Bioaccumulation and Human Health Risk Assessment of Arsenic and Chromium Species in Water–Soil–Vegetables System in Lephalale, Limpopo Province, South Africa
Abstract
1. Introduction
2. Experiment
2.1. Study Area and Sample Collection
2.2. Sample Pre-Treatment
2.3. Instrumentation
2.4. Chemicals, Standards and Certified Reference Materials
2.5. Preparation of Samples
2.6. Analytical Figures of Merit
2.7. Statistical Analysis
2.8. Human Health Risk Assessment
3. Results and Discussion
3.1. Evaluation of Analytical Figures of Merit
3.1.1. Total Quantification Method
3.1.2. Speciation Analysis Method
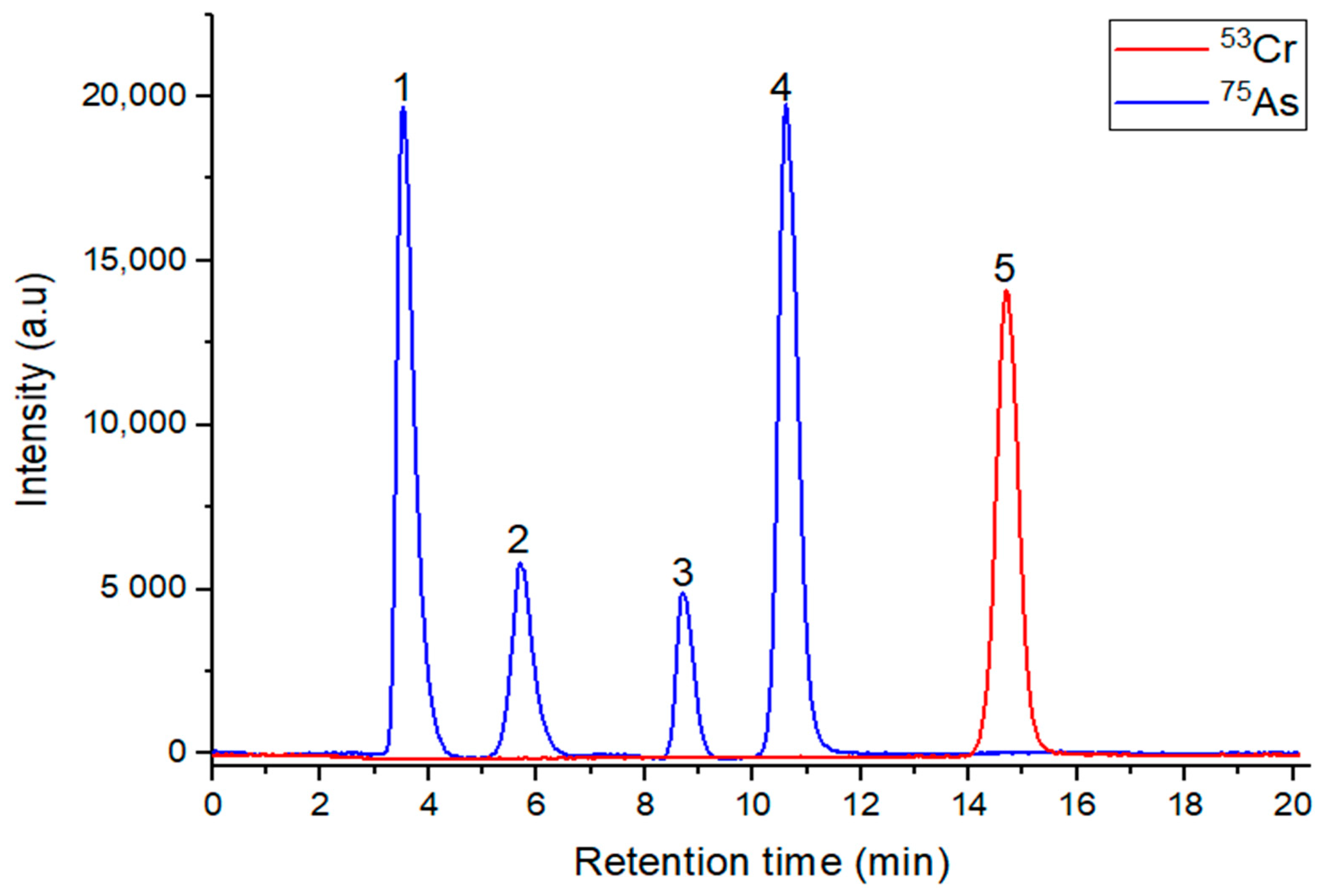
3.2. Species Identification and Correction of Polyatomic Interferences
3.3. Total Concentrations of As and Cr in Water Samples
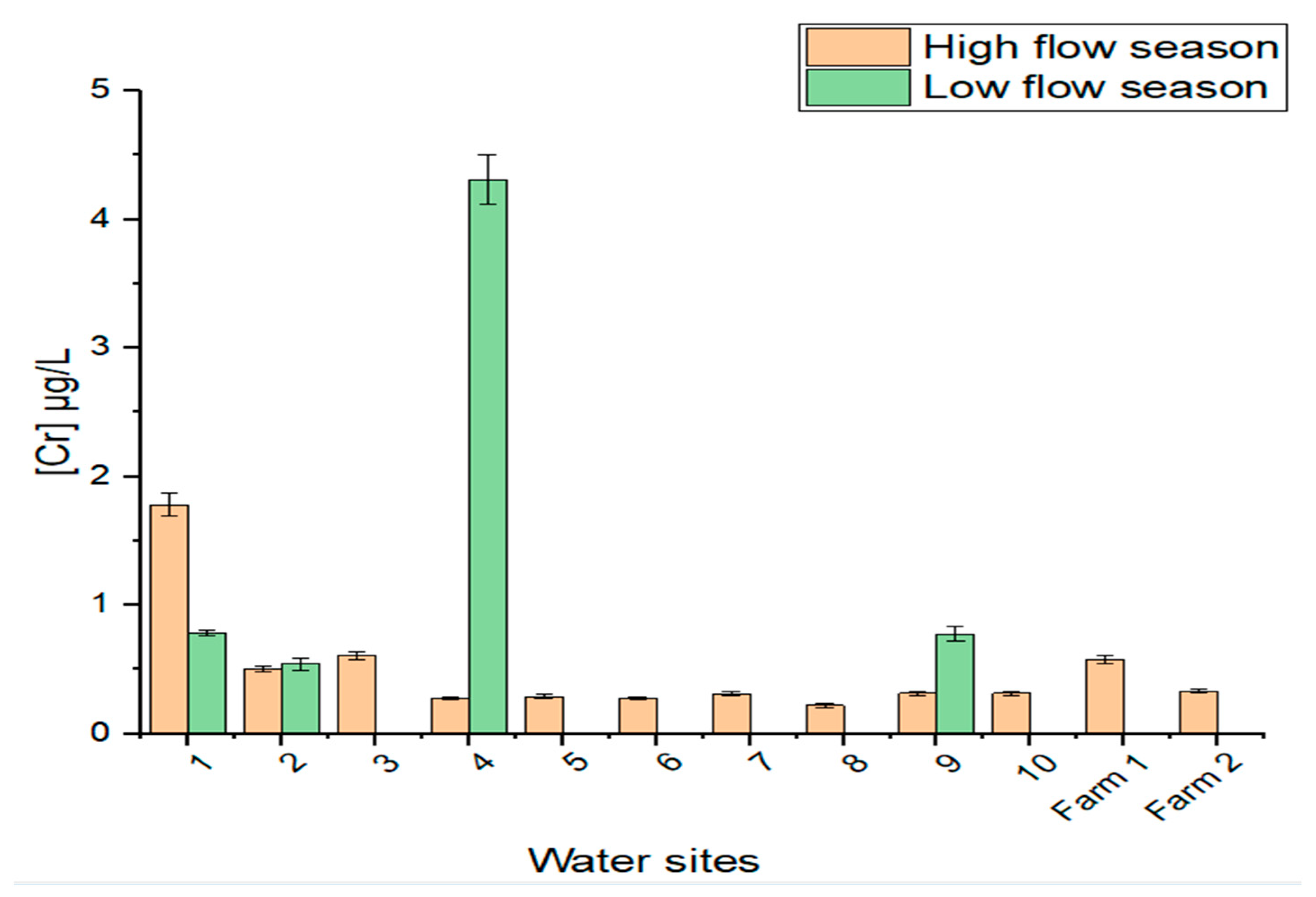
3.3.1. Chromium in Water Samples
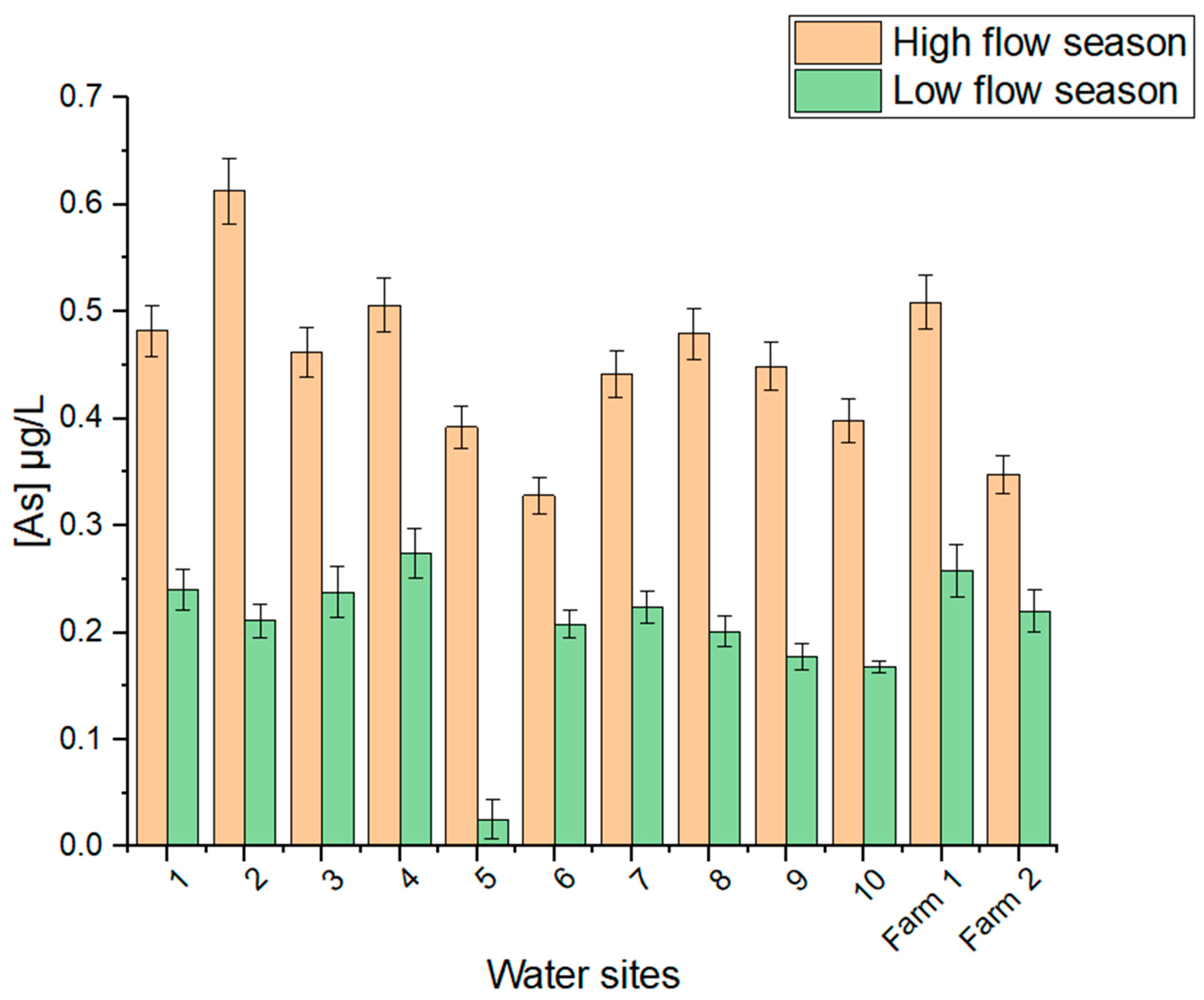
3.3.2. Arsenic in Water Samples
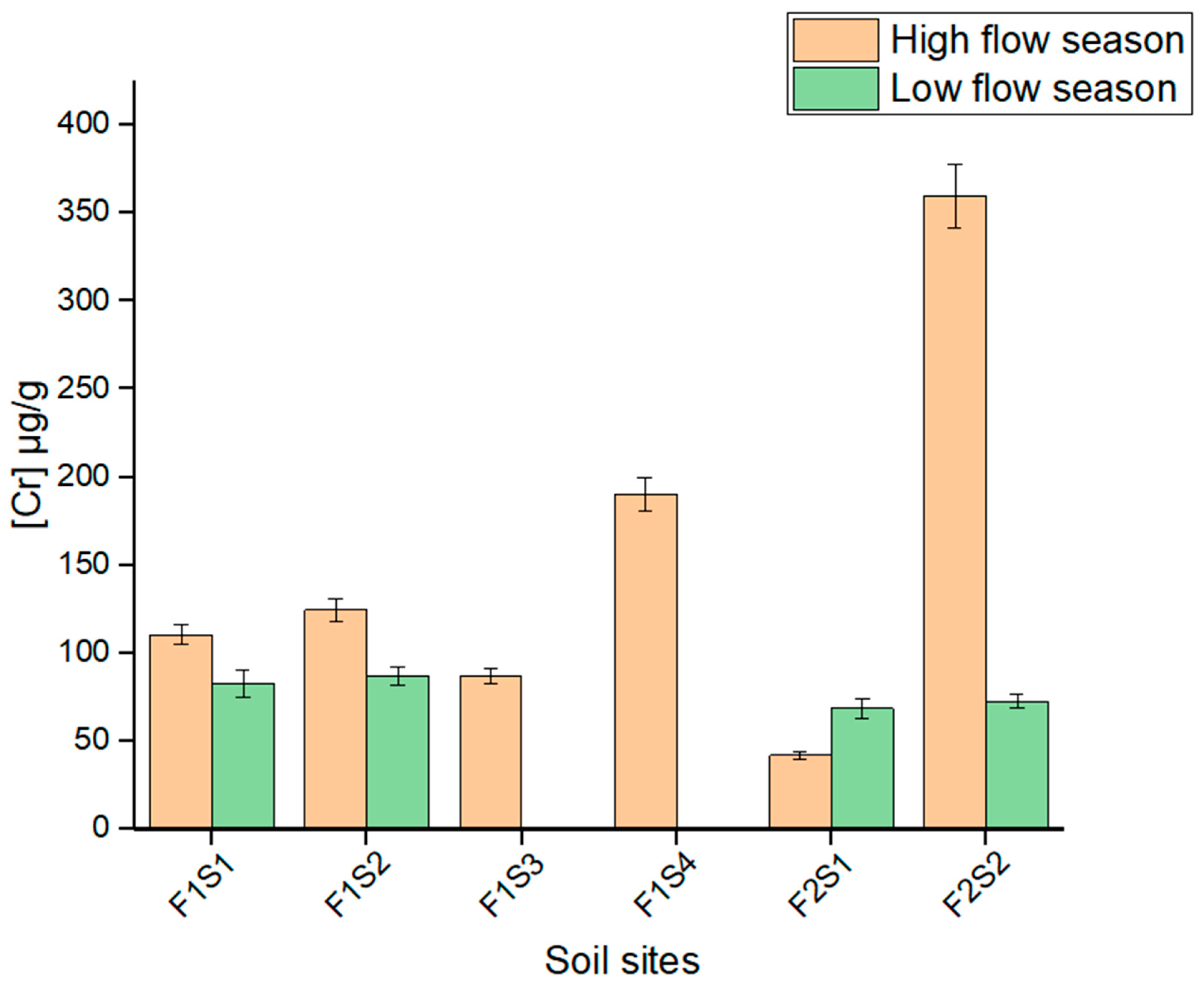
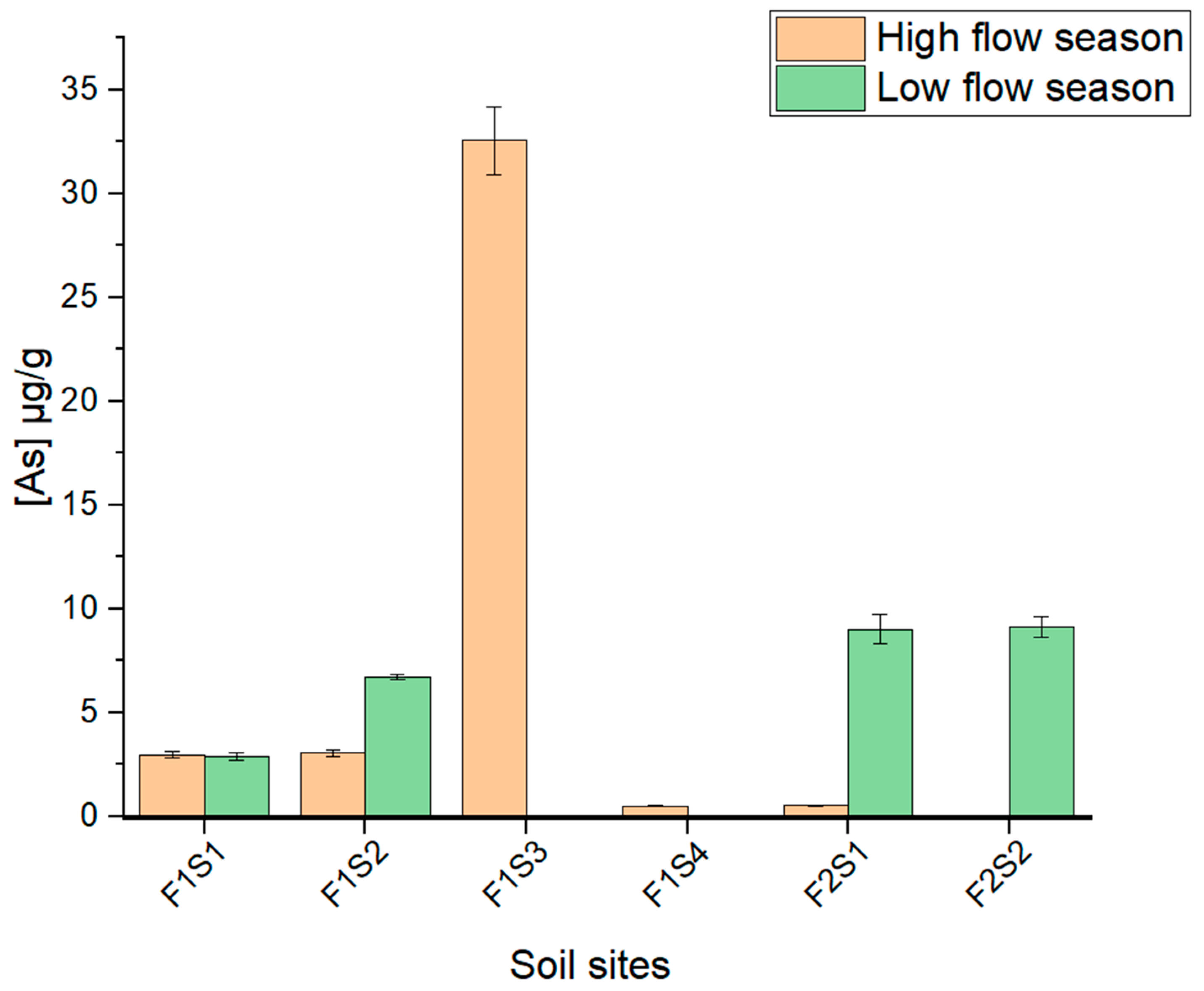
3.4. Total Concentrations of As and Cr in Soil Samples
3.5. Speciation Analysis of As and Cr in Soil Samples
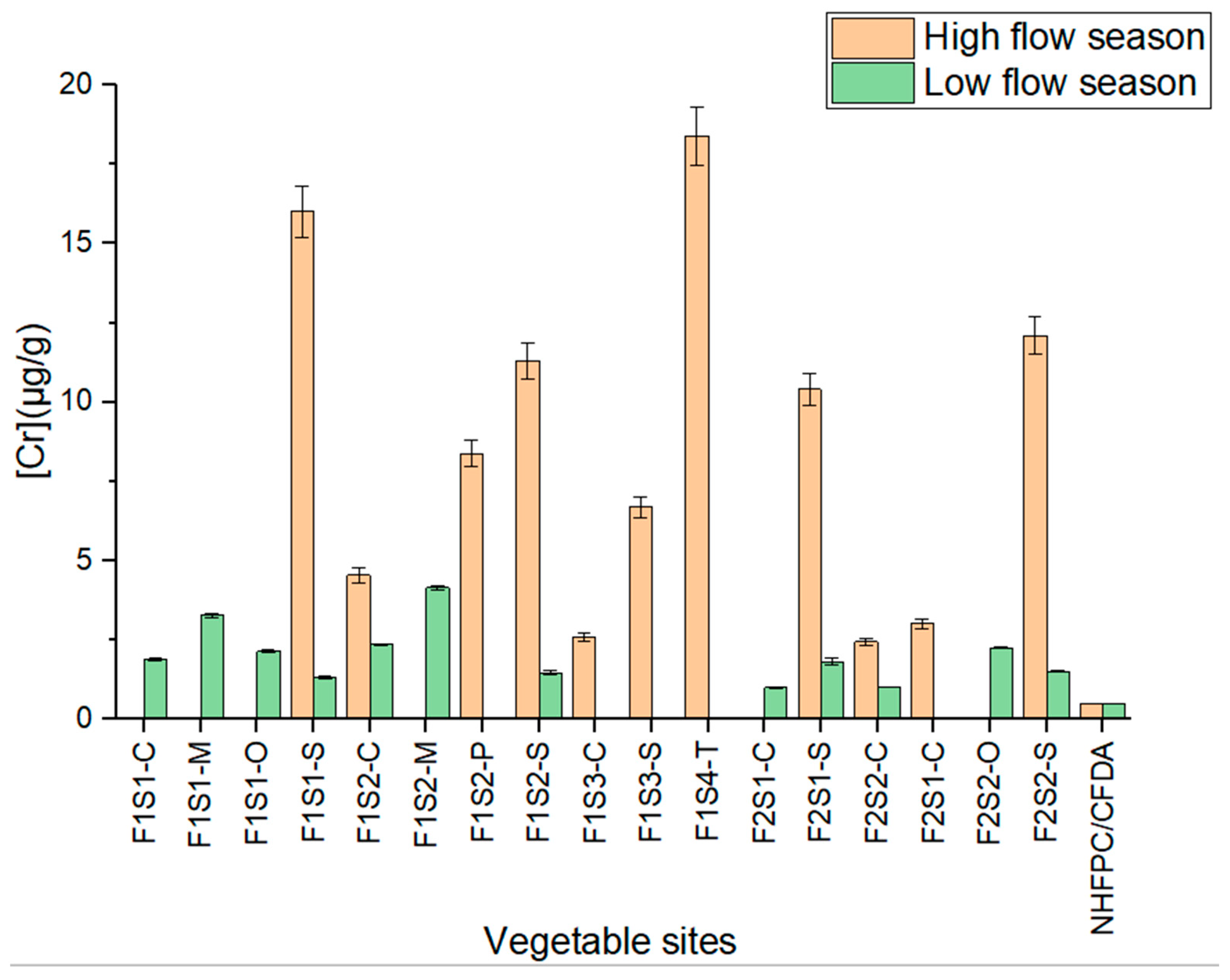
3.6. Total Concentrations of Cr in Vegetable Samples
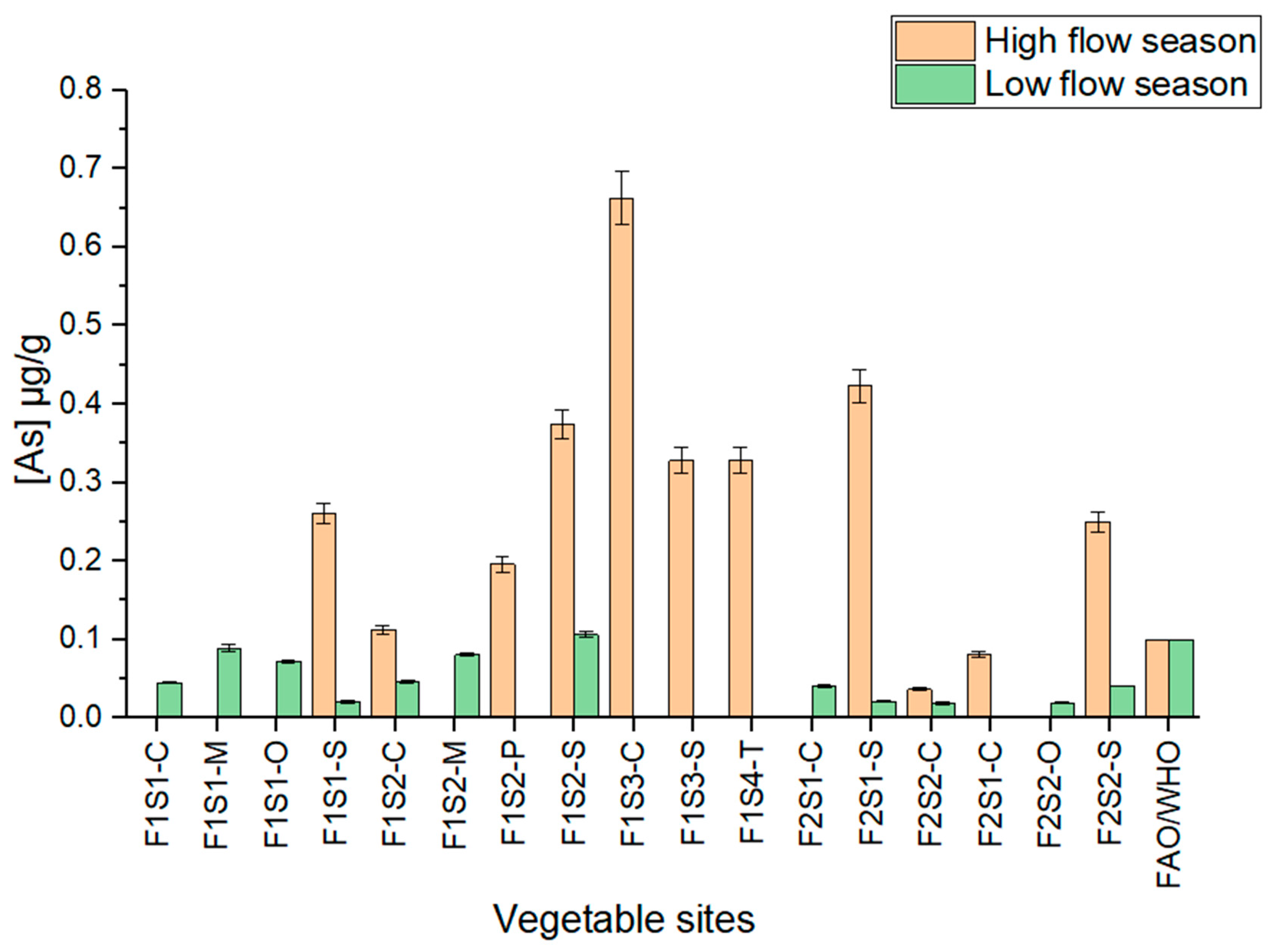
3.7. Total Concentrations of As in Vegetable Samples
3.8. Speciation Analysis of As and Cr in Vegetables
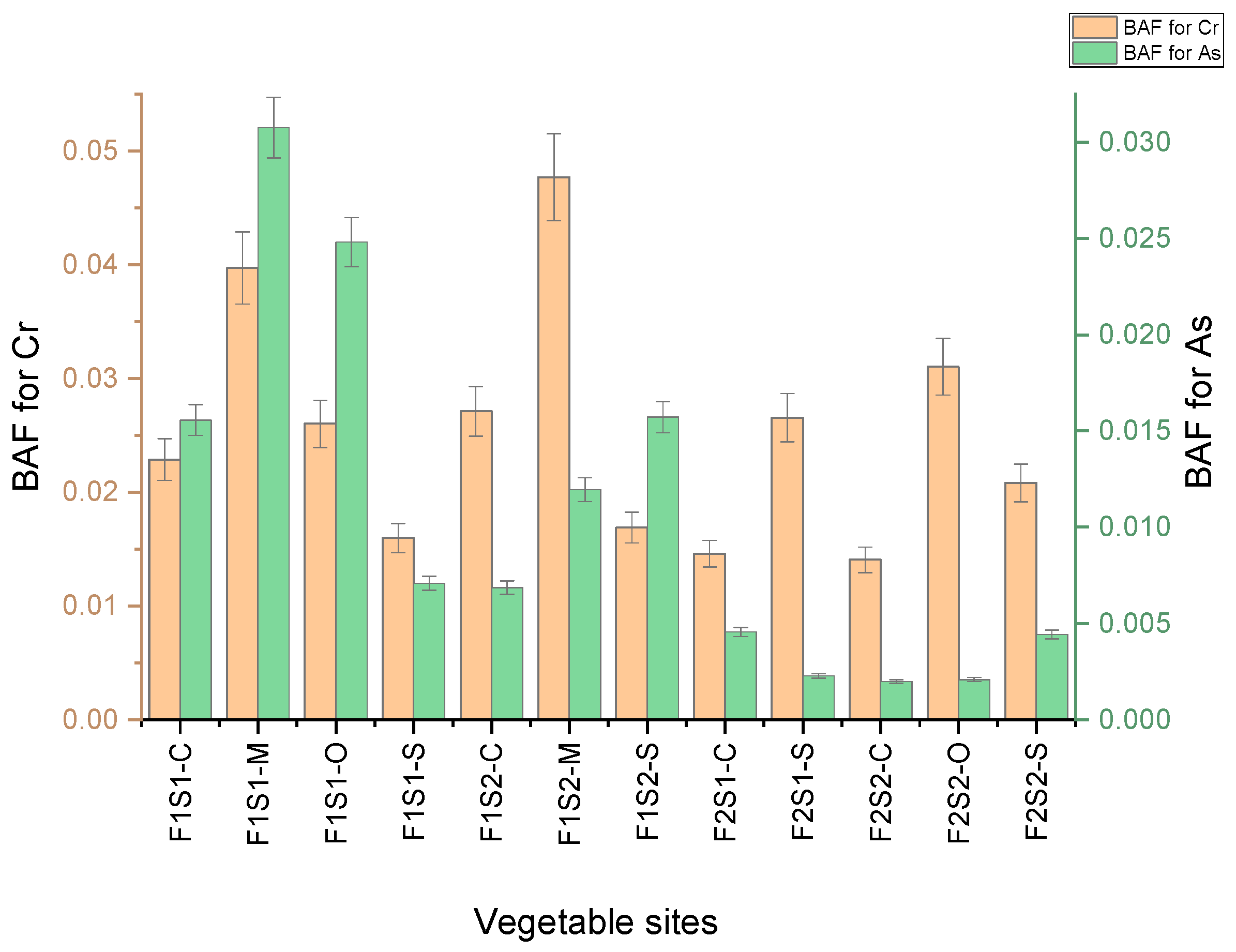
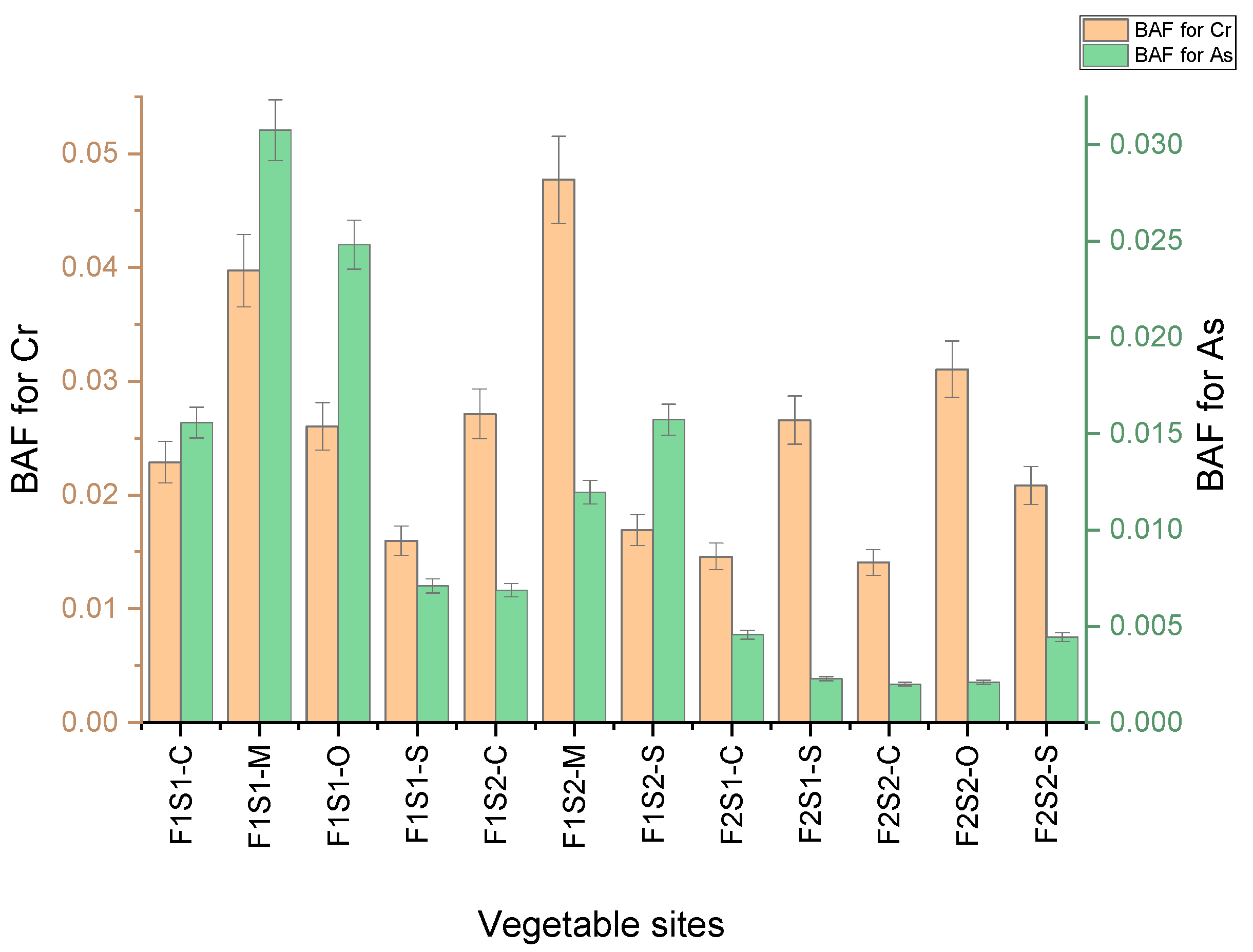
3.9. Bioaccumulation Factor
3.10. The Human Health Risk Assessment
3.10.1. Non-Carcinogenic Effects
Estimated Daily Intake
Total Hazard Quotient
3.10.2. Carcinogenic Effects Based on As(III), As(V) and Cr(VI)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayed, A.M.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef]
- Pan, L.; Fang, G.; Wang, Y.; Wang, L.; Su, B.; Li, D.; Xiang, B. Potentially toxic element pollution levels and risk assessment of soils and sediments in the upstream river, miyun reservoir, china. Int. J. Environ. Res. Public Health 2018, 15, 2364. [Google Scholar] [CrossRef] [PubMed]
- Nde, S.C.; Mathuthu, M. Assessment of Potentially Toxic Elements as Non-Point Sources of Contamination in the Upper Crocodile Catchment Area, North-West Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 576. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Fereydoni, M. The potential risk of heavy metals on human health due to the daily consumption of vegetables. Environ. Health Eng. Manag. 2019, 6, 11–16. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Tamjidi, S. The role of bentonite clay and bentonite clay@MnFe2O4 composite and their physico-chemical properties on the removal of Cr(III) and Cr(VI) from aqueous media. Environ. Sci. Pollut. Res. 2020, 27, 14044–14057. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Liu, Y.; Guo, J. Research on the adsorption of Cr3+ and Cr6+ by the cracked products of β-cyclodextrin. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 12159. [Google Scholar] [CrossRef]
