δ-MnO2 Drives the Green Decomposition of Arsenopyrite by Mediating the Fate of Arsenic to Generate FeAsO4
Abstract
1. Introduction
2. Materials and Methods
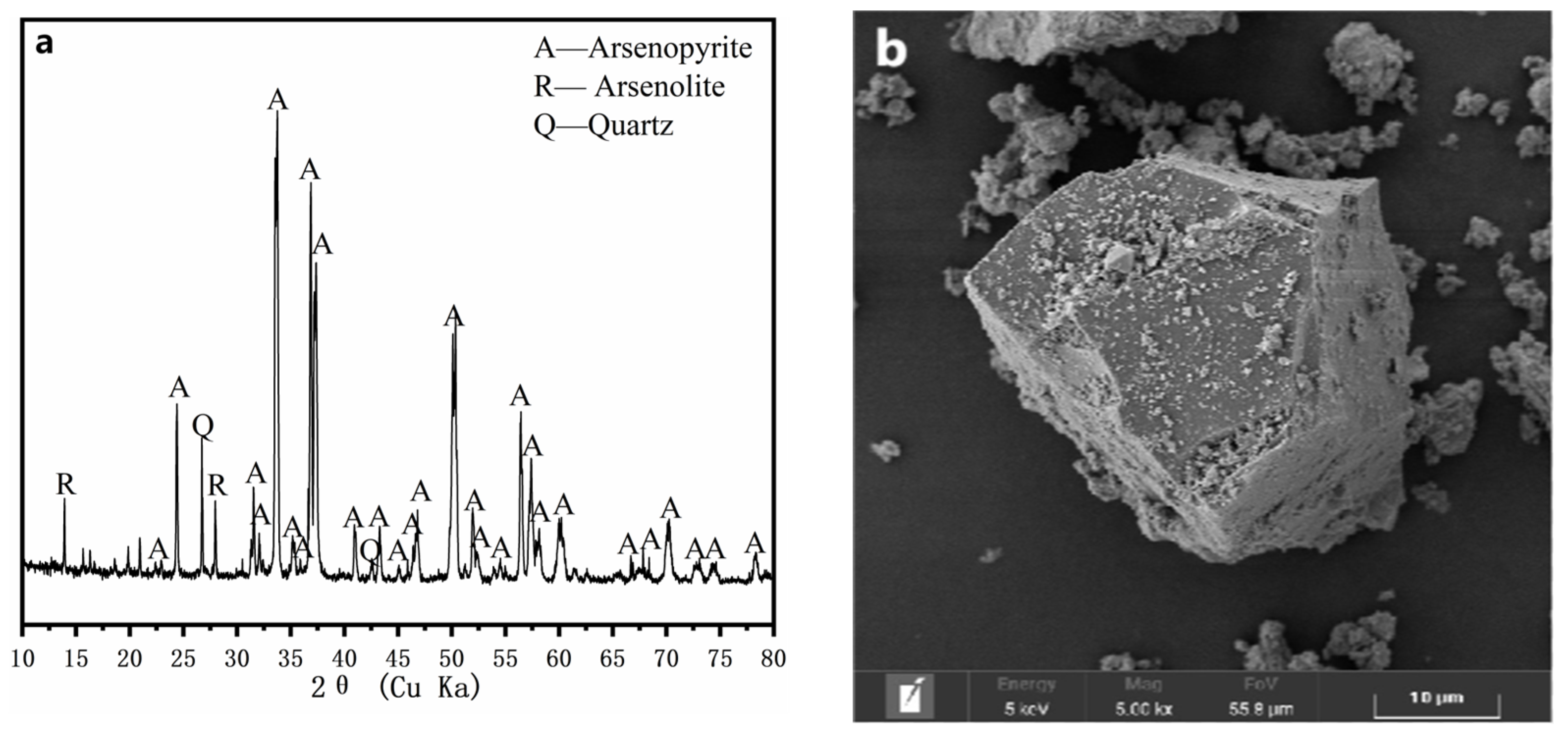
