Abstract
Sphalerite is a common sulfide mineral, and xanthate is usually used as the collector of sphalerite flotation. In order to realize the simultaneous flotation of sphalerite- and zinc-containing oxide ores, in this paper, dodecylamine was used as the collector and sodium sulfide as the regulator to explore the flotation behavior of sphalerite in a sulfuration–amine system through single mineral flotation tests, and several analytical methods, including contact angle measurement, adsorption capacity test, FT-IR, and XPS, were employed to discuss the interaction mechanism of dodecylamine on the surface of sphalerite. The results confirm that dodecylamine had a strong collecting effect on sphalerite throughout almost the entire pH range, and the flotation recovery increased with the increase in pH, reaching 86.81% at pH 12. It was found that the effect of sodium sulfide on the floatability of sphalerite was relatively weak, and a weak activation effect, of about 2% improved recovery, was achieved. The results of the mechanism analysis further show that dodecylamine underwent both physical adsorption and chemical adsorption on the surface of sphalerite, and the physical adsorption was enhanced with the increase in pH. The above findings provide evidence for the simultaneous flotation of zinc-containing sulfide-oxide mixed minerals.
1. Introduction
Zinc is an important industrial metal raw material, mainly used in the fields of machinery and metallurgy, and the chemical industry and light industry [1,2]. China is rich in zinc resources, and its geological reserves rank first in the world. The provinces in China with the largest reserves are Yunnan, Guangdong, Hunan, Gansu, Guangxi, Hunan, Gansu, Inner Mongolia, Sichuan, and Qinghai. Chinese zinc resources suffer from a lower amount of rich ore and more low-grade ore, and less large-scale ore and more small and medium-sized mines, and mining is quite difficult [3]. Zinc is a sulfophilic element, which usually exists in the form of sulfide minerals in the Earth’s crust. There are many zinc oxide ore resources, mainly because the shallow zinc sulfide deposits have been subjected to the long-term effects of oxygen, water and microorganisms, and after a series of weathering and metamorphism effects, the zinc-bearing minerals near the surface take the form of smithsonite, hemimorphite and other oxide minerals. Further, the distribution of sulfide ore and oxide ore is not clearly stratified, so it is difficult to find a single zinc sulfide ore and lead–zinc oxide ore [4,5,6]. With the gradual depletion of sulfide ore resources that are easy to mine and process, the efficient utilization of oxidized mineral resources is now of great significance if we are to meet the strategic demands for zinc metal. The sulfuration–amine method, fatty acid method, sulfuration–metal ion activation–xanthate method, and chelating collector method are mainly used to float the smithsonite and hemimorphite [7,8,9]. The sulfuration–amine method uses sodium sulfide to sulfurate zinc oxide minerals, and uses a cationic collector (such as primary amine) as a collector for flotation. Since amine collectors cannot collect calcite and dolomite, and excessive sodium sulfide will not significantly inhibit the flotation of zinc oxide minerals, the sulfuration–amine flotation method is widely used in zinc oxide mineral flotation [10]. For the flotation of zinc ore with a high degree of oxidation (containing some zinc sulfides), oxide ore collectors such as amines are generally used to recover zinc oxide minerals. It is of great significance to investigate the flotation behavior and mechanisms of amine collectors in relation to zinc sulfides so as to improve the comprehensive recovery of zinc from zinc ore with a high degree of oxidation. Sulfuration is an important process in the flotation of zinc oxide minerals. However, the excessive sodium sulfide added to the xanthate system commonly used in sulfide ore flotation will inhibit the sulfide minerals due to the competitive adsorption between sulfhydryl ions produced by the hydrolysis of sodium sulfide and xanthate ions [11].
The structures of amine collectors can be divided into four types, which are primary amines, secondary amines, tertiary amines, and quaternary amines [12,13,14], and the primary amine represented by dodecylamine is the most widely used [15]. Amine molecules are easily hydrolyzed and ionized in water, and hydrolysis equilibrium and ionization equilibrium can be achieved. Due to the existence of hydrogen bonds, ammonia molecules also undergo molecular association, so the existing forms of amine salts in aqueous solution may be , , or . As the pH of the solution changes, the contents of these molecules or ions also change. In most cases, the interaction between amine collectors and mineral surfaces is mainly due to the cation or dimer of amines adsorbing on the negatively charged mineral surface via electrostatic attraction on the electric double layer of the mineral surface [10]. Laskowski [16] investigated the flotation behavior of dodecylamine on three single minerals, sphalerite, quartz and pyrite, and found that dodecylamine could be used to separate quartz from sphalerite in the high pH range (pH > 12.0), and also found that using lime and hydrogen peroxide as a combined inhibitor could inhibit pyrite effectively, thereby achieving the separation of sphalerite and pyrite. S.G. Malghan [10] investigated the mechanisms of sodium sulfide in the flotation of willemite and hemimorphite via the “sulfuration–amine” method. The results show that sodium sulfide plays the two roles of the sulfuration and adjustment of pulp pH, and sodium hydroxide could also be used to replace some of the sodium sulfide to adjust pulp pH, thereby reducing the adverse impact of sodium sulfide on the environment. Li [17] studied the solution chemistry mechanism of smithsonite–calcite flotation. It is shown that the main mechanism for the adsorption of dodecylamine on the surface of smithsonite should be the formation of zinc amine complexes while at high pH, and when at low pH, the main mechanism should be the formation of ammonium salts between dodecylamine and carbonate ions. According to the characteristics of refractory hemimorphite, the synergistic effects of and in the flotation solution during the flotation of hemimorphite by the “sulfuration–amine” method were studied. The results show that both and dissociated from sodium sulfide in the solution could activate hemimorphite, and the activation effect of HS− is stronger than that of OH−. When the pH is between 9.0 and 11.0, the and have an obvious synergistic effect, and a relatively high recovery of hemimorphite could be obtained at a relatively low dosage of sodium sulfide [18].