- Ramjukadh, C.L.; Silberbauer, M.; Taljaard, S. An anomaly in pH data in South Africa’s national water quality monitoring database—Implications for future use. Water SA 2018, 44, 760–763. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, C. Evaluation of Calibration Equations by Using Regression Analysis: An Example of Chemical Analysis. Sensors 2022, 22, 447. [Google Scholar] [CrossRef]
- Thi Hue, N.; Van Hop, N.; Thai Long, H.; Hai Phong, N.; Uyen, T.H.; Quoc Hung, L.; Nhi Phuong, N. Determination of Chromium in Natural Water by Adsorptive Stripping Voltammetry Using in Situ Bismuth Film Electrode. J. Environ. Public Health 2020, 2020, 1347836. [Google Scholar] [CrossRef]
- Baig, J.A.; Kazi, T.G.; Shah, A.Q.; Kandhro, G.A.; Afridi, H.I.; Arain, M.B.; Jamali, M.K.; Jalbani, N. Speciation and evaluation of Arsenic in surface water and groundwater samples: A multivariate case study. Ecotoxicol. Environ. Saf. 2010, 73, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-C.; Somanna, Y.; Kim, H. Source, Distribution, Toxicity and Remediation of Arsenic in the Environment—A review. Int. J. Appl. Environ. Sci. 2016, 11, 973–6077. [Google Scholar]
- Genthe, B.; Kapwata, T.; Le Roux, W.; Chamier, J.; Wright, C.Y. The reach of human health risks associated with metals/metalloids in water and vegetables along a contaminated river catchment: South Africa and Mozambique. Chemosphere 2018, 199, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.L.; Smith, E.; Weber, J.; Rees, M.; Rofe, A.; Kuchel, T.; Sansom, L.; Naidu, R. In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ. Health Perspect. 2006, 114, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, M.; Wright, C.; Matooane, M.; Phala, N. Human health risk assessment of airborne metals to a potentially exposed community: A screening exercise. Clean Air J. 2015, 25, 51–57. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- World Health Organization. WHO Human Health Risk Assessment Toolkit: Chemical Hazards; IPCS Harmonization Project Document Xv; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Ali, M.A.; Badruzzaman, A.B.M.; Jalil, M.A.; Hossain, M.D.; Ahmed, M.F.; Masud, A.A.K.; Kamruzzaman, M. Fate of Arsenic in the Environment. In Arsenic Contamination: Bangladesh Perspective; Ahmed, M.F., Ed.; ITN-Bangladesh: Dhaka, Bangladesh, 2003; pp. 7–8. [Google Scholar]
