Estimation of Carbon Content in High-Ash Coal Using Mid-Infrared Fourier-Transform Infrared Spectroscopy
Abstract
1. Introduction
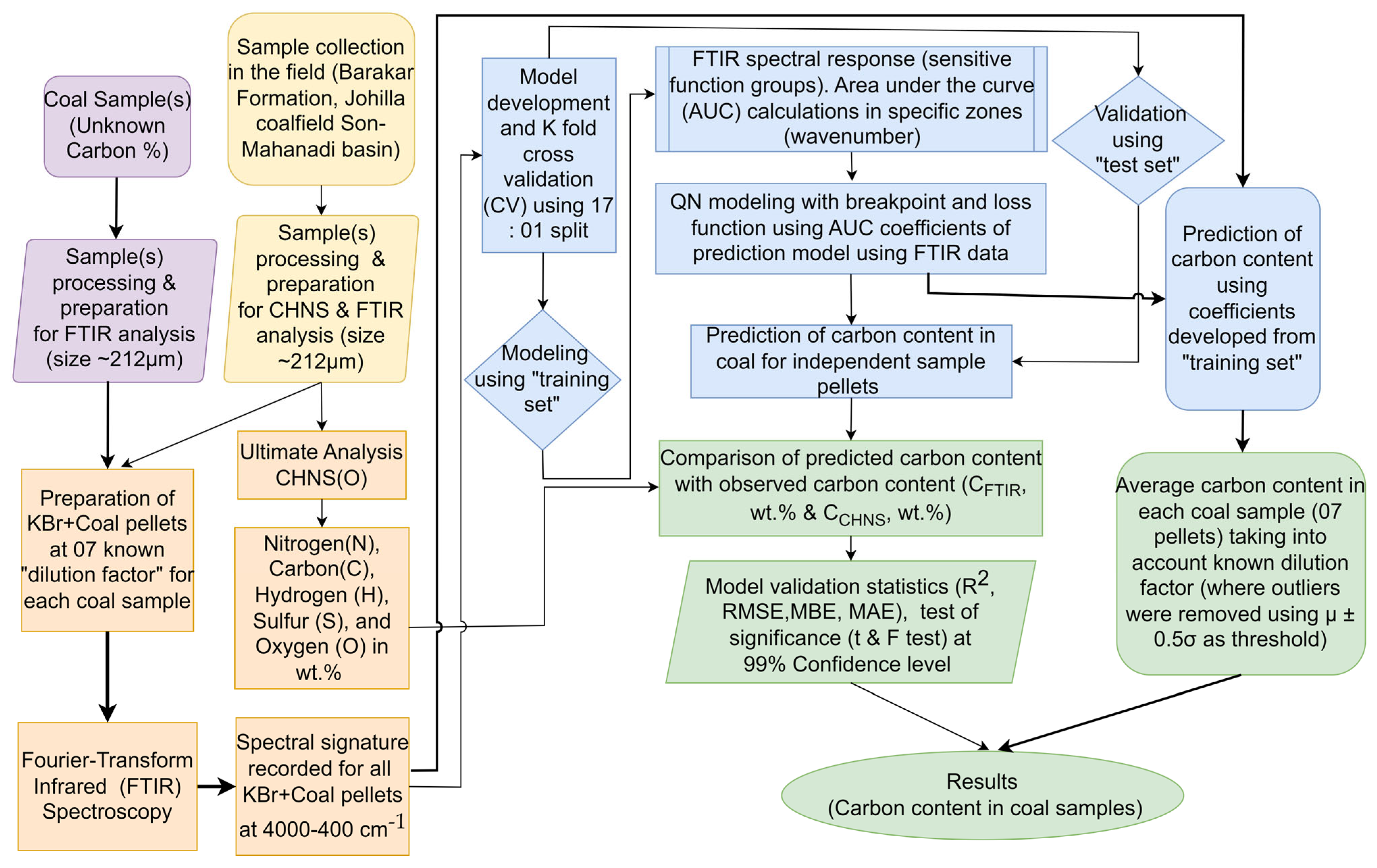
2. Materials and Methods
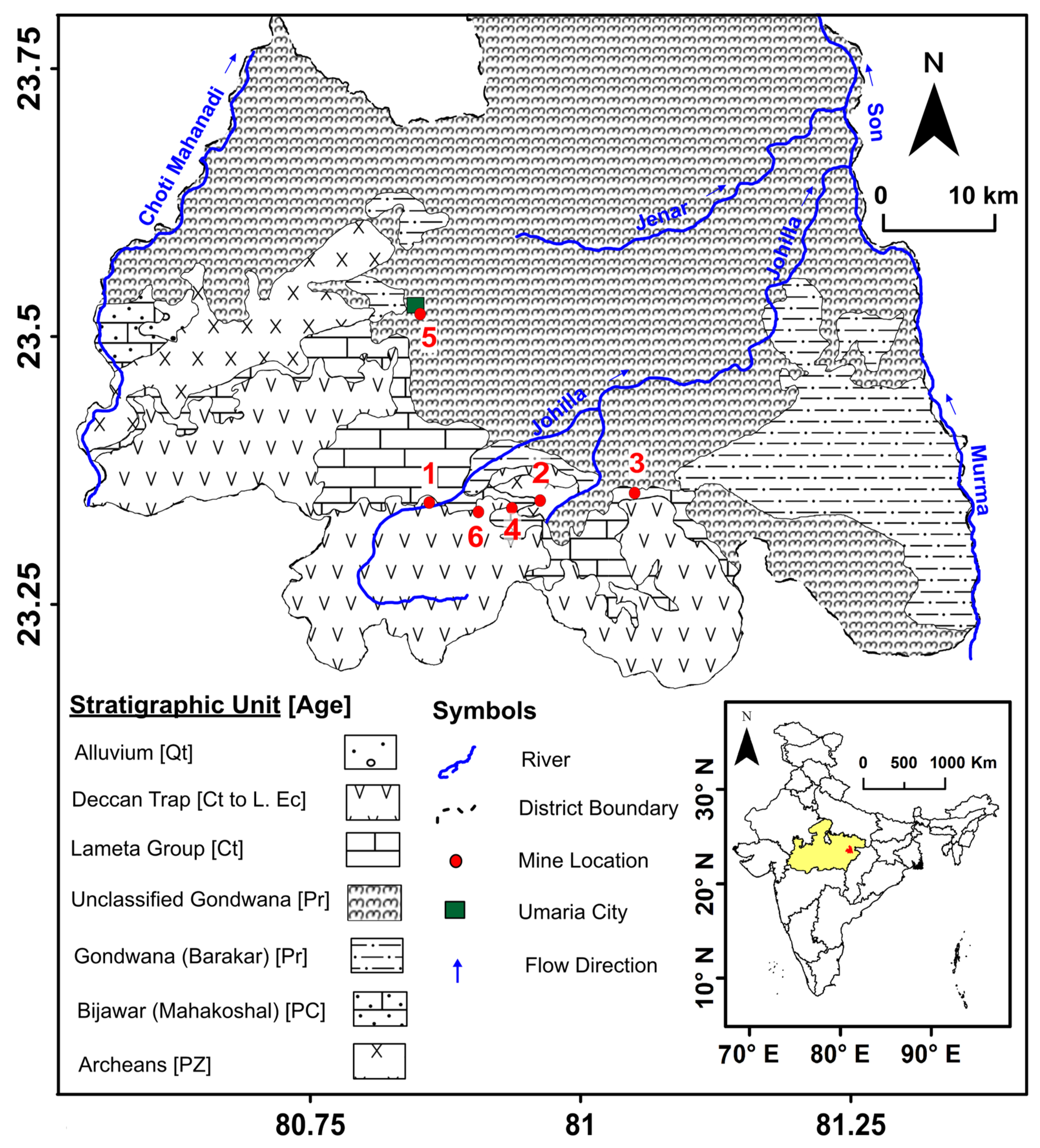
2.1. Geology of the Study Area
2.2. Ultimate Analysis
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. Quasi-Newton (QN) Method
3. Results and Discussion
3.1. Elemental Composition of Coal
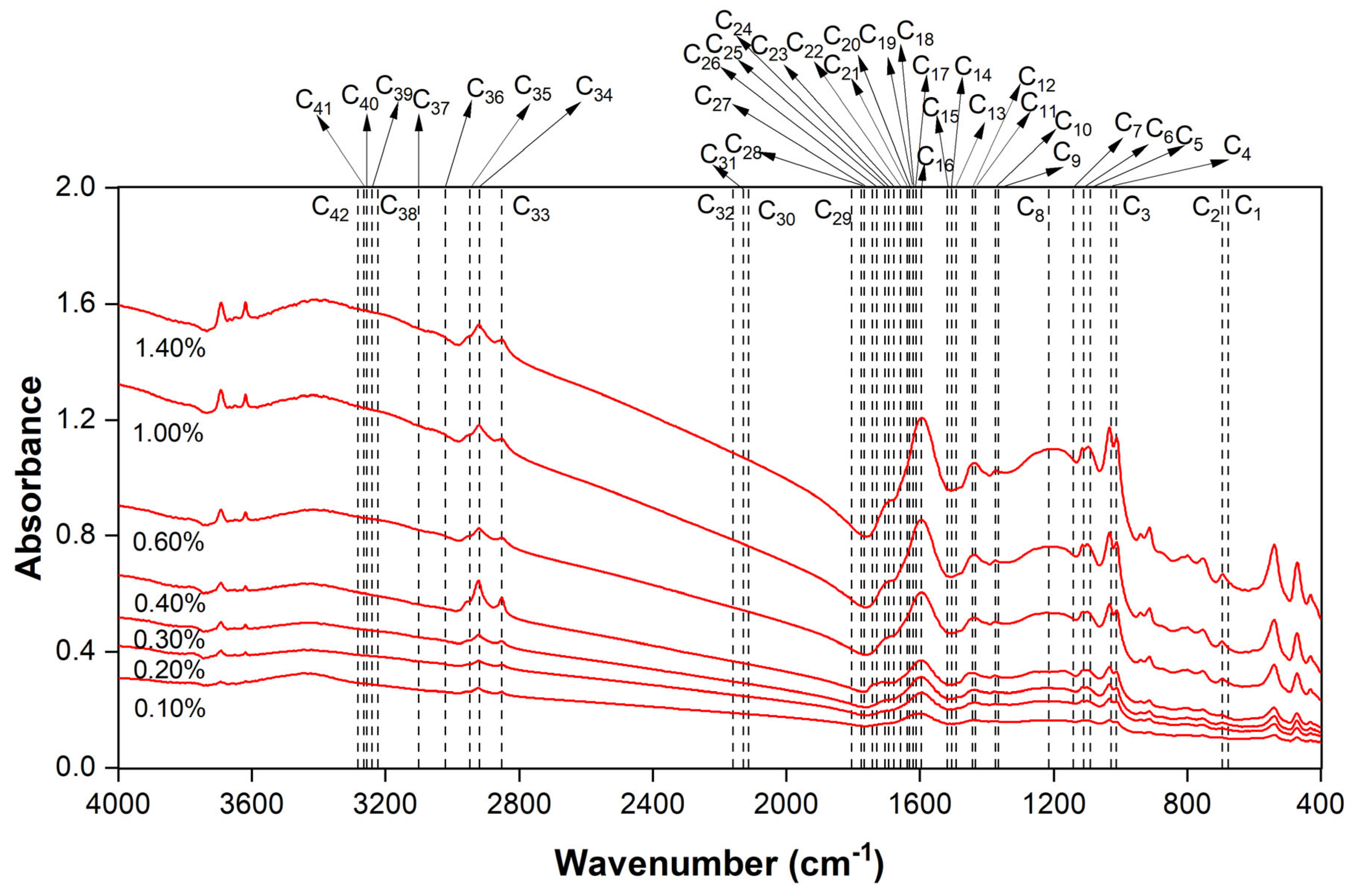
3.2. Identification of Functional Group
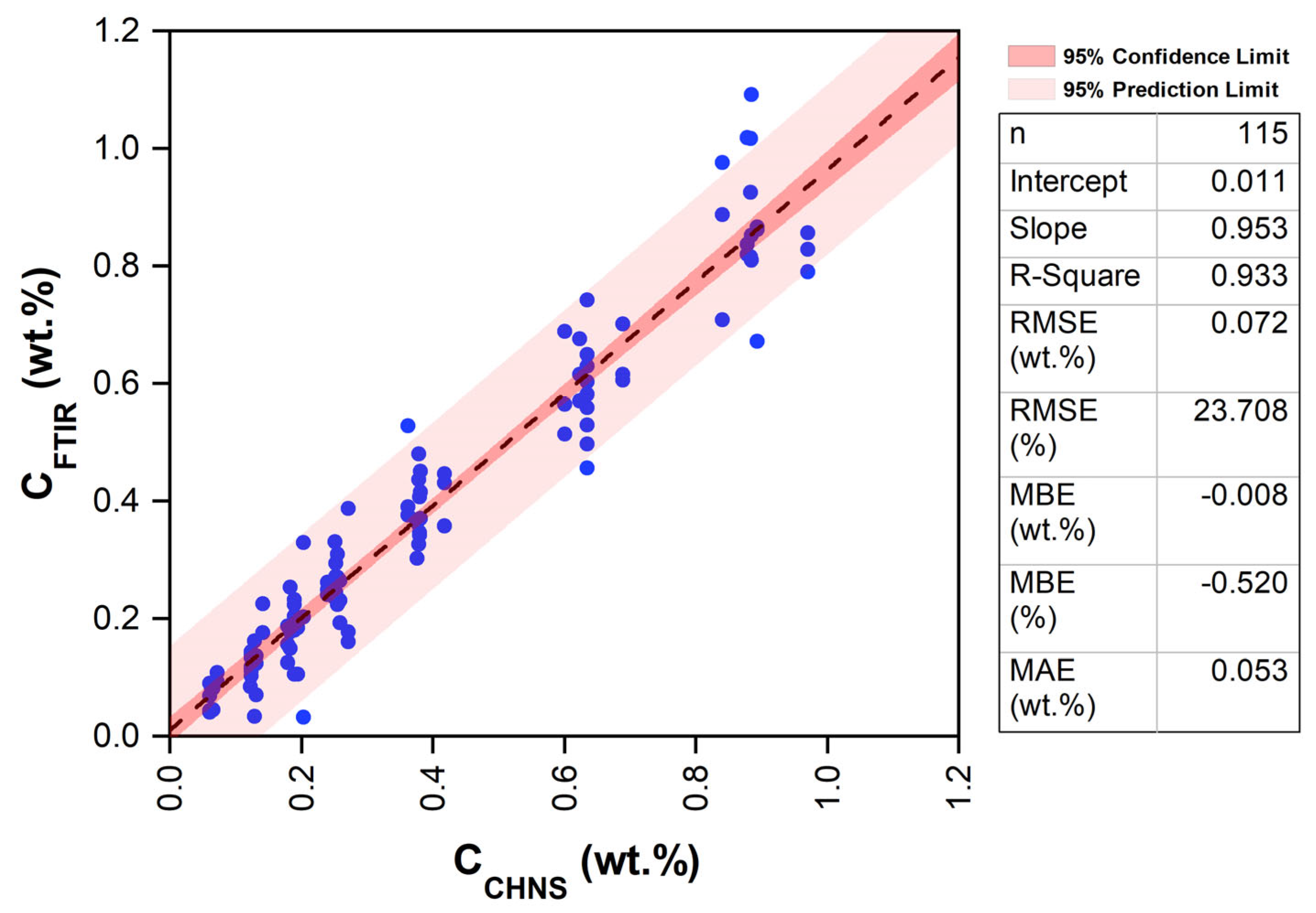
3.3. Model Estimation
3.4. Comparison with Previously Developed Models
4. Conclusions
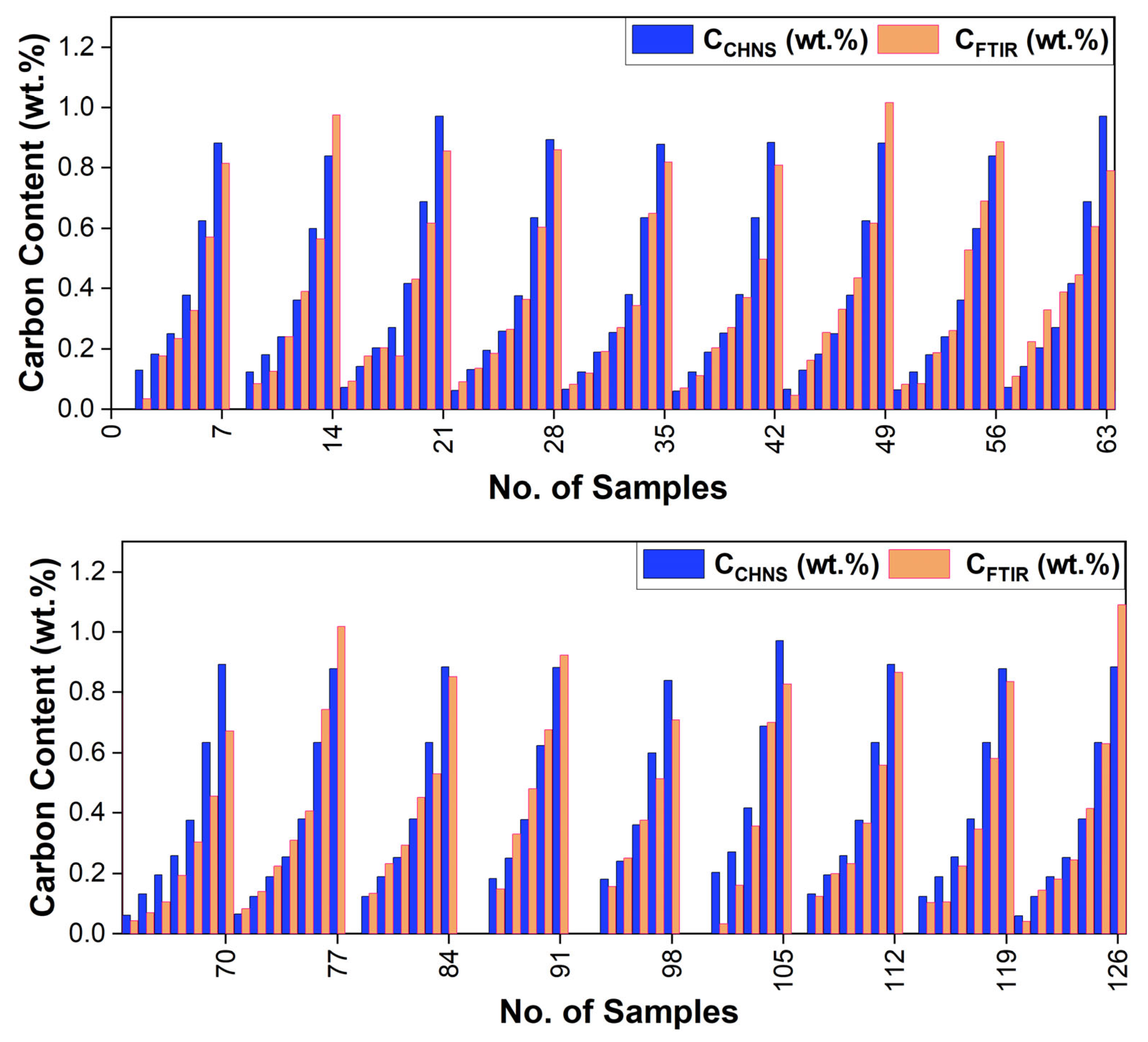
- A strong linear relationship was found between the observed (CCHNS, wt.%) and model-estimated (CFTIR, wt.%) carbon content, with a high coefficient of determination (R2 of 0.93), low RMSE (23.71%), and low MBE (−0.52%).
- The mean carbon contents estimated from CCHNS and CFTIR were 0.383 and 0.391, respectively. Similarly, the variance was 0.075 for the observed carbon using the CHNSO analyzer (CCHNS) and 0.077 for the FTIR data-based model (CFTIR).
- The t-test and F-test of significance, conducted for mean and variance, respectively, clearly show that there is no significant difference between the CCHNS (wt.%, observed) and CFTIR (wt.%, model-estimated) values.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saptoro, A.; Vuthaluru, H.B.; Tadé, M.O. A Comparative Study of Prediction of Elemental Composition of Coal Using Empirical Modelling. IFAC Proc. Vol. 2006, 39, 747–752. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Coal-Water Slurries Containing Petrochemicals to Solve Problems of Air Pollution by Coal Thermal Power Stations and Boiler Plants: An Introductory Review. Sci. Total Environ. 