1. Introduction
The solid and sludge by-products generated during steel production represent a source of secondary resources due to their considerable content of valuable materials. The usage of technologies for the valorization of residues could, therefore, lower the demand for raw materials while reducing landfilling, resulting in economic savings for the steel industry and closing material cycles [
1]. Despite the existence of several methods for the recovery of metals from metallurgical subproducts [
2,
3,
4], limited literature references where bioleaching processes were used as a metal recovery technique from steel mill by-products were found [
5,
6,
7,
8]. The current study is, to our knowledge, the first to focus on the cast house dust treatment through the utilization of extremely acidophilic bacteria to remove the element zinc, while preventing the leaching of iron. For this reason, the leaching efficiency, as well as the influence of the sulfur in the leaching process, together with the composition of the solid cast house gas dust, were also investigated during this study.
Zinc is naturally present in iron ores (loaded into the blast furnace in the form of ferrites, silicates, or sulfides), as well as in the recycled materials. The zinc compounds which enter the blast furnace react with the reducing gas at temperatures exceeding the boiling point of zinc, forming metallic zinc vapor which ascends with the gas to the colder parts of the blast furnace where it is re-oxidized again to form zinc oxide (ZnO). While part of the condensed zinc phases leave the furnace together with the gas, the remaining amount descends, forming a cyclical pattern as illustrated in
Figure 1 [
9,
10].
The accumulation of zinc in the blast furnace has some detrimental effects, such as the increase of the reductant rate and the reduction of the lining life of carbon-based brick materials or scaffold formation, which may lead to deteriorating the furnace performance [
9,
10]. Since the restriction of the zinc content in the blast furnace is mainly achieved by regulating the input levels of this element, a pre-separation of the zinc present in the by-products entering the blast furnace or upstream processes would be very interesting to avoid the abovementioned problems.
The by-product cast house dust, with an iron content of around 65% (
w/
w), is an interesting source of iron. Therefore, the removal of the zinc content for its further reutilization in the steel mill was analyzed during this research. The cast house dust is emitted into the cast house of a blast furnace as a consequence of the casting of liquid iron and slag [
11]. Conventionally, around 2.8 kg of cast house dust are produced per ton of hot metal [
12]. Since cast house dust is commonly recycled to the sinter process, elevated zinc concentrations in the cast house dust would make it difficult to fully recirculate blast furnace flue gas dust, as its recirculation will favor the zinc enrichment in the system. Consequently, part of the blast furnace flue gas dust ends up in landfills. Therefore, separation of the zinc present in the cast house dust would enhance the recycling rates of the blast furnace dust.
The current processes focused on the recovery of metals from steel mill dust can be classified into the following categories: physical, hydrometallurgical, and pyrometallurgical processes [
2]. As acid leaching occurs at low pH (1.5–2), traditionally, it represents the predominant method for the removal of metals from wastes. However, other methods such as microbial leaching have emerged in recent years for the removal of heavy metals such as zinc [
13], since it is environmentally friendlier, has lower energy necessities, and fewer chemical-associated costs when compared to chemical leaching [
14]. The usage of microbial processes in metal extraction is usually known as bioleaching [
13]. The microorganisms commonly used for bioleaching applications are extremely acidophilic (pH < 3) and mesophilic, which means that those organisms grow optimally in relatively acid environments and at moderate temperature. Bacterial leaching of metal oxides from solid materials is mainly facilitated by biogenic production of inorganic and organic acids and the secretion of complexing agents [
15]. Some microorganisms, especially those which belong to the
Acidithiobacillus genus, can improve the leaching of metal sulfides [
13].
Acidithiobacillus ferrooxidans is an acidophilic bacterium which obtains energy from the oxidation of sulfide minerals (e.g., FeS
2, CuFeS
2), ferrous oxide (FeO), and elemental sulfur [
13,
15].
A. ferrooxidans converts insoluble metal sulfides or oxides into water soluble metal sulfates through biochemical oxidation reactions [
16]. The optimal growth conditions for
A. ferrooxidans are a pH between 2 and 2.5 and a temperature in the range of 28–35 °C [
17].
Acidithiobacillus thiooxidans obtains energy derived from the oxidation of elemental sulfur and reduced inorganic sulfur compounds to support its autotrophic growth.
A. thiooxidans grows in the optimum pH range of 2.0–3.5, being the optimum temperature 28–30 °C [
18]. The mechanisms proposed for biological metal leaching are the following:
In the first mechanism,
A. ferrooxidans and
A. thiooxidans produce sulfuric acid according to Equation (1) [
15]. This sulfuric acid is responsible for the dissolution of the waste matrix surrounding the metal sulfides or oxides and facilitates the production of soluble metal sulfates [
19].
In the second mechanism, shown in Equation (2),
A. ferrooxidans interacts with the insoluble metal sulfides (MeS) causing the release of the metal, which is a free metal ion (Me
2+). The microorganisms adhere to the surface of sulfide mineral and solubilize the metal directly [
20].
