Natural Experiment on the Extraction and Quenching of Rapakivi-like Magmas: Traces of Interaction with the Mafic Melts and Their Derivatives, Salmi Batholith (Karelia, Russia)
Abstract
1. Introduction
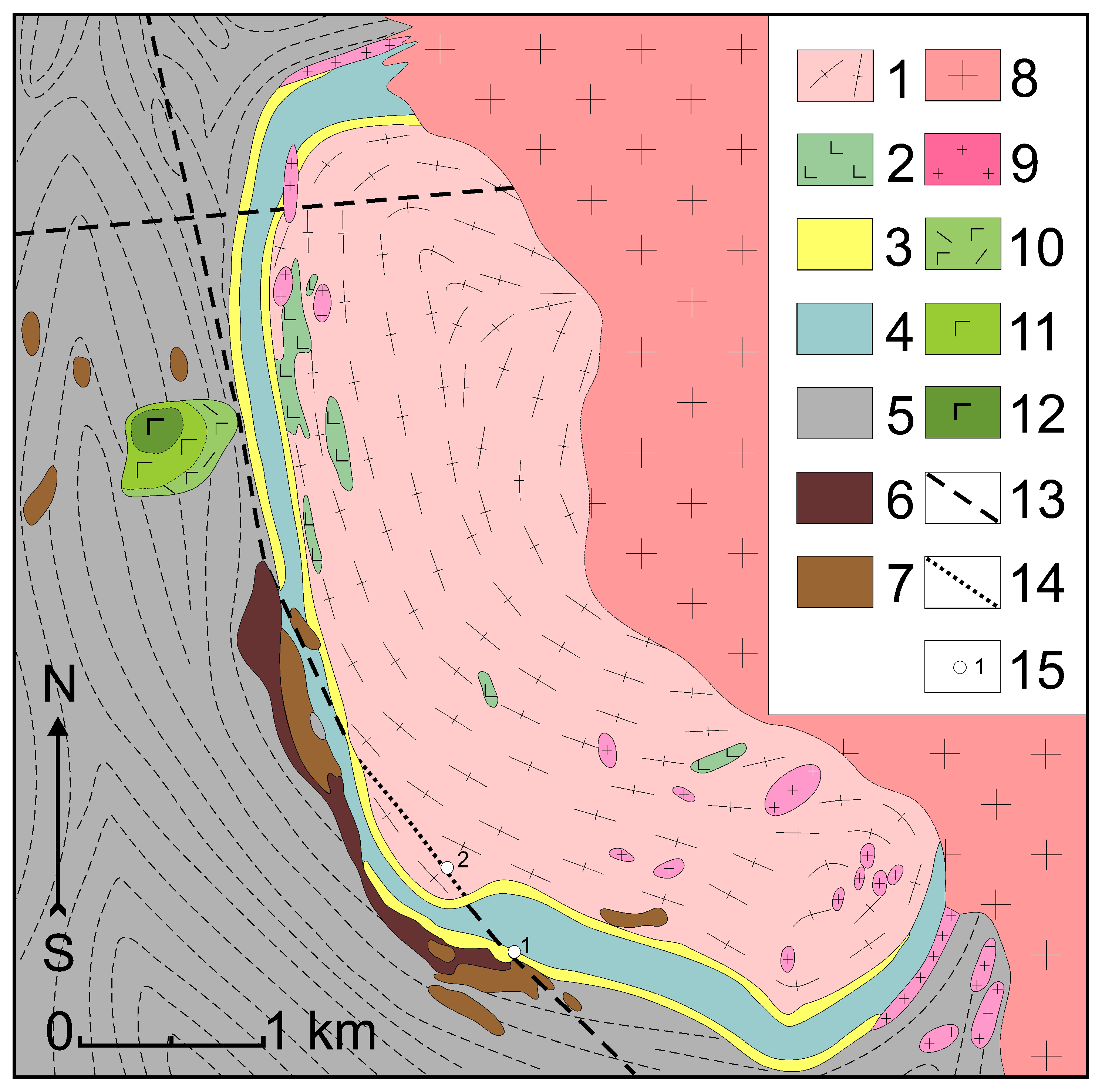
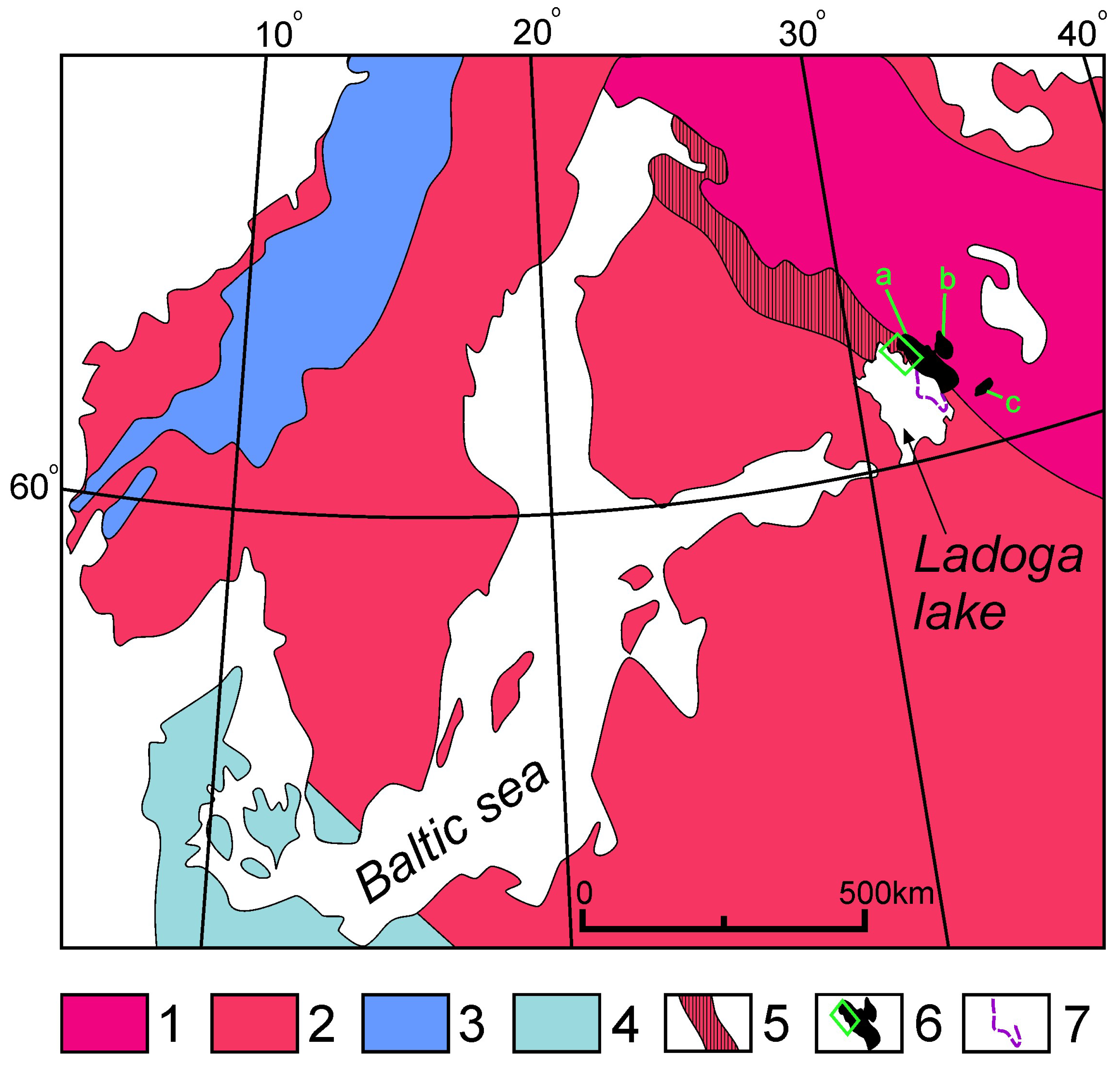
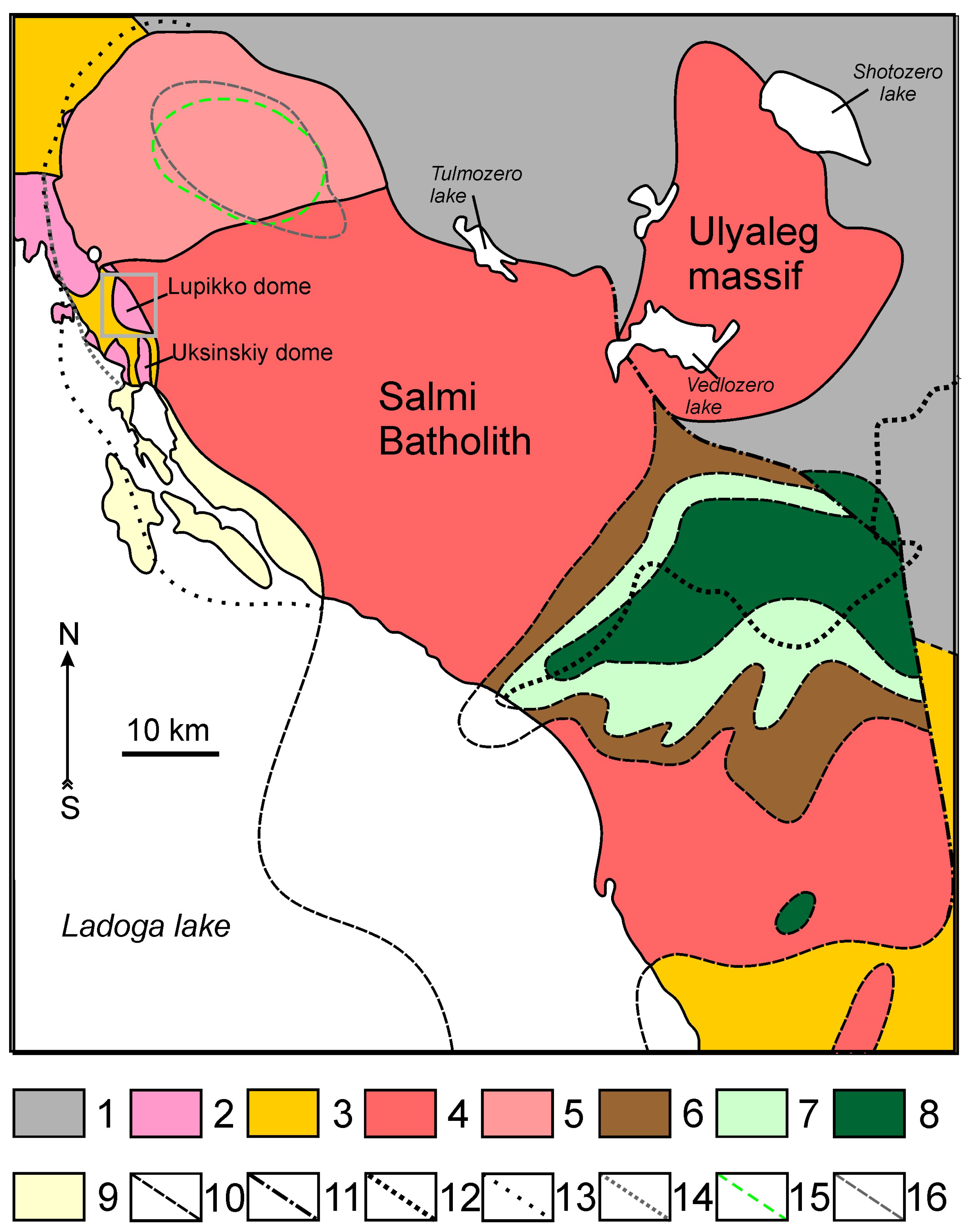
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Brief Characteristics of the Geological Setting of the Granite Porphyry Dikes
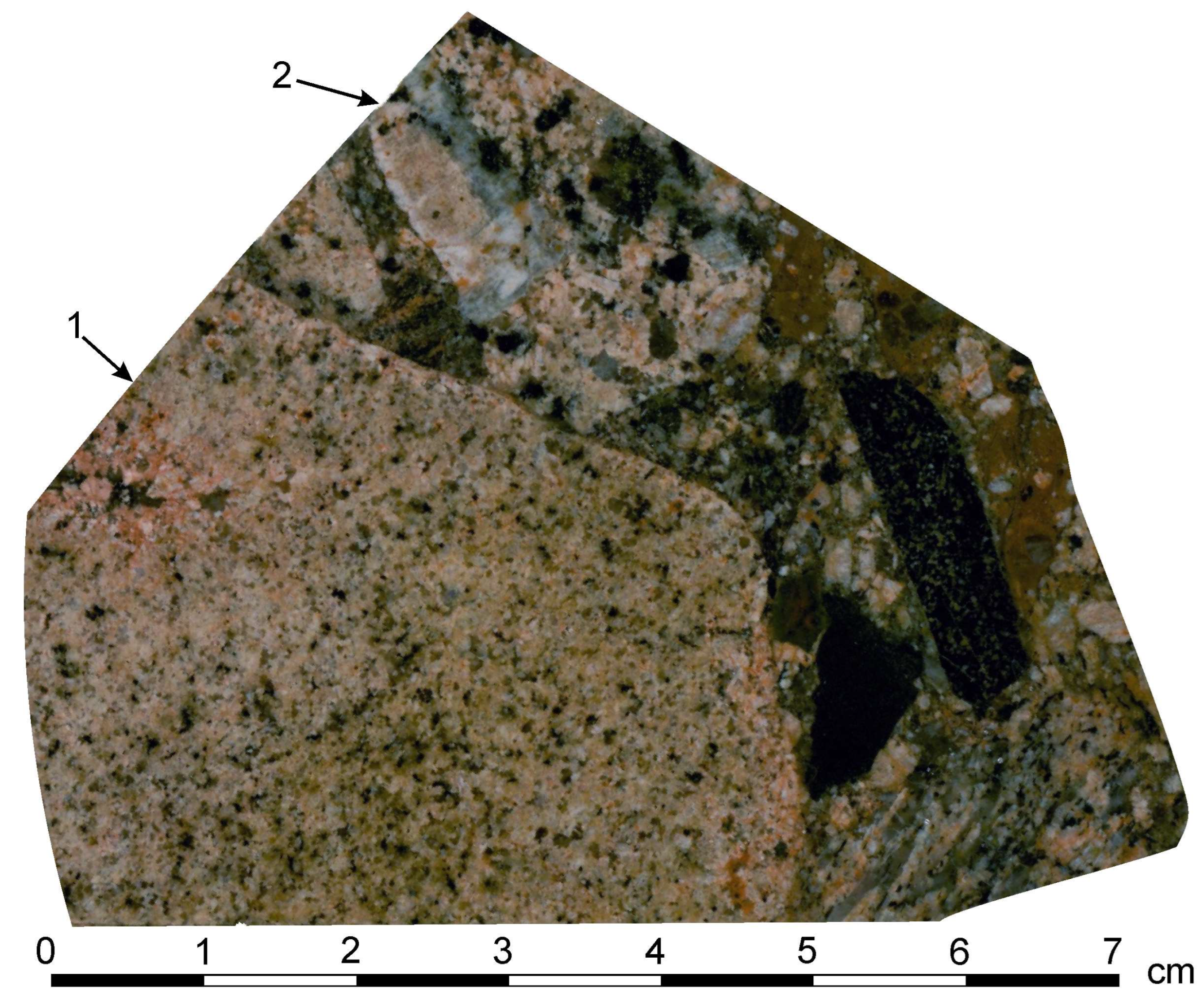
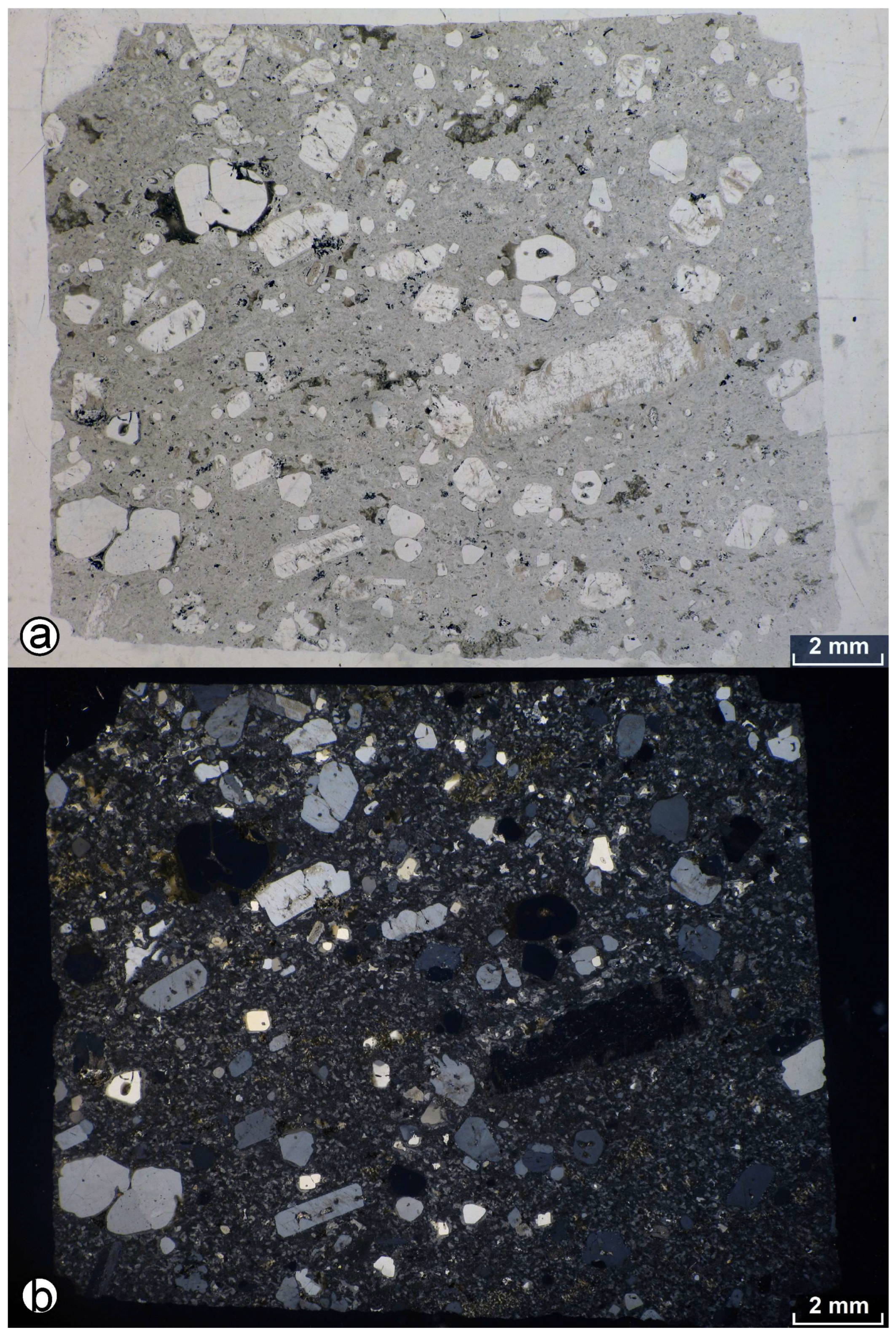
4.2. Petrography of the Granite Porphyry Dikes
4.3. Geochemistry of the Rocks
4.3.1. Major Oxides
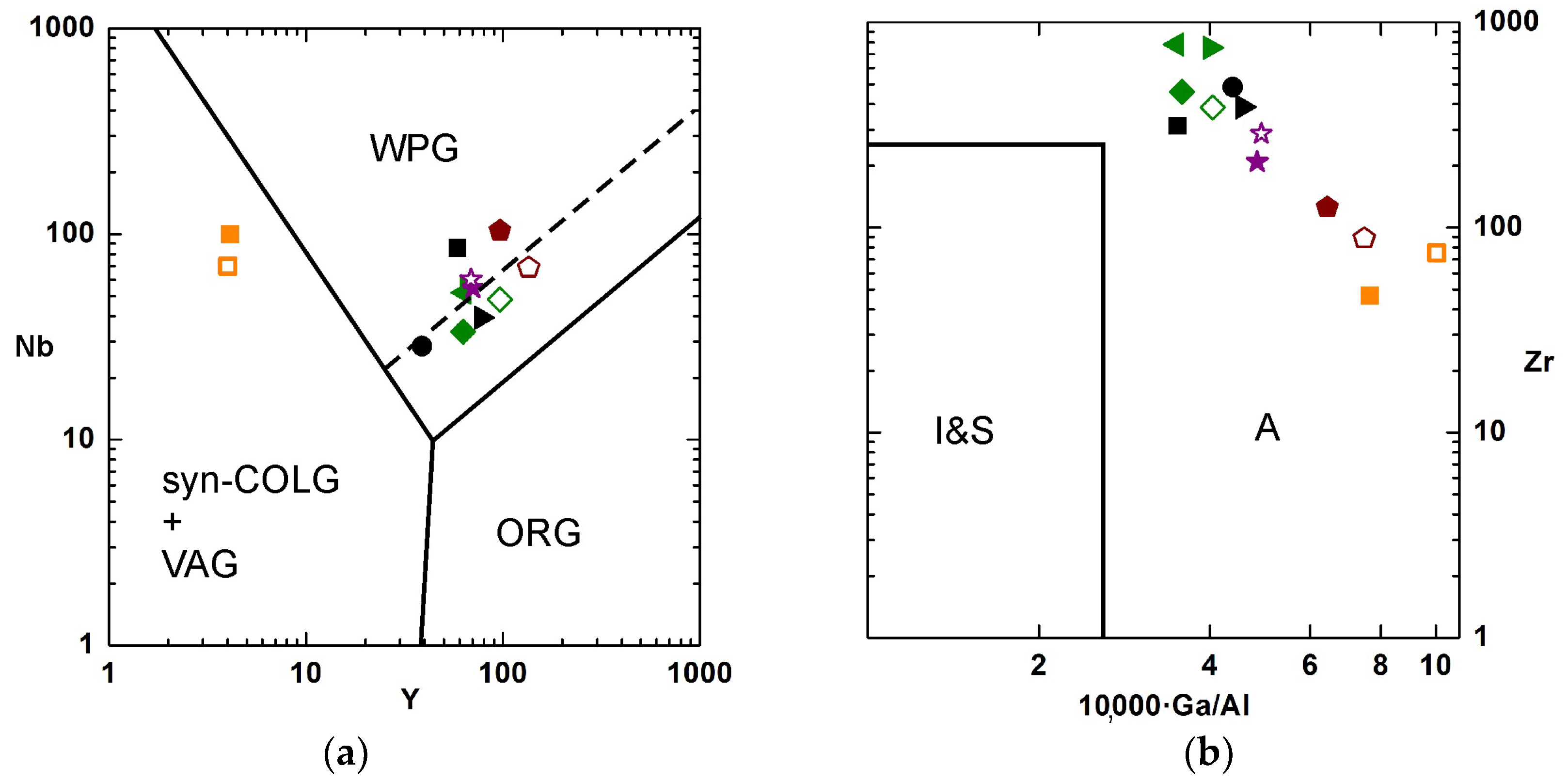
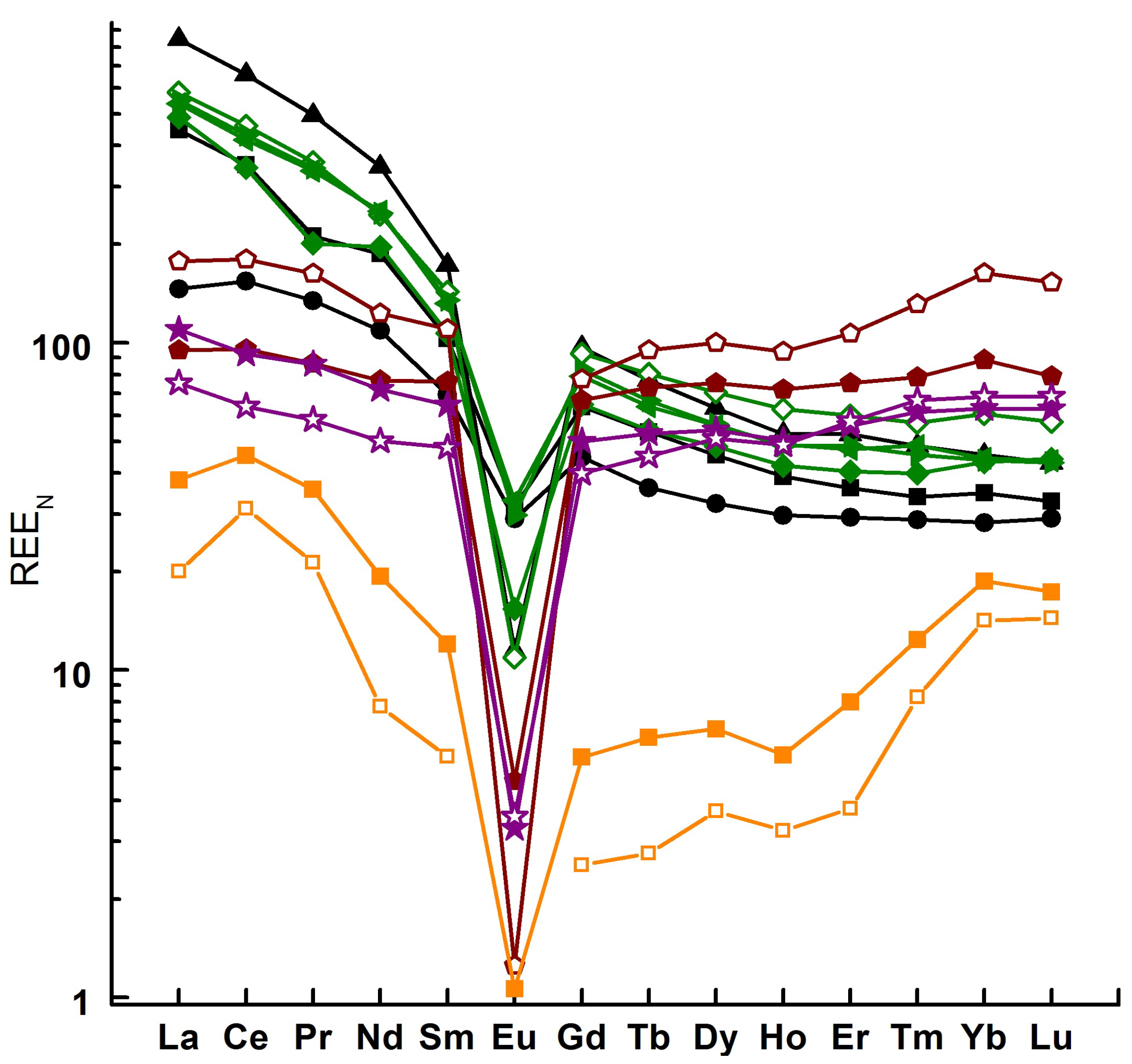
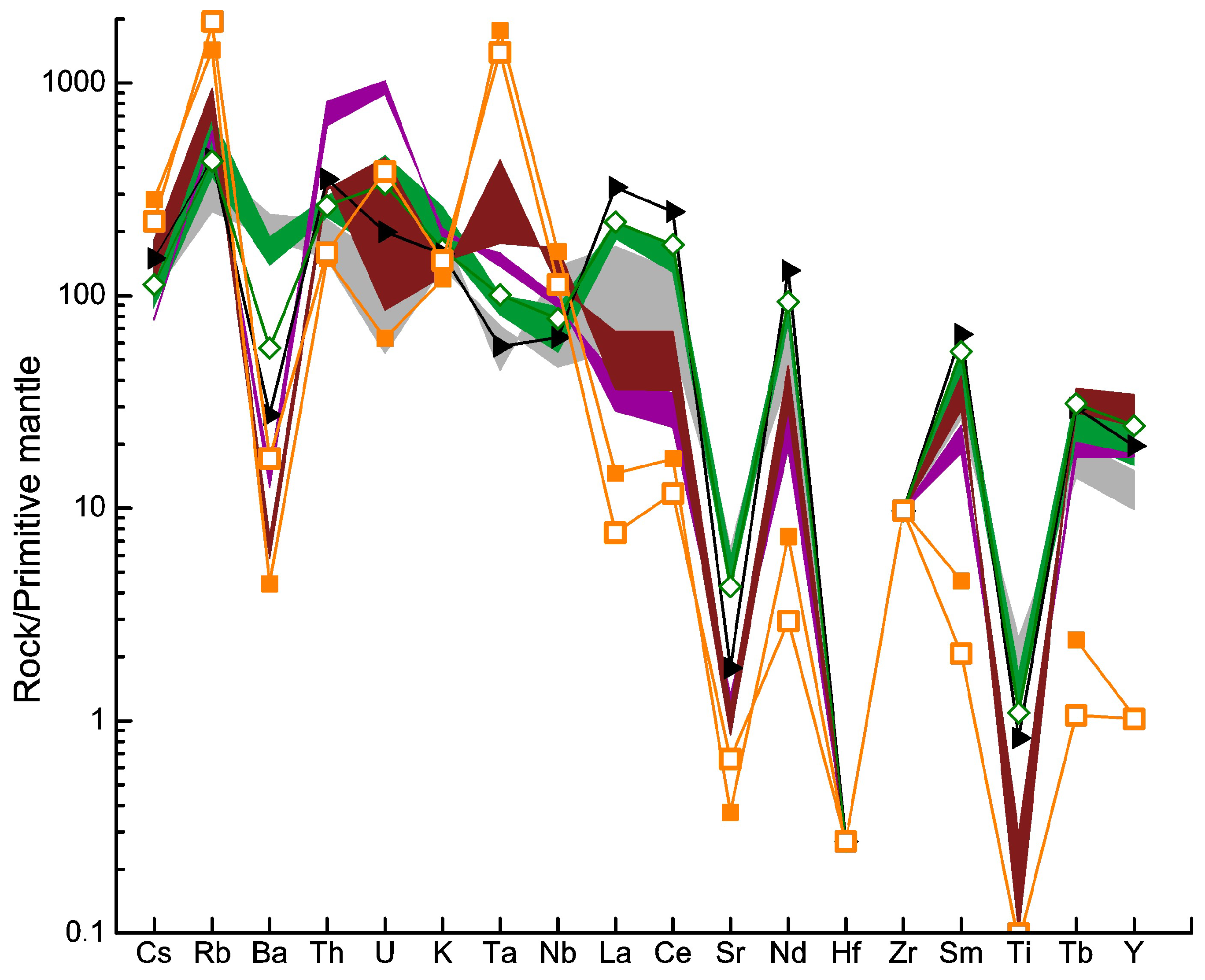
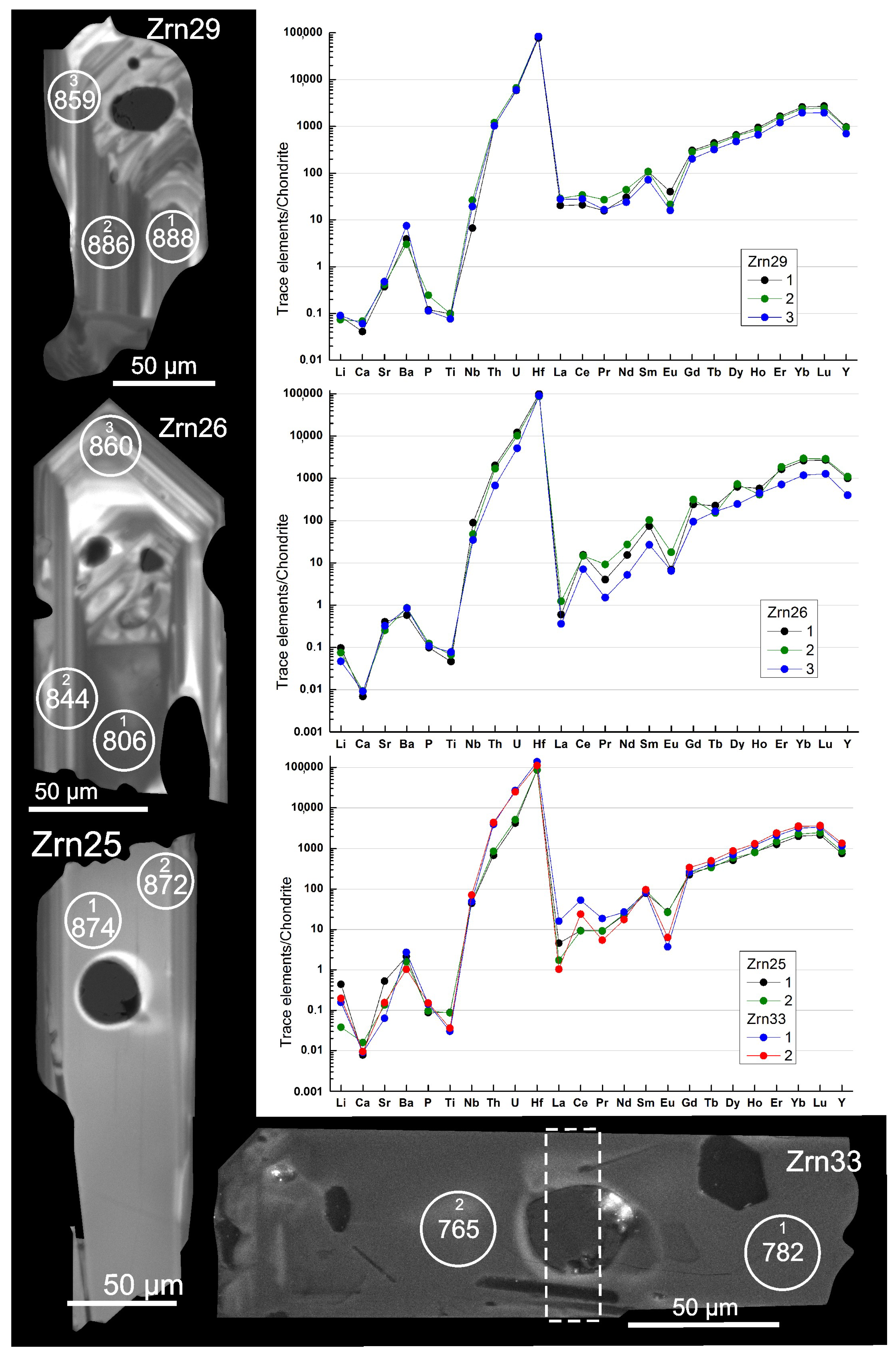
