Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Samples
2.2. X-ray Diffractometry Detection (XRD)
2.3. Scanning Electron Microscope Test
2.4. Gas Chromatography—Mass Spectroscopy Test
2.5. Flotation Kinetic Experiment
2.6. Fourier Transform Infrared Spectroscopy Test
2.7. X-ray Photoelectron Spectroscopy Test
2.8. Contact Angle Measurement
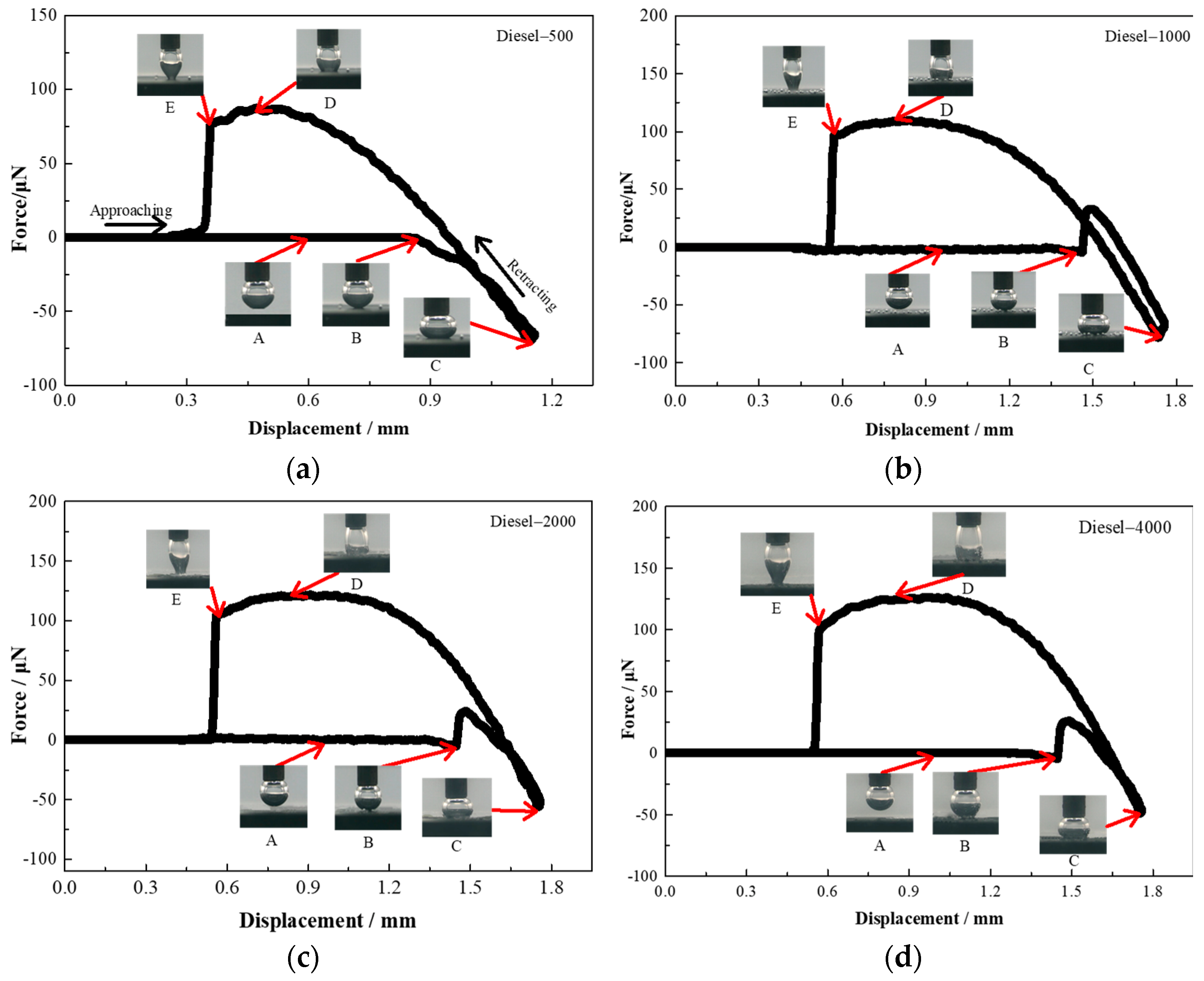
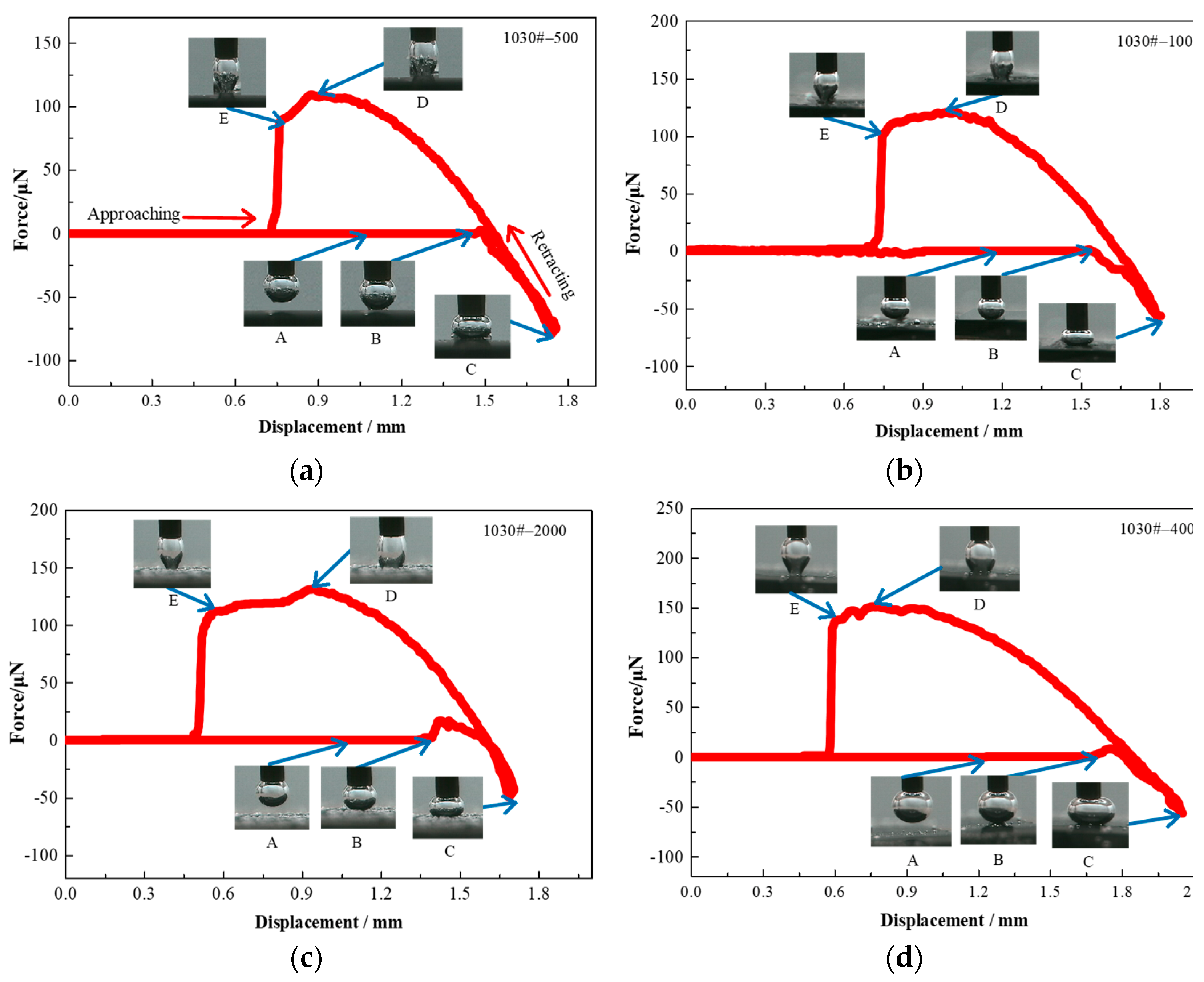
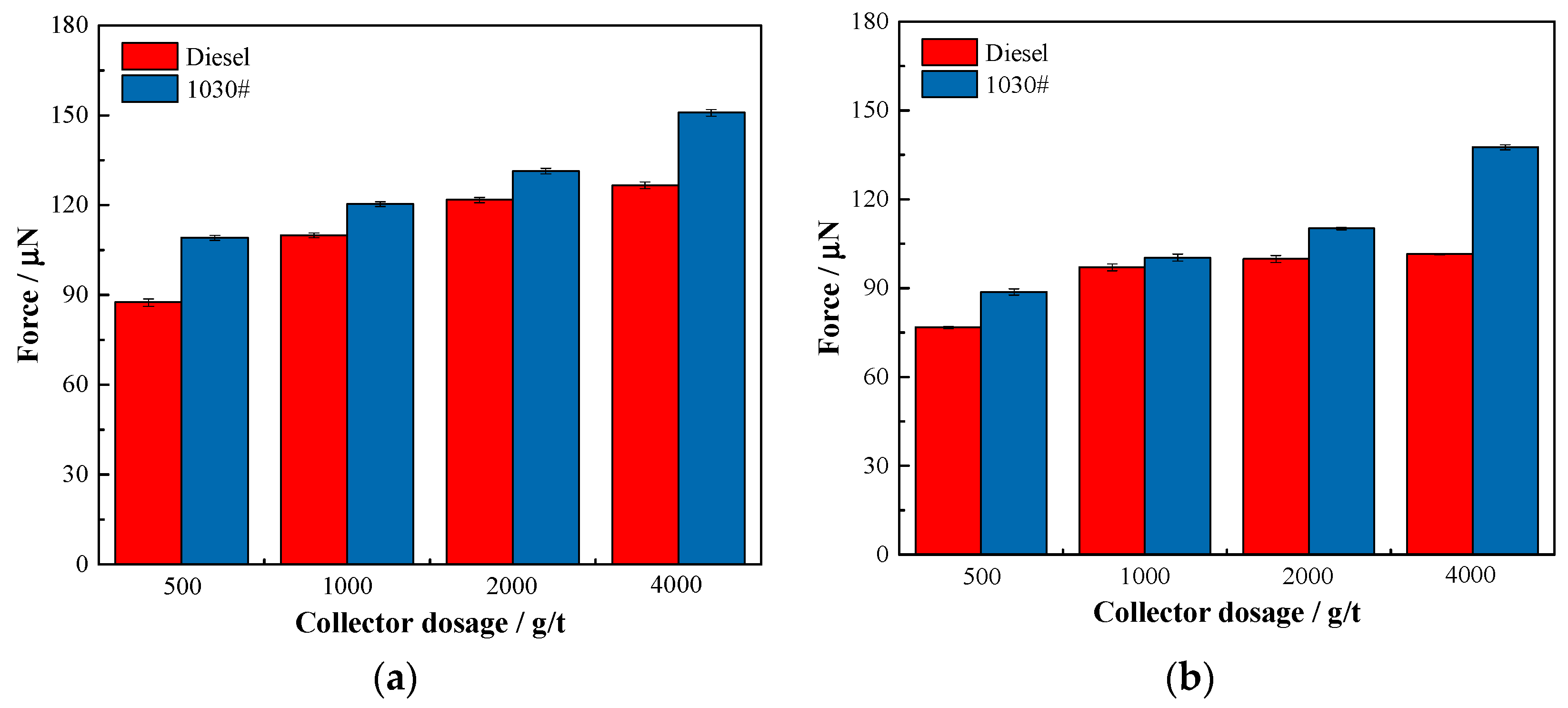
2.9. Bubble—Particle Interaction Measurement
3. Results and Discussion
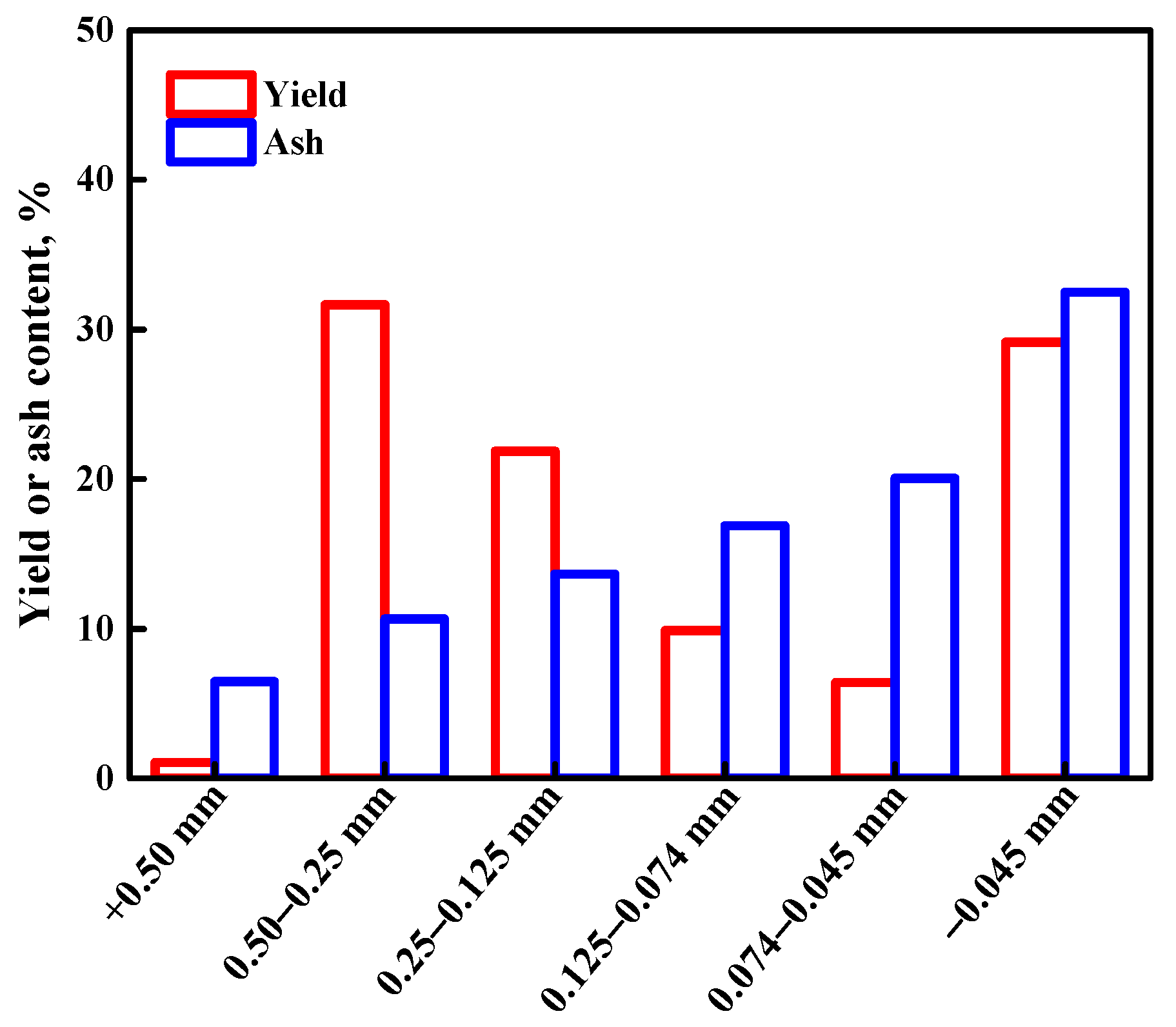
3.1. Analysis of Properties for Raw Coal
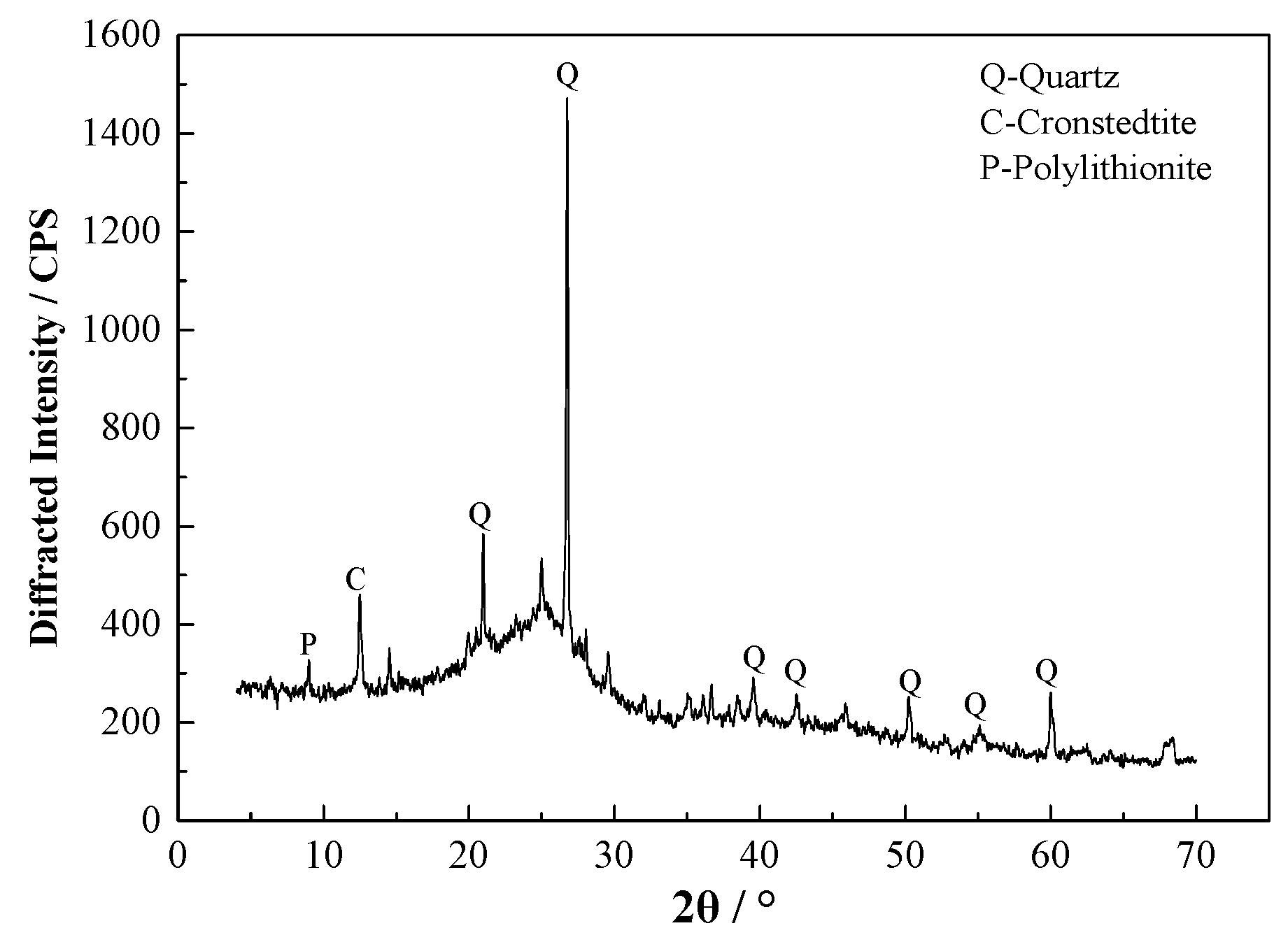
3.1.1. XRD Result of Raw Coal
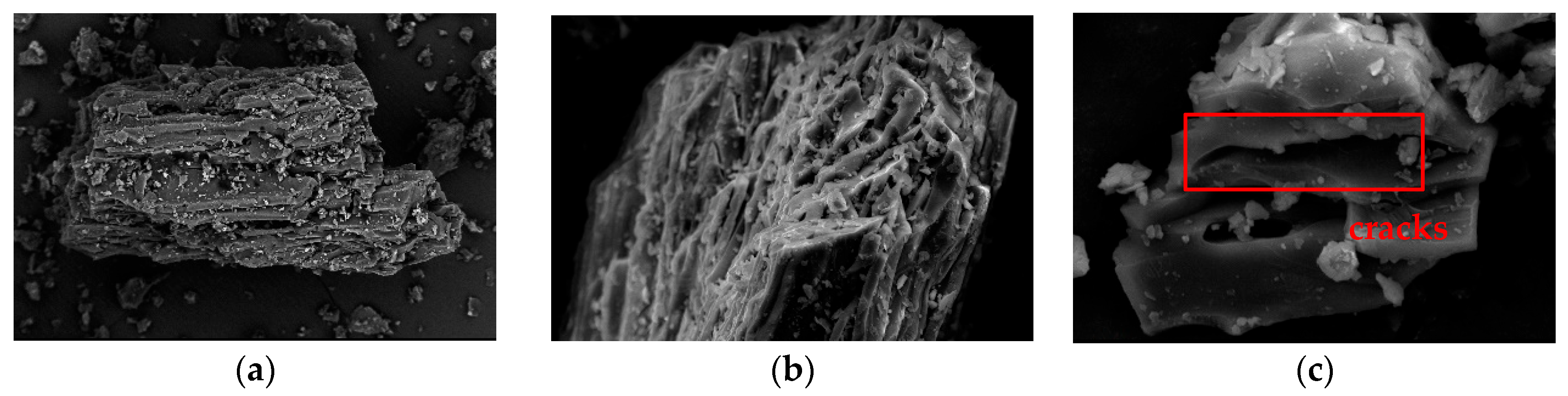
3.1.2. Surface Morphology of Raw Coal
3.2. GC-MS Results of Homemade Vegetable Oil 1030#
3.3. Flotation Results
3.4. Research Results of Enhanced Flotation Mechanism of Vegetable Oil
3.4.1. FTIR Results
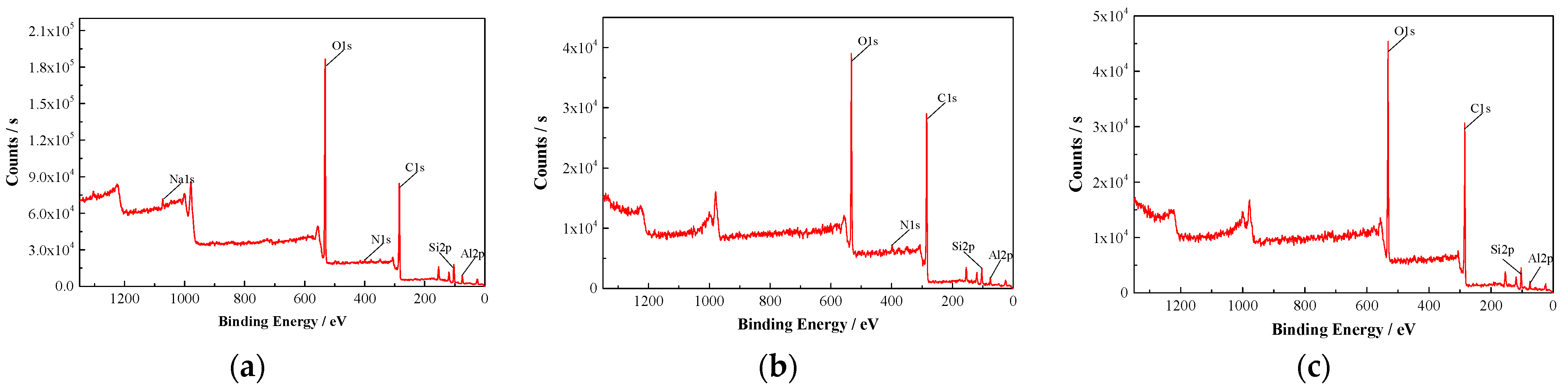
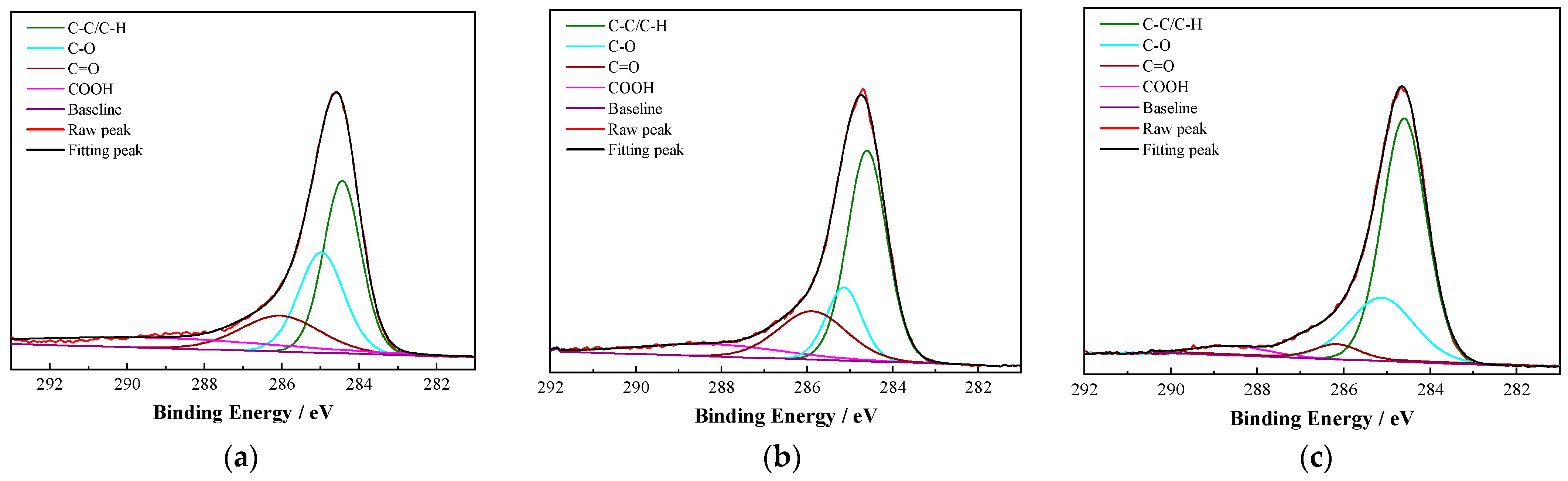
3.4.2. XPS Results
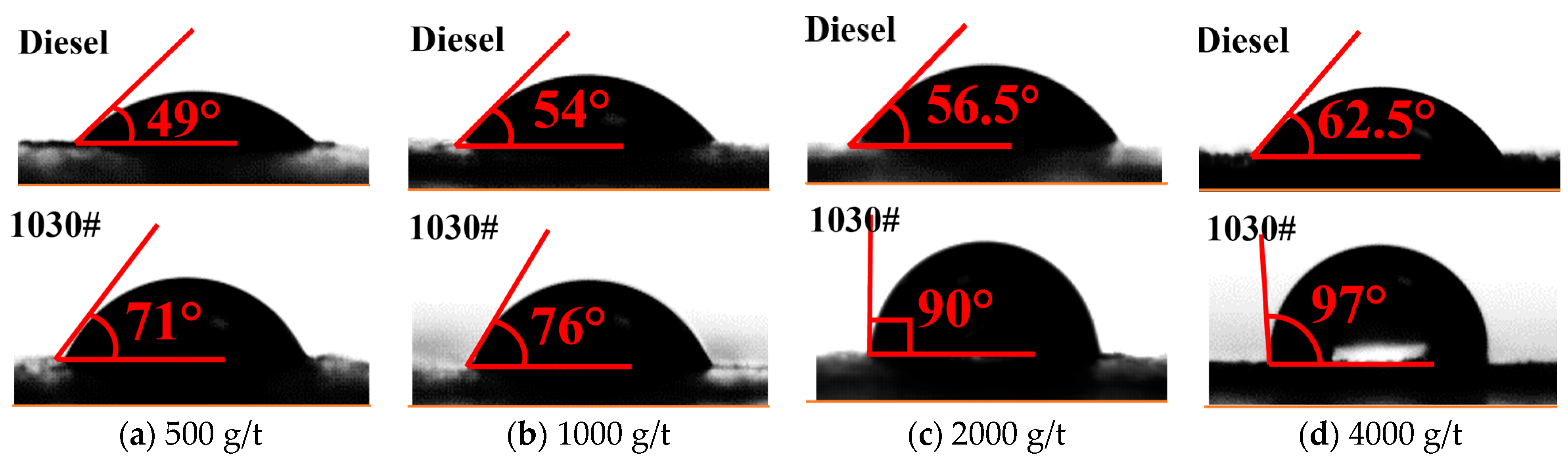
3.4.3. Surface Wettability Test Results
3.4.4. Surface Wettability Test Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, B.; Yao, X.; Liu, X. China’s energy structure adjustment of under the constraint of energy saving and carbon emission strategy. Soc. Sci. China 2010, 31, 91–110. [Google Scholar]