In the third mechanism,
A. ferrooxidans oxidizes Fe
2+ to form Fe
3+, as presented in Equation (3). The oxidation of Fe
2+ to form Fe
3+ by iron-oxidizing bacteria leads to the dissolution of acid-soluble and acid non-soluble metal sulfides [
19], since Fe
3+ subsequently causes the chemical leaching of metal-bearing minerals, as seen in Equation (4), which is a purely chemical process [
20].
In this study, elemental sulfur (S0) was added, since it acts as a substrate for the production of sulfuric acid (Equation (1)). During the sulfur dosage, care was taken not to exceed the amount of sulfur needed for the microorganisms to avoid the accumulation of sulfur in the cast house dust, as the solid residue, once zinc is reduced, is going to be reinjected in the steel mill.
2. Materials and Methods
2.1. Materials and Chemicals
The cast house dust composition was analyzed, with iron resulting as the main component at ~64% (w/w) and zinc at ~3% (w/w), respectively. Other elements as aluminum, lead, chromium, or sulfur were also detected, being the sum of the overall concentrations lower than 0.6% (w/w).
All chemicals were of analytical grade, purchased from Sigma-Aldrich (St. Louis, MI, USA) and all solutions were prepared using deionized water.
2.2. Bacterial Strain and Medium
Cultivation of the microbial population was performed in a sulfur medium, according to Wakeman et al. [
21]. The composition of the growth medium contained (mg/L) Na
2SO
4·10H
2O (150), (NH
4)
2SO
4 (450), KCl (50), MgSO
4·7H
2O (500), KH
2PO
4 (50), Ca (NO
3)
2·4H
2O (14), and 5, 7.5, or 15 (g/L) of sulfur powder. Those components (except the sulfur) were dissolved in deionized water and sterilized for 20 min at 121 °C. The pre-cultivation phase was performed at 30 °C and 160 rpm, using a co-culture of
A. thiooxidans (DSM No. 504) and
A. ferrooxidans (DSM No. 583). The bacteria were purchased from DSMZ.
2.3. Stirred Tank Reactor Setup
A double jacketed continuous stirred tank reactor (CSTR) (Schmizo AG, Oftringen, Switzerland), with a working volume of 3 L, was used for the experiments. The temperature, pH, and redox potential were continuously monitored with an AwiComb automated control unit (Awite Bioenergy GmbH, Langenbach, Germany). Two electrodes (Mettler Toledo GmbH, Vienna, Austria) were used to measure the redox potential and the pH. The reactor was heated to 30 °C using a water bath (Proline P 8, LAUDA, Regau, Austria) and the solution was mixed with the help of a motor-stirrer set up to 160 rpm. Aeration of the system was achieved using an air pump (Schego M2K3, SCHEGO Schemel & Goetz GmbH & Co KG, Offenbach, Germany) which was set to 75 L/h, measured by a rotameter. A schematic representation of the stirred tank system can be seen in
Figure 2.
2.4. Methodology
Prior to each bioleaching experiment, the CSTR, containing the growth medium, was inoculated with a co-culture of
A. ferrooxidans and
A. thiooxidans (10% (
v/
v)), and was pre-cultivated for one week at 30 °C, 160 rpm, and 75 L/h air, adding the corresponding sulfur amount to each run. Three experiments with different sulfur concentrations and cast house dust concentrations were carried out (see
Table 1). The goals of this experiments were: (I) the determination of the ideal sulfur concentration to cover the microorganisms’ necessities and, therefore, produce the highest amount of sulfuric acid, and (II) the leaching of the highest achievable zinc share while obtaining the lowest possible remaining sulfur in the already treated solid residue.
Since the exposure of the microorganisms to a residue could possibly affect the microorganisms [
22], batch experiments were conducted with (10, 30, 40 and 50 g/L) in 250 mL shaker flasks prior to the CSTR tests. The results of these experiments showed that no inhibition occurred. Additionally, some literature sources show high Zn tolerance (up to 1071 mmol/L) of
Acidithiobacillus ferrooxidans [
23,
24].
2.5. Bioleaching Experiments in CSTR
After a one-week pre-cultivation period, the evaporation losses were compensated with deionized water and, afterwards, the corresponding amount of cast house dust and elemental sulfur was added (as indicated in
Table 1). To measure the concentration of the metals dissolved in the lixiviant, a liquid sample of the leachate was taken at the beginning (zero), middle (1 week), and end (2 weeks) of each cycle. Prior to elemental analyses, these samples were filtered through a 0.2 µm Chromafil filter (Macherey-Nagel, Düren, Germany). At the end of each cycle (2 weeks), a sample of the undissolved solid in the leachate was taken as well. The solid sample was filtered (through a 0.2 µm filter) and rinsed with deionized water until the pH of the permeate was close to neutral to prevent further oxidation reactions. The pH of the leachate was analyzed with a pH meter (Knick pH Meter 765, Berlin, Germany) and the microorganism population from the leachate was qualitatively quantified using a Carl Zeiss microscope (Axio Lab A1, Jena, Germany).
