Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia
Abstract
1. Introduction
2. Background
2.1. Geological Setting and Ore Genesis
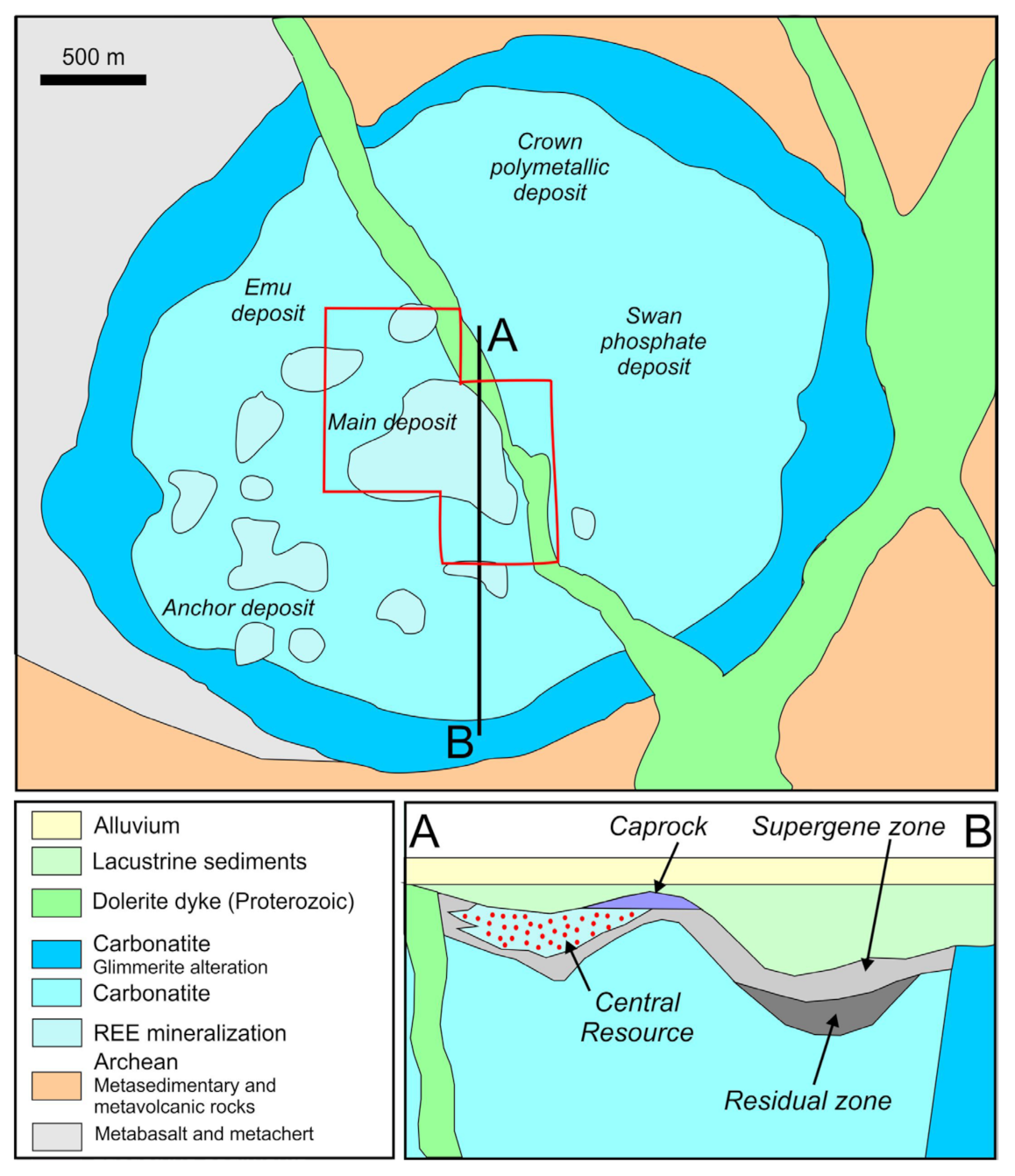
2.2. The Mount Weld Orefield
3. Sample Suite
4. Analytical Methodology
5. Results
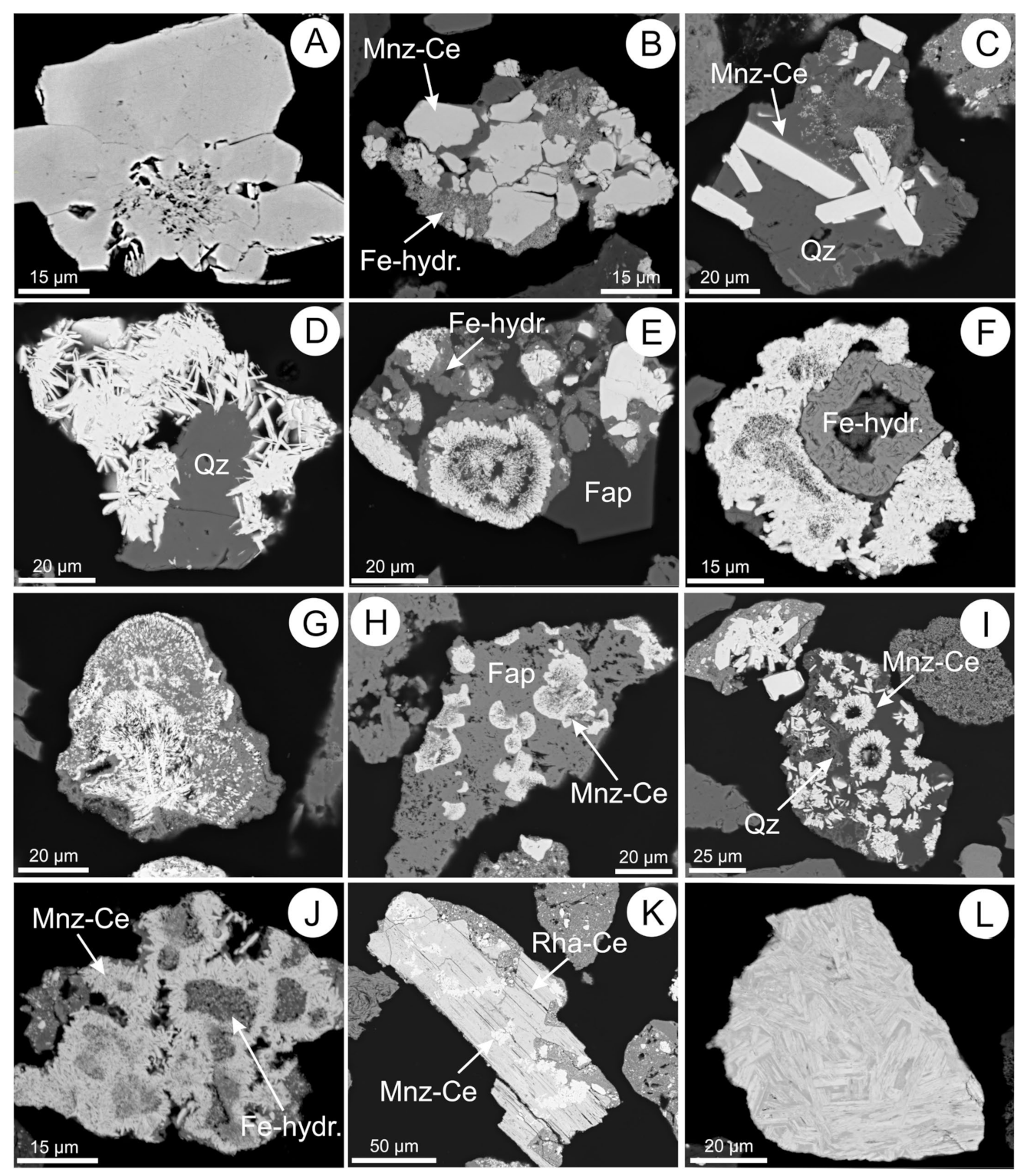
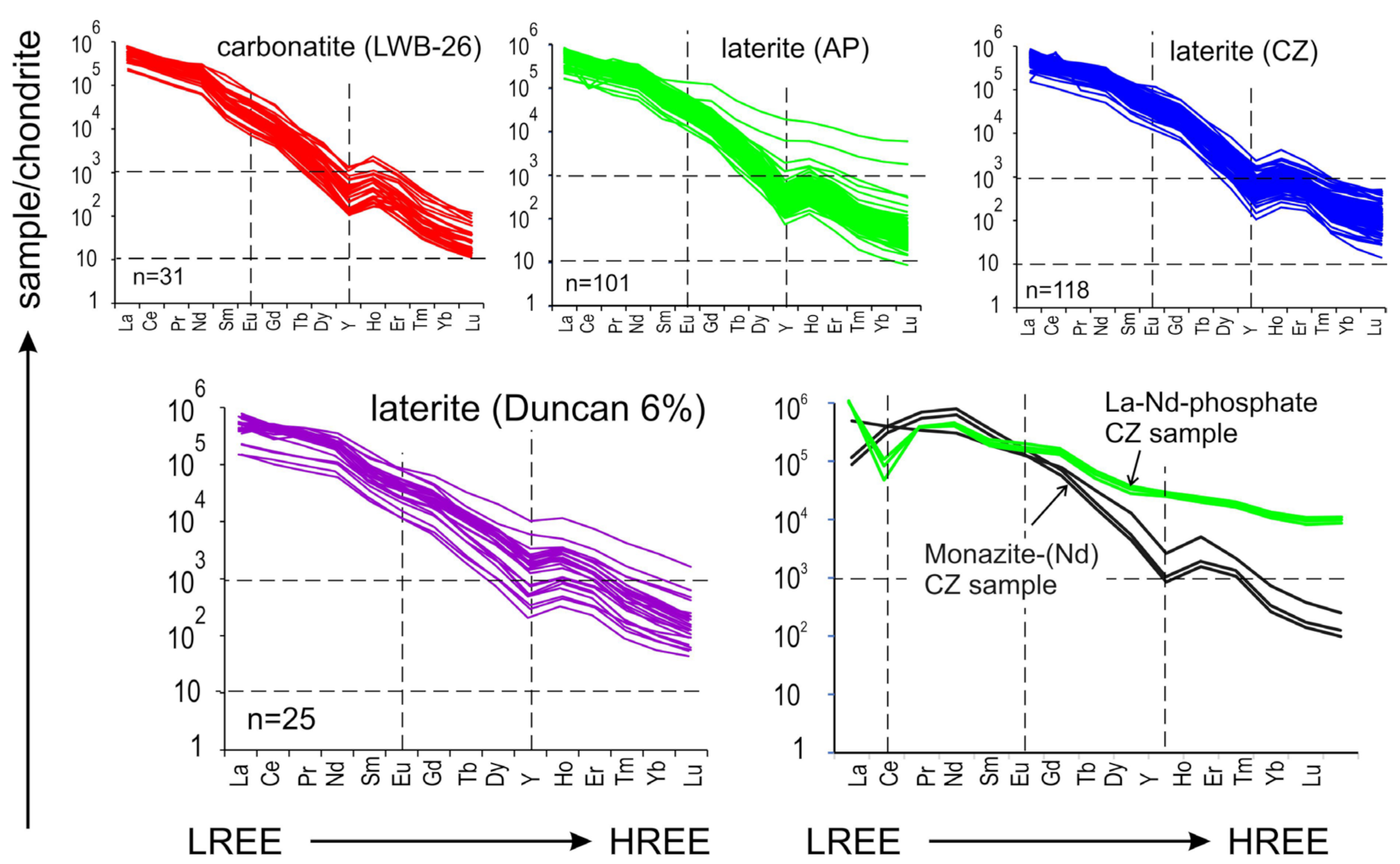
5.1. Monazite
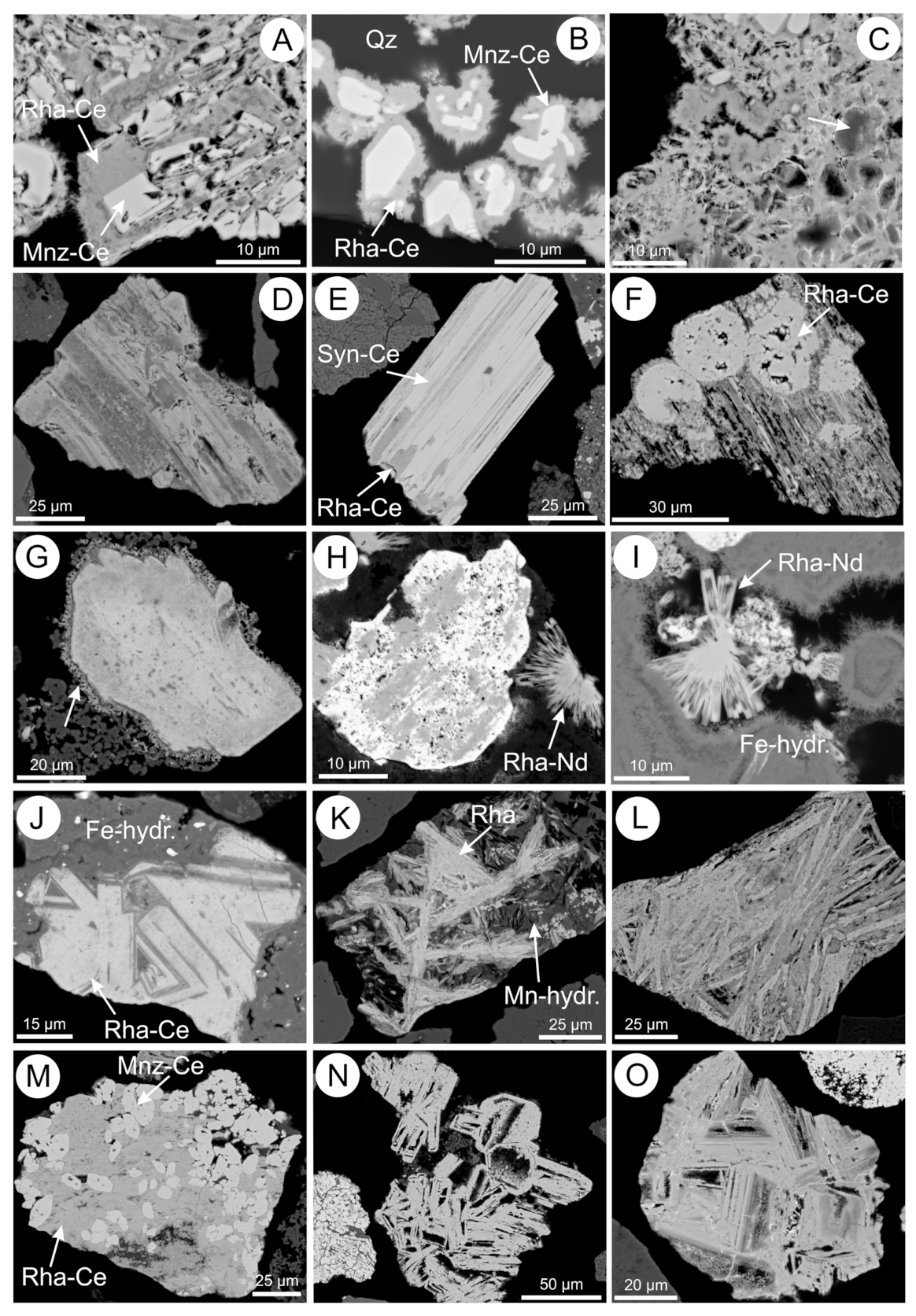
5.2. Rhabdophane
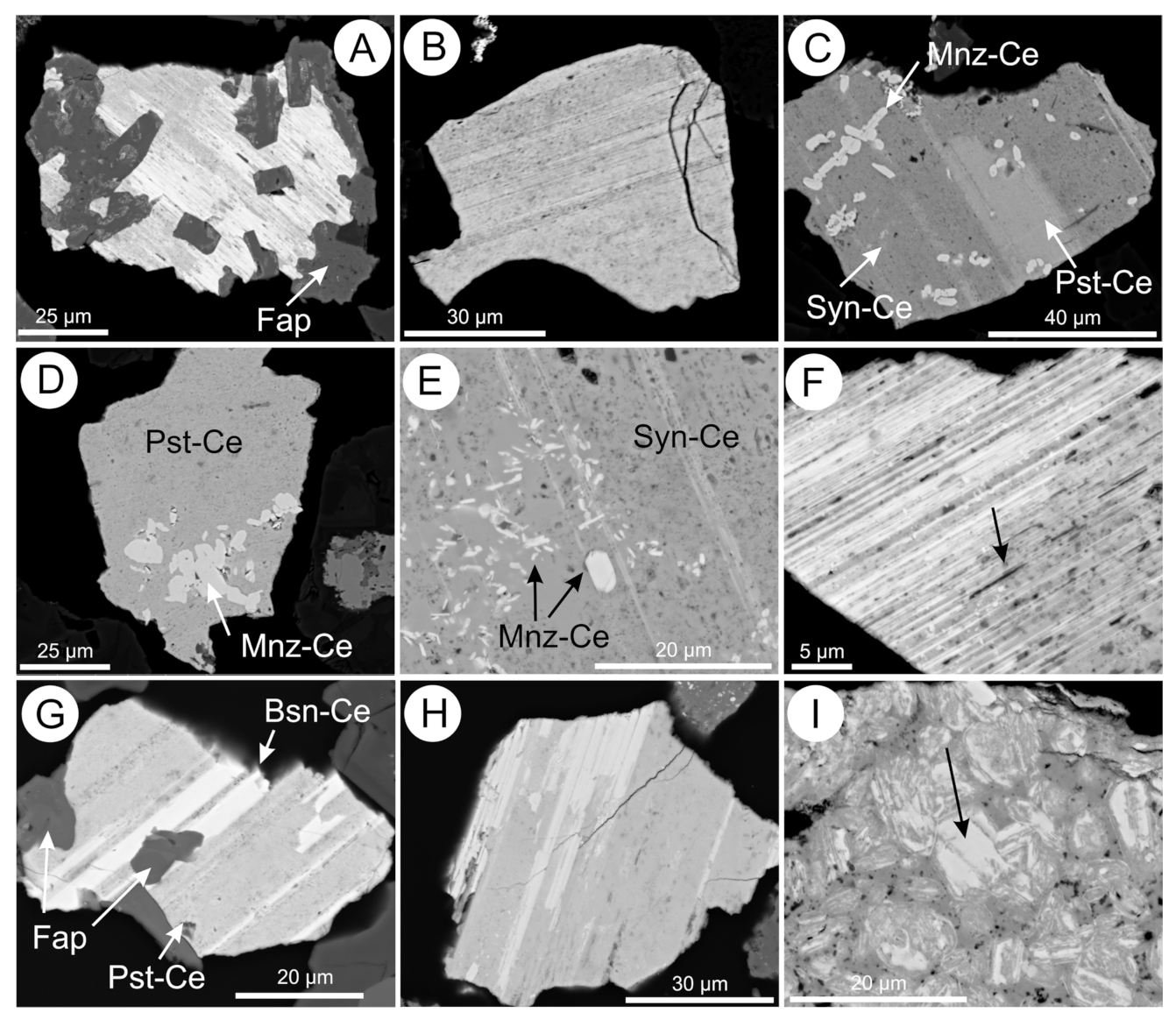
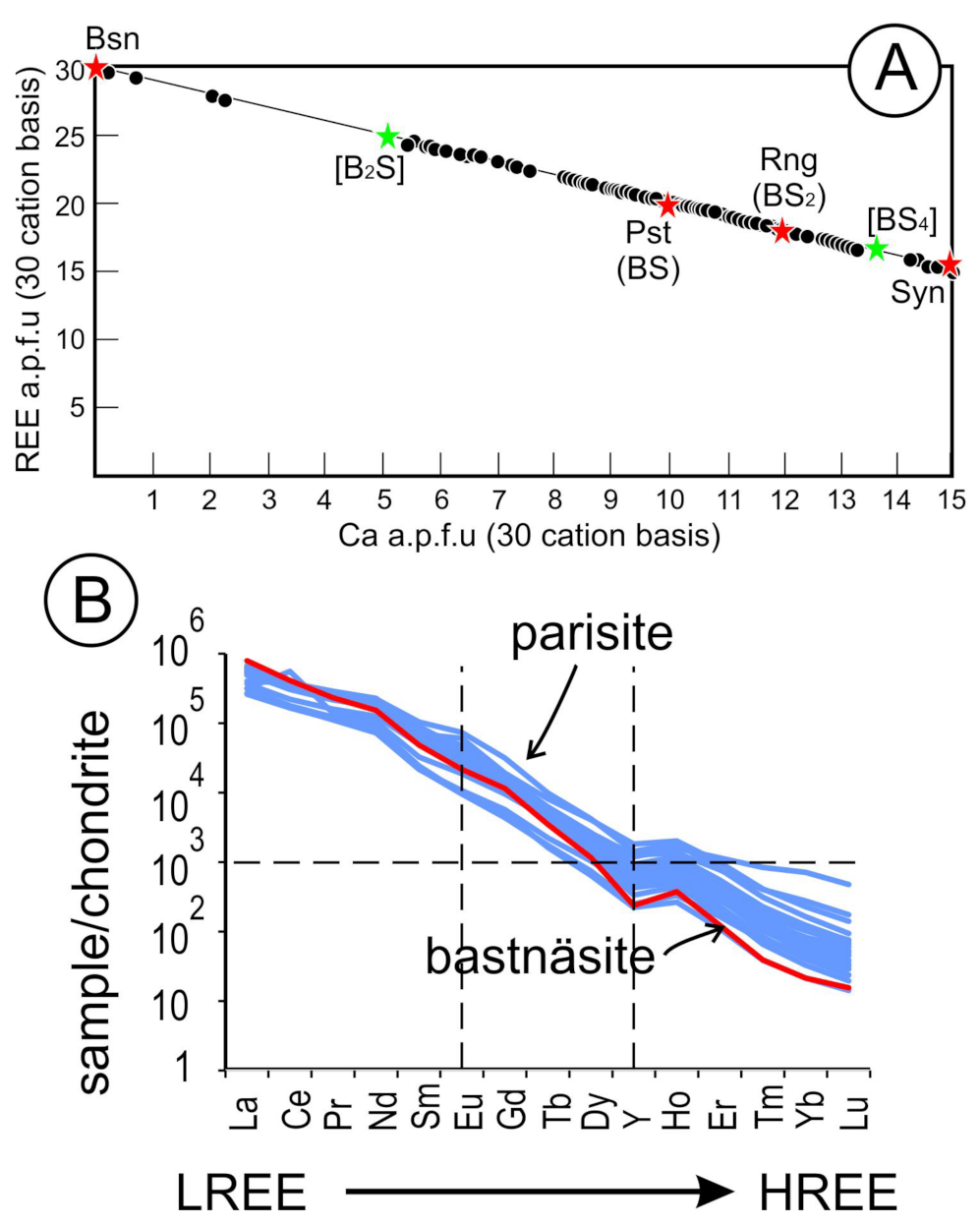
5.3. REE-Fluorocarbonates
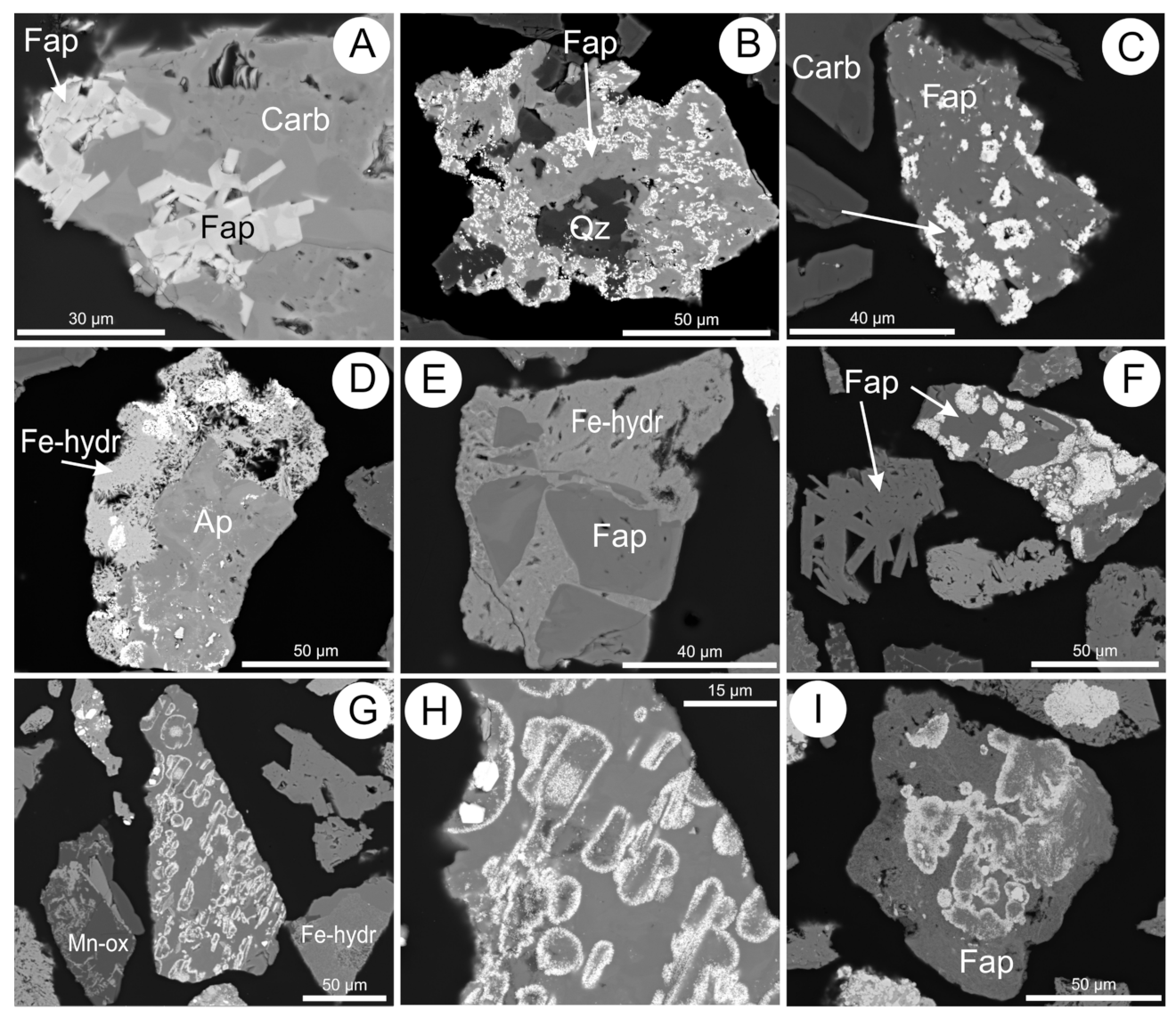
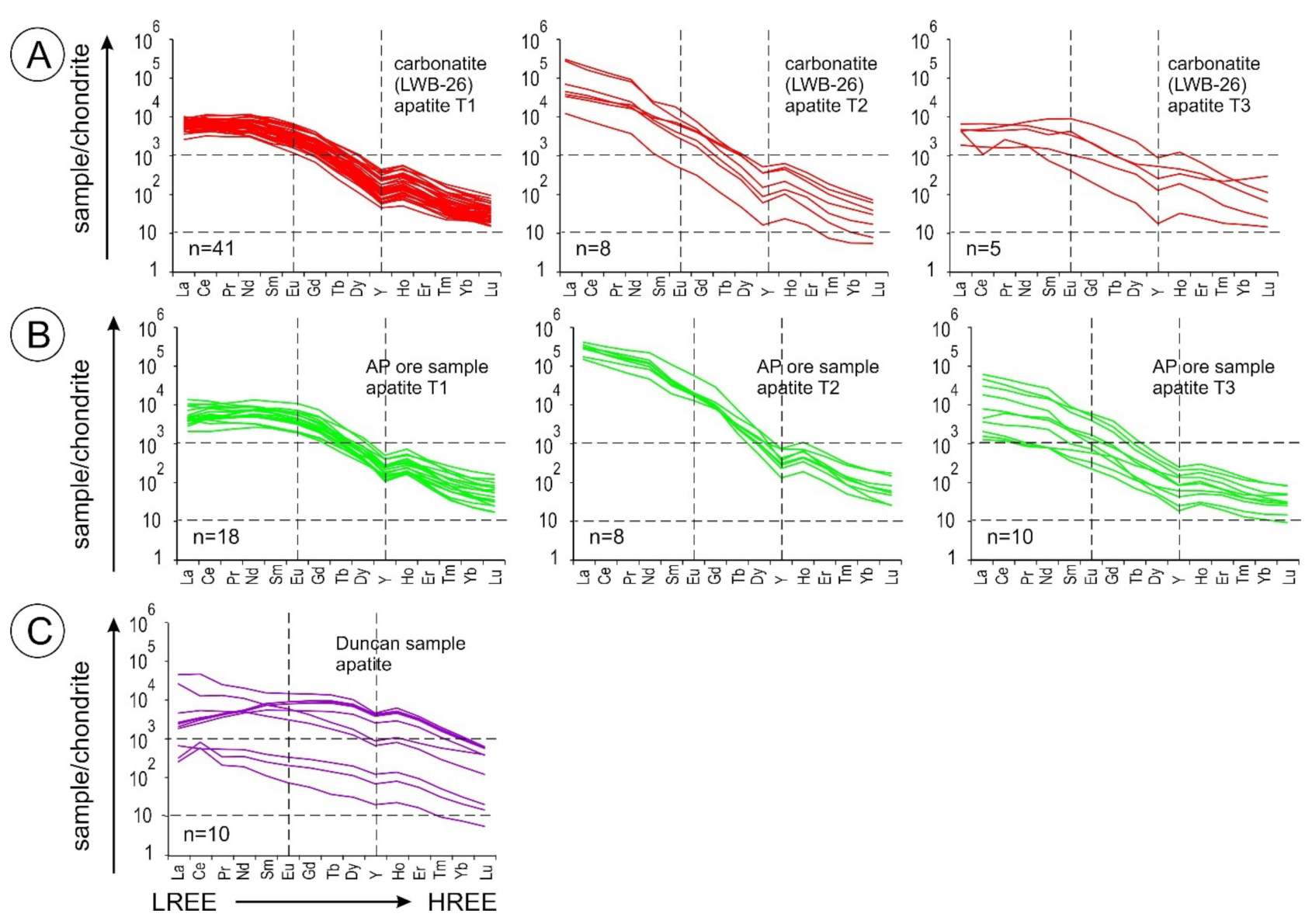
5.4. Apatite Group Minerals
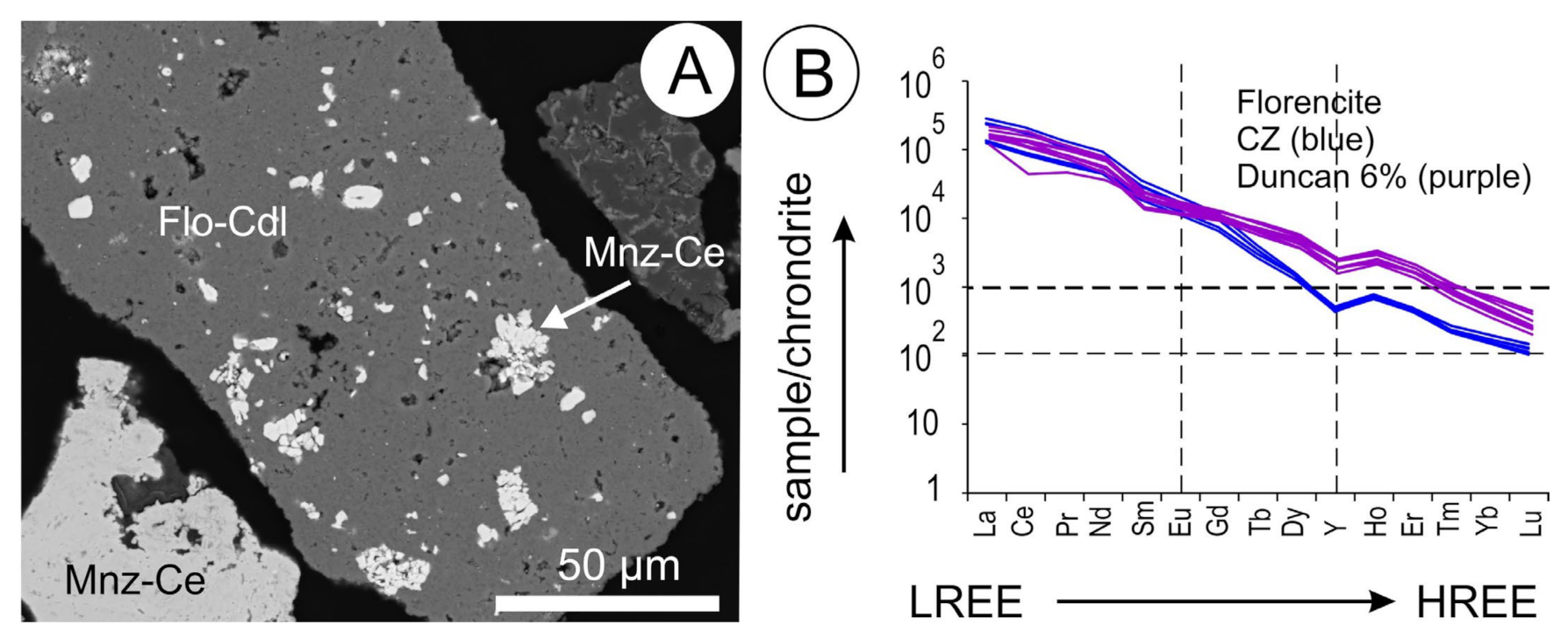
5.5. Florencite
5.6. Fluocerite
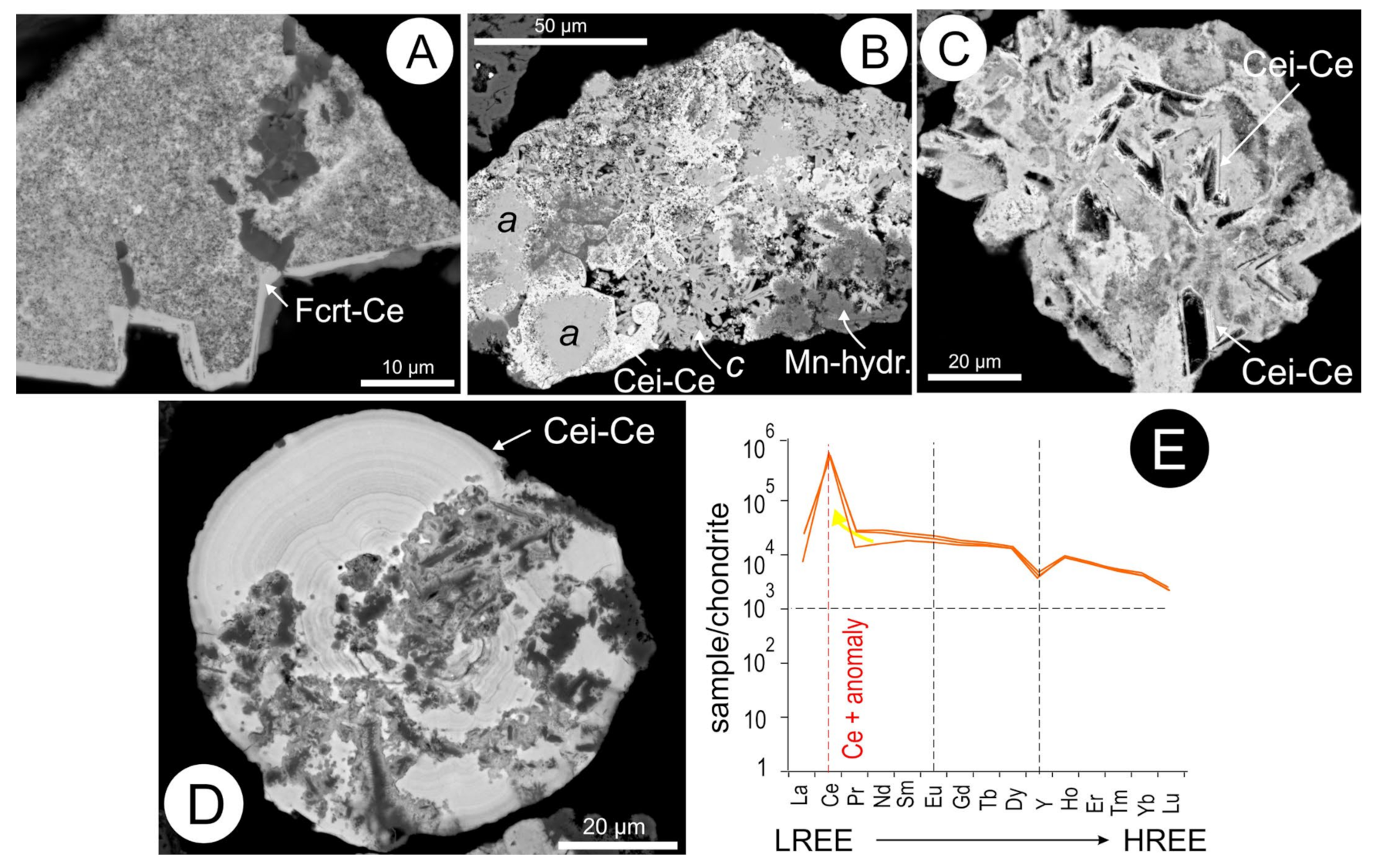
5.7. Cerianite and Cerite
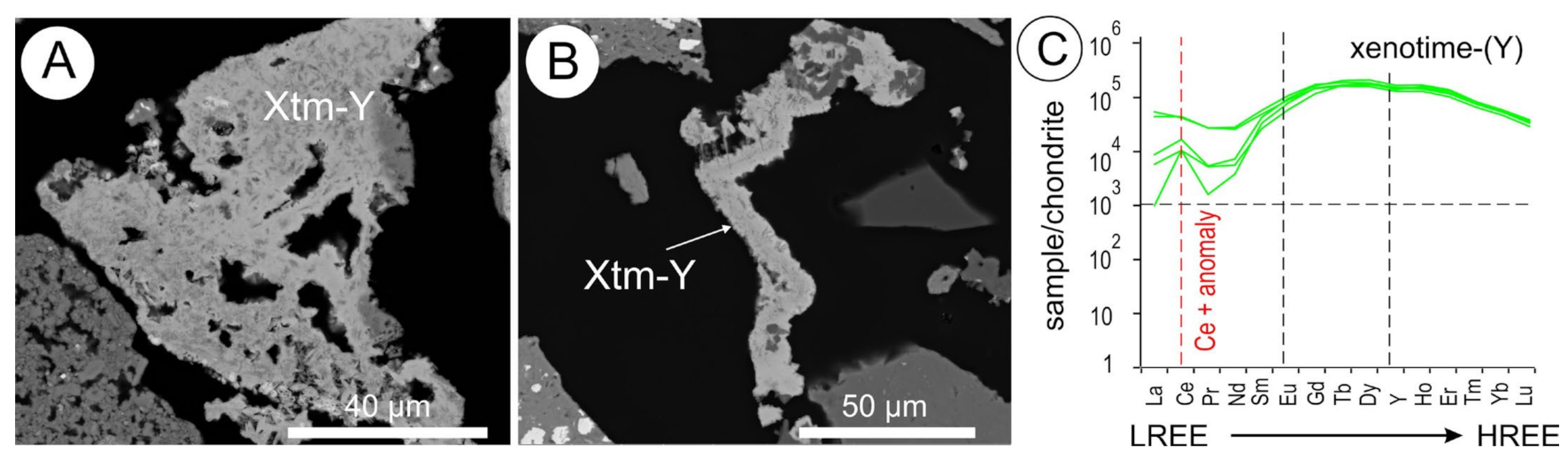
5.8. Xenotime
5.9. Zircon
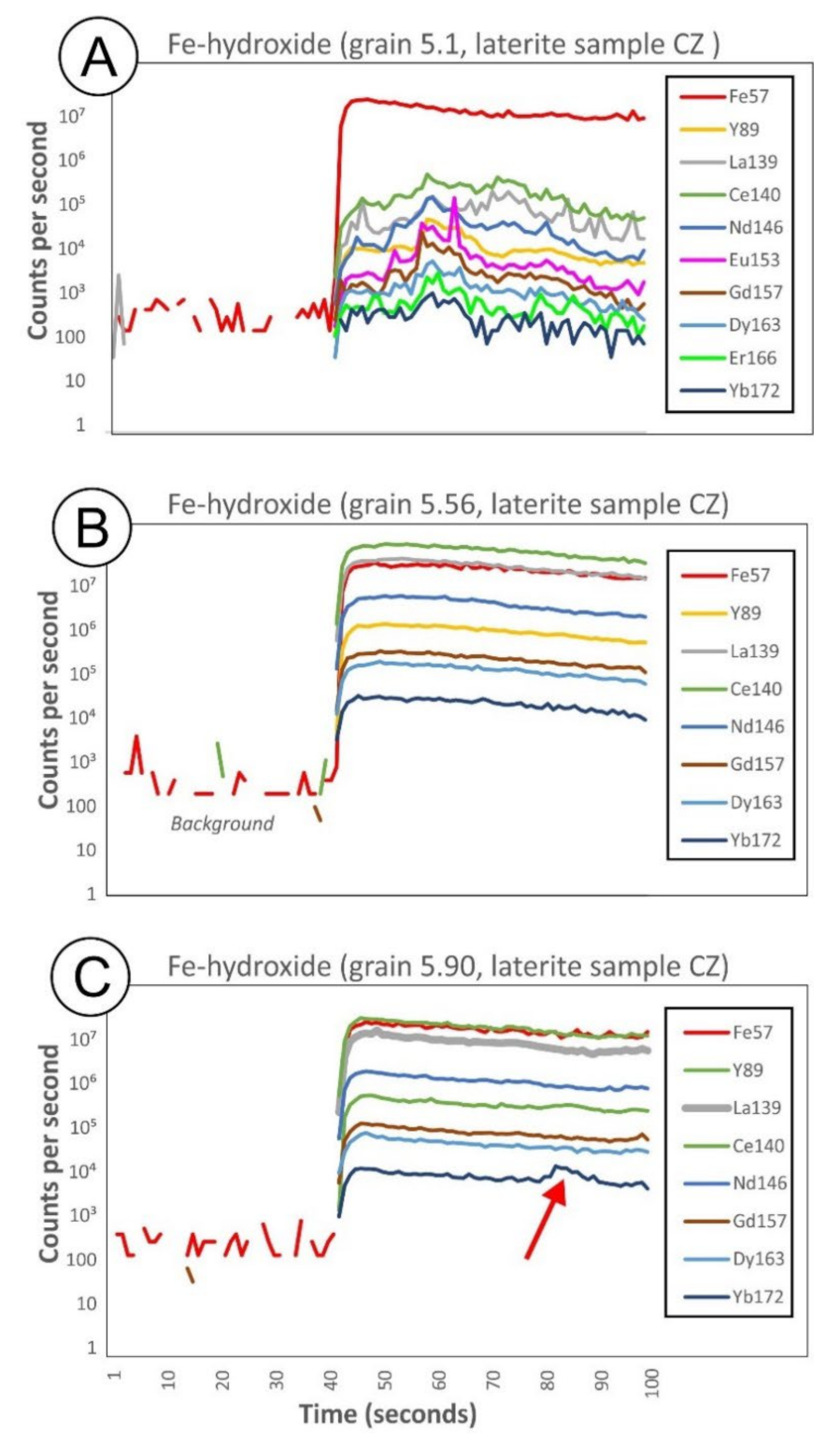
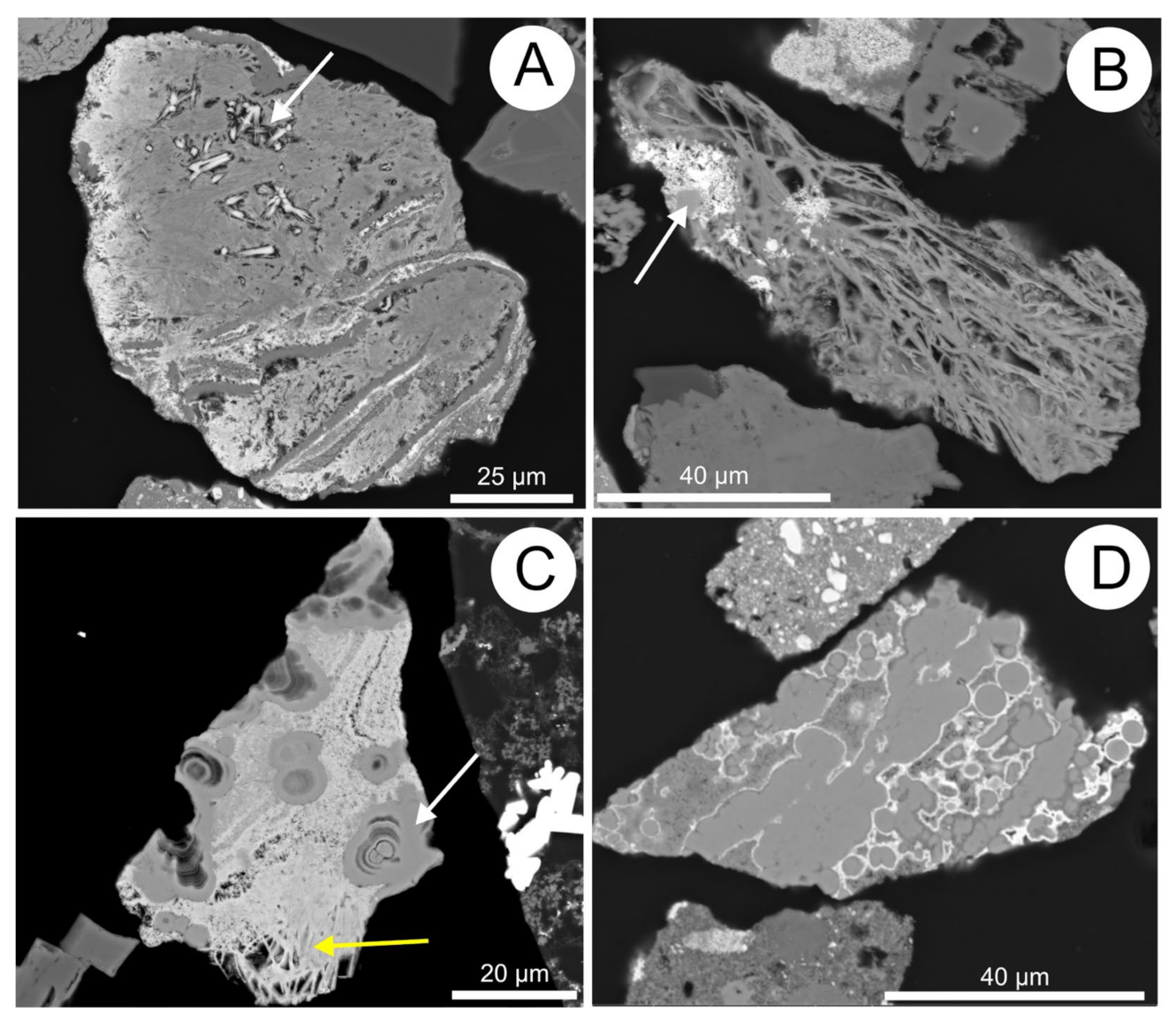
5.10. Fe-(hydr)oxides
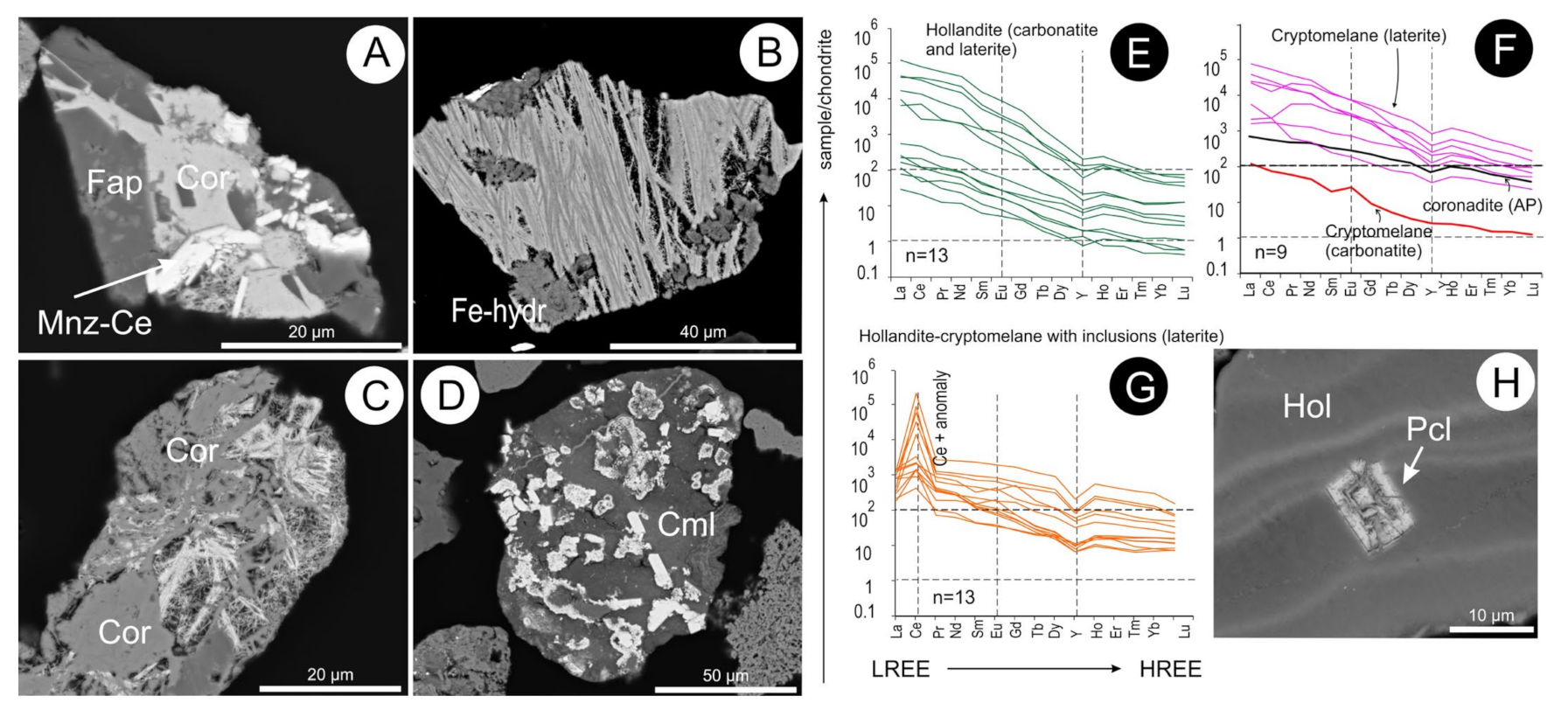
5.11. Mn- and Mn-Fe-(hydr)oxides
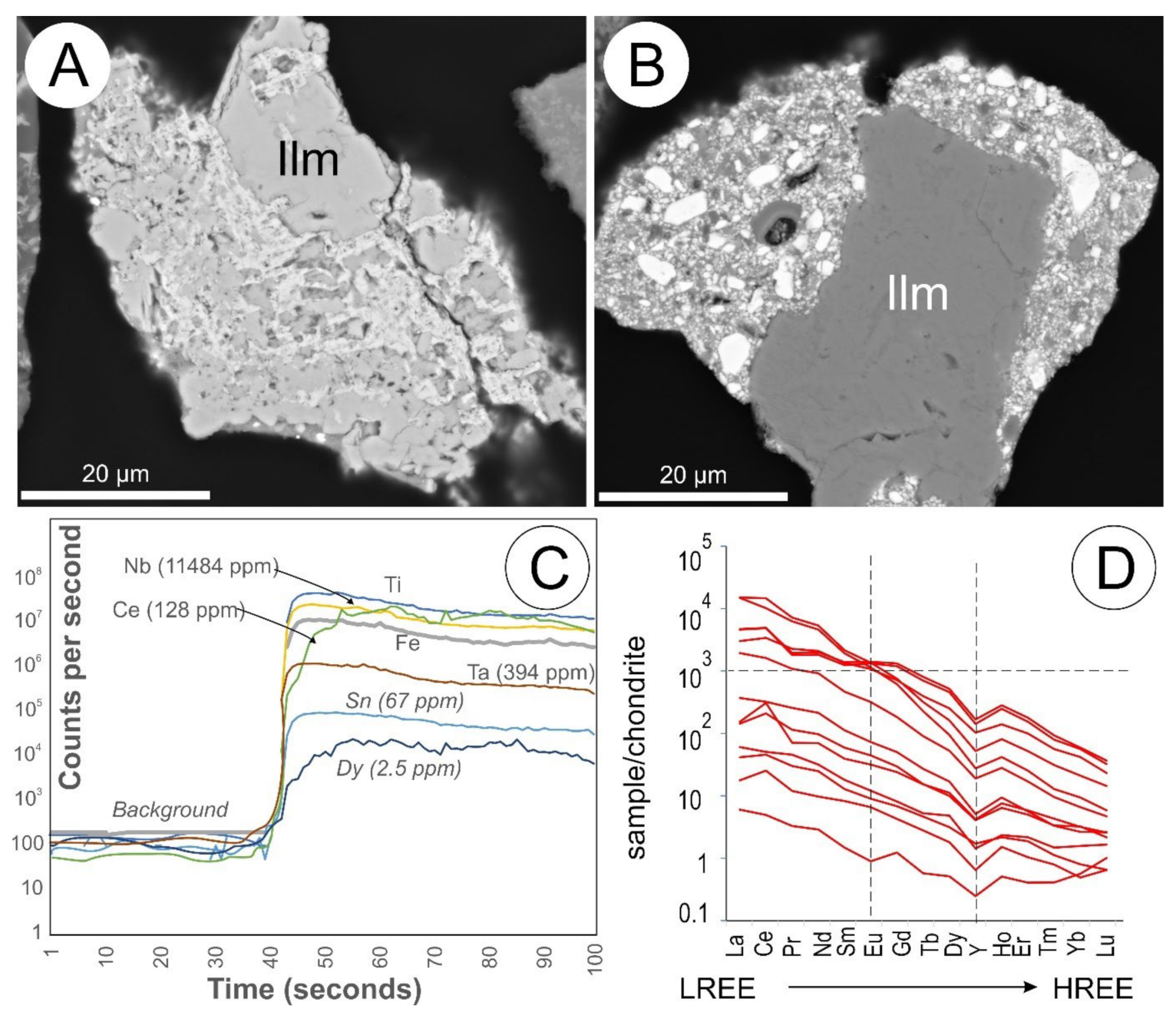
5.12. Ilmenite
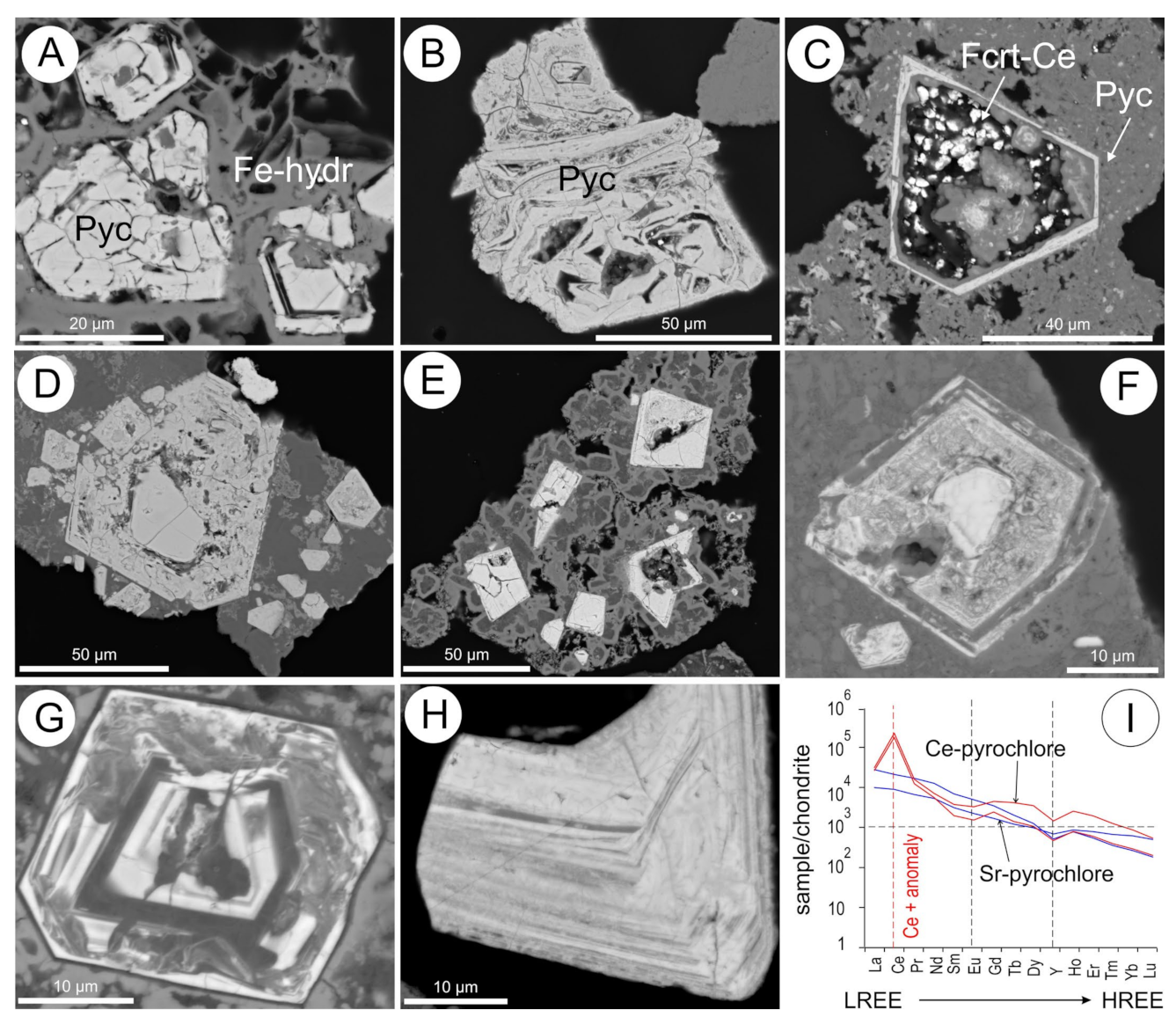
5.13. Pyrochlore Group Minerals
5.14. Hollandite, Cryptomelane and Coronadite
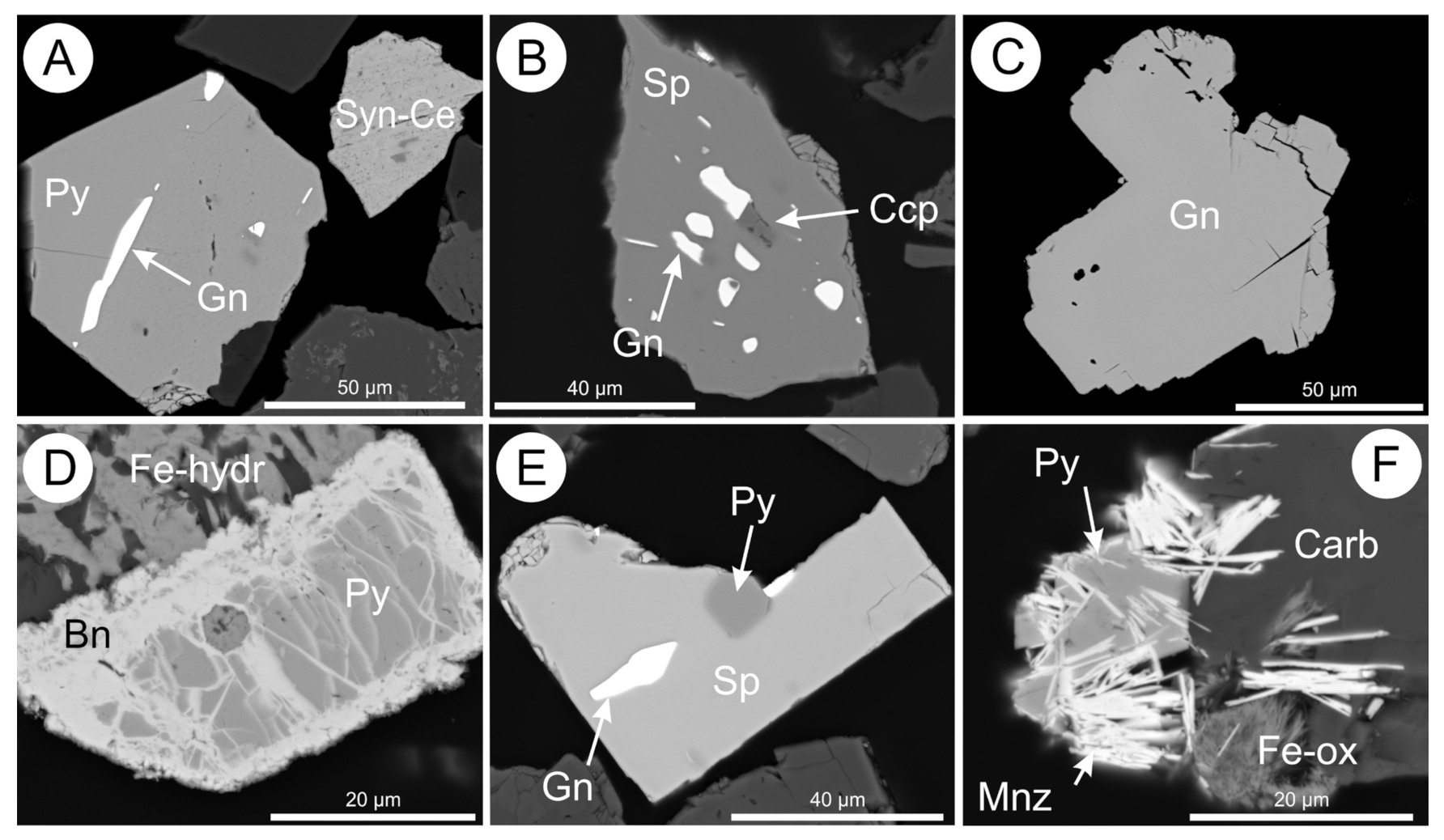
5.15. Sulphides and Sulphates
5.16. Carbonate Minerals, Quartz, and Other Minerals
6. Discussion
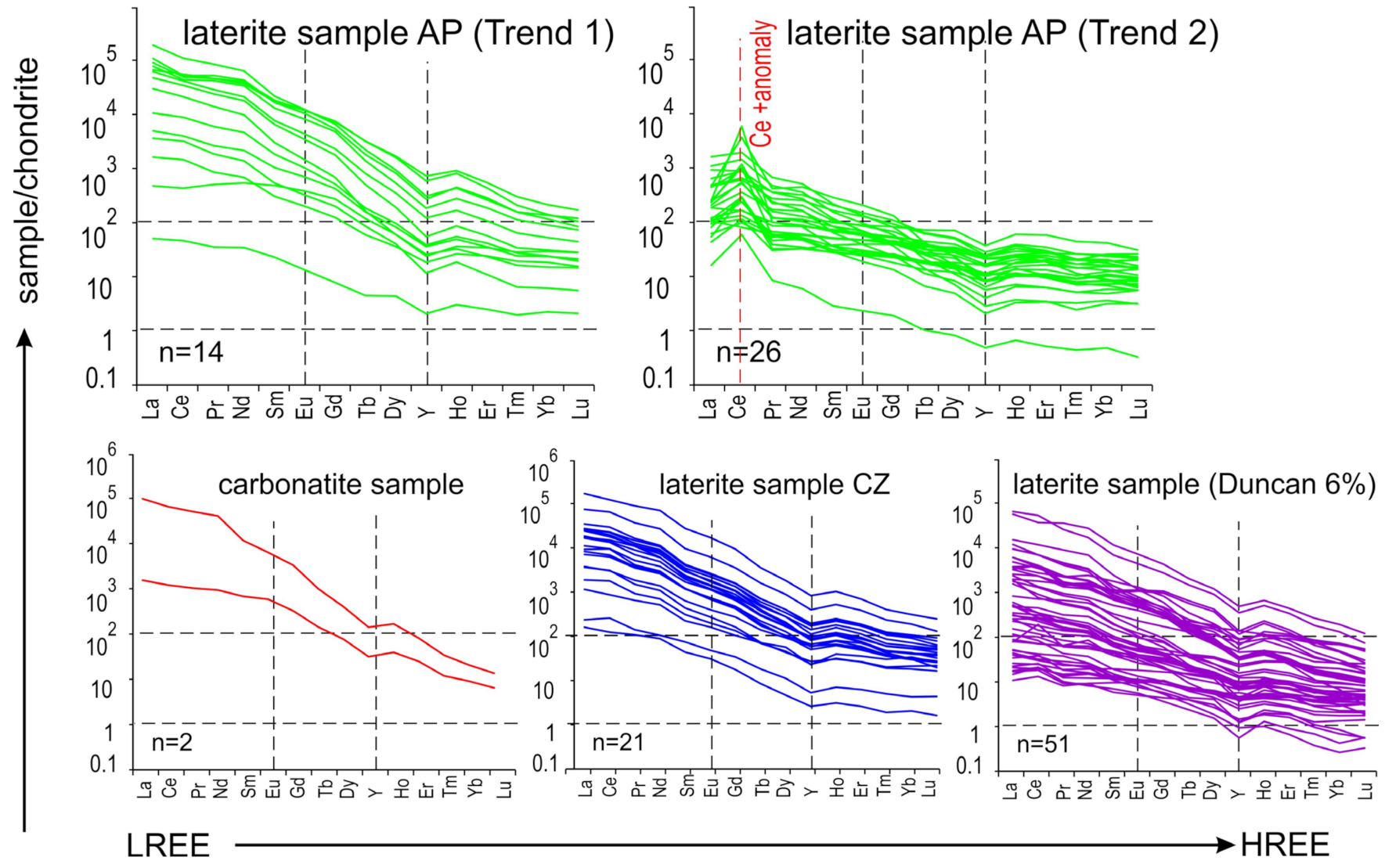
6.1. REE Deportment: The Role of Gangue Minerals
6.2. The Imperative of Nanoscale Characterisation, and the Hunt for HREE-Specific Minerals
6.3. Deposit-Scale Differences in REE Distributions and Mechanisms of REE Re-Distribution
7. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Geological Survey. Mineral Commodity Summaries. 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 10 May 2022).
