Abstract
China, Vietnam, Brazil, and Russia have the largest deposits of rare earths. However, in recent works, the occurrence of light rare earth elements has been demonstrated in an exhalative sedimentary type mineral (SEDEX) in Mexico, with adequate Ce and Nd contents. Additionally, it is this mineral that has been used to study the cation exchange capacity of non-metallic minerals and organic materials, such as bentonite, diatomite, and eggshell. To carry out this work, the crushed and ground SEDEX ore was leached using HCl, H2SO4, and HNO3. Subsequently, the liquid containing the Ce and Nd ions extracted from the mineral was put in contact with the respective ion exchangers, evaluating the effect of temperature and pH to determine the cation exchange efficiency of each exchanger tried. It was found that the best leaching results were achieved with the H2SO4, obtaining an extraction of Ce and Nd of 97.6% and 95.7%, respectively. On the other hand, in the case of cation exchange, the best results found for the extraction of Ce and Nd were using diatomite at a temperature of 323 K and a pH of 3, obtaining an extraction of 99.06% Ce and 99.07% Nd.
1. Introduction
China has been the major producer and exporter of rare earth elements (REE) in the world, previously having a production of almost 97% of the market [1], but recently other countries have increased their production, placing China at 70% of the global production [2]. Some years ago, the country issued some policies with the purpose of reducing exports [3]. Additionally, increasing the price and demand of REE has caused a greater interest (including in Mexico) in research on issues related to finding deposits of these elements and their processing to recover them [4].
In Mexico, there are some preliminary works related to the exploration and prospection of mineral deposits containing REE [5,6,7]. Recently, some researchers [8] reported a Sedimentary Exhalative ore deposit (SEDEX) containing some positive contents of REE, which is the result of hydrothermal sedimentation, that also has other concentrated elements of interest such as Pt, Au, and Pd, among others.
However, with the discovery of this mineral, there is now a need to find the best method for processing and extracting the REE contained, according to the particular characteristics of the ore, which have to be viable, of low cost, and environmentally friendly.
The cationic exchange method is a technique that implies a reversible ion change between a solid and a liquid phase [9]. This separation technique is used constantly in the industry due to its great efficiency, selectivity, low cost (traditionally, the process is carried out at room temperature), ease of use, and automation [10,11,12]. In the case of efficiency, the cation exchange with polymeric membranes has been used to recover elements such as Ce, Nd, and Pr, obtaining a recovery of 86.5% for each one. With the use of resins, elements such as Ce (15.2%), Nd (12.4%), La (11.5%), Pr (14.6%), Sm (14.6%), Eu (14.3%), and Gd (14.0%), could be recovered, previously eliminating Fe(III) [13,14].
The principal factors that affect the cationic exchange are the solution medium, the particle size of the mineral or material, pH, temperature, and the type of cationic exchanger [15]. Generally, the cationic exchange technique is used in the recovery and purification of REE, which are of importance due to their applications in modern life [16,17,18].
For instance, some works have probed the cationic exchange technique for the separation of REE [19,20,21,22], including the study of clay-type exchangers [23,24]. However, there is a lack of works related to the study of the cationic exchange of REE using exchangers such as bentonite (with a general formula of SiO3·H2O), diatomite (with a general formula of SiO2·nH2O), or eggshell (composed basically of CaCO3) [25].
In the case of bentonite, it is a non-metallic mineral whose structure contains clay minerals. Additionally, due to its properties, such as high porosity, abundance in the earth’s crust, and low cost, this mineral could be tested as a cationic exchanger [26]. On the other hand, diatomite is a sedimentary rock composed of fossilized diatom skeletons [27], and these skeletons are commonly composed of amorphous silica, carbonate, clay minerals, quartz, and feldspars. Diatomite also has a high capacity for absorption and adequate superficial area, chemical stability, and low apparent density due to its honeycomb structure formed by the skeletons of diatoms [28]. Finally, eggshell is composed mainly of calcium carbonate. When calcium is eliminated from its surface, organic material remains, and this can react with metallic ions; this means that eggshells could serve as adsorbent material for the recovery of metallic ions [29].
Around the world, there are few studies related to this topic using the above exchangers mentioned, but the following works are the basis for this study. First, Vander Watt et al., in 2012, studied the desorption of REE (Nd, Sm, and Dy) using sulfuric and chloride acid mediums for dissolution and bentonite as a cationic exchanger, getting good results and validating this mineral as an exchanger [30]. Other researchers analyzed the adsorption of La contained in an artificial solution using bentonite, evaluating the effect of the temperature in Langmuir and Freundlich adsorption isotherms, concluding that this mineral served as a low-cost and effective exchanger when working between 293 and 303 K with higher La concentrations in solution [31].
On the other hand, Agaard et al. studied the REE adsorption by cationic exchange using clay minerals such as kaolinite (whose general formula is [Al2Si2O5(OH)4], illite (with the general formula [(K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2, (H2O)]], and smectite (which general formula is [(Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O)], and they also concluded that the clay minerals were adequate for the cationic exchange of REE due to their good adsorption and structural characteristics [32]. In the same way, other work described the effect of pH and temperature during the cationic exchange between diatomite and thorium, concluding that this mineral was efficient for the process of exchange, being the most notorious pH effect [33].
Similarly, other researchers studied the viability and efficiency in the separation of La and Ce using animal products as exchangers, such as eggshells, and finding that the best adsorption conditions were obtained at a pH value of 6 during 4 h of reaction at a temperature of 323 K, getting adequate recoveries of Ce and La [25].
Additionally, also was carried out a preliminary study of cationic exchange using bentonite, phosphorite, and diatomite, finding that the best results were obtained with the phosphorite for the cationic exchange of precious metals and REE [34].
According to the above-mentioned studies, the innovation of this work will be to discover the efficiency of the cationic exchange of bentonite, diatomite, and eggshell to achieve adequate adsorption of REE contained in a SEDEX type ore mineral, evaluating the effect of pH and temperature during the adsorption of Ce and Nd, previously leached.
2. Materials and Methods
2.1. Materials
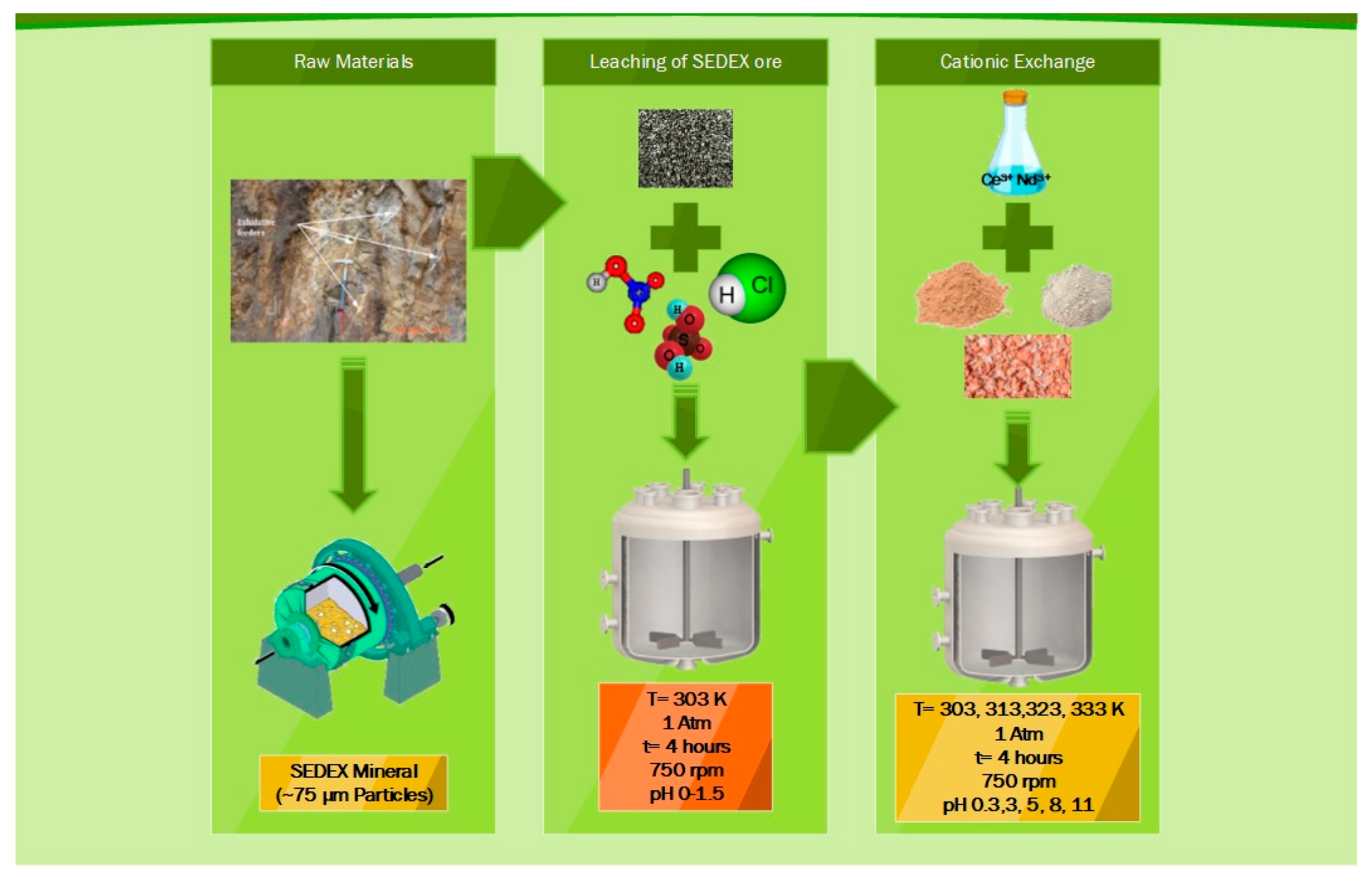
The mineral used for the Ce and Nd extraction was described and reported in previous work [8] and corresponds to a SEDEX-type mineral located in Mexico, which has different thermodynamic characteristics of formation and was also studied in other research work for leaching of precious metals contained in it [35,36]. This material, once collected, was crushed, ground, and sieved to a particle size of 75 μm (80% passing), which was used in the leaching experiments to get the Ce and Nd ions into solution, determined using spectrophotometry of emission by plasma inductively coupled (ICP).