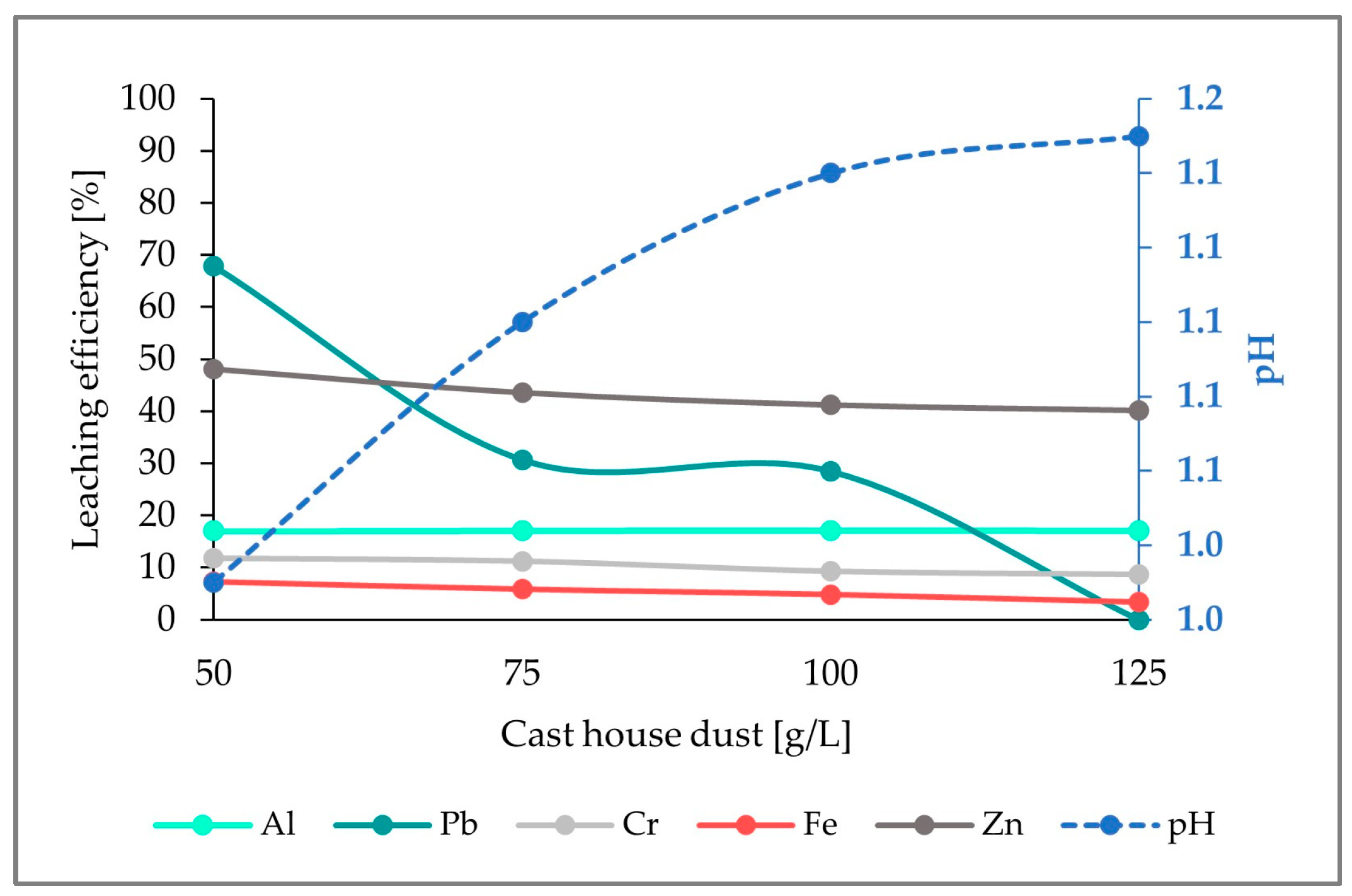
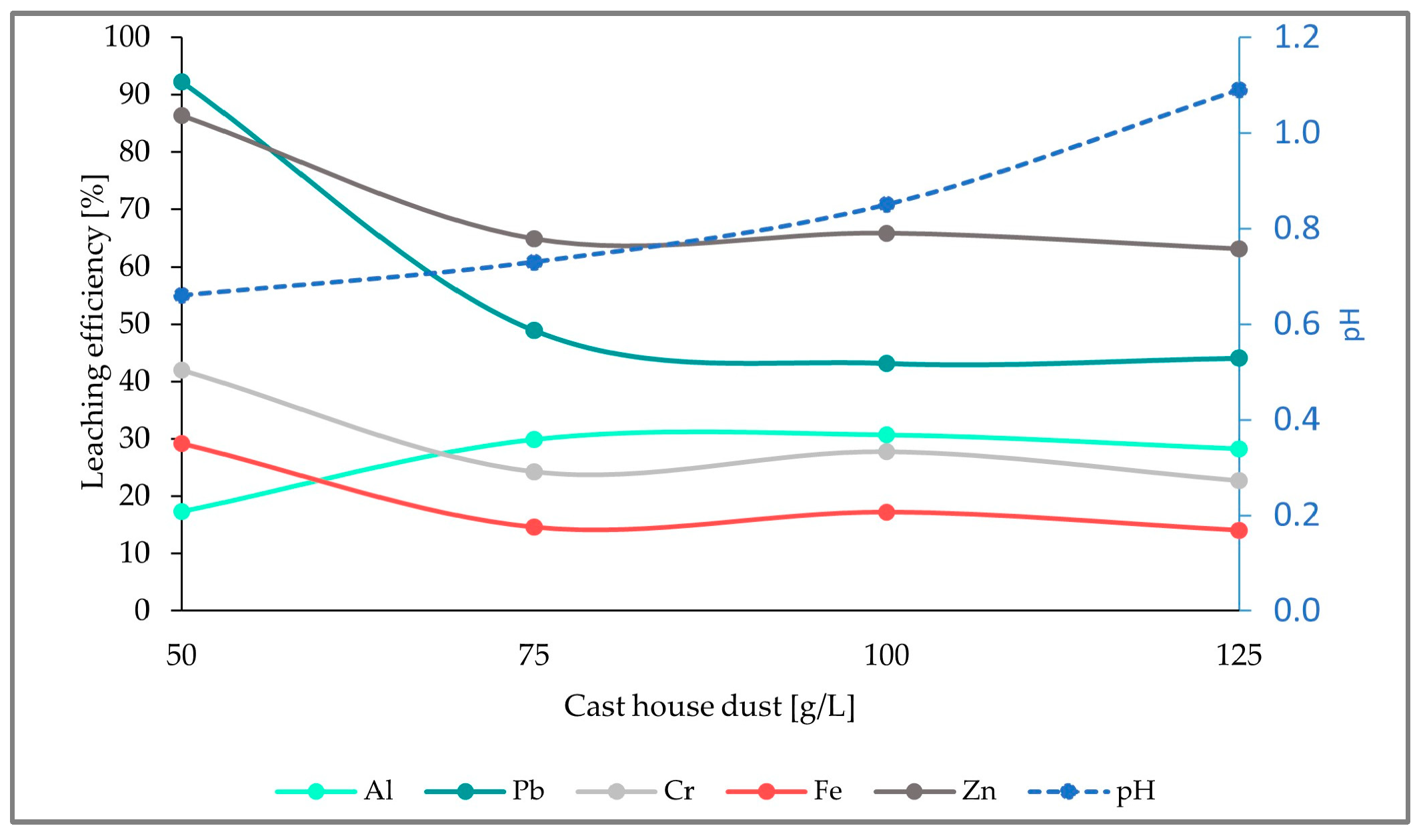
The first run started with 50 g/L cast house dust and 5 g/L sulfur. Increasing amounts of dust were added every 2 weeks as specified in
Table 1, in order to study the behavior of the leaching efficiency with increasing amounts of dust. However, the addition of this material was not arbitrary. To start a new stage, two thirds of the stirred tank content had to be pumped out and replaced with 2 L of sterile growth medium with its corresponding amount of sulfur (5, 7.5, or 15 g/L, for the runs 1, 2, and 3, respectively). The process was performed in this manner to maintain, in the reactor, a part of the microbial population which was already adapted to lower dust concentrations. The required amount of dust was added to an aliquot and the dust concentration was increased in the following steps: 50-, 75-, 100-, and 125 g/L.
2.6. Chemical and Crystallographic Analysis
The chemical composition of the samples was determined as follows: sulfur was analyzed according to DIN EN ISO 15350 (Leco CS-744). To measure chromium, zinc, and lead, the sample was decomposed in a high-pressure autoclave (Ultraclave MLS) with a mixture of nitric and hydrofluoric acid. Afterwards, the decomposed liquid samples were analyzed using ICP-OES (Spectro Arcos) or ICP-MS (Thermo Scientific iCAP RQ, Waltham, MA, USA) for Cr and Zn and Pb, respectively. For iron and aluminium determination, the sample was decomposed in a mixture of hydrochloric and perchloric acid according to OENORM EN ISO 439, and afterwards measured with ICP-OES.
