Effects of Steel Slag Powder as A Cementitious Material on Compressive Strength of Cement-Based Composite
Abstract
1. Introduction
2. Cementitious Properties of SSP
3. Effect of SSP on Compressive Strength
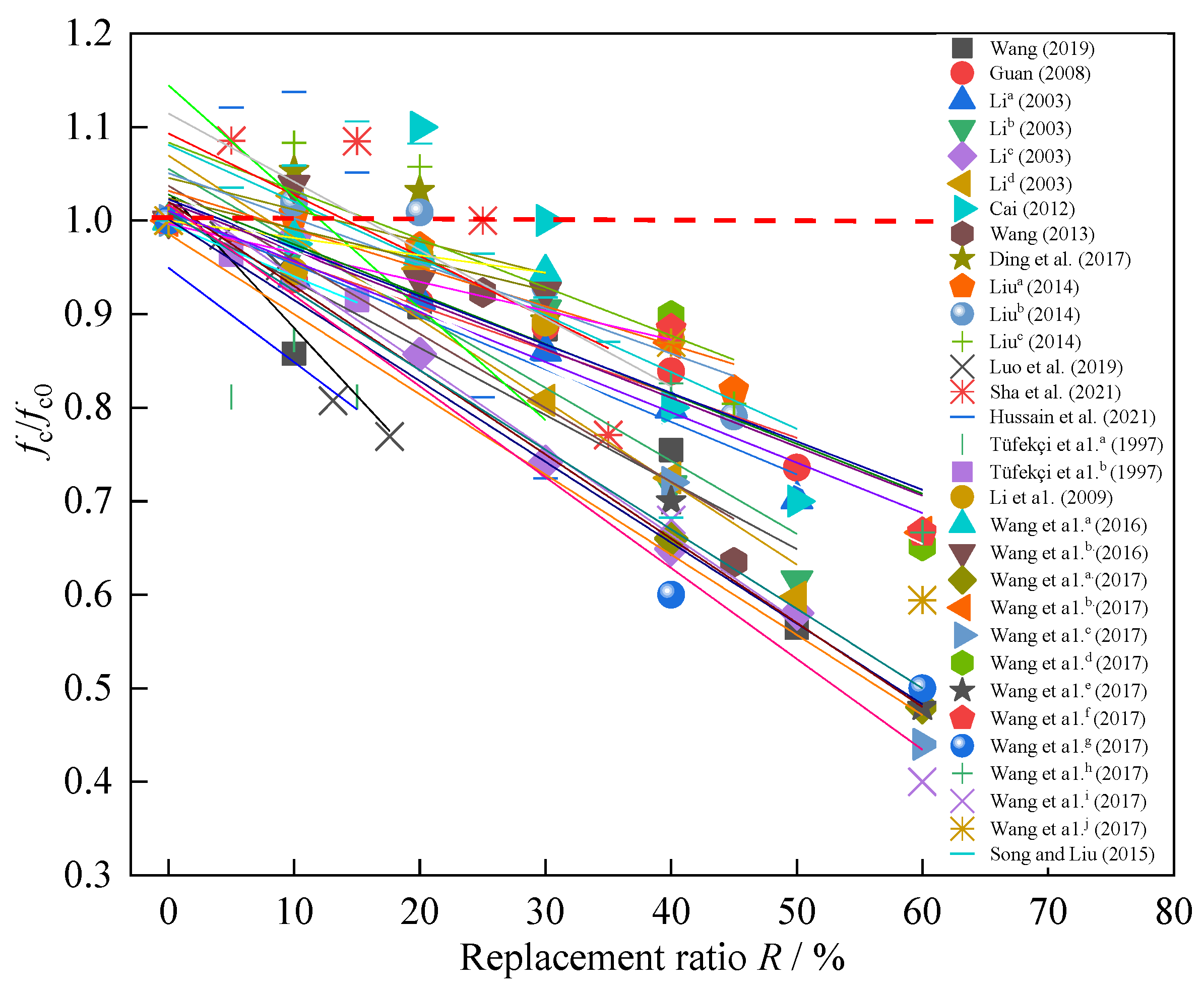
3.1. Replacing Cement with SSP
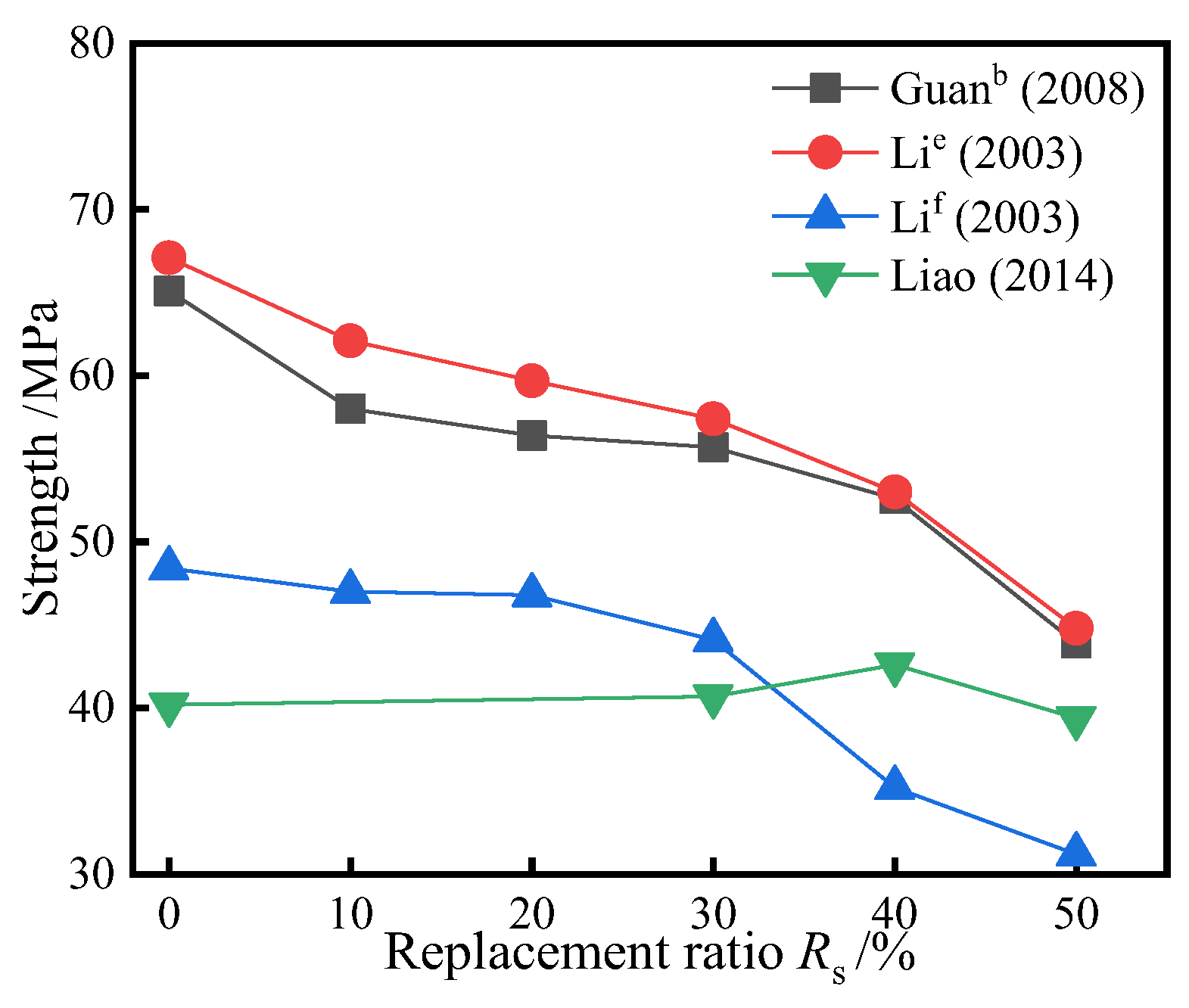
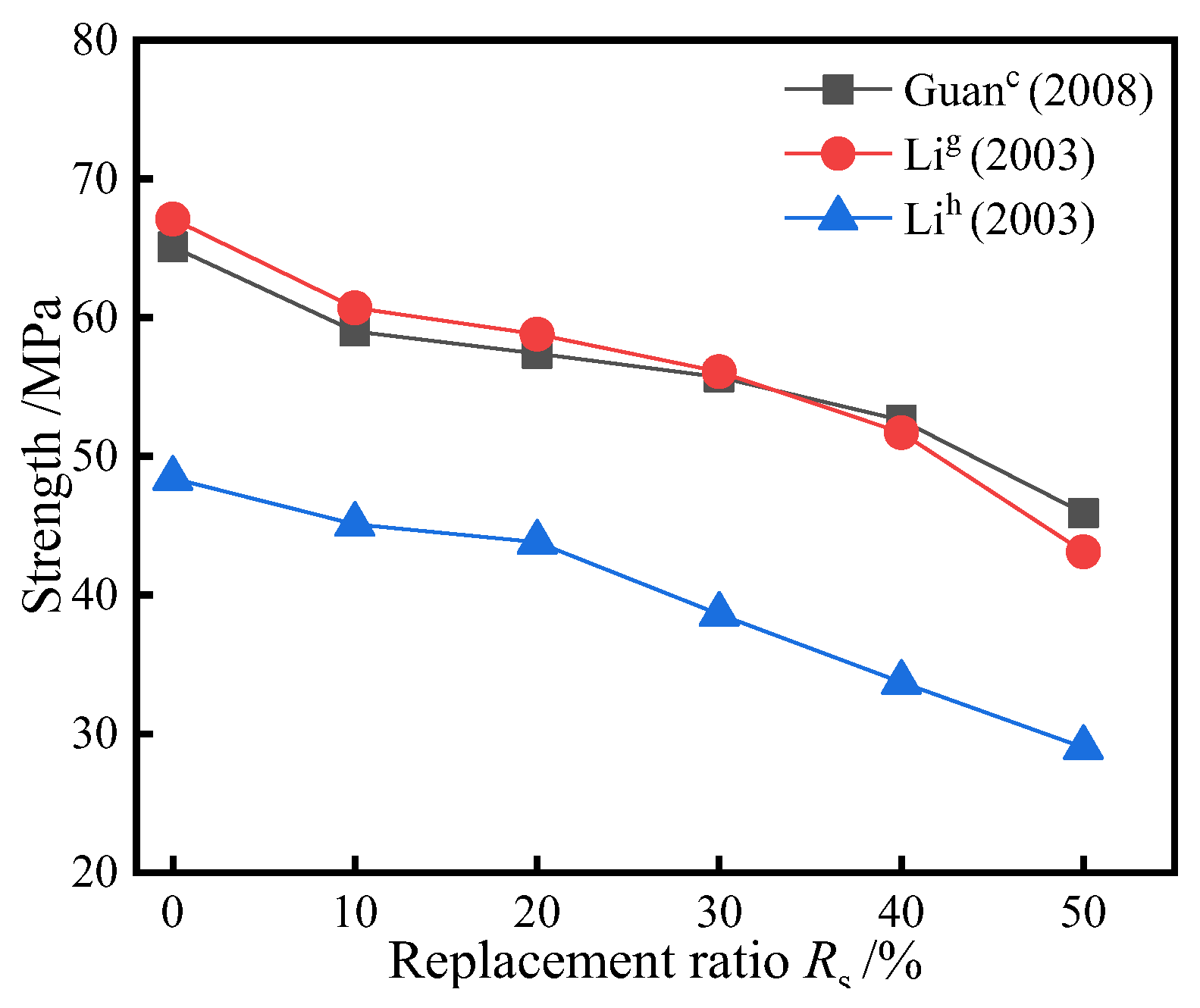
3.2. Replacing Cement with the Mixture of SSP and Slag/Fly Ash
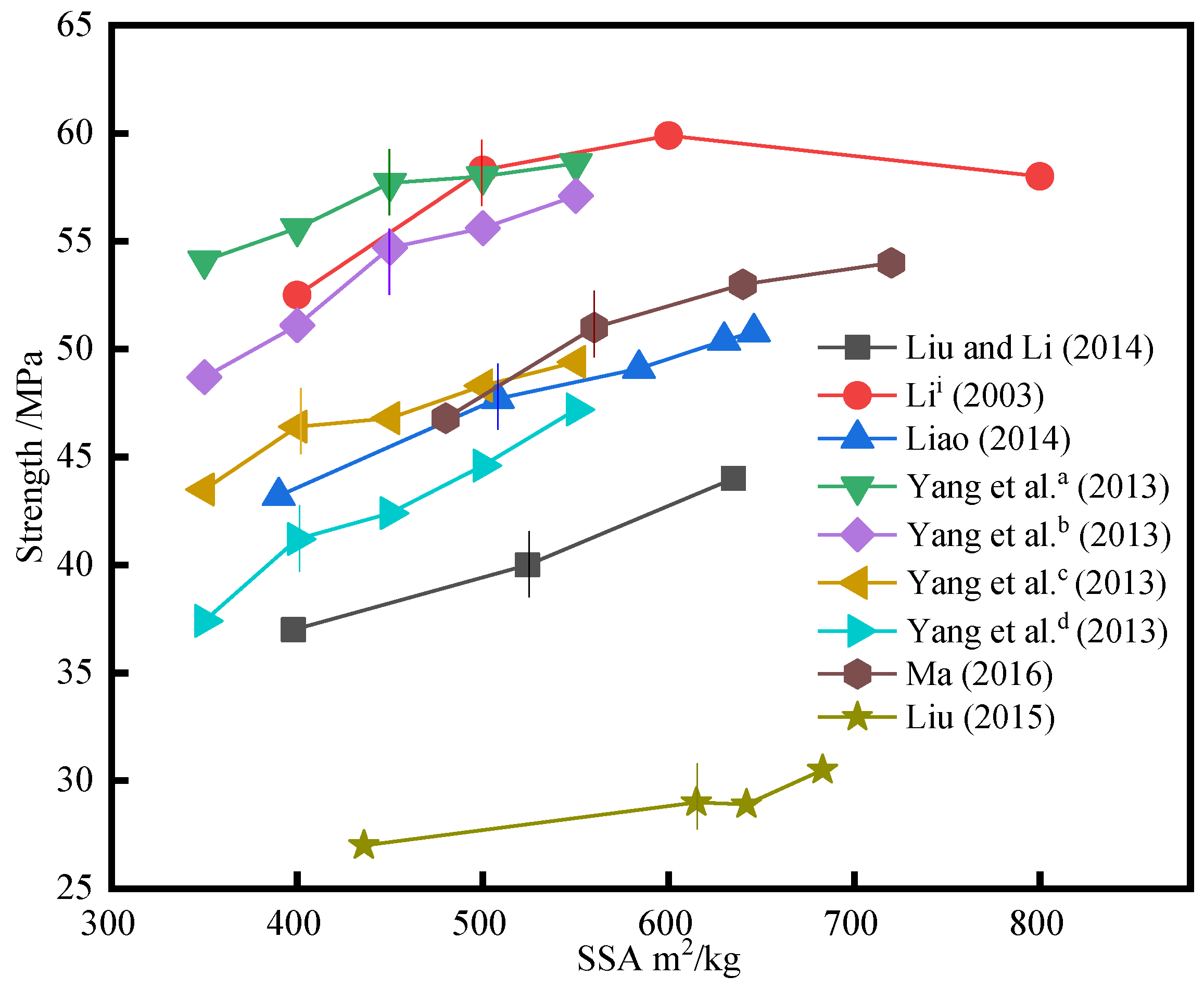