- European Commission. Study on the EU’s List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-21050-4. Available online: https://rmis.jrc.ec.europa.eu/uploads/CRM_2020_Report_Final.pdf (accessed on 10 May 2022).
- Australian Government, Department of Industry, Science, Energy and Resources. Critical Minerals Strategy. 2022. Available online: https://www.industry.gov.au/sites/default/files/March%202022/document/2022-critical-minerals-strategy.pdf (accessed on 10 May 2022).
- Lynas Corporation Ltd. Annual Report. 2020. Available online: https://www.lynascorp.com/wp-content/uploads/2020/10/LYC_AR20-30Sep20-LODGE-2122450.pdf (accessed on 4 February 2023).
- Lottermoser, B.G. Rare earth element mineralisation within the Mt. Weld carbonatite laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- Smith, M.P.; Moore, K.; Kavecsánszki, D.; Finch, A.A.; Kynicky, J.; Wall, F. From mantle to critical zone: A review of large and giant sized deposits of the rare earth elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef]
- Hellman, P.L.; Duncan, R.K. Evaluation of rare earth element deposits. Appl. Earth Sci. 2014, 123, 107–117. [Google Scholar]
- Middlemost, E. Mineralogy and petrology of the rauhaugites of the Mt Weld carbonatite complex of Western Australia. Mineral. Petrol. 1990, 41, 145–161. [Google Scholar] [CrossRef]
- Duncan, R.K.; Willett, G.C. Mount Weld Carbonatite. In Geology of the Mineral Deposits of Australia & Papua New Guinea; Hughes, F.E., Ed.; Australasian Institute of Mining & Metallurgy Monograph 14: Carlton, VIC, Australia, 1990; pp. 591–597. [Google Scholar]