- Ma, H.W.; Hung, M.L.; Chen, P.C. A systemic health risk assessment for the chromium cycle in Taiwan. Environ. Int. 2007, 33, 206–218. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Godeto, T.W.; Magadzu, T.; Ambushe, A.A. Quantitative Speciation of Arsenic in Water and Sediment Samples from the Mokolo River in Limpopo Province, South Africa. Anal. Lett. 2018, 51, 2761–2775. [Google Scholar] [CrossRef]
- Cui, W.; Meng, Q.; Feng, Q.; Zhou, L.; Cui, Y.; Li, W. Occurrence and release of cadmium, chromium, and lead from stone coal combustion. Int. J. Coal Sci. Technol. 2019, 6, 586–594. [Google Scholar] [CrossRef]
- Mekonnen, K.N.; Ambushe, A.A.; Chandravanshi, B.S.; Redi-Abshiro, M. Assessment of potentially toxic elements in Swiss chard and sediments of Akaki River, Ethiopia. Toxicol. Environ. Chem. 2015, 96, 1501–1515. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Mamo, M.A.; Ambushe, A.A. Simultaneous quantitative speciation of selected toxic elements in water using high performance liquid chromatography coupled to inductively coupled plasma-mass spectrometry (HPLC-ICP-MS). Phys. Chem. Earth Parts A/B/C 2021, 124, 103011. [Google Scholar] [CrossRef]
- Onyedikachi, U.B.; Belonwu, C.D.; Wegwu, M.O. Health Risk Assessment of Chromium, Manganese and Arsenic through the Consumption of Food from Industrial Areas in South Eastern States of Nigeria. Annu. Res. Rev. Biol. 2019, 31, 1–20. [Google Scholar] [CrossRef]
- Sharma, S. Das Risk assessment via oral and dermal pathways from heavy metal polluted water of Kolleru lake—A Ramsar wetland in Andhra Pradesh, India. Environ. Anal. Health Toxicol. 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Diet Compositions. 2017. Available online: https://ourworldindata.org/diet-compositions (accessed on 21 August 2021).
- Amwele, H.R.; Motsei, L.; Kalumbu, G.; Kgabi, N.; Limen Njinga, R.; Tshivhase, V.M. Investigation of possible human exposure to metals concentration in vegetables. J. Toxicol. Environ. Health Sci. Full Length Res. Pap. 2017, 9, 66–72. [Google Scholar] [CrossRef][Green Version]
- Department of Environmental Affairs (DEA). Framework for the Management of Contaminated Land; Department of Environmental Affairs (DEA): Pretoria, South Africa, 2010.
- Fakhri, Y.; Mousavi Khaneghah, A.; Hadiani, M.R.; Keramati, H.; Hosseini Pouya, R.; Moradi, B.; da Silva, B.S. Non-carcinogenic risk assessment induced by heavy metals content of the bottled water in Iran. Toxin Rev. 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Edlund, K.K.; Killman, F.; Molnár, P.; Boman, J.; Stockfelt, L.; Wichmann, J. Health Risk Assessment of PM2.5 and PM2.5-Bound Trace Elements in Thohoyandou, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 1359. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, W.; Markiewicz, B.; Kruszka, D.; Kachlicki, P.; Barałkiewicz, D. Study on speciation of As, Cr, and Sb in bottled flavored drinking water samples using advanced analytical techniques IEC/SEC-HPLC/ICP-DRC-MS and ESI-MS/MS. Molecules 2019, 24, 668. [Google Scholar] [CrossRef]
