Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review
Abstract
1. Introduction
2. Materials and Methods
3. Geological Outline
4. Characteristic Features of Bauxite Deposits
4.1. Mineralogy and Texture
4.2. Geochemistry
4.2.1. Critical Metals in Bauxite Ores
4.2.2. A Comparison between the Parnassos–Ghiona and Other Bauxite Deposits
4.2.3. The Greek Bauxite Metallurgical Residue (Red Mud)
5. Discussion
5.1. Organic Matter as Driving Force of Redox Processes Reactions
5.2. Source and Economic and Genetic Significance of Lithium
5.3. Implications of Critical Metals for the Exploration for Bauxites
5.4. Knowledge Gaps
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bárdossy, G. Karst Bauxites. Bauxite Deposits in Carbonate Rocks. Developments in Economic Geology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 14, 441p, ISBN 9780444597533. [Google Scholar]
- USGS. 2018 Minerals Yearbook—Bauxite and Alumina [Advance Release]; USGS: Reston, VA, USA, 2022; pp. 1–15.
- Aronis, G. Geographical distribution, geological placing and aspects on the genesis of the Greek bauxite. Bull. Geol. Soc. Greece 1955, 2, 55–79. (In Greek) [Google Scholar]
- Valeton, I.; Bierman, M.; Reche, R.; Rosenberg, F.F. Genesis of nickel laterites and bauxites in Greece during the Jurassic and the Cretaceous and their relation to ultrabasic rocks. Ore Geol. Rev. 1987, 2, 359–404. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Bio-mineralization and potential biogeochemical processes in bauxite deposits: Genetic and ore quality significance. Miner. Petrol. 2013, 407, 171–186. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Siavalas, G.; Skarpelis, N.; Araujo, C.V.; Christanis, C. Late Cretaceous coal overlying karstic bauxite deposits in the Parnassus-Ghiona Unit, Central Greece: Coal characteristics and depositional environment. Int. J. Coal Geol. 2010, 81, 211–226. [Google Scholar] [CrossRef]
- Radusinović, S.; Papadopoulos, A. The Potential for REE and Associated Critical Metals in Karstic Bauxites and Bauxite Residue of Montenegro. Minerals 2021, 11, 975. [Google Scholar] [CrossRef]