4.3.2. Trace Elements
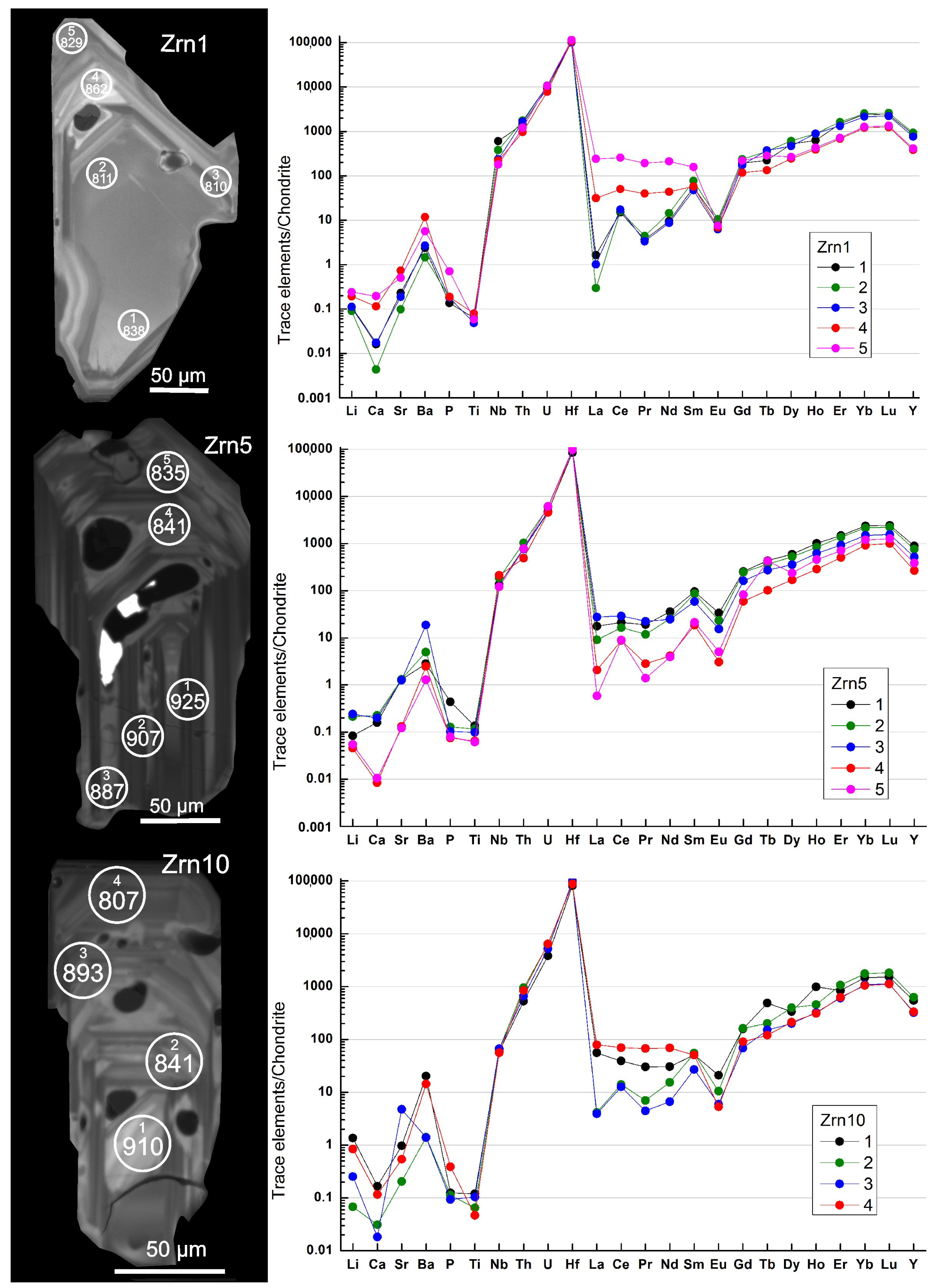
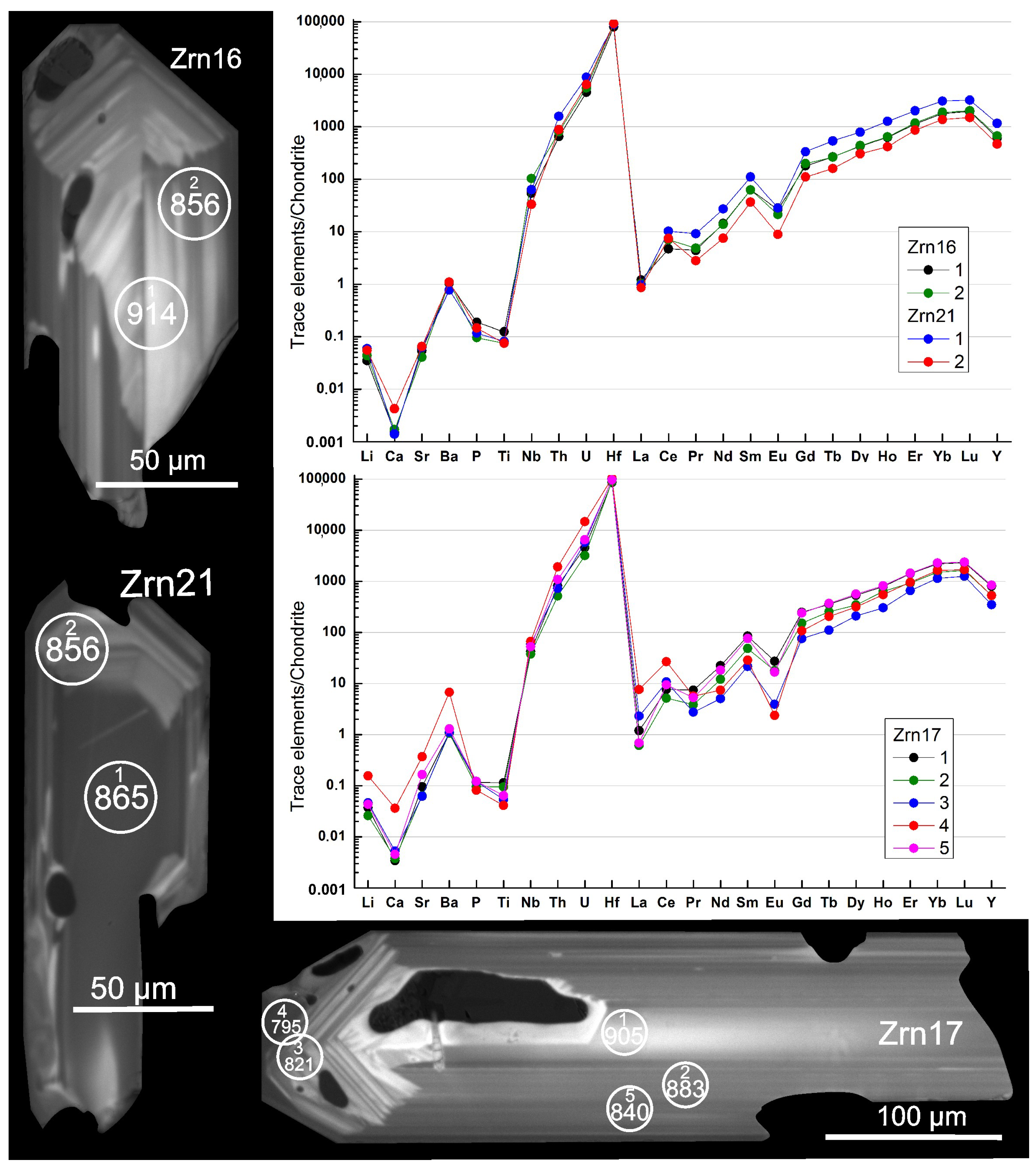
4.4. Zircon: Morphology, Internal Structure, Composition, Ti in Zircon Thermometry, and Inclusions
4.4.1. Characteristics of Zircon Grains
- Strictly two-zonal crystals with a homogeneous inner part and a thinly zoned outer part (Zrn21 and Zrn25);
- Crystals with complex combinations of growth zonality expressed in thin layers of dark and light bands (Zrn1, Zrn5, Zrn10, Zrn26);
- Crystals without a homogeneous central part but with a thinly overlapping growth zonality (Zrn16 and Zrn17). The sectoriality is particularly pronounced in these grains. This is reflected in the difference in color intensity between growth zones on different growth planes.
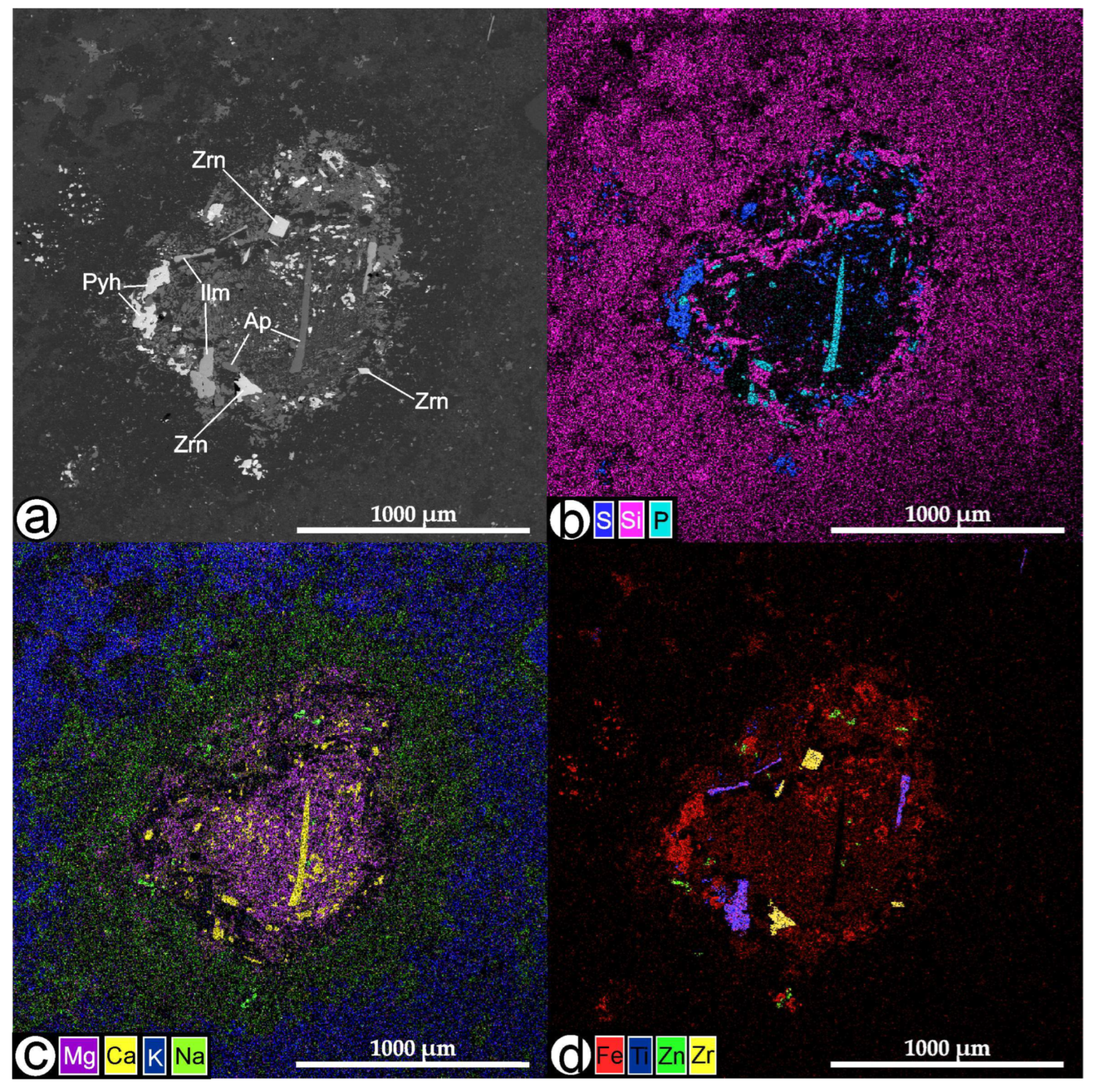