3.3. Effect of Specific Surface Area (SSA) on Strength
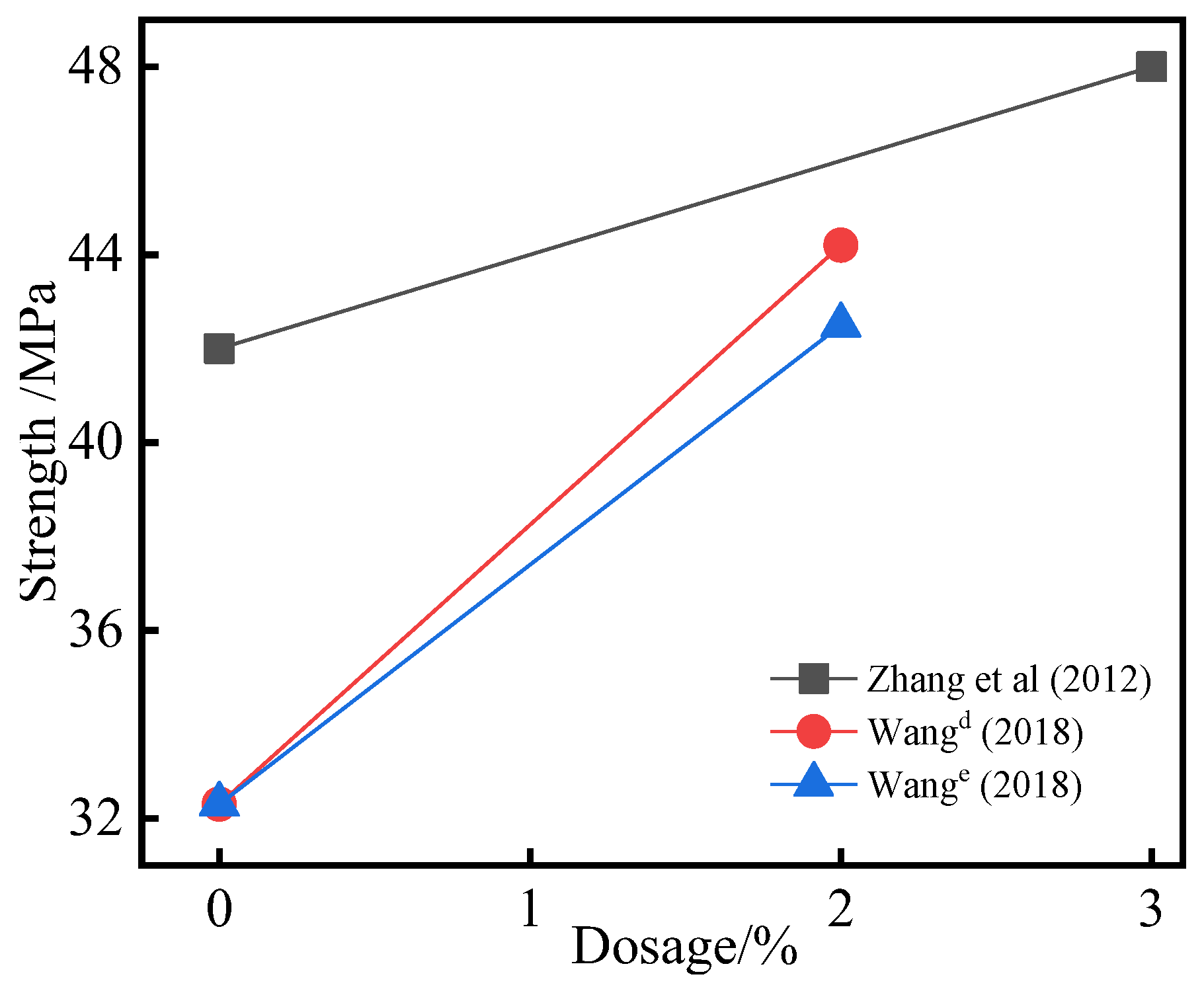
3.4. Effect of Chemical Activated SSP on Strength
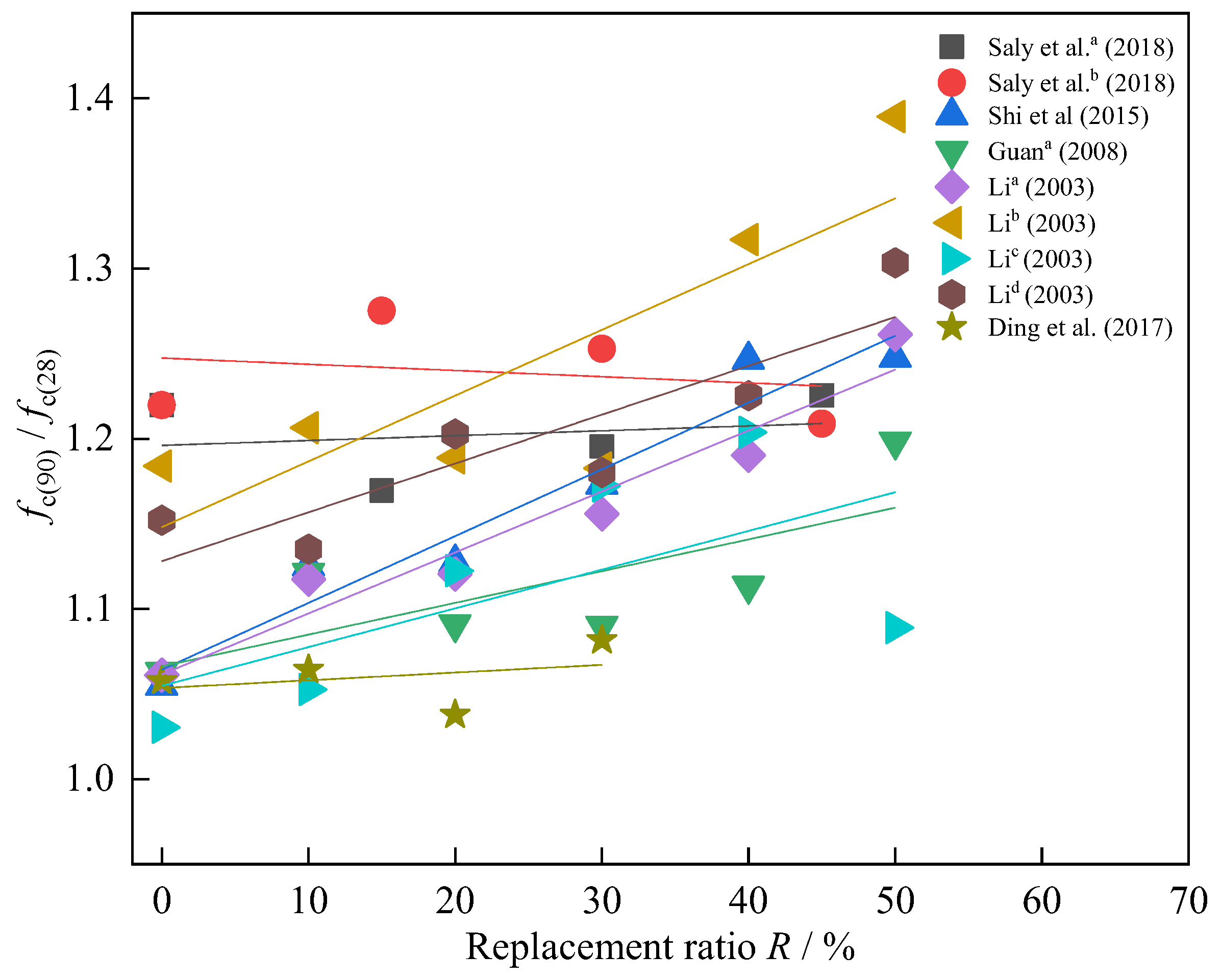
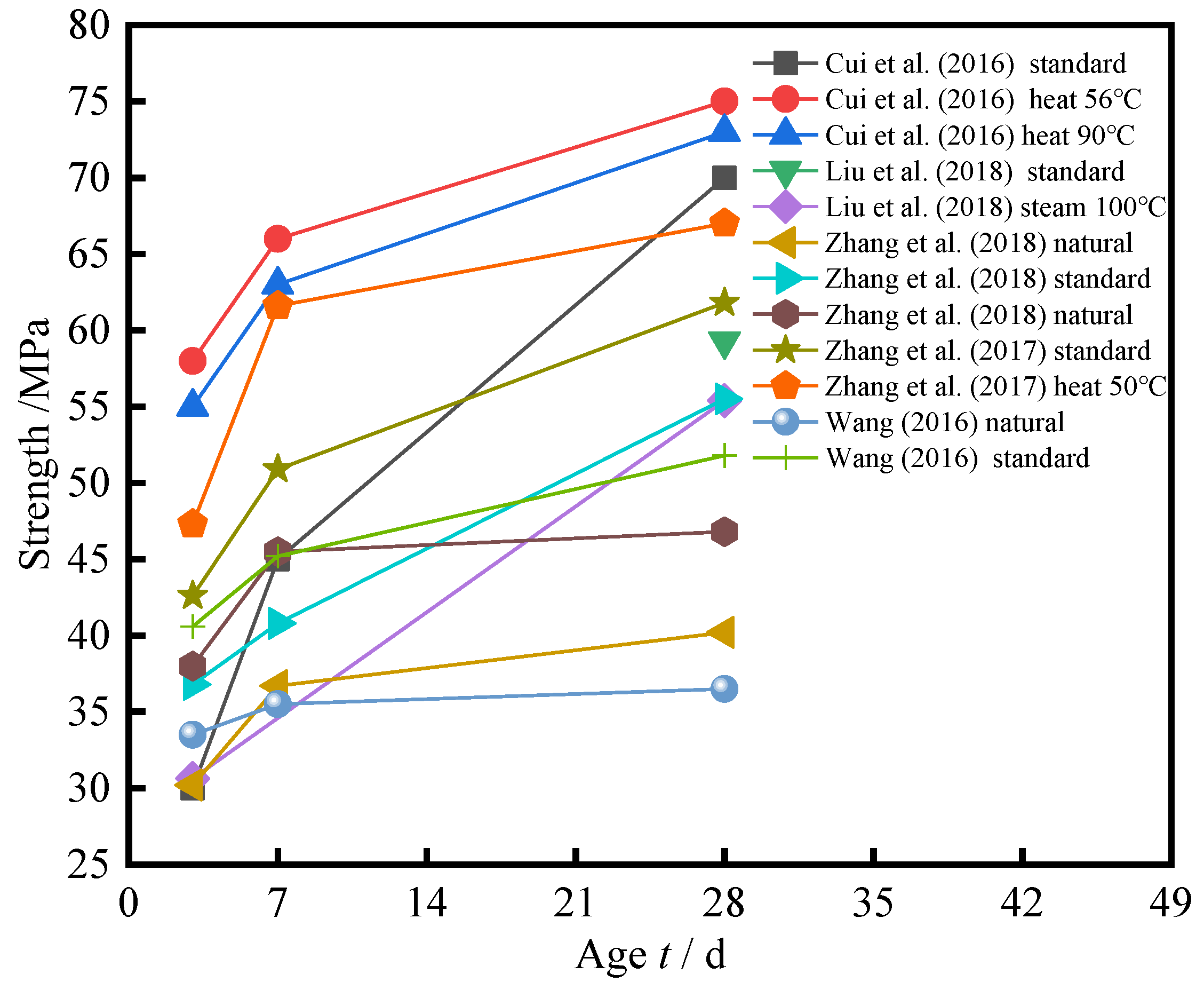
3.5. Effect of Different Curing and Age t on Strength
3.6. Effect of Different R on Heat of Hydration
4. Conclusions
- (1)
- When using SSP to replace cement as a cementitious material, the strength shows an overall decreasing trend with the increase of R. In terms of mineral composition, compared to cement, the active substances in SSP are relatively less, which limits the hydration reaction and leads to insufficient cementitious composition, resulting in a decrease in strength. In terms of physical properties, Due to the particles of SSP are mostly in the form of flakes, which affects fluidity and is unfavorable for forming a denser slurry structure, resulting in a decrease in strength.