When processing sulfide–zinc oxide mixed ore, the flotation method of “sulfide first and oxide later” is usually used, but there are disadvantages such as the complicated technological process, and the effect of flotation is greatly affected by backwater. In order to realize the simultaneous flotation of sphalerite- and zinc-containing oxide ores, in this paper, aiming at the sulfuration–amine system, the effects of dodecaylamine as collector and sodium sulfide as a regulator on the flotation behavior of sphalerite were investigated. The mechanism of the effect of dodecylamine on the surface of sphalerite was analyzed by the solution chemistry of flotation, contact angle measurement, adsorption capacity tests, FT-IR and XPS. The results will provide theoretical guidance for further research on the simultaneous flotation of zinc-containing sulfide oxide mixed minerals.
2. Materials and Methods
2.1. Mineral Samples and Reagents
The sample used in this study was high-grade sphalerite. Samples were manually selected and ground in a ceramic ball mill, and the ground samples were dry-sieved to obtain samples with particle size of −74 + 38 μm, which were used for the single-mineral flotation test, contact angle measurement, adsorption capacity test, XRD, FT-IR and XPS. The X-ray diffraction patterns of sphalerite particles were recorded by an X-ray diffractometer (D/MAX-2600, Rigaku International Corporation, Tokyo, Japan) with Cu kα radiation at 40 kV and 20 mA. Jade software (Livermore, CA, USA) was used to analyze the detection results. The XRD pattern of sphalerite is shown in Figure 1, and the XRF result is displayed in Table 1. The XRD pattern shows that sphalerite was the overwhelming component in samples, and the small amounts of impurities were galena and quartz. Combined with the XRF results, it could be inferred that the mineral purity of this sphalerite was greater than 95%, which meets the requirements of the pure mineral flotation test.
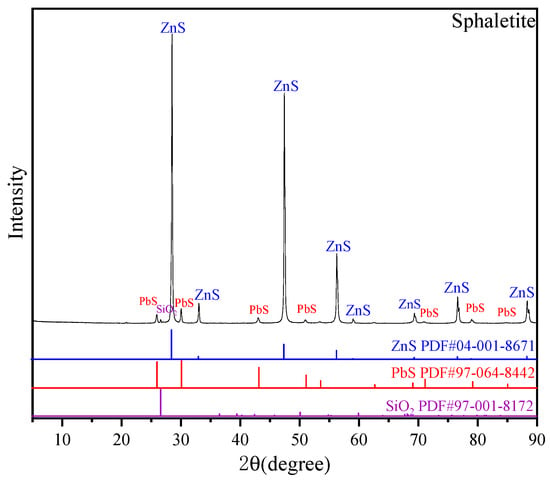
Figure 1.
X-ray diffraction (XRD) pattern of pure sphalerite particles.

Table 1.
Multi-element analysis results of pure sphalerite particles by X-ray fluorescence (XRF).
In the experiment, dodecylamine (C12H27N) was used as the collector, and sodium sulfide (Na2S), HCl and NaOH as the regulator. All the reagents were of analytical grade. Deionized (DI) water, with resistivity of 18.2 MΩ, was prepared with an Elix 5 and a Millipore-UV plus water purification system (Millipore Inc., Canada), which was used throughout the microflotation tests and other tests.
2.2. Flotation Tests
The pure mineral flotation tests were carried out in an XFG 5-35 flotation machine (Jilin Exploration Machinery Plant, Jilin, China). The effective volume of the flotation cell is 40 mL, and the stirring speed was set at 1754 r·min−1.
For the single-mineral flotation test, 2.0 g of pure sample with a particle size of −74 + 38 μm was immersed in 30 mL of distilled water and fully dispersed with ultrasound for 5 min. Then, sodium sulfide, pH regulator and dodecylamine were added into the pulp in sequence, and the flotation time was 3 min. The froth products and tailings were dried and weighed to calculate the recovery. Each test was repeated three times to ensure the accuracy.
2.3. Contact Angle Measurement
A JY-82B Kruss DSA goniometer (Dataphysics, Stuttgart, Germany) was used to measure the contact angle of samples using the static drop method. The sphalerite powders pretreated with DI water, dodecylamine, or sodium sulfide + dodecylamine were used as the samples. In total, 4 g of sphalerite powder was pressed into a circular plate for 60 s under 15 MPa pressure using a compressor (ZHY-401B, Beijing Zhonghe Venture Technology Development Co., Ltd., Beijing, China). Then, the plates were placed on the platform of the contact angle instrument and adjusted horizontally. The tip was controlled by a computer program to drop a droplet above the sample. After the droplet was stable, the image was captured and the contact angle was analyzed. The contact angle test of each sample was repeated 3 times to reduce errors.
