Water–Rock Interactions Driving Groundwater Composition in the Pra Basin (Ghana) Identified by Combinatorial Inverse Geochemical Modelling
Abstract
:1. Introduction
2. Materials and Methods
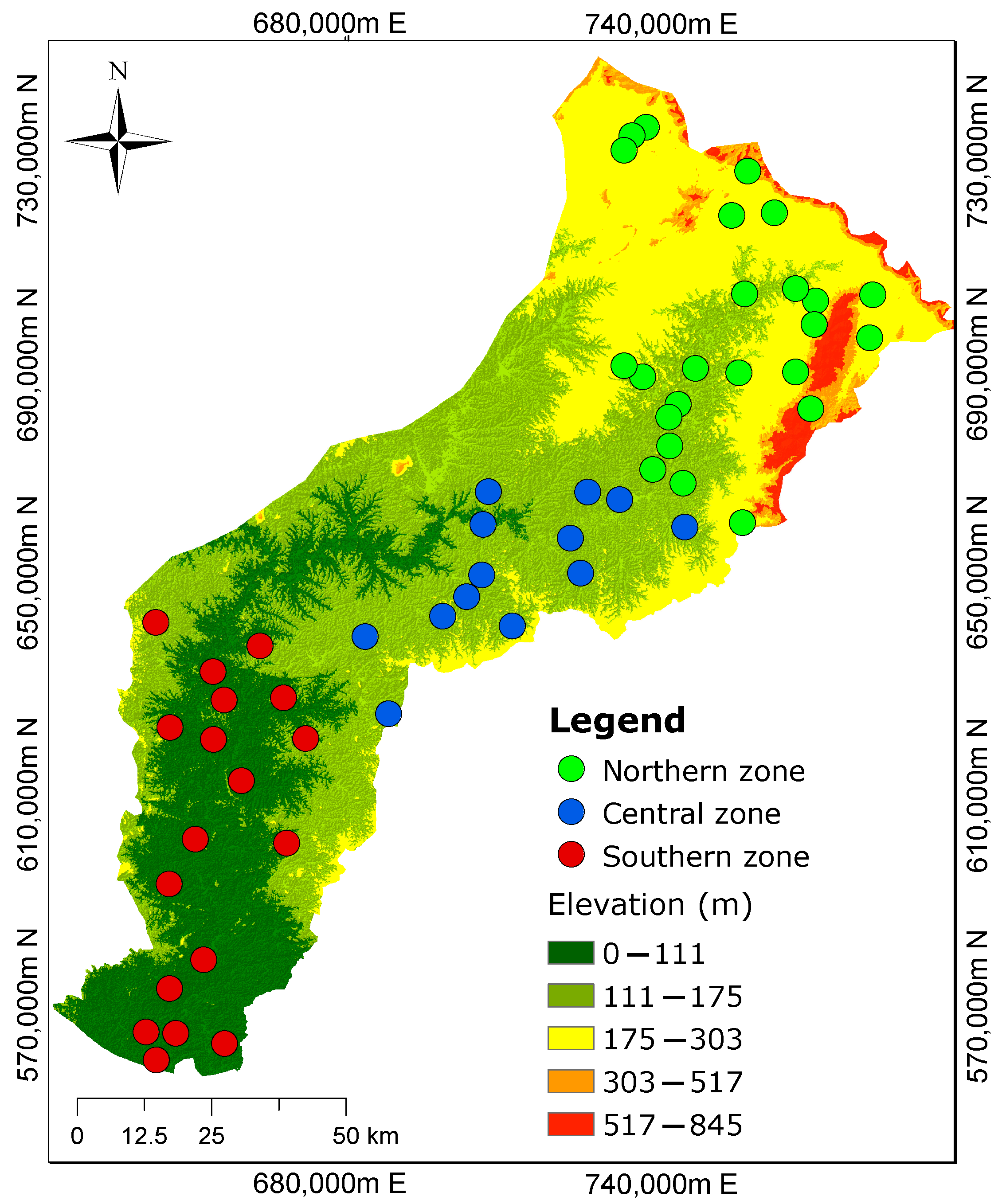
2.1. Study Area
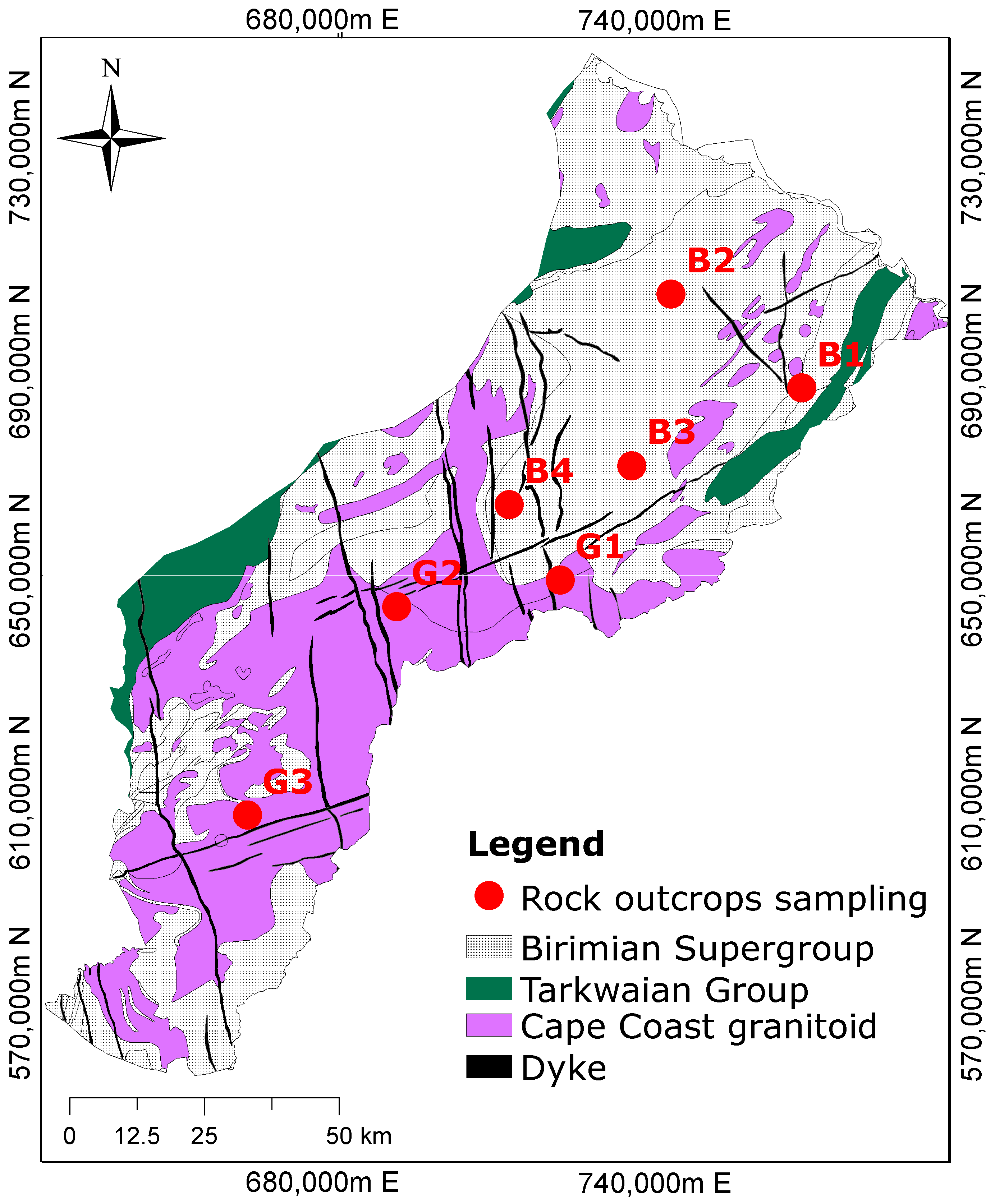
2.2. Geological Setting
2.3. Hydrogeologic Conditions
2.4. Field Work
2.5. Modelling Input Data Sources
2.6. Geochemical Code and Thermodynamic Data
2.7. Geochemical Modelling
2.7.1. Combinatorial Inverse Modelling Approach