- Yu, J.; Tahmasebi, A.; Han, Y.; Yin, F.; Li, X. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Process. Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Xu, M.; Guo, F.; Zhang, Y.; Yang, Z.; Cao, Y.; Gui, X.; Xing, Y. Effect of hydrothermal pretreatment on surface physicochemical properties of lignite and its flotation response. Powder Technol. 2021, 386, 81–89. [Google Scholar] [CrossRef]
- Sivrikaya, O. Cleaning study of a low-rank lignite with DMS, Reichert spiral and flotation. Fuel 2014, 119, 252–258. [Google Scholar] [CrossRef]
- Tu, Q.; Cheng, Y.; Xue, S.; Ren, T.; Cheng, X. Energy-limiting factor for coal and gas outburst occurrence in intact coal seam. Int. J. Min. Sci. Technol. 2021, 31, 729–742. [Google Scholar] [CrossRef]
- Pan, J.; Hassas, B.; Rezaee, M.; Zhou, C.; Pisupati, S. Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J. Clean. Prod. 2021, 284, 124725. [Google Scholar] [CrossRef]
- Hu, P.; Liang, L.; Xie, G.; Zhou, S.; Peng, Y. Effect of slurry conditioning on flocculant-aided filtration of coal tailings studied by low-field nuclear magnetic resonance and X-ray micro-tomography. Int. J. Min. Sci. Technol. 2020, 30, 859–864. [Google Scholar] [CrossRef]
- Behera, S.; Meena, H.; Chakraborty, S.; Meikap, B. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Stańczyk, K.; Bajerski, A.; Czny, M. Negative-pressure pneumatic separator:a new solution for hard-coal beneficiation. Int. J. Min. Sci. Technol. 2021, 8, 103–123. [Google Scholar]