- Combes, P.-J. Regards sur la géologie des bauxites; aspects récents sur la genése de quelques gisements à substratum carbonate–A look at the geology of bauxite; recent data on the genesis of some deposits in carbonate rock. Bull. Cent. Rech. Explor.-Prod. Elf-Aquitaine 1984, 8, 251–274. [Google Scholar]
- Šinkovec, B.; Sakac, K.; Durn, G. Pyritized bauxites from Minjera, Istria, Croatia. Nat. Croat. 1994, 3, 41–65. [Google Scholar]
- Öztürk, H.; Hein, J.R.; Hanilçi, N. Genesis of the Doğankuzu and Mortaş bauxite deposits, Taurides, Turkey: Separation of Al, Fe, and Mn and implication for passive margin metallogeny. Econ. Geol. 2002, 97, 1063–1077. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Kasama, T.; Church, N.S.; Douvalis, A.P.; Göttlicher, J.; Steininger, R.; Boubnov, A.; Pontikes, Y.; Tzamos, E.; et al. Nano-mineralogy and geochemistry of high-grade diasporic karst-type bauxite from Parnassos-Ghiona mines, Greece. Ore Geol. Rev. 2017, 84, 228–244. [Google Scholar] [CrossRef]
- Abedini, A.; Mehr, M.H.; Khosravi, M.; Calagari, A.A. Geochemical characteristics of the karst-type bauxites: An example from the Kanirash deposit, NW Iran. Arab. J. Geosci. 2019, 12, 475. [Google Scholar] [CrossRef]
- Mondillo, N.; Di Nuzzo, M.; Kalaitzidis, S.; Boni, M.; Santoro, L.; Balassone, G. Petrographic and geochemical features of the B3 bauxite horizon (Cenomanian-Turonian) in the Parnassos-Ghiona area: A contribution towards the genesis of the Greek karst bauxites. Ore Geol. Rev. 2022, 143, 104759. [Google Scholar] [CrossRef]
- Laskou, M.; Andreou, G. Rare earth element distribution and REE minerals from the Parnassos-Ghiona bauxite deposits, Greece. In Mineral Exploration and Sustainable Development, Proceedings of the 7th Biennial SGA Meeting, Athens, Greece, 24–28 August 2003; Eliopoulos, D., Baker, T., Barriga, F., Beaudoin, G., Benardos, A., Boni, M., Borg, G., Bouchot, V., Brown, A., Christidis, G., et al., Eds.; Millpress: Rotterdam, The Netherlands, 2003; pp. 89–92. [Google Scholar]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The rare earth elements potential of Greek bauxite active mines in the light of a sustainable REE demand. J. Sustain. Metall. 2019, 5, 20–47. [Google Scholar] [CrossRef]