- Graham, S.; Lambert, D.; Shee, S. The petrogenesis of carbonatite, melonite and kimberlite from the Eastern Goldfield Province, Yilgarn Craton. Lithos 2004, 76, 519–533. [Google Scholar] [CrossRef]
- Hoatson, D.M.; Jaireth, S.; Miezitis, Y. The Major Rare-Earth-Element Deposits of Australia: Geological Setting, Exploration, and Resources; Geoscience Australia: Symonston, ACT, Australia, 2011; 204p. [Google Scholar]
- Zhukova, I.A.; Stepanov, A.S.; Jiang, S.-Y.; Murphy, D.; Mavrogenes, J.; Allen, C.; Chen, W.; Bottrill, R. Complex REE systematics of carbonatites and weathering products from uniquely rich Mount Weld REE deposit, Western Australia. Ore Geol. Rev. 2021, 139B, 104539. [Google Scholar] [CrossRef]
- Jaireth, S.; Hoatson, D.M.; Miezitis, Y. Geological setting and resources of the major rare-earth-element deposits in Australia. Ore Geol. Rev. 2014, 62, 72–128. [Google Scholar] [CrossRef]
- Pirajno, F.; Gonzalez-Alvarez, I.; Border, A.; Porter, M. Mount Weld and Gifford Creek rare earth elements carbonatites. In Australian Ore Deposits, 6th ed.; Phillips, N., Ed.; Monograph 32; Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2017; pp. 163–166. [Google Scholar]
- Lottermoser, B. Churchite from the Mt Weld Carbonatite Laterite Western Australia. Mineral. Mag. 1987, 51, 468–469. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; England, B.M. Compositional variation in pyrochlores from the Mt Weld carbonatite laterite, Western Australia. Mineral. Petrol. 1988, 38, 37–51. [Google Scholar] [CrossRef]
- Zhukova, I.A.; Stepanov, A.S.; Korsakov, A.V.; Jiang, S.-Y. Application of Raman spectroscopy for the identification of phosphate minerals from REE supergene deposit. J. Raman Spectr. 2022, 53, 485–496. [Google Scholar] [CrossRef]