2018, 613–614, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Mcfarland, J.; Herzog, H.; Jacoby, H. The Future of Coal Consumption in a Carbon Constrained World. In Greenhouse Gas Control Technologies 7; Elsevier: Amsterdam, The Netherlands, 2005; Volume II, pp. 1563–1568. ISBN 978-0-08-044704-9. [Google Scholar]
- Yan, C.; Qi, J.; Ma, J.; Tang, H.; Zhang, T.; Li, H. Determination of Carbon and Sulfur Content in Coal by Laser Induced Breakdown Spectroscopy Combined with Kernel-Based Extreme Learning Machine. Chemom. Intell. Lab. Syst. 2017, 167, 226–231. [Google Scholar] [CrossRef]
- Li, X.; Yin, H.; Wang, Z.; Fu, Y.; Li, Z.; Ni, W. Quantitative Carbon Analysis in Coal by Combining Data Processing and Spatial Confinement in Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2015, 111, 102–107. [Google Scholar] [CrossRef]
- Fowler, N.O.; McCall, D.; Chou, T.C.; Holmes, J.C.; Hanenson, I.B. Electrocardiographic Changes and Cardiac Arrhythmias in Patients Receiving Psychotropic Drugs. Am. J. Cardiol. 1976, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Noli, F.; Tsamos, P. Concentration of Heavy Metals and Trace Elements in Soils, Waters and Vegetables and Assessment of Health Risk in the Vicinity of a Lignite-Fired Power Plant. Sci. Total Environ. 2016, 563–564, 377–385. [Google Scholar] [CrossRef]
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Cappelaere, P. Prolactin and breast cancers. Pathol. Biol. 1975, 23, 161–170. [Google Scholar]
- Badman, D.G.; Jaffé, E.R. Blood and Air Pollution; State of Knowledge and Research Needs. Otolaryngol. Neck Surg. 1996, 114, 205–208. [Google Scholar] [CrossRef]