On the other hand, the materials used as exchangers were obtained from deposits located in the state of Hidalgo in Mexico, for the case of diatomite [37], and bentonite obtained from the mining region of “Ejido Severino Ceniceros” in the state of Durango, Mexico, which were also crushed, ground, and sieved to obtain particle sizes lower than 75 μm. In the case of eggshells, they were collected domestically, then dehydrated, ground, and sieved to obtain similar particle sizes.
Characterization
The characterization for minerals and exchangers was executed to determine mainly the Ce and Nd contents in minerals before and after the extraction and cationic exchange procedures, and the principal characteristics of exchangers to evaluate their possible capabilities and efficiency before and after the cationic exchange procedure.
In addition, for all materials involved in this study, the analytical techniques used for the basic characterization were the following: X-ray diffraction (XRD), which was executed using a diffractometer of brand INEL, model EQUINOX 2000, located at the Autonomous University of the State of Hidalgo (UAEH), Mexico. For this case, the diffractograms were obtained at room temperature in an interval of 2θ from 20 to 100°, using Co kα1 radiation (λ = 1.789010 Å). The acquisition time was from 25 to 45 min, and the evaluation and indexation of the so obtained diffraction spectrums were carried out using the MATCH 3 software, which contains the base data COD-Inorg REV 1842382016.07.05 (Powder Diffraction Data Base).
The morphological analysis, and the semi-quantitative and punctual characterization, were performed using scanning electron microscopy (SEM) of brand JEOL, model JSM-6300 (located at the UAEH, Mexico), with a probe current of 102 to 105 Amperes (A), and a voltage of 30 kV. For the case of the morphological analysis, were employed the following conditions; acceleration voltage of 30 kV, capacitor current of 1 × 109 A, image mode in secondary electrons (SE), work distance of 15 mm, and opening of 600 μm.
On the other hand, the determination of the semi-quantitative and punctual chemical composition of materials was executed by energy dispersive spectrometry of X-rays (EDS), which was carried out at 15 KeV of energy using a spectrophotometer of brand OXFORD, which is attached to the SEM equipment. Additionally, the porosity of the three exchangers was measured using porosity measuring equipment by BET Micrometrics, model ASAP 2020 (located at the UAEH), which was carried out with 1 g of corresponding sample (bentonite, diatomite, and eggshell).
Finally, for the quantitative determination of Ce and Nd after leaching experiments and during cationic exchange procedure, was done using a spectrophotometer of brand PERKIN ELMER, model OPTIMA 3000 XL, which was employed using plasma of argon as a source for the producing of ionized atoms, also located at the UAEH, Mexico.
2.2. Experimental Procedure
Leaching of Mineral and Cationic Exchange
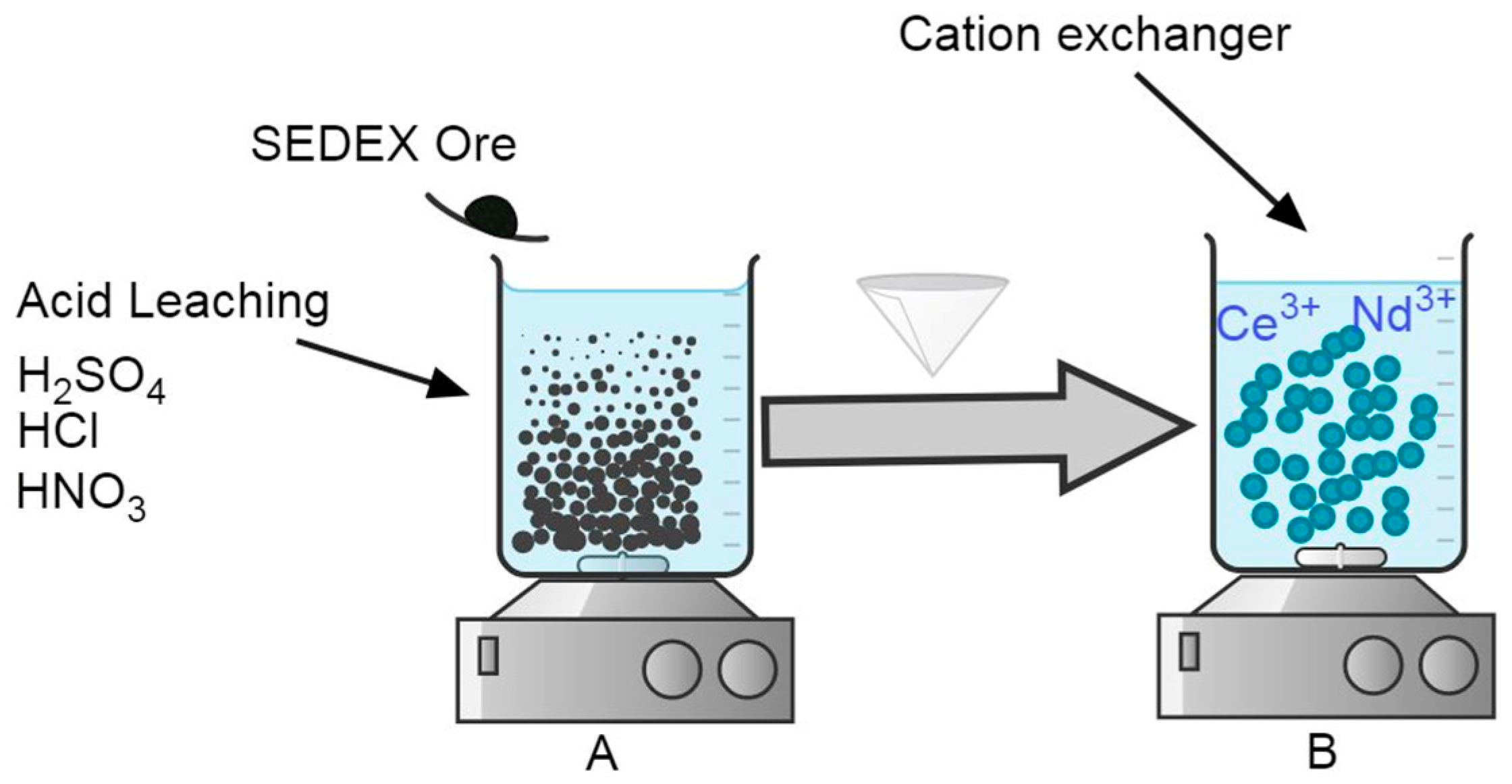
The leaching experiments to extract Ce and Nd from the SEDEX mineral were carried out in a 1 L conventional flat bottom glass reactor, with 500 mL of acid solution using H2SO4, HCl, or HNO3 1 M in each stage. The reactor was mounted on a heating plate with magnetic stirring and control of temperature. Additionally, pH measurement equipment was also used with a corresponding electrode of pH suitable for working in extreme conditions (pH range from 0 to 14). This experimental apparatus is shown in Figure 1A, and the experimental conditions used are shown in Table 1.
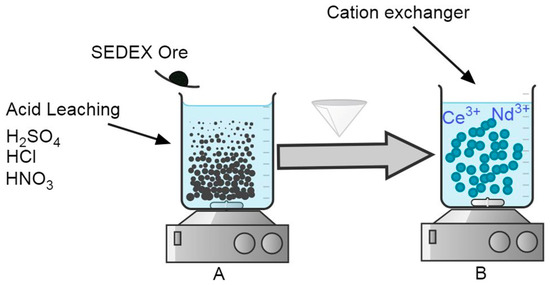
Figure 1.
Experimental setup designed for (A) the acid leaching of the SEDEX ore mineral and (B) the cationic exchange procedure using different cationic exchangers (bentonite, diatomite, and eggshell).

Table 1.
Experimental conditions used for the leaching of Ce and Nd contained in the SEDEX ore mineral.
For the case of cationic exchange experiments, the same experimental apparatus was used (Figure 1B). Here, the solution used was the obtained liquid after leaching experiments executed with 1 M H2SO4, because with this reagent, were obtained the maximum extractions of Ce and Nd. Table 2 shows the experimental conditions used in this procedure for the use of bentonite, diatomite, and eggshell during the cationic exchange experiments.