2.1. Minerals and Particle Preparation
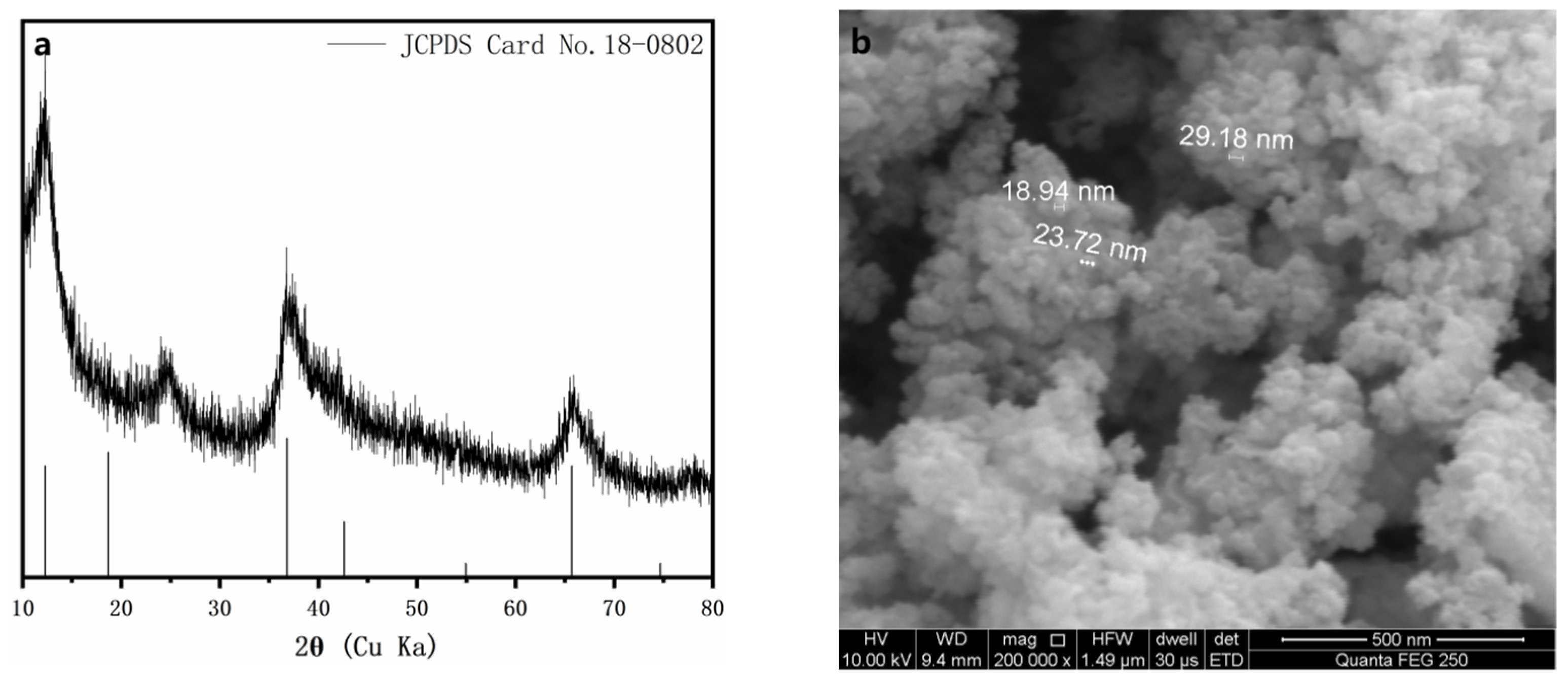
2.2. Preparation of δ-MnO2 Nanoparticles and Their Characterization
2.3. Experimental Procedure
2.4. Analytical Method
3. Results and Discussion
