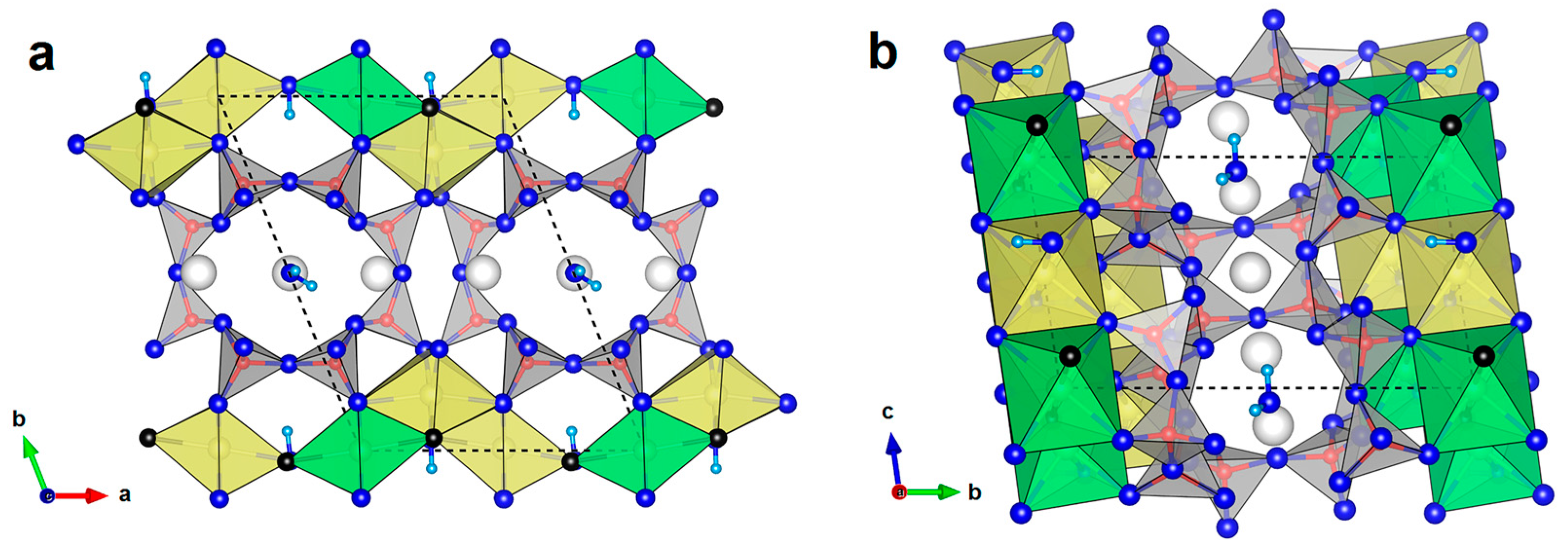
3.1. Crystal Chemistry and Structure Description
3.2. Ab Initio Calculations
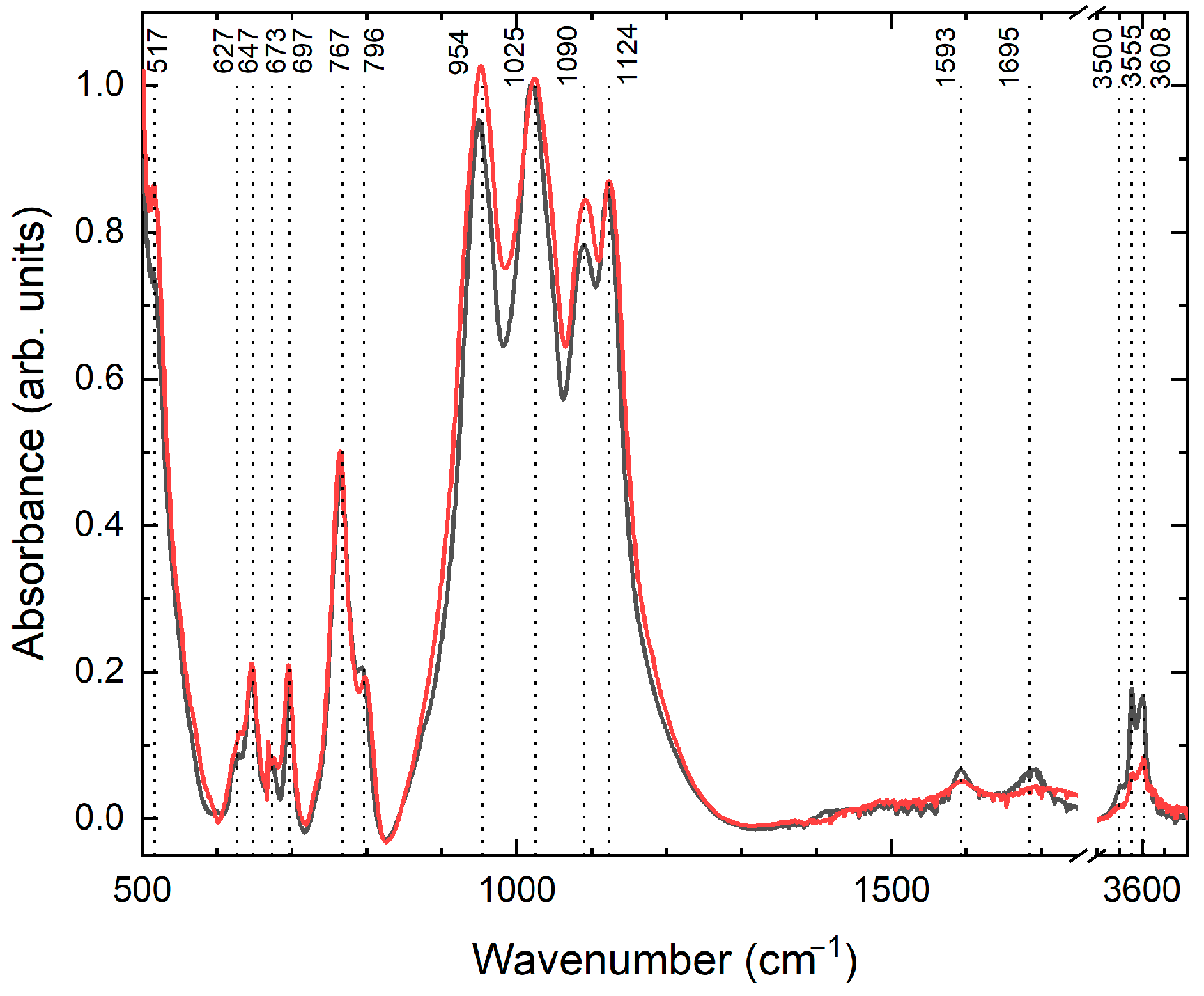
3.3. Infrared and Raman Spectroscopy
3.4. Optical Absorption and Luminescence
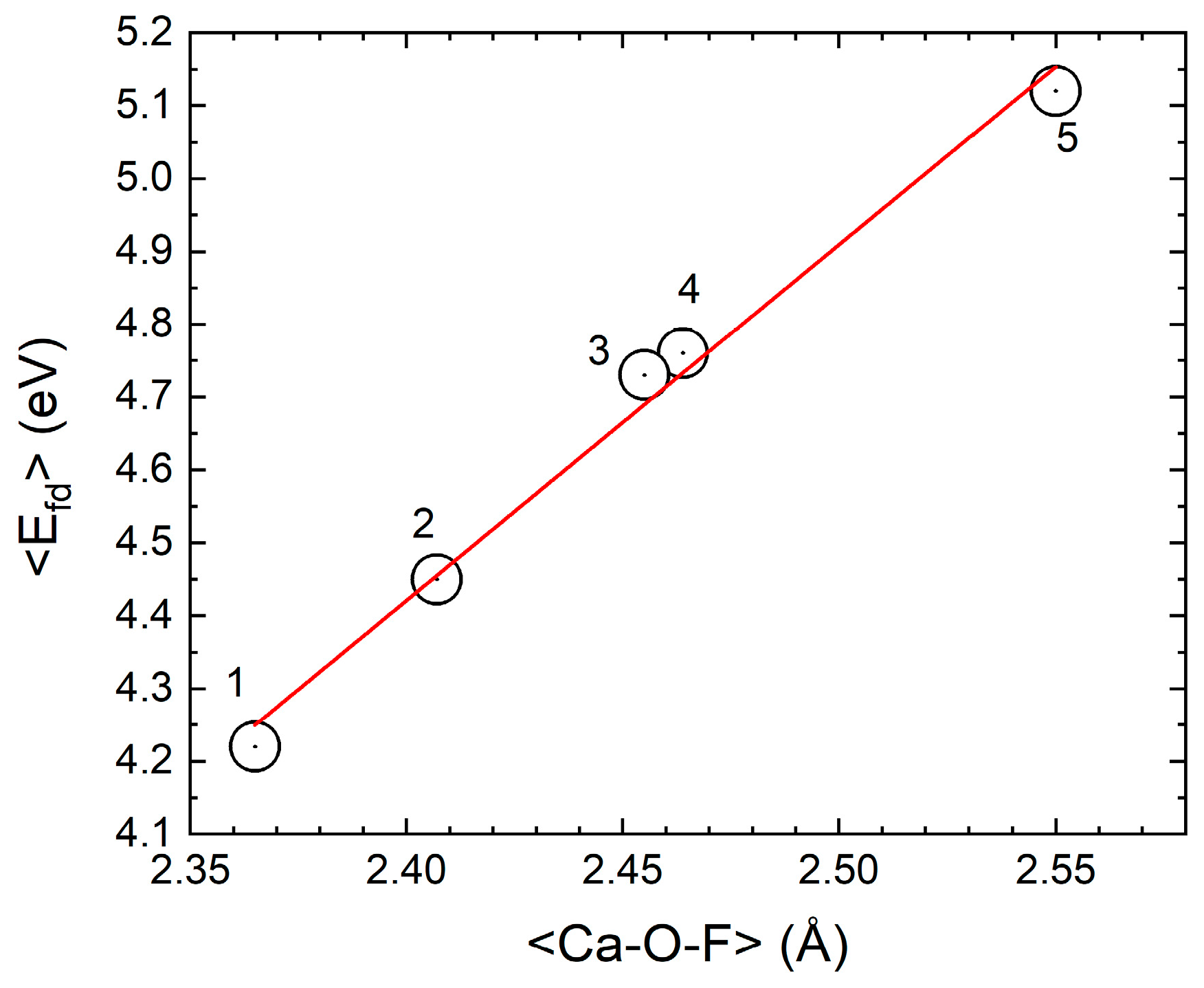
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, M.; Hawthorne, F.C. A structure hierarchy for silicate minerals: Chain, ribbon, and tube silicates. Mineral. Mag. 2020, 84, 165–244. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.; Mugnaioli, E.; Czank, M.; Depmeier, W.; Kolb, U.; Merlino, S. Essential features of the polytypic charoite-96 structure compared to charoite-90. Mineral. Mag. 2011, 75, 2833–2846. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Bannova, I.I.; Lazebnik, Y.D. Canasite: Refinement of structure and structural typomorphism. Miner. Zh. 1988, 10, 31–44. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Krivokoneva, G.K.; Rastsvetaeva, R.K.; Rozenberg, K.A.; Rozhdestvenskaya, I.V. Fluorcanasite, K3Na3Ca5Si12O30(F,OH)4·H2O, a new mineral species from the Khibiny alkaline pluton, Kola peninsula, Russia, and new data on canasite. Geol. Ore Depos. 2009, 51, 757–766. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Rogachev, D.L.; Goroshchenko, Z.I.; Uspenskaya, E.I. Canasite: A new mineral. Tr. Mineral. Muzeya Akad. Nauk SSSR. 1959, 9, 158–166. (In Russian) [Google Scholar]
- Chiragov, M.I.; Mamedov, K.S.; Belov, N.V. Crystal structure of canasite, Ca5Na4K2[Si12O30](OH,F)4. Dokl. Akad. Nauk SSSR. 1969, 185, 672–674. (In Russian) [Google Scholar]
- Evdokimov, M.D.; Regir, E.P. Canasite in charoite of the Murun alkaline complex (Sirenevy Kamen deposit). Zap. Vseross. Mineral. O-va 1994, 123, 104–118. (In Russian) [Google Scholar]
- Nikishova, L.V.; Lazebnik, K.A.; Rozhdestvenskaya, I.V.; Emel’yanova, N.N.; Lazebnik, Y.D. Triclinic canasite from charoitites of Yakutia. Mineral. Zh. 1992, 14, 71–77. (In Russian) [Google Scholar]
- Nikishova, L.V.; Lazebnik, K.A.; Rozhdestvenskaya, I.V.; Emel’yanova, N.N.; Lazebnik, Y.D. Frankamenite K3Na3Ca5(Si12O30)F3(OH)·nH2O—A new mineral species. Triclinic analogue of canasite from charoitite. Zap. Vseross. Mineral. O-va 1996, 125, 106–108. (In Russian) [Google Scholar]
- Konev, A.A.; Vorob’ev, E.I.; Lazebnik, K.A. Mineralogy of the Alkaline Murun Massif; SB RAS, SPC JIGGM: Novosibirsk, Russia, 1996. (In Russian) [Google Scholar]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, K.A. The crystal structure of frankamenite. Mineral. Mag. 1996, 60, 897–905. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Rozenberg, K.A.; Khomyakov, A.P.; Rozhdestvenskaya, I.V. Crystal structure of F-canasite. Dokl. Chem. 2003, 391, 177–180. [Google Scholar] [CrossRef]