2.7.2. Calibration Based on Reaction Path Scheme
3. Results
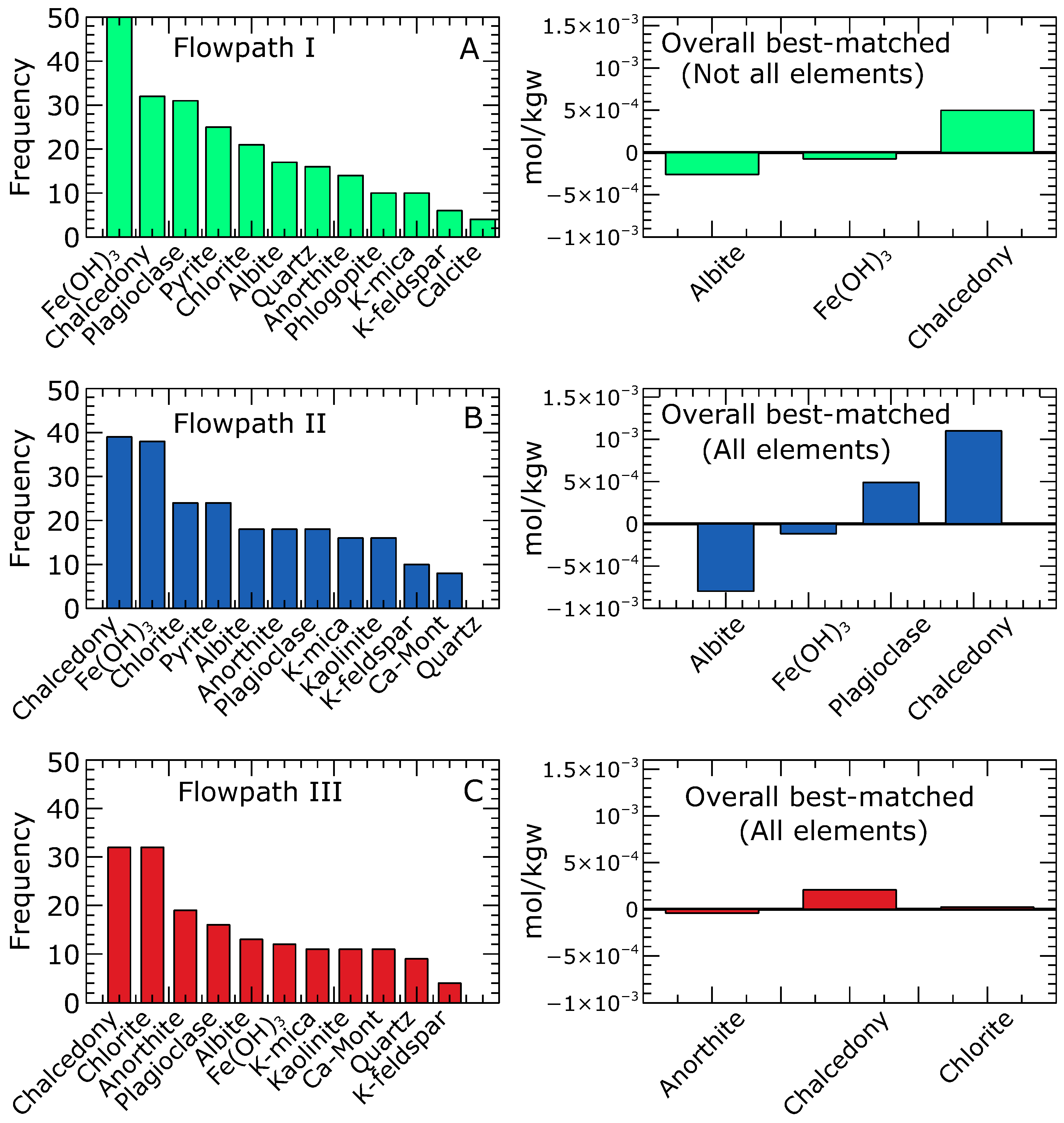
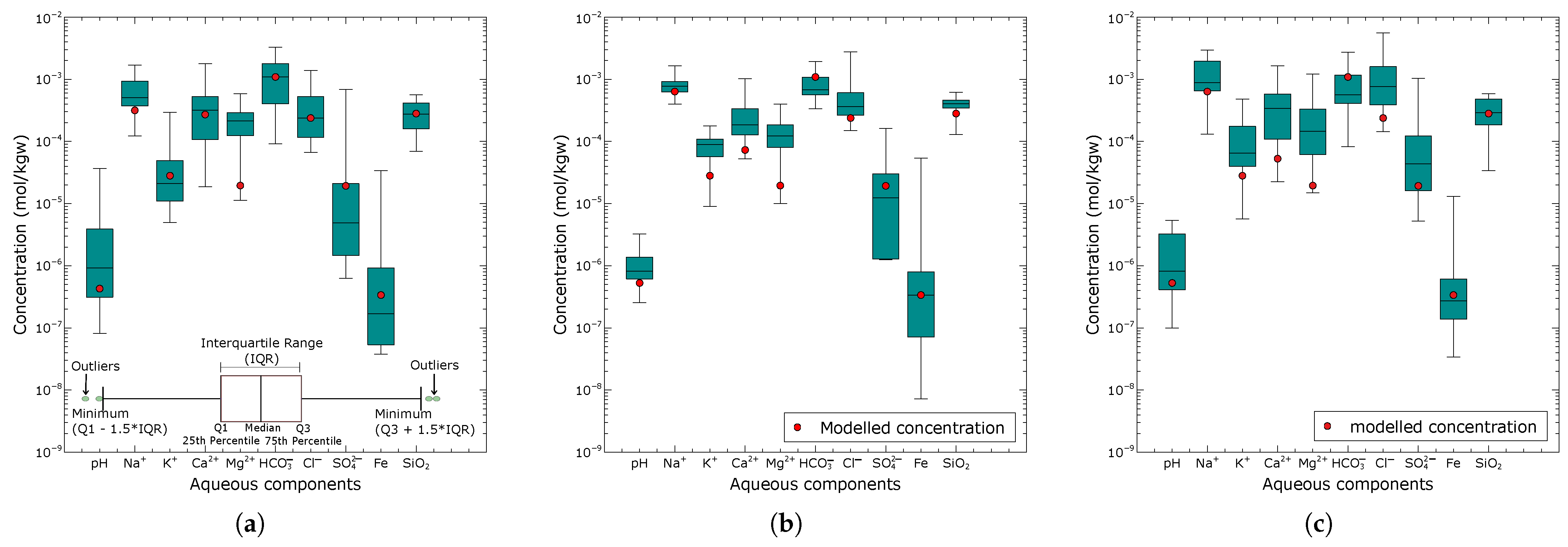
3.1. Mineral Assemblages Identified from the Combinatorial Inverse Modelling
3.2. Reaction Path Modelling
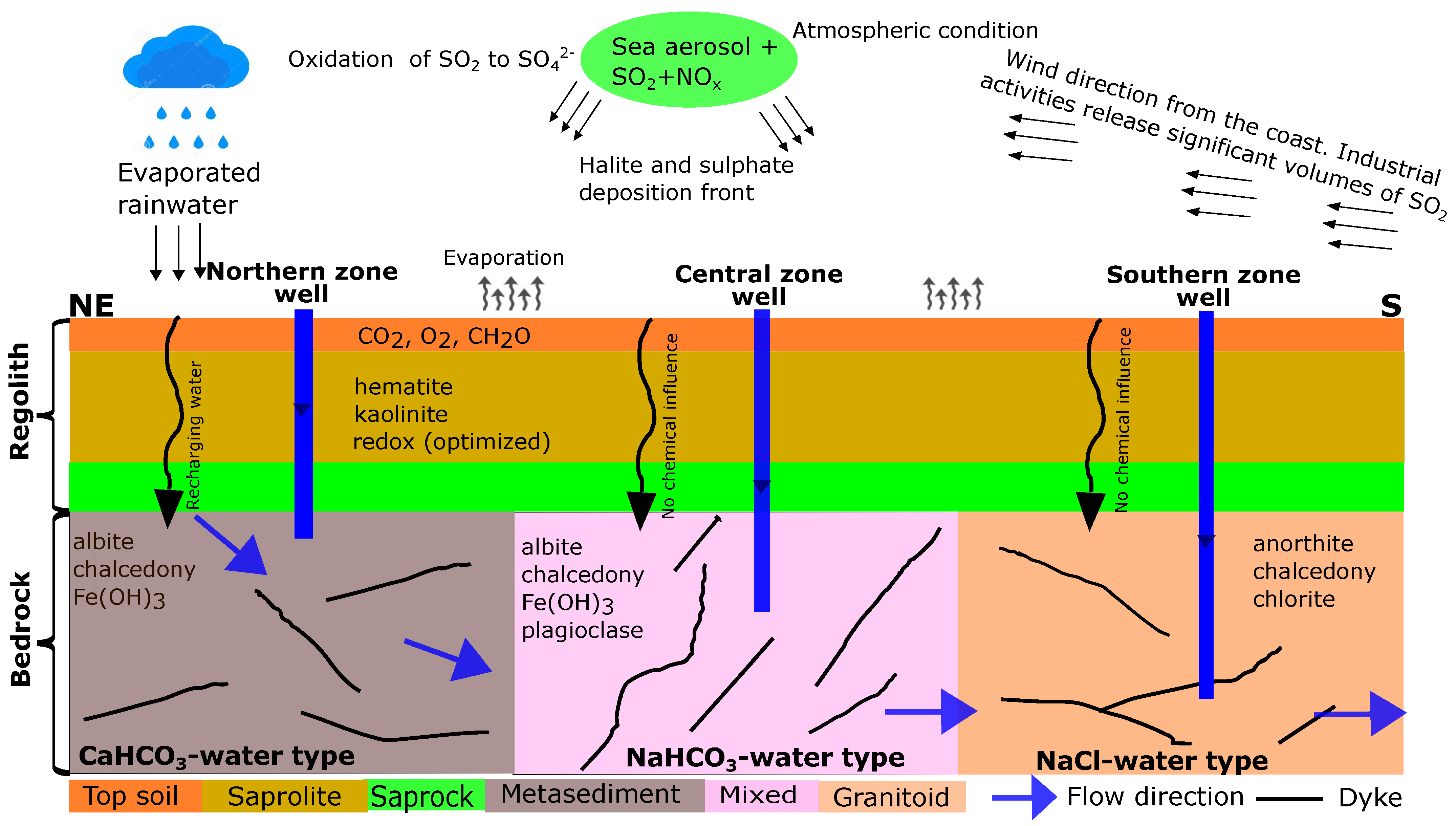
4. Discussion
Model Limitations
5. Conclusions
- The plausible mineral assemblages that best explain the chemical composition of the groundwater in the Pra Basin have been identified. These mineral assemblages, including albite, chalcedony, and for the northern zone, albite, chalcedony, , and plagioclase for the central zone, and anorthite, chalcedony, and chlorite for the southern zone, were found as plausible mineral assemblages governing the dissolved ions in the groundwater, and these assemblages align with petrographic information from outcrops.
- Groundwater chemistry is governed by silicate mineral weathering, with the dissolution of carbonate minerals playing a subordinate role. Based on the results of the combinatorial inverse modelling, it is clear that the majority of the mineral phases commonly found in the models are silicates, including primary albite, anorthite, plagioclase, phlogopite, K-mica, and K-feldspar, as well as secondary chlorite, kaolinite, chalcedony, and quartz. Our model results rarely predicted the occurrence of calcite in the 50 best-matched solutions, thus suggesting that carbonate minerals have less impact on the basin’s groundwater composition.