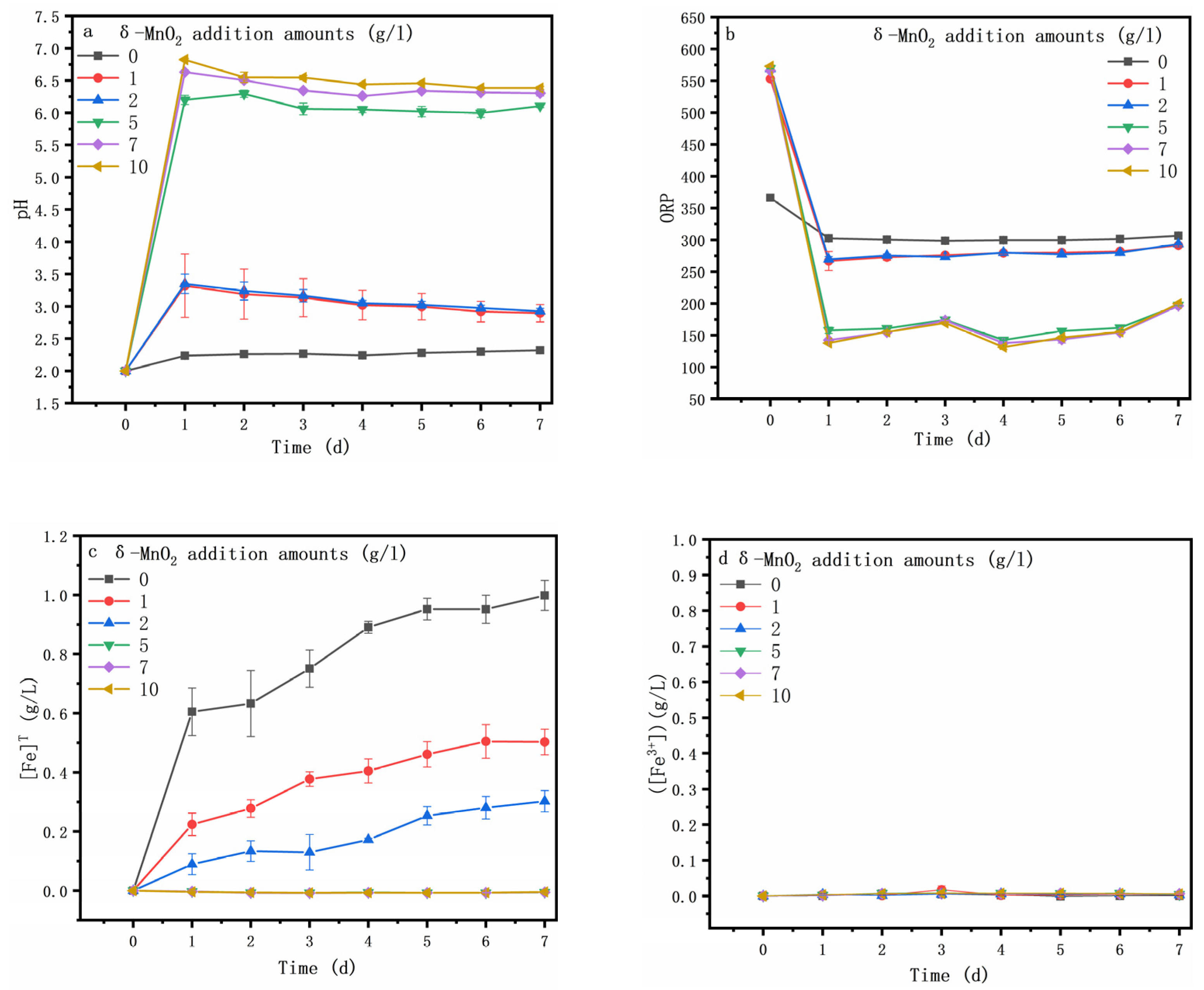
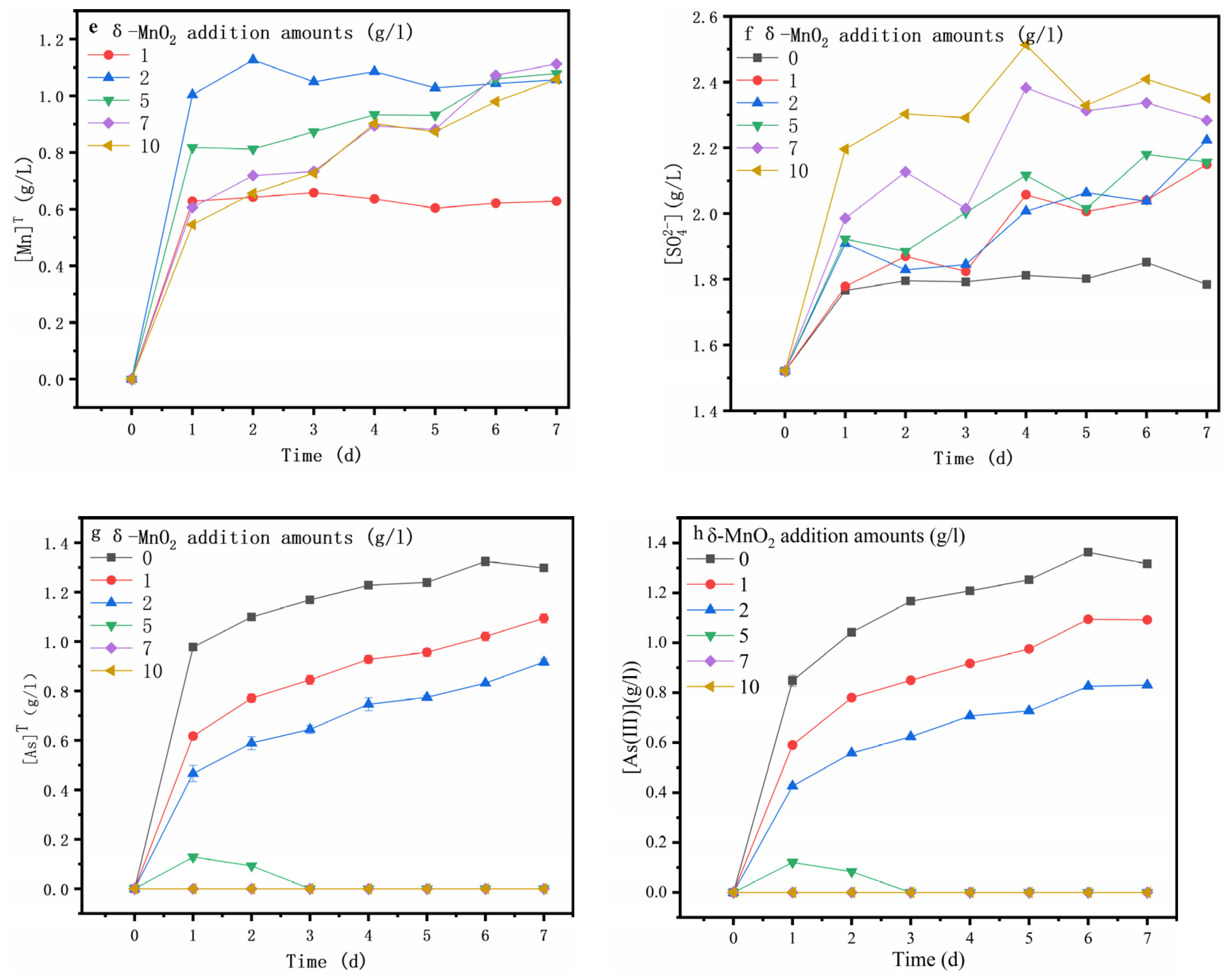
3.1. Oxidative Dissolution of Arsenopyrite Mediated by δ-MnO2
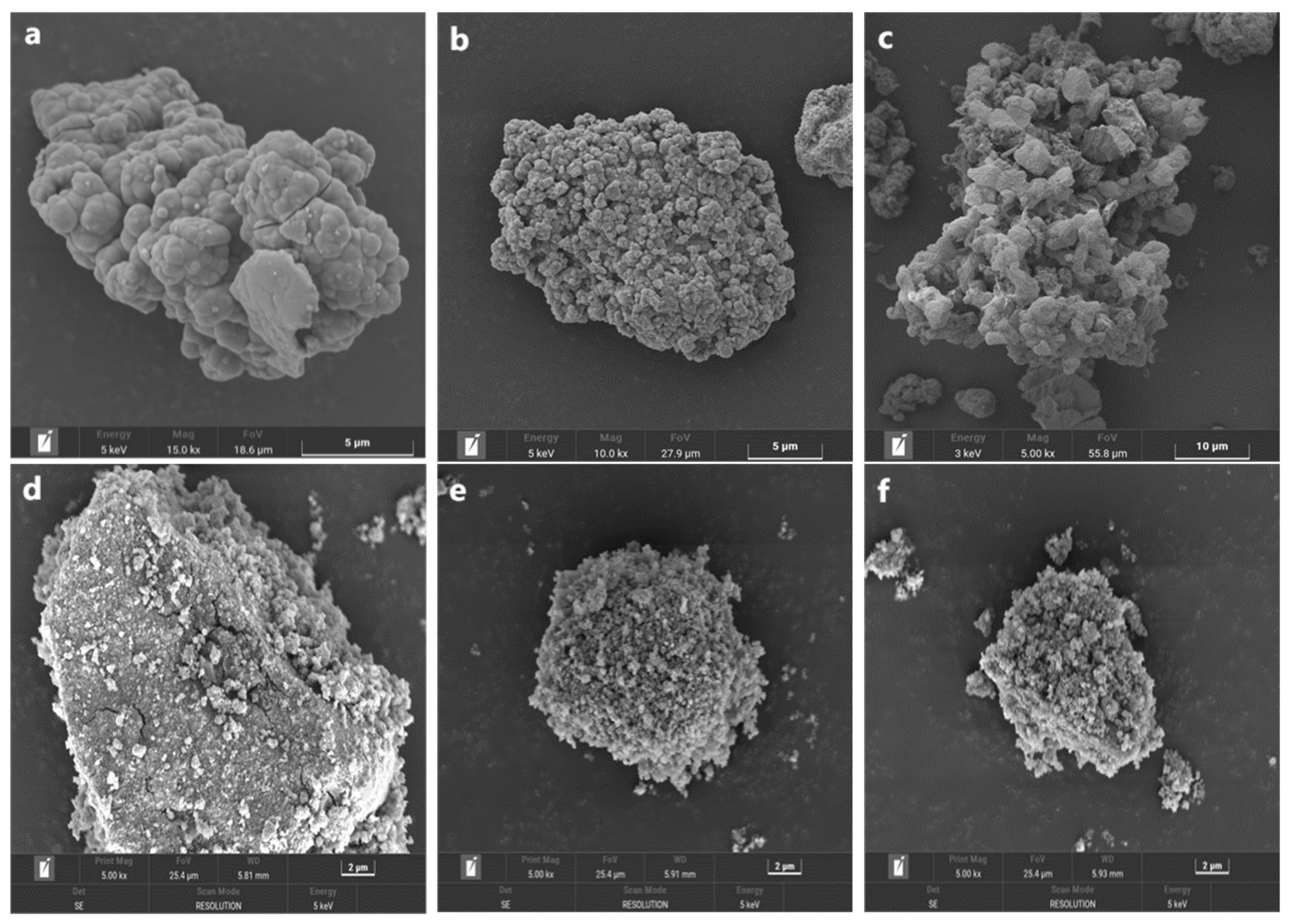