2.4. Adsorption Capacity Test
In this study, a UV-3600 ultraviolet–visible spectrophotometer was used to measure the adsorption capacity of dodecylamine on the sphalerite surface in the wavelength range of 100 nm to 600 nm. Dodecylamine solution with a concentration of 15.0 mg·L−1 was prepared for spectrum scanning to find the wavelength of its characteristic absorption peak, and the result is shown in Figure 2. The subsequent measurements were all carried out at a wavelength of 204 nm. Dodecylamine solutions with concentrations of 3.0 mg·L−1, 6.0 mg·L−1, 9.0 mg·L−1, 12.0 mg·L−1, and 15.0 mg·L−1 were prepared and we measured the absorbance at a pH of around 12.0. These data were linearly fitted to obtain the standard curve of absorbance and concentration of the dodecylamine solution. As shown in Figure 3, the standard absorption equation is:
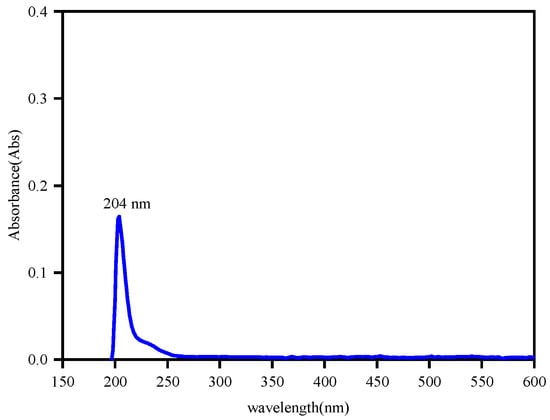
Figure 2.
UV spectrum of dodecylamine solution of 15.0 mg·L−1.
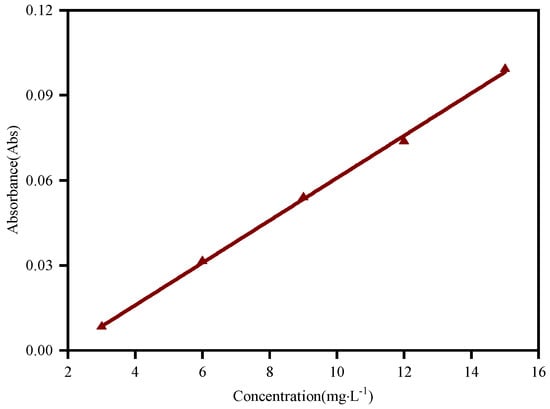
Figure 3.
Standard curve of dodecylamine solution by linear fitting.
The correlation coefficient R2 of the equation was 0.99846, and the fitting met the test requirements.
2.5. FT-IR
The infrared spectra of the samples were measured with an iS10 FT-IR spectrometer (Thermo Nicolet Corporation, Waltham, MA, USA) with wavenumbers ranging from 4000 cm−1 to 500 cm−1 using 16 scans every 10 s. The sphalerite powders pretreated with DI water, dodecylamine, and sodium sulfide + dodecylamine were used as the samples, respectively.
2.6. XPS
A 250 Xi X-ray photoelectron spectrometer from ThermoFisher Scientific in the United States was used, with an Al Ka radiation source (hv = 1486.6 eV), for XPS detection. The vacuum was about 5.0 × 10−9 mbar, and the scanning range was 1400 eV to 0 eV. The samples were pretreated with DI water, dodecylamine and sodium sulfide + dodecylamine, respectively. The C1s peak of the results was corrected to 284.8 eV, and other binding energy peaks were corrected according to the C1s peak. The detected spectra were fitted and plotted using XPS Peak 4.1 software.
3. Results and Discussion
3.1. Flotation Results
3.1.1. The Effect of Dodecylamine Dosage on the Flotation of Sphalerite
The effect of dodecylamine dosage on the flotation of sphalerite was explored and the results are shown in Figure 4; the pH was 6.36 when any agent was not added. It was concluded that the recovery of sphalerite increased obviously with an increase in the dosage of dodecylamine. When the dosage of dodecylamine reached 15.0 mg·L−1 or above, the recovery of sphalerite was stable at around 81.63%. It could be proposed that dodecylamine could be used as an effective collector for sphalerite flotation.
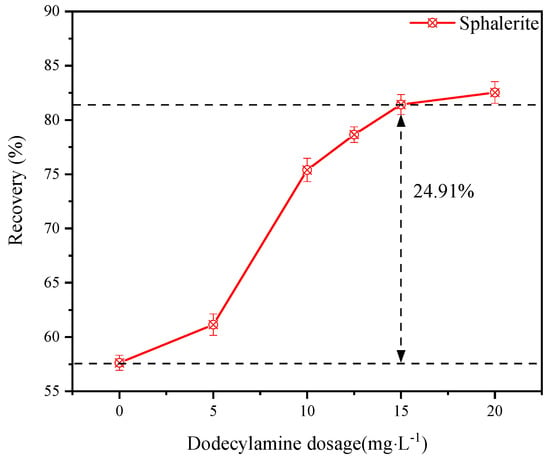
Figure 4.
The effect of dodecylamine dosage on the flotation of sphalerite.
3.1.2. The Effect of pH on Flotation of Sphalerite
When the dosage of dodecylamine was set at 15.0 mg·L−1, the effect of pH on the flotation recovery of sphalerite was explored, and the results are shown in Figure 5. It could be deduced that a good floatability of sphalerite was achieved when the pH was changed within a wide pH range from 2.0 to 12.0. The flotation recovery of sphalerite tended to increase with the increase in pH, and a recovery of 86.81% was reached when the pH was about 12. It could be inferred that the adsorption of dodecylamine was basically not affected by the pH of the pulp. Therefore, when the pH of the solution was low, even below the point of zero charge (the mineral surface was positively charged), sphalerite still maintained good floatability, and the recovery of sphalerite was relatively high even in a strongly acidic environment. It could be concluded that dodecylammonium ions () or dodecylamine molecules () in solution were chemisorbed on the sphalerite’s surface [19].