- (2)
- When using a mixture of SSP and slag/fly ash instead of cement, the strength of most groups decreases with the increase of Rs, but the downward trend is not significant; This is due to the secondary hydration reaction between silica and alumina in slag and fly ash and calcium hydroxide to generate hydrated calcium silicate. Compared to slag and fly ash, the contribution of SSP to strength is relatively poor.
- (3)
- When only one activator is used for excitation, the compressive strength usually increases first and then decreases within the 4% range as the dosage of a single activator increases. It indicates that there is an optimal amount of SSP excited using a single activator. Within the range of 3%, the effect of mixing multiple activators is better than that of a single activator.
- (4)
- The compressive strength increases with the increase of SSA. This is because mechanical force crushes the active glass crystals in SSP, increasing the contact area between the active substance and water, and improving the hydration reaction. However, when SSA is too high, it will reduce its strength, which is caused by the aggregation effect of SSP. Considering the preparation cost, the optimal range of SSA is 400 m2/kg to 500 m2/kg.
- (5)
- With the increase of age t, the compressive strength of cement-based composite with SSP increases. The increase rate of strength in the first 7 days is significantly greater than that from the 7th to 28th days. The effect of curing methods on compressive strength is in the following order: hot and heat curing > standard curing > natural curing. Due to the possibility of insufficient moisture in steam curing, its effectiveness may be worse than standard curing, and more experiments are needed to verify.
- (6)
- SSP has a significant impact on the hydration heat of composite cementitious systems. With the increase of R, the cumulative hydration heat significantly decreases, which is significantly different from the hydration heat of pure cement. The hydration heat evolution rate decreases with increase of R and also with the increase of age.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faraone, N.; Tonello, G.; Furlani, E.; Maschio, S. Steelmaking Slag as Aggregate for Mortars: Effects of Particle Dimension on Compression Strength. Chemosphere 2009, 77, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Furlani, E.; Tonello, G.; Maschio, S. Recycling of Steel Slag and Glass Cullet from Energy Saving Lamps by Fast Firing Production of Ceramics. Waste Manag. 2010, 30, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Fang, G.; Xia, Y.; Wang, H.Y. Study on Compressive Strength of Concrete Mixed by SSP and Fly Ash. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 508, p. 012183. [Google Scholar]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Salazar, K. Mineral Commodity Summaries 2013: US Geological Survey (USGS); J. US Geological Survey: Reston, VA, USA, 2013.
- Summaries, U.M.C.; Series, U.U. Mineral Commodity Summaries 2019: US Geological Survey (USGS); J. US Geological Survey: Reston, VA, USA, 2019.
- Yang, X.B.; Dong, F.S.; Zhang, X.Z.; Li, C.Z.; Gao, Q. Review on Comprehensive Utilization of Magnesium Slag and Development Prospect of Preparing Backfilling Materials. Minerals 2022, 12, 1415. [Google Scholar] [CrossRef]
- Cheng, X.X.; Yang, Q.B. Comprehensive Utilization of Steel Slag. Chin. Fly Ash Compr. Util. 2010, 5, 45–49. [Google Scholar]
- Motz, H.; Geiseler, J. Products of Steel Slags an Opportunity to Save Natural Resources. Waste Manag. 2001, 3, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, W.; Hu, Y.; Wu, J.Q.; Li, M.K.; Yang, X.W.; Wang, W.Y.; Xu, M. Enhanced Performance of Extruded-spheronized Carbide Slag Pellets for High Temperature CO2 Capture. Chem. Eng. J. 2016, 285, 293–303. [Google Scholar] [CrossRef]
- Shi, C.; Qian, J. High Performance Cementing Materials from Industrial Slags—A Review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Phan, D.H.; Mai, H.H.; Nguyen, D.L. Investigation on Compressive Characteristics of Steel-slag Concrete. Materials 2020, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A.G. Accelerated Carbonation and Performance of Concrete Made with Steel Slag as Binding Materials and Aggregates. Cem. Concr. Comp. 2017, 83, 138–145. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z. Effect of Silicate Modulus of Water Glass on the Hydration of Alkali-activated Converter Steel Slag. J. Therm. Anal. Calorim. 2019, 138, 47–56. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration Properties and Microstructure Characteristics of Alkali–activated Steel Slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Sheng, G.H.; Jin, S.J.; Li, C.; Bai, Q.; Wang, X.Y. Compressive-Tensile Mechanics and Energy Consumptions of a Cementitious Composite with High Utilization of Steel Slag. KSCE J. Civ. Eng. 2023, 27, 1236–1248. [Google Scholar] [CrossRef]
- Khater, A.M.; Soliman, A.; Ahmed, T.S.; Ismail, I.M. Power Generation in White Cement Plants from Waste Heat Recovery Using Steam-organic Combined Rankine cycle. Case Stud. Chem. Environ. Eng. 2021, 4, 100138. [Google Scholar] [CrossRef]
- Sahoo, N.; Kumar, A.; Samsher, S. Review on Energy Conservation and Emission Reduction Approaches for Cement Industry. Environ. Dev. 2022, 44, 100767. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Kucharski, P.; Smolinski, A. Conventional and Alternative Sources of Thermal Energy in the Production of Cement—An Impact on CO2 Emission. Energies 2021, 14, 1539. [Google Scholar] [CrossRef]
- Dinga, C.D.; Wen, Z. China’s Green Deal: Can China’s Cement Industry Achieve Carbon Neutral Emissions by 2060. Renew. Sust. Energ. Rev. 2022, 155, 111931. [Google Scholar] [CrossRef]
- Shi, C. Steel Slag—Its Production, Processing, Characteristics, and Cementitious Properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Zhang, G.; Ding, Q.J. Bonding and Abrasion Resistance of Geopolymeric Repair Material Made with Steel Slag. Cem. Concr. Comp. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Tüfekçi, M.; Demirbaş, A.; Genc, H. Evaluation of Steel Furnace Slags as Cement Additives. Cem. Concr. Res. 1997, 27, 1713–1717. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration Properties of Basic Oxygen Furnace Steel Slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Wang, D. Research on Mineral Characteristics of Converter Steel Slag and its Comprehensive Utilization of Internal and External Recycle. J. Clean Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Influence of Fineness on the Cementitious Properties of Steel Slag. J. Therm Anal. Calorim. 2014, 117, 629–634. [Google Scholar] [CrossRef]
- Oluwasola, E.A.; Hainin, M.R.; Aziz, M.M.A.; Yaacob, H.; Warid, M.N.M. Potentials of Steel Slag and Copper Mine Tailings as Construction Materials. Mater. Res. Innov. 2014, 18, S6-250–S6-254. [Google Scholar] [CrossRef]
- Pyzalski, M.; Dabek, J.; Adamczyk, A.; Brylewski, T. Physicochemical Study of the Self-Disintegration of Calcium Orthosilicate (β→γ) in the Presence of the C12A7 Aluminate Phase. Materials 2021, 14, 6459. [Google Scholar] [CrossRef]
- Chai, C.; Cheng, Y.C.; Zhang, Y.W.; Zhu, B.; Liu, H. Mechanical Properties of Crumb Rubber and Basalt Fiber Composite Modified Porous Asphalt Concrete with Steel Slag as Aggregate. Polymers 2020, 12, 2552. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, H.; Hou, X.K.; Li, H.S. Study on Steel Slag and Fly Ash Composite Portland Cement. Cem. Concr. Res. 1999, 29, 1103–1106. [Google Scholar]