3.2. Changes in the Morphology and Mineralogical Phase during the Oxidative Dissolution of Arsenopyrite
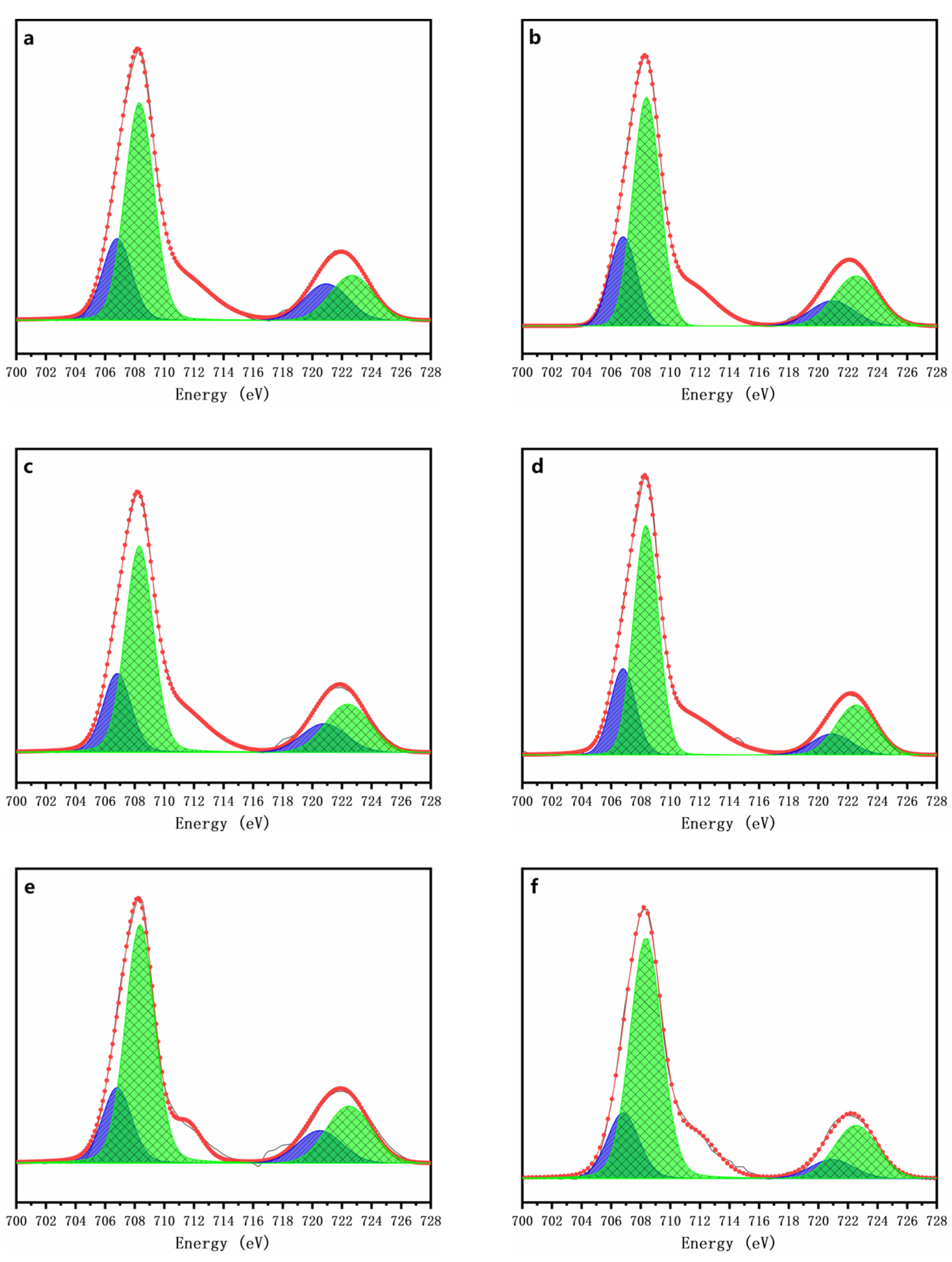
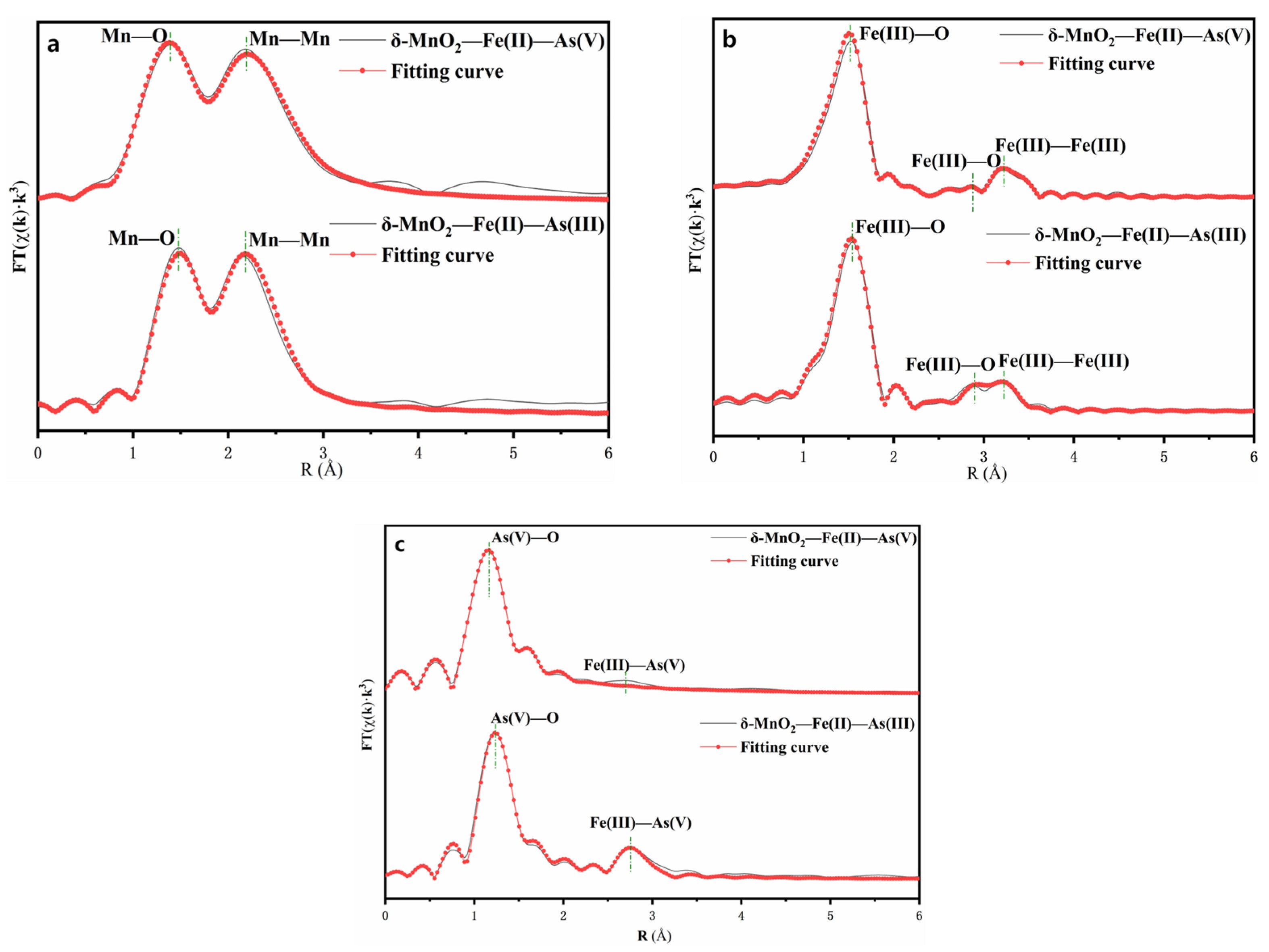
3.3. Speciation Transformation of As, Fe