- US_EPA Data Validation Standard Operating Procedures for Contract Laboratory Program US-EPA, Region 4, SESD, Athens, Georgia Inorganic Data By Inductively Coupled Plasma—Atomic Emission Spectroscopy and Inductively Coupled Plasma—Mass Spectroscopy. 2011; pp. 1–35. Available online: https://www.epa.gov/quality/data-validation-standard-operating-procedures-contract-laboratory-program-routine (accessed on 14 March 2022).
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25. [Google Scholar] [CrossRef]
- King, M. Correlation and regression. In Fisheries Biology, Assessment and Management; John Wiley & Sons, Ltd.: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Mattusch, J.; Wennrich, R.; Schmidt, A.C.; Reisser, W. Determination of arsenic species in water, soils and plants. Fresenius J. Anal. Chem. 2000, 366, 200–203. [Google Scholar] [CrossRef]
- Leśniewska, B.; Gontarska, M.; Godlewska-Żyłkiewicz, B. Selective Separation of Chromium Species from Soils by Single-Step Extraction Methods: A Critical Appraisal. Water. Air. Soil Pollut. 2017, 228, 274. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. Parameters that Characterize HPLC Analysis. Essentials Mod. HPLC Sep. 2013, 53–83. [Google Scholar] [CrossRef]
- Robards, K.; Ryan, D. Analyses. In Principles and Practice of Modern Chromatographic Methods; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Mamo, M.A.; Ambushe, A.A. Synchronous Extraction and Quantitative Speciation of Arsenic and Chromium in Sediments by High-Performance Liquid Chromatography—Inductively Coupled Plasma—Mass Spectrometry (HPLC-ICP-MS). Anal. Lett. 2021, 54, 1943–1967. [Google Scholar] [CrossRef]
- Low, G.K.C.; Batley, G.E.; Buchanan, S.J. Interference of chloride in the speciation of arsenic by ion chromatography. Chromatographia 1986, 22, 292–298. [Google Scholar] [CrossRef]
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines, 2nd ed.; Domestic Use; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996; Volume 1.
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- SANS South African National Standard 241-1; Drinking water. Part 1: Microbiological, Physical, Aesthetic and Chemical Determinands. South African Bureau of Standards (SABS) Standards Division: Pretoria, South Africa, 2015.
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines, 2nd ed.; Agricultural Use: Irrigation; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996; Volume 4.