- Aral, H.; Bruckard, W.J. Characterisation of the Mt Weld (Western Australia) niobium ore. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. Sect. C 2008, 117, 193–204. [Google Scholar] [CrossRef]
- Armstrong, J.T. Quantitative analysis of silicate and oxide minerals: Comparison of Monte Carlo, ZAF, and φ(ρz) procedures. In Microbeam Analysis; Newbury, D.E., Ed.; San Francisco Press: San Francisco, CA, USA, 1988; pp. 239–246. [Google Scholar]
- Donovan, J.J.; Tingle, T.N. An Improved Mean Atomic Number Background Correction for Quantitative Microanalysis. Microsc. Microanal. 1996, 1, 1–7. [Google Scholar] [CrossRef]
- Donovan, J.J.; Singer, J.W.; Armstrong, J.T. A new EPMA method for fast trace element analysis in simple matrices. Am. Mineral. 2016, 101, 1839–1853. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare earth element fluorocarbonate minerals from the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 202. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kametetsky, V.S. Rare earth element phosphate minerals from the Olympic Dam Cu-U-Au-Ag deposit, South Australia: Recognizing temporal-spatial controls on REE mineralogy in an evolved IOCG systems. Can. Mineral. 2019, 57, 1–22. [Google Scholar] [CrossRef]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Berger, A.; Gnos, E.; Janots, E.; Fernandez, A.; Giese, J. Formation and composition of rhabdophane, bastnäsite and hydrated thorium minerals during alteration: Implications for geochronology and low-temperature processes. Chem. Geol. 2008, 254, 238–248. [Google Scholar] [CrossRef]