- Teichert, Z.; Bose, M.; Williams, L.B. Lithium isotope compositions of U.S. coals and source rocks: Potential tracer of hydrocarbons. Chem. Geol. 2020, 549, 119694. [Google Scholar] [CrossRef]
- Ling, K.Y.; Wen, H.Z.; Zhang, Q.Z.; Luo, C.G.; Gu, H.N.; Du, S.J.; Yu, W.X. Super-enrichment of lithium and niobium in the upper Permian Heshan Formation in Pingguo, Guangxi, China. Sci. China Earth Sci. 2021, 64, 753–772. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, Q.; Liu, X.F.; Zhou, G.F.; Xu, H.; Zhu, Y.G. Provenance and ore-forming process of Permian lithium-rich bauxite in central Yunnan, SW China. Ore Geol. Rev. 2022, 145, 104862. [Google Scholar] [CrossRef]
- S&P Global Market Intelligence. Lithium M&A Involving Assets With Resources (2012–2019). 2021. Available online: https://www.spglobal.com/marketintelligence (accessed on 1 April 2023).
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Laskou, M. Concentrations of rare earths in Greek bauxites. Acta Geol. Hung. 1991, 3, 395–404. [Google Scholar]
- Laskou, M. Geochemical and mineralogical characteristics of the bauxite deposits of western Greece. In Mineral Exploration and Sustainable Development, Proceedings of the 7th Biennial SGA Meeting, Athens, Greece, 24–28 August 2003; Eliopoulos, D., Baker, T., Barriga, F., Beaudoin, G., Benardos, A., Boni, M., Borg, G., Bouchot, V., Brown, A., Christidis, G., et al., Eds.; Millpress: Rotterdam, The Netherlands, 2003; pp. 93–96. [Google Scholar]
- Pajović, M. Genesis and genetic types of karst bauxites. Iran. J. Earth Sci. 2009, 1, 44–56. [Google Scholar]
- Pajović, M.; Radusinović, S. Stratigraphy of bauxites in Montenegro; Geological Survey of Montenegro, Podgorica. Geol. Bull. 2015, 16, 27–57. [Google Scholar]
- Radusinović, S. Metallogeny of Jurassic Karstic Bauxites of Vojnik-Maganik and Prekornica Mining Areas, Montenegro. Ph.D. Thesis, University of Belgrade, Faculty of Mining and Geology, Belgrade, Serbia, 2017; pp. 1–349. [Google Scholar]
- Economou-Eliopoulos, M.; Laskou, M.; Eliopoulos, D.G.; Megremi, I.; Kalatha, S.; Eliopoulos, G.D. Origin of Critical Metals in Fe-Ni Laterites from the Balkan Peninsula: Opportunities and Environmental Risk. Minerals 2021, 11, 1009. [Google Scholar] [CrossRef]