- Xia, W.; Xie, G.; Peng, Y. Recent advances in beneficiation for low rank coals. Powder Technol. 2015, 277, 206–221. [Google Scholar] [CrossRef]
- Ozkan, S. Effects of simultaneous ultrasonic treatment on flotation of hard coal slimes. Fuel 2012, 93, 576–580. [Google Scholar] [CrossRef]
- Liu, J.; Bao, X.; Hao, Y.; Liu, J.; Cheng, Y.; Zhang, R.; Xing, Y.; Gui, X.; Li, J.; Avid, B. Research on mechanisms of improving flotation selectivity of coal slime by adding sodium polyphosphate. Minerals 2022, 12, 1392. [Google Scholar]
- Rong, G.; Xia, Y.; Zhang, Y.; Guo, F.; Wang, D.; Zhang, R.; Xing, Y.; Gui, X. Effect of Comminution methods on low-rank coal bubble–particle attachment/detachment: Implications for flotation. Minerals 2019, 9, 452. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Yu, J.; Han, Y.; Zhao, H.; Bhattacharya, S. A kinetic study of microwave and fluidized-bed drying of a Chinese lignite. Chem. Eng. Res. Des. 2014, 92, 54–65. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.; Fuerstenau, D. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Min. Sci. Technol. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Atesok, G.; Celik, M. A new flotation scheme for a difficult-to-float coal using pitch additive in dry grinding. Fuel 2000, 79, 1509–1513. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.; Fuerstenau, D. Chemical reagents for enhanced coal flotation. Int. J. Coal Prep. Util. 2002, 22, 123–149. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Wang, T.; Cao, Y.; Miao, Z.; Xu, M. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector α-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Performance of used lubricating oil as flotation collector for the recovery of clean low-rank coal. Fuel 2018, 239, 717–725. [Google Scholar] [CrossRef]
- Zhu, C.; Xing, Y.; Xia, Y.; Wang, Y.; Li, G.; Gui, X. Flotation intensification of low-rank coal using a new compound collector. Powder Technol. 2020, 370, 197–205. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. Improving oxidized coal flotation using biodiesel as a collector. Int. J. Coal Prep. Util. 2013, 33, 181–187. [Google Scholar] [CrossRef]
- Hacifazlioglu, H.; Senol-Arslan, D. Sunflower oil as green collector in bituminous coal flotation. Energy Sources Part A 2017, 39, 1602–1609. [Google Scholar] [CrossRef]
- Alonso, M.; Castano, C.; Garcia, A. Performance of vegetable oils as flotation collectors for the recovery of coal from coal fines wastes. Int. J. Coal Prep. Util. 2000, 21, 411–420. [Google Scholar] [CrossRef]
- Alonso, M.; Valdés, A.; MartíNez-Tarazona, R.; Garcia, A. Coal recovery from coal fines cleaning wastes by agglomeration with vegetable oils: Effects of oil type and concentration. Fuel 1999, 78, 753–759. [Google Scholar] [CrossRef]
- Das, B.; Reddy, P. The utilization of non-coking coal by flotation using non-conventional reagents. Energy Sources Part A 2010, 32, 1784–1793. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Cao, Y.; Gui, X. Waste colza oil used as renewable collector for low rank coal flotation. Powder Technol. 2019, 344, 611–616. [Google Scholar] [CrossRef]
- Chen, P. Coal classification in China: A complete system (part 1). China Coal 2000, 26, 5–8. [Google Scholar]
- Zhu, C.; Li, G.; Xing, Y.; Gui, X. Adhesion forces for water/oil droplet and bubble on coking coal surfaces with different roughness. Int. J. Min. Sci. Technol. 2021, 31, 681–687. [Google Scholar] [CrossRef]
- Li, M.; Xing, Y.; Zhu, C.; Liu, Q.; Yang, Z.; Zhang, R.; Zhang, Y.; Xia, Y.; Gui, X. Effect of roughness on wettability and floatability: Based on wetting film drainage between bubbles and solid surfaces. Int. J. Min. Sci. Technol. 2022, 32, 1389–1396. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic-static microbubble flotation column with collector emulsification. J. Clean. Prod. 2016, 153, 657–672. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, G.; Xia, Y.; He, Q.; Zeng, H.; Xing, Y.; Gui, X. Utilization of waste cooking oil for highly efficient recovery of unburned carbon from coal fly ash. J. Clean. Prod. 2020, 282, 124547. [Google Scholar] [CrossRef]