- Belogub, E.V.; Shilovskikh, V.V.; Novoselov, K.A.; Blinov, I.A.; Filippova, K.A. Authigenic rhabdophane from brown iron ore of the oxidation zone of the Babaryk massive sulfide occurrence (South Urals): Scanning electron microscope (SEM) and electron backscattered diffraction (EBSD) study. Eur. J. Mineral. 2021, 33, 605–620. [Google Scholar] [CrossRef]
- Van Landuyt, J.; Amelinckx, S. Multiple Beam Direct Lattice Imaging of New Mixed-Layer Compounds of the Bastnaesite-Synchisite Series. Am. Mineral. 1975, 60, 351–358. [Google Scholar]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in mixed-layer compounds: A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Slattery, A.; Ehrig, K.; Liu, W.Y. Nanoscale intergrowths in the bastnäsite-synchysite series record transition towards thermodynamic equilibrium. MRS Bull. 2022, 47, 250–257. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Rare earth element behaviour in apatite from the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 135. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.J. Numerical modelling of REE fractionation patterns in fluorapatite from the Olympic Dam deposit (South Australia). Minerals 2018, 8, 342. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Kozmenko, O.A.; Nekipelova, A.V.; Rudmin, M.; Khvorov, P.V.; Artemyev, D.A. Geochemistry and mineralogy of rare earth elements in high-phosphorus ooidal ironstones: A case study of the Kamysh-Burun deposit (Azov–Black Sea iron Province). Ore Geol. Rev. 2020, 127, 103827. [Google Scholar] [CrossRef]
- Atencio, G. Pyrochlore-Supergroup Minerals Nomenclature: An Update. Front. Chem. 2021, 9, 713368. [Google Scholar] [CrossRef]