- Gan, W.G.; Zhou, G.M. Comparison of Components and Structural Characteristics of Different Types of Steel Slag and Technical Characteristics of Steel Slag Powder. Chin. Cem. Guide New Epoch. 2022, 28, 1–5. [Google Scholar]
- Guo, X.; Shi, H. Modification of SSP by Mineral Admixture and Chemical Activators to Utilize in Cement-based Materials. Mater. Struct. 2013, 46, 1265–1273. [Google Scholar] [CrossRef]
- Muhmood, L.; Vitta, S.; Venkateswaran, D. Cementitious and Pozzolanic Behavior of Electric Arc Furnace Steel Slags. Cem. Concr. Res. 2009, 39, 102–109. [Google Scholar] [CrossRef]
- Saly, F.; Guo, L.P.; Ma, R.; GU, C.P.; Sun, W. Properties of Steel Slag and Stainless Steel Slag as Cement Replacement Materials: A Comparative Study. J. Wuhan. Univ. Technol. 2018, 33, 1444–1451. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, S.; Ding, Q. Preparation of Reactive Powder Concrete Using Fly Ash and Steel Slag Powder. J. Wuhan. Univ. Technol. 2010, 25, 349–354. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R. Applications of SSP and Steel Slag Aggregate in Ultra-high Performance Concrete. Adv. Civ. Eng. 2018, 2018, 1–8. [Google Scholar]
- Shi, Y.; Chen, H.; Wang, J.; Feng, Q.M. Preliminary Investigation on the Pozzolanic Activity of Superfine Steel Slag. Constr. Build. Mater. 2015, 82, 227–234. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D.; Luo, D. Enhanced Hydration and Mechanical Properties of Cement-based Materials with Steel Slag Modified by Water Glass. J. Mater. Res. Technol. 2022, 21, 1830–1842. [Google Scholar] [CrossRef]
- Xu, G.; He, X.; He, Y. Effect of Steel Slag and Granulated Blast-furnace Slag on the Mechanical Strength and Pore Structure of Cement Composites. J. Wuhan. Univ. Technol. 2018, 33, 1186–1192. [Google Scholar] [CrossRef]
- Gan, L.; Wang, H.; Li, X.; Qi, Y.; Zhang, C. Strength Activity Index of Air Quenched Basic Oxygen Furnace Steel Slag. J. Iron. Steel. Res. Int. 2015, 22, 219–225. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J. Investigation on Mechanical Properties, Durability and Micro-structural Development of Steel Slag Blended Cements. J. Therm. Anal. Calorim. 2012, 110, 633–639. [Google Scholar] [CrossRef]
- Altun, I.A.; Yılmaz, İ. Study on Steel Furnace Slags with High MgO as Additive in Portland Cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Da Silva Magalhães, M.; Faleschini, F.; Pellegrino, C.; Pellegrino, C.; Brunelli, K. Influence of alkali addition on the setting and mechanical behavior of cement pastes and mortars with electric arc furnace dust. Constr. Build. Mater. 2019, 214, 413–419. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Feng, J. A Discussion on Improving Hydration Activity of Steel Slag by Altering its Mineral Compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, P.; Mi, G. Effect of Blended Steel Slag–GBFS Mineral Admixture on Hydration and Strength of Cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Wang, R. Influence of Steel Slag Powder on Concrete Performance. Chin. J. Shandong Agric. Univ. (Nat. Sci. Ed.). 2019, 50, 221–224. [Google Scholar]
- Guan, S.P. Research on the Active & Cementitious Capacity of Steel-Making Slag and its Concrete Properties; China Wuhan University of Technology: Wuhan, China, 2008. [Google Scholar]
- Li, Y.X. Study on the Composition, Structure and Performance of Cement and Concrete with Steel-Making Slag Powder Mineral Additive; China Building Materials Academy: Beijing, China, 2003. [Google Scholar]
- Cai, Q.Y. Study on the Effect of Ground Steel Slag Powder on the Properties of Cement Concrete. Chin. China Concr. Cem. Prod. 2012, 5, 5–8. [Google Scholar]
- Wang, Q.; Yang, J.; Yan, P. Cementitious Properties of Super-fine Steel Slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Ding, T.T.; Li, Q.H.; Cheng, H.D. Effect of Steel Slag on the Mechanical Properties and Durability of Concrete. Chin. Bull. Chin. Ceram. Soc. 2017, 36, 1723–1727. [Google Scholar]
- Liu, P. Study on the Compressive Strength and the Early Crack Resistance of Steel Slag Concrete; China Xinjiang Agricultural University: Urumqi, China, 2014. [Google Scholar]
- Luo, X.; Si, Y.; Gu, W. Effect of Silica Fume on Mechanical Properties of Concrete Incorporating SSP. J. Wuhan Univ. Technol. 2019, 24, 86–92. [Google Scholar] [CrossRef]
- Sha, F.; Liu, P.; Ding, Y. Application Investigation of High-phosphorus Steel Slag in Cementitious Material and Ordinary Concrete. J. Mater. Res. Technol. 2021, 11, 2074–2091. [Google Scholar] [CrossRef]
- Hussain, I.; Ali, B.; Rashid, M.U.; Amir, M.T.; Riaz, S.; Ali, A. Engineering Properties of Factory Manufactured Paving Blocks Utilizing Steel Slag as Cement Replacement. Case. Stud. Constr. Mat. 2021, 15, e00755. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Wang, L. Recycling of Industrial Waste and Performance of Steel Slag Green Concrete. J. Cent. South Univ. T 2009, 16, 768–773. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, M.X.; Yang, J. Influence of Classified Steel Slag with Particle Sizes Smaller than 20 μm on the Properties of Cement and Concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar]
- Wang, Q.; Wang, D.; Zhuang, S. The Soundness of Steel Slag with Different Free CaO and MgO Contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Song, K.; Liu, F.T. Research on the Influence of Steel Slag Powder, Slag Power, and Fly Ash on the Performance of Concrete. In Proceedings of the 2015 4th International Conference on Mechatronics, Materials, Chemistry and Computer Engineering, Xi’an, China, 12–13 December 2015; Atlantis Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and Hydration of Blended Cements with Steel-making Slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Young, J.F.; Berger, R.L.; Breese, J. Accelerated Curing of Compacted Calcium Silicate Mortars on Exposure to CO2. J. Am. Ceram. Soc. 1974, 57, 394–397. [Google Scholar] [CrossRef]
- Monshi, A.; Asgarani, M.K. Producing Portland Cement from Iron and Steel Slags and Limestone. Cem. Concr. Res. 1999, 29, 1373–1377. [Google Scholar] [CrossRef]
- Dunstan, E.R. How Does Pozzolanic Reaction Make Concrete Green. In Proceedings of the 2011 World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011; pp. 1–14. [Google Scholar]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A Hydration Study of Various Calcium Sulfoaluminate Cements. Cement Concrete Comp. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Jörg Müller, C. Beneficial Use of Limestone Filler with Calcium Sulphoaluminate Cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, X.B.; Yao, Y.H.; Cui, S.P.; Wei, Q.; Hao, L.W. Research Progress of Slag Structure and Hydration Activity. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2021; Volume 1035, pp. 972–979. [Google Scholar]
- Hayet, C.; Ammar, N.; Nacira, S.; Khedidja, A.M. Mechanical Properties of Slag Sand Mixture used in Road Pavements. Mag. Civ. Eng. 2021, 8, 10806. [Google Scholar]
- Erlin, B.; Jana, D. Forces of Hydration That Can Cause Havoc in Concrete. Concr. Int. 2003, 25, 51–57. [Google Scholar]
- Li, Z.; Zhao, S.; Zhao, X.; He, T. Cementitious property modification of basic oxygen furnace steel slag. Constr. Build. Mater. 2013, 48, 575–579. [Google Scholar] [CrossRef]
- Liao, L. Study on the Gelation Properties of Composite of Steel Slag and Blast Furnace Slag, and the Reach of its Application for Marine Environment; China Jinan University: Jinan, China, 2014. [Google Scholar]
- Shi, Y. Research on the Cementitious Properties of Superfine Steel Slag and the Utilization in Concrete; Southwest University of Science and Technology: Mianyang, China, 2015. [Google Scholar]