Table 2.
Experimental conditions used during cationic exchange carried out with bentonite (B), diatomite (D), and eggshell (E).
For both cases mentioned above, the beginning of reaction (leaching and cationic exchange) was determined when the mineral or exchanger came into contact with the respective solution.
All the aqueous products obtained after the leaching of minerals and during the cationic exchange were wholly analyzed by ICP to determine Ce and Nd contents after leaching and cationic exchange.
Finally, the efficiency of each one of the cationic exchangers used was obtained using the following equation:
where C1 is the Ce or Nd concentration in the liquid before the cationic exchange process, and C0 is the Ce or Nd concentration in the liquid after the cationic exchange process.
3. Results
3.1. Characterization of SEDEX-Type Ore
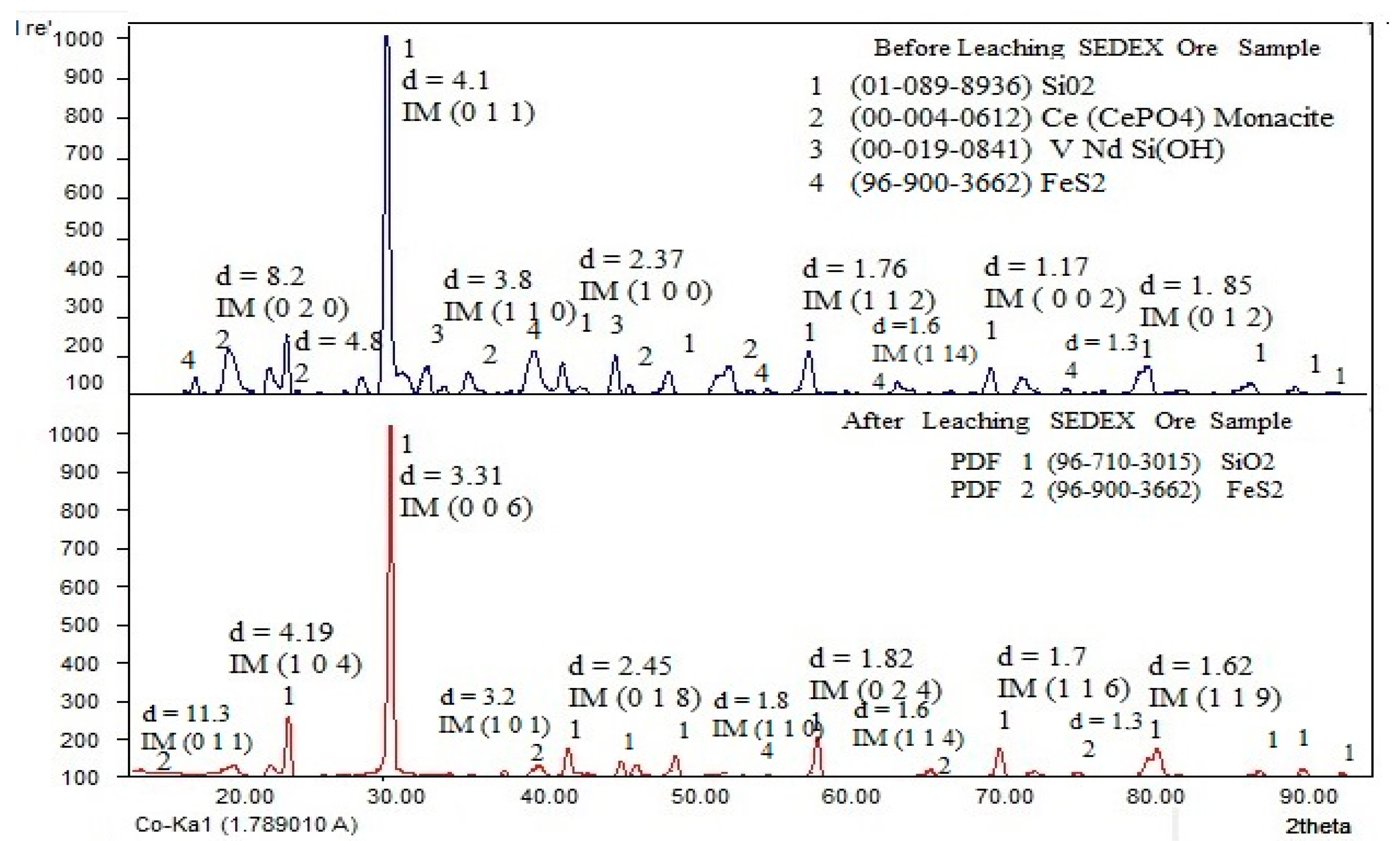
According to the obtained results found during the X-ray diffraction analysis performed on the ore, Figure 2 shows the XRD spectra from the SEDEX ore before and after leaching. The presence of SiO2 can be observed as major mineral species, but also, the ore has contents of some metals sulfides of Fe, Cu, and Ag. Additionally, the mineral has contents of Pt, Au, Pd, Eu, Dy, Ce, Nd, Y, and Gd in minor contents. After leaching (bottom of the spectrum), the absence of Ce and Nd can be observed, confirming that the leaching process adequately dissolved these elements, leaving in the residue the silica and the rest of the sulfides that did not react with sulfuric acid. In this figure, were identified and indexed the corresponding peaks using the following PDFs: [01-089-8936] for the SiO2; [00-004-0612] for the Monacite (general formula CePO4LaPO4NdPO4SmPO4); and [00-19-0814] for VNdSi(OH), which is the result of natural leaching by percolation of Vanadite [Pb5Cl(VO4)3], Monacite forming, and hydroxide, which associates V with Nd. Preliminarily, this result can confirm the presence of mineral phases that contain LREEs, such as Ce and Nd, which are the target of this work.
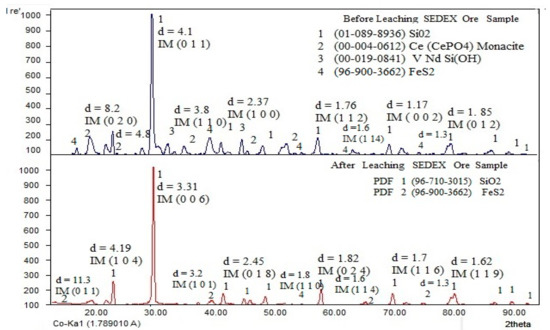
Figure 2.
XRD spectra of the SEDEX-type ore, before and after the extraction of Ce and Nd by leaching.
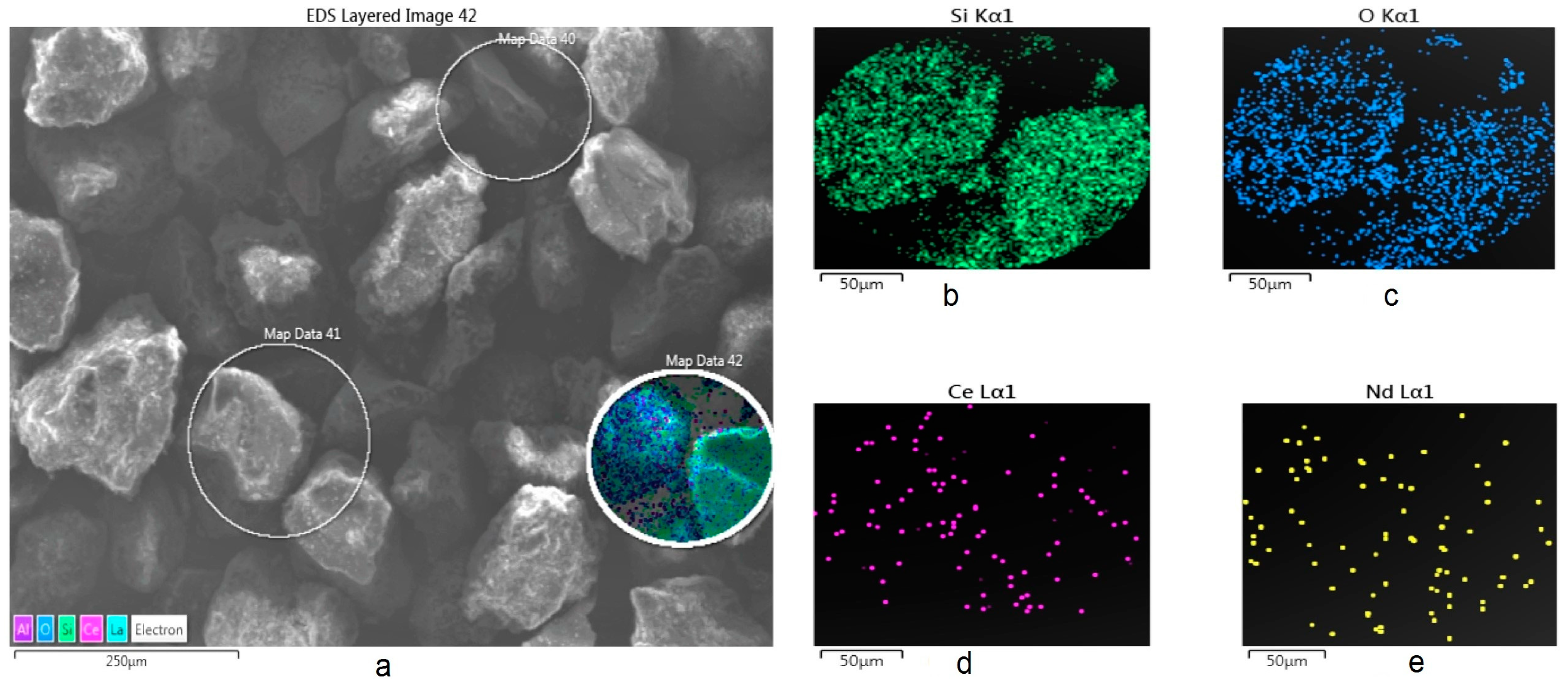
On the other hand, Figure 3 shows an image obtained by SEM-SE of some particles of the mineral, showing also the zones were punctual and semi-quantitative analysis was performed using EDS. The image also shows the corresponding X-ray mappings obtained for Ce and Nd (done on the Map Data 42 zone, while results for the Map Data 40–41 are not shown, because were similar), again confirming the presence of these elements.