- Kroumova, E.; Aroyo, M.I.; Perez-Mato, J.M.; Kirov, A.; Capillas, C.; Ivantchev, S.; Wondratschek, H. Bilbao Crystallographic Server: Useful Databases and Tools for Phase-Transition Studies. Phase Transit. A Multinat. J. 2003, 76, 155–170. [Google Scholar] [CrossRef]
- Farag, M.M.; El-Rashedi, A.M.I.; Rüssel, C. In vitro biocompatibility evaluation of canasite-calcium phosphate glass-ceramics. J. Non-Cryst. Solids 2018, 488, 24–35. [Google Scholar] [CrossRef]
- Basaran, N.; Capoglu, A. The effect of LiF, CaF2 and MgF2 addition on the sintering and crystallisation behaviour of a base glass containing calcined bone ash. J. Non-Cryst. Solids 2021, 561, 120752. [Google Scholar] [CrossRef]
- Miller, C.A.; Kokubo, T.; Reaney, I.M.; Hatton, P.V.; James, P.F. Formation of apatite layers on modified canasite glass–ceramics in simulated body fluid. J. Biomed. Mater. Res.–B Appl. Biomater. 2002, 59, 473–480. [Google Scholar] [CrossRef]
- El-Meliegy, E.; van Noort, R. Models of Bioactive Glass Ceramics. In Glasses and Glass Ceramics for Medical Applications; Springer: New York, NY, USA, 2012; pp. 229–238. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Reaney, I.M.; Hurrell-Gillingham, K.; Brook, I.M.; Hatton, P.V. Evaluation of modified fluorcanasite glass-ceramics for bone tissue augmentation. Key Eng. Mater. 2005, 284–286, 557–560. [Google Scholar] [CrossRef]
- Johnson, A.; Shareef, M.Y.; Van Noort, R.; Walsh, J.M. Effect of furnace type and ceramming heat treatment conditions on the biaxial flexural strength of a canasite glass-ceramic. Dent. Mater. 2000, 16, 280–284. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Faria, P.E.P.; Johnson, A.; Felipucci, D.N.B.; Reaney, I.M.; Salata, L.A.; Brook, I.M.; Hatton, P.V. Osteoconductivity of modified fluorcanasite glass–ceramics for bone tissue augmentation and repair. J. Biomed. Mater. Res. A 2010, 94, 760–768. [Google Scholar] [CrossRef]
- Kraipok, A.; Intawin, P.; Kamnoy, M.; Bintachitt, P.; Leenakul, W.; Panyata, S.; Eitssayeam, S.; Tunkasiri, T.; Pengpat, K. Preparation and characterization of lithium disilicate-fluorcanasite glass-ceramics for dental applications. J. Mech. Behav. Biomed. Mater. 2023, 137, 105548. [Google Scholar] [CrossRef]