- The degradation of organic matter primarily controls the reduction in sulfate in the groundwater. This is supported by the observed foul smell of rotten eggs in some of the sampled wells, which indicated the presence of hydrogen sulfide (). Additionally, our modelling results indicate the formation of pyrite, which occurs when produced from organic matter degradation reacts with the dissolved Fe(II) in the groundwater.
- The simulation results revealed that accurate knowledge of aquifer mineralogy is less important than aquatic chemistry, which fortunately is easier to sample and measure. Average water compositions are sufficient to successfully “bridge” the knowledge gap on the large basin scale to come up with distinct “best” mineral assemblages. By leveraging aquatic chemistry, we were able to produce plausible predictions of the mineralogy on a large basin scale with limited knowledge of the mineral compositions obtained from the petrographic analysis of some outcrops in the study area.
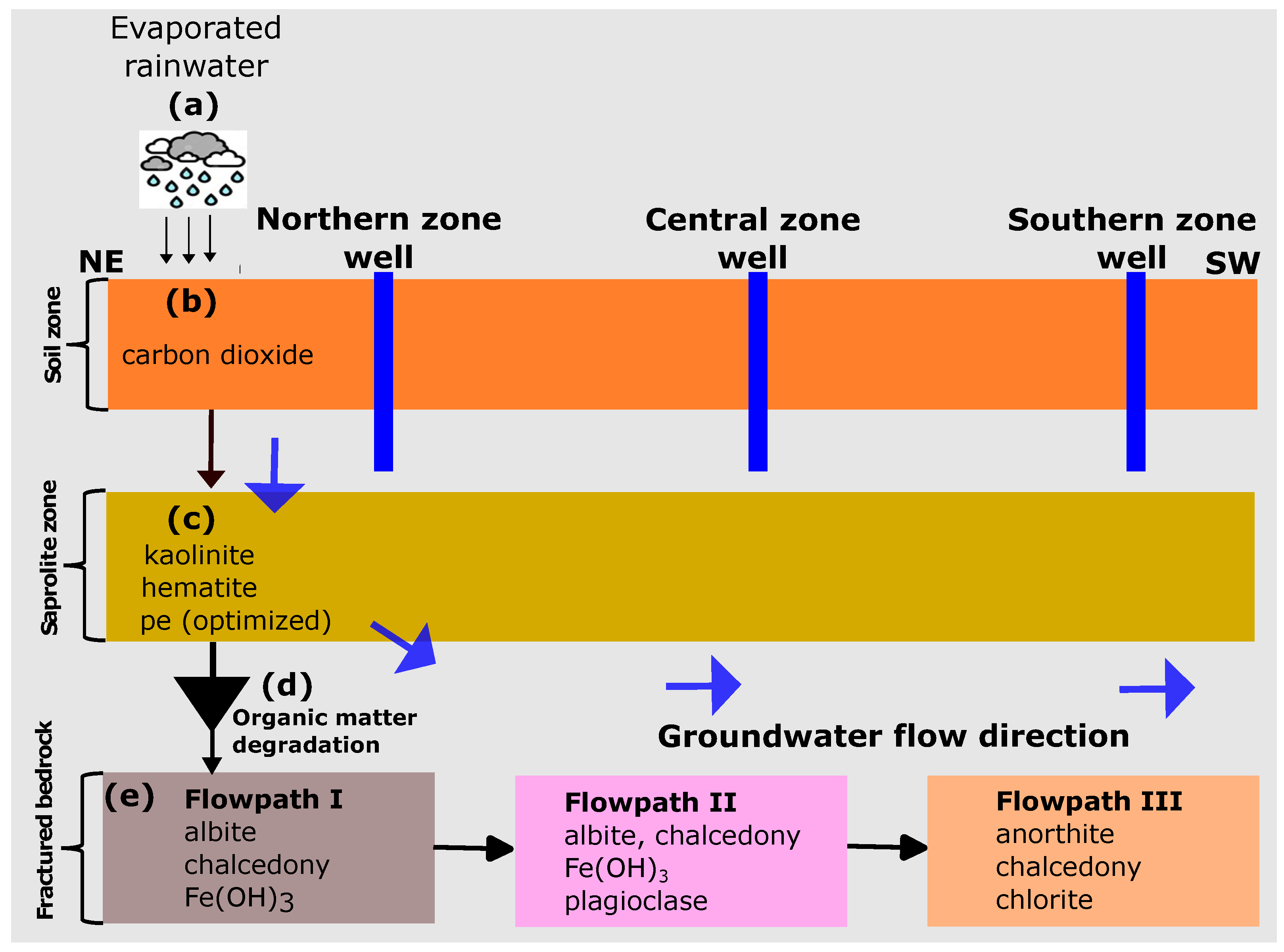
- Equilibrium-based thermodynamic concepts of water–rock interactions were used to quantify the observed hydrochemical variations. However, we acknowledge that the northern zone (Flowpath I), which is assumed to be the recharge area, required additional hypotheses to match the observed composition, thus pointing towards kinetic effects during water–rock interactions. The results of our models emphasized the equilibration of the initial rainwater with the partial pressure of ( atm), followed by the subsequent equilibration of the resulting solution with kaolinite, hematite, and redox optimization (pe = 3.5). Additionally, the calibrated model accounted for the reaction with moles of organic matter.
