Abstract
A database consisting of 163 data on the uranium content and 234U/238U initial activity ratio of 15 Italian travertine and calcareous tufa sites was created using data from the relevant literature. Using a graphical method, data were interpreted considering the U geochemistry in natural environments as well as the geological, hydrogeological and hydrogeochemical settings of each site. The U content and 234U/238U initial activity ratio in travertine and tufa appear to be affected by different factors, such as the availability of U in the aquifer rocks, the redox state of the waters, and the alpha-active radionuclide recoil phenomenon. The data allow the identification of four groups of travertines/tufas: (i) those precipitated from circulating groundwater, with a short/fast flow path, in volcanic rocks with a high radionuclide content; (ii) those precipitated from circulating groundwater, with a long, deep flow path in carbonate/evaporite formations with a relatively low radionuclide content; and (iii) those precipitated from cold waters associated with riverine systems, which are characterized by oxidizing conditions and fed by high-discharge springs recharged by carbonate aquifers. The fourth group represents the intermediate situations frequently occurring due to the mixing of waters from different aquifers. The results suggest an interpretative model that might contribute to the paleo-environmental reconstruction of fossil travertine and calcareous tufa depositing systems.
1. Introduction
Travertine and calcareous tufa are lithological terms generally used to describe continental carbonates that form in subaerial environments through the precipitation of calcite/aragonite from waters ranging in temperature from ambient to boiling, around groundwater seepages, springs, and along streams and rivers. These carbonate deposits are commonly found in the Quaternary deposits in central and southern Italy [1,2]. They are particularly abundant along the western sector of the Italian peninsula, where they can form large deposits of remarkable thickness. The most notable example of this type is represented by the travertine of Tivoli (a town near Rome), a continental tabular carbonatic plateau that reaches in its depocenter a thickness of 85 m [3]. It has been exploited as a building material since the Roman period; even the word ‘travertine’ probably originated from Lapis Tiburtinus, i.e., the stone of Tibur, from the Latin name of Tivoli.
Travertines and calcareous tufas are important terrestrial archives of past climate and environmental changes [4,5], tectonic activity [6], and paleohydrological–hydrothermal circulations [7,8]. They may also indicate the possible location of geothermal resources [9,10] and contribute to defining the carbon cycle and budget [11,12]. Therefore, during the last twenty years, many studies have been conducted on these deposits. Many of these studies rely on U-series geochronology, because they can be reliably dated through the U-Th method, which allows the establishment of a precise chronology of up to 600 thousand years (ka) [13].
Radiometric dating techniques require the measurement of uranium content (U) and the determination of the 234U/238U initial activity ratio (i.e., at time of deposition) in the precipitated carbonate; these data are generally published in scientific papers and are used to calculate the age, but they are rarely discussed in relation to travertine/tufa formation and are not considered for their geochemical significance. In this study, we created a dataset of 163 published data on the U content and 234U/238U initial activity ratio (herein referred to as (234U/238U)i), of travertine and calcareous tufa samples from the relevant literature, in an effort to investigate the relationship between travertine/tufa deposition, groundwater circulation and mixing, and subsurface geology. The dataset includes fifteen Italian travertine and tufa deposits originating from cold and thermal waters. Data were interpreted using a graphical method. The data interpretation also made use of the related information on the groundwaters, the aquifers and the geological settings of the various deposits.
Introduction to Uranium Geochemistry and Rationale of This Study
The behavior of uranium in the environment has been studied by many authors ([14,15], and reference therein). In nature, U mainly exists in two oxidation states, U(IV) (tetravalent) and U(VI) (hexavalent). In surface environments, U(VI) is the dominant form; in reducing environments and in the mantle, tetravalent U is dominant and insoluble in water, and is generally less mobile than hexavalent U.
In aqueous solutions, the uranium concentration depends on several factors, including pH, Eh, temperature, ionic strength, and the ability to complex other ions (fluorides, chlorides, phosphates, and carbonates) and organic ligands [16,17].
Under oxidizing conditions, hexavalent uranium can be highly mobile, as it forms the uranyl ion (UO2)2+ which forms compounds that are soluble in water in a wide range of pH conditions. The ability to form complexes with inorganic (carbonate, bicarbonate and phosphate) and organic ligands further enhances the uranium’s mobility, because these complexes do not participate in sorption processes. Under near-neutral conditions, uranium forms soluble complexes with carbonate and phosphates, while at lower pH values and saline waters, it forms soluble complexes with fluorides, chlorides and sulphates [15].
Under reducing conditions, tetravalent uranium tends to be insoluble and precipitates as insoluble uraninite (UO2). At a low pH, uranium (IV)’s solubility tends to increase due to the formation of complexes with fluoride, and at pH > 7–8 it tends to increase due to complexation with hydroxyl ions [15].
In continental carbonates, such as travertine/tufa, traces of U, Ra and other decayed products of the 238U series are common [18]. Uranium is generally present in carbonate because of its incorporation in the crystal structure of calcite [19], but the way in which uranium is incorporated into the calcite lattice is poorly understood [20]. Generally, uranium is not present in the carbonate as a separate phase, as evidenced by the experimental study of [21]. Uranium can be adsorbed on mineral surfaces and particulate impurities and associated with organic matter [22].
Naturally occurring uranium is an isotopic mixture of three long-lived isotopes: 238U (t1/2 = 4.5 billion years), 235U (t1/2 = 0.7 billion years), and 234U (t1/2 = 245,000 years). In a closed system, 238U and 234U are in secular radioactive equilibrium, which corresponds to the steady state reached by the whole decay series (i.e., their activity is equal) [23]. However, physical and chemical processes occurring during water–rock interactions can preferentially remove different uranium isotopes from the system, altering the secular equilibrium and changing the activity ratios of uranium isotopes. The preferential dissolution of 234U is mainly caused by leaching owing to crystal lattice instability after alpha emission during 238U decay, and by the direct recoil ejection of the 234Th nucleus into the water via the alpha recoil effect or via the etching of alpha recoil tracks [24]. Other reasons for isotope fractionation could be associated with adsorption, changes in U speciation or redox chemistry, including microbially mediated uranium reduction [25]. As a consequence, natural waters are generally enriched in 234U, and their 234U/238U activity ratios can deviate from the radioactive equilibrium by up to more than 10% [26].
Based on the studies of [27,28], it is possible to argue that the total U content and 234U/238U initial activity ratio, generally measured on continental carbonates for U-Th dating determinations, can provide information about the groundwaters from which they precipitated and the respective geological nature of the aquifer. Three basic assumptions are needed: (i) the U concentration in continental carbonates reflects the U content in the groundwater at the time of deposition, (ii) the 234U/238U initial activity ratio in continental carbonates is the same occurring in the groundwater, and (iii) the carbonates maintain a closed-system behavior (i.e., uranium and the products of its decay used in the dating are neither added nor removed from the carbonate system after deposition).
The mobilization of U and fractionation of 234U/238U take place in different geological contexts through different processes. Groundwater dissolution preferentially mobilizes U in a carbonate aquifer over a silicate aquifer; however, its content in the former is generally lower than in the latter, so it is more frequently possible to find more U in groundwaters flowing through silicate aquifers. 234U/238U fractionation is a process due to the alpha-active radionuclide recoil phenomenon that preferentially leaches 234U into the circulating waters. This phenomenon is directly related to the groundwater residence time, which in hydrogeological Italian contexts is generally longer in the carbonate aquifers that constitute the Meso-Cenozoic bedrock of the Plio-Quaternary volcanic and alluvial formations.
Therefore, it can be stated that a relatively high 234U/238U initial activity ratio measured in continental carbonate may be indicative of precipitating groundwaters that circulated through a Meso-Cenozoic carbonate aquifer, in which the recoil enrichment of 234U was more pronounced; on the contrary, a low 234U/238U initial activity ratio may be indicative of waters that circulated through a silicate (volcanic) aquifer. In summary, U content, used in conjunction with the 234U/238U ratio, contributes to distinguishing groundwaters that flowed deep into the crust versus those that resided at shallower levels.
2. Materials and Methods
2.1. Travertine and Calcareous Tufa Deposits
Fifteen travertine and calcareous tufa deposits are considered in this study (Figure 1). Most of these deposits are located in the western sector of central Italy: in the southern Tuscany (sites 4—Bagno Vignoni, 11—Rapolano, 13—Sarteano, 14—Semproiano and 15—Saturnia) and Lazio (sites 2—Acque Albule, 3—Aniene Valley, 5—Bolsena lake, 6—Canino, 9—Middle Velino valley and 10—Prima Porta) regions. Two deposits (sites 1—Acquasanta T. and 12—Rocchetta al Volturno) are located in the eastern regions of central Italy (Marche and Molise, respectively). The northern deposit is located in the Veneto region (site 8—Euganean Hill), whereas the southern one is in Sicily (site 7—Mt. Etna). As a consequence of the different geological contexts and genetic processes, these deposits show different morphological and geochemical features, which are synthetized in Table 1.
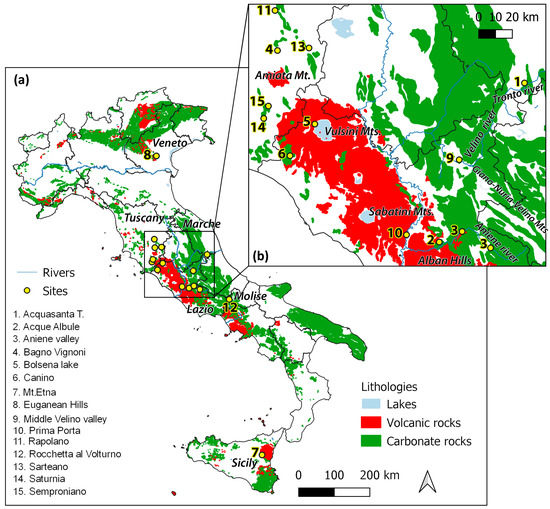
Figure 1.