- Slattery, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K. iDPC STEM as a tool for atomic-scale imaging of light elements and beam-sensitive minerals. In Proceedings of the 27th Australian Conference on Microscopy and Microanalysis, Perth, Australia, 29 January–2 February 2023; p. 134. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Slattery, A.D.; Wade, B.P. Cerian-, strontian- and sodium-calcium pyrochlores from Mount Weld, Western Australia: A nanoscale study. Mineral. Mag. 2023. to be submitted. [Google Scholar]
- Biagioni, C.; Capalbo, C.; Pasero, M. Nomenclature tunings in the hollandite supergroup. Eur. J. Mineral. 2013, 25, 85–90. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup. Am. Mineral. 1995, 80, 732–743. [Google Scholar] [CrossRef]
- Geisler, T.; Berndt, J.; Meyer, H.-W.; Pollok, K.; Putnis, A. Low-temperature aqueous alteration of crystalline pyrochlore: Correspondence between nature and experiment. Mineral. Mag. 2004, 68, 905–922. [Google Scholar] [CrossRef]
- Ochiao, A.; Utsunomiya, S. Crystal chemistry and stability of hydrated rare-earth phosphates formed at room temperature. Minerals 2017, 8, 84. [Google Scholar] [CrossRef]
- Ondrejka, M.; Bačík, P.; Sobocký, T.S.; Uher, P.; Škoda, R.; Mikuš, T.; Luptáková, J.; Konečný, P. Minerals of the rhabdophane group and the alunite supergroup in microgranite: Products of low-temperature alteration in a highly acidic environment from the Velence Hills, Hungary. Mineral. Mag. 2018, 82, 1277–1300. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Siegfried, P.R.; Wall, F.; O’Neill, M.; Brooker, R.A.; Fallon, E.K.; Pickles, J.R.; Banks, D.A. The origin and composition of carbonatite-derived carbonate-bearing fluorapatite deposits. Min. Depos. 2021, 56, 863–884. [Google Scholar] [CrossRef]
- Berger, A.; Janots, E.; Gnos, E.; Frei, R.; Bernier, F. Rare earth element mineralogy and geochemistry in a laterite profile from Madagascar. Appl. Geochem. 2014, 41, 218–228. [Google Scholar] [CrossRef]