- Pajović, M.; Radusinovićc, S. Mineral resources of Montenegro. Montenegro in the XXI century—In the Era of Competitiveness. The living environment and sustainable development. Montenegrin Acad. Sci. Arts (Spec. Ed.) 2010, 73, 237–282, (In Serbian, with English Abstract). [Google Scholar]
- Gamaletsos, P. Mineralogy and Geochemistry of Bauxites from Parnassos-Ghiona Mines and the Impact on the Origin of the Deposits. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2014; p. 347. [Google Scholar]
- Williams, R.J. Karst-Associated Bauxite Deposits of Parnassos-Ghiona, Central Greece: Ore Genesis and Structural Evolution. Ph.D. Thesis, University of Brighton, Brighton, UK, 2014. [Google Scholar]
- Laskou, M.; Economou, M. Palladium, Pt, Rh and Au Contents in Some Bauxite Occurrences of Greece. In Proceedings of the Balkan-Carpathian Congress, Sofia, Bulgaria, 11–13 December 1989; pp. 1367–1371. [Google Scholar]
- Laskou, M. Pyrite-rich bauxites from the Parnassos-Ghiona zone, Greece. In Mineral Deposit Research: Meeting the Global Challenge; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1007–1010. [Google Scholar]
- Lu, F.H.; Xiao, T.F.; Lin, J.; Li, A.J.; Long, Q.; Huang, F.; Xiao, L.H.; Li, X.; Wang, J.W.; Xiao, Q.X.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. The Economic Geology of Scandium, the Runt of the Rare Earth Element Litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef]
- Dragovic, D. The red and white karstic bauxites of Montenegro (Yugoslavia). Travaux 1989, 19, 249–257. [Google Scholar]
- Tang, B.; Fu, Y.; Yan, S.; Chen, P.-W.; Cao, C.; Guo, C.; Wu, P.; Long, Z.; Long, K.-S.; Wang, T.-S.; et al. The source, host minerals, and enrichment mechanism of lithium in the Xinmin bauxite deposit, northern Guizhou, China: Constraints from lithium isotopes. Ore Geol. Rev. 2022, 141, 104653. [Google Scholar] [CrossRef]
- Putzolu, F.; Papa, A.P.; Mondillo, N.; Boni, M.; Balassone, G.; Mormone, A. Geochemical Characterization of Bauxite Deposits from the Abruzzi Mining District (Italy). Minerals 2018, 8, 298. [Google Scholar] [CrossRef]