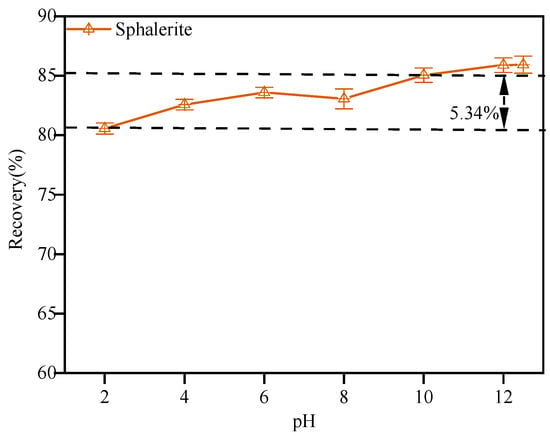
Figure 5.
The effect of pH on the flotation of sphalerite.
3.1.3. The Effect of Sodium Sulfide Dosage on the Flotation of Sphalerite
The effect of sodium sulfide dosage on the flotation of sphalerite was explored under the following conditions: the dosage of dodecylamine was 15.0 mg·L−1 and the pH value of the slurry was controlled at about 12.0. It could be seen from Figure 6 that the dosage of sodium sulfide had little effect on the flotation recovery of sphalerite, and a weak activation effect was obtained. When the dosage of sodium sulfide was greater than 2000 mg·L−1, the flotation recovery of sphalerite was basically stable at about 88.87%. In the flotation system with dodecylamine as a collector, excessive sodium sulfide did not inhibit sphalerite as in the xanthate flotation system. This provided a prerequisite for the simultaneous flotation of zinc-containing mixed sulfide and oxide minerals.
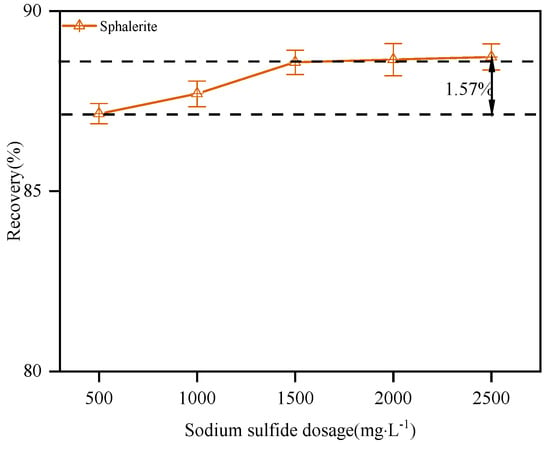
Figure 6.
The effect of sodium sulfide dosage on the flotation of sphalerite.
3.2. Solution Chemistry Analysis
According to the flotation solution chemical calculation, the changes of species on the sphalerite’s surface with pH are shown in Figure 7, and the lgC-pH curves of dodecylamine are shown in Figure 8. As the pH increased, the dominant species on the sphalerite surface gradually transitioned from cations to , , and anions, and the electronegativity gradually increased [20]. When the surface of sphalerite was negatively charged, the cationic collector dodecylamine could be physically adsorbed on the surface of sphalerite, and the recovery of sphalerite increased gradually. When the surface of sphalerite was positively charged, dodecylamine still had a strong collection effect on sphalerite. It was proposed that chemical adsorption occurred because the N atom (bonding atom) in or was covalently bonded with the in sphalerite to form a relatively stable complex [8]. In addition, the non-polar groups of and were more conducive to the mutual association, leading to co-adsorption on the surface of sphalerite, or the semi-micelle adsorption of the dimer . This greatly enhanced the collection effect of dodecylamine on sphalerite.
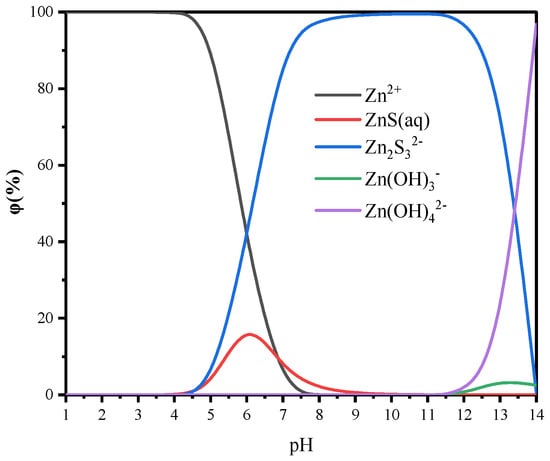
Figure 7.
The effect of pH on the distribution of dominating species on the dissolved sphalerite’s surface.
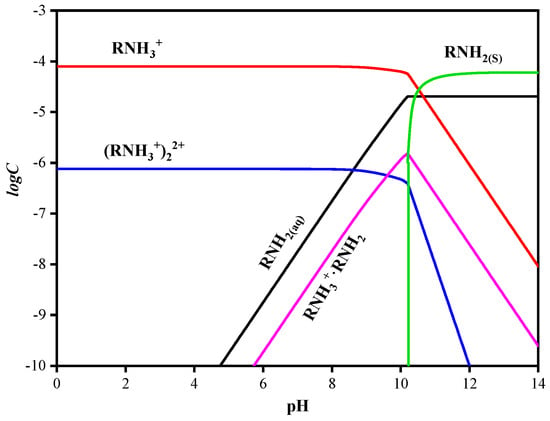
Figure 8.
The lgC-pH curves of dodecylamine at the concentration of 0.8 × 10−4 mol/L.