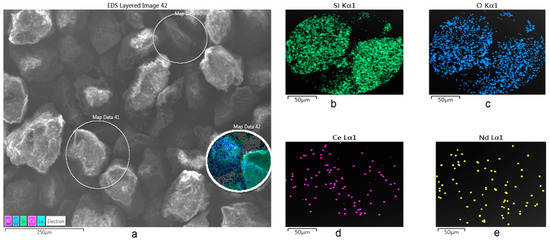
Figure 3.
SEM X-ray mapping analysis of the mineral used for the extraction and cationic exchange of Ce and Nd. (a) General image of particles; and X-ray mappings for (b) Si, (c) O, (d) Ce, and (e) Nd (zone Map Data 42).
3.2. Acid Leaching of the SEDEX Ore Mineral for Ce and Nd Extraction
For the case of acid leaching, Table 3 shows the corresponding extraction of Ce and Nd before and after leaching using H2SO4, HCl, or HNO3, respectively. The adequate extraction of both elements can be observed for all cases. However, for the case of H2SO4, were obtained higher contents of Ce and Nd dissolved in solution, corresponding to 97.6% for Ce and 95.7% for Nd.

Table 3.
Results of the acid leaching of SEDEX ore mineral using H2SO4, HCl, and HNO3.
3.3. Cationic Exchange
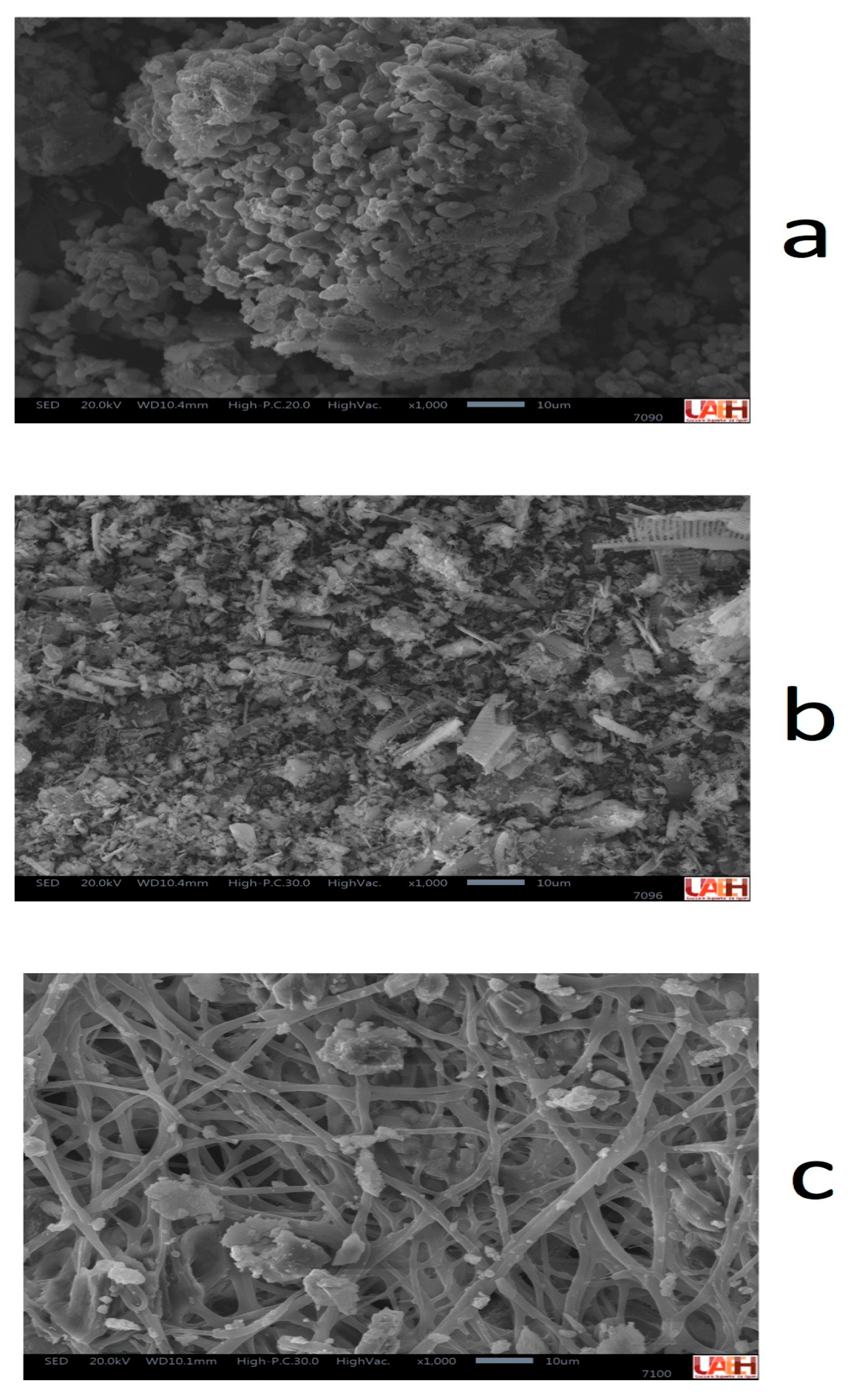
Before the cationic exchange procedure, the corresponding exchangers were characterized to determine if they had contents of Ce and Nd to effectively determine the extraction efficiency by cation exchange. So, Figure 4 shows the morphological characteristics of exchangers used during the cationic exchange experiments obtained by SEM (SE). The high porosity of all exchangers can be observed: bentonite (Figure 4a), diatomite (Figure 4b), and eggshell (Figure 4c). In all cases, this porosity is of importance for the cationic exchange process in the extraction of Ce and Nd.
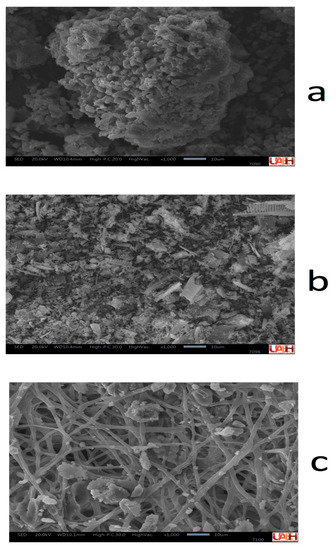
Figure 4.
Image of cationic exchangers; (a) bentonite, (b) diatomite, and (c) eggshell, obtained by SEM (SE) × 1000.
The results obtained for the BET-specific area surface for all exchangers are shown in Table 4, where it can be observed that both diatomite and bentonite have higher specific areas than eggshell, which offers more active sites for the interchange.

Table 4.
Results obtained from the BET analysis of the diatomite, bentonite, and eggshell.
On the other hand, Table 5 shows the concentration of Ce and Nd before experiments of cationic exchange. From the table, it can be observed that minerals used as exchangers did not have Ce and Nd before the exchange procedure. Additionally, the table shows the initial content of these elements found in the leachate.

Table 5.
Content of Ce and Nd in the leachate, bentonite, diatomite, and eggshell before cationic exchange experiments.