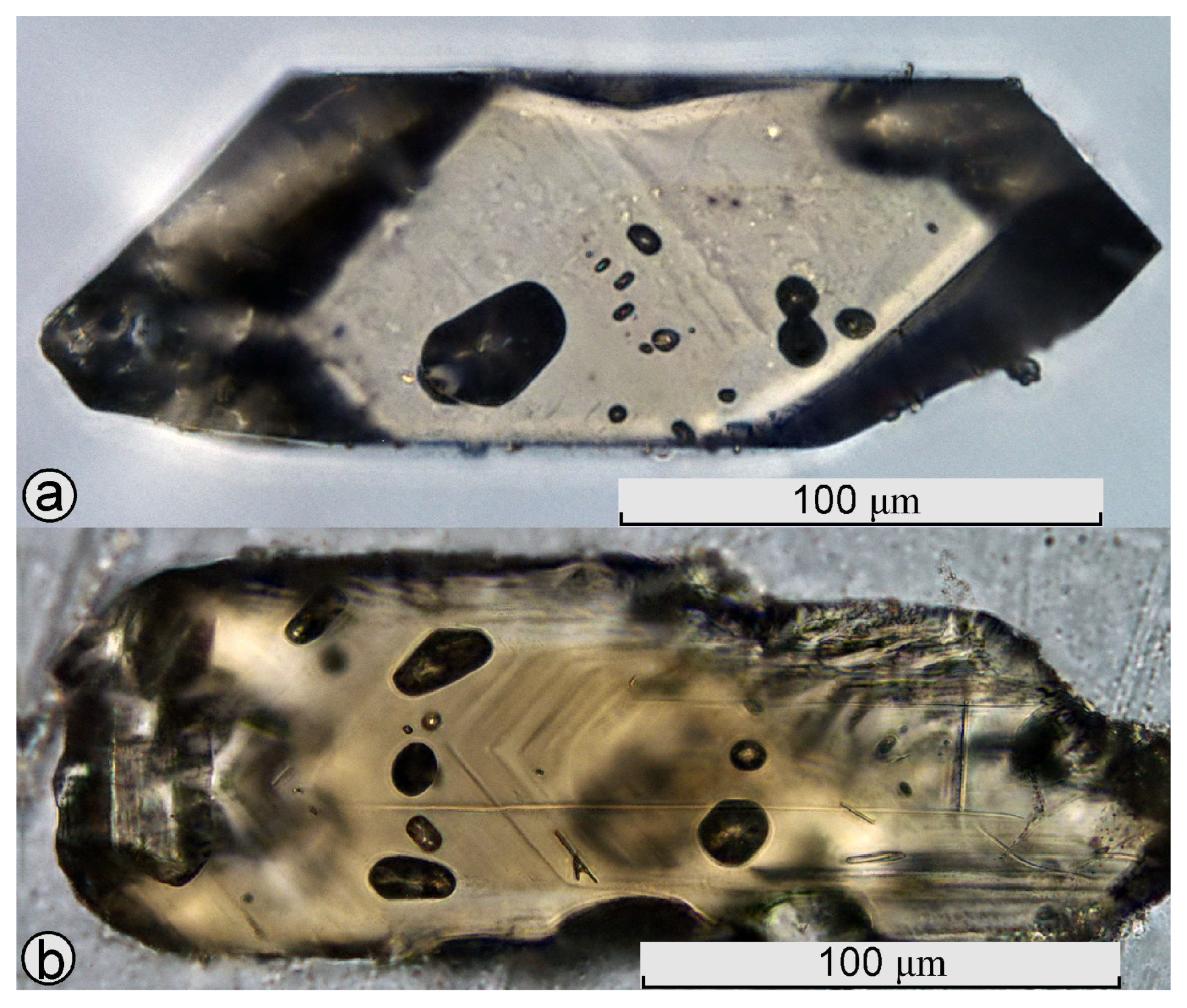
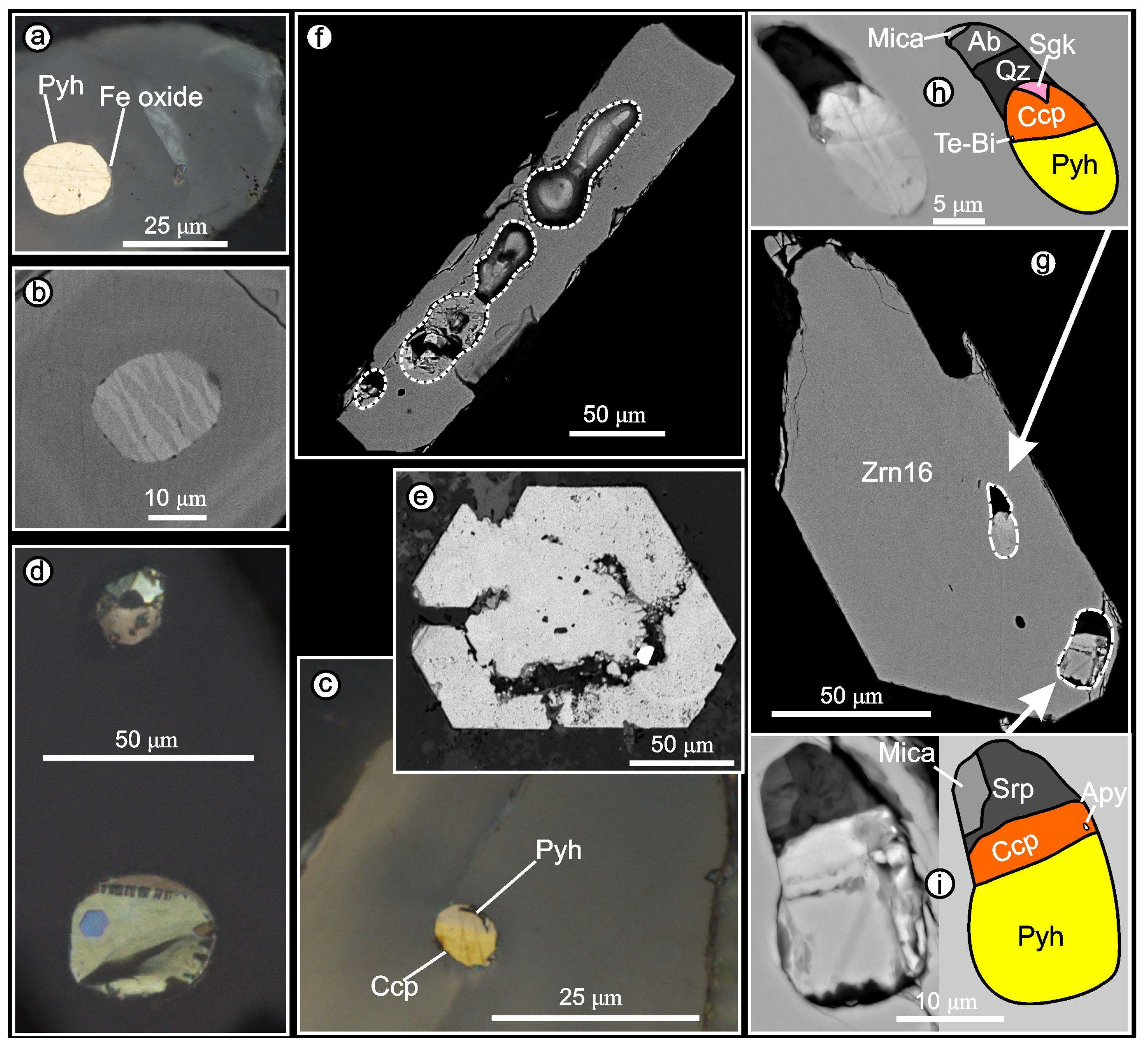
4.4.2. Inclusions in Zircon: Phase Composition, Morphology, and Relationships
Solid-Phase Inclusions
Melt Inclusions
Inclusions of Heterophase Capture: Silicate-Sulfide
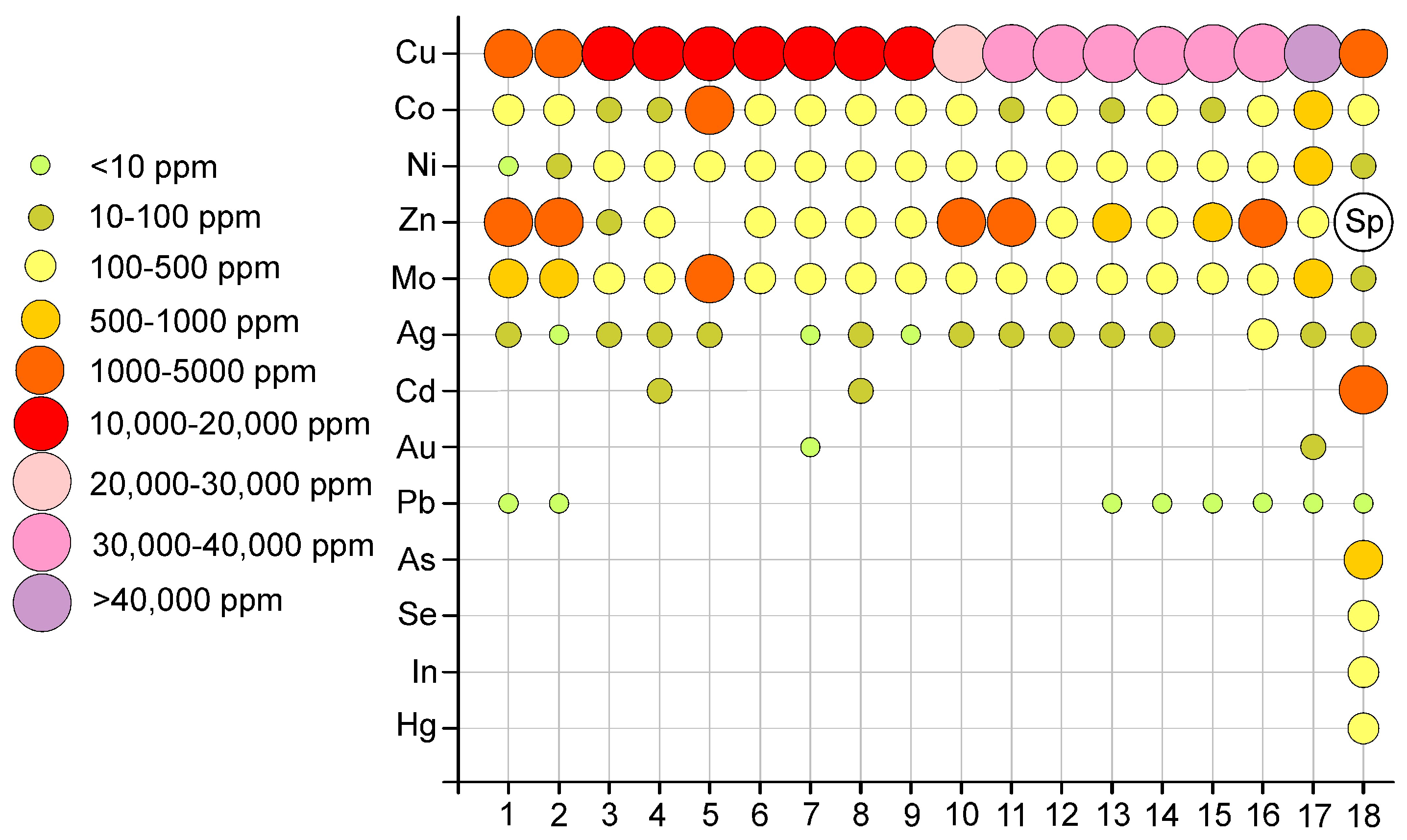
4.4.3. Composition of the Homogenized Sulfide Inclusions
5. Discussion
5.1. Geochemistry and Petrology
5.2. Zircon
5.3. Solid-Phase Inclusions
5.4. Inclusions of Heterophase Capture: Silicate-Sulfide
5.5. Sulfides
5.6. Dynamic Aspects of Implementation
6. Conclusions
- The relative time position of the studied granite porphyry dikes relative to other rocks associated with the Salmi batholith, an ARG complex, was clarified in this paper. Granite porphyry dikes were formed after amphibole-biotite granite (rapakivi) and fine equigranular granite, but before the introduction of rare-metal granite dikes;