- Sankhla, M.S.; Kumar, R.; Prasad, L. Seasonal variations of lead and chromium concentrations in the water samples from yamuna river in Delhi, India. Iran. J. Toxicol. 2021, 15, 109–114. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, E.O.; Msagati, T.A.M. Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa. Open Chem. 2017, 15, 272–282. [Google Scholar] [CrossRef]
- Loock, M.M.; Beukes, J.P.; van Zyl, P.G. A survey of Cr(VI) contamination of surface water in the proximity of ferrochromium smelters in South Africa. Water SA 2014, 40, 709–716. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment (CCME). Canadian Council of Ministers of the Environment (CCME). Canadian soil quality guidelines for the protection of environmental and human health: Chromium (total 1997) (VI 1999). In Canadian Environmental Quality Guidelines, 1999; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Lombard, M.A.; Daniel, J.; Jeddy, Z.; Hay, L.E.; Ayotte, J.D. Assessing the Impact of Drought on Arsenic Exposure from Private Domestic Wells in the Conterminous United States. Environ. Sci. Technol. 2021, 55, 1822–1831. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment (CCME). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health—Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999.
- Kumar, B.L.; Gopal, D.V.R.S. Effective role of indigenous microorganisms for sustainable environment. 3 Biotech 2015, 5, 867–876. [Google Scholar] [CrossRef]
- Kamal, M.Z.U.; Miah, M.Y. Arsenic Speciation Techniques in Soil Water and Plant: An Overview. In Arsenic Monitoring, Removal and Remediation; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Esteban, E.; Peñalosa, J.M. The fate of arsenic in soil-plant systems. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2012; Volume 215, pp. 1–37. [Google Scholar] [CrossRef]
- Owolabi, I.A.; Mandiwana, K.L.; Panichev, N. Speciation of chromium and vanadium in medicinal plants. S. Afr. J. Chem. 2016, 69, 67–71. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Li, T.; Chen, B.; Wang, H.; He, Z.; Yang, X. Reduction Kinetics of Hexavalent Chromium in Soils and Its Correlation with Soil Properties. J. Environ. Qual. 2012, 41, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Venter, A.D.; Beukes, J.P.; Gideon van Zyl, P.; Josipovic, M.; Jaars, K.; Vakkari, V. Regional atmospheric Cr(VI) pollution from the Bushveld Complex, South Africa. Atmos. Pollut. Res. 2016, 7, 762–767. [Google Scholar] [CrossRef][Green Version]
- Ontario Ministry of Environment (OME). Rules For Soil Management and Excess Soil Quality Standards; Queen’s Printer for Ontario: Toronto, ON, Canada, 2020.