- Yi, L.; Feng, J.; Qin, Y.-H.; Li, W.-Y. Prediction of Elemental Composition of Coal Using Proximate Analysis. Fuel 2017, 193, 315–321. [Google Scholar] [CrossRef]
- Liu, F. A Comparison between Multivariate Linear Model and Maximum Likelihood Estimation for the Prediction of Elemental Composition of Coal Using Proximate Analysis. Results Eng. 2022, 13, 100338. [Google Scholar] [CrossRef]
- Čechák, T.; Thinová, L. Sulfur Content Measurement in Coal by X-Ray Fluorescence Method. Radiat. Phys. Chem. 2001, 61, 759–761. [Google Scholar] [CrossRef]
- Suarez-Fernandez, G.P.; Vega, J.M.G.; Fuertes, A.B.; Garcia, A.B.; Martinez-Tarazona, M.R. Analysis of Major, Minor and Trace Elements in Coal by Radioisotope X-Ray Fluorescence Spectrometry. Fuel 2001, 80, 255–261. [Google Scholar] [CrossRef]
- Pereira, J.S.F.; Mello, P.A.; Moraes, D.P.; Duarte, F.A.; Dressler, V.L.; Knapp, G.; Flores, É.M.M. Chlorine and Sulfur Determination in Extra-Heavy Crude Oil by Inductively Coupled Plasma Optical Emission Spectrometry after Microwave-Induced Combustion. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 554–558. [Google Scholar] [CrossRef]
- Chaves, E.S.; de Loos-Vollebregt, M.T.C.; Curtius, A.J.; Vanhaecke, F. Determination of Trace Elements in Biodiesel and Vegetable Oil by Inductively Coupled Plasma Optical Emission Spectrometry Following Alcohol Dilution. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 733–739. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Heilmann, J.; Prohaska, T.; Heumann, K.G. Development of an Accurate, Sensitive, and Robust Isotope Dilution Laser Ablation ICP-MS Method for Simultaneous Multi-Element Analysis (Chlorine, Sulfur, and Heavy Metals) in Coal Samples. Anal. Bioanal. Chem. 2007, 389, 697–706. [Google Scholar] [CrossRef]