X-ray diffraction analysis (XRD) was implemented for the crystallographic analysis. The XRD analyses were performed using a Panalytical X’Pert PRO MPD diffractometer with Bragg-Brentano geometry and Cobalt Kα1 radiation with a wavelength of 1.789010 ångström at 35 kV and 40 mA. A 1-D RTMS detector (X’Celerator) and a Kβ (Fe)-filter were also used to achieve high sensitivity and noise reduction. The sample preparation was conducted manually using a mortar to achieve a fine pulverization, thereby reducing the effects of any preferred orientation and obtaining a better signal due to smaller particle sizes.
The measurements were performed with a step size of 0.0167° 2 Θ and the angular range extended from 15–130° 2 Θ. The total measurement time per sample was three hours and four minutes, during which the samples were continuously rotated to achieve better statistical accuracy of the measurements. The collected data were processed using Panalytical HighScore Plus 3.0.5 software. Quantitative phase analyses were performed using the Rietveld method.
2.7. Calculation of the Leaching Efficiency and Metal Removal Efficiency
The leaching efficiency (
) from the leachate collected was calculated as follows:
where
is the actual measured metal concentration in [mg/L],
is the total volume of the reactor in [L],
is the final metal concentration of the previous stage measured in [mg/L],
is the rest volume of the previous stage which remains in the reactor in [L],
is the initial metal concentration in the untreated dust in [mg/kg], and
is the dust mass used in the present stage in [kg].