- A combined interpretation of the combinatorial inverse and reaction path models allowed for the successful development of a conceptual framework of the hydrochemical evolution for the Pra Basin. Based on our models, the main source of groundwater recharge in the Pra Basin is rainwater that has undergone some degree of evaporation. Our hypothesis of geochemical equilibrium among specific mineral assemblages explains the chemical evolution of groundwater from the point of recharge to the point of discharge. Our modelling results indicate that specific reactions play a crucial role in controlling the groundwater evolution in different zones of the basin. In the northern zone, the equilibration of albite, , and chalcedony is highlighted as the primary reaction influencing the groundwater chemistry. The equilibration involving albite, , plagioclase, and chalcedony is identified as an important factor for the central zone. In the southern zone, the equilibrium of anorthite, chalcedony, and chlorite is significant for understanding the groundwater composition.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mineral | Chemical Equation | |
|---|---|---|
| Phlogopite | + 10 = 1 + 1 + 3 + 3 | 41.08 |
| Plagioclase | + 8 = 0.62 + 0.38 + 1.38 + 2.62 | −18.65 |
| Organic matter | + = 4 + 4 + | 4.80 |
References
- Manu, E.; De Lucia, M.; Kühn, M. Hydrochemical characterization of groundwater in the crystalline basement aquifer system in the Pra Basin (Ghana). Water 2023, 15, 1325. [Google Scholar] [CrossRef]
- Tay, C.K.; Kortatsi, B.K.; Hayford, E.; Hodgson, I.O. Origin of major dissolved ions in groundwater within the Lower Pra Basin using groundwater geochemistry, source-rock deduction and stable isotopes of 2 H and 18 O. Environ. Earth Sci. 2014, 71, 5079–5097. [Google Scholar] [CrossRef]
- Affum, A.O.; Dede, S.O.; Nyarko, B.J.B.; Acquaah, S.O.; Kwaansa-Ansah, E.E.; Darko, G.; Dickson, A.; Affum, E.A.; Fianko, J.R. Influence of small-scale gold mining and toxic element concentrations in Bonsa river, Ghana: A potential risk to water quality and public health. Environ. Earth Sci. 2016, 75, 178. [Google Scholar] [CrossRef]
- Bempah, C.K.; Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ. Monit. Assess. 2016, 188, 261. [Google Scholar] [CrossRef]
- Armah, F.A.; Quansah, R.; Luginaah, I. A systematic review of heavy metals of anthropogenic origin in environmental media and biota in the context of gold mining in Ghana. Int. Sch. Res. Not. 2014, 2014, 252148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golow, A.; Mingle, L. Mercury in river water and sediments in some rivers near Dunkwa-on-Offin, an alluvial goldmine, Ghana. Bull. Environ. Contam. Toxicol. 2003, 70, 0379–0384. [Google Scholar] [CrossRef] [PubMed]
- Amonoo-Neizer, E.H.; Amekor, E. Determination of total arsenic in environmental samples from Kumasi and Obuasi, Ghana. Environ. Health Perspect. 1993, 101, 46–49. [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Fynn, O.F.; Manu, E.; Afrifa, G.Y.; Addai, M.O.; Akurugu, B.A.; Yidana, S.M. Groundwater-surface water interactions: Application of hydrochemical and stable isotope tracers to the lake bosumtwi area in Ghana. Environ. Earth Sci. 2022, 81, 518. [Google Scholar] [CrossRef]
- Tay, C.K.; Hayford, E.; Hodgson, I.O.; Kortatsi, B.K. Hydrochemical appraisal of groundwater evolution within the Lower Pra Basin, Ghana: A hierarchical cluster analysis (HCA) approach. Environ. Earth Sci. 2015, 73, 3579–3591. [Google Scholar] [CrossRef]
- Banoeng-Yakubo, B.; Yidana, S.M.; Anku, Y.; Akabzaa, T.; Asiedu, D. Water quality characterization in some Birimian aquifers of the Birim Basin, Ghana. KSCE J. Civ. Eng. 2009, 13, 179–187. [Google Scholar] [CrossRef]
- Loh, Y.S.A.; Addai, M.O.; Fynn, O.F.; Manu, E. Characterisation and quality assessment of surface and groundwater in and around Lake Bosumtwi impact craton (Ghana). Sustain. Water Resour. Manag. 2021, 7, 1–18. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Bloise, L.; Calabrese, E.; Marini, L.; Vespasiano, G.; Muto, F. Geochemical modeling of water-rock interaction processes in the Pollino National Park. Geofluids 2021, 2021, 6655711. [Google Scholar] [CrossRef]
- Elango, L.; Kannan, R. Rock–water interaction and its control on chemical composition of groundwater. Dev. Environ. Sci. 2007, 5, 229–243. [Google Scholar]
- Plummer, L.N.; Prestemon, E.C.; Parkhurst, D.L. An interactive code (NETPATH) for modeling net geochemical reactions along a flow path, version 2.0. Water-Resour. Investig. Rep. 1994, 94, 4169. [Google Scholar]
- Parkhurst, D.L.; Appelo, C. User’s guide to PHREEQC (Version 2): A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Parkhurst, D.L. User’s Guide to PHREEQC: A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations; US Geological Survey; US Department of the Interior: Washington, DC, USA, 1995; pp. 95–4227.