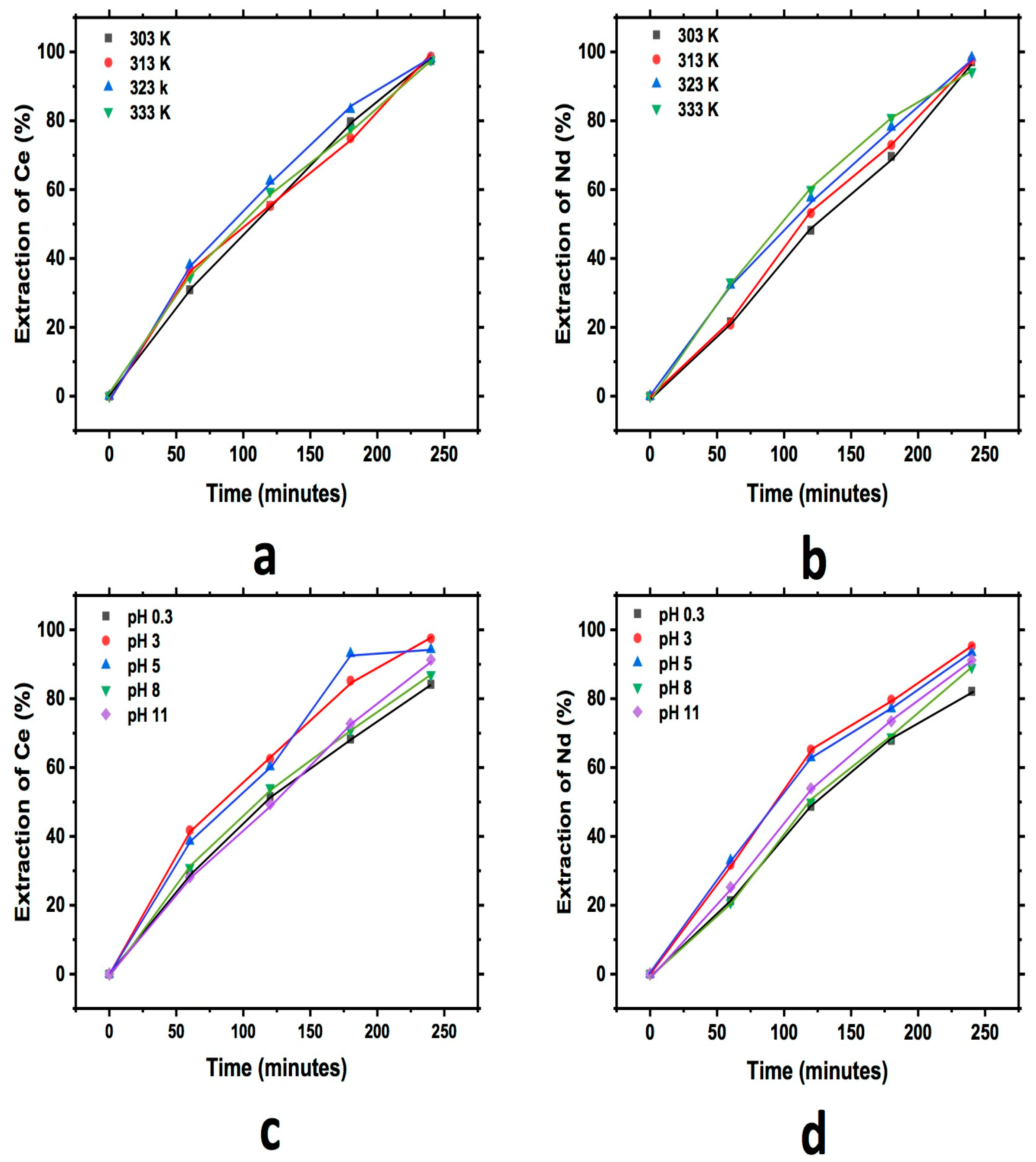
3.3.1. Bentonite
Figure 5 shows a graph that describes the extraction behavior for Ce and Nd during the study of cationic exchange, evaluating the effect of pH and temperature using bentonite. In each of the graphs, an increase in the extraction of both elements into the exchanger with respect to the reaction time can be observed until a maximum extraction for both elements is reached. A similar behavior for the case of temperature for Ce and Nd can be observed, while in the pH study, a little more extraction at a pH of 3 for Ce and Nd can be observed; a similar rate of reaction can also be seen in all cases.
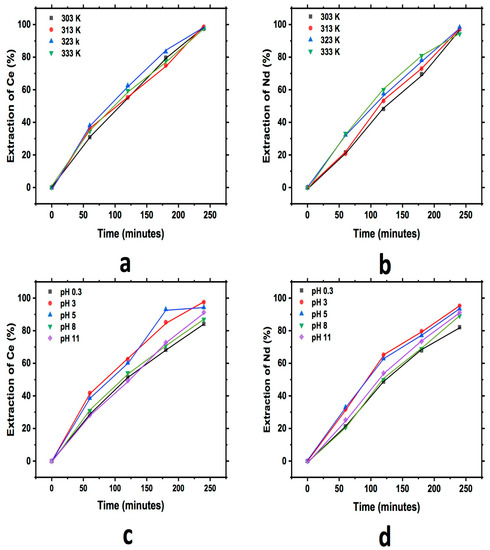
Figure 5.
Extraction behavior of Ce and Nd by cationic exchange using bentonite as exchanger; effect of temperature Ce (a) and Nd (b), and effect of pH Ce (c) and Nd (d), executed at a pH of 3 (temperature effect) and 303 K (pH effect).
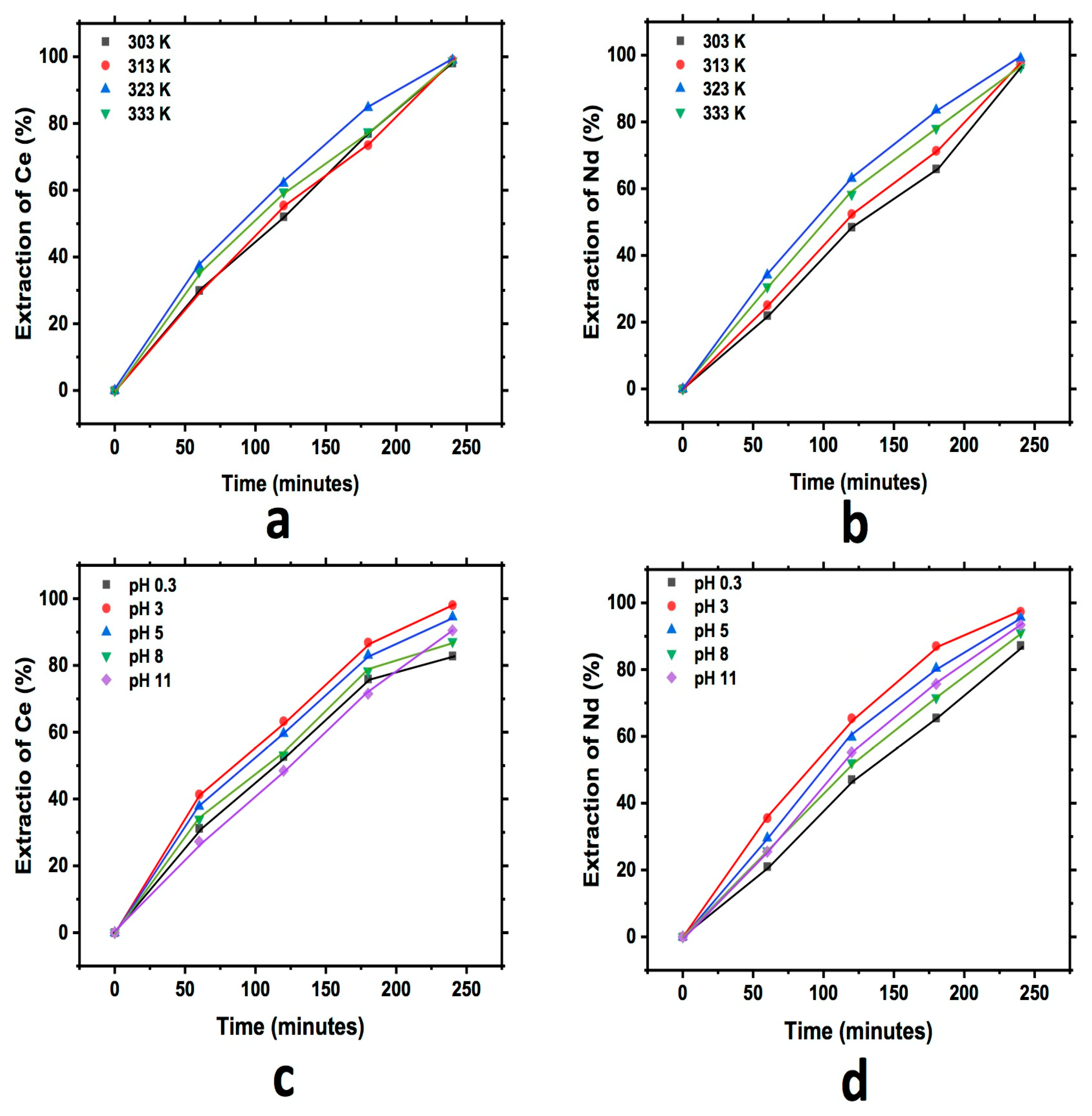
3.3.2. Diatomite
Figure 6 shows the graph that describes the extraction behavior for Ce and Nd during the study of the effect of pH and temperature in the cationic exchange using diatomite. In each of the graphs, an increase in the content of both elements in the diatomite with respect to the reaction time can be observed, as in the previous case. Additionally, as was described, a little more extraction for Ce and Nd at a pH of 3 was also observed in this case, while the effect of temperature has similar behavior for all temperatures tested.