- Weritz, F.; Ryahi, S.; Schaurich, D.; Taffe, A.; Wilsch, G. Quantitative Determination of Sulfur Content in Concrete with Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 1121–1131. [Google Scholar] [CrossRef]
- Gaft, M.; Nagli, L.; Fasaki, I.; Kompitsas, M.; Wilsch, G. Laser-Induced Breakdown Spectroscopy for on-Line Sulfur Analyses of Minerals in Ambient Conditions. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1098–1104. [Google Scholar] [CrossRef]
- Yang, X.; Ingham, D.; Ma, L.; Srinivasan, N.; Pourkashanian, M. Ash Deposition Propensity of Coals/Blends Combustion in Boilers: A Modeling Analysis Based on Multi-Slagging Routes. Proc. Combust. Inst. 2017, 36, 3341–3350. [Google Scholar] [CrossRef]
- Qin, H.; Lu, Z.; Yao, S.; Li, Z.; Lu, J. Combining Laser-Induced Breakdown Spectroscopy and Fourier-Transform Infrared Spectroscopy for the Analysis of Coal Properties. J. Anal. At. Spectrom. 2019, 34, 347–355. [Google Scholar] [CrossRef]
- Yao, S.; Qin, H.; Wang, Q.; Lu, Z.; Yao, X.; Yu, Z.; Chen, X.; Zhang, L.; Lu, J. Optimizing Analysis of Coal Property Using Laser-Induced Breakdown and near-Infrared Reflectance Spectroscopies. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 239, 118492. [Google Scholar] [CrossRef]
- Gazeli, O.; Stefas, D.; Couris, S. Sulfur Detection in Soil by Laser Induced Breakdown Spectroscopy Assisted by Multivariate Analysis. Materials 2021, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.R.; Serio, M.A.; Carangelo, R.M.; Bassilakis, R.; Gravel, D.; Baillargeon, M.; Baudais, F.; Vail, G. Analysis of the Argonne Premium Coal Samples by Thermogravimetric Fourier Transform Infrared Spectroscopy. Energy Fuels 1990, 4, 319–333. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Blesa, M.J.; Juan, R.; Vandenberghe, R.E. Characterisation of an Egyptian Coal by Mossbauer and FT-IR Spectroscopy. Fuel 2003, 82, 1825–1829. [Google Scholar] [CrossRef]
- Kaihara, M.; Takahashi, T.; Akazawa, T.; Sato, T.; Takahashi, S. Application of Near Infrared Spectroscopy to Rapid Analysis of Coals. Spectrosc. Lett. 2002, 35, 369–376. [Google Scholar] [CrossRef]
- Orrego-Ruiz, J.A.; Cabanzo, R.; Mejía-Ospino, E. Study of Colombian Coals Using Photoacoustic Fourier Transform Infrared Spectroscopy. Int. J. Coal Geol. 2011, 85, 307–310. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Tewari, R.; Agnihotri, D.; Kar, R.; Pillai, S.S.K.; Bajpai, S.; Tripathi, S.C. Field Guide Book: Gondwana Formations of South Rewa and Upper Narmada Basins, Central India; Birbal Sahni Institute of Palaeobotany: Lucknow, India, 2015; p. 40. [Google Scholar]
- Mukherjee, D.; Ray, S.; Chandra, S.; Pal, S.; Bandyopadhyay, S. Upper Gondwana Succession of the Rewa Basin, India: Understanding the Interrelationship of Lithologic and Stratigraphic Variables. J. Geol. Soc. India 2012, 79, 563–575. [Google Scholar] [CrossRef]
- Dutta, P.K.; Acharyya, S.K.; Jha, N.; Khangar, R.; Khasdeo, L.; Misra, K.G.; Ramanamurty, B.V. Resolving Kamthi-Related Problems in Gondwana Stratigraphy of Peninsular India. Indian J. Geosci. 2015, 69, 85–102. [Google Scholar]
- ASTM D2234/D2234M−17 2017; American Society for Testing and Materials (ASTM) Standard Practice for Collection of a Gross Sample of Coal. ASTM: Philadelphia, PA, USA, 2017.