and S during Oxidative Dissolution of Arsenopyrite
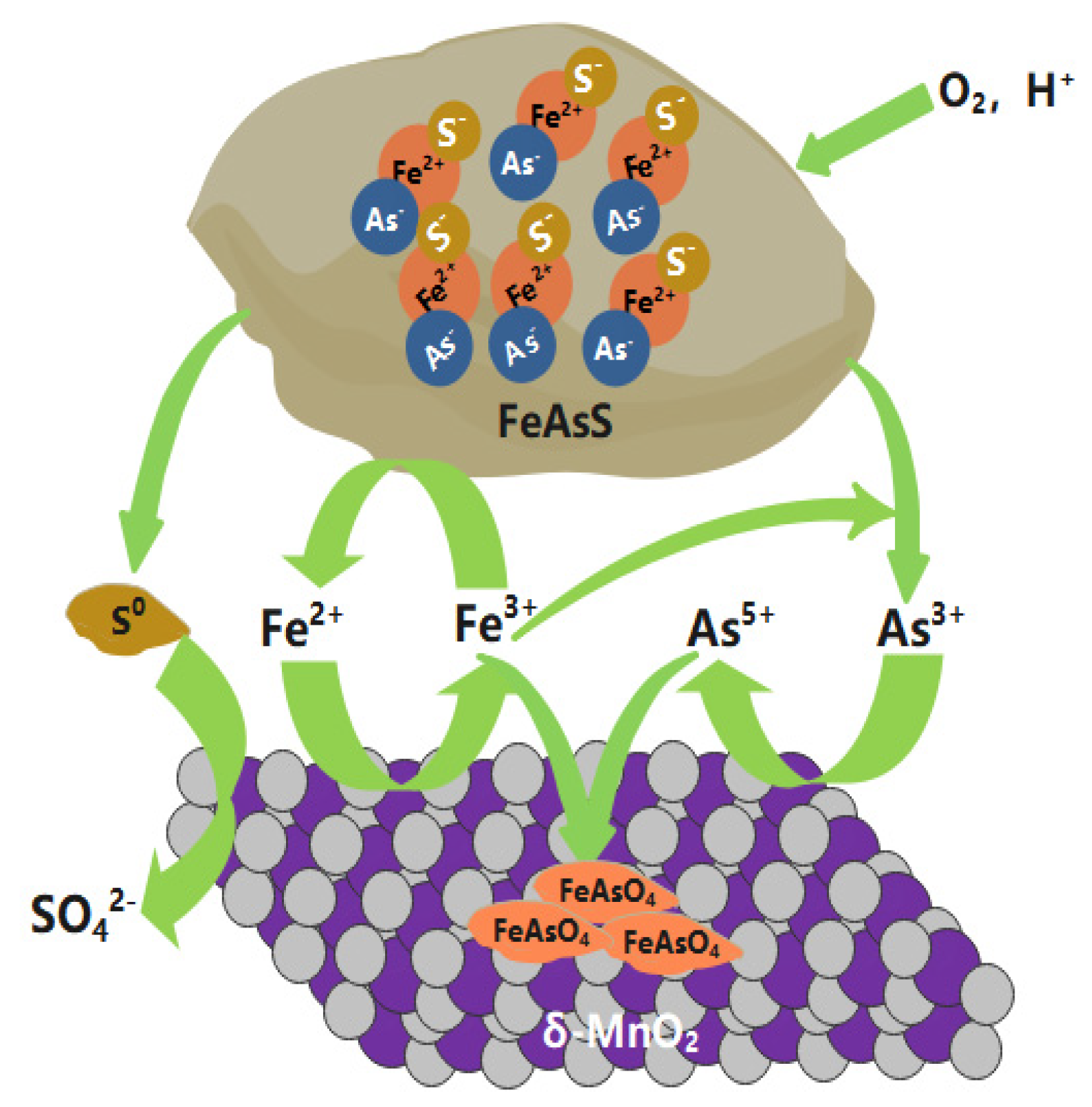
3.4. Mechanisms of Arsenopyrite Oxidative Dissolution and Arsenic Immobility Mediated by δ-MnO2
4. Conclusions
- i.