- Kanchanarat, N.; Bandyopadhyay-Ghosh, S.; Reaney, I.M.; Brook, I.M.; Hatton, P.V. Microstructure and mechanical properties of fluorcanasite glass-ceramics for biomedical applications. J. Mater. Sci. 2008, 43, 759–765. [Google Scholar] [CrossRef]
- Omar, A.A.; Hamzawy, E.M.A.; Farag, M.M. Crystallization of fluorcanasite–fluorrichterite glasses. Ceram. Int. 2009, 35, 301–307. [Google Scholar] [CrossRef]
- Vyas, A.; Vijay Kumawat, S.; Bandhu Ghosh, S.; Bandyopadhyay-Ghosh, S. Microstructural analysis and bioactive response of selectively engineered glass-ceramics in simulated body fluid. Mater. Technol. 2020, 36, 451–459. [Google Scholar] [CrossRef]
- Abo-Mosallam, H.A.; Mahdy, E.A. Crystallization behavior and properties of fluorcanasite–lithium disilicate glasses for potential use in dental application. Ceram. Int. 2019, 45, 21144–21149. [Google Scholar] [CrossRef]
- Kraipok, A.; Intawin, P.; Bintachitt, P.; Leenakul, W.; Khamman, O.; Eitssayeam, S.; Tunkasiri, T.; Pengpat, K. Influence of heat treatment temperature on the properties of the lithium disilicate-fluorcanasite glass-ceramics. Int. J. Appl. Ceram. 2022, 19, 1415–1427. [Google Scholar] [CrossRef]
- Vyas, A.; Bandhu Ghosh, S.; Bandyopadhyay-Ghosh, S.; Agrawal, A.K.; Khare, D.; Dubey, A.K. Digital light processing mediated 3D printing of biocomposite bone scaffolds: Physico-chemical interactions and in-vitro biocompatibility. Polym. Compos. 2022, 43, 3175–3188. [Google Scholar] [CrossRef]
- Takav, P.; Banijamali, S.; Sedaghat Ahangari Hossein Zadeh, A.; Mobasherpour, I. Influence of TiO2 content on phase evolution, microstructure and properties of fluorcanasite glass-ceramics prepared through sintering procedure for dental restoration applications. Ceram. Int. 2018, 44, 7057–7066. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, W.; Zheng, W.; Tian, P.; Lin, M.; Ji, S. Phase transition of different crystals in canasite-based glass-ceramics. J. Chin. Ceram. Soc. 2012, 40, 1415–1419. [Google Scholar]
- Kanchanarat, N.; Miller, C.A.; Hatton, P.V.; James, P.F.; Reaney, I.M. Early stages of crystallization in canasite-based glass ceramics. J. Am. Ceram. 2005, 88, 3198–3204. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Reaney, I.M.; Brook, I.M.; Hurrell-Gillingham, K.; Johnson, A.; Hatton, P.V. In vitro biocompatibility of fluorcanasite glass-ceramics for bone tissue repair. J. Biomed. Mater. Res. A 2007, 80, 175–183. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Tricot, G.; Kothiyal, G.P.; Montagne, L. A comparative overview of glass-ceramic characterization by MAS-NMR and XRD. Crit. Rev. Solid State Mater. Sci. 2011, 36, 229–241. [Google Scholar] [CrossRef]