- Mongelli, G.; Buccione, R.; Gueguen, E.; Langone, A.; Sinisi, R. Geochemistry of the apulian allochthonous karst bauxite, Southern Italy: Distribution of critical elements and constraints on Late Cretaceous Peri-Tethyan palaeogeography. Ore Geol. Rev. 2016, 77, 246–259. [Google Scholar] [CrossRef]
- Hampl, F.J.; Melcher, F. The formation of the Unterlaussa karst bauxite (Austria)—A re-evaluation of the established model. J. Geochem. Explor. 2023, 245, 107141. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, Y.; Zhu, S.; Zhang, S.; Cao, J. Study on the properties of bauxite modified by acid leaching and calcination for improving fluorine removal. Asia-Pac. J. Chem. Eng. 2023, 18, e2839. [Google Scholar] [CrossRef]
- Economou-eliopoulos, M.; Kontou, M.; Mrgremi, I. Biogeochemical Redox Processes Controlling the Element Cycling: Insights from Karst-Type Bauxite, Greece. Minerals 2022, 12, 446. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Direct determination of lanthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Wagh, A.S.; Pinnock, W.R. Occurrence of scandium and rare earth elements in Jamaican bauxite waste. Econ. Geol. 1987, 82, 757–761. [Google Scholar] [CrossRef]
- Boni, M.; Rollinson, G.; Mondillo, N.; Balassone, G.; Santoro, L. Quantitative mineralogical characterization of karst bauxite deposits in the Southern Apennines, Italy. Econ. Geol. 2013, 108, 813–833. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Roosen, J.; Van Roosendael, S.; Borra, C.R.; Van Gerven, T.; Mullens, S.; Binnemans, K. Recovery of scandium from leachates of Greek bauxite residue by adsorption on functionalized chitosan-silica hybrid materials. Green Chem. 2016, 18, 2005–2013. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. 100th Anniversary special paper: On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005, 100, 419–438. [Google Scholar]
- Baskar, S.; Baskar, R.; Kaushik, A. Role of microorganisms in weathering of the Konkan-Goa laterite formation. Curr. Sci. 2003, 85, 1129–1134. [Google Scholar]
- Southam, G.; Saunders, J. The geomicrobiology of ore deposits. Econ. Geol. 2005, 100, 1067–1084. [Google Scholar] [CrossRef]