- Fonge, B.A.; Larissa, M.T.; Egbe, A.M.; Afanga, Y.A.; Fru, N.G.; Ngole-Jeme, V.M. An assessment of heavy metal exposure risk associated with consumption of cabbage and carrot grown in a tropical Savannah region. Sustain. Environ. 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Department of Health. Foodstuffs, Cosmetics and Disinfectants Act: Regulations: Maximum Levels of Metals in Foodstuffs (English/Setswana); Department of Health: Pretoria, South Africa, 2018.
- NHFPC/CHFA. National Food Safety Standard of Maximum Levels of Contaminants in Foods; Global Agricultural Information Network: Beijing China, 2017. [Google Scholar]
- Joint FAO/WHO Codex Alimentarius Commission; Joint FAO/WHO Food Standards Programme; WHO. Codex Alimentarius Commission: Procedural Manual; Electronic Publishing Policy and Support Branch: Rome, Italy, 2007. [Google Scholar]
- Laizu, J. Speciation of arsenic in vegetables and their correlation with inorganic phosphate level. Bangladesh J. Pharmacol. 2008, 2, 88–94. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Mitra, K.; Sarkhel, S.; Hobbes, M.; Burló, F.; De Groot, W.T.; Carbonell-Barrachina, A.A. Arsenic speciation in food and estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. J. Agric. Food Chem. 2008, 56, 9469–9474. [Google Scholar] [CrossRef]
- EFSA, E. Scientific Opinion on Arsenic in Food. Sci. Opin. Arsen. Food EFSA J. 2009, 7, 1351–1355. [Google Scholar] [CrossRef]
- Gezahegn, A.M.; Feyessa, F.F.; Tekeste, E.A.; Beyene, E.M. Chromium Laden Soil, Water, and Vegetables nearby Tanning Industries: Speciation and Spatial Distribution. J. Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L. Toxicological Profile for Chromium. Atlanta (GA): US Department of Health and Human Services; Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Benford, D.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Dogliotti, E.; Edler, L.; Farmer, P.; Fürst, P.; Hoogenboom, L.; Katrine Knutsen, H.; et al. Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J. 2014, 12, 3595. [Google Scholar] [CrossRef]
- Koleli, N.; Demir, A.; Kantar, C.; Atag, G.A.; Kusvuran, K.; Binzet, R. Heavy Metal Accumulation in Serpentine Flora of Mersin-Findikpinari (Turkey)—Role of Ethylenediamine Tetraacetic Acid in Facilitating Extraction of Nickel. Soil Remediat. Plants Prospect. Chall. 2015, 629–659. [Google Scholar] [CrossRef]
- Huang, W.L.; Chang, W.H.; Cheng, S.F.; Li, H.Y.; Chen, H.L. Potential risk of consuming vegetables planted in soil with lead and cadmium and the influence on vegetable antioxidant activity. Appl. Sci. 2021, 11, 3761. [Google Scholar] [CrossRef]
- Busato, B.; de Almeida Abreu, E.C.; de Oliveira Petkowicz, C.L.; Martinez, G.R.; Rodrigues Noleto, G. Pectin from Brassica oleracea var. italica triggers immunomodulating effects in vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Hewage, C.M.; Thomson, A.R.; Bailey, K.; Sadler, I.H.; Fry, S.C. Patterns of methyl and O-acetyl esterification in spinach pectins: New complexity. Phytochemistry 2002, 60, 67–77. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Rizwan, M.; Ahmed, T.; Al Jabri, H. The Carcinogenic and Non-Carcinogenic Health Risks of Metal(oid)s Bioaccumulation in Leafy Vegetables: A Consumption Advisory. Front. Environ. Sci. 2021, 9, 380. [Google Scholar] [CrossRef]
- Barbillon, A.; Aubry, C.; Nold, F.; Besancon, S.; Manouchehri, N. Health Risks Assessment in Three Urban Farms of Paris Region for Different Scenarios of Urban Agricultural Users: A Case of Soil Trace Metals Contamination. Agric. Sci. 2019, 10, 352–370. [Google Scholar] [CrossRef][Green Version]
- WHO/IPCS. Dietary Exposure Assessment of Chemicals in Food. In Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety; Environmental Health Criteria: Geneva, Switzerland, 2009; Volume 240. [Google Scholar]
- Taylor, A.A.; Tsuji, J.S.; McArdle, M.E.; Adams, W.J.; Goodfellow, W.L. Recommended Reference Values for Risk Assessment of Oral Exposure to Copper. Risk Anal. 2022, 43, 211–218. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, W.; Zheng, H.; Wu, C.; Zhang, Q.; Bu, Y. Comparison of Health Risk Assessments of Heavy Metals and As in Sewage Sludge from Wastewater Treatment Plants (WWTPs) for Adults and Children in the Urban District of Taiyuan, China. Int. J. Environ. Res. Public Health 2017, 14, 1194. [Google Scholar] [CrossRef]
- Ssemugabo, C.; Bradman, A.; Ssempebwa, J.C.; Sillé, F.; Guwatudde, D. An assessment of health risks posed by consumption of pesticide residues in fruits and vegetables among residents in the Kampala Metropolitan Area in Uganda. Int. J. Food Contam. 2022, 9, 4. [Google Scholar] [CrossRef]
- Pronczuk-Garbino, J. Childrens Health and the Environment: A Global Perspective. Int. J. Health Care Qual. Assur. 2006, 19, 3–4. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Bello, S.; Nasiru, R.; Garba, N.N.; Adeyemo, D.J. Carcinogenic and non-carcinogenic health risk assessment of heavy metals exposure from Shanono and Bagwai artisanal gold mines, Kano state, Nigeria. Sci. Afr. 2019, 6, e00197. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Qi, Y.; Hong, C. Seasonal variations of mercury levels and human health risk in vegetables from Arid Oasis (Shihezi city), Xinjiang, Northwest China. Hum. Ecol. Risk Assess. 2018, 24, 122–136. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1–17. [Google Scholar] [CrossRef]

| Parameter | Setting |
|---|---|
| Analytical column | Hamilton PRP-X100 |
| Column dimension | 4.6 mm × 250 mm, 5 µm |
| Guard column | 4.6 mm × 150 mm, PEEK |
| Pump flow rate | 1 mL/min |
| Pump pressure | 2520 psi |
| Injection volume | 100 µL |
| Mobile phase A | NH4NO3 (10 mM) and NH4H2PO4 (0.5 mM) at pH 8.7 |
| Mobile phase B | NH4NO3 (70 mM) at pH 8.7 |
| Nebulizer gas flow | 1.18 L/min |
| Auxiliary has flow | 1.20 L/min |
| Plasma gas flow | 15 L/min |
| ICP RF power | 1450 V |
| Lens voltage | 12 |
| Parameter | Unit | Adult | Children | Adult | Children |
|---|---|---|---|---|---|