3.3. Wettability Analysis
Figure 9 shows the contact angle changes of sphalerite powders pretreated with DI water, dodecylamine, and/or sodium sulfide + dodecylamine, respectively. The contact angle of natural sphalerite powders was 75.58°, which proved that sphalerite had a certain hydrophobicity [21,22]. The contact angle of the sphalerite surface pretreated with dodecylamine was 115.11°, which meant that the addition of dodecylamine improved the hydrophobicity of sphalerite, and the dodecylamine adsorbed on the surface of sphalerite increased the floatability of minerals. The contact angle of sphalerite pretreated with sodium sulfide + dodecylamine was 117.83°, which indicates that the addition of sodium sulfide improved the hydrophobicity of the sphalerite’s surface, which is also consistent with the flotation results for sphalerite.
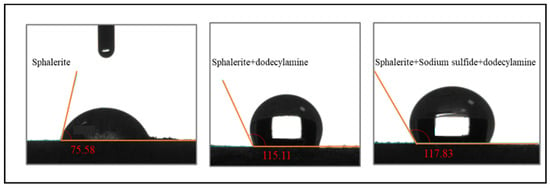
Figure 9.
Variation of sphalerite contact angles under different test conditions.
3.4. Adsorption Analysis of Dodecylamine
The amount of dodecylamine absorbed on the sphalerite surface was measured at the dodecylamine concentrations of 3.0 mg·L−1, 6.0 mg·L−1, 9.0 mg·L−1, 12.0 mg·L−1, and 15.0 mg·L−1, respectively. The results are shown in Figure 10. This indicated that with the increase in dodecylamine concentration, the amount of dodecylamine adsorbed on the surface of sphalerite gradually increased, but when the dodecylamine concentration increased to 15.0 mg·L−1, the adsorption amount curve tended to flatten. This result is consistent with the results of the effect of dodecylamine dosage on the flotation of sphalerite, as shown in Figure 4.
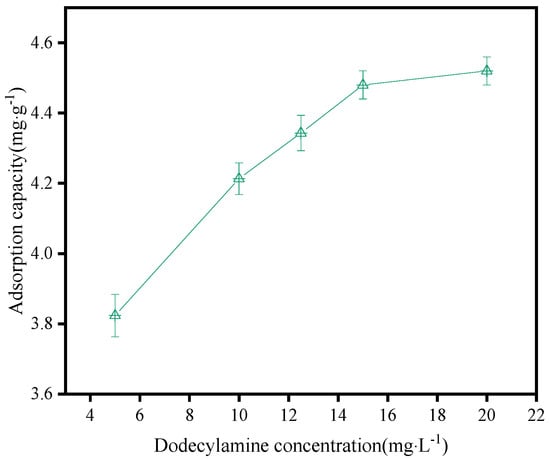
Figure 10.
Adsorbed quantity of dodecylamine on the sphalerite surface.
The effect of the sodium sulfide dosage on the adsorption quantity of dodecylamine on the surface of sphalerite was also studied with a concentration of dodecylamine of 15.0 mg·L−1, and the results are shown in Figure 11. The adsorption quantity of dodecylamine on the sphalerite surface increased slightly with the increase in sodium sulfide dosage. The adsorption quantity did not change significantly when the sodium sulfide dosage increased to 2000 mg·L−1. Sodium sulfide had no depressing effect on the adsorption of dodecylamine on the sphalerite’s surface, which is consistent with the results of the flotation tests for sphalerite.
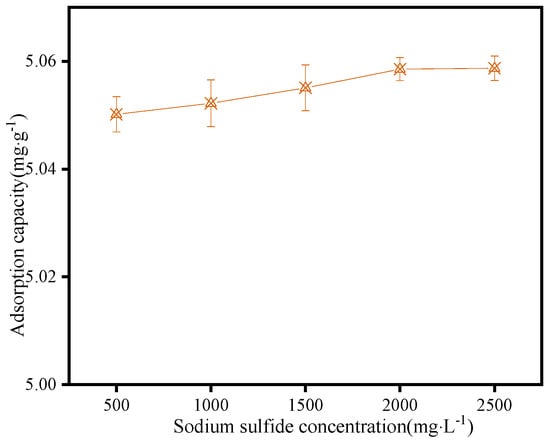
Figure 11.
The effect of sodium sulfide concentrations on the adsorption quantity of dodecylamine on the sphalerite’s surface.
3.5. FT-IR
The infrared spectra of sphalerite under different chemical conditions in a highly alkaline environment are shown in Figure 12. No characteristic peaks were found on the natural sphalerite’s surface in the wavenumber range from 4000 cm−1 to 1000 cm−1. The obvious characteristic absorption peaks appeared at 2870 cm−1 and 2926 cm−1 when the sphalerite adsorbed the dodecylamine. The characteristic peak at a wavenumber of 2870 cm−1 was caused by symmetric stretching vibration, and the characteristic peak at a wavenumber of 2926 cm−1 was the absorption peak of antisymmetric stretching vibration [23,24]. The absorption peak in the infrared spectra after the interaction between dodecylamine and sphalerite was characteristic of physical adsorption. It could be proposed that dodecylamine is physically adsorbed onto the surface of sphalerite in a highly alkaline environment. The infrared spectroscopy showed that an absorption peak caused by amino stretching vibration appeared at around 3336 cm−1 when dodecylamine interacted with sphalerite, which may have been caused by the change in electron density in dodecylamine, indicating that dodecylamine underwent strong physical adsorption on the surface of the sphalerite. This adsorption may have been caused by the hemimicelle adsorption of amine molecules and their ionic dimers; in addition to the electrostatic attraction, there was also a certain degree of van der Waals force [25]. After adding sodium sulfide, both absorption peak areas of and stretching vibration showed a certain increase, indicating that the electron density of N in the amine molecule had changed, which may have been caused by the bonding between S and the Zn site on the surface of the sphalerite.