- δ-MnO2, as a strong oxidant, could oxidize arsenopyrite under acid conditions, while controlling arsenic release.
- ii.
- The addition of δ-MnO2 significantly altered arsenic precipitation and the solution’s chemistry, thereby influencing the fate of arsenic.
- iii.
- The addition of δ-MnO2 significantly accelerated the oxidation dissolution of arsenopyrite, and the formation of amorphous ferric arsenate, which is advantageous for the immobilization of arsenic.
- iv.
- The addition of an increased amount of δ-MnO2 (≥5 g/L) resulted in a significant increase in the solution pH, and the iron and arsenic concentrations decreased to almost zero. Small amounts of elemental sulfur and large amounts of amorphous ferric arsenate formed a porous structure on the surface of the mineral precipitate. Consequently, the dissolution of arsenopyrite continued, and the released arsenic was completely precipitated.
- v.
- δ-MnO2 rapidly oxidized As(III) and Fe2+ on the adsorbed surface to As(V) and Fe3+, and As(V) and Fe3+ further rapidly generated amorphous ferric arsenate precipitates.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudd, G.M. Global trends in gold mining: Towards quantifying environmental and resource sustainability. Resour. Policy 2007, 32, 42–56. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Gonzalez, R.; Gentina, J.C.; Acevedo, F. Biooxidation of a gold concentrate in a continuous stirred tank reactor: Mathematical model and optimal configuration. Biochem. Eng. J. 2004, 19, 33–42. [Google Scholar] [CrossRef]
- Nan, X.Y.; Cai, X.; Kong, J. Pretreatment Process on Refractory Gold Ores with As. Isij Int. 2014, 54, 543–547. [Google Scholar] [CrossRef][Green Version]
- Akcil, A.; Ciftci, H. Pretreatments Applied to Refractory Gold Ores. Madencilik 2009, 48, 17–30. [Google Scholar]
- Koslides, T.; Ciminelli, V.S.T. Pressure oxidation of arsenopyrite and pyrite in alkaline solutions. Hydrometallurgy 1992, 30, 87–106. [Google Scholar] [CrossRef]
- Michelis, I.D.; Olivieri, A.; Ubaldini, S.; Ferella, F.; Beolchini, F.; Vegliò, F. Roasting and chlorine leaching of gold-bearing refractory concentrate: Experimental and process analysis. Int. J. Min. Sci. Technol. 2013, 23, 709–715. [Google Scholar] [CrossRef]
- Bowden, W.; Grey, C.P.; Hackney, S.; Wang, F.; Paik, Y.; Iltchev, N.; Sirotina, R. Lithiation of ramsdellite–pyrolusite MnO2; NMR, XRD, TEM and electrochemical investigation of the discharge mechanism. J. Power Sources 2006, 153, 265–273. [Google Scholar] [CrossRef]
- Ouvrard, S.; Donato, P.D.; Simonnot, M.O.; Begin, S.; Ghanbaja, J.; Alnot, M.; Duval, Y.B.; Lhote, F.; Barres, O.; Sardin, M. Natural manganese oxide: Combined analytical approach for solid characterization and arsenic retention. Geochim. Cosmochim. Acta 2005, 69, 2715–2724. [Google Scholar] [CrossRef]
- Dawadi, S.; Gupta, A.; Khatri, M.; Budhathoki, B.; Lamichhane, G.; Parajuli, N. Manganese dioxide nanoparticles: Synthesis, application and challenges. B. Mater. Sci. 2020, 43, 277. [Google Scholar] [CrossRef]
- Yang, R.J.; Fan, Y.Y.; Ye, R.Q.; Tang, Y.X.; Cao, X.H.; Yin, Z.Y.; Zeng, Z.Y. MnO2-Based Materials for Environmental Applications. Adv. Mater. 2021, 33, 2004862. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cui, P.X.; Liu, C.; Peng, S.M.; Alves, M.E.; Zhou, D.M.; Shi, Z.Q.; Wang, Y.J. Antimony oxidation and sorption behavior on birnessites with different properties (δ-MnO2 and triclinic birnessite). Environ. Pollut. 2019, 246, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Owings, S.M.; Luther, G.W.; Taillefert, M. Development of a rate law for arsenite oxidation by manganese oxides. Geochim. Cosmchim. Acta 2019, 250, 251–267. [Google Scholar] [CrossRef]
- Oscarson, D.W.; Huang, P.M.; Liaw, W.K.; Hammer, U.T. Kinetics of oxidation of arsenite by various manganese dioxides [Soil chemistry]. Soil Sci. Soc. Am. J. 1983, 47, 644–648. [Google Scholar] [CrossRef]
- Su, C.M.; Puls, R.W. Arsenate and Arsenite Sorption on Magnetite: Relations to Groundwater Arsenic Treatment Using Zerovalent Iron and Natural Attenuation. Water Air Soil Poll. 2008, 193, 65–78. [Google Scholar] [CrossRef]
- Scott, M.J.; Morgan, J.J. Reactions at Oxide Surfaces. 1. Oxidation of As(III) by Synthetic Birnessite. Environ. Sci. Technol. 1995, 29, 1898–1905. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Ginder-Vogel, M.; Sparks, D.L. Arsenite Oxidation by a Poorly Crystalline Manganese-Oxide 1. Stirred-Flow Experiments. Environ. Sci. Technol. 2010, 44, 8460–8466. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Ginder-Vogel, M.; Zhu, M.; Livi, K.J.T.; Sparks, D.L. Arsenite Oxidation by a Poorly Crystalline Manganese-Oxide. 2. Results from X-ray Absorption Spectroscopy and X-ray Diffraction. Environ. Sci. Technol. 2010, 44, 8467–8472. [Google Scholar] [CrossRef][Green Version]