(a) Location of travertine and calcareous tufa sites and simplified lithological map: carbonate rocks include carbonate platform and pelagic formations, limestone-marls (Mesozoic-Cenozoic), volcanic rocks include extrusive volcanites (Miocene, Plio-Quaternary, and Holocene), (b) location of deposits in central Italy. The site 3—Aniene valley includes two outcrops, the first at Subiaco (the eastern site) and the second at Vicovaro Mandela (the western site).
According to a widely used classification which considers the genetic environment and the presence of biota [5,29,30], the term “travertine” indicates the hard and crystalline lithotype, related to abiotic processes in waters which are typically hydrothermal in origin, whereas the term “calcareous tufa” indicate a material more porous than travertine, rich in microphytes, macrophytes, invertebrate and bacterial remains, generally formed in waters of an ambient temperature. This classification implies some uncertainties, especially when carbonate deposition occurs from cooled thermal waters, where tufa-like deposits may be precipitated when the water temperature decreases with distance from the spring orifice. In Table 1, we use the terms “travertine” and “calcareous tufa” for the deposits based on the description and terminology used in the reference papers.
The travertine and tufa deposition in the sites included in this study occurred during the last 600 ka [3,12,27,28,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. At present, carbonate deposition is still active (at sites 1, 2, 3, 4, 6, 7, 11, 14), but on a very small scale [2]; for example, in the Sarteano site, the deposition is restricted to the discharge channel of the local thermal spa [44]. Some exceptions are the Rapolano and Saturnia sites, where the carbonate still precipitates abundantly.
Several morphologies were recognized: mounds, fissure ridges, plateaus, terraces, cascades, and dams. Travertine/tufa deposits are complex systems, and therefore such morphologies coexist frequently in the same deposits. Different geometries depend on multiple factors—firstly the substrate geometry and topographic gradient, but also the spring orifice position and its change during time, as well as the water geochemistry. Mounds and fissure ridges are depositional types typical of vent environments, i.e., associated with spring orifices. Circular mounds, consisting of domes situated at the spring orifices, can be identified at sites 1, 4 and 8 [32,35,39]. Linear-to-arcuate fissure ridges are elongated mounds with a central fissure along the long axis, developing along fractures; this morphology is recognizable at sites 4 and 15 [12,35,45]. Moving away from the spring orifice, different depositional systems can occur, varying from gentle inclines to steep slopes, depending on the underlying morphology. Variably inclined lobate bodies characterized by smooth to well-developed terraced slopes in their frontal part can be formed by waters flowing away from the vent. Terraced pools, aprons, and self-building channels are distinct forms developing on steep and smooth slopes, identifiable at sites 1, 2, 4, 11, 12 and 14 [3,28,31,32,35,43,45]. At sites 1, 3, 9 and 12, deposits are developed along the course of the fluvial valley, i.e., the Tronto, Aniene, Velino and Volturno rivers, respectively. In these cases, dams, terraces and slope systems, grading from a proximal feeding system into flat marginal deposits, are recognized [31,32,34,40,43]. Finally, in a low relief topography, a tabular plateau can be formed, as in the case of sites 2 and 15 [3,12,33,45]. With the exception of mounds and fissure ridges, spring orifices are rarely recognizable in fossil deposits, hence the difficulty of reconstructing the temporal and spatial evolution of travertine/tufa deposits.
Groundwaters from which the travertine/tufa precipitated vary significantly in temperature and chemical composition [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. At sites 3, 5, 7, 9 and 12, the travertine/tufa precipitated from cold waters (T < 15 °C) and Ca-HCO3 and Na-HCO3 chemical composition, typical of cold karstic springs [49,53,55,57,63]. In the other cases, the travertine precipitated from thermal (T > 20 °C) or intermediate (15 < T < 20 °C) waters, whose chemical compositions include Ca-HCO3 (sites 4, 10, 11 [51,59,61]) and are sometimes enriched in Mg (site 2 [48]) or Na (site 7 [55]), Ca-Cl (sites 14 and 15 [66,67]), Ca-SO4 (site 13 [64,65]), Ca-SO4 and Na-Cl (sites 1, 6 and 8 [47,54,56]) hydrochemical facies. Their chemistry and temperature are due to the hydrothermal origins of the groundwaters. In several sites (1, 2, 5, 6, 9, 10, 11, 13), the hydrochemistry of groundwater and hydrogeological data suggest a mixing process of groundwater from deep and shallow aquifers [27,47,48,54,58,60,61,64,65]. In general, Meso-Cenozoic carbonate formations act as deep, sometimes confined, aquifers that can be hydraulically connected with shallow, unconfined aquifers through faults and fractures [27,47,48,60].
Indeed, most of the deposits are located in active hydrothermal settings, in tectonically active areas, where active faults and fractures offer possible pathways for the circulation and mixing of deep and shallow fluids and lead to diffuse hydrothermal manifestations, such as thermal springs and gas vents, next to continental carbonate deposition.
In southern Tuscany, several travertine deposits (sites 4, 11, 13, 14 and 15) occur at the periphery of the Mt. Amiata volcanic center [12,28,35,42,44,45,46]. Its magmatism is characterized by intrusive acidic and volcanic products with associated high-temperature metamorphism [68]. The location of travertine/tufa deposits and parental thermal springs is tectonically controlled by regional and local faults; for example, the fluid upwelling connected to the deposition of Sarteano was favored by the intersection of transcurrent faults with pre-existing regional normal faults [44], while in the case of the Bagno Vignoni site, travertine deposition was tectonically controlled by WSW–ENE striking, oblique and normal faults, associated with a main fault (named as the Violante Fault) [28].
In the Lazio region, magmatism generated several volcanic centers, the activity of which started approximately 600 ka, with the emplacement of large volumes of potassic and ultrapotassic lavas and pyroclastics [69]. Several travertine/tufa deposits are associated with the volcanic centers of the Roman Magmatic Province, such as the Vulsini Mts. (sites 5 and 6 [27]), the Sabatini Mts. (site 10 [41,59,60]) and Alban Hills (site 2 [3,33]) volcanic complexes. Additionally, in these cases, tectonics controlled hydrothermal setting and travertine deposits: examples are the transverse faults (NE–SW and N–S) identified in the Acque Albule basin [3], or the normal faulting that controls the rising of water from the deep aquifer in the Canino area [70].
In the Veneto region, a travertine deposit (site 8) is located in the Euganean Hills district, an isolated volcanic body within the Veneto alluvial plain and the most important geothermal field of northern Italy [56]. This area is within the complex Lessini-Euganei structural domain, which is part of the transcurrent western margin of the Adria Indenter and, at the same time, the western boundary of the foreland of the South Alpine thrust and fold belt [71]. Travertine crops out in the spa town of Abano Terme, and is closely associated with a fault buried under the Quaternary alluvial deposits [39].
Finally, in eastern Sicily, a travertine deposit (site 7) crops out on the lower southwestern flank of Mt. Etna., the largest strato-volcano in Europe and one of the major emitters of magmatic CO2 on the Earth’s surface [37]. This volcano grew in proximity to the collision boundary of the African and Eurasian continental plates due to repeated eruptions of alkali basalts–hawaiites over the last 200 ka [69].
In the inner and eastern sectors of central Italy, the travertine/tufa deposits crop out far from the main volcanic centers of the peninsula. Some deposits occur within the Apennine chain, the Neogene fold and thrust belt formed by the convergence of the European and Adria-African continental blocks during the late Cretaceous. These deposits (sites 1, 3, 9 and 12) developed along the course of fluvial valleys, and formed depositional terraces [31,32,34,40,43]; their formation in some cases was directly connected to hydrothermal systems and tectonically active areas (sites 1 and 9), and not in other cases (sites 3 and 12). In the inner sector of central Apennine, several deposits occur along the Middle Velino valley (site 9); they crop out along the northern boundary of the San Vittorino plain, an intramontane depression that is the result of extensional and/or transtensive tectonics, displacement along major fault planes and regional uplift [40]. The plain is characterized by the occurrence of mineralized springs, gas emissions and sinkholes, as well as recent and historical seismicity. The travertine here likely formed due to the emergence of mineralized water [72] following intense tectonic activity at the end of the Middle Pleistocene. Other travertine/tufa deposits, in the inner sector of central Apennine, crop out along the course of Aniene river (site 3); their formation was due to fluvial barrage that developed in correspondence with a morphological step, possibly of tectonic origin [34].
In the eastern part of central Apennine, deposit of Acquasanta T. is located (site 1) [31,32,47,73,74]. This deposit is associated with an important compressional feature, the Acquasanta Terme anticline, which was cross-cut by a local transtensional tectonic regime resulting in dextral strike-slip and minor normal faulting during the Neogene-Quaternary, that may have controlled the hydrothermal circulation system [73]. Additionally, the Acquasanta Terme system is sustained by a low temperature geothermal field probably due to a normal geothermal gradient [74], a consequence of long and deep circulation of meteoric fluids infiltrating nearby mountain ranges, such as the Sibillini mountains to the east [74] or the Laga mountains to the south [47].
Finally, in the southeastern sector of central Apennine, the Rocchetta al Volturno tufa (site 12) crops out along the Volturno river valley. This deposit was formed by waterfall that, aggrading and prograding, acted as barrier behind which small accumulation basins developed [43].
2.2. The Dataset
The dataset is listed in Appendix A. It consists of 163 data on the uranium content (in ppb) and (234U/238U)i at 15 Italian travertine and calcareous tufa sites, reviewed from the following studies: [3,12,27,28,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. The dataset includes only uranium data from travertine and tufa samples, and not from groundwater, because such data are not available.
The uranium content data from travertines and calcareous tufas are, in principle, not directly comparable, since the respective depositional contexts differ mainly in terms of carbonate accumulation rate, temperature and ionic strength of the precipitating waters. The uranium concentrations in the carbonate precipitates may be influenced by these variables, and reflect the uranium concentrations in the parent waters. It should also be noted that there is a considerable uncertainty in the correct classification of tufas or travertines in the sites reviewed, and travertines can often belong to depositional facies that recall those typical of tufa, especially if deposited far from the spring orifice. It is therefore difficult to identify depositional features in each sample. For these reasons, in this study, it is assumed that the uranium concentration in carbonate precipitates mainly reflects the concentration in groundwater, irrespective of their attribution to travertines or tufas.