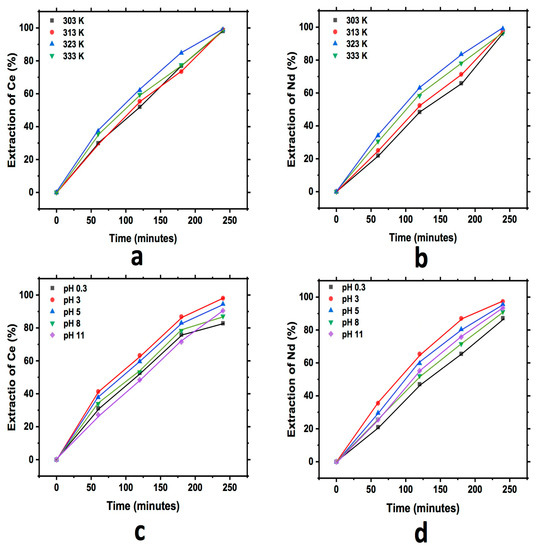
Figure 6.
Extraction behavior of Ce and Nd by cationic exchange using diatomite as exchanger; effect of temperature Ce (a) and Nd (b), and effect of pH Ce (c) and Nd (d), executed at a pH of 3 (temperature effect) and 303 K (pH effect).
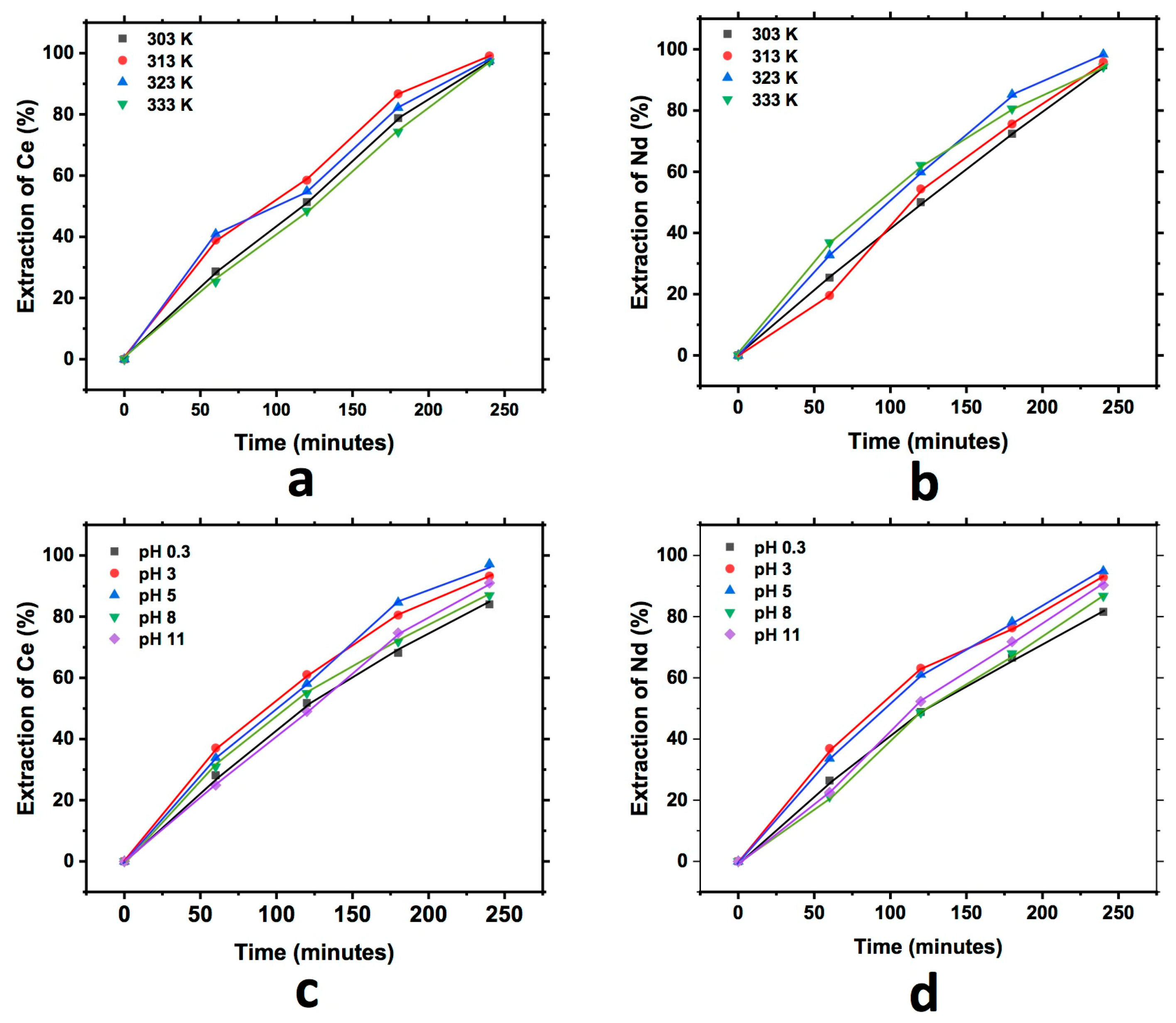
3.3.3. Eggshell
Similarly, Figure 7 shows the graph that describes the extraction behavior of Ce and Nd during the study of the effect of pH and temperature in the cationic exchange using the eggshell. In each of the graphs, an increase in the extraction of both elements into the exchanger with respect to the reaction time can be observed. The behavior for this case was similar to that observed in the two exchangers analyzed before, finding similar Nd and Ce extraction for all the temperatures tested, and in the case of the effect of pH, the best results obtained were at a pH of 5 for both elements, although the difference was minimal.
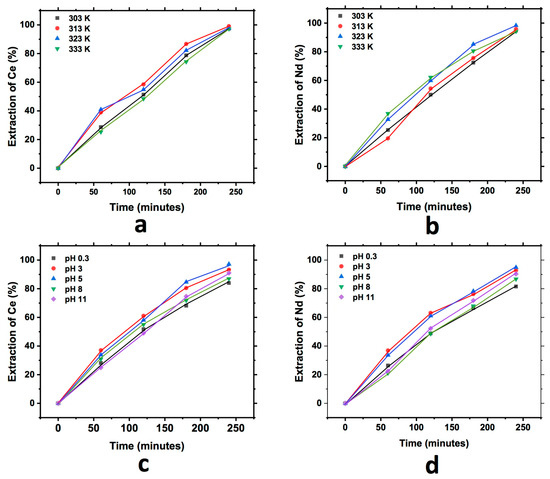
Figure 7.
Extraction behavior of Ce and Nd by cationic exchange using eggshell as exchanger; effect of temperature Ce (a) and Nd (b), and effect of pH Ce (c) and Nd (d), executed at a pH of 3 (temperature effect) and 303 K (pH effect).
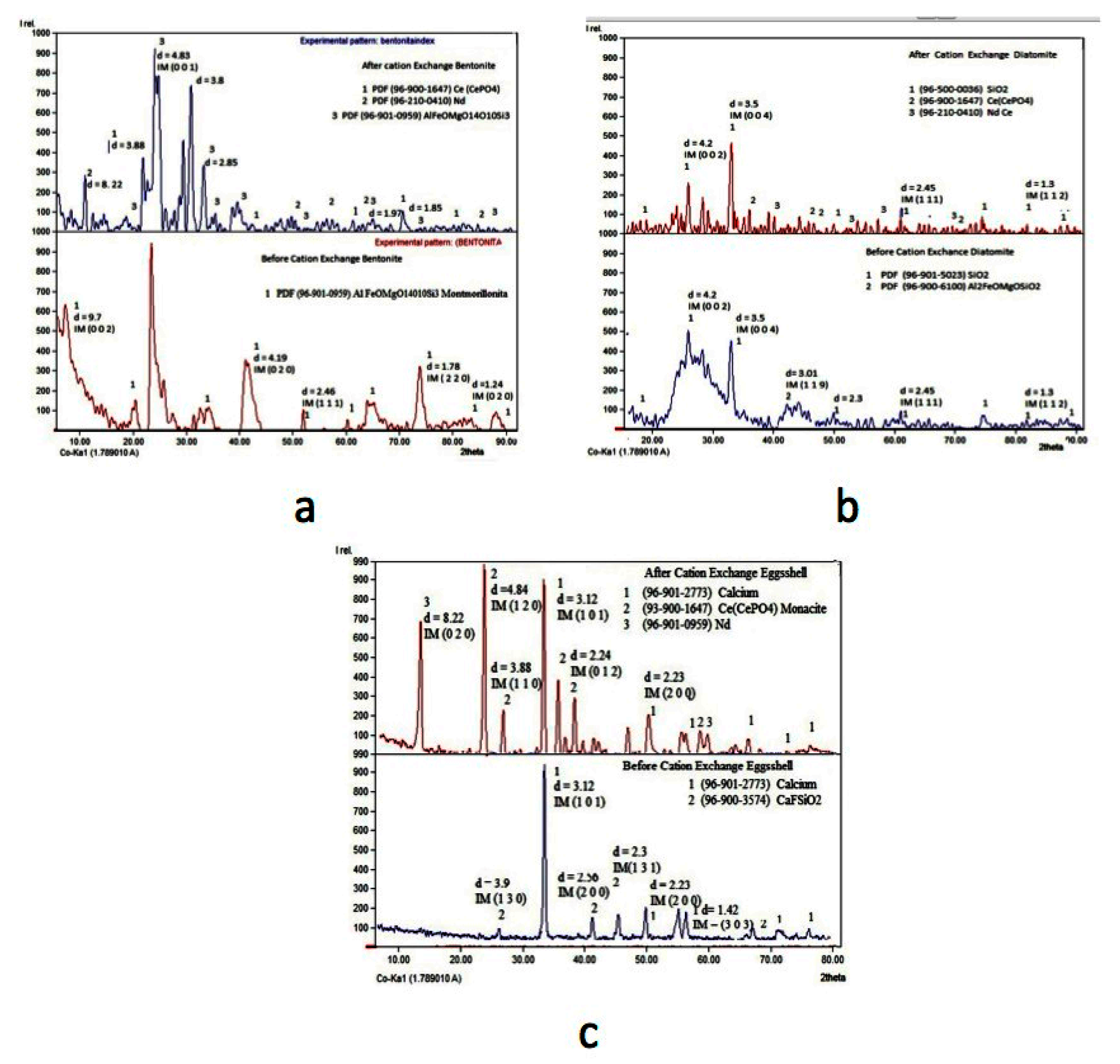
Finally, once the cationic exchange finished, the resulting solids obtained with the Ce and Nd adsorbed were characterized by XRD to disclose the presence of these elements in the structure of the corresponding exchangers. Figure 8 shows the XRD spectra for the bentonite, diatomite, and eggshell before and after the procedure.