- Ehrlich, H.; Witkowski, A. Biomineralization in Diatoms: The Organic Templates. In Evolution of Lightweight Structures. Biologically-Inspired Systems; Hamm, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 6. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press, Inc.: Boca Raton, FL, USA, 2000; p. 550. [Google Scholar]
- Finkelman, R.B. Trace elements in coal. Environmental and health significance. Biol. Trace Element. Res. 1999, 67, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Christanis, K.; Georgakopoulos, A.; Fernandez-Turiel, J.L.; Bouzinos, A. Geological factors influencing the concentration of trace elements in the Philippi peatland, eastern Macedonia, Greece. Int. J. Coal Geol. 1998, 36, 295–313. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment; Springer: Berlin/Heidelberg, Germany, 1998; p. 398. [Google Scholar]
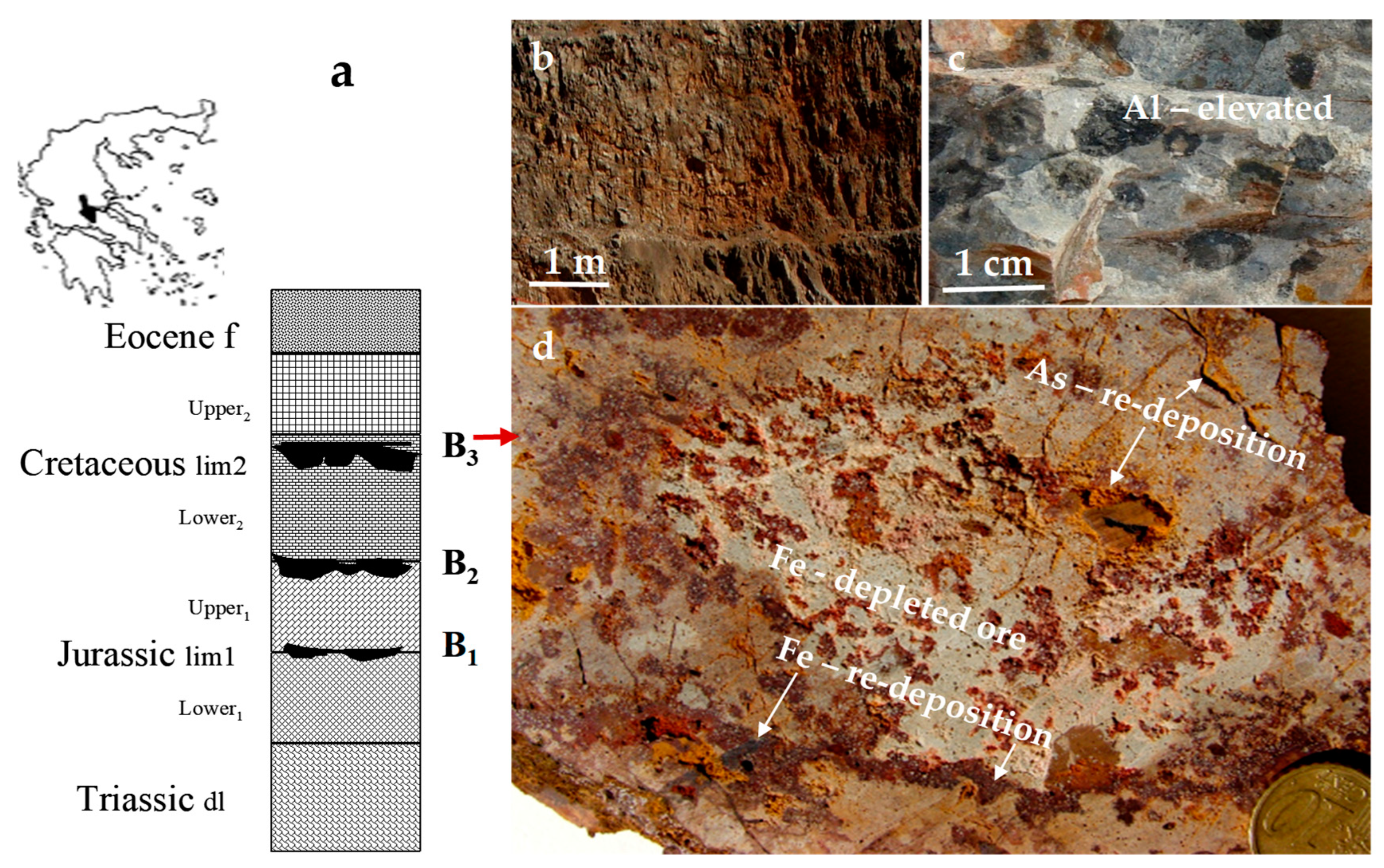
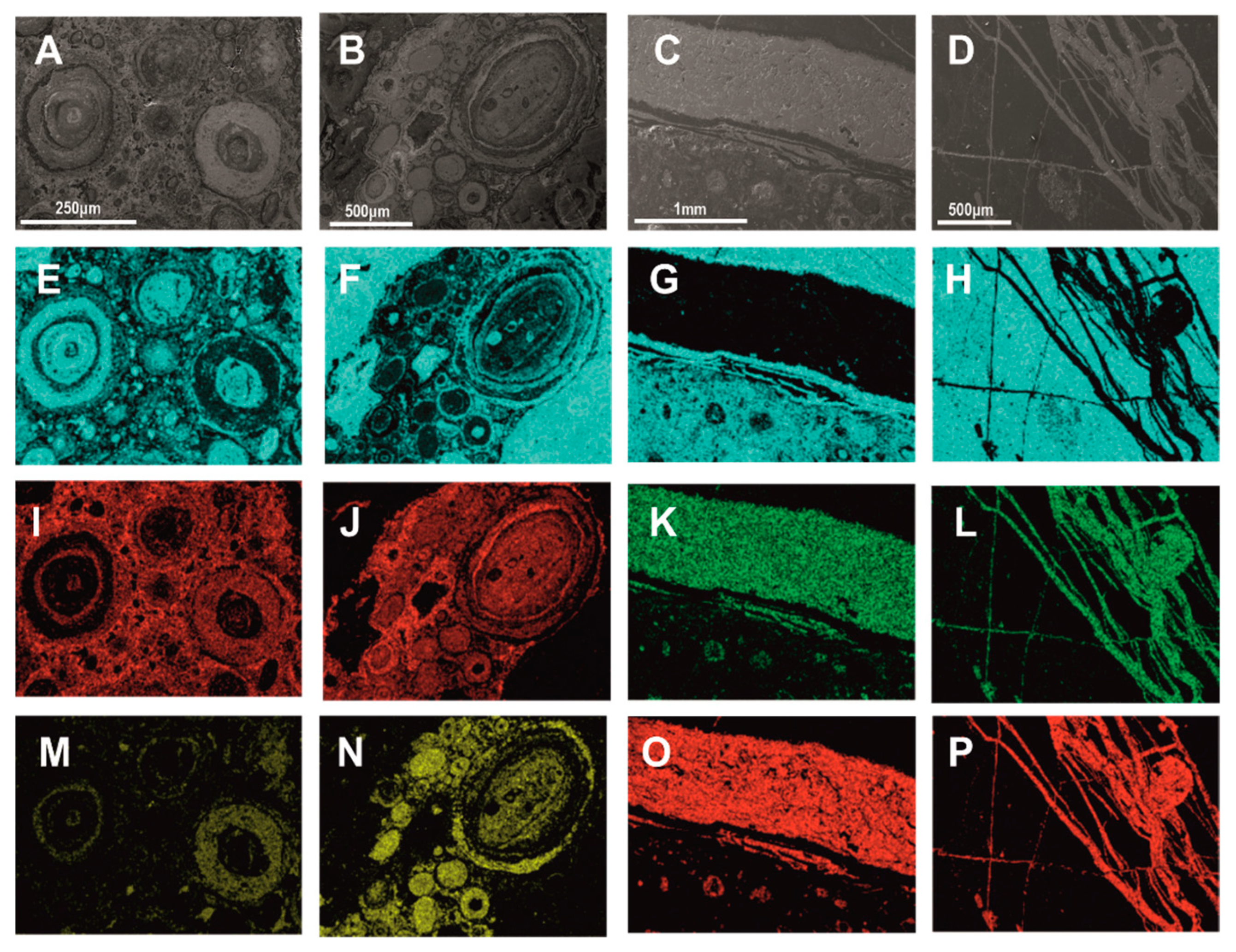

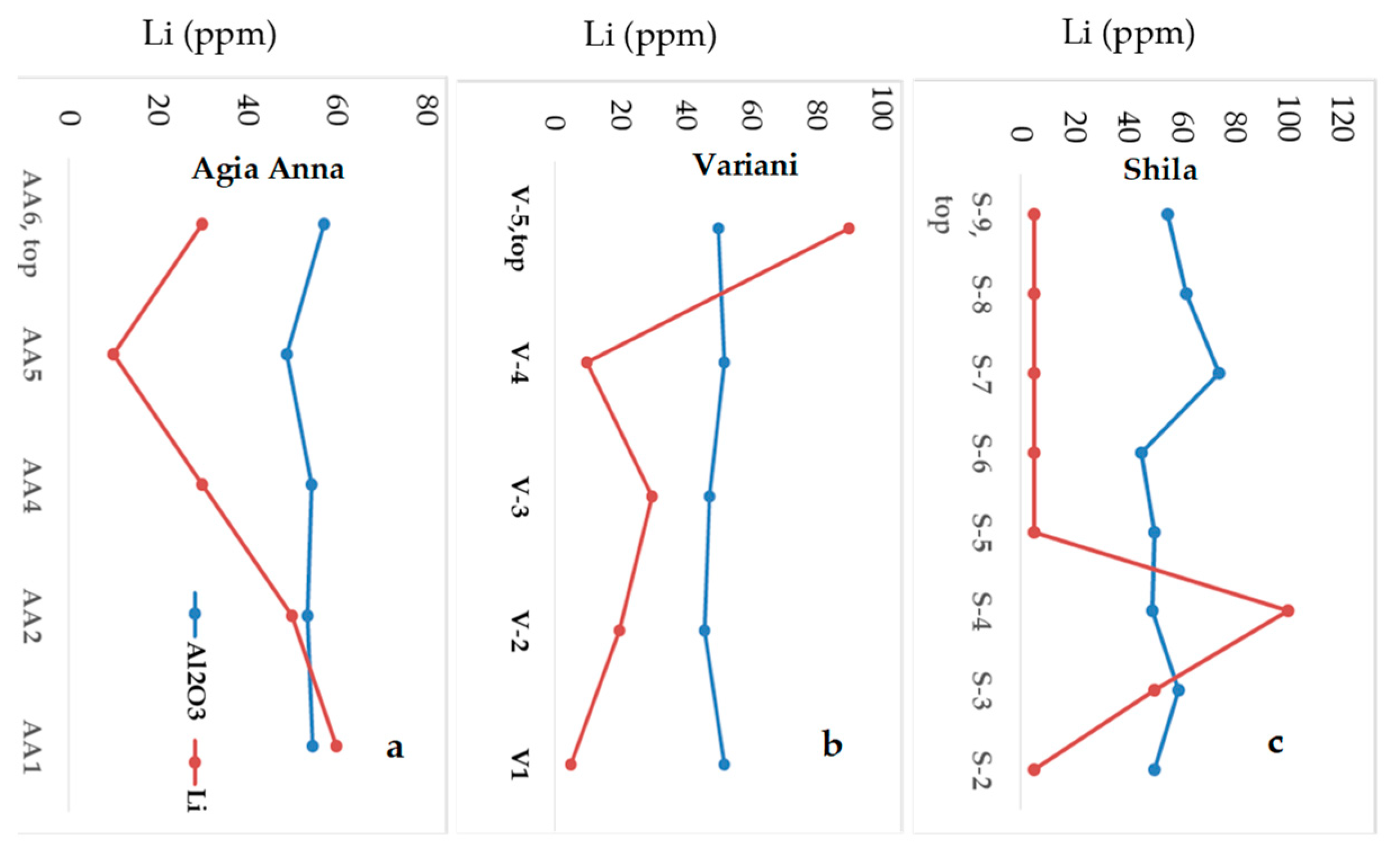
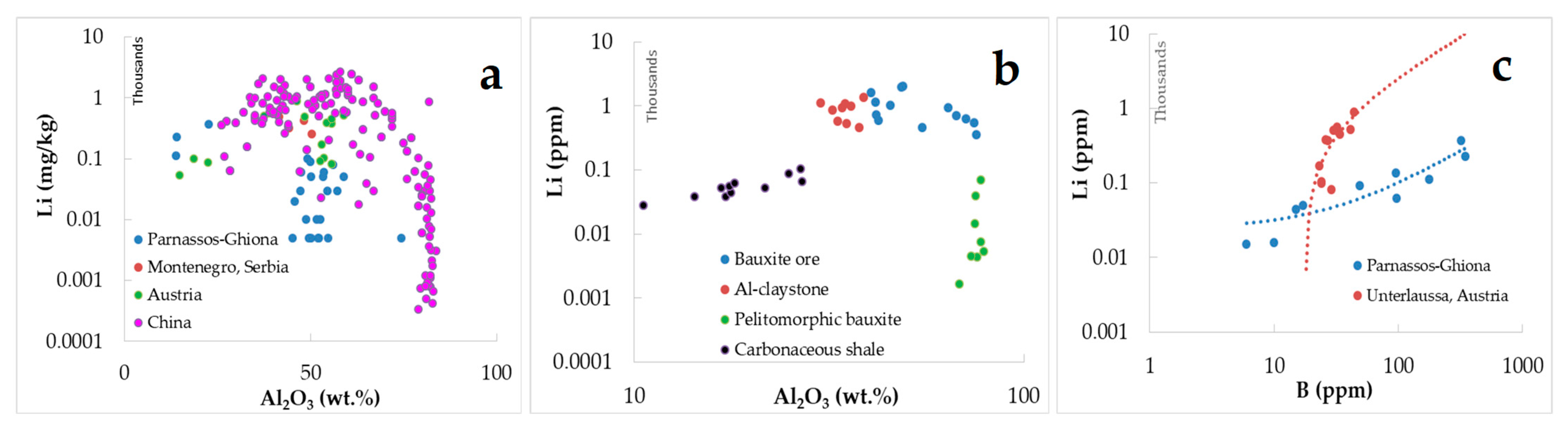
| Bauxite Ores | Carbonaceous Shale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | B2 | B2 | B3 | B3 | B3 | B3 | B3 | B3 | B3 | B3 | B3 |
| Location | Proussorema | Frousia | Pera Lakkos | Agia Anna | Variani | Pera Lakkos | |||||
| wt.% | PR-12 | PR-5 | Ff17 | F20 | PL-grey | PL-red-brown | PS1-1 | PS-1-4 | PSS2-5 | 1-3cs | 2-11cs |
| SiO2 | 9.6 | 15.0 | 32.1 | 2.24 | 0.08 | 0.75 | 4.4 | 1.7 | 5.1 | 19.64 | 24.65 |
| Al2O3 | 39.11 | 60.1 | 41.4 | 60.64 | 79.2 | 61.7 | 53.54 | 54.5 | 49.96 | 14.1 | 22.7 |
| Fe2O3T | 21.2 | 4.1 | 10.58 | 21.2 | 1.7 | 21.95 | 23.0 | 25.9 | 24.52 | 3.7 | 0.9 |
| MnO | 0.43 | 0.27 | n.d. | 0.01 | n.d. | 0.01 | n.d. | n.d. | 0.01 | n.a. | n.a. |
| MgO | 1.25 | 0.43 | 0.28 | 0.03 | 0.11 | n.d. | 0.9 | n.d. | 0.87 | 0.66 | 0.56 |
| K2O | 2.39 | 1.25 | 0.14 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.02 | 1.96 | 3.01 |
| TiO2 | 0.13 | 2.37 | 1.8 | 2.35 | 3.35 | 2.63 | 4.3 | 3.1 | 3.5 | 0.63 | 1.1 |
| CaO | 0.06 | 0.13 | 0.22 | 0.92 | n.d. | n.d. | n.d. | n.d. | 0.19 | 14.3 | 6.36 |
| Na2O | 0.11 | 0.06 | 0.19 | 0.06 | n.d. | n.d. | 0.01 | 0.01 | 0.02 | 0.24 | 0.18 |