- Bethke, C.M. Geochemical and Biogeochemical Reaction Modeling; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT User’s Guide: A Simulation Program for Non-Isothermal Multiphase Reactive Geochemical Transport in Variable Saturated Geologic Media; Technical Report; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2004.
- De Lucia, M.; Kühn, M. Geochemical and reactive transport modelling in R with the RedModRphree package. Adv. Geosci. 2021, 56, 33–43. [Google Scholar] [CrossRef]
- Dzigbodi-Adjimah, K. Geology and geochemical patterns of the Birimian gold deposits, Ghana, West Africa. J. Geochem. Explor. 1993, 47, 305–320. [Google Scholar] [CrossRef]
- Kesse, G. The Manganese Ore Deposits of Ghana; Ghana Geological Survey Bulletin: Accra, Ghana, 1976; Volume 44. [Google Scholar]
- Kesse, G.O. The Mineral and Rock Resources of Ghana; Ghana Geological Survey Bulletin: Accra, Ghana, 1985. [Google Scholar]
- Leube, A.; Hirdes, W.; Mauer, R.; Kesse, G.O. The early Proterozoic Birimian Supergroup of Ghana and some aspects of its associated gold mineralization. Precambrian Res. 1990, 46, 139–165. [Google Scholar] [CrossRef]
- Nyame, F.K. Origins of Birimian (ca 2.2 Ga) mafic magmatism and the P aleoproterozoic greenstone belt metallogeny: A review. Isl. Arc 2013, 22, 538–548. [Google Scholar] [CrossRef]
- Manu, J.; Hayford, E.; Anani, C.; Kutu, J.M.; Armah, T. Aspects of the chemical composition of the Birimian gold fluid. J. Earth Sci. Geotech. Eng. 2013, 3, 87–106. [Google Scholar]
- Banoeng-Yakubo, B.; Yidana, S.; Ajayi, J.; Loh, Y.; Asiedu, D. Hydrogeology and groundwater resources of Ghana: A review of the hydrogeology and hydrochemistry of Ghana. In Potable Water and Sanitation; McMann, J.M., Ed.; Nova Science: New York, NY, USA, 2010; Volume 142. [Google Scholar]
- Yidana, S.M.; Banoeng-Yakubo, B.; Sakyi, P.A. Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J. Earth Syst. Sci. 2012, 121, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Leube, A.; Hirdes, W. The early Proterozoic (Birimian and Tarkwaian) of Ghana and some aspects of its associated gold mineralizations. Ext. Abstr. Geocongress 1986, 86, 315–319. [Google Scholar]
- Ganyaglo, S.Y.; Banoeng-Yakubo, B.; Osae, S.; Dampare, S.B.; Fianko, J.R.; Bhuiyan, M.A. Hydrochemical and isotopic characterisation of groundwaters in the eastern region of Ghana. J. Water Resour. Prot. 2010, 2, 199. [Google Scholar] [CrossRef] [Green Version]
- Manu, E.; Vieth-Hillebrand, A.; Rach, O.; Schleicher, A.M.; Trumbull, R.; Stammeier, J.A.; Gottsche, A.; Kühn, M. Hydrochemistry and stable oxygen (δ18O) and hydrogen (δ2H) isotopic composition of surface water and groundwater and mineralogy, in the Pra Basin (Ghana) West Africa. GFZ Data Services. 2023. [Google Scholar] [CrossRef]
- Akoto, O.; Darko, G.; Nkansah, M. Chemical composition of rainwater over a mining area in Ghana. Int. J. Environ. Res. 2011, 5, 847–854. [Google Scholar]
- Akoto, O.; Adiyiah, J. Chemical analysis of drinking water from some communities in the Brong Ahafo region. Int. J. Environ. Sci. Technol. 2007, 4, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Thyne, G.; Güler, C.; Poeter, E. Sequential analysis of hydrochemical data for watershed characterization. Groundwater 2004, 42, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Circone, S.; Navrotsky, A. Substitution of [6, 4] Al in phlogopite: High-temperature solution calorimetry, heat capacities, and thermodynamic properties of the phlogopite-eastonite join. Am. Mineral. 1992, 77, 1191–1205. [Google Scholar]
- Appelo, C.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Rotterdam, B., Ed.; CRC Press: London, UK, 2005. [Google Scholar]
- Pitkaenen, P.; Luukkonen, A.; Ruotsalainen, P.; Leino-Forsman, H.; Vuorinen, U. Geochemical Modelling of Groundwater Evolution and Residence Time at the Kivetty Site; Technical Report; Posiva Oy: Eurajoki, Finland, 1998. [Google Scholar]
- Asiedu, D.K.; Dampare, S.B.; Sakyi, P.A.; Banoeng-Yakubo, B.; Osae, S.; Nyarko, B.J.B.; Manu, J. Geochemistry of Paleoproterozoic metasedimentary rocks from the Birim diamondiferous field, southern Ghana: Implications for provenance and crustal evolution at the Archean-Proterozoic boundary. Geochem. J. 2004, 38, 215–228. [Google Scholar] [CrossRef]
- Adiaffi, B.; Marlin, C.; Coulibaly, Y.; Oga, Y.M.S.; Pichon, R. Hydrogeochemistry of Bedrock Groundwater in SE Ivory Coast. Int. J. Emerg. Technol. Adv. Eng. 2016, 6, 1–14. [Google Scholar]
- Appelo, C.; Dimier, A. Geochemistry, Groundwater and Pollution: Learning by Modeling; US Federal Agency Workshop: Albuquerque, NM, USA, 2004.