- Jones, R.A.; Koval, S.F.; Nesbitt, H.W. Surface alteration of arsenopyrite (FeAsS) by Thiobacillus ferrooxidans. Geochim. Cosmchim. Ac. 2003, 67, 955–965. [Google Scholar] [CrossRef]
- Yu, Y.M.; Zhu, Y.X.; Gao, Z.M.; Gammons, C.H.; Li, D.X. Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8-12.6 and 15–45 °C. Environ. Sci. Technol. 2007, 41, 6460–6464. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.H.; Wu, Z.T.; Xu, X.Y. Bioleaching of arsenopyrite by mixed cultures of iron-oxidizing and sulfur-oxidizing microorganisms. Chemosphere 2017, 185, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, D.R.; Xia, J.L.; Nie, Z.Y.; Xue, Z. Enhancement of arsenopyrite bioleaching by different Fe(III) compounds through changing composition and structure of passivation layer. J. Mater. Res. Technol. 2020, 9, 12364–12377. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.L.; Yin, H.Q.; Yang, Y.B.; Xu, B.; Jiang, T.; He, Y.H. The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium. J. Clean. Pro. 2020, 256, 120391. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Sun, S.K.; Liu, X.L.; Jiang, T.; Lyu, X.J.; He, Y.H. Electrochemical behaviour of the oxidative dissolution of arsenopyrite catalysed by Ag+ in 9K culture medium. Colloid. Surf. A. 2021, 614, 126169. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.L.; Li, H.R. Enhancement of bio-oxidation of refractory arsenopyritic gold ore by adding pyrolusite in bioleaching system. T. Nonferr. Metals Soc. 2016, 26, 2479–2484. [Google Scholar] [CrossRef]
- Deng, S.; He, G.S.; Wu, B.C.; Gu, G.H. Pyrite-promoted dissolution of arsenopyrite in the presence of Sulfobacillus thermosulfidooxidans. J. Mater. Res. Technol. 2020, 9, 9362–9371. [Google Scholar] [CrossRef]
- Zhang, D.R.; Chen, H.R.; Xia, J.L.; Nie, Z.Y.; Fan, X.L.; Liu, H.C.; Zheng, L.; Zhang, L.J.; Yang, H.Y. Humic acid promotes arsenopyrite bio-oxidation and arsenic immobilization. J. Hazard. Mater. 2020, 384, 121359. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Qin, Y.W.; Zheng, B.H.; Zhang, L.; Zhao, Y.M. Arsenic release from the abiotic oxidation of arsenopyrite under the impact of waterborne H2O2: A SEM and XPS study. Environ. Sci. Pollut. Res. 2016, 23, 1381–1390. [Google Scholar] [CrossRef]
- Dong, Z.Z.; Zhu, Y.M.; Han, Y.X.; Gao, P.; Gu, X.T.; Sun, Y.S. Chemical oxidation of arsenopyrite using a novel oxidant—Chlorine dioxide. Miner. Eng. 2019, 139, 105863. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.W.; Vajtai, R.; Ajayan, P.M.; Wei, B.Q. Hydrothermal Synthesis and Pseudocapacitance Properties of MnO2 Nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef]
- Karamanev, D.G.; Nikolov, L.N.; Mamatarkova, V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions. Miner. Eng. 2002, 15, 341–346. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, J.L.; Yang, Y.; Nie, Z.Y.; Zheng, L.; Ma, C.Y.; Zhang, R.Y.; Peng, A.A.; Tang, L.; Qiu, G.Z. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite. Bioresource Technol. 2011, 102, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, H.B.; Wang, J.; Gan, X.W.; Hu, M.H.; Tao, L.; Qin, W.Q.; Qiu, G.Z. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential. Hydrometallurgy 2016, 164, 159–165. [Google Scholar] [CrossRef]
- Gu, G.H.; Hu, K.T.; Zhang, X.; Xiong, X.X.; Yang, H.S. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum. Electrochim. Acta 2013, 103, 50–57. [Google Scholar] [CrossRef]
- Liu, H.C.; Xia, J.L.; Nie, Z.Y.; Ma, C.Y.; Zheng, L.; Hong, C.; Zhao, Y.D.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Philip, L.; Pradeep, T. As(III) removal from drinking water using manganese oxide-coated-alumina: Performance evaluation and mechanistic details of surface binding. Chem. Eng. J. 2009, 153, 101–107. [Google Scholar] [CrossRef]
- Carlson, L.; Bigham, J.M.; Schwertmann, U.; Kyek, A.; Wagner, F. Scavenging of As from Acid Mine Drainage by Schwertmannite and Ferrihydrite:? A Comparison with Synthetic Analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef]
- Jia, Y.F.; Xu, L.Y.; Wang, X.; Demopoulos, G.P. Infrared spectroscopic and X-ray diffraction characterization of the nature of adsorbed arsenate on ferrihydrite. Geochim. Cosmchim. Ac. 2007, 71, 1643–1654. [Google Scholar] [CrossRef]