- Yang, J.W. Influence of Steel Slag and Composite Mineral Admixture Containing Steel Slag on the Properties of Concrete; China Tsinghua University: Beijing, China, 2013. [Google Scholar]
- Song, K.Q. Study on the Effect of Steel Slag Powder on the Performance of Road Concrete; China Jinan of University: Jinan, China, 2016. [Google Scholar]
- Wang, Q.; Li, M.Y.; Shi, M.X. Hydration Properties of Cement-Steel Slag-Ground Granulated Blast Furnace Slag Complex Binder. Chin. J. Chin. Ceram. Soc. 2014, 42, 629–634. [Google Scholar]
- Liu, F. Effect of Steel Slag Powder on Performance of Cement Based Materials; China Wuhan University of Technology: Wuhan, China, 2015. [Google Scholar]
- Li, Y.F.; Wang, L.; Lin, H. Workability and Mechanical Properties of Concrete with Steel Slag Powder. Chin. Concrete 2008, 09, 38–40. [Google Scholar]
- Song, K.Q.; Liu, F.T.; Zhao, T.J.; Liu, L.Y. Influence of Steel Slag and Fly Ash on Concrete Characteristic and Its Hydration Mechanism. In Proceedings of the Energy, Environmental & Sustainable Ecosystem Development: International Conference on Energy, Environmental & Sustainable Ecosystem Development (EESED2015), Kunming, China, 26–28 August 2016. [Google Scholar]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation Mechanisms of Three Types of Industrial by-product Gypsums on Steel Slag–granulated Blast Furnace Slag-based Binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Guilmeau, E.; Funahashi, R.; Mikami, M.; Chong, K.; Chateigner, D. Thermoelectric Properties–texture Relationship in Highly Oriented Ca3Co4O9 Composites. Appl. Phys. Lett. 2004, 85, 1490–1492. [Google Scholar] [CrossRef]
- Ren, Q. Influence of Steel Slag Powder and Iron Oxide on the Performance of Concrete; China Zhejiang University: Hangzhou, China, 2018. [Google Scholar]
- Celik, I.B. The Effects of Particle Size Distribution and Surface Area upon Cement Strength Development. Powder. Technol. 2009, 188, 272–276. [Google Scholar] [CrossRef]
- Li, Q.L.; Chen, M.Z.; Liu, F.; Wu, S.P.; Sang, Y. Effect of Superfine Blast Furnace Slag Powder on Properties of Cement-based Materials. Mater. Res. Innov. 2015, 19, S1-168–S1-171. [Google Scholar] [CrossRef]
- Yang, X.J.; Zhou, H.Q.; Han, C.J.; Tang, Y.; He, C.M. Study on the Effect of Specific Surface Area and Content of Steel Slag on Cement Properties. Chin. Dev. Guide Build. Mater. 2013, 11, 42–45. [Google Scholar]
- Ma, X.M.; Ni, W.; Liu, X. Experimental Study on Performance Optimization of Steel Slag Powder and Preparation of Non-Clinker Concrete. Chin. Mater. Rev. 2016, 30, 135–140. [Google Scholar]
- Liu, F.; Chen, M.Z.; Li, F.Z.; Li, Q.L.; Wu, S.P.; Sang, Y. Effect of Ground Steel Slag Powder on Cement Properties. Mater. Res. Innov. 2015, 19, S1-150–S1-153. [Google Scholar] [CrossRef]
- Singh, S.K.; Vashistha, P. Development of Newer Composite Cement Through Mechano-chemical Activation of Steel Slag. Constr. Build. Mater. 2021, 268, 121147. [Google Scholar] [CrossRef]
- Hu, J. Comparison between the Effects of Superfine Steel Slag and Superfine Phosphorus Slag on the Long-term Performances and Durability of Concrete. J. Therm. Anal. Calorim. 2017, 128, 1251–1263. [Google Scholar] [CrossRef]
- Wang, K.X.; Long, H.M.; Meng, Q.M.; Wei, R.F.; Zhang, H. Steel slag Cementitious Activity and Mechanism based on Physical Excitation. Chin. Iron Steel. 2018, 53, 82–86. [Google Scholar]
- Zhao, F.C.; Ju, J.T.; Liao, J.L.; Kong, W.M.; Dang, Y.J. Analysis of Comprehensive Utilization and Basic Properties of Converter Slag processed. Chin. J. Iron Steel Res. 2013, 25, 23–28. [Google Scholar]
- Wang, Z. Research on Steel Slag Used as Cement Mixture; China Hebei University of Engineering: Handan, China, 2020. [Google Scholar]
- Davidovits, J. Geopolymers and Geopolymeric Materials. J. Therm. Anal Calorim. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated Binders: A Review: Part 1. Historical Background, Terminology, Reaction Mechanisms and Hydration Products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated Fly Ashes: A cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Wang, Y. Study on Active Excitation of Steel Slag and Application of Building Materials; China Anhui University of Science and Technology: Hefei, China, 2018. [Google Scholar]
- Dong, L.R. Preparation of Low-Clinker Cement with Steel Slag and Application in Concrete; China Inner Mongolia University of Science & Technology: Baotou, China, 2020. [Google Scholar]
- Ionescu, D.; Meadowcroft, T.R.; Barr, P.V. Early-age Hydration Kinetics of Steel Slags. Adv. Cem. Res. 2001, 13, 21–30. [Google Scholar] [CrossRef]
- Cerulli, T.; Pistolesi, C.; Maltese, C.; Salvioni, D. Durability of Traditional Plasters with Respect to Blast Furnace Slag-based Plaster. Cem. Concr. Res. 2003, 33, 1375–1383. [Google Scholar] [CrossRef]
- Hu, S.G.; Wei, J.X.; Ding, Q.J. Study on the Excitation Mechanism of Water Glass on Steel Slag Cement. Chin. Cem. Eng. 2001, 5, 4–6+52. [Google Scholar]
- Feldman, R.F.; Carette, G.G.; Malhotra, V.M. Studies on Mechanics of Development of Physical and Mechanical Properties of High-volume Fly Ash-cement Pastes. Cement. Concr. Comp. 1990, 12, 245–251. [Google Scholar] [CrossRef]
- Wu, F.H.; Wang, Y.; Zhang, C.S.; Yang, F.L.; Hu, Z.C.; Sheng, D. Effect of Activator on Gelling Activity and Microstructure of Steel Slag. Chin. Concr. 2019, 12, 99–102. [Google Scholar]
- Cui, X.W.; Di, Y.Q.; Xu, C.Y. Preparation of High-Performance Concrete Mixed with Steel Slag Powder and Tailings. Chin. China Concr. Cem. Prod. 2016, 9, 78–81. [Google Scholar]
- Liu, H.; Shen, Y.C.; Deng, Z.; Zhou, H.W.; Jin, Q.; Zhang, Z.Z. Effect of Mineral Admixtures and Steam Curing Methods on The Mechanical Properties of Steel Slag Concrete with Pipe Segments. Chin. China Concr. Cem. Prod. 2018, 6, 35–38. [Google Scholar]
- Zhang, Z.Z.; Jin, Q.; He, J.C.; Liu, H.; Li, H.G. Different Ways of Curing Effect on the Properties of Steel Slag Concrete Compressive Strength. Chin. Fly Ash Compr. Util. 2017, 2, 12–16. [Google Scholar]
- Zhang, Z.Z.; Feng, Y.; Jin, Q.; He, J.C.; Liu, H. Mechanical Properties of Steel-Slag Concrete Contained Different Mineral Admixtures. Chin. Concr. 2017, 11, 110–113. [Google Scholar]
- Wang, F.H. The Experimental Research on Physical Mechanical Properties and Frost Resistance of Mineral Admixture to Steel Slag Concrete; China Xinjiang Agricultural University: Urumqi, China, 2016. [Google Scholar]
- Han, F.H.; Zhang, Z.Q.; Wang, D.M.; Yan, P.Y. Hydration Heat Evolution and Kinetics of Blended Cement Containing Steel Slag at Different Temperatures. Thermochim Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Zhuang, S.Y.; Wang, Q. Inhibition Mechanisms of Steel Slag on the Early-Age Hydration of Cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]




| CaO | SiO2 | Al2O3 | FeO | Fe2O3 | MgO |
|---|---|---|---|---|---|
| 45~60 | 10~15 | 1~5 | 7~20 | 3~9 | 3~13 |
| Reference | R (%) | Strength (Mpa) | Type | Key Conclusions of Different Replacement Ratio R |
|---|---|---|---|---|
| Guo and Shi [32] | 0~50 | 60~32 | paste | With the increase of R, the strength decreases. |
| Muhmood et al. a [33]. | 0~30 | 66.5~53.4 | paste | With the increase of R, the strength decreases. |
| Muhmood et al. b [33]. | 0~30 | 66.5~51.4 | ||
| Liu and Li [26] | 0~45 | 57~37 | mortar | With the increase of R, the strength decreases. |
| Saly et al. a [34] | 0~45 | 48.7~31.5 | mortar | The strengths are lower than that of the control group. The strength decreases linearly with the increase of R. |
| Saly et al. b [34] | 0~45 | 48.7~35.9 | mortar | The strengths are lower than that of the control group. When R ≥ 30%, they decrease rapidly. |