Table 1.
Overview of the Italian travertine and calcareous tufa deposits examined for this study, with ages, description of sites, hydrogeochemistry of waters associated with carbonate precipitation and hydrogeological settings of the sites.
Table 1.
Overview of the Italian travertine and calcareous tufa deposits examined for this study, with ages, description of sites, hydrogeochemistry of waters associated with carbonate precipitation and hydrogeological settings of the sites.
| Site Code | Site Name | Lithotype | Age (ka) | Deposit Description | Hydrogeochemistry | Hydrogeology | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Acquasanta T. | Travertine | 221 ± 5.8 to 26 ± 3.0 | Slope system grading from a proximal feeding system into flat marginal deposits bordered by waterfall travertine through smoothly sloping deposits and eventually a more distal system. Active. | Acquasanta Terme spring: Na-Cl to Ca-SO4 thermal waters (T = 51 to 73 °C), associated with CO2 emission; pH = 6.8 | A deep circulation within Triassic CaSO4-rich evaporitic formations; close to surface, dilution with HCO3 waters. Recharge area of deep aquifer: Laga Mts. (30 km far). | [31,32,47] |
| 2 | Acque Albule | Travertine | 219 ± 26 to 28 ± 16 | Cycles of travertine aggradational deposition in sub-horizontal pools and terraces occurring over the inclined erosional surface. Active. | Lago Regina spring: Ca-Mg-HCO3 intermediate waters (T = 23–24 °C), associated with CO2 emission; pH = 6.24; Eh = −9 mV | A deep confined carbonate aquifer, and a shallow travertine aquifer, connected through tectonic discontinuities. Recharge area of deep aquifer: Cornicolani Mts. (10 km far). | [3,33,48] |
| 3 | Aniene valley | Calcareous tufa | 112 ± 7 to 5.5 ± 0.3 | From palustrine to fluvio-lacustrine environment. Three-order depositional terraces. Active. | Agosta spring: Ca-HCO3 cold water (T = 13 °C); pH = 7.30. Pertuso spring: Ca-HCO3 cold water (T = 8–9.5 °C); pH = 6.9–10.8; Eh = +110 to +481 mV. | Recharge area: carbonate unit of Simbruini Mts. | [34,49,50] |
| 4 | Bagno Vignoni | Travertine | 160.6 ± 6.6 to 95 ± 1.3 | Fissure ridges; cone-shape mounds; slope depositional system that passed to a distal zone characterized by sub-horizontal morphology and low-energy environment. Active. | Bagno Grande pool: Ca-HCO3 thermal water (T = 33 °C); pH = 7.06; Eh = +180 mV. Borehole: Ca-SO4 thermal water (T = 48.4 °C), Eh = −106 mV. | Deep circulation in a Mesozoic evaporite carbonate reservoir. Residence time of deep carbonate aquifer > 40 and up to 70 years. | [35,51,52] |
| 5 | Bolsena Lake | Travertine | 218 ± 39 to 3.6 ± 2 | Travertine plates, from the bottom of the lake and along the Fiora river. Fossil. | Bolsena lake water: Na-HCO3 cold water (T = 9–15 °C); pH = 8.5 | One aquifer connected to deep carbonatic formations, and another to the surface volcanic products. | [27,53] |
| 6 | Canino | Travertine | 57 ± 5.5 to 1.2 ± 1.8 | Depressional environments representing the distal portion the thermal springs, related to normal faulting of Mesozoic sediments on Mt. Canino. Active. | Canino-Torlonia springs: Ca-SO4 and Na-Cl thermal waters (T up to 50 °C), associated with CO2 emission; pH = 6.30 | Hydrothermal system (located within buried carbonate structures) is mede up of shallower zone (Ca-SO4 water type) and deeper zone (Na-Cl brines). Recharge area: partly by carbonate and partly by regional volcanic aquifer. | [27,36,54] |
| 7 | Mt. Etna | Travertine | 24 ± 4 to 4 ± 0.7 | Stratified and massive in the lower part, porous and with clay lens in the upper part. Active. | Adrano spring-borehole: Ca-Na-HCO3 cold water (T = 7–14 °C); pH = 7.98–8.48 | Volcanic aquifer. Recharge area: Western flank of Mount Etna volcano. | [37,38,55] |
| 8 | Euganean Hills | Travertine | 30 ± 4 to 25 ± 5 | Sub-circular mound, up to 15 m high. Fossil. | Abano Terme springs: Na-Cl thermal waters (T = 53.5 to 86.5 °C), associated with CO2 emission; pH = 6.3–7.3 | Recharge area: carbonatic complex of Prealps (70 km far). | [39,56] |
| 9 | Middle Velino Valley | Travertine–Calcareous tufa | 113.6 ± 18 to 5.3 ± 1.1 | Dams along the course of fluvial valley. Fossil. | Cotilia spa spring: Ca-HCO3 cold water (T = 14.2 °C), associated with CO2 emission; pH = 6.2; Eh = −198 mV | Multi-layered aquifer of the plain is recharged by inflows from the carbonate aquifers on the two sides of the valley and affected by input of deep CO2-rich fluids. Recharge area: carbonate units of the Giano-Nuria-Velino Mts. hydrogeological system and in part of the Mt. Paterno-Canetra hydrogeological unit. | [40,57,58] |
| 10 | Prima Porta | Travertine | 53.5 ± 10 to 24.2 ± 2.7 | Buried body, probably pool. Fossil. | Prima Porta well: Ca-HCO3, intermediate water (T = 18 °C), associated with CO2 emission; pH = 6.15; Eh = −7.4 mV | Probably a mixing between volcanic and alluvial aquifers. Recharge area: Sabatini volcanic complex and alluvial Tiber Valley. | [41,59,60] |
| 11 | Rapolano | Travertine | 157 ± 15 to 6.7 ± 1.4 | Fan-like high spring-water flow depositional systems (N–NW) and flat, low spring-water flow depositional system (E–SE). Active. | Terme Querciolaie spring: Ca-HCO3 thermal water (T = 35 °C), associated with CO2 emission; pH = 6.43 | Mesozoic limestone and associated travertine hosts shallow cold Ca-HCO3 waters and deep thermal saline Ca-SO4(HCO3) waters. Cold saline Na-Cl waters are hosted in confined aquifer in Neogene sediments. Recharge area of deep aquifer: pre-Apenninic belt. | [28,42,61] |
| 12 | Rocchetta al Volturno | Calcareous tufa | 75 ± 8 to 4.4 ± 0.5 | Slope system. Fossil. | Capo Volturno spring: Ca-HCO3 cold water (T = 11 °C); pH = 7.2; Eh = +200 to +400 mV | Recharge area: Mt. Genzana and Mt. Greco. | [43,62,63] |
| 13 | Sarteano | Travertine–Calcareous tufa | 268 ± 22 to 16 ± 3 | Tectonically controlled, mature perched-spring system. Active. | Le Canalette springs: Ca-SO4 thermal water (T = 24 °C); pH = 6.7 | Groundwater circulates in a highly fractured, quasi-continuous reservoir constituted by the Mesozoic limestone and the underlying Burano anhydrite formation. Mixing between deep and shallow fluids. Recharge area: Mt. Cetona. | [44,64,65] |
| 14 | Saturnia | Travertine | 142 ± 23 to 40 ± 7 | Cascades, pools, and terraced slopes. Active. | Saturnia Terme spring: Ca-Cl thermal waters (T = 34 °C), associated with CO2 emission; pH = 6.3; Eh = −212 mV | A deep carbonate aquifer, residence time c.a. 30 years. Recharge area of deep aquifer: south of Roccalbegna (10 km far). | [45,66,67] |
| 15 | Semproniano | Travertine | 613 ± 200 to 33 ± 4 | Fissure ridge; travertine plateau. Fossil. | Saturnia Terme spring: Ca-Cl thermal waters (T = 34 °C), associated with CO2 emission; pH = 6.3; Eh = −212 mV | A deep carbonate aquifer, residence time c.a. 30 years. Recharge area of deep aquifer: south of Roccalbegna (10 km far). | [12,45,46,66,67] |
3. Results
The U content of the sites of this study ranges from 3 to 6390 ppb (Appendix A), with a median value of 77 ppb. Among the sites, U varies significantly (Figure 2a). Very low median values (<15 ppb) are calculated for the sites 1, 4, and 8, while very high values are calculated (>500 ppb) for the sites 5 and 7. Intra-site variability is sometimes recognized, e.g., sites 2, 5, 6, and 7. Occasionally, outliers are detected (sites 1, 2, 3, 11, 15). Figure 2b shows the variation of the (234U/238U)i ratio: it varies between 0.91 and 2.879 (Appendix A), with a median value of 1.106. In most of the sites, the isotopic ratio is between 1 and 1.5, except for sites 4, 8, and 11, where the ratio is higher, and in general, it is correlated with a marked intra-site variability. Outliers are statistically detected in sites 2 and 9.
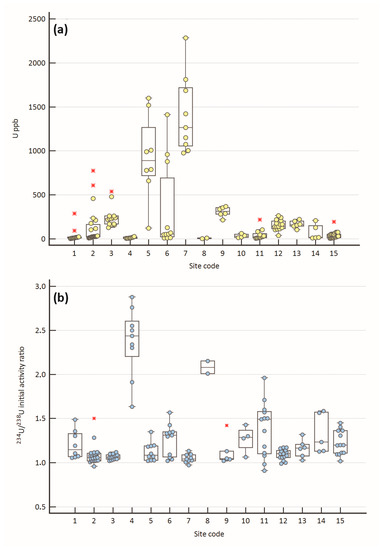
Figure 2.