The metal removal efficiency of the solid samples was calculated as follows:
where
is the mass loss or mass increase in [%],
is the actual measured metal concentration in [%],
is the total dust mass (dry) present in the reactor in the present stage in [g],
is the fresh dust mass added in the present stage (dry) in [g],
is the measured metal concentration in the untreated dust in [%], and
is the metal concentration measured in the previous stage in [%]. The remaining sulfur content (S) in the solid samples was additionally calculated as follows (Equation (6)).
where
is the final sulfur amount in [g] present in the reactor in the present stage after 2 weeks of experiments,
is the initial sulfur in [g] in the reactor in the present stage initially, right after the addition of fresh sulfur and dust,
is the measured sulfur concentration in the present stage in [%],
is the total dust mass (dry) present in the reactor in the present stage in [g],
is the fresh dust mass added in the present stage (dry) in [g],
is the measured sulfur concentration in the untreated dust in [%], and
is the sulfur concentration measured in the previous stage in [%].
4. Influence of Sulfur Concentration on Bioleaching of Zinc and Iron
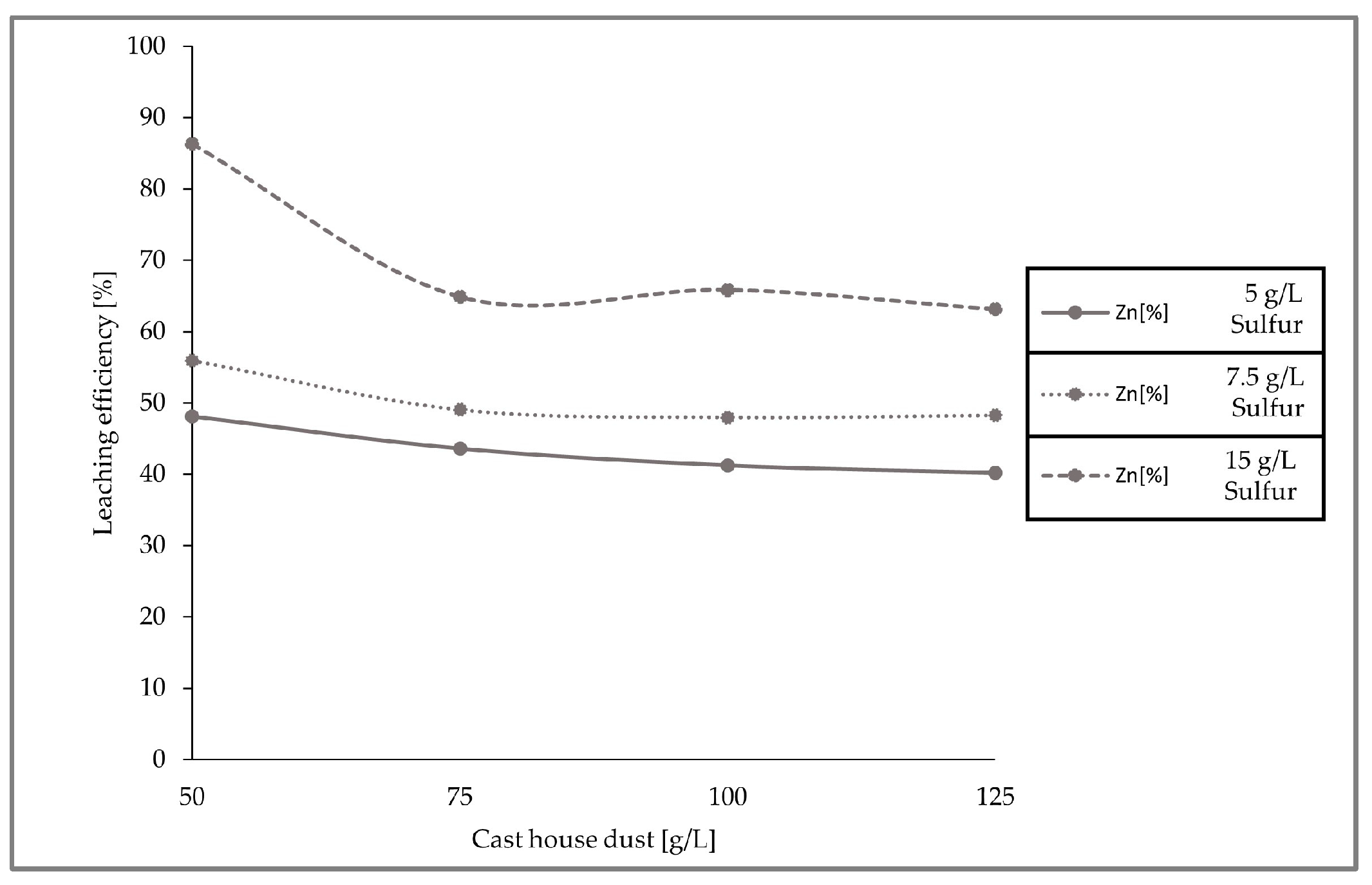
The leaching efficiencies for zinc for the three set of experiments with different sulfur and cast house dust concentrations were calculated and are presented in the figure below (
Figure 10), concluding that higher leaching efficiencies can be achieved with higher sulfur concentrations.