- Guven, O.; Kaymakoğlu, B.; Ehsani, A.; Hassanzadeh, A.; Sivrikaya, O. Effects of grinding time on morphology and collectorless flotation of coal particles. Powder Technol. 2021, 339, 117010. [Google Scholar] [CrossRef]
- Yin, W.; Zhu, Z.; Yang, B.; Fu, Y.; Yao, J. Contribution of particle shape and surface roughness on the flotation behavior of low-ash coking coal. Energy Sources Part A 2019, 41, 636–644. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Bradford, S.; Hwang, G.; Heyes, G.; Kim, H. Particle–bubble interaction energies for particles with physical and chemical heterogeneities. Miner. Eng. 2020, 155, 106472. [Google Scholar] [CrossRef]
- Xia, Y.; Rong, G.; Xing, Y.; Gui, X. Synergistic adsorption of polar and nonpolar reagents on oxygen-containing graphite surfaces: Implications for low-rank coal flotation. J. Colloid Interf. Sci. 2019, 557, 276–281. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, L.; Qu, J.; Tao, X.; He, H. Exploration on the mechanism of enhancing low-rank coal flotation with cationic surfactant in the presence of oily collector. Fuel 2018, 227, 190–198. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, F.; Xia, Y.; Xing, Y.; Gui, X. Improved floatability of low-rank coal through surface modification by hydrothermal pretreatment. J. Clean. Prod. 2020, 246, 119025. [Google Scholar] [CrossRef]