| P2O5 | 15.7 | 0.11 | 0.05 | 0.05 | 0.01 | 0.02 | 0.03 | 0.02 | 0.07 | n.a. | n.a. |
| LOI | 20.5 | 15.7 | 13.5 | 12.9 | 14.91 | 12.18 | 13.85 | 15.05 | 13.6 | n.a. | n.a. |
| Total | 99.1 | 99.5 | 100.26 | 100.4 | 99.36 | 99.24 | 100.0 | 100.28 | 97.86 | ||
| ppm | |||||||||||
| Li | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 60 | 30 | 90 | 226 | 367 |
| Ga | n.a. | n.a. | n.a. | n.a. | 84 | 66 | 75 | 66 | 65 | 22 | 46 |
| Sc | n.a. | n.a. | n.a. | n.a. | 30 | 51 | 28 | 28 | 25 | n.a. | n.a. |
| V | n.a. | n.a. | n.a. | n.a. | 520 | 620 | 330 | 380 | 480 | 350 | 240 |
| La | 390 | 220 | 42 | 58 | 6.1 | 83 | 69 | 41 | 9.6 | 16 | 69 |
| Ce | 98 | 830 | 67 | 36 | 106 | 99 | 260 | 26 | 217 | 38 | 228 |
| Pr | 870 | 6.3 | 203 | 125 | 1.8 | 12 | 14 | 11 | 2.3 | 4.2 | 17 |
| Nd | 27 | 70 | 19 | 7.2 | 6.3 | 35 | 47 | 44 | 8.5 | 15 | 69 |
| Sm | 230 | 250 | 68 | 28 | 2.3 | 7.8 | 8 | 9.2 | 2.1 | 2.7 | 14.5 |
| Eu | 75 | 49 | 15 | 7.7 | 0.4 | 1.9 | 1.7 | 2 | 0.54 | 0.5 | 3.3 |
| Gd | 17 | 7.8 | 2.9 | 1.8 | 1.03 | 9.1 | 7.7 | 8.3 | 3.32 | 2.8 | 16.6 |
| Tb | 1700 | 1430 | 420 | 260 | 0.7 | 2.8 | 1.5 | 1.6 | 0.8 | 0.4 | 2.2 |
| Dy | 83 | 39 | 12 | 8.6 | 4.7 | 18 | 12 | 10 | 5.5 | 2.2 | 13 |
| Ho | 12 | 5.3 | 1.7 | 1.5 | 1.05 | 3.9 | 2.6 | 2.3 | 1.24 | 0.5 | 2.6 |
| Er | 64 | 31 | 10 | 9.4 | 3.5 | 12.5 | 8.8 | 7.3 | 4 | 1.5 | 7.1 |
| Tm | 12 | 6.8 | 1.8 | 2.1 | 0.6 | 2 | 1.5 | 1.2 | 0.6 | 0.2 | 0.9 |
| Yb | 30 | 18 | 5.9 | 6.3 | 3.05 | 13 | 10.4 | 8.6 | 4.1 | 1.5 | 5.6 |
| Lu | 3.9 | 2.5 | 0.9 | 1 | 0.7 | 2.3 | 1.6 | 1.4 | 0.6 | 0.2 | 0.8 |
| Y | 21 | 15 | 7 | 8 | 31 | 71 | 70 | 57 | 34 | 11 | 72 |
| Σ REE | 3630 | 2980 | 880 | 560 | 170 | 370 | 576 | 230 | 384 | 323 | 890 |
| Age | Range and Mean | Thickness of Overlying Organic-Rich Sediments | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | (Ma) | Al2O3 (wt.%) | ΣREE (ppm) | Li (ppm) | Ga (ppm) | Sc (ppm) | V (ppm) | |||
| Greece | ||||||||||
| Parnassos-Ghiona | Cretaceous | n = 22 | 45–74 (52.5) | 123–444 (252) | 5–90 (35) | 58–108 (67) | 12–38 (26) | 330–720 (460) | Bauxite ore | [14] |
| Parnassos-Ghiona | Cretaceous | n = 3 | 14–22.7 (17) | 83–345 (247) | 111–526 (335) | 22–46 (33) | n.a. | 244–427 (343) | Carbonaceous shale | [7] |