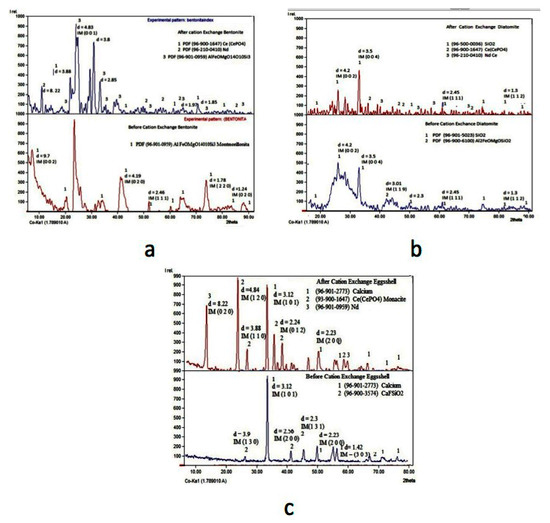
Figure 8.
XRD spectra for the cationic exchangers, bentonite (a), diatomite (b), and eggshell (c); before (bottom spectrum) and after (upper spectrum) the Ce and Nd extraction.
It can be observed for the three exchangers that, before the cationic exchange procedure, neither Ce nor Nd is present, but at the end of the exchange process, these elements were extracted from the liquid to each exchanger.
3.3.4. Efficiency of the Cationic Exchange
According to the obtained results, Table 6 shows the efficiency of extraction for each exchanger. Although the results are very similar, it can be observed that the best condition for the extraction of Ce and Nd was when using diatomite at a pH of 3.

Table 6.
Recovery efficiency of Ce and Nd, using different cationic exchangers; effect of pH at 303 K in all experiments.
Similarly, Table 7 shows the values of the elements extracted in the experiments of cationic exchange regarding the temperature (at a pH of 3); it can be observed that the Ce and Nd extraction were quite similar at all temperatures tested using the diatomite as an exchanger, which is of importance to reduce energy in the heat solution during the process.

Table 7.
Recovery efficiency of Ce and Nd, using different cationic exchangers; effect of temperature at a pH of 3 in all experiments.
4. Discussion
In the first place, the obtained results showed the presence of Ce and Nd in the SEDEX-type ore. Then, the extraction of these elements using acid leaching gave good results using H2SO4, confirming the adequate effect of this reagent for the dissolution of LREEs, as was reported by other authors [38,39] who pointed that sulfuric acid has the best capacity to solubilize the LREEs instead HCl or even HNO3. On the other hand, in this work, it was possible to corroborate the viability of ionic exchange between Ce and Nd with diatomite, bentonite, and eggshell. These results partially coincide with other studies [30,34,40]. The results obtained from the respective graphs show that there is a strong relationship between the change in the value of pH in the ion exchange experiment between diatomite and Ce and Nd; these results coincide with the tests of Sheng et al. [33], which obtained similar results for the element thorium. On the other hand, this was unlike Anastopoulos et al. [25], who achieved their best results for Ce recovery using the eggshell as a cationic exchanger at a temperature of 323 K and a pH of 6. In this work, the best conditions of temperature and pH were all temperatures (because there were minimal differences versus energy required) and a value of 5, respectively. Additionally, in comparison with the maximum yield obtained by Anastopoulos et al., which was 16.6%, in this work, the maximum yield obtained from Ce was 98.36%. In the case of comparing the results of this work with those of Juan Hernández et al. [34], it can be said that the results are similar since they also obtained yields above 99%. The best results for the cationic exchange found here were when using diatomite as the exchanger at all temperatures and a pH of 3 to obtain an extraction of Ce ranging from 98.13 to 99.06% and Nd ranging from 96.39 to 99.07%. On the other hand, according to the above, these results are of importance, due to how the cationic procedure could be carried out at room temperature, avoiding the use of additional energy for heating solutions, obtaining important savings, and reducing the adverse effects to the environment.
The results also show that there is a directly proportional relationship between the change in the concentration of Ce and Nd in each experiment, both for the temperature effect and for the pH effect, so for future studies, it is recommended to realize a kinetic study, in which the purpose of the work will be to obtain a model that describes the reaction process.
Finally, the processes involved during the extraction of Ce and Nd from the SEDEX ore are leaching and cationic exchange, which can be described with the flowsheet shown in Figure 9. Additionally, it can be mentioned that to obtain the final product of Ce and Nd oxides, this could be carried out with the stripping (HCl or HNO3) of the exchangers containing said elements. Subsequently, the precipitation can be performed with oxalates (using oxalic acid) and, finally, a calcination stage at 800 °C. Another alternate way is to leach exchangers with Ce and Nd using ammonium nitrate or sulfate, carry out precipitation with ammonium carbonate, and finally, recover the oxides by electro-flotation.
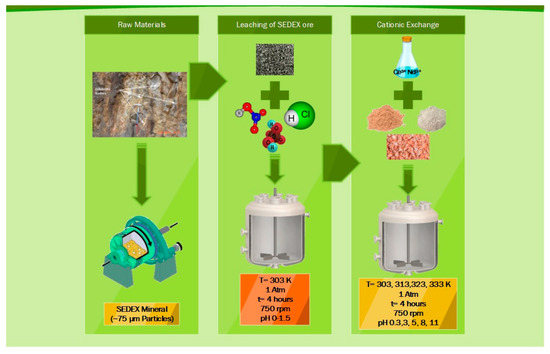
Figure 9.
Flowsheet for the leaching of the SEDEX ore for the extraction of Ce and Nd by cationic exchange.
5. Conclusions
The results obtained showed the presence of Ce and Nd in the SEDEX ore used for the leaching and cationic exchange processes.
During acid leaching, the best extractions for Ce and Nd were obtained when using H2SO4 as a leaching reagent, getting 97.6% of Ce and 95.7% of Nd at room temperature.
During the analysis of results, it was determined that there were minimal differences during the recovery of Ce and Nd at all temperatures tested for the three exchangers used. However, the most important results are described next.
For the case of bentonite, this exchanger showed better recoveries of Ce with a temperature of 313 K, getting a maximum of 98.63%, and with a pH of 3, of 97.57%, while for the case of Nd, the best conditions used for the recovery of a higher content of this element were at a temperature of 323 K (98.23%) and a pH of 3 (95.24%).
Similarly, the eggshell had good behavior as a cationic exchanger, showing better recoveries of Ce at a temperature of 313 K (99.11%) and a pH value of 5 (97.18%). On the other hand, for the case of Nd, the best recoveries were obtained with a temperature of 323 K (98.36%) and a pH of 5 (94.87%).
Finally, the highest extraction of Ce and Nd obtained was using diatomite as a cationic exchanger at a temperature of 323 K and a pH value of 3. The values obtained for Ce and Nd were 99.06 and 99.07%, respectively.
Author Contributions
Conceptualization, E.S.R., J.H.-Á. and E.C.-S.; methodology, E.A.C.-R.; software, M.P.G.-A., A.S.-C. and O.A.A.-S.; validation, E.S.R., F.R.B.-H. and J.F.-B.; formal analysis, E.S.R. and J.H.-Á.; investigation, E.S.R., E.C.-S. and J.H.-Á.; resources, O.A.A.-S. and E.A.C.-R.; data curation, M.P.G.-A. and E.S.R.; writing—original draft preparation, E.S.R., E.A.C.-R., J.H.-Á. and E.C.-S.; writing—review and editing, E.S.R.; visualization, F.R.B.-H., J.F.-B. and O.A.A.-S.; supervision, E.S.R. and E.C.-S.; project administration, J.H.-Á. and E.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data Sharing is not applicable to this article.
Acknowledgments
The authors are thankful to CONAHCyT of the Mexican Government for the support of the scholarship awarded to the doctorate student (E.A.C.-R.) with CVU 778782.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daly, J.C.K. Mongolia’s rare earth reserves draw foreign investor interest. Centr. Asia-Cauc. Anal. 2011, 13, 6–8. [Google Scholar]
- USGS (U.S. Geological Survey). Mineral Commodity Summaries (2018–2022e) [R/LO]. Available online: https://pubs..usgs.gov/periodicals/mcs2023/msc2023-rare-earths.pdf (accessed on 15 May 2023).