| FIR [26] | g/person/day | 114.17 | 114.17 | 114.17 | 114.17 |
| EF | days/year | 365 [27] | 350 [28] | 365 | 350 |
| ED | year | 30 [27] | 6 [28] | 30 | 6 |
| RfD [27,29] | mg/kg/day | 0.003 | 0.003 | 0.0003 | 0.0003 |
| ABW [30] | kg | 68.1 | 13.8 | 68.1 | 13.8 |
| AT | days/year | 10950 [27] | 2190 [28] | 10950 | 2190 |
| Sample | Analyte | LOD | LOQ | Certified Value | Measured Value | Percentage Recovery (%) |
|---|---|---|---|---|---|---|
| Water | Cr (µg/L) | 0.193 | 0.643 | 40.2 ± 0.28 | 40.5 ± 1.7 | 101 |
| As (µg/L) | 0.117 | 0.390 | 8.08 ± 0.070 | 8.70 ± 0.19 | 108 | |
| Soil | Cr (ng/g) | 2.93 | 9.77 | 130 ± 4.0 | 130 ± 15 | 100 |
| As (ng/g) | 0.942 | 3.14 | 10.5 ± 0.30 | 10.1 ± 0.95 | 96.3 | |
| Vegetable | Cr (ng/g) | 1.30 | 4.33 | 1.99 ± 0.034 | 2.13 ± 0.16 | 107 |
| As (ng/g) | 0.418 | 1.39 | 0.113 ± 0.0024 | 0.131 ± 0.011 | 116 |
| Samples | Analyte | LOD (ng/g) | LOQ (ng/g) | Measured Concentration at 3 × LOQ (ng/g) | Measured Concentration at 20 × LOQ (ng/g) | Percentage Recovery (3 × LOQ) (%) | Percentage Recovery (20 × LOQ) (%) |
|---|---|---|---|---|---|---|---|
| Soil | As(III) | 0.161 | 0.537 | 26.3 ± 0.90 | 30.4 ± 3.3 | 99.2 | 85.3 |
| DMA | 0.453 | 1.51 | 237 ± 22 | 245 ± 28 | 103 | 95.5 | |
| MMA | 0.343 | 1.14 | 109 ± 9.2 | 116 ± 3.0 | 114 | 101 | |
| As(V) | 1.59 | 5.30 | 188 ± 8.5 | 241 ± 18 | 108 | 91.6 | |
| Cr(VI) | 0.670 | 2.23 | 120 ± 7.5 | 177 ± 16 | 98.1 | 110 | |
| Vegetable | As(III) | 0.152 | 0.507 | 67.7 ± 3.2 | 64.2 ± 6.1 | 103 | 87.8 |
| DMA | 0.0932 | 0.311 | 66.2 ± 4.9 | 66.8 ± 5.6 | 104 | 98.0 | |
| MMA | 1.35 | 4.50 | 55.4 ± 5.3 | 129 ± 8.3 | 97.6 | 104 | |
| As(V) | 0.458 | 1.53 | 117 ± 10 | 123 ± 6.9 | 109 | 94.1 | |
| Cr(VI) | 0.598 | 1.99 | 1030 ± 74 | 1130 ± 47 | 105 | 113 |
| Soil | As(III) (ng/g) | DMA (ng/g) | MMA (ng/g) | As(V) (ng/g) | Cr(VI) (ng/g) | Cr (III) (ng/g) |
|---|---|---|---|---|---|---|
| F1S1 | 2010 ± 197 | 1.79 ± 0.15 | <0.343 | 868 ± 69 | 235 ± 15 | 2740 ± 250 |
| F1S2 | 241 ± 19 | 348 ± 25 | 1200 ± 20 | 1190 ± 100 | 2120 ± 170 | 949 ± 77 |
| F1S3 | 3960 ± 240 | 20,600 ± 530 | 3110 ± 150 | 4850 ± 350 | 3000 ± 170 | 30,500 ± 1800 |
| F1S4 | 24.9 ± 1.3 | 226 ± 20 | 92.0 ± 8.4 | 158 ± 0.30 | 116 ± 10 | 385 ± 33 |
| F2S1 | 359 ± 22 | <0.453 | 71.4 ± 0.13 | <1.59 | 236 ± 22 | 284 ± 26 |
| F2S2 | 1.51 ± 0.088 | 43.4 ± 0.62 | 0.471 ± 0.053 | <1.59 | 11.2 ± 0.99 | 34.1 ± 3.0 |
| Soil | As(III) (ng/g) | DMA (ng/g) | MMA (ng/g) | As(V) (ng/g) | Cr(VI) (ng/g) | Cr (III) (ng/g) |
|---|---|---|---|---|---|---|
| F1S1 | 373 ± 16 | 860 ± 18 | 438 ± 12 | 1130 ± 43 | 2260 ± 47 | 635 ± 13 |
| F1S2 | 452 ± 46 | 2560 ± 220 | 1070 ± 59 | 2570 ± 190 | 2200 ± 73 | 4540 ± 14 |
| F2S1 | <0.161 | 6550 ± 360 | 1160 ± 65 | 1330 ± 160 | 2890 ± 120 | 6150 ± 250 |
| F2S2 | 1600 ± 71 | 2490 ± 150 | 1390 ± 140 | 3570 ± 120 | 2370 ± 59 | 6770 ± 170 |
| Vegetable | As(III) (ng/g) | DMA (ng/g) | MMA (ng/g) | As(V) (ng/g) | Cr(VI) (ng/g) | Cr (III) (ng/g) |
|---|---|---|---|---|---|---|
| F1S1-S | 63.0 ± 5.8 | 62.0 ± 0.52 | 34.3 ± 0.19 | 99.8 ± 6.7 | 963 ± 57 | 15,000 ± 840 |
| F1S2-C | 25.6 ± 0.65 | 40.1 ± 3.1 | 35.3 ± 0.73 | 8.97 ± 0.51 | 722 ± 62 | 3820 ± 330 |
| F1S2-P | 67.5 ± 6.5 | <0.0932 | 34.3 ± 0.87 | 92.3 ± 4.1 | 602 ± 19 | 7780 ± 250 |
| F1S2-S | 49.1 ± 2.5 | <0.0932 | 61.3 ± 6.6 | 262 ± 6.7 | 1090 ± 55 | 10,200 ± 520 |
| F1S3-C | 189 ± 4.3 | 131 ± 6.8 | 292 ± 3.6 | 48.9 ± 4.2 | 731 ± 24 | 1850 ± 60 |
| F1S3-S | 160 ± 11 | <0.0932 | 70.7 ± 8.7 | 95.7 ± 4.5 | 764 ± 40 | 5930 ± 310 |
| F1S4-T | 100 ± 1.4 | 119 ± 6.1 | 107 ± 3.5 | 1.05 ± 0.090 | 956 ± 40 | 17,400 ± 740 |
| F2S1-C | 16.1 ± 1.5 | 53.4 ± 3.1 | 9.48 ± 0.36 | <0.458 | 1060 ± 87 | 1960 ± 120 |
| F2S1-S | 502 ± 31 | <0.0932 | <1.35 | 81.3 ± 3.5 | 1180 ± 97 | 9220 ± 480 |
| F2S2-C | 1.55 ± 0.15 | <0.0932 | 31.7 ± 0.24 | 0.91 ± 0.070 | 594 ± 18 | 1850 ± 55 |
| F2S2-S | 54.7 ± 1.2 | 153 ± 0.095 | 27.7 ± 1.8 | 12.1 ± 0.91 | 962 ± 88 | 11,100 ± 1000 |
| Vegetables | As(III) (ng/g) | DMA (ng/g) | MMA (ng/g) | As(V) (ng/g) | Cr(VI) (ng/g) | Cr (III) (ng/g) |
|---|---|---|---|---|---|---|
| F1S1-C | 41.8 ± 2.1 | 0.338 ± 0.032 | <1.35 | 0.870 ± 0.087 | 197 ± 13 | 1690 ± 110 |
| F1S1-M | 52.9 ± 2.6 | 6.17 ± 0.57 | <1.35 | 27.8 ± 0.99 | 687 ± 8.7 | 2590 ± 33 |
| F1S1-O | 34.0 ± 1.5 | <0.0932 | 15.7 ± 0.36 | 19.9 ± 1.1 | 603 ± 23 | 1550 ± 58 |
| F1S1-S | 7.83 ± 0.62 | 7.47 ± 0.55 | 2.48 ± 0.096 | 0.720 ± 0.045 | 668 ± 22 | 652 ± 22 |
| F1S2-C | 28.1 ± 2.7 | 6.54 ± 0.097 | 5.42 ± 0.075 | 4.28 ± 0.071 | 566 ± 38 | 1790 ± 100 |
| F1S2-M | 37.8 ± 1.6 | 30.3 ± 2.9 | 3.66 ± 0.23 | 6.86 ± 0.40 | 608 ± 18 | 3540 ± 110 |
| F2S1-C | 31.5 ± 1.7 | 1.38 ± 0.091 | 4.89 ± 0.24 | 1.45 ± 0.12 | 517 ± 9.3 | 483 ± 8.6 |
| F2S1-S | 8.60 ± 0.30 | 3.85 ± 0.19 | 3.74 ± 0.34 | 2.40 ± 0.062 | 585 ± 8.7 | 1240 ± 18 |
| F2S2-C | 7.03 ± 0.44 | <0.0932 | 6.97 ± 0.00042 | 2.20 ± 0.13 | 646 ± 31 | 374 ± 18 |
| F2S2-O | 13.8 ± 1.0 | <0.0932 | <1.35 | 3.29 ± 0.20 | 500 ± 28 | 1750 ± 98 |
| F2S2-S | 28.6 ± 0.86 | 1.09 ± 0.054 | 3.55 ± 0.20 | 5.30 ± 0.31 | 579 ± 8.7 | 931 ± 51 |
| Adult Cr | Children Cr | Adult As | Children As | |
|---|---|---|---|---|
| Vegetables | EDI | |||
| F1S1-S | 0.0270 | 0.1276 | 0.000440 | 0.00312 |
| F1S2-C | 0.00765 | 0.0362 | 0.000189 | 0.00134 |
| F1S2-P | 0.0141 | 0.0668 | 0.000330 | 0.00234 |
| F1S2-S | 0.0190 | 0.0901 | 0.000630 | 0.00447 |
| F1S3-C | 0.00435 | 0.0206 | 0.00112 | 0.00793 |
| F1S3-S | 0.0113 | 0.0534 | 0.000553 | 0.00392 |
| F1S4-T | 0.0310 | 0.147 | 0.000554 | 0.00394 |
| F2S1-C | 0.00411 | 0.0195 | 0.000061 | 0.000433 |
| F2S1-S | 0.0175 | 0.0829 | 0.000713 | 0.00506 |
| F2S2-C | 0.00507 | 0.0240 | 0.000137 | 0.00097 |
| F2S2-S | 0.0204 | 0.0965 | 0.000421 | 0.00299 |
| RfD (mg/kg/day) | 0.003 | 0.003 | 0.0003 | 0.0003 |
| Adult Cr | Children Cr | Adult As | Children As | |
|---|---|---|---|---|
| Vegetables | EDI | |||
| F2S2-C | 0.00172 | 0.00813 | 0.0000307 | 0.000145 |
| F1S1-C | 0.00319 | 0.0151 | 0.0000758 | 0.000359 |
| F1S1-M | 0.00553 | 0.0262 | 0.000150 | 0.000709 |