- The physical–chemical parameters of zircon formation from granite-porphyry dikes were determined for the first time. According to the Ti-zircon thermometer [24], the maximum temperature was 925 °C. The ƒO2 values for the early stages of rock formation were in the region below the boundary of the FMQ buffer. It was qualitatively determined that the formation occurred in the field of fluorite stability in the presence of CO2-forming carbonates during the crystallization of silicate melt inclusions;
- The composition of a sulfide melt coexisting with magma of granite porphyry based on the melt inclusions of sulfide melts in zircon was characterized for the first time. The presence of two sulfide melts at the time of capture for the sulfide inclusions by the zircon, pyrrhotite-chalcopyrite, and sphalerite-chalcopyrite compositions was established;
- The connection between the formation of granite porphyry with mafic melts that formed rocks of the gabbro and anorthosite types was established. It is likely that the injection area of the material was an acidic magmatic chamber connected with a magmatic melt with a mafic composition.
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larin, A.M. Rapakivi Granites and Associated Rocks; Nauka: St. Petersburg, Russia, 2011; p. 410. (In Russian) [Google Scholar]
- Rämö, O.T.; Haapala, I. One hundred years of Rapakivi Granite. Mineral. Petrol. 1995, 52, 129–185. [Google Scholar] [CrossRef]