(a) Box plot of the uranium content measured in travertine and calcareous tufa samples; (b) box plot of the (234U/238U)i ratio. The middle line of the plots represents the median; red asterisks are the outlier values. Site code: 1—Acquasanta T., 2—Acque Albule, 3—Aniene valley, 4—Bagno Vignoni, 5—Bolsena Lake, 6—Canino, 7—Mt. Etna, 8—Euganean Hills, 9—Middle Velino Valley, 10—Prima Porta, 11—Rapolano, 12—Rocchetta al Volturno, 13—Sarteano, 14—Saturnia, 15—Semproniano. See also Figure 1 for the site location.
Figure 3 shows the plots of the (234U/238U)i ratio versus the inverse of total U content of the reviewed sites. In this type of plot, it is assumed that the (234U/238U)i ratio of travertine and tufa is the same of the parental groundwater, and also that U content would reflect the U content in the groundwater. In the reviewed sites, different patterns are recognized, and related to their hydrogeological setting (summarized also in Table 1):
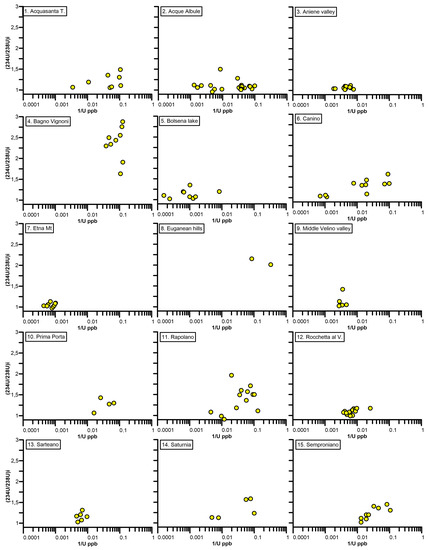
Figure 3.
For each site, the 234U/238U initial activity ratio is plotted against the uranium content, expressed as 1/U in logarithmic scale.
- -
- Site 1—Acquasanta T. The plot shows intermediate to low values of U and a (234U/238U)i ratio < 1.5; the U-ratio tends to increase as the U content decreases. According to [47], travertines of Acquasanta are deposited by thermal waters that rise from an aquifer hosted in a carbonate sequence. Chemical and isotope data suggest the co-existence of a deep circuit, involving Triassic evaporitic formations, and a variable amount of cold water coming from fast surface circuits. The recharge area of the deep aquifer could be identified in the Laga Mts., about 30 km away from the site.
- -
- Site 2—Acque Albule. The plot shows intermediate to low values of U and a (234U/238U)i ratio < 1.5. According to [48], in the Acque Albule area, a deep confined carbonate aquifer is connected through tectonic discontinuities to a shallow travertine aquifer. The recharge area of this deep aquifer could be identified in the Cornicolani Mts., about 10 km away from the site.
- -
- Site 3—Aniene valley. The plot shows intermediate values of U and a (234U/238U)i ratio < 1.2. The calcareous tufa of the Aniene valley is deposited by the cold waters of the Aniene river, fed by many tributaries and the Agosta spring that, with a discharge rate of 5400 l/s, has a recharge area corresponding to the carbonate unit of Simbruini Mts. [49].
- -
- Site 4—Bagno Vignoni. The plot shows low values of U and a (234U/238U)i ratio > 1.5; the U-ratio tends to increase as the U content decreases. Groundwater chemistry suggests a deep circulation in a Mesozoic evaporite carbonate reservoir [51]. Tritium data point to a residence time for the deep carbonate aquifer of between 40 and 70 years [52].
- -
- Site 5—Bolsena lake. The plot shows a high U concentration (>1000 ppb) and a (234U/238U)i ratio < 1.2. According to [27], the groundwaters are hosted in part in an aquifer connected to deep carbonatic formations, and in part in a surficial aquifer consisting of volcanic products, from the Vulsini volcanic complex, particularly enriched in uranium (up to 22.9 ppm; [69]).
- -
- Site 6—Canino. The plot shows a wide range of U values, from low to high, and a (234U/238U)i ratio < 1.5; the U-ratio tends to increase as the U content decreases. According to [54], the hydrothermal system, located within buried carbonate structures, is made up of a shallower zone (where Ca-SO4 waters are prevalent) and a deeper zone (where Na-Cl brines are present). The recharge area can be identified partly in carbonate structures and partly in the regional volcanic aquifer. As the small carbonate outcrop of Mt. Canino is not sufficient to explain the flow rate of thermal waters, the authors of [54] hypothesize that the carbonate structures are locally in contact with the regional volcanic aquifer that recharges the deep aquifer.
- -
- Site 7—Mt. Etna. The plot shows a high U concentration (>1000 ppb) and a (234U/238U)i ratio < 1.2. The recharge area of the spring related to carbonate precipitation is identified in the western flank of Etna Mt volcano [55]. The U content of volcanic products is up to 4.9 ppm [69].
- -
- Site 8—Euganean Hills. The plot shows a very low U concentration (<20 ppb) and a (234U/238U)i ratio >2. According to [56], the recharge area of the thermal fluids can be identified in the Venetian Prealps, situated about 70 km north of the Euganean Hills. A magmatic contribution, possibly related to vestiges of the volcanic activity that occurred in the Abano area during the Tertiary age, can be ruled out, on the basis of the chemical and isotope compositions of gases associated with the thermal manifestations [56].
- -
- Site 9—Middle Velino valley. The plot shows intermediate values of U content and a (234U/238U)i ratio < 1.5. According to [58], the springs of the middle valley of Velino river and of the San Vittorino plain are recharged by the carbonate units of Giano-Nuria-Velino Mts. hydrogeological system and by the Mt. Paterno-Canetra hydrogeological unit. Mineralized fluids of deep origin affect groundwater, which have particular hydrochemical characteristics (sulphurous water, ferruginous water with slightly hydrothermal character) in some cases [57].
- -
- Site 10—Prima Porta. The plot shows low values of U content and a (234U/238U)i ratio < 1.5. The fossil travertine system of Prima Porta is located at the border of the Paleo-Tiber Graben, a tectonic depression filled by an aquifer, comprising a thin layer of volcanic rocks, overlying sandy, gravelly terrains and a thin and discontinuous sandy, gravelly layer at its base [41]. The chemistry of present-day groundwater suggests the circulation in a volcanic aquifer, and probably mixing with the alluvial plain aquifer [59,60].
- -
- Site 11—Rapolano. The plot shows intermediate to low values of U content and a (234U/238U)i ratio increasing up to 2 as the U content decreases. According to [61], in the Rapolano area, Mesozoic limestones of the pre-Apenninic belt and associated travertines are the recharge areas of two well-mixed water types, shallow cold Ca-HCO3 waters and deep thermal saline Ca-SO4(HCO3) waters; further aquifers confined in Neogene sediments of the Siena basin host cold saline Na-Cl waters, that appear relatively isolated and are not mixed with the thermal waters.
- -
- Site 12—Rocchetta al V. The plot shows intermediate values of U content and a (234U/238U)i ratio < 1.2. The carbonates of Rocchetta al V. are deposited by the cold waters of the Volturno river, fed by the Capo Volturno spring that, with a discharge rate of 6600 L/s, has a recharge area corresponding to the carbonate unit of Mt. Genzana and Mt. Greco [62,63].
- -
- Site 13—Sarteano. The plot shows intermediate values of U content and a (234U/238U)i ratio < 1.5. In the Sarteano area, mixing between deep and shallow fluids, initially of meteoric origin, occurs. Groundwater circulation occurs in a highly fractured, quasi-continuous reservoir constituted by the Mesozoic limestone and the underlying Burano anhydrite formation; the recharge area is in Cetona Mt ridge [44].
- -
- Site 14—Saturnia. The plot shows intermediate to low values of U content and a (234U/238U)i ratio just over 1.5. Thermal waters currently discharged at Saturnia are hosted in a deep aquifer occurring within the Mesozoic carbonate units of the Tuscan nappe. The residence time, calculated on the basis of tritium data, is about 30 years. The recharge area is located south of Roccalbegna, 10 km away from the site [67].
- -
- Site 15—Semproniano. The plot shows low values of U content and a (234U/238U)i ratio < 1.5; the U-ratio tends to increase as the [U] decreases. The fossil system of Semproniano is genetically connected to the hydrothermal system of Saturnia [45], and therefore the hydrogeological setting can be considered not different.
4. Discussion
On the basis of data collected and plots of Figure 3, the U content and (234U/238U)i ratio in the travertine/tufa were interpreted to be the results of the not-trivial interaction between different factors: (a) the U content of the aquifers where groundwater circulated; (b) the redox conditions of the groundwaters; and (c) the alpha-active radionuclide recoil phenomenon.
The main factor affecting the uranium in the travertine/tufa seems to be U enrichment in groundwater through mineral dissolution, which depends firstly on the availability of U in the aquifer rocks. Volcanic aquifers are generally characterized by rocks with high radionuclide contents, up to 22.9 ppm of U in the Vulsini volcanic complex (which hosts the surficial aquifer for site 5—Bolsena lake) and up to 4.9 ppm in Mt. Etna (which is the recharge area of the groundwater precipitating travertine in site 7) [69]. On the contrary, the carbonate rocks are characterized by radionuclide contents generally lower than the volcanic rocks, although data on the U content in carbonate formations are quite scarce. A value of 2.19 ppm of U is reported for limestones, the Meso-Cenozoic carbonate bedrocks of central Italy [75], which represent the aquifer of several sites (1—Acquasanta, 2—Acque Albule, 3—Aniene valley, 6—Canino and 9—Middle Velino valley).
Secondly, the redox state of the waters controls the speciation of uranium and therefore the presence of uranyl compound which is the U mobile phase. For example, uranyl-carbonates are the most common complexes in equilibrium with Mt. Etna oxidizing groundwaters (site 7) [76]. Waters characterized by oxidizing conditions include those of the sites 3—Aniene valley (Eh = +110 to +481 mV [50]) and 12—Rocchetta al V. (Eh = +200 to +400 mV [63]) (Table 1). This could explain the relatively high content of U. Under more reduced conditions, U is very insoluble, and this behavior could explain the lowest contents of U in the sites 14—Saturnia (Eh = −212 mV [66]) and 4—Bagno Vignoni (Eh = −106 mV [51]) (Table 1). The residence time of groundwater could also influence the redox conditions; travertines/tufas will show a lower or higher U content depending on whether groundwaters are deep with long residence times, and thus reducing, or shallow with short residence times, and thus oxidizing.