| Parnassos-Ghiona | Cretaceous | n = 6 | 1.2–4.4 (2.2) | 44–290 (87) | 16–137 (67) | 4.7–10 (7.8) | n.a. | 21–121 (77) | Up to 50 cm coal seams | [7] |
| Serbia | ||||||||||
| Montenegro | Cretaceous to Triassic | n = 16 | 41–62 (47.2) | 310–1180 (770) | 257–485 (370) | 42–71 (49) | 25–59 (44) | 93–730 (346) | Bauxite ore | [8,27] |
| S. Italy | Cretaceous | |||||||||
| Otranto | n = 20 | 54–62 (58) | 3150480 (340) | n.a. | 52–68 (58) | 41–54 (44) | 190–249 (210) | Bauxite ore | [39] | |
| Austria | n = 13 | 37.5–59 (51) | 380–1550 (600) | 99–900 (354) | 35–60 (50) | 31–44 (35) | 464–920 (690) | Bauxite ore | [40] | |
| Unterlaussa | Upper Cretaceous | n = 4 | 15–5-26 (20.5) | 115–235 (220) | 53–354 (150) | 16–36 (25) | 12–24 (18) | 155–33 (220) | More than 1400 m mostly | [40] |
| Unterlaussa | organic-rich sediments | |||||||||
| China | ||||||||||
| Yunnan | Upper Permian | n = 9 | 27–60 (48) | 73–280 (156) | 108–2060 (955) | 30–115 (80) | 21–50 (32) | 110–206 (154) | Bauxite ore and Claystone | [20] |
| Guangxi | Upper Permian | n = 14 | 55–80 (74) | 23–174 (64) | 3–25.4 (10.4) | n.a. | n.a. | n.a. | Bauxite ore | [19] |
| Guangxi | Upper Permian | n = 11 | 37–54 (40) | 26–116 (78) | 1030–3388 (2590) | n.a. | n.a. | n.a. | Carbonaceous clays | |
| ~10 m carbonaceous rocks and coal seams | [19] | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Economou-Eliopoulos, M.; Kanellopoulos, C. Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review. Minerals 2023, 13, 962. https://doi.org/10.3390/min13070962
Economou-Eliopoulos M, Kanellopoulos C. Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review. Minerals. 2023; 13(7):962. https://doi.org/10.3390/min13070962
Chicago/Turabian StyleEconomou-Eliopoulos, Maria, and Christos Kanellopoulos. 2023. "Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review" Minerals 13, no. 7: 962. https://doi.org/10.3390/min13070962
APA StyleEconomou-Eliopoulos, M., & Kanellopoulos, C. (2023). Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review. Minerals, 13(7), 962. https://doi.org/10.3390/min13070962