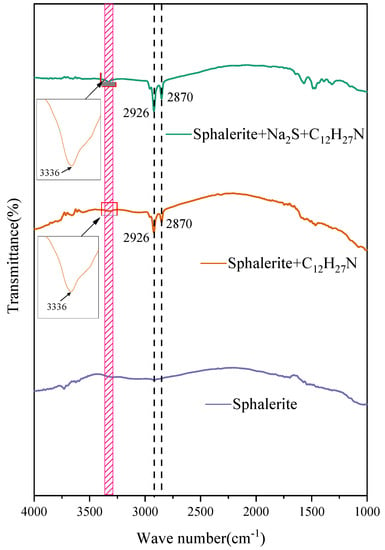
Figure 12.
Infrared spectra of sphalerite under different conditions.
3.6. XPS
XPS tests were used to verify the interaction mechanism between dodecylamine and the sphalerite’s surface. The relevant measured spectra and relative atomic amounts on the sphalerite surfaces, with binding energies ranging from 0 to 1200 eV, are shown in Figure 13 and Table 2, respectively. The results show that the main elements on the surface of the sphalerite powders studied in this paper were C, S, Zn, and N, with no obvious impurities. The contents of C and N obviously increased after adding dodecylamine, which indicated that the adsorption of dodecylamine molecules on the sphalerite’s surface occurred. The amount of S on the sphalerite’s surface decreased by 1.81%, and the amount of Zn decreased by 3.74% after adding sodium sulfide and dodecylamine, compared with the natural sphalerite powders. The combination of and anions dissolved from the sphalerite with and cations dissolved from the dodecylamine under alkaline conditions mainly caused in this phenomenon [26]. The change in the amount of N shows that dodecylamine was adsorbed on the surface of sphalerite. The amine adsorbed on the surface of sphalerite increased with the increase in dodecylamine concentration under highly alkaline conditions. To further identify the surface differences between the samples before and after the adsorption of sodium sulfide and dodecylamine, the fitting of Zn, S, and C peaks was conducted.
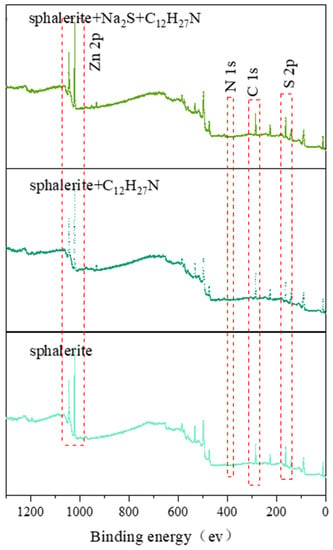
Figure 13.
XPS spectra of sphalerite surface under different conditions.

Table 2.
Relative atomic amounts on the sphalerite surface.
It can be seen from Figure 14, Figure 15 and Figure 16 that the characteristic peak of C 1s on the sphalerite surface at 284.82 eV was caused by the hydrocarbon pollution that was pre-adsorbed on the surface of the sphalerite [27], and this was also the standard peak used for calibrating all the XPS data. Only one S 2p doublet was found on the surface of the sphalerite, with an S 2p3/2 binding energy of 161.80 eV and an S 2p1/2 binding energy of 162.98 eV. This suggests that sulfur is only present in the S2− species on natural sphalerite [28]. The binding energy of the Zn 2p3/2 characteristic peak was 1022.07 eV [29]. The C 1s of the sphalerite pretreated with dodecylamine changed slightly due to the absorption of dodecylamine molecules [30]. The chemical shift of sulfur in the sphalerite pretreated with dodecylamine indicates that these two sulfur components were present in different chemical environments. The loss of electrons or bonding to more electronegative atoms led to this phenomenon [25]. The shift in Zn 2p could have arisen because the chemical state of Zn changed, and the N atom in dodecylamine formed a relatively stable complex with Zn2+ on the sphalerite’s surface, after which chemical adsorption occurred. Zn 2p, S 2p, and C 1s shifted slightly after the addition of sodium sulfide, indicating that sodium sulfide had a certain activation effect on the sphalerite in the dodecylamine system, instead of an inhibiting effect.
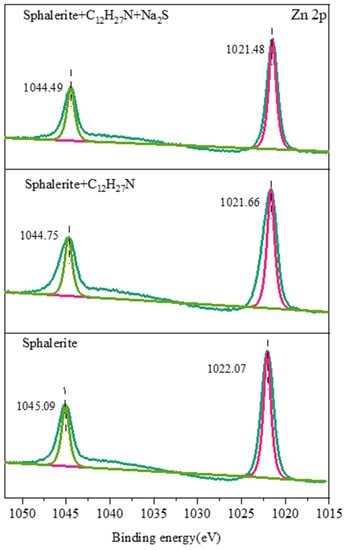
Figure 14.
Zn 2p XPS spectra of sphalerite’s surface.
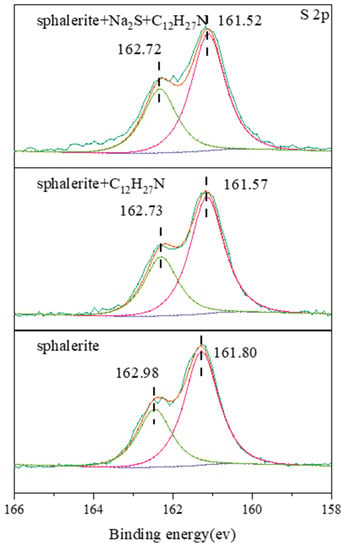
Figure 15.
S 2p XPS spectra of sphalerite’s surface.