- Buckley, A.N.; Walker, G.W. The surface composition of arsenopyrite exposed to oxidizing environments. Appl. Surf. Sci. 1988, 35, 227–240. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air. Miner. Petrol. 1998, 62, 123–144. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J.; Prarr, A.R. Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim. Cosmchim. Acta 1995, 59, 1773–1786. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. X-ray photoelectron spectroscopy of oxidised pyrrhotite surfaces. Appl. Surf. Sci. 1985, 22, 280–287. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef][Green Version]
- Mikhlin, Y.; Tomashevich, Y. Pristine and reacted surfaces of pyrrhotite and arsenopyrite as studied by X-ray absorption near-edge structure spectroscopy. Phys. Chem. Miner. 2005, 32, 19–27. [Google Scholar] [CrossRef]
- Godehusen, K.; Richter, T.; Zimmermann, P.; Wernet, P. Iron L-Edge Absorption Spectroscopy of Iron Pentacarbonyl and Ferrocene in the Gas Phase. J. Phys. Chem. A 2017, 121, 66–72. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.W.; Spiro, T.G.; Tebo, B.M. Mn(II,III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef][Green Version]
- Zhang, G.S.; Liu, F.D.; Liu, H.J.; Qu, J.H.; Liu, R.P. Respective Role of Fe and Mn Oxide Contents for Arsenic Sorption in Iron and Manganese Binary Oxide: An X-ray Absorption Spectroscopy Investigation. Environ. Sci. Technol. 2014, 48, 10316–10322. [Google Scholar] [CrossRef]
- Arčon, I.; van Elteren, J.T.; Glass, H.J.; Kodre, A.; Šlejkovec, Z. EXAFS and XANES study of arsenic in contaminated soil. X-ray Spectrom. 2005, 34, 435–438. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Sparks, D.L. Effect of Iron(II) on Arsenic Sequestration by δ-MnO2: Desorption Studies Using Stirred-Flow Experiments and X-Ray Absorption Fine-Structure Spectroscopy. Environ. Sci. Technol. 2015, 49, 13360–13368. [Google Scholar] [CrossRef]





| Samples | Dv(10), μm | Dv(50), μm | Dv(90), μm |
|---|---|---|---|
| Original arsenopyrite | 3.46 | 30.94 | 176.06 |
| 0 | 9.21 | 21.65 | 50.94 |
| 1 | 3.24 | 9.66 | 56.44 |
| 2 | 4.59 | 14.78 | 90.33 |
| 5 | 0.87 | 8.52 | 35.91 |
| 7 | 0.82 | 7.07 | 26.66 |
| 10 | 1.31 | 10.51 | 35.53 |
| Samples | Fe | As | S | Fe/S | Fe/As | As/S |
|---|---|---|---|---|---|---|
| Original arsenopyrite | 25.32 | 36.05 | 38.63 | 0.66 | 0.70 | 0.93 |
| 0 | 26.28 | 43.09 | 30.63 | 0.86 | 0.61 | 1.41 |
| 1 | 34.66 | 48.20 | 17.10 | 0.72 | 0.72 | 2.03 |
| 2 | 26.60 | 42.56 | 30.80 | 0.86 | 0.63 | 1.38 |
| 5 | 24.33 | 61.90 | 13.80 | 1.76 | 0.39 | 4.49 |
| 7 | 28.38 | 59.60 | 12.00 | 2.37 | 0.48 | 4.97 |
| 10 | 26.80 | 61.00 | 12.20 | 2.20 | 0.44 | 5.00 |
| Element | Samples | Path | N | R (Å) | σ2 (Å2) | ΔE0 (eV) | R factor |
|---|---|---|---|---|---|---|---|
| Mn | δ-MnO2–Fe(II)–As(V) | Mn–O | 3.3 | 1.95 | 0.002 | 5.23 | 0.024 |
| Mn–Mn | 3.9 | 2.94 | 0.003 | ||||
| δ-MnO2–Fe(II)–As(III) | Mn–O | 3.1 | 1.95 | 0.001 | 4.78 | 0.018 | |
| Mn–Mn | 3.7 | 2.94 | 0.002 | ||||
| Fe | δ-MnO2–Fe(II)–As(V) | Fe(III)–O | 2.6 | 1.92 | 0.0009 | 6.11 | 0.032 |
| Fe(III)–O | 0.7 | 3.29 | 0.002 | ||||
| Fe(III)–Fe(III) | 0.8 | 3.37 | 0.001 | ||||
| δ-MnO2–Fe(II)–As(III) | Fe(III)–O | 2.6 | 1.92 | 0.001 | 5.47 | 0.027 | |
| Fe(III)–O | 0.7 | 3.27 | 0.003 | ||||
| Fe(III)–Fe(III) | 0.8 | 3.36 | 0.004 | ||||
| As | δ-MnO2–Fe(II)–As(V) | As(V)–O | 3.3 | 1.68 | 0.0008 | 3.94 | 0.019 |
| As(V)–Fe(III) | 2.4 | 3.38 | 0.002 | ||||
| δ-MnO2–Fe(II)–As(III) | As(V)–O | 3.2 | 1.71 | 0.003 | 5.18 | 0.017 | |
| As(V)–Fe(III) | 2.1 | 3.40 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Liu, L.-Z.; Nie, Z.-Y.; Xia, J.-L. δ-MnO2 Drives the Green Decomposition of Arsenopyrite by Mediating the Fate of Arsenic to Generate FeAsO4. Minerals 2023, 13, 657. https://doi.org/10.3390/min13050657
Pan X, Liu L-Z, Nie Z-Y, Xia J-L. δ-MnO2 Drives the Green Decomposition of Arsenopyrite by Mediating the Fate of Arsenic to Generate FeAsO4. Minerals. 2023; 13(5):657. https://doi.org/10.3390/min13050657
Chicago/Turabian StylePan, Xuan, Li-Zhu Liu, Zhen-Yuan Nie, and Jin-Lan Xia. 2023. "δ-MnO2 Drives the Green Decomposition of Arsenopyrite by Mediating the Fate of Arsenic to Generate FeAsO4" Minerals 13, no. 5: 657. https://doi.org/10.3390/min13050657
APA StylePan, X., Liu, L.-Z., Nie, Z.-Y., & Xia, J.-L. (2023). δ-MnO2 Drives the Green Decomposition of Arsenopyrite by Mediating the Fate of Arsenic to Generate FeAsO4. Minerals, 13(5), 657. https://doi.org/10.3390/min13050657