- Verdugo-Ihl, M.R.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L.; Gilbert, S. Textures and U-W-Sn-Mo signatures in hematite from the Cu-U-Au-Ag orebody at Olympic Dam, South Australia: Defining the archetype for IOCG deposits. Ore Geol. Rev. 2017, 91, 173–195. [Google Scholar] [CrossRef]
- Ilton, E.S.; Collins, R.N.; Ciobanu, C.L.; Cook, N.J.; Verdugo-Ihl, M.; Slattery, A.D.; Paterson, D.J.; Mergelsberg, S.T.; Bylaska, E.J.; Ehrig, K. Pentavalent Uranium Incorporated in the Structure of Proterozoic Hematite. Environm. Sci. Technol. 2022, 56, 11857–11864. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.D.; Gilbert, S.E. Micron- to atomic-scale investigation of rare earth elements in iron oxides. Front. Earth Sci. 2022, 10, 967189. [Google Scholar] [CrossRef]
- Owen, N.D.; Cook, N.J.; Rollog, M.; Ehrig, K.; Schmandt, D.S.; Ram, R.; Brugger, J.; Ciobanu, C.L.; Wade, B.; Guagliardo, P. REE-, Sr- Ca-aluminum-phosphate-sulfate minerals of the alunite supergroup and their role as hosts for radionuclides. Am. Mineral. 2019, 104, 1806–1819. [Google Scholar] [CrossRef]




| Sample | SiO2 % | Fe2O3 % | MnO % | MgO % | P2O5 % | Th % | K2O % | SO3 % | Na2O % | BaO % | TiO2 % | Zn % | Zr % | ΣREO % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP RC | 4.05 | 37.48 | 3.72 | 0.90 | 15.2 | 0.060 | 0.12 | 0.082 | 0.18 | 0.15 | 0.723 | 0.626 | 0.04 | 12.25 |
| CZ RC | 2.76 | 52.18 | 2.61 | 0.46 | 8.4 | 0.115 | 0.05 | 0.155 | 0.11 | 0.29 | 2.070 | 0.404 | 0.08 | 15.22 |
| Duncan 6% | 6.50 | 51.41 | 2.76 | 1.01 | 7.0 | 0.040 | 0.16 | 0.679 | 0.27 | 0.41 | 1.090 | 0.176 | 0.03 | 7.44 |