- ASTM D4749−12 2012; American Society for Testing and Materials (ASTM) Standard Test Method for Performing the Sieve Analysis of Coal and Designating Coal Size. ASTM: Philadelphia, PA, USA, 2012.
- ASTM D5373−16 2016; American Society for Testing and Materials (ASTM) Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM: Philadelphia, PA, USA, 2016.
- ASTM D4239−18 2018; American Society for Testing and Materials (ASTM) Standard Test Method for Sulfur in the Analysis Sample of Coal and Coke Using HighTemperature Tube Furnace Combustion. ASTM: Philadelphia, PA, USA, 2018.
- Organic Elemental Analyzer Vario MACRO Cube—Elementar. Available online: https://www.elementar.com/en-in/products/organic-elemental-analyzers/vario-macro-cube (accessed on 15 February 2023).
- Othman, N. IR Spectroscopy in Qualitative and Quantitative Analysis. In Infrared Spectroscopy-Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Prasad, A.K.; Singh, R.P.; Tare, V.; Kafatos, M. Use of Vegetation Index and Meteorological Parameters for the Prediction of Crop Yield in India. Int. J. Remote Sens. 2007, 28, 5207–5235. [Google Scholar] [CrossRef]
- Singh, R.P.; Prasad, A.K.; Tare, V.; Kafatos, M. Crop Yield Prediction Using Piecewise Linear Regression with a Break Point and Weather and Agricultural Parameters. U.S. Patent No. 7,702,597, 20 April 2010. [Google Scholar]
- Speight, J.G. Handbook of Coal Analysis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-1-119-03832-0. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, 5th ed.; Cengage Learning: Boston, MA, USA, 2014; ISBN 978-1-305-17782-6. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-01113-3. [Google Scholar]
- Preety, K.; Prasad, A.K.; Varma, A.K.; El-Askary, H. Accuracy Assessment, Comparative Performance, and Enhancement of Public Domain Digital Elevation Models (ASTER 30 m, SRTM 30 m, CARTOSAT 30 m, SRTM 90 m, MERIT 90 m, and TanDEM-X 90 m) Using DGPS. Remote Sens. 2022, 14, 1334. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, M.; Cheng, L.; Wei, L.; Cai, J.; Lu, J. Improved Measurement in Quantitative Analysis of Coal Properties Using Laser Induced Breakdown Spectroscopy. J. Anal. At. Spectrom. 2020, 35, 810–818. [Google Scholar] [CrossRef]
- Dong, M.; Wei, L.; Lu, J.; Li, W.; Lu, S.; Li, S.; Liu, C.; Yoo, J.H. A Comparative Model Combining Carbon Atomic and Molecular Emissions Based on Partial Least Squares and Support Vector Regression Correction for Carbon Analysis in Coal Using LIBS. J. Anal. At. Spectrom. 2019, 34, 480–488. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.; West, L.; Li, Z.; Ni, W. A PLS Model Based on Dominant Factor for Coal Analysis Using Laser-Induced Breakdown Spectroscopy. Anal. Bioanal. Chem. 2011, 400, 3261–3271. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Z.; Li, L.; Li, Z.; Ni, W. A Nonlinearized Multivariate Dominant Factor–Based Partial Least Squares (PLS) Model for Coal Analysis by Using Laser-Induced Breakdown Spectroscopy. Appl. Spectrosc. 2013, 67, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, A.; Wang, X.; Ding, C.; Qiu, S.; He, Y.; Lu, T.; He, F.; Zou, B.; Liu, R. The High-Accuracy Prediction of Carbon Content in Semi-Coke by Laser-Induced Breakdown Spectroscopy. J. Anal. At. Spectrom. 2020, 35, 984–992. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Fu, Y.; Li, Z.; Ni, W. A Model Combining Spectrum Standardization and Dominant Factor Based Partial Least Square Method for Carbon Analysis in Coal Using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 82–86. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, T.-B.; Lui, S.-L.; Hou, Z.-Y.; Li, X.-W.; Li, Z.; Ni, W.-D. Major Elements Analysis in Bituminous Coals under Different Ambient Gases by Laser-Induced Breakdown Spectroscopy with PLS Modeling. Front. Phys. 2012, 7, 708–713. [Google Scholar] [CrossRef]