- Yang, H.; Xing, Y.; Sun, L.; Cao, Y.; Gui, X. Kinetics of bubble-particle attachment and detachment at a single-bubble scale. Powder Technol. 2020, 370, 251–258. [Google Scholar] [CrossRef]
- Sherman, H.; Nguyen, A.; Bruckard, W. An analysis of bubble deformation by a sphere relevant to the measurements of bubble-particle contact interaction and detachment forces. Langmuir 2016, 32, 12022–12030. [Google Scholar] [CrossRef] [PubMed]


| Mad/% | Aad/% | Vdaf/% | FCdaf/% |
|---|---|---|---|
| 11.47 | 17.68 | 35.02 | 64.98 |
| Retention Time/min | Component | Content/% | Retention Time/min | Component | Content/% |
|---|---|---|---|---|---|
| 18.608 | Trans-methyl oleate; | 40.22 | 23.671 | Methyl erucic acid | 2.86 |
| 20.386 | Eicosonaic acid methyl ester | 8.83 | 23.872 | Methyl tetradecanoate | 2.42 |
| 20.533 | Eicosanoic acid methyl ester | 6.93 | 24.604 | Palmitic acid methyl acetate | 1.5 |
| 21.078 | Methyl docoxate | 6.6 | 25.176 | Methyl wax | 1.35 |
| 22.057 | Methyl laurate | 5.25 | 25.348 | Methyl twenty-three acid vinegar | 1.29 |
| Coal Sample | C-O-C | C=O | -CH3 | -CH2 | -OH |
|---|---|---|---|---|---|
| Raw coal | 9.90 | 2.14 | 0.67 | 0.70 | 2.14 |
| Diesel oil treatment | 6.96 | 1.48 | 0.72 | 0.66 | 1.78 |