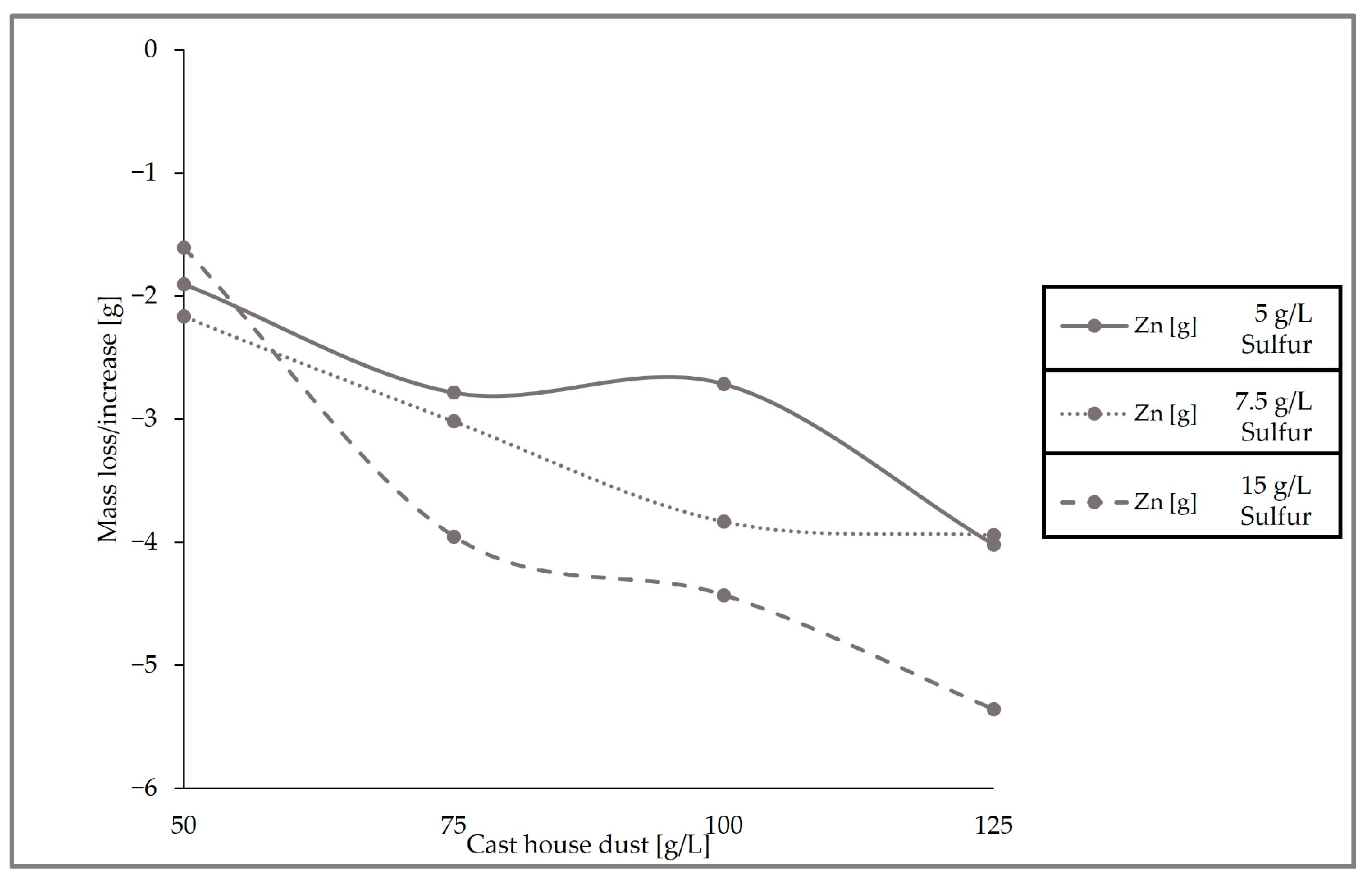
Figure 11, which presents the removal behavior of zinc in the solid sample for the three runs conducted with different sulfur and cast house dust concentrations, exhibits identical results with respect to the leaching efficiencies, showing higher zinc removal with higher sulfur concentrations. For example, for a cast house dust concentration of 125 g/L, the zinc removal achieved for sulfur concentrations of 7.5 and 5 g/L was ≤4 g, while when using 15 g/L of sulfur approximately 5.5 g of zinc was removed from the dust.