- Kaneva, E.V.; Shendrik, R.Y.; Vladykin, N.V.; Radomskaya, T.A. Crystal-chemical features of rare and complex silicates from charoite rocks of the Malyy Murun volcano-plutonic alkaline complex. In Alkaline Rocks, Kimberlites and Carbonatites: Geochemistry and Genesis. Springer Proceedings in Earth and Environmental Sciences; Vladykin, N., Ed.; Springer: Cham, Switzerland, 2021; pp. 115–129. [Google Scholar] [CrossRef]
- Hanus, R.; Štubňa, J.; Jungmannová, K. Frankamenite as an ornamental gem material. J. Gemmol. 2020, 37, 132–133. [Google Scholar] [CrossRef]
- Zhang, R.X.; Yang, S.Y. A mathematical model for determining carbon coating thickness and its application in electron probe microanalysis. Microsc. Microanal. 2016, 22, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Bruker APEX2, version 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Bruker SAINT, version 6.0; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. XPREP, version 2008/2; Bruker-AXS: Madison, WI, USA, 2008. [Google Scholar]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. Crystals version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Copper, R.I.; Gould, R.O.; Parsons, S.; Watkin, D.J. The derivation of non-merohedral twin laws during refinement by analysis of poorly fitting intensity data and the refinement of non-merohedrally twinned crystal structures in the program CRYSTALS. J. Appl. Cryst. 2002, 35, 168–174. [Google Scholar] [CrossRef]
- Kaneva, E.V.; Shendrik, R.Y.; Radomskaya, T.A.; Suvorova, L.F. Fedorite from Murun alkaline complex (Russia): Spectroscopy and crystal chemical features. Minerals 2020, 10, 702. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Makovicky, E. Determination of the centroid or ‘the best centre’ of a coordination polyhedron. Acta Crystallogr. 1996, B52, 78–81. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Vicković, I. IVTON—Program for the calculation of geometrical aspects of crystal structures and some crystal chemical applications. J. Appl. Cryst. 1996, 29, 305–306. [Google Scholar] [CrossRef]
- Breese, N.E.; O’Keeffe, M. Bond-valence parameters for solid. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Gagnè, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W.J.P.R. Density functional theory (DFT). Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, E.; Bogdanov, A.; Shendrik, R. Structural and vibrational properties of agrellite. Sci. Rep. 2020, 10, 15569. [Google Scholar] [CrossRef]