The recoil phenomenon in the aquifer constitutes the factor controlling the (234U/238U)i in travertine and tufa deposits. This ratio is significantly influenced by water–rock interactions and by the alpha recoil phenomenon. A long residence time of groundwater in the aquifer, due to deep circulation and/or remote recharge area, may determine intense water–rock interactions and a selective extraction of 234U, increasing the (234U/238U)i ratio. 234U enrichment is especially evident in the reducing groundwaters that precipitated the travertine deposits of site 1—Acquasanta T., 4—Bagno Vignoni, 8—Euganean Hills, 14—Saturnia and 15—Semproniano (Table 1); correspondingly, they show low values of U and a (234U/238U)i ratio that increases as the U content decreases. Their long residence times are also confirmed by the related hydrogeological studies. Conversely, the low (234U/238U)i ratio could suggest a short residence time (as generally occurs in the shallow, generally oxidizing, groundwaters), determining a scarce 234U recoil enrichment. The mixing process between different groundwaters can occur, determining intermediate values of U and a (234U/238U)i ratio in the precipitated carbonates. These characteristics can be observed in some samples of sites 1—Acquasanta, 2—Acque Albule, 5—Bolsena lake, 6—Canino, 9—Middle Velino valley, 10—Prima Porta, 11—Rapolano and 13—Sarteano. These sites demonstrate mixing between deep-circulating fluids from carbonate/evaporite aquifers and shallow-cold groundwaters, that circulate through volcanic (e.g., sites 10, 5 and 6), alluvial (e.g., site 10) and travertine (e.g., site 2) aquifers. In these sites, the mixing processes of groundwater from deep and shallow aquifers are confirmed by geochemical and hydrogeological studies [27,47,48,60].
Figure 4a shows an idealized cross section that summarizes the occurrence of different travertine/tufa deposits and the interaction of the factors that influence the U behavior. In Figure 4b, the samples of travertines/tufas are grouped into four clusters that correspond to the various occurrences of the travertine/tufa deposits in Figure 4a.
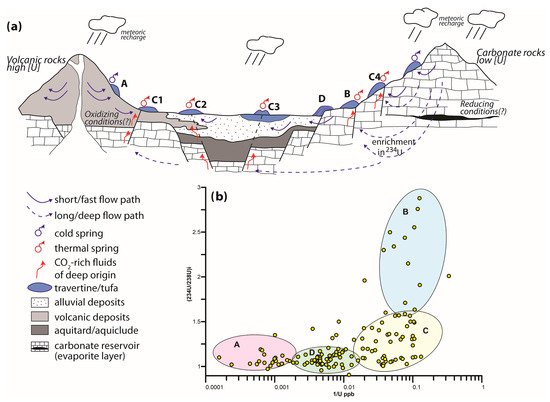
Figure 4.
(a) Idealized section in which the occurrence of different travertine and calcareous tufa deposits is summarized; (b) the 234U/238U initial activity ratio versus 1/U values with all samples reviewed from literature. Samples are grouped into four clusters (A, B, C and D) which correspond to the different occurrences of travertine/tufa in the idealized section. See text for explanation.
Cluster A groups travertine samples from sites 5 and 7, and partly from 6. The data show very high U concentration (>1000 ppb) and a (234U/238U)i ratio of ~1.2. According to our interpretation, groundwater, circulating in volcanic rocks with high radionuclide content, deposited travertines with the highest U contents. The residence time of groundwaters can be short in the volcanic aquifers, and therefore the 234U recoil enrichment is scarce, explaining the relatively low (234U/238U)i ratio. Oxidizing groundwater conditions may have favored the U mobility; data on Eh are generally not available to fully confirm this condition.
Cluster B includes travertine samples from sites 4, 8 and partly from 11, 14 and 15. These data show a low U concentration (<50 ppb) and an increasing (234U/238U)i ratio from 1.5 to 3. In this case, groundwaters circulating in carbonate/evaporite formations are not particularly enriched in U due to the relatively low content of U in the reservoir rocks. The long residence time of the groundwaters in these aquifers, related to deep circulation, allows a strong 234U recoil enrichment and consequently an increase in the (234U/238U)i ratio. In addition, the reducing groundwater conditions, due to a slow and presumably anoxic deep circulation, may have hindered the U mobility.
Cluster C groups travertine/tufa samples from several sites (1, 2, 6, 10, 11, 13, 14, 15). They are characterized by intermediate to low U concentration and a (234U/238U)i ratio lower than 1.5, implying mixing processes of the parent groundwaters; mixing can occur between groundwaters from deep/carbonate aquifers and groundwaters from the other types of aquifers as displayed in Figure 4a: a shallow/volcanic aquifer (C1), a shallow/alluvial aquifer (C2), and a shallow/travertine aquifer (C3). C4 represents the mixing between groundwaters from a deep/carbonate aquifer and surface waters from streams and rivers (C4).
Cluster D exclusively groups tufa samples from sites 3, 9 and 12, which are characterized by intermediate values of U content and a (234U/238U)i ratio < 1.2. In these cases, carbonate is precipitated from cold waters associated with river systems, which are characterized by oxidizing conditions and fed by high-discharge springs recharged by carbonate aquifers. The oxidizing conditions for these waters are confirmed by the data for redox potential.
In summary, the residence time of groundwater has been found to be crucial for every factor affecting the U content and (234U/238U)i ratio: (i) a long residence time, due to deep circulation and/or a remote recharge area, may determine intense water–rock interactions that may increase the uranium content and (234U/238U)i ratio; meanwhile, the slow, deep circulation that characterized such conditions may cause anoxia, hindering the uranium mobility and decreasing its concentration; the natural cases observed in this study (Figure 4b) demonstrate that the 234U shows a non-significant depletion due to uranium reduction, thus not showing an evident effect on the fractionation of the (234U/238U)i ratio; (ii) a short residence time, due to a shallow and fast circulation, may determine a decrease in the (234U/238U)i ratio, and possibly oxidizing conditions that favor the U mobility.
5. Conclusions
This paper reviews the available data on the uranium content and 234U/238U initial activity ratio from 15 Italian travertine and calcareous tufa deposits reported in the relevant literature. Using the graphical method previously proposed by [27,28], data have been interpreted considering the U geochemistry in natural environments as well as the geological, hydrogeological and hydrogeochemical setting of each site.
The U content and (234U/238U)i ratio in travertines and tufas appear to be affected by different factors, such as the availability of U in the aquifer rocks, the redox state of the groundwaters, and the alpha-active radionuclide recoil phenomenon. These factors interact with each other and determine the U content and isotope ratio of waters responsible for carbonate precipitation. Four groups of travertines/tufas were identified: (i) those that precipitated from groundwater circulating, with a short/fast flow path, in volcanic rocks with a high radionuclide content; these carbonates have a very high U concentration (>1000 ppb) and a (234U/238U)i ratio of ~1.2; (ii) those that precipitated from groundwater circulating, with a long and deep flow path, in carbonate/evaporite formations with a relatively low radionuclide content; these continental carbonates have a low U concentration (<50 ppb) and an increasing (234U/238U)i ratio (> 1.5, up to 3); (iii) those that precipitated from cold waters associated with river systems, which are characterized by oxidizing conditions and fed by high-discharge springs recharged by carbonate aquifers; these continental carbonates have intermediate values of U content and a (234U/238U)i ratio < 1.2; and (iv) those that precipitated in hydrogeological settings where mixing between groundwaters from different aquifers occurs.
The graphical method of [27,28], suitably implemented with a large amount of data reviewed from the literature, has proved to be effective for investigating the characteristics of parental waters and the related aquifers of travertines and calcareous tufas. This approach might be useful to infer paleo-environmental characteristics of fossil continental carbonate depositing systems.
Author Contributions
Conceptualization, F.G. and M.B.; methodology, F.G. and M.B.; investigation, F.G. and M.B.; writing—original draft preparation, F.G.; writing—review and editing, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are available in the article and Appendix A.
Acknowledgments
We are grateful to Mario Voltaggio for the constructive discussions and long-standing collaboration. We are indebted to all the authors who published uranium data of travertine and calcareous tufa that we have used in this study. We kindly thank the anonymous reviewers for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Uranium content (in ppb) and 234U/238U initial activity ratio from 15 Italian travertine and calcareous tufa deposits reviewed from the literature.
Table A1.
Uranium content (in ppb) and 234U/238U initial activity ratio from 15 Italian travertine and calcareous tufa deposits reviewed from the literature.