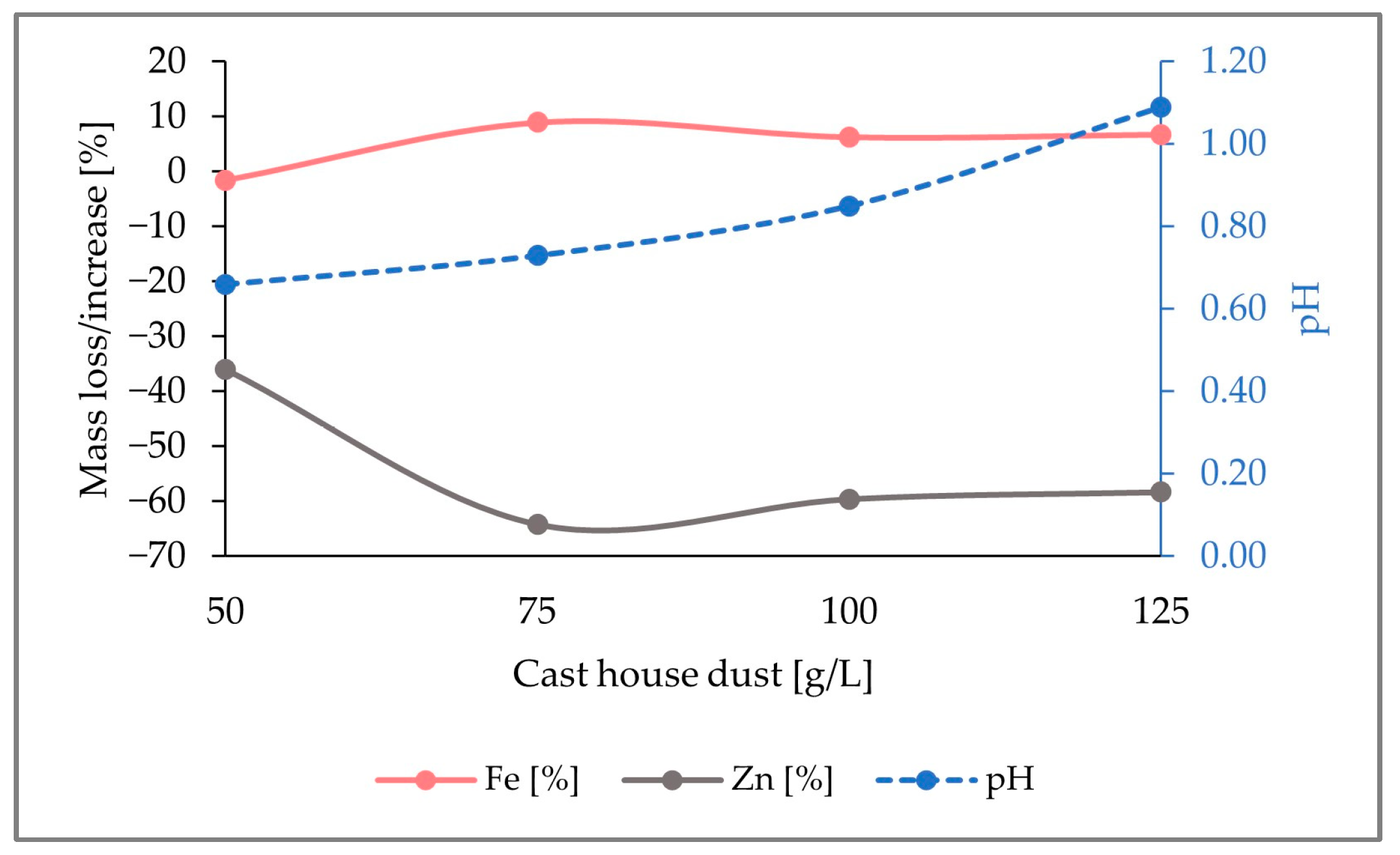
When looking at the loss of zinc and the iron content enrichment in the solid sample (
Figure 12), it can be observed that the highest zinc removal efficiencies were reached with 15 g/L of sulfur. With dust concentrations above 75 g/L, the average removal efficiency of zinc from the cast house dust is 60% (
w/
w) when using 15 g/L of sulfur, 47% (
w/
w) for sulfur concentrations of 7.5 g/L, and around 40% (
w/
w) for 5 g/L of sulfur. On the other hand, the highest iron enrichment with dust concentrations higher than 75 g/L was also linked to higher sulfur concentrations, reaching beyond 7% (
w/
w), on average, iron enrichment for sulfur concentrations of 15 g/L. While results from other studies with
A. ferrooxidans and similar metal concentrations showed iron losses of 30% (
w/
w) [
13], the present study found rather low or no iron leaching at all during the three runs, an advantageous fact since iron loss is prevented, enhancing the further reintroduction of the treated dust in the steel mill.