| Peng et al. [35] | 0~47.1 | 113.1~87.8 | mortar | All the strengths are lower than that of the control group. The optimal mixing ratio R = 30%. |
| Liu and Guo [36] | 0~20 | 156~140 | mortar | With the increase of R, the strength decreases, and the decrease rate is gradually increased. |
| Shi et al. [37] | 0~50 | 53~56~36 | mortar | The strength first increases and then decreases with the increase of R. The early hydration rate of SSP is lower than that of cement. |
| Zhang et al. [38] | 0~40 | 44~16 | mortar | With the increase of R, the strength decreases. When R ≥ 20%, the strength decreases rapidly. |
| Xu et al. [39] | 0~40 | 54~44 | mortar | The compressive strength decreases with the increase of R, but except R = 10%. |
| Gan et al. a [40] | 0~10 | 37.65~31.25 | mortar | With the increase of R, the strength decreases. |
| Zhang et al. a [41] | 0, 63 | 51~34 | mortar | With the increase of R, the strength decreases. |
| Altun and Yılmaz a [42] | 0~45 | 58~35.7 | mortar | With the increase of R, the compressive strength decreases linearly. |
| Altun and Yılmaz b [42] | 0~45 | 58~43.7 | ||
| da Silva et al. [43]. | 0~20 | 55~53 | mortar | When R ≤ 20%, SSP has little effect on strength. |
| Wang et al. [44] | 0~40 | 48.3~28.1 | mortar | With the increase of R, the strength decreases. |
| Wang et al. a [45] | 0~60 | 54~27 | mortar | With the increase of R, the strength decreases. |
| Wang et al. b [45] | 0~60 | 60~35 | ||
| Wang [46] | 0~50 | 37.9~21.4 | concrete | When R = 20%, the strength is the highest but lower than the control group. When R ≥ 30%, the strength decreases rapidly. |
| Guan a [47] | 0~50 | 65.1~47.9 | concrete | With the increase of R, the strength decreases. When R ≥ 30%, the strength decreases rapidly. |
| Li a [48] | 0~50 | 67.1~47.1 | concrete | With the increase of R, the strength decreases When R ≥ 30%, the strength decreases rapidly. |
| Li b [48] | 0~50 | 48.4~29.8 | concrete | All the strengths are lower than that of the control group. The optimal mixing ratio R = 20%~30%. |
| Li c [48] | 0~50 | 65.8~38.2 | concrete | All the strengths are lower than that of the control group. When R = 20%~50%, the strength decreases significantly with the increase of R. |
| Li d [48] | 0~50 | 44.7~26.7 | concrete | All the strengths are lower than that of the control group. When R = 30%~50%, the strength decreases significantly with the increase of R. |
| Cai a [49] | 0~50 | 40~44~28 | concrete | When 0 < R ≤ 30%, the strengths are higher than that of the control group, and when R > 30%, the strengths are decrease. |
| Wang [50] | 0~45 | 52~48~33 | concrete | The strength decreases with the increase of R. |
| Ding et al. [51] | 0~30 | 47~49.5~42 | concrete | When R ≤ 20%, the strengths are higher than the control group. When R = 30% is lower than the base group. |
| Liu a [52] | 0~45 | 60.1~60.5~49.2 | concrete | When R ≤ 10%, the strengths are higher than that of the control group. When R > 30%, the strengths decreases rapidly. |
| Liu b [52] | 0~45 | 57.8~58.9~45.7 | ||
| Liu c [52] | 0~45 | 43.4~47~34.9 | ||
| Luo et al. [53] | 0~17.6 | 39.9~30.7 | concrete | The strengths are lower than that of the control group. When R ≥ 10%, they decrease rapidly. |
| Sha et al. [54] | 0~35 | 48~52.1~37 | concrete | When R ≤ 15%, the strengths are higher than that of the control group. When R ≥ 25%, the strengths decreases. |
| Hussain et al. [55] | 0~30 | 60.3~68.6~43.7 | concrete | With the increase of R, the strength first increases and then decreases, and the highest strength is 68.6 MPa when R = 10%. |
| Tüfekçi et a1. a [23] | 0~15 | 32.9~26.7 | concrete | With the increase of R, the strength decreases, SSP A has greater effect on strength than B. |
| Tüfekçi et a1. b [23] | 0~15 | 32.9~30.1 | ||
| Li et a1. a [56] | 0~40 | 55~48 | concrete | With the increase of R, the strength decreases. |
| Wang et a1. a [57] | 0~30 | 54~51 | concrete | With the increase of R, the strength decreases. |
| Wang et a1. b [57] | 0~30 | 79~82~73 | concrete | The strength first increases and then decreases with the increase of R. and the highest strength is 82 MPa when R = 10% |
| Wang et a1. a [58] | 0~60 | 50~24 | concrete | With the increase of R, the strength decreases. |
| Wang et a1. b [58] | 69~46 | |||
| Wang et a1. c [58] | 50~22 | |||
| Wang et a1. d [58] | 69~45 | |||
| Wang et a1. e [58] | 50~24 | |||
| Wang et a1. f [58] | 69~46 | |||
| Wang et a1. g [58] | 50~25 | |||
| Wang et a1. h [58] | 69~46 | |||
| Wang et a1. i [58] | 50~20 | |||
| Wang et a1. j [58] | 69~41 | |||
| Song and Liu [59] | 0~40 | 42.5~47~29 | concrete | The strength first increases and then decreases with the increase of R. The highest strength is 47 MPa when R = 15. |
| Reference | Rc (%) | Rs (%) | Strength (Mpa) | Type | Key Conclusions of Different Rc and Rs |
|---|---|---|---|---|---|
| Guo and Shi b [32] | 0~100 | 0, 50 | 60, 58~33 | paste | The strength decreases with the increase of Rs. |
| Muhmood et al. c [33] | 0, 40 | 0, 50 | 66.5, 58.6~58 | paste | The strength decreases by adding SSP and slag. |
| Muhmood et al. d [33] | 0, 40 | 0, 50 | 66.5, 58.6~61 | ||
| Gan et al. b [40] | 0~40 | 0, 50 | 53, 64~57 | mortar | The strength decreases with the increase of Rs and the slag improves it. |
| Zhang et al. b [41] | 0, 100 | 0, 63 | 51, 41, 34 | mortar | SSP reduces the strength more compare slag. |
| Wang et al. c [45] | 30, 50 | 0, 50 | 46, 45, 42 | concrete | The strength decreases with the increase of Rs from 30% to 50%. |
| Guan b [47] | 0~50 | 50 | 65.1~43.9 | concrete | The strength decreases with the increase of Rc when Rs = 50%. |
| Li e [48] | 0~50 | 50 | 67.1~44.8 | concrete | The strength decreases with the increase of Rc when Rs = 50%. |
| Li f [48] | 0~50 | 50 | 48.4~31.2 | ||
| Li et a1 b [56] | 0~67 | 0, 30 | 55, 53~45 | concrete | The strength decreases with the increase of Rs. |
| Liao [70] | 0~50 | 40 | 40.2~42.6~39 | concrete | With the increase of Rc, the strength first increases and then decreases, and the highest strength is 42.6 MPa when Rc = 40%. |
| Shi a [71] | 0~100 | 30 | 67~52 | concrete | Compared with SSP, slag contributes more to the strength. The strength decreases with the increase of Rs. |
| Yang a [72] | 0~40 | 40 | 56~52 | concrete | At high water–cement ratio, the strength decreases with the increase of Rs, which increases at a low–water cement ratio. The strength increases with the increase of Rs at a low water–cement ratio, and has a mutual effect with slag. |
| Yang b [72] | 0~40 | 30 | 77~82 | concrete | |
| Song a [73] | 0~100 | 30 | 44.0~35.1 | paste | The strength decreases with the increase of Rs, and the strength is the highest when the Rs = 20%~30%. |
| Wang [74] | 20~80 | 0, 50 | 64, 62.8~39.4 | concrete | The strength decreases with the increase of Rs. |
| Liu [75] | 0~50 | 50 | 55~33.5 | concrete | The strength decreases with the increase of Rs. The slag contributed more to the strength. |
| Li [76] | 0~100 | 0, 30 | 56,46~54~45 | concrete | The strength first increases and then decreases with the increase of Rs. The highest compressive strength is 54MPa, when Rs = 67%. |
| Reference | Rc (%) | Rs (%) | Strength (Mpa) | Type | Key Conclusions of Different Rc and Rs |
|---|---|---|---|---|---|
| Zhang c [41] | 0, 100 | 0, 63 | 51, 40, 34 | mortar | The strength decreases with the increase of Rs. |
| Guan c [47] | 50 | 0~50 | 65.1~45.9 | concrete | The strength decreases with the increase of Rc. When Rc ≥ 40%, it decreases quickly. |