| ID | Site | U ppb | (234U/238U)i | Ref. | ID | Site | U ppb | (234U/238U)i | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1— Acquasanta T. | 4.5937 | [31] | 82 | 7— Etna Mt | 1266 | 0.974 | [38] | ||
| 2 | 16.379 | [31] | 83 | 1422 | 1.133 | [38] | ||||
| 3 | 13.108 | [31] | 84 | 2286 | 1.03 | [38] | ||||
| 4 | 6.09 | [31] | 85 | 978 | 1.096 | [38] | ||||
| 5 | 15.837 | [31] | 86 | 1685 | 1.057 | [38] | ||||
| 6 | 8.545 | [31] | 87 | 1151 | 1.008 | [38] | ||||
| 7 | 93.1 | 1.191 | [32] | 88 | 1073 | 1.043 | [38] | |||
| 8 | 288.2 | 1.065 | [32] | 89 | 1006 | 1.086 | [38] | |||
| 9 | 9.5 | 1.489 | [32] | 90 | 1812 | 1.025 | [38] | |||
| 10 | 23.2 | 1.354 | [32] | 91 | 8—Euganean hills | 11.7 | 2.15 | [39] | ||
| 11 | 9.2 | 1.105 | [32] | 92 | 3 | 2.01 | [39] | |||
| 12 | 20.1 | 1.056 | [32] | 93 | 9— Middle Velino Valley | 216 | 1.05 | [40] | ||
| 13 | 17.7 | 1.074 | [32] | 94 | 339 | 1.04 | [40] | |||
| 14 | 10.2 | 1.304 | [32] | 95 | 289 | 1.04 | [40] | |||
| 15 | 2— Acque Albule | 106 | 1.02 | [33] | 96 | 282 | 1.42 | [40] | ||
| 16 | 118 | 1.5 | [33] | 97 | 368 | 1.02 | [40] | |||
| 17 | 238 | 1.11 | [33] | 98 | 356 | 1.13 | [40] | |||
| 18 | 774 | 1.12 | [33] | 99 | 10— Prima Porta | 21 | 1.275 | [41] | ||
| 19 | 21 | 1.05 | [33] | 100 | 62.3 | 1.065 | [41] | |||
| 20 | 28 | 1.02 | [33] | 101 | 39 | 1.43 | [41] | |||
| 21 | 27 | 1.02 | [33] | 102 | 15 | 1.3 | [41] | |||
| 22 | 179 | 1.02 | [33] | 103 | 11— Rapolano | 27.9 | 1.49 | [28] | ||
| 23 | 10 | 1.1 | [33] | 104 | 35.1 | 1.18 | [28] | |||
| 24 | 610 | 1.06 | [33] | 105 | 17.3 | 1.36 | [28] | |||
| 25 | 31.25 | 1.056 | [3] | 106 | 10.8 | 1.5 | [28] | |||
| 26 | 12.25 | 1.026 | [3] | 107 | 15.9 | 1.57 | [28] | |||
| 27 | 26 | 1.115 | [3] | 108 | 24.8 | 1.6 | [28] | |||
| 28 | 34.25 | 1.281 | [3] | 109 | 12.9 | 1.71 | [28] | |||
| 29 | 14.75 | 1.094 | [3] | 110 | 9.7 | 1.5 | [28] | |||
| 30 | 14 | 1.084 | [3] | 111 | 7.6 | 1.108 | [42] | |||
| 31 | 27.25 | 1.093 | [3] | 112 | 84 | 0.91 | [42] | |||
| 32 | 24.667 | 1.054 | [3] | 113 | 103 | 0.983 | [42] | |||
| 33 | 25.25 | 1.081 | [3] | 114 | 220 | 1.08 | [42] | |||
| 34 | 211 | 0.958 | [3] | 115 | 50 | 1.96 | [42] | |||
| 35 | 26.5 | 1.027 | [3] | 116 | 12— Rocchetta al Volturno | 166 | 1.06 | [43] | ||
| 36 | 27 | 1.006 | [3] | 117 | 139 | 1 | [43] | |||
| 37 | 458.5 | 1.107 | [3] | 118 | 143 | 1.12 | [43] | |||
| 38 | 3— Aniene valley | 260 | 1.06 | [34] | 119 | 128 | 1.16 | [43] | ||
| 39 | 130 | 1.028 | [34] | 120 | 138 | 1.13 | [43] | |||
| 40 | 240 | 1.1 | [34] | 121 | 209 | 1.02 | [43] | |||
| 41 | 260 | 1.059 | [34] | 122 | 243 | 1.1 | [43] | |||
| 42 | 230 | 1.037 | [34] | 123 | 177 | 1.08 | [43] | |||
| 43 | 230 | 1.086 | [34] | 124 | 164 | 0.99 | [43] | |||
| 44 | 220 | 1.098 | [34] | 125 | 265 | 1.07 | [43] | |||
| 45 | 240 | 1.021 | [34] | 126 | 115 | 1.14 | [43] | |||
| 46 | 170 | 1.082 | [34] | 127 | 39 | 1.17 | [43] | |||
| 47 | 160 | 1.12 | [34] | 128 | 113 | 1.1 | [43] | |||
| 48 | 160 | 1.066 | [34] | 129 | 103 | 1.17 | [43] | |||
| 49 | 170 | 1.078 | [34] | 130 | 219 | 1.06 | [43] | |||
| 50 | 540 | 1.042 | [34] | 131 | 13— Sarteano | 105 | 1.157 | [44] | ||
| 51 | 480 | 1.042 | [34] | 132 | 146 | 1.316 | [44] | |||
| 52 | 4— Bagno Vignoni | 8 | 2.879 | [35] | 133 | 203 | 1.028 | [44] | ||
| 53 | 9.4 | 1.634 | [35] | 134 | 221 | 1.17 | [44] | |||
| 54 | 8.5 | 2.759 | [35] | 135 | 155 | 1.077 | [44] | |||
| 55 | 7.9 | 1.91 | [35] | 136 | 167 | 1.206 | [44] | |||
| 56 | 18.768 | 2.34 | [35] | 137 | 14— Saturnia | 129 | 1.125 | [45] | ||
| 57 | 26.421 | 2.3 | [35] | 138 | 208 | 1.133 | [45] | |||
| 58 | 9.521 | 2.553 | [35] | 139 | 18 | 1.565 | [45] | |||
| 59 | 21.394 | 2.498 | [35] | 140 | 13 | 1.585 | [45] | |||
| 60 | 13.049 | 2.436 | [35] | 141 | 10 | 1.234 | [45] | |||
| 61 | 5— Bolsena Lake | 990 | 1.35 | [27] | 142 | 15— Semproniano | 15.063 | [12] | ||
| 62 | 1600 | 1.19 | [27] | 143 | 29.783 | [12] | ||||
| 63 | 1520 | 1.18 | [27] | 144 | 7.287 | [12] | ||||
| 64 | 780 | 1.03 | [27] | 145 | 12.908 | [12] | ||||
| 65 | 790 | 1.03 | [27] | 146 | 7.451 | [12] | ||||
| 66 | 660 | 1.07 | [27] | 147 | 22.212 | [12] | ||||
| 67 | 1010 | 1.07 | [27] | 148 | 65.13 | [12] | ||||
| 68 | 6390 | 1.1 | [27] | 149 | 30.647 | [12] | ||||
| 69 | 120 | 1.195 | [27] | 150 | 77 | 1.017 | [12] | |||
| 70 | 4210 | 1.021 | [27] | 151 | 53 | 1.094 | [12] | |||
| 71 | 6— Canino | 128 | 1.349 | [36] | 152 | 53 | 1.198 | [12] | ||
| 72 | 54 | 1.311 | [36] | 153 | 77 | 1.112 | [12] | |||
| 73 | 51 | 1.426 | [36] | 154 | 22 | 1.361 | [46] | |||
| 74 | 71 | 1.297 | [36] | 155 | 196 | [46] | ||||
| 75 | 11 | 1.566 | [36] | 156 | 53 | 1.094 | [45] | |||
| 76 | 10 | 1.34 | [36] | 157 | 53 | 1.198 | [45] | |||
| 77 | 14 | 1.33 | [36] | 158 | 77 | 1.112 | [45] | |||
| 78 | 50 | 1.09 | [36] | 159 | 31 | 1.403 | [45] | |||
| 79 | 880 | 1.02 | [27] | 160 | 12.1 | 1.449 | [45] | |||
| 80 | 960 | 1.06 | [27] | 161 | 22 | 1.361 | [45] | |||
| 81 | 1410 | 1.04 | [27] | 162 | 45 | 1.196 | [45] | |||
| 163 | 9.5 | 1.307 | [45] |
References
- Panichi, C.; Tongiorgi, E. Carbon isotopic composition of CO2 from springs, fumaroles, mofettes and travertines of central and southern Italy: A preliminary prospection method of geothermal area. In Proceedings of the 2nd UN Symposium on the Development and Use of Geothermal Energy, San Francisco, CA, USA, 20–29 May 1975; pp. 815–825. [Google Scholar]
- Minissale, A. Origin, transport and discharge of CO2 in central Italy. Earth-Sci. Rev. 2004, 66, 89–141. [Google Scholar] [CrossRef]
- Faccenna, C.; Soligo, M.; Billi, A.; De Filippis, L.; Funiciello, R.; Rossetti, C.; Tuccinei, P. Late Pleistocene depositional cycles of the lapis Tiburtinus travertine (Tivoli, Central Italy): Possible influence of climate and fault activity. Glob. Planet. Change 2008, 63, 299–308. [Google Scholar] [CrossRef]
- Andrews, J.E. Palaeoclimatic records from stable isotopes in riverine tufas: Synthesis and review. Earth-Sci. Rev. 2006, 75, 85–104. [Google Scholar] [CrossRef]
- Capezzuoli, E.; Gandin, A.; Pedley, H.M. Decoding tufa and travertine (freshwater carbonates) in the sedimentary record: The state of the art. Sedimentology 2014, 61, 1–21. [Google Scholar] [CrossRef]
- Hancock, P.L.; Chalmers, R.M.L.; Altunel, E.; Çakir, Z. Travitonics: Using travertines in active fault studies. J. Struct. Geol. 1999, 21, 903–916. [Google Scholar] [CrossRef]
- Crossey, L.J.; Karlstrom, K.E.; Springer, A.E.; Newell, D.; Hilton, D.R.; Fischer, T. Degassing of mantle-derived CO2 and He from springs in the southern Colorado Plateau region—Neotectonic connections and implications for groundwater systems. Geol. Soc. Am. Bull. 2009, 121, 1034–1053. [Google Scholar] [CrossRef]
- Priewisch, A.; Crossey, L.J.; Karlstrom, K.E.; Polyak, V.J.; Asmerom, Y.; Nereson, A.; Ricketts, J.W. U-series geochronology of large-volume Quaternary travertine deposits of the southeastern Colorado Plateau: Evaluating episodicity and tectonic and paleohydrologic controls. Geosphere 2014, 10, 401–423. [Google Scholar] [CrossRef]
- Navarro, A.; Font, X.; Viladevall, M. Geochemistry and groundwater contamination in the La Selva geothermal system (Girona, Northeast Spain). Geothermics 2011, 40, 275–285. [Google Scholar] [CrossRef]
- Brogi, A.; Alçiçek, M.C.; Yalçıner, C.Ç.; Capezzuoli, E.; Liotta, D.; Meccheri, M.; Rimondi, V.; Ruggieri, G.; Gandin, A.; Boschi, C.; et al. Hydrothermal fluids circulation and travertine deposition in an active tectonic setting: Insights from the Kamara geothermal area (western Anatolia, Turkey). Tectonophysics 2016, 680, 211–232. [Google Scholar] [CrossRef]
- Frery, E.; Gratier, J.P.; Ellouz-Zimmerman, N.; Deschamps, P.; Blamart, D.; Hamelin, B.; Swennen, R. Geochemical transect through a travertine mount: A detailed record of CO2-enriched fluid leakage from Late Pleistocene to present-day—Little Grand Wash fault (Utah, USA). Quat. Int. 2017, 437, 98–106. [Google Scholar] [CrossRef]