- Dokuchits, E.Y.; Jiang, S.-Y.; Stepanov, A.S.; Zhukova, I.A.; Radomskaya, T.A.; Marfin, A.E.; Vishnevskiy, A.V. Geochemistry of Ca-(K)-(Na) silicates from charoitites in the Sirenevyi Kamen gemstone deposit, Murun Complex, Eastern Siberia. Ore Geol. Rev. 2022, 143, 104787. [Google Scholar] [CrossRef]
- Hoppe, R.; Voigt, S.; Glaum, H.; Kissel, J.; Müller, H.P.; Bernet, K. A new route to charge distributions in ionic solids. J. Less-Common. Met. 1989, 156, 105–122. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G.; Ohashi, H. Charge distribution as a tool to investigate structural details: Meaning and application to pyroxenes. Acta Crystallogr. 1999, B55, 902–916. [Google Scholar] [CrossRef]
- Renner, B.; Lehmann, G. Correlation of angular and bond length dis tortions in TO4 units in crystals. Z. Kristallogr. 1986, 175, 43–59. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts. Pure Appl. Chem. 2001, 73, 381–394. [Google Scholar] [CrossRef]
- Cadoni, M.; Ferraris, G. Synthesis and crystal structure of Na2MnSi4O10: Relationship with the manaksite group. Rend. Fis. Acc. Lincei 2011, 22, 225–234. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H…O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Beckenkamp, K.; Lutz, H.D. Lattice vibration spectra Part LXXII. OH stretching frequencies of solid hydroxides—Correlation with structural and bonding data. J. Mol. Struct. 1992, 270, 393–405. [Google Scholar] [CrossRef]
- Bogdanov, A.; Kaneva, E.; Shendrik, R. New insights into the crystal chemistry of elpidite, Na2Zr[Si6O15]·3H2O and (Na1+yCax□(1-x-y))Σ=2Zr[Si6O15]·(3 − x)H2O, and ab initio modeling of IR spectra. Materials 2021, 14, 2160. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Boldyreva, E.V. Difference in the dynamic properties of chiral and racemic crystals of serine studied by raman spectroscopy at 3−295 K. J. Phys. Chem. B 2007, 111, 14387–14397. [Google Scholar] [CrossRef]
- Kaneva, E.; Bogdanov, A.; Radomskaya, T.; Belozerova, O.; Shendrik, R. Crystal-chemical characterizationand spectroscopyof fluorcarletonite and carletonite. Mineral. Mag. 2023, 87, 356–368. [Google Scholar] [CrossRef]
- Kaneva, E.; Shendrik, R.; Mesto, E.; Bogdanov, A.; Vladykin, N. Spectroscopy and crystal chemical properties of NaCa2[Si4O10]F natural agrellite with tubular structure. Chem. Phys. Lett. 2020, 738, 136868. [Google Scholar] [CrossRef]