| Li g [48] | 50 | 0~50 | 67.1~43.1 | concrete | The strength decreases with the increase of Rc and that of low water cement ratio decreases more obviously. |
| Li h [48] | 50 | 0~50 | 48.4~29.0 | ||
| Cai b [49] | 20~80 | 50 | 50~56 | concrete | The strength slightly increases with the increase of Rs. |
| Shi b [71] | 0~100 | 30 | 62.5~52 | concrete | The strength decreases with the increase of Rs. When the Rs ≥ 40%, it decreases quickly. |
| Yang c [72] | 60~100 | 0, 35 | 50, 45~35 | concrete | The strength decreases with the increase of Rc and Rs. The strength of low water cement ratio is significantly higher than that of high water cement ratio. |
| Yang d [72] | 60~100 | 0, 20 | 50, 47~42 | ||
| Yang e [72] | 60~100 | 35 | 75, 68~54 | ||
| Yang f [72] | 60~100 | 20 | 75, 81~64 | ||
| Song b [73] | 67~100 | 30 | 39.1~35.1 | paste | The strength decreases with the increase of Rs. |
| Song a [77] | 25~100 | 20 | 42.5~46 | concrete | Rs has a greater impact on compressive strength with the increase of Rc. |
| Song b [77] | 17~100 | 30 | 36~43~39 | ||
| Song c [77] | 13~100 | 40 | 40~45~29 |
| Reference | R (%) | SSA (m2/kg) | Strength (Mpa) | Type | Key Conclusions of Strength–SSA |
|---|---|---|---|---|---|
| Liu and Li [26] | 45 | 398~635 | 37~44 | concrete | The strength increases with the increase of SSA. |
| Li i [48] | 30 | 400~800 | 52.5~59.5~58 | concrete | The strength increases first and then decreases with the increase of SSA. |
| Liao [70] | 25 | 390~646 | 43.2~50.8 | concrete | The strength increases with the increase of SSA. |
| Yang et al. a [83] | 10 | 350~550 | 54.1~58.6 | concrete | The strength increases with the increase of SSA and decreases with the increase of R. |
| Yang et al. b [83] | 20 | 350~550 | 48.7~57.1 | ||
| Yang et al. c [83] | 30 | 350~550 | 43.5~49.4 | ||
| Yang et al. d [83] | 40 | 350~550 | 37.4~47.2 | ||
| Ma [84] | 17.6 | 480~720 | 46.8~54 | concrete | The strength increases with the increase of SSA. |
| Liu [85] | 30 | 615~683 | 29~30.5 | concrete | The strength increases slightly with the increase of SSA. |
| References | 1st Part (m2/kg) | Strength Growth Rate of 1st Part % | 2nd Part (m2/kg) | Strength Growth Rate of 2nd Part % |
|---|---|---|---|---|
| Liu and Li [26] | 398~524 | 2.4 | 524~635 | 3.6 |
| Li i [48] | 400~500 | 5.8 | 500~600 | 1.6 |
| Liao [70] | 390~508 | 3.0 | 508~646 | 2.2 |
| Yang et al. a [83] | 350~450 | 3.6 | 450~550 | 0.9 |
| Yang et al. b [83] | 350~450 | 6.0 | 450~550 | 2.4 |
| Yang et al. c [83] | 350~400 | 5.8 | 400~550 | 2.0 |
| Yang et al. d [83] | 350~400 | 7.6 | 400~550 | 4.0 |
| Ma [84] | 480~560 | 5.3 | 560~720 | 1.9 |
| Liu [85] | 436~615 | 1.1 | 615~683 | 2.2 |
| Activated Types | Activators | Characteristic(s) |
|---|---|---|
| alkali activators | NaOH, Ca(OH)2, etc. | By directly providing an alkaline environment, the vitreous hydration of SSP is activated. |
| salt activators | Na2SO4, Na2CO3, NaAlO2, Na2SiO3, etc. | The provided negative ions can combine with the positive ions contained in SSP to generate the substance contributing to the cementitious material. |
| acidic activators | H2SO4, H2CO3, etc. | By providing acidic environment, the glass body depolymerization of SSP is promoted and the hydration reaction is improved. |
| Reference | Activators | Dosages (%) | R (%) | Strength (MPa) | Key Conclusions of Strength Dosages with Different Activators |
|---|---|---|---|---|---|
| Zhang et al. a [38] | Na2SiO3 | 0, 4 | 10 | 41.5~44 | The strength increases with the increase of dosages, except R = 40% |
| Zhang et al. b [38] | Na2SiO3 | 0, 4 | 20 | 32~39 | |
| Zhang et al. c [38] | Na2SiO3 | 0, 4 | 30 | 24~30 | |
| Zhang et al. d [38] | Na2SiO3 | 0, 4 | 40 | 26~25 | |
| Zhang et al. [41] | Na2SiO3 + CaO+ KAl(SO4)2 | 0, 3 | 32 | 42~48 | The strength increased from 42 MPa to 48 MPa with the addition of composite activator. |
| Wang a [95] | Na2SO4 | 0~2.5 | 100 | 32.3~43~33.1 | The strength increases first and then decreases with the increase of Na2SO4. |
| Wang b [95] | Na2SiO3 | 0~2.5 | 100 | 32.3~46.3~42.6 | The strength increases first and then decreases with the increase of Na2SiO3 |
| Wang c [95] | NaAlO2, | 0~2.5 | 100 | 32.3~46.7 | The strength increases with the increase of NaAlO2. |
| Wang d [95] | Na2SO4 + NaAlO2 | 0, 2 | 100 | 32.3~44.2 | The strength increases with the increase of Na2SO4 + NaAlO2. |
| Wang e [95] | Na2SO4 + Na2SiO3 | 0, 2 | 100 | 32.3~42.5 | The strength increases with the increase of Na2SO4 + Na2SiO3. |
| Dong a [96] | Na2SO4 | 0~4 | 100 | 33.4~42.2~38.9 | The strength is the highest at a dosage of 2% with Na2SO4 |
| Dong b [96] | Ca2SO4 | 0~4 | 100 | 33.5~42.3~40.9 | The strength is the highest at a dosage of 3% with Ca2SO4 |
| Dong c [96] | NaOH | 0~2 | 100 | 33.5~39.4~35.7 | The strength is the highest at a dosage of 1.5% with NaOH. |
| Dong d [96] | Ca(OH)2 | 0~2 | 100 | 33.5~42~41.1 | The strength is the highest at a dosage of 1% with Ca (OH)2 |
| Dong e [96] | Na2SiO3 | 0~4 | 100 | 33.5~38.2~35.6 | The strength is the highest at a dosage of 2% with Na2SiO3. |
| Dong f [96] | NaAlO2 | 0~4 | 100 | 33.5~43.7~40.2 | The strength is the highest at a dosage of 3% with NaAlO2. |
| Sun [14] | Na2SiO3 | 0.5~2 | 100 | 10~14 | With the increase of Na2SiO3, the strength increases. |
| Reference | Curing Condition | R (%) |
|---|---|---|
| Cui et al. [102] | Standard curing, hot and heat curing (56 °C and 90 °C) | 20 |
| Liu et al. [103] | Standard curing, steam curing (100 °C) | 5 |
| Zhang et al. [104] | Natural curing, standard curing | 20 |
| Zhang et al. [105] | Natural curing, standard curing, hot and heat curing (50 °C) | 5 |
| Wang [106] | Natural curing, standard curing | 7.5 |
| Reference | R (%) | Type | Hydration Heat | Description/Unit | ||
|---|---|---|---|---|---|---|
| 1st d | 3rd d | 7th d | ||||
| Liu and Li [26] | 0, 20, 45 | paste | – | – | 282.0, 224.9, 162.1 | Cumulative/(J/g) |
| – | – | 282.0, 230.1, 164.2 | ||||
| – | – | 282.0, 236.0, 162.7 | ||||
| Wang et al. a [45] | 0, 20 | paste | 5.3, 5.2 | 2.2, 1.5 | – | Rate/ J/(gh) |
| 0, 40 | 5.3, 4.0 | 2.2, 1.2 | – | |||
| 0, 60 | 5.3, 2.4 | 2.2, 1.0 | – | |||
| Guan a [47] | 0, 30 | paste | 187.6, 117.6 | 240.0, 169.8 | 271.3, 206.1 | Cumulative/(J/g) |
| Li a [48] | 0, 40 | paste | 176.3, 112.0 | 228.0, 159.9 | 255.0, 193.4 | Cumulative/(J/g) |
| Song a [73] | 0, 30 | paste | 77.3, 54.1 | 113.0, 79.1 | 133.4, 93.4 | Cumulative/(J/g) |
| Han et al. [107] | 0~50 | paste | 220~100 | 275~140 | 290~150 | Cumulative/(J/g) |
| Zhuang and Wang [108] | 0, 30 | paste | 210, 75 | 260, 150 | 290, 205 | Cumulative/(J/g) |
| Sun et al. [15] | 0, 100 | mortar | 210, 95 | 260, 120 | 280, 130 | Cumulative/(J/g) |
| Zhang et al. [42] | 0, 63 | paste | 180,50 | 199,75 | 205,85 | Cumulative/(J/g) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, G.; Li, C.; Jin, S.; Bai, Q. Effects of Steel Slag Powder as A Cementitious Material on Compressive Strength of Cement-Based Composite. Minerals 2023, 13, 869. https://doi.org/10.3390/min13070869
Sheng G, Li C, Jin S, Bai Q. Effects of Steel Slag Powder as A Cementitious Material on Compressive Strength of Cement-Based Composite. Minerals. 2023; 13(7):869. https://doi.org/10.3390/min13070869
Chicago/Turabian StyleSheng, Guohua, Chao Li, Shengji Jin, and Quan Bai. 2023. "Effects of Steel Slag Powder as A Cementitious Material on Compressive Strength of Cement-Based Composite" Minerals 13, no. 7: 869. https://doi.org/10.3390/min13070869
APA StyleSheng, G., Li, C., Jin, S., & Bai, Q. (2023). Effects of Steel Slag Powder as A Cementitious Material on Compressive Strength of Cement-Based Composite. Minerals, 13(7), 869. https://doi.org/10.3390/min13070869