- Andrés, J.M.; Bona, M.T. Analysis of Coal by Diffuse Reflectance Near-Infrared Spectroscopy. Anal. Chim. Acta 2005, 535, 123–132. [Google Scholar] [CrossRef]

| Sample | Ultimate Analysis (wt.%) | |||||
|---|---|---|---|---|---|---|
| Nad | Cad | Had | Sad | Odif | Ashad | |
| Johilla_S01 | 1.29 | 65.29 | 4.15 | 0.83 | 28.45 | 8.40 |
| Johilla_S02 | 1.12 | 53.38 | 3.54 | 5.71 | 36.25 | 12.20 |
| Johilla_S03 | 1.32 | 67.98 | 3.56 | 2.19 | 24.95 | 9.20 |
| Johilla_S04 | 1.18 | 63.92 | 3.89 | 1.35 | 29.66 | 17.50 |
| Johilla_S05 | 1.26 | 63.83 | 3.82 | 0.74 | 30.35 | 13.00 |
| Johilla_S06 | 1.35 | 59.38 | 3.61 | 1.57 | 34.10 | 13.00 |
| Johilla_S07 | 1.39 | 63.80 | 3.84 | 0.72 | 30.26 | 10.30 |
| Johilla_S08 | 1.09 | 61.06 | 3.84 | 0.70 | 33.31 | 9.40 |
| Johilla_S09 | 1.28 | 68.89 | 3.51 | 0.96 | 25.36 | 9.50 |
| Johilla_S10 | 1.17 | 56.42 | 3.86 | 1.01 | 37.54 | 10.30 |
| Johilla_S11 | 1.36 | 64.53 | 3.77 | 0.64 | 29.70 | 17.80 |
| Johilla_S12 | 1.39 | 59.60 | 3.53 | 1.67 | 33.80 | 5.00 |
| Johilla_S13 | 1.28 | 62.98 | 4.04 | 0.78 | 30.92 | 9.80 |
| Johilla_S14 | 1.25 | 60.14 | 3.66 | 0.72 | 34.23 | 10.40 |
| Johilla_S15 | 1.23 | 68.95 | 3.50 | 2.19 | 24.13 | 11.10 |
| Johilla_S16 | 1.06 | 64.10 | 4.10 | 0.70 | 30.04 | 11.70 |
| Johilla_S17 | 1.29 | 63.25 | 3.78 | 0.62 | 31.06 | 12.00 |
| Johilla_S18 | 1.26 | 63.32 | 3.53 | 0.92 | 30.97 | 11.40 |
| Mean | 1.25 | 62.82 | 3.75 | 1.33 | 30.84 | 11.22 |
| Med | 1.27 | 63.56 | 3.78 | 0.88 | 30.64 | 10.75 |
| SD | 0.10 | 4.04 | 0.21 | 1.20 | 3.68 | 2.99 |
| Var | 0.01 | 16.35 | 0.04 | 1.45 | 13.56 | 8.96 |
| Min | 1.06 | 53.38 | 3.5 | 0.62 | 24.13 | 5.00 |
| Max | 1.39 | 68.95 | 4.15 | 5.71 | 37.54 | 17.80 |
| Peaks | Center | Start | End | Functional Group and Nature of Bond | |
|---|---|---|---|---|---|
| C1 | 671.33 | 664.192 | 674.19 | Mercaptans and thioethers | C-S |
| C2 | 694.187 | 674.19 | 718.47 | ||
| C3 | 1011.285 | 1001.287 | 1021.284 | Alcohols, ethers, esters, carboxylic acids, anhydrides | C-O |
| C4 | 1032.711 | 1021.284 | 1069.849 | ||
| C5 | 1099.844 | 1069.849 | 1108.415 | ||
| C6 | 1114.128 | 1108.415 | 1138.41 | ||
| C7 | 1164.121 | 1138.41 | 1179.833 | ||
| C8 | 1229.826 | 1179.833 | 1299.816 | ||
| C9 | 1368.378 | 1359.808 | 1374.091 | Alkanes | -CH3 |
| C10 | 1378.377 | 1374.091 | 1385.518 | ||
| C11 | 1451.223 | 1445.51 | 1456.937 | Alkanes and aromatic | H-C-H & C-C=C |
| C12 | 1462.65 | 1456.937 | 1472.649 | ||
| C13 | 1492.646 | 1486.933 | 1495.503 | ||
| C14 | 1501.216 | 1495.503 | 1505.501 | ||
| C15 | 1512.643 | 1505.501 | 1518.357 | ||
| C16 | 1598.346 | 1585.49 | 1608.344 | Alkenes and amides | C-C=C &C=O |
| C17 | 1611.201 | 1608.344 | 1615.486 | ||
| C18 | 1619.771 | 1615.486 | 1624.056 | ||
| C19 | 1628.341 | 1624.056 | 1634.055 | Amide, ketones, aldehydes, carboxylic acids, ester | C=O |
| C20 | 1638.34 | 1634.055 | 1648.338 | ||
| C21 | 1652.624 | 1648.338 | 1671.192 | ||
| C22 | 1675.477 | 1671.192 | 1682.619 | ||
| C23 | 1691.19 | 1682.619 | 1696.903 | ||
| C24 | 1701.188 | 1696.903 | 1716.9 | ||
| C25 | 1721.185 | 1716.9 | 1732.612 | ||
| C26 | 1736.897 | 1732.612 | 1748.324 | ||
| C27 | 1775.463 | 1769.75 | 1779.748 | Anhydrides and acyl halides | C=O |
| C28 | 1785.462 | 1779.748 | 1791.175 | ||
| C29 | 1802.602 | 1791.175 | 1808.316 | ||
| C30 | 2115.415 | 2108.274 | 2119.701 | Alkyne | C≡C |
| C31 | 2136.841 | 2128.271 | 2142.554 | ||
| C32 | 2162.552 | 2156.838 | 2165.408 | ||
| C33 | 2851.026 | 2821.303 | 2876.737 | Alkanes | H-C-H |