| Mineral | Ideal Structural Formula [1] | Type Locality | Sp. gr. |
|---|---|---|---|
| Canasite | K3Na3Ca5[Si12O30](OH)4 | Khibiny Massif | C2/m |
| Frankamenite | K3Na3Ca5[Si12O30]F3(OH)(H2O) | Murun Massif | P1 |
| Fluorcanasite | K3Na3Ca5[Si12O30]F3(OH)(H2O) | Khibiny Massif | Cm |
| Constituent | wt.% | Range | Stand. Dev. | Atom | apfu |
|---|---|---|---|---|---|
| SiO2 | 55.78 | 54.81–57.19 | 0.15 | Si | 11.99 |
| Al2O3 | 0.04 | 0–0.29 | 0.05 | Al | 0.01 |
| Na2O | 6.57 | 6.09–6.91 | 0.26 | Na | 2.74 |
| MgO | 0.01 | 0–0.03 | 0.01 | Mg | – |
| K2O | 10.82 | 10.62–11.08 | 0.14 | K | 2.97 |
| CaO | 21.83 | 21.45–22.15 | 0.21 | Ca | 5.03 |
| MnO | 0.42 | 0.34–0.48 | 0.04 | Mn | 0.08 |
| FeO | 0.07 | 0.03–0.12 | 0.04 | Fe | 0.01 |
| SrO | 0.28 | 0.17–0.39 | 0.06 | Sr | 0.03 |
| BaO | 0.10 | 0–0.26 | 0.09 | Ba | 0.01 |
| F | 4.41 | 4.30–4.61 | 0.16 | F | 3.00 |
| Sum | 100.33 | ||||
| –O=F2 | 1.86 | ||||
| H2O | 1.56 | ||||
| Total | 100.03 |
| Crystal Data | |||
|---|---|---|---|
| a (Å) | 10.093(1) | α (°) | 89.954(4) |
| b (Å) | 12.695(1) | β (°) | 111.043(4) |
| c (Å) | 7.2347(8) | γ (°) | 110.244(4) |
| V (Å3) | 803.6(2) | Space group, Z | P1, 1 |
| Crystal dimensions (mm) | 0.194 × 0.089 × 0.046 | ||
| Data collection | Refinement | ||
| Reflections measured | 40,780 | Reflections used in the refinement (I > 3σ(I)) | 7437 |
| Independent reflections | 11,451 | No. of refined parameters | 515 |
| Rmerging (R(int)) (%) | 4.28 | R a (on F) (%) | 3.67 |
| hmin, hmax | −15, 15 | Rw b (on F) (%) | 4.75 |
| kmin, kmax | −19, 19 | Goof c | 1.0653 |
| lmin, lmax | −10, 11 | Δρmin/Δρmax (e−/Å3) | −0.48/0.61 |
| Thetamin/Thetamax | 2.328/32.883 | Twin element ratio | 0.496:0.504 |
| Si1 | Si2 | Si3 | Si4 | Si5 | Si6 | |
| ECoN | 3.916 | 3.996 | 3.906 | 3.924 | 3.823 | 3.966 |
| Vp (Å3) | 2.119 | 2.117 | 2.261 | 2.200 | 2.068 | 2.170 |
| r (Å) | 1.608 | 1.609 | 1.644 | 1.626 | 1.596 | 1.621 |
| rv (Å) | 1.606 | 1.608 | 1.642 | 1.625 | 1.593 | 1.620 |
| ΔV (Å) | 0.061 | 0.016 | 0.063 | 0.059 | 0.081 | 0.040 |
| rs (Å) | 1.605 | 1.606 | 1.641 | 1.625 | 1.593 | 1.618 |
| Δ (Å) | 0.100 | 0.087 | 0.104 | 0.071 | 0.112 | 0.096 |
| Vs (Å3) | 17.362 | 17.423 | 18.554 | 17.989 | 16.941 | 17.818 |
| ECCv | 0.1090 | 0.0293 | 0.1112 | 0.1050 | 0.1450 | 0.0724 |
| SPHv | 0.9999 | 0.9999 | 1.0000 | 0.9999 | 1.0000 | 0.9999 |
| BLD (%) | 1.79 | 0.47 | 2.01 | 1.93 | 2.59 | 1.23 |
| A (°) | 109.3 | 109.3 | 109.4 | 109.4 | 109.3 | 109.3 |