- Berardi, G.; Vignaroli, G.; Billi, A.; Rossetti, F.; Soligo, M.; Kele, S.; Baykara, M.O.; Bernasconi, S.M.; Castorina, F.; Tecce, F.; et al. Growth of a Pleistocene giant carbonate vein and nearby thermogene travertine deposits at Semproniano, southern Tuscany, Italy: Estimate of CO2 leakage. Tectonophysics 2016, 690, 219–239. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wu, C.-C.; Cheng, H.; Edwards, R.L.; Hsieh, Y.-T.; Gallet, S.; Chang, C.-C.; Li, T.-Y.; Lam, D.D.; Kano, A.; et al. High-precision and high resolution carbonate 230Th dating by MC-ICP-MS with SEM protocols. Geochim. Cosmochim. Acta 2012, 99, 71–86. [Google Scholar] [CrossRef]
- Bourdon, B.; Turner, S.; Henderson, G.M.; Lundstrom, C.C. Introduction to U-series geochemistry. Rev. Mineral. Geochem. 2003, 52, 1–21. [Google Scholar] [CrossRef]
- Chabaux, F.; Riotte, J.; Dequincey, O. U-Th-Ra fractionation during weathering and river transport. Rev. Mineral. Geochem. 2003, 52, 533–576. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium-solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Murphy, W.M.; Shock, E.L. Environmental aqueous geochemistry of actinides. In Uranium: Mineralogy, Geochemistry and the Environment; Burns, P.C., Finch, R., Eds.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 1999; Volume 38, pp. 221–254. [Google Scholar]
- Pentcost, A. Travertine, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005; p. 446. [Google Scholar]
- Sturchio, N.C.; Antonio, M.R.; Soderholm, L.; Sutton, S.R.; Brannon, J.C. Tretravalent uranium in calcite. Science 1998, 281, 971–973. [Google Scholar] [CrossRef]
- Rihs, S.; Condomines, M.; Sigmarsson, O. U, Ra and Ba incorporation during precipitation of hydrothermal carbonates: Implications for 226Ra-Ba dating of impure travertines. Geochim. Cosmochim. Acta 2000, 64, 661–671. [Google Scholar] [CrossRef]
- Reeder, R.J.; Nugent, M.; Tait, D.; Morris, D.E.; Heald, S.M.; Beck, K.M.; Hess, W.P.; Lanzirotti, A. Coprecipitation of Uranium (VI) with Calcite: XAFS, micro-XAS, and luminescence characterization. Geochim. Cosmochim. Acta 2001, 65, 3491–3503. [Google Scholar] [CrossRef]
- Khoury, H.N.; Salameh, E.M.; Clark, I.D. Mineralogy and origin of surficial uranium deposits hosted in travertine and calcrete from central Jordan. Appl. Geochem. 2014, 43, 49–65. [Google Scholar] [CrossRef]
- Ivanovich, M.; Latham, A.G.; Ku, T.-L. Uranium-series disequilibrium applications in geochronology. In Uranium-Series Disequilibrium Applications to Earth, Marine and Environmental Sciences; Ivanovich, M., Harmon, R.S., Eds.; Oxford Science Publications; Clarendon Press: Oxford, UK, 1992; pp. 62–94. [Google Scholar]
- Fleischer, R.L. Alpha-recoil damage and solution effects in minerals: Uranium isotopic disequilibrium and radon release. Geochim. Cosmochim. Acta 1982, 46, 2191–2201. [Google Scholar] [CrossRef]
- Weyer, S.; Anbar, A.D.; Gerdes, A.; Gordon, G.W.; Algeo, T.J.; Boyle, E.A. Natural fractionation of 238U/235U. Geochim. Cosmochim. Acta 2008, 72, 345–359. [Google Scholar] [CrossRef]
- Rovan, L.; Lojen, S.; Zuliani, T.; Kanduč, T.; Petrič, M.; Horvat, B.; Rusjan, S.; Štrok, M. Comparison of Uranium Isotopes and Classical Geochemical Tracers in Karst Aquifer of Ljubljanica River catchment (Slovenia). Water 2020, 12, 2064. [Google Scholar] [CrossRef]
- Taddeucci, A.; Voltaggio, M. Th-230 dating of the travertines connected to the Vulsini Mts. volcanism (Northern Latium, Italy): Neotectonics and hydrogeology. Period. Mineral. 1987, 56, 295–302. [Google Scholar]
- Brogi, A.; Capezzuoli, E.; Aquè, R.; Branca, M.; Voltaggio, M. Studying travertines for neotectonics investigations: Middle–Late Pleistocene syn-tectonic travertine deposition at Serre di Rapolano (Northern Apennines, Italy). Int. J. Earth Sci. 2010, 99, 1383–1398. [Google Scholar] [CrossRef]
- Özkul, M.; Kele, S.; Gökgöz, A.; Shen, C.-C.; Jones, B.; Baykara, M.O.; Fόrizs, I.; Nemeth, T.; Chang, Y.-W.; Alçiçek, M.C. Comparison of the Quaternary travertine sites in the Denizli Extensional Basin based on their depositional and geochemical data. Sedim. Geol. 2013, 294, 179–204. [Google Scholar] [CrossRef]
- Gandin, A.; Capezzuoli, E. Travertine: Distinctive depositional fabrics of carbonates from thermal spring systems. Sedimentology 2014, 61, 264–290. [Google Scholar] [CrossRef]
- Janssens, N.; Capezzuoli, E.; Claes, H.; Muchez, F.; Yu, T.-L.; Shen, C.-C.; Ellam, R.M.; Swennen, R. Fossil travertine system and palaeofluid provenance, migration and evolution through time: Example from the geothermal area of Acquasanta Terme (Central Italy). Sediment. Geol. 2020, 398, 105580. [Google Scholar] [CrossRef]
- Sembroni, A.; Molin, P.; Soligo, M.; Tuccimei, P.; Anzalone, E.; Billi, A.; Franchini, S.; Ranaldi, M.; Tarchini, L. The uplift of the Adriatic flank of the Apennines since the Middle Pleistocene: New insights from the Tronto River basin and the Acquasanta Terme Travertine (central Italy). Geomorphology 2020, 352, 106990. [Google Scholar] [CrossRef]
- Faccenna, C.; Funiciello, R.; Montone, P.; Parotto, M.; Voltaggio, M. Late Pleistocene strike–slip tectonics in the Acque Albule Basin (Tivoli, Latium). Mem. Descr. Carta Geol. d’It. 1994, 49, 37–50. [Google Scholar]
- Carrara, C.; Branca, M.; Pisegna, E.; Verrubbi, V.; Voltaggio, M. Calcareous tufa deposits of the Aniene valley between Vallepietra and Mandela-Vicovaro (Latium, Central Italy). Il Quaternario 2006, 19, 19–44. [Google Scholar]
- Brogi, A.; Liotta, D.; Capezzuoli, E.; Matera, P.F.; Kele, S.; Soligo, M.; Tuccimei, P.; Ruggieri, G.; Yu, T.-L.; Shen, C.-C.; et al. Travertine deposits constraining transfer zone neotectonics in geothermal areas: An example from the inner Northern Apennines (Bagno Vignoni-Val d’Orcia area, Italy). Geothermics 2020, 85, 101763. [Google Scholar] [CrossRef]
- Carrara, C. I travertini di Canino (Viterbo, Italia Centrale): Elementi di Cronolitostratigrfia, di Geochimica Isotopica e loro significato ambientale e climatico. Il Quaternario 1994, 7, 73–90. [Google Scholar]
- D’Alessandro, W.; Glammanco, S.; Bellomo, S.; Parello, F. Geochemistry and mineralogy of travertine deposits of the SW flank of Mt. Etna (Italy): Relationships with past volcanic and degassing activity. J. Volcanol. Geotherm. Res. 2007, 165, 64–70. [Google Scholar] [CrossRef]
- Romano, R.; Taddeucci, A.; Voltaggio, M. Datazione col metodo del Th-130 di alcuni travertini deposti sul versante sudoccidentale del M.te Etna. Rend. Soc. Ital. Mineral. Petrol. 1987, 42, 294. [Google Scholar]
- Pola, M.; Gandin, A.; Tuccimei, P.; Soligo, M.; Deiana, R.; Fabbri, P.; Zampieri, D. A multidisciplinary approach to understanding carbonate deposition under tectonically controlled hydrothermal circulation: A case study from a recent travertine mound in the Euganean hydrothermal system, northern Italy. Sedimentology 2014, 61, 172–199. [Google Scholar] [CrossRef]
- Soligo, M.; Tuccimei, P.; Barberi, R.; Delitala, M.C.; Miccadei, E.; Taddeucci, A. U/Th dating of freshwater travertine from Middle Velino Valley (Central Italy): Geological and paleoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 184, 147–161. [Google Scholar] [CrossRef]
- Giustini, F.; Brilli, M.; Di Salvo, C.; Mancini, M.; Voltaggio, M. Multidisciplinary characterization of the buried travertine body of Prima Porta (Central Italy). Quat. Int. 2020, 568, 65–78. [Google Scholar] [CrossRef]
- Carrara, C.; Ciuffarella, L.; Paganin, G. Inquadramento geomorfologico e climatico -ambientale dei travertini di Rapolano Terme (SI). Il Quaternario 1998, 11, 319–329. [Google Scholar]
- Brancaccio, L.; D’Argenio, B.; Ferreri, V.; Stanzione, D.; Taddeucci, D.; Voltaggio, M. I travertini di Rocchetta a Volturno (Molise): Datazioni con 230Th e modello deposizionale. Mem. Soc. Geol. Ital. 1988, 41, 673–683. [Google Scholar]