| C34 | 2921.016 | 2876.737 | 2948.155 | ||
| C35 | 2958.154 | 2948.155 | 2982.436 | ||
| C36 | 3040.999 | 3008.147 | 3072.423 | Alkenes and aromatic | C=C-H |
| C37 | 3100.991 | 3072.423 | 3129.558 | ||
| C38 | 3219.545 | 3205.262 | 3223.831 | Alkynes | ≡C-H |
| C39 | 3233.829 | 3223.831 | 3239.543 | ||
| C40 | 3245.256 | 3239.543 | 3250.97 | ||
| C41 | 3255.255 | 3250.97 | 3263.825 | ||
| C42 | 3275.252 | 3263.825 | 3282.394 | ||
| Pair | S2 | n | df | t-Test | F-Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tstat | p-Value | tcritical | H0: μd = 0 | Fstat | p-Value | CI | H0: σo2 =σp2 | |||||
| CFTIR (wt.%) | 0.391 | 0.077 | 115 | 114 | 1.139 | 0.257 | 2.620 | T | 1.027 | 0.887 | (0.615–1.626) | T |
| CCHNS (wt.%) | 0.383 | 0.075 | ||||||||||
| Sl. No. | Method | Sample Location [Reference] | Nature; Number of Samples | R2 | RMSE, (MBE) |
|---|---|---|---|---|---|
| 1 | Correlation with proximate analysis | China [11] | Lignite; N= 66 | 0.92 | NA |
| Sub-bituminous; N= 74 | 0.86 | NA | |||
| Bituminous; N= 94 | 0.93 | NA | |||
| Anthracite; N= 66 | 0.95 | NA | |||
| 2 | Correlation with proximate analysis (MLM) | China [12] | Coal (blend); N = 755 | NA | 2.72, (0.26%) |
| Correlation with proximate analysis (MLE) | NA | 1.91, (0.63%) | |||
| 3 | LIBS with SVR | China [43] | Coal; N = 44 | 0.99 | 1.08%, NA |
| 4 | LIBS with MLR and SVR | China [44] | Coal; N = 44 | 0.99 | 1.43%, NA |
| LIBS with MLR and PLSR | 0.99 | 2.46%, NA | |||
| LIBS with MLR | 0.86 | 3.41%, NA | |||
| 5 | LIBS with PLS based on dominant factor | China [45] | Bituminous; N = 33 | 0.99 | 4.47%, NA |
| 6 | LIBS with nonlinearized multivariate dominant factor | China [46] | Bituminous; N = 33 | 0.94 | 3.28%, NA |
| LIBS with nonlinearized multivariate dominant factor-based PLS | 0.97 | 3.13%, NA | |||
| 7 | LIBS with PLS–SVM | China [47] | Bituminous (semi coke); N = 79 | 0.94 | 0.90%, NA |
| 8 | LIBS with K-ELM | China [4] | Coal; N = 26 | 0.99 | 0.37, NA |
| 9 | LIBS with spectrum standardization and dominant factor-based PLS with spatial confinement | China [48] | Bituminous; N = 24 | 0.99 | 1.35%, (-) |
| 10 | LIBS with PLS (Ambience: Air) | China [49] | Bituminous; N = 24 | 0.87 | 3.91%, (-) |
| LIBS with PLS (Ambience: Ar) | 0.95 | 2.69%, (-) | |||
| LIBS with PLS (Ambience: He) | 0.96 | 2.43%, (-) | |||
| 11 | DRIFTS and NIRS with PCA | Germany, Poland, Czech Republic, Russia, China, North-America, Australia, and Spain [50] | Coal (blend); N = 142 | - | 2.58%, (-) |
| 12 | FTIR with QN | Johilla, India [Present Study] | Coal; N = 115 | 0.93 | 23.71%, (−0.52%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.; Prasad, A.K.; Shukla, A.; Vinod, A.; Preety, K.; Varma, A.K. Estimation of Carbon Content in High-Ash Coal Using Mid-Infrared Fourier-Transform Infrared Spectroscopy. Minerals 2023, 13, 938. https://doi.org/10.3390/min13070938
Mishra S, Prasad AK, Shukla A, Vinod A, Preety K, Varma AK. Estimation of Carbon Content in High-Ash Coal Using Mid-Infrared Fourier-Transform Infrared Spectroscopy. Minerals. 2023; 13(7):938. https://doi.org/10.3390/min13070938
Chicago/Turabian StyleMishra, Sameeksha, Anup Krishna Prasad, Anubhav Shukla, Arya Vinod, Kumari Preety, and Atul Kumar Varma. 2023. "Estimation of Carbon Content in High-Ash Coal Using Mid-Infrared Fourier-Transform Infrared Spectroscopy" Minerals 13, no. 7: 938. https://doi.org/10.3390/min13070938
APA StyleMishra, S., Prasad, A. K., Shukla, A., Vinod, A., Preety, K., & Varma, A. K. (2023). Estimation of Carbon Content in High-Ash Coal Using Mid-Infrared Fourier-Transform Infrared Spectroscopy. Minerals, 13(7), 938. https://doi.org/10.3390/min13070938