| Sample | Nb2O5 | Ta2O5 | CaO % | Al2O3 % | SrO % | PbO % | Sc | U | Cu | Zn | SrO | As2O3 | Sb | Y2O3 |
| AP RC | 0.269 | 0.005 | 14.5 | 0.68 | 0.514 | 0.068 | 144 | 21.6 | 32 | 8160 | 4890 | 40 | 0.6 | 404 |
| CZ RC | 0.525 | 0.005 | 0.75 | 3.34 | 0.435 | 0.056 | 222 | 22.7 | 122 | 5060 | 4070 | 60 | 1.2 | 790 |
| Duncan 6% | 0.361 | −0.005 | 3.65 | 5.15 | 0.388 | 0.029 | 146 | 61.4 | 176 | 2250 | 3770 | 60 | 1 | 3090 |
| Sample | La2O3 % | CeO2 % | Pr6O11 % | Nd2O3 % | Sm2O3 | Eu2O3 | Gd2O3 | Tb2O3 | Dy2O3 | Ho2O3 | Er2O3 | Tm2O3 | Yb2O3 | Lu2O3 |
| AP RC | 3.10 | 5.82 | 0.631 | 2.17 | 0.289 | 587 | 1050 | 68.4 | 174 | 18.8 | 31.4 | 3.09 | 14.5 | 1.83 |
| CZ RC | 3.68 | 7.25 | 0.794 | 2.75 | 0.373 | 804 | 1530 | 111 | 315 | 36.4 | 61.5 | 5.94 | 27.8 | 3.24 |
| Duncan 6% | 1.48 | 3.16 | 0.387 | 1.45 | 0.242 | 661 | 1700 | 225 | 983 | 137 | 263 | 25.1 | 106 | 9.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, N.J.; Ciobanu, C.L.; Wade, B.P.; Gilbert, S.E.; Alford, R. Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia. Minerals 2023, 13, 656. https://doi.org/10.3390/min13050656
Cook NJ, Ciobanu CL, Wade BP, Gilbert SE, Alford R. Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia. Minerals. 2023; 13(5):656. https://doi.org/10.3390/min13050656
Chicago/Turabian StyleCook, Nigel J., Cristiana L. Ciobanu, Benjamin P. Wade, Sarah E. Gilbert, and Robert Alford. 2023. "Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia" Minerals 13, no. 5: 656. https://doi.org/10.3390/min13050656
APA StyleCook, N. J., Ciobanu, C. L., Wade, B. P., Gilbert, S. E., & Alford, R. (2023). Mineralogy and Distribution of REE in Oxidised Ores of the Mount Weld Laterite Deposit, Western Australia. Minerals, 13(5), 656. https://doi.org/10.3390/min13050656