| TAV | 22.747 | 24.649 | 27.532 | 10.076 | 28.264 | 23.021 |
| TQE | 1.005 | 1.006 | 1.007 | 1.003 | 1.007 | 1.006 |
| Si7 | Si8 | Si9 | Si10 | Si11 | Si12 | |
| ECoN | 3.901 | 3.935 | 3.967 | 3.947 | 3.972 | 3.970 |
| Vp (Å3) | 2.197 | 2.239 | 2.090 | 2.137 | 2.211 | 2.183 |
| r (Å) | 1.628 | 1.639 | 1.601 | 1.616 | 1.630 | 1.626 |
| rv (Å) | 1.625 | 1.637 | 1.599 | 1.614 | 1.629 | 1.625 |
| ΔV (Å) | 0.072 | 0.059 | 0.039 | 0.048 | 0.038 | 0.043 |
| rs (Å) | 1.625 | 1.635 | 1.598 | 1.611 | 1.628 | 1.622 |
| Δ (Å) | 0.099 | 0.115 | 0.095 | 0.129 | 0.083 | 0.099 |
| Vs (Å3) | 17.982 | 18.362 | 17.122 | 17.597 | 18.114 | 17.987 |
| ECCv | 0.1271 | 0.1046 | 0.0717 | 0.0874 | 0.0687 | 0.0766 |
| SPHv | 0.9999 | 1.0000 | 0.9999 | 1.0000 | 0.9999 | 1.0000 |
| BLD (%) | 1.99 | 1.62 | 1.19 | 1.50 | 1.12 | 1.25 |
| A (°) | 109.3 | 109.3 | 109.3 | 109.2 | 109.4 | 109.3 |
| TAV | 21.892 | 28.672 | 21.739 | 45.552 | 17.988 | 29.384 |
| TQE | 1.005 | 1.007 | 1.005 | 1.010 | 1.004 | 1.007 |
| M1 | M2 | M3 | M4 | M5 | M6 | |
| ECoN | 5.983 | 5.984 | 5.965 | 5.929 | 5.966 | 5.956 |
| Vp (Å3) | 18.912 | 17.442 | 17.787 | 17.225 | 17.023 | 17.769 |
| r (Å) | 2.438 | 2.368 | 2.392 | 2.372 | 2.362 | 2.392 |
| rv (Å) | 2.438 | 2.368 | 2.391 | 2.373 | 2.363 | 2.392 |
| ΔV (Å) | 0.023 | 0.030 | 0.046 | 0.049 | 0.045 | 0.010 |
| rs (Å) | 2.438 | 2.367 | 2.391 | 2.370 | 2.359 | 2.390 |
| Δ (Å) | 0.031 | 0.020 | 0.074 | 0.084 | 0.084 | 0.071 |
| Vs (Å3) | 60.678 | 55.608 | 57.251 | 55.970 | 55.250 | 57.335 |
| ECCv | 0.0286 | 0.0372 | 0.0568 | 0.0608 | 0.0564 | 0.0121 |
| SPHv | 0.9779 | 0.9872 | 0.9818 | 0.9602 | 0.9791 | 0.9546 |
| BLD (%) | 0.78 | 0.67 | 1.13 | 1.06 | 1.00 | 1.18 |
| A (°) | 90.0 | 90.0 | 90.0 | 90.1 | 90.1 | 89.9 |
| OAV | 46.555 | 31.905 | 54.943 | 73.448 | 71.895 | 56.354 |
| OQE | 1.014 | 1.010 | 1.017 | 1.022 | 1.021 | 1.018 |
| M7 | M8 | K1 | K2 | K3 | ||
| ECoN | 5.893 | 5.990 | 8.947 | 9.017 | 8.651 | |
| Vp (Å3) | 17.248 | 16.944 | 49.004 | 50.087 | 50.491 | |
| r (Å) | 2.373 | 2.357 | 3.100 | 2.978 | 2.980 | |
| rv (Å) | 2.373 | 2.358 | 3.098 | 2.987 | 2.980 | |
| ΔV (Å) | 0.049 | 0.021 | 0.067 | 0.103 | 0.024 | |
| rs (Å) | 2.370 | 2.355 | 3.075 | 2.969 | 2.966 | |
| Δ (Å) | 0.098 | 0.095 | 0.355 | 0.186 | 0.256 | |
| Vs (Å3) | 55.986 | 54.890 | 124.499 | 111.606 | 110.834 | |
| ECCv | 0.0609 | 0.0270 | 0.0632 | 0.1003 | 0.0237 | |
| SPHv | 0.9455 | 0.9864 | 0.9804 | 0.8517 | 0.8525 | |
| BLD (%) | 1.77 | 0.44 | 1.17 | 3.63 | 3.55 | |
| A (°) | 90.1 | 90.0 | – | – | – | |