| F1S1-O | 0.00362 | 0.0171 | 0.000121 | 0.000572 |
| F1S1-S | 0.00222 | 0.0105 | 0.0000345 | 0.000163 |
| F1S2-C | 0.00398 | 0.0188 | 0.0000780 | 0.000369 |
| F1S2-M | 0.00699 | 0.0331 | 0.000136 | 0.000643 |
| F1S2-S | 0.00248 | 0.0117 | 0.000179 | 0.000845 |
| F2S1-C | 0.00169 | 0.00797 | 0.0000694 | 0.000329 |
| F2S1-S | 0.00307 | 0.0145 | 0.0000347 | 0.000164 |
| F2S2-C | 0.00172 | 0.00813 | 0.0000307 | 0.000145 |
| F2S2-O | 0.00379 | 0.0179 | 0.0000322 | 0.000152 |
| F2S2-S | 0.00254 | 0.0120 | 0.0000683 | 0.000323 |
| RfD | 0.003 | 0.003 | 0.0003 | 0.0003 |
| Cr | As | |||
|---|---|---|---|---|
| Adult | Children | Adult | Children | |
| Vegetables | THQ | THQ | ||
| F1S1-S | 8.99 | 42.5 | 1.47 | 6.94 |
| F1S2-S | 6.35 | 30.0 | 2.10 | 9.94 |
| F1S2-C | 2.55 | 12.1 | 0.629 | 2.98 |
| F1S2-P | 4.71 | 22.3 | 1.10 | 5.21 |
| F1S3-C | 1.45 | 6.86 | 3.72 | 17.6 |
| F1S3-S | 3.76 | 17.8 | 1.84 | 8.72 |
| F1S4-T | 10.3 | 48.9 | 1.85 | 8.75 |
| F2S1-S | 5.84 | 27.6 | 2.38 | 11.2 |
| F2S2-S | 6.80 | 32.2 | 1.40 | 6.65 |
| F2S1-C | 1.37 | 6.49 | 0.20 | 0.962 |
| F2S2-C | 1.69 | 8.00 | 0.46 | 2.15 |
| Cr | As | |||
|---|---|---|---|---|
| Adult | Children | Adult | Children | |
| Vegetables | THQ | THQ | ||
| F1S1-C | 1.06 | 5.02 | 0.253 | 1.20 |
| F1S1-M | 1.84 | 8.72 | 0.499 | 2.36 |
| F1S1-O | 1.21 | 5.72 | 0.403 | 1.91 |
| F1S1-S | 0.742 | 3.51 | 0.115 | 0.545 |
| F1S2-C | 1.33 | 6.27 | 0.260 | 1.23 |
| F1S2-M | 2.33 | 11.0 | 0.453 | 2.14 |
| F1S2-S | 0.826 | 3.91 | 0.595 | 2.82 |
| F2S1-C | 0.562 | 2.66 | 0.231 | 1.10 |
| F2S1-S | 1.02 | 4.84 | 0.116 | 0.548 |
| F2S2-C | 0.573 | 2.71 | 0.102 | 0.484 |
| F2S2-O | 1.26 | 5.98 | 0.107 | 0.508 |
| F2S2-S | 0.848 | 4.01 | 0.228 | 1.08 |
| Vegetable | As(III) | As(V) | Cr(VI) | Total Risk (As(III) + As(V) + Cr(VI)) | ||||
|---|---|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | Adults | Children | Adults | Children | |
| ILCR | ||||||||
| F1S1-S | 1.59 × 10−4 | 7.53 × 10−4 | 2.52 × 10−4 | 1.19 × 10−3 | 2.43 × 10−3 | 1.15 × 10−2 | 2.84 × 10−3 | 1.34 × 10−2 |
| F1S2-C | 6.48 × 10−5 | 3.06 × 10−4 | 2.27 × 10−5 | 1.07 × 10−4 | 1.83 × 10−3 | 8.64 × 10−3 | 1.92 × 10−3 | 9.05 × 10−3 |
| F1S2-P | 1.71 × 10−4 | 8.07 × 10−4 | 2.33 × 10−4 | 1.10 × 10−3 | 1.52 × 10−3 | 7.20 × 10−3 | 1.92 × 10−3 | 9.11 × 10−3 |
| F1S2-S | 1.24 × 10−4 | 5.87 × 10−4 | 6.61 × 10−4 | 3.13 × 10−3 | 2.75 × 10−3 | 1.30 × 10−2 | 3.54 × 10−3 | 1.67 × 10−2 |
| F1S3-C | 4.78 × 10−4 | 2.26 × 10−3 | 1.24 × 10−4 | 5.84 × 10−4 | 1.85 × 10−3 | 8.75 × 10−3 | 2.45 × 10−3 | 1.16 × 10−2 |
| F1S3-S | 4.04 × 10−4 | 1.91 × 10−3 | 2.42 × 10−4 | 1.14 × 10−3 | 1.93 × 10−3 | 9.14 × 10−3 | 2.58 × 10−3 | 1.22 × 10−2 |
| F1S4-T | 2.53 × 10−4 | 1.20 × 10−3 | 2.65 × 10−6 | 1.25 × 10−5 | 2.42 × 10−3 | 1.14 × 10−2 | 2.68 × 10−3 | 1.26 × 10−2 |
| F2S1-C | 4.07 × 10−5 | 1.93 × 10−4 | N/A * | N/A * | 2.67 × 10−3 | 1.26 × 10−2 | 2.71 × 10−3 | 1.28 × 10−2 |
| F2S1-S | 1.27 × 10−3 | 6.01 × 10−3 | 2.05 × 10−4 | 9.72 × 10−4 | 2.97 × 10−3 | 1.41 × 10−2 | 4.45 × 10−3 | 2.11 × 10−2 |
| F2S2-C | 3.92 × 10−6 | 1.85 × 10−5 | 2.31 × 10−6 | 1.09 × 10−5 | 1.50 × 10−3 | 7.11 × 10−3 | 1.51 × 10−3 | 7.14 × 10−3 |
| F2S2-S | 1.38 × 10−4 | 6.54 × 10−4 | 3.07 × 10−5 | 1.45 × 10−4 | 2.43 × 10−3 | 1.15 × 10−2 | 2.60 × 10−3 | 1.23 × 10−2 |
| Vegetables | As(III) | As(V) | Cr(VI) | Total Risk (As(III) + As(V) + Cr(VI)) | ||||
|---|---|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | Adults | Children | Adults | Children | |
| ILCR | ||||||||
| F1S1-C | 1.06 × 10−4 | 5.00 × 10−4 | 2.20 × 10−6 | 1.04 × 10−5 | 4.98 × 10−4 | 7.47 × 10−4 | 6.06 × 10−4 | 1.26 × 10−3 |
| F1S1-M | 1.34 × 10−4 | 6.33 × 10−4 | 7.03 × 10−5 | 3.33 × 10−4 | 1.74 × 10−3 | 2.61 × 10−3 | 1.94 × 10−3 | 3.58 × 10−3 |
| F1S1-O | 8.61 × 10−5 | 4.07 × 10−4 | 5.04 × 10−5 | 2.38 × 10−4 | 1.52 × 10−3 | 2.29 × 10−3 | 1.66 × 10−3 | 2.94 × 10−3 |
| F1S1-S | 1.98 × 10−5 | 9.37 × 10−4 | 1.82 × 10−5 | 8.61 × 10−6 | 1.69 × 10−3 | 2.53 × 10−3 | 1.71 × 10−3 | 2.63 × 10−3 |
| F1S2-C | 7.09 × 10−5 | 3.36 × 10−4 | 1.08 × 10−5 | 5.12 × 10−5 | 1.43 × 10−3 | 2.15 × 10−3 | 1.51 × 10−3 | 2.54 × 10−3 |
| F1S2-M | 9.56 × 10−5 | 4.52 × 10−4 | 1.74 × 10−5 | 8.21 × 10−5 | 1.54 × 10−3 | 2.31 × 10−3 | 1.65 × 10−3 | 2.84 × 10−3 |
| F2S1-C | 7.96 × 10−5 | 3.77 × 10−4 | 3.67 × 10−6 | 1.74 × 10−5 | 1.31 × 10−3 | 1.96 × 10−3 | 1.39 × 10−3 | 2.35 × 10−3 |
| F2S1-S | 2.18 × 10−5 | 1.03 × 10−4 | 6.07 × 10−6 | 2.87 × 10−5 | 1.48 × 10−3 | 2.22 × 10−3 | 1.51 × 10−3 | 2.35 × 10−3 |
| F2S2-C | 1.78 × 10−5 | 8.40 × 10−5 | 5.57 × 10−6 | 2.64 × 10−5 | 1.63 × 10−3 | 2.45 × 10−3 | 1.65 × 10−3 | 2.56 × 10−3 |
| F2S2-O | 3.49 × 10−5 | 1.65 × 10−4 | 8.32 × 10−6 | 3.94 × 10−5 | 1.26 × 10−3 | 1.90 × 10−3 | 1.30 × 10−3 | 2.10 × 10−3 |
| F2S2-S | 7.22 × 10−5 | 3.42 × 10−4 | 1.34 × 10−5 | 6.35 × 10−5 | 4.88 × 10−4 | 2.44 × 10−4 | 5.74 × 10−4 | 6.50 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dintsi, B.S.; Letsoalo, M.R.; Ambushe, A.A. Bioaccumulation and Human Health Risk Assessment of Arsenic and Chromium Species in Water–Soil–Vegetables System in Lephalale, Limpopo Province, South Africa. Minerals 2023, 13, 930. https://doi.org/10.3390/min13070930
Dintsi BS, Letsoalo MR, Ambushe AA. Bioaccumulation and Human Health Risk Assessment of Arsenic and Chromium Species in Water–Soil–Vegetables System in Lephalale, Limpopo Province, South Africa. Minerals. 2023; 13(7):930. https://doi.org/10.3390/min13070930
Chicago/Turabian StyleDintsi, Bennett Siphiwe, Mokgehle Refiloe Letsoalo, and Abayneh Ataro Ambushe. 2023. "Bioaccumulation and Human Health Risk Assessment of Arsenic and Chromium Species in Water–Soil–Vegetables System in Lephalale, Limpopo Province, South Africa" Minerals 13, no. 7: 930. https://doi.org/10.3390/min13070930
APA StyleDintsi, B. S., Letsoalo, M. R., & Ambushe, A. A. (2023). Bioaccumulation and Human Health Risk Assessment of Arsenic and Chromium Species in Water–Soil–Vegetables System in Lephalale, Limpopo Province, South Africa. Minerals, 13(7), 930. https://doi.org/10.3390/min13070930