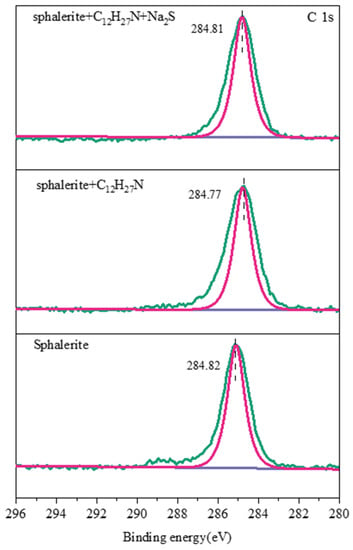
Figure 16.
C 1s XPS spectra of sphalerite’s surface.
3.7. Discussion on Mechanism
Based on the XPS spectra, it could be inferred that there were many components on the sphalerite’s surface. This might be because various redox reactions occurred on the sphalerite’s surface, and this resulted in different products when the pH was adjusted. Combined with the flotation results, it could be seen that the floatability of sphalerite was enhanced when the pH was greater than 10. It is speculated that this is because dodecylamine played a role in both chemical adsorption and physical adsorption on the sphalerite’s surface [31]. In addition to the electrostatic adsorption on the electric double layer, the association between the amine ion and the non-polar group of the molecule as well as the van der Waals force between the hydrocarbon chains caused by the hemimicelle adsorption also played important roles in the physical adsorption. The adsorption mechanism is shown in Figure 17.
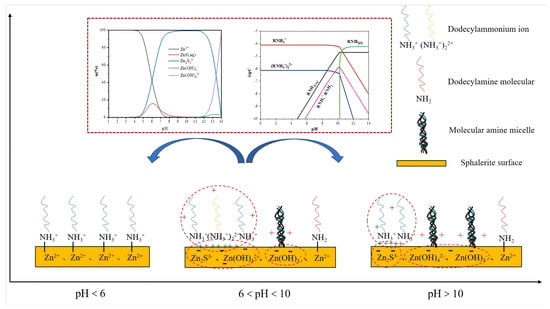
Figure 17.
Schematic diagram of the adsorption mechanism of dodecylamine on sphalerite.
Dodecylamine had a strong collection effect on sphalerite when the surface of the sphalerite was positively charged in the acidic pulp. This was because the N atom (bonding atom) in was covalently bonded with the in sphalerite to form a relatively stable complex, and chemical adsorption occurred. The cationic collector dodecylamine could become physically adsorbed on the negatively charged sphalerite’s surface with the increase in pH, and the recovery gradually increased. The sphalerite surface displayed co-adsorption with the and molecules, or the semi-micelle adsorption of the dimer . The static electricity and chemical adsorption simultaneously significantly enhanced the collection effect of dodecylamine on the sphalerite.
Regarding the mechanism of the weak promoting effect of sodium sulfide on the flotation of sphalerite, there are two possible explanations. Firstly, the addition of alkaline sodium sulfide enhances the electronegativity of the sphalerite’s surface, which makes cationic collector dodecylamine more likely to be physically adsorbed on the surface of sphalerite; secondly, sodium sulfide inhibits the surface oxidation of sphalerite samples, or causes the reforming of zinc sulfide film, which means dodecylamine is more likely to be chemically adsorbed on the surface of the sphalerite.
4. Conclusions
In this paper, we investigated the effects of dodecylamine as a collector and sodium sulfide as a regulator on the flotation behavior of sphalerite. The mechanism of interaction of dodecylamine with the surface of sphalerite was analyzed via the solution chemistry of flotation, contact angle measurement, adsorption capacity tests, FT-IR and XPS. The main conclusions are as follows:
- ●
- The single-mineral flotation tests, contact angle measurements and adsorption capacity tests show that dodecylamine had a good collecting effect on sphalerite in the pH range of 2.0–12.0, and the flotation recovery of sphalerite increased with the increase in pH. Sodium sulfide had a negligible effect on sphalerite flotation in the amine flotation system, which differs from the depressing effect of excessive sodium sulfide on sphalerite in a xanthate flotation system;
- ●
- The solution chemistry, FT-IR and XPS analyses showed that the chemical adsorption and physical adsorption of dodecylamine occurred simultaneously on the surface of the sphalerite. The chemical adsorption was present throughout almost the whole range of pH values, and physical adsorption mainly occurred when the pH of the solution was greater than the isoelectric point of sphalerite. The combination of physical adsorption and chemical adsorption enhanced the collecting power of dodecylamine in relation to sphalerite under alkaline conditions. The N atom of dodecylamine formed a complex with the in sphalerite, which was the main avenue of the chemical adsorption of dodecylamine onto the surface of the sphalerite. In addition to electrostatic attraction, the van der Waals force between hydrocarbon bonds generated by semi-micelle adsorption, and the association and co-adsorption between amine ions and molecular non-polar groups, also played important roles in physical adsorption;
- ●
- This study has demonstrated that it is feasible to float sphalerite via the sulfuration–amine method, but out studies into the flotation activation of sodium sulfide were not sufficient. In the future, further study should be undertaken on the flotation separation of sphalerite and other sulfide minerals (such as pyrite), as well as the simultaneous flotation of zinc-containing sulfide oxide mixed minerals in a sulfuration–amine system.