| OAV | 72.134 | 70.168 | – | – | – | |
| OQE | 1.022 | 1.020 | – | – | – |
| Site | This Study | [10] | ||||
|---|---|---|---|---|---|---|
| Cation Distribution | e−X-ray | e−EPMA | Δ e− | Cation Distribution | e– | |
| K1 | K0.99 | 18.20 | 18.81 | 0.61 | K0.87 | 16.53 |
| K2 | K0.99 | 18.38 | 18.81 | 0.43 | K | 19.00 |
| K3 | K0.99Ba0.01 | 19.82 | 19.37 | 0.45 | K | 19.00 |
| M1 | Na0.85Ca0.10Sr0.03 | 12.87 | 12.49 | 0.38 | Na | 11.00 |
| M2 | Ca0.99 | 20.00 | 19.80 | 0.20 | Ca | 20.00 |
| M3 | Ca0.56Na0.43 | 16.20 | 15.93 | 0.27 | Na0.52Ca0.48 | 15.32 |
| M4 | Ca0.76Na0.23 | 18.04 | 17.73 | 0.31 | Ca0.54Na0.46 | 15.86 |
| M5 | Ca0.73Na0.25 | 17.81 | 17.35 | 0.46 | Ca0.70Na0.30 | 17.30 |
| M6 | Ca0.56Na0.43 | 16.25 | 15.93 | 0.32 | Ca0.69Na0.31 | 17.21 |
| M7 | Ca0.75Na0.23 | 17.99 | 17.53 | 0.46 | Ca0.73Na0.27 | 17.57 |
| M8 | Ca0.58Na0.32Mn0.08Fe0.01 | 17.72 | 17.38 | 0.34 | Ca0.72Na0.28 | 17.48 |
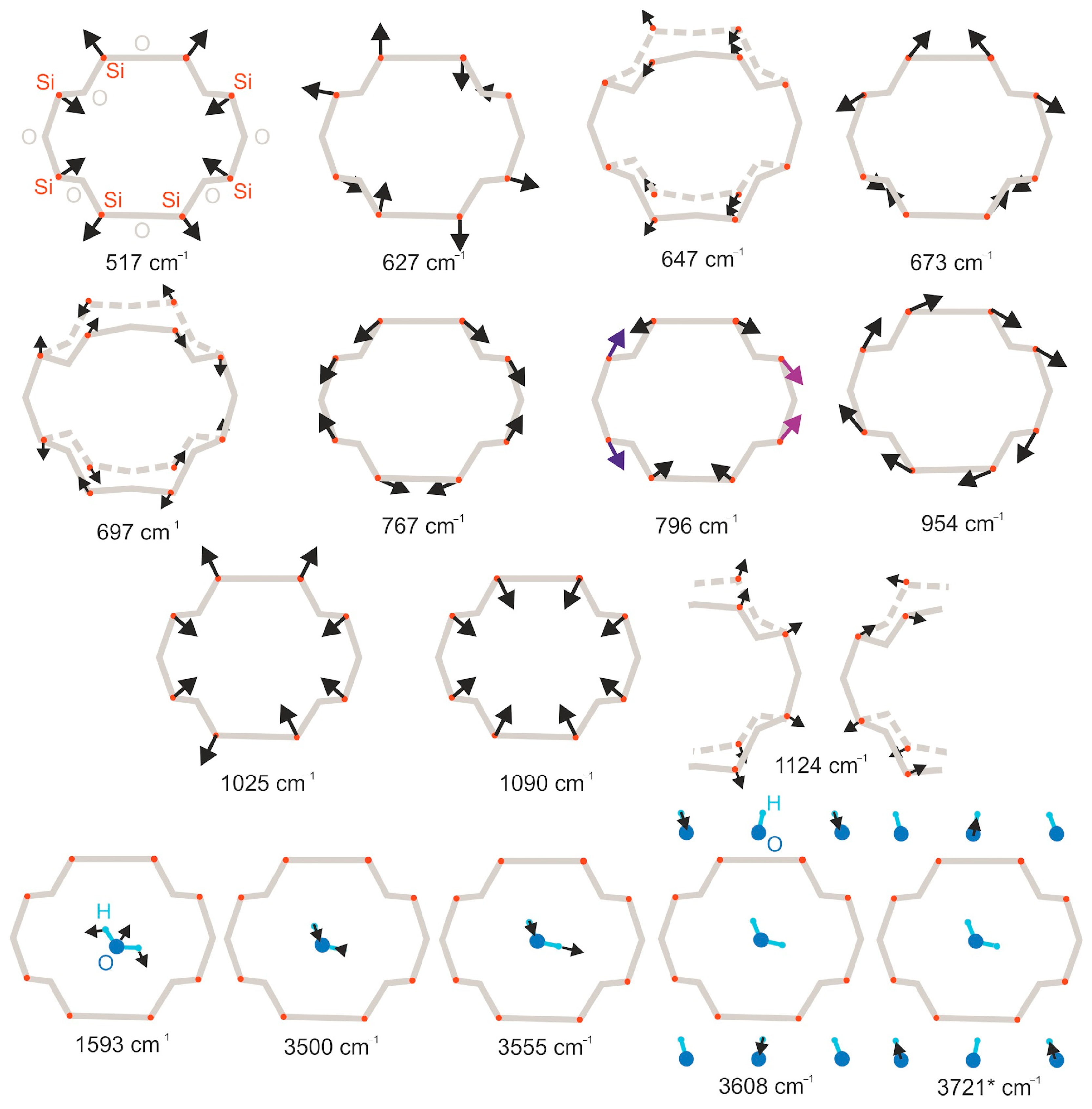
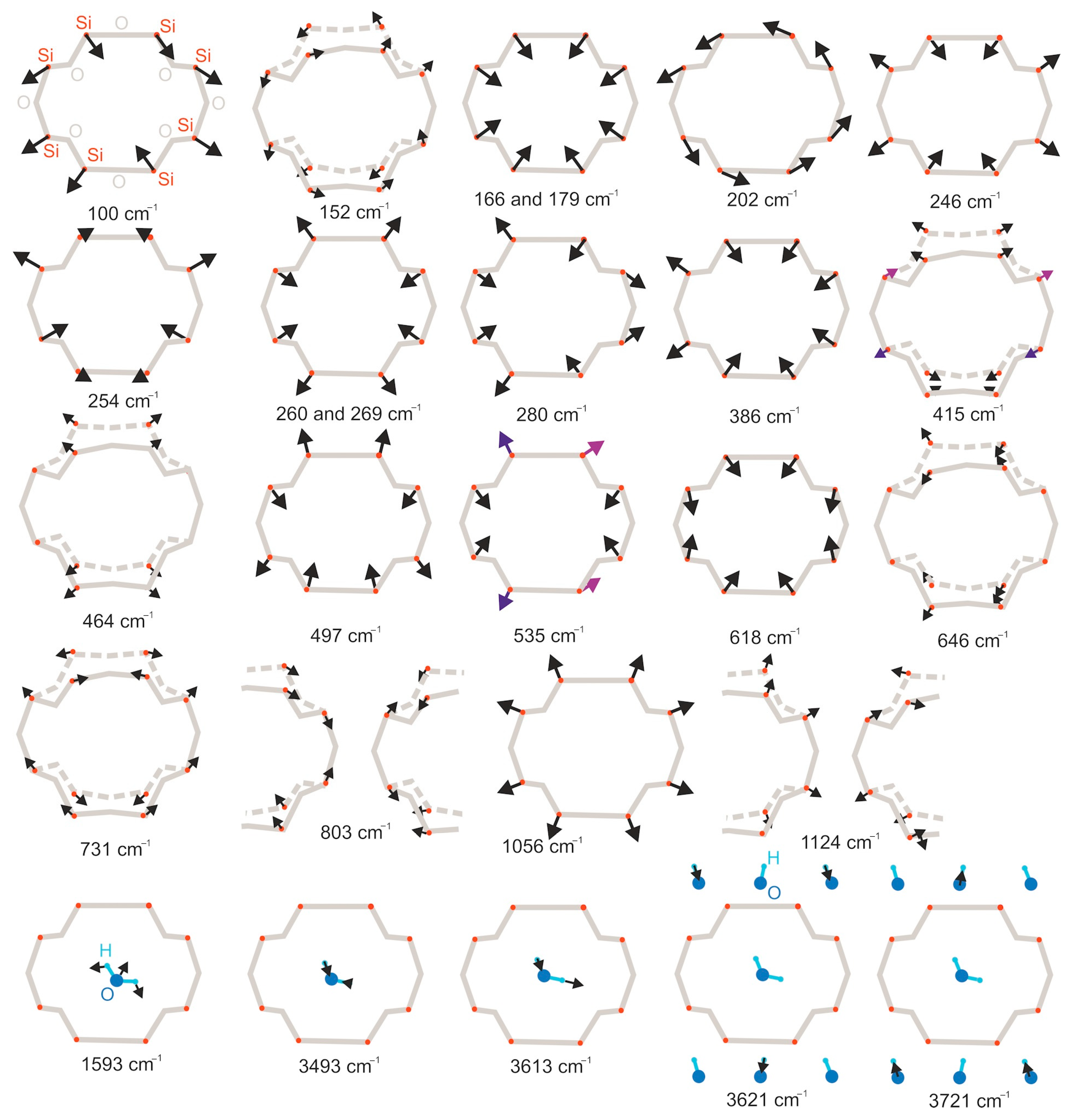
| Raman Modes, cm−1 | IR Modes, cm−1 | Assignment |
|---|---|---|
| 415, 464, 646, 731 | 647, 697 | Asymmetrical ring motion |
| 166, 179, 1056 | Breathing modes | |
| 202 | 954 | Ring rotation around the c-axis |
| 166, 179, 202, 246, 254, 260, 269, 280, 386, 497, 535, 618, 1056 | 517, 627, 673, 767, 796, 964, 1025, 1090 | Symmetrical ring motion |
| 1593, 1695 | Bending H–O–H | |
| 3557 | 3500, 3555 | Stretching H–O–H modes (ν1 and ν3) |
| 3608 | 3608 | Stretching O–H vibrations (ν1). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneva, E.; Shendrik, R.; Pankrushina, E.; Dokuchits, E.; Radomskaya, T.; Pechurin, M.; Ushakov, A. Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties. Minerals 2023, 13, 1017. https://doi.org/10.3390/min13081017
Kaneva E, Shendrik R, Pankrushina E, Dokuchits E, Radomskaya T, Pechurin M, Ushakov A. Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties. Minerals. 2023; 13(8):1017. https://doi.org/10.3390/min13081017
Chicago/Turabian StyleKaneva, Ekaterina, Roman Shendrik, Elizaveta Pankrushina, Emilia Dokuchits, Tatiana Radomskaya, Mikhail Pechurin, and Aleksey Ushakov. 2023. "Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties" Minerals 13, no. 8: 1017. https://doi.org/10.3390/min13081017
APA StyleKaneva, E., Shendrik, R., Pankrushina, E., Dokuchits, E., Radomskaya, T., Pechurin, M., & Ushakov, A. (2023). Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties. Minerals, 13(8), 1017. https://doi.org/10.3390/min13081017