- Freeze, R.A.; Cherry, J. Groundwater; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Tay, C.K. Hydrochemistry of groundwater in the Savelugu–Nanton District, northern Ghana. Environ. Earth Sci. 2012, 67, 2077–2087. [Google Scholar] [CrossRef]
- Okofo, L.B.; Anderson, N.A.; Bedu-Addo, K.; Armoo, E.A. Hydrochemical peculiarities and groundwater quality assessment of the Birimian and Tarkwaian aquifer systems in Bosome Freho District and Bekwai Municipality of the Ashanti Region, Ghana. Environ. Earth Sci. 2021, 80, 818. [Google Scholar] [CrossRef]

| Prameter | Units | RW | Median | Range | ||||
|---|---|---|---|---|---|---|---|---|
| Northern | Central | Southern | Northern | Central | Southern | |||
| pH | - | 4.7 | 6.5 | 6.1 | 6.0 | 5.9–7.0 | 5.6–6.4 | 5.3–6.4 |
| Temp | °C | ND | 28.1 | 28.4 | 29 | 26.0–29.0 | 28.1–29.6 | 27.8–31.0 |
| mg/L | 0.4 | 11.9 | 17.1 | 44.3 | 3.1–32.4 | 9.2–21.8 | 16.4–67.4 | |
| mg/L | 0.7 | 0.7 | 3.7 | 6.1 | 0.3–8.2 | 0.4–6.3 | 1.2–16.3 | |
| mg/L | 0.8 | 26.4 | 7.2 | 14.8 | 12.6–51.5 | 2.1–13.5 | 3.1 -66.0 | |
| mg/L | 0.3 | 6.4 | 2.5 | 7.2 | 1.6–12.8 | 0.9–4.6 | 1.5–29.4 | |
| mg/L | 6.7 | 108.5 | 3.7 | 28.0 | 37.8–191.0 | 29.3–67.1 | 5.0–134.0 | |
| mg/L | 4.5 | 8.4 | 12.7 | 58.1 | 2.4–49.0 | 6.0–21.5 | 17.5–196.7 | |
| mg/L | 11.1 | 1.1 | 1.6 | 8.6 | 0.4–66.0 | 0.1–4.3 | 1.6–99.4 | |
| mg/L | ND | 0.2 | 0.01 | 0.02 | 0.008–0.8 | 0.001–0.2 | 0.006–0.09 | |
| mg/L | ND | 23.9 | 23.4 | 17.5 | 7.2–33.7 | 13.6–37.3 | 11.8–29.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manu, E.; De Lucia, M.; Kühn, M. Water–Rock Interactions Driving Groundwater Composition in the Pra Basin (Ghana) Identified by Combinatorial Inverse Geochemical Modelling. Minerals 2023, 13, 899. https://doi.org/10.3390/min13070899
Manu E, De Lucia M, Kühn M. Water–Rock Interactions Driving Groundwater Composition in the Pra Basin (Ghana) Identified by Combinatorial Inverse Geochemical Modelling. Minerals. 2023; 13(7):899. https://doi.org/10.3390/min13070899
Chicago/Turabian StyleManu, Evans, Marco De Lucia, and Michael Kühn. 2023. "Water–Rock Interactions Driving Groundwater Composition in the Pra Basin (Ghana) Identified by Combinatorial Inverse Geochemical Modelling" Minerals 13, no. 7: 899. https://doi.org/10.3390/min13070899
APA StyleManu, E., De Lucia, M., & Kühn, M. (2023). Water–Rock Interactions Driving Groundwater Composition in the Pra Basin (Ghana) Identified by Combinatorial Inverse Geochemical Modelling. Minerals, 13(7), 899. https://doi.org/10.3390/min13070899