Author Contributions
Conceptualization, Y.P. and Z.P.; methodology, Y.P. and F.H.; software, Y.S.; validation, Y.Z. and X.Z.; formal analysis, F.H.; investigation, Y.P., F.H. and K.L.; data curation, Y.S. and X.G.; writing—original draft preparation, Y.P.; writing—review and editing, Y.P., F.H. and X.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of China under grant number U20A20269 and the National Natural Science Foundation of China (No. 51974030).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseini, S.H.; Forssberg, E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector. Miner. Eng. 2007, 20, 621–624. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Smithsonite flotation using mixed anionic/cationic collector. Miner. Process. Extr. Metall. 2013, 118, 186–190. [Google Scholar] [CrossRef]
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Deng, J.; Lai, H.; Chen, M.; Glen, M.; Wen, S.; Zhao, B.; Wang, P. Effect of iron concentration on the crystallization and electronic structure of sphalerite/marmatite: A DFT study. Miner. Eng. 2019, 136, 168–174. [Google Scholar] [CrossRef]
- Shi, S.; Fang, Z.; Ni, J. Comparative study on the bioleaching of zinc sulphides. Process Biochem. 2006, 41, 438–446. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Ma, W.; Meng, Q.; Chen, Y. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 89, 163–167. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Navidi Kashani, A.H.; Rashchi, F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents. Miner. Eng. 2008, 21, 967–972. [Google Scholar] [CrossRef]
- Malghan, S.G. Role of sodium sulfide in the flotation of oxidized copper, lead, and zinc ores. Min. Metall. Explor. 1986, 3, 158–163. [Google Scholar] [CrossRef]
- Wu, S.; Pan, T.; Cao, J.; He, D.; Xiao, Z. Upgrade of three municipal wastewater treatment lagoons using a high surface area media. Front. Environ. Sci. Eng. 2012, 6, 288–293. [Google Scholar]
- Luo, Y.; Zhang, G.; Mai, Q.; Liu, H.; Li, C.; Feng, H. Flotation separation of smithsonite from calcite using depressant sodium alginate and mixed cationic/anionic collectors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124227. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite, and Quartz. Minerals 2018, 8, 141. [Google Scholar] [CrossRef]
- Selective separation of silica from a siliceous-calcareous phosphate rock. Min. Sci. Technol. 2011, 21, 135–139.
- Samet-Meziou, A.; Etter, A.; Baudin, T.; Penelle, R. TEM study of recovery and recrystallization mechanisms after 40% cold rolling in an IF-Ti steel. Scr. Mater. 2005, 53, 1001–1006. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Yuan, X.-M. Flotation of Sulfide Minerals with the use of a Cationic Collector. Can. Metall. Q. 2002, 41, 381–390. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.-K.; Wei, C.; Liu, C.-X.; Jiang, J.-B.; Wang, F. Sulfidation roasting of low grade lead–zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Marabini, A.M.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating reagents for flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Marabini, A.; Ciriachi, M.; Plescia, P.; Barbaro, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar]
- Xu, S.; Kou, J.; Sun, T.; Jong, K. A study of adsorption mechanism of dodecylamine on sphalerite. Colloids Surf. A Physicochem. Eng. Asp. 2015, 486, 145–152. [Google Scholar] [CrossRef]
- Xu, S.; Kou, J.; Sun, T.; Jong, K. Synergistic depression mechanism of zinc sulfate and sodium dimethyl dithiocarbamate on sphalerite in Pb Zn flotation system-ScienceDirect. Trans. Nonferrous Met. Soc. China 2020, 30, 2547–2555. [Google Scholar]
- Fosu, S.; Skinner, W.; Zanin, M. Detachment of coarse composite sphalerite particles from bubbles in flotation: Influence of xanthate collector type and concentration. Miner. Eng. 2015, 71, 73–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Liu, S.; Lu, Z.; Niu, X.; Ahmed, M.M.M.; Liu, G. The selective flotation separation of galena from sphalerite with a novel collector of 5-amyl-1, 2, 4-triazole-3-thione. J. Mol. Liq. 2021, 332, 115902. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Liu, G.; Huang, Y.; Zhang, Z. Selective Flotation of Copper Oxide Minerals with A Novel Amino-Triazole-Thione Surfactant: A Comparison to Hydroxamic Acid Collector. Miner. Process. Extr. Metall. Rev. 2020, 41, 96–106. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Rao, K.H.; Vidyadhar, A.; Shchukarev, A.V. Mechanism of Adsorption of Long-Chain Alkylamines on Silicates: A Spectroscopic Study. 2. Albite. Langmuir 2001, 17, 775–785. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger Parameter Measurements of Zinc Compounds Relevant to Zinc Transport in the Environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Islam, N.; Ghosh, T.; Chopra, K.; Acharya, H. XPS and X-ray diffraction studies of aluminum-doped zinc oxide transparent conducting films. Thin Solid Film. 1996, 280, 20–25. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Skinner, W.M.; Ralston, J.; Smart, R.S.C. Copper(II) activation and cyanide deactivation of zinc sulphide under mildly alkaline conditions. Appl. Surf. Sci. 1997, 108, 333–344. [Google Scholar]
- Santhiya, D.; Subramanian, S.; Natarajan, K.A. Surface chemical studies on galena and sphalerite in the presence of thiobacillus thiooxjdans with reference to mineral beneficiation. Miner. Eng. 2000, 13, 747–763. [Google Scholar] [CrossRef]
- Khmeleva, T.N.; Georgiev, T.V.; Jasieniak, M.; Skinner, W.M.; Beattie, D.A. XPS and ToF-SIMS study of a chalcopyrite-pyrite-sphalerite mixture treated with xanthate and sodium bisulphite. Surf. Interface Anal. 2005, 37, 699–709. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R.; Wouterlood, H.J. An XPS Investigation of the Surface of Natural Sphalerites under Flotation-Related Conditions. Int. J. Miner. Process. 1989, 26, 29–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