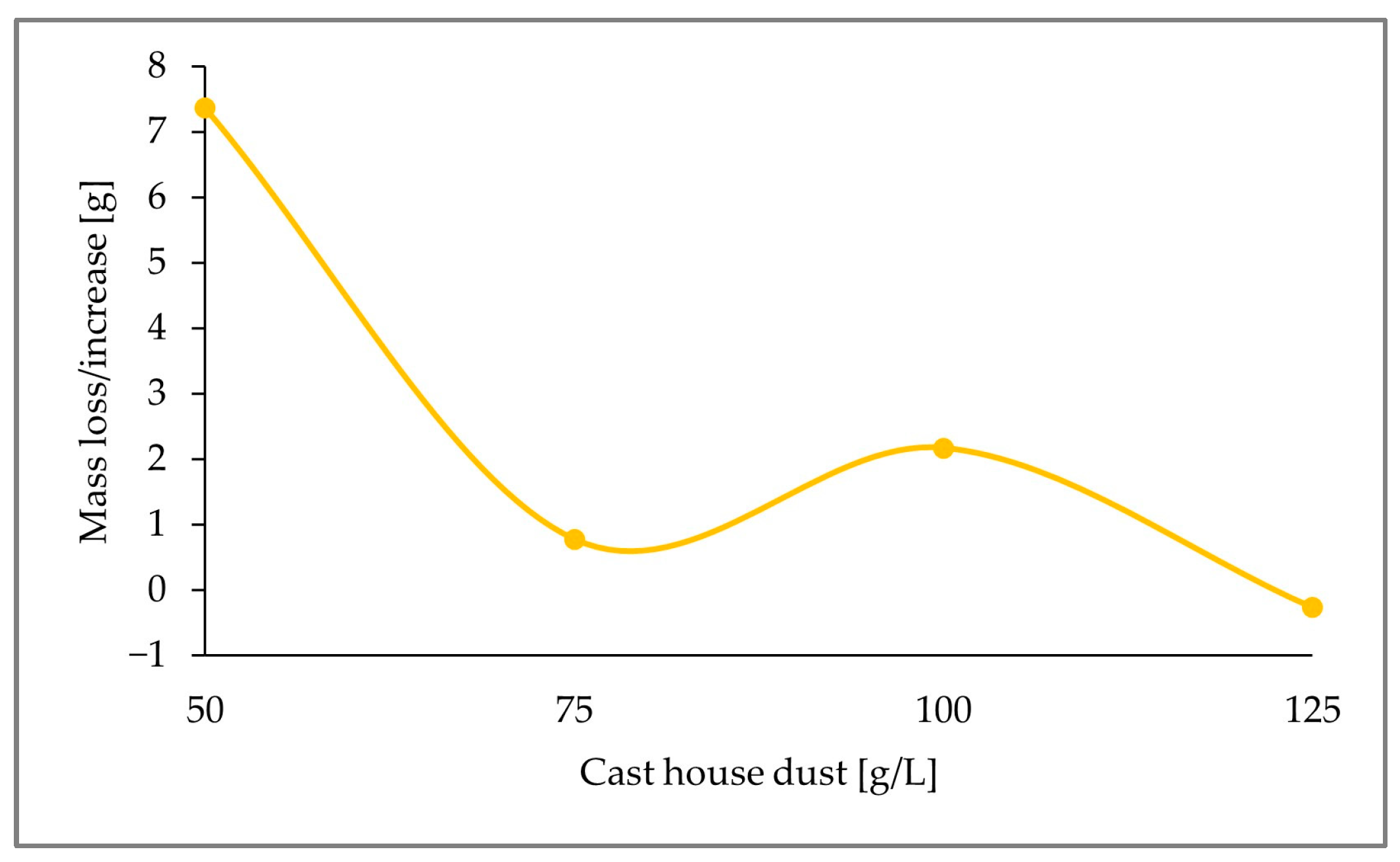
Despite residual amounts of sulfur in the leached dust being undesired for further recirculation in the steel mill, sufficient sulfur (either in the solid sample or as elemental sulfur) must be available for the microorganisms to catalyze the bioleaching of zinc. Additionally, higher dust concentrations are preferred to increase the amount of dust treated per batch. For this reason, the remaining sulfur in the leached dust was calculated as a function of the concentration of the cast house dust to determine the maximum allowable dust concentration that can be added for a determined added sulfur concentration.
Due to the promising results afforded by sulfur concentrations of 15 g/L, and considering that one of the premises for the recirculation of the treated dust is the absence of additional sulfur, the remaining sulfur content in the leached cast house dust was analyzed for run3 (15 g/L S). The change in mass content of elemental sulfur present in the solid residue with increasing cast house dust concentrations was calculated according to Equation (3) for run3, as displayed in
Figure 13. This shows the total consumption of the added sulfur for dust concentrations of 125 g/L.
Additional X-ray diffraction (XRD) analyses were carried out to confirm the absence of sulfur and to check the evolution of the mineralogical composition of the leached dust through the experiments. For this purpose, the untreated cast house dust, as well as the leached dust after the consecution of the experiments with a sulfur content of 15 g/L and a dust concentration of 125 g/L, were analyzed. Therefore, one sample of the dust suspended in the leachate (run3, 125 g/L dust) was collected, filtered, dried, and analyzed. The results of the analyses are shown in
Figure 14. Looking at the final composition and comparing it with the initial substrate’s structure, the absence of sulfur or zinc compounds in the final leached substrate, together with an enrichment of hematite (Fe
3+), can be observed, which might confirm the
A. ferrooxidans oxidation mechanism from Fe
2+ to Fe
3+, since this bacterium is known to be capable of oxidizing both iron and sulfur, as confirmed by previous studies [
20].