- Brogi, A.; Capezzuoli, E.; Buracchi, E.; Branca, M. Tectonic Control on Travertine and Calcareous Tufa Deposition in a Low-Temperature Geothermal System (Sarteano, Central Italy). J. Geol. Soc. London 2012, 169, 461–476. [Google Scholar] [CrossRef]
- Vignaroli, G.; Berardi, G.; Billi, A.; Kele, S.; Rossetti, F.; Soligo, M.; Bernasconi, S.M. Tectonics, hydrothermalism, and paleoclimate recorded by Quaternary travertines and their spatio-temporal distribution in the Albegna basin, central Italy: Insights on Tyrrhenian margin neotectonics. Lithosphere 2016, 8, 335–358. [Google Scholar] [CrossRef]
- Billi, A.; Berardi, G.; Gratier, J.P.; Rossetti, F.; Vignaroli, G.; Baykara, M.O.; Bernasconi, S.; Kele, S.; Soligo, M.; De Filippis, L.; et al. First records of syn-diagenetic non-tectonic folding in quaternary thermogene travertines caused by hydrothermal incremental veining. Tectonophysics 2017, 700–701, 60–79. [Google Scholar] [CrossRef]
- Fusari, A.; Carroll, M.R.; Ferraro, S.; Giovannetti, R.; Giudetti, G.; Invernizzi, C.; Mussi, M.; Pennisi, M. Circulation path of thermal waters within the Laga foredeep basin inferred from chemical and isotopic (δ18O, δD, 3H, 87Sr/86Sr) data. Appl. Geochem. 2017, 78, 23–34. [Google Scholar] [CrossRef]
- Carucci, V.; Petitta, M.; Aravena, R. Interaction between shallow and deep aquifers in the Tivoli Plain (Central Italy) enhanced by groundwater extraction: A multiisotope approach and geochemical modeling. Appl. Geochem. 2012, 27, 266–280. [Google Scholar] [CrossRef]
- Chiodini, G.; Frondini, F.; Cardellini, C.; Parello, F.; Peruzzi, L. Rate of diffuse carbon dioxide Earth degassing estimated from carbon balance of regional aquifers: The case of central Apennine, Italy. J. Geophys. Res. 2000, 105, 8423–8434. [Google Scholar] [CrossRef]
- Sappa, G.; Ferranti, F. Pertuso Spring discharge assessment in the Upper Valley of Aniene River (Central Italy). Int. J. Energy Environ. 2016, 10, 33–40. [Google Scholar]
- Cioni, R.; Guidi, M.; Pierotti, L.; Scozzari, A. An automatic monitoring network installed in Tuscany (Italy) for studying possible geochemical precursory phenomena. Nat. Hazards Earth Syst. Sci. 2007, 7, 405–416. [Google Scholar] [CrossRef]
- Magi, F. Isotope geochemistry of rainfall, thermal and non-thermal waters from the Mt. Amiata area (northern-central Italy). Plinius 2020, 46, 56–63. [Google Scholar]
- Mosello, R.; Arisci, S.; Bruni, P. Lake Bolsena (Central Italy): An updating study on its water chemistry. J. Limnol. 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Chiodini, G.; Giaquinto, S.; Frondini, F.; Santucci, A. Hydrogeochemistry and hydrogeology of the Canino hydrothermal system (Italy). Geothermics 1991, 20, 329–342. [Google Scholar] [CrossRef]
- Bellia, C.; Gallardo, A.H.; Yasuhara, M.; Kazahaya, K. Geochemical characterization of groundwater in a volcanic system. Resources 2015, 4, 358–377. [Google Scholar] [CrossRef]
- Gherardi, F.; Panichi, C.; Caliro, S.; Magro, G.; Pennisi, M. Water and gas geochemistry of the Euganean and Berician thermal district (Italy). Appl. Geochem. 2000, 15, 455–474. [Google Scholar] [CrossRef]
- Giustini, F.; Blessing, M.; Brilli, M.; Lombardi, S.; Voltattorni, N.; Widory, D. Determining the origin of carbon dioxide and methane in the gaseous emissions of the San Vittorino plain (Central Italy) by means of stable isotopes and noble gas analysis. Appl. Geochem. 2013, 34, 90–101. [Google Scholar] [CrossRef]
- Petitta, M. Idrogeologia della media valle del fiume Velino e della piana di S. Vittorino (Rieti, Italia centrale) / Hydrogeology of the middle valley of the Velino river and of the S. Vittorino plain (Rieti, Central Italy). Ital. J. Eng. Geol. Environ. 2009, 1, 157–181. [Google Scholar]
- Giustini, F.; Brilli, M.; Mancini, M. Geochemical study of travertines along middle-lower Tiber valley (central Italy): Genesis, palaeo-environmental and tectonic implications. Int. J. Earth Sci. 2018, 107, 1321–1342. [Google Scholar] [CrossRef]
- Brilli, M.; Giustini, F. Geochemical stratigraphy of the Prima Porta travertine deposit (Roma, Italy). Minerals 2023. this Special Issue. [Google Scholar]
- Minissale, A.; Vaselli, O.; Tassi, F.; Magro, G.; Grechi, G.P. Fluid mixing in carbonate aquifers near Rapolano (central Italy): Chemical and isotopic constraints. Appl. Geochem. 2002, 17, 1329–1342. [Google Scholar] [CrossRef]
- Celico, P. Idrogeologia dei Massicci Carbonatici, Delle Piane Quaternarie e Delle Aree Vulcaniche Dell’italia Centro-Meridionale (Marche e Lazio Meridionali, Abruzzo, Molise e Campania); Cassa per il Mezzogiorno, stampa Grafiche Magliana: Roma, Italy, 1983; p. 236. [Google Scholar]
- Cuoco, E.; Colombani, N.; Darrah, T.H.; Mastrocicco, M.; Tedesco, D. Geolithological and anthropogenic controls on the hydrochemistry of the Volturno River (Southern Italy). Hydrol. Process. 2016, 31, 627–638. [Google Scholar] [CrossRef]
- Fancelli, R.; Nuti, S. Studio sulle acque termali e minerali del graben di Siena. Boll. Soc. Geol. Ital. 1975, 94, 135–155. [Google Scholar]
- Panichi, C.; D’Amore, F.; Fancelli, R.; Noto, P.; Nuti, S. Geochemical Survey of the Siena Province. In Proceedings of the Seminar on Geothermal Energy, Bruxelles, Belgium, 6–8 December 1977; Volume 2, pp. 481–503. [Google Scholar]
- Bertrami, R.; Cameli, G.M.; Lovari, F.; Rossi, U. Discovery of Latera geothermal field, problems of the exploration and research. Proceedings of Seminar on Utilization of Geothermal Energy for Electric Power Production and Space Heating, Florence, Italy, 14–17 May 1984; pp. 1–18. [Google Scholar]
- Barbagli, A.; Brogna, F.N.A.; Callegari, I.; Guastaldi, E.; Liali, G.; Marsico, N.; Rezza, C.; Trotta, M. Approccio multi-isotopico ed idrogeochimico per la caratterizzazione di acque termali: Il caso di Saturnia (GR). Ital. J. Groundw. 2013, AS07029, 025–040. [Google Scholar]
- Barberi, F.; Innocenti, F.; Ricci, C.A. Il magmatismo dell’Appennino Centro Settentrionale. Rend. Della Soc. Ital. Di Mineral. Petrol. 1971, 27, 169–210. [Google Scholar]
- Peccerillo, A. Cenozoic Volcanism in the Tyrrhenian Sea region, 2nd ed.; Springer: Cham, Switzerland, 2017; p. 399. [Google Scholar]
- Cocozza, T. Nuovi dati stratigrafici e tettonici sul monte Canino (Viterbo). Geol. Rom. 1963, 2, 15–40. [Google Scholar]
- Viganò, A.; Zampieri, D.; Rossato, S.; Martin, S.; Selli, L.; Prosser, G.; Ivy-Ochs, S.; Campedel, P.; Fedrizzi, F.; Franceschi, M.; et al. Past to present deformation of the central-eastern Southern Alps: From the foreland to the Giudicarie belt. Geol. Field Trips Maps 2018, 10, 2–78. [Google Scholar] [CrossRef]
- Minissale, A.; Kerrick, D.M.; Magro, G.; Murrell, M.T.; Paladini, M.; Rihs, S.; Sturchio, N.C.; Tassi, F.; Vaselli, O. Geochemistry of Quaternary travertines in the region north of Rome (Italy): Structural, hydrologic and paleoclimatic implications. Earth Planet. Sci. Lett. 2002, 203, 709–728. [Google Scholar] [CrossRef]
- Maggi, M.; Cianfarra, P.; Salvini, F.; Coelho de Lima, C. Staircase fractures in microbialites and the role of lamination-related mechanical anisotropy: The example of the Acquasanta Terme travertine deposits (central Italy). GSA Bull. 2015, 127, 879–896. [Google Scholar] [CrossRef]
- Menichetti, M. Assetto strutturale del sistema geotermico di Acquasanta Terme (Ascoli Piceno). Rend. Online Soc. Geol. Ital. Note Brevi 2008, 1, 118–122. [Google Scholar]
- Verdelocco, S.; Turkowsky, P.; Walzer, D. L’incidenza dei fattori geologici e delle variazioni climatiche nell’individuazione di aree ad alto potenziale di radon. Geol. Tec. Ambient. 2000, 1, 45–52. [Google Scholar]
- Aiuppa, A.; Allard, P.; D’Alessandro, W.; Michel, A.; Parello, F.; Treuil, M.; Valenza, M. Mobility and fluxes of major, minor and trace metals during basalt weathering and groundwater transport at Mt. Etna volcano (Sicily). Geochim. Cosmochim. Acta 2000, 64, 1827–1841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).