- Kim, R.; Cho, H.; Han, K.N.; Kim, K.; Mun, M. Optimization of acid leaching of rare-earth elements from mongolian apatite-based ore. Minerals 2016, 6, 63. [Google Scholar] [CrossRef]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A review on the cracking, baking and leaching processes of rare earth element concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Rodriguez-Figueroa, G.M. Geochemistry of Trace Elements, Major Elements and Rare Earth Elements, in the Marine Sediments of the Mining District of Santa Rosalía, B.C.S; Instituto Politecnico Nacional: Mexico City, Mexico, 2004. [Google Scholar]
- Campa Uranga, M.F.; Torres de León, R.; Iriondo, A.; Premo, W.R. Caracterización geológica de los ensambles metamórficos de Taxco y Taxco el Viejo, Guerrero (In Spanish), México. Boletín La Soc. Geológica Mex. 2012, 64, 369–385. [Google Scholar] [CrossRef]
- Badillo, A.L.; Flores, K.; Esquivel, C.; Duran, J.C.; Noé, P. Organic Geochemistry applied to Middle Jurassic sedimentary rocks in the Aztlan outcrop in Huhuetla, Hidalgo (Mexico): Paleoenvironmental implications. XXII Congr. Nac. Geoquimica 2012, 18, 181. [Google Scholar]
- Cerecedo-Sáenz, E.; Rodríguez-Lugo, V.; Hernández-Ávila, J.; Mendoza-Anaya, D.; Reyes-Valderrama, M.I.; Moreno-Pérez, E.; Salinas-Rodríguez, E. Mineralization of Rare Earths, Platinum and Gold in a Sedimentary Deposit, Found Using an Indirect Method of Exploration. Asp. Min. Miner. Sci. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan; Hong, H.J.; Chung, K.W.; Kim, S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep. Purif. Technol. 2020, 246, 116896. [Google Scholar] [CrossRef]
- Walls, D.; Walker, J.M. Protein Chromatography. Protein Chromatogr. 2017, 1485, 423. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Chapter 22 Ion-Exchange Chromatography. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 463, pp. 349–371. [Google Scholar]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, A. Recovery of rare earths elements from electronic waste by diffusion dialysis. Separ. Pufic. Tech. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Hermassi, M.; Granados, M.; Valderrama, C.; Skoglund, N.; Ayora, C.; Cortina, J.L. Impact of functional group types in ion exchange resins on rare earths elements recovery fromtreated acid mine waters. J. Clean. Prod. 2022, 379 Pt 2, 134742. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Xin, C.; Zhu, J.; Xinghu, W.; Zhu, R.; Wang, H. Understanding the role of natural clay minerals as effective adsorbents and alternative source of rare earth elements: Adsorption operative parameters. Hydrometallurgy 2019, 185, 149–161. [Google Scholar] [CrossRef]
- Avdibegović, D.; Zhang, W.; Xu, J.; Regadío, M.; Koivula, R.; Binnemans, K. Selective ion-exchange separation of scandium(III) over iron(III) by crystalline A-zirconium phosphate platelets under acidic conditions. Sep. Purif. Technol. 2019, 215, 81–90. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yan, Y.; Chen, J.; Dai, J.; Dai, X. Separation of adjacent heavy rare earth Lutetium (III) and Ytterbium (III) by task-specific ionic liquid Cyphos IL 104 embedded polymer inclusion membrane. J. Memb. Sci. 2020, 610, 118263. [Google Scholar] [CrossRef]
- Shu, Q.; Khayambashi, A.; Wang, X.; Wei, Y. Studies on adsorption of rare earth elements from nitric acid solution with macroporous silica-based bis(2-ethylhexyl)phosphoric acid impregnated polymeric adsorbent. Adsorpt. Sci. Technol. 2018, 36, 1049–1065. [Google Scholar] [CrossRef]
- Khawassek, Y.M.; Eliwa, A.A.; Haggag, E.S.A.; Omar, S.A.; Abdel-Wahab, S.M. Adsorption of rare earth elements by strong acid cation exchange resin thermodynamics, characteristics and kinetics. SN Appl. Sci. 2019, 1, 51. [Google Scholar] [CrossRef]
- Bohác, P.; Delaverhe, L.; Zervas, E.; Königer, F.; Schuhmann, R.; Emmerich, K. Cation exchange capacity of bentonite in a saline environment. Appl. Geochem. 2019, 100, 407–413. [Google Scholar] [CrossRef]
- da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar] [CrossRef]
- Cristiani, C.; Bellotto, M.; Dotelli, G.; Latorrata, S.; Ramis, G.; Stampino, P.G.; Zubiani, E.M.I.; Finocchio, E. Rare earths (La, y, and nd) adsorption behaviour towards mineral clays and organoclays: Monoionic and trionic solutions. Minerals 2021, 11, 30. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Pelosato, R.; Mesto, E.; Schingaro, E.; Lacalamita, M. Use of natural clays as sorbent materials for rare earth ions: Materials characterization and set up of the operative parameters. Waste Manag. 2015, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Freiherr, G. Conventional x-ray still tops in vascular imaging. Diagn. Imaging 1994, 16, 33–34, 37, 39. [Google Scholar]
- Cheng, Y.; Tao, J.; Jiao, Y.; Guo, Q.; Li, C. Influence of diatomite and mineral powder on thermal oxidative ageing properties of asphalt. Adv. Mater. Sci. Eng. 2015, 2015, 947834. [Google Scholar] [CrossRef]
- Al-Degs, Y.; Khraisheh, M.A.M.; Tutunji, M.F. Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res. 2001, 35, 3724–3728. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption of Cr(III) ions by eggshells. J. Hazard. Mater. 2005, 121, 167–173. [Google Scholar] [CrossRef]
- Van Der Watt, J.G.; Waanders, F.B. Leaching of rare earth elements from bentonite clay. J. S. Afr. Inst. Min. Metall. 2012, 112, 281–285. [Google Scholar]
- Chegrouche, S.; Mellah, A.; Telmoune, S. Removal of lanthanum from aqueous solutions by natural bentonite. Water Res. 1997, 31, 1733–1737. [Google Scholar] [CrossRef]
- Aagaard, P. Rare earth elements adsorption on clay minerals. Bull. Groupe Français Argiles 1974, 26, 193–199. [Google Scholar] [CrossRef]
- Sheng, G.; Hu, J.; Wang, X. Sorption properties of Th(IV) on the raw diatomite-Effects of contact time, pH, ionic strength and temperature. Appl. Radiat. Isot. 2008, 66, 1313–1320. [Google Scholar] [CrossRef]
- Hernandez-Avila, J.; Serrano-Mejía, E.O.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Isabel Reyes-Valderrama, M.; del Pilar Gutiérrez-Amador, M.; Rodríguez-Lugo, V. Use of Porous no Metallic Minerals to Remove Heavy Metals, Precious Metals and Rare Earths, by Cationic Exchange. In Trace Metals in the Environment-New Approaches and Recent Advances; IntechOpen: London, UK, 2021; Chapter 11; pp. 1–18. [Google Scholar] [CrossRef]
- Roldán-Contreras, E.; Salinas-Rodríguez, E.; Hernández-Ávila, J.; Cerecedo-Sáenz, E.; Rodríguez-Lugo, V.; Jeldres, R.I.; Toro, N. Leaching of Silver and Gold Contained in a Sedimentary Ore, Using Sodium Thiosulfate; A Preliminary Kinetic Study. Metals 2020, 10, 159. [Google Scholar] [CrossRef]
- Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Amador-Ortega, C.A.; del Pilar Gutiérrez-Amador, M.; Sánchez-Trujillo, M.G.; Hernández-Ávila, J. Recuperación de Tierras Raras Mediante Intercambio Catiónico, Usando bentonita Natural: Estudio Preliminar (In Spanish). Pädi 2019, 7, 98–103. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.I.; Arenas-Flores, A.; Román-Gutiérrez, A.D.; Rodríguez-Lugo, V. Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals 2017, 7, 169. [Google Scholar] [CrossRef]
- Echeverry-Vargas, L.; Rojas-Reyes, N.R.; Ocampo-Carmona, L.M. Recovery of light rare earths elements, cerium, lanthanum, and neodymium from alluvial gold mining waste from the Bagre-Nechí mining district in Colombia using acid leaching, oxalate precipitation and calcination. Hydrometallurgy 2023, 216, 106009. [Google Scholar] [CrossRef]
- Xie, C.; Xiao, Y.; He, C.; Lui, W.-S.; Tang, Y.-T.; Wang, S.; van der Ent, A.; Morel, J.L.; Simonnot, M.-O.; Qiu, R.-L. Selective recoveryof rare earths elements and value-added chemicals from de Dicranopteris linearis bio-ore produced by agromining using green fractionation. J. Hazard. Mater. 2023, 443, 130253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Xie, Z.; Li, Y.; Tang, W.; Yu, J. Calcium Hydroxide recycled from waste eggshell resources for the effective recovery of flouride from wastwater. RSC Adv. 2022, 12, 28264–28278. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).