| 1030# oil treatment | 5.40 | 0.74 | 0.77 | 0.71 | 1.37 |
| Relative Contents/% | Types of Elements | ||||
|---|---|---|---|---|---|
| C | O | Si | Al | Na | |
| Raw coal | 43.92 | 39.05 | 8.16 | 6.53 | 1.29 |
| Diesel oil treatment | 53.79 | 33.11 | 7.53 | 5.57 | - |
| 1030# oil treatment | 55.67 | 29.48 | 6.28 | 4.92 | - |
| Relative Contents/% | Types of Functional Group | |||
|---|---|---|---|---|
| C-C/C-H | C-O | C=O | C=O-O | |
| Low-rank coal | 42.69 | 32.07 | 19.34 | 5.9 |
| Diesel oil treatment | 51.99 | 16.31 | 20.68 | 11.01 |
| 1030# oil treatment | 64.28 | 28.55 | 3.65 | 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhou, Y.; Hao, Y.; Cao, Y.; Xing, Y.; Gui, X. Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil. Minerals 2023, 13, 717. https://doi.org/10.3390/min13060717
Xu M, Zhou Y, Hao Y, Cao Y, Xing Y, Gui X. Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil. Minerals. 2023; 13(6):717. https://doi.org/10.3390/min13060717
Chicago/Turabian StyleXu, Mengdi, Ying Zhou, Yesheng Hao, Yijun Cao, Yaowen Xing, and Xiahui Gui. 2023. "Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil" Minerals 13, no. 6: 717. https://doi.org/10.3390/min13060717
APA StyleXu, M., Zhou, Y., Hao, Y., Cao, Y., Xing, Y., & Gui, X. (2023). Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil. Minerals, 13(6), 717. https://doi.org/10.3390/min13060717