- Liverton, T.; Botelho, N.F. Fractionated alkaline rare-metal granites: Two examples. J. Asian Earth Sci. 2001, 19, 399–412. [Google Scholar] [CrossRef]
- Konopelko, D.; Biske, G.; Belyatsky, B.; Eklund, O.; Seltmann, R. Hercynian post-collisional magmatism of the SE Tien Shan, Kyrgyzstan: Timing and metallogenic potential. In Proceedings of the International Field Symposium “Paleozoic Geodynamic Processes and Metallogeny of Chinese Altay and Tianshan”, Urumqi, China, 9–21 August 2003; pp. 10–15. [Google Scholar]
- Vorma, A. On the petrochemistry of rapakivi granites with special reference to the Laitila massif, southwestern Finland. Geol. Surv. Finland Bull. 1976, 285, 98. [Google Scholar]
- Sharkov, E.V. Middle-Proterozoic anorthosite–rapakivi granite complexes: An example of within-plate magmatism in abnormally thick crust: Evidence from the East European Craton. Precambr. Res. 2010, 183, 689–700. [Google Scholar] [CrossRef]
- Vaasjoki, M.; Rämö, O.T.; Sakko, M. New U-Pb ages from the Wiborg rapakivi area: Constraints on the temporal evolution of the rapakivi granite-anorthosite-diabase dyke association of southeastern Finland. Precambr. Res. 1991, 51, 227–243. [Google Scholar] [CrossRef]
- Rämö, O.T.; Nironen, M.; Kosunen, P.; Elliot, B.A. Proterozoic Granites of South-Central Finland—Travel Across a Paleoproterozoic Terrane Boundary. Field Trip Guide; Helsinki University Press: Helsinki, Finland, 1999; p. 106. [Google Scholar]
- Neymark, L.A.; Amelin, Y.V.; Larin, A.M. Pb-Nd-Sr isotopic and geochemical constraints on the origin of the 1.54–1.56 Ga Salmi rapakivi granite–Anorthosite batholith (Karelia, Russia). Contrib. Mineral. Petrol. 1994, 50, 173–193. [Google Scholar] [CrossRef]
- Rämö, O.T. Petrogenesis of the Proterozoic rapakivi granites and basic rocks of Southeastern Fennoscandia Nd and Pb isotopic and general geochemical constraints. Geol. Surv. Finland Bull. 1991, 355, 161. [Google Scholar]
- Larin, A.M.; Amelin, Y.; Neymark, L.; Krymsky, R.; Ovchinnikova, G.; Belyaev, A.; Shebanov, A. The origin of Salmi and Uljalegi anorthosite rapakivi granite massifs. Constraints from precise U-Pb geochronology and Pb-Sr-Nd isotopic data. In Proceedings of the 7th International Symposium on Rapakivi Granites and Related Rocks, Helsinki, Finland, 24–26 July 1996; p. 47. [Google Scholar]
- Eklund, O.; Frojdo, S.; Lindberg, B. Magma mixing, the petrogenetic link between anorthositic suites and rapakivi granites, Aland, SW Finland. Mineral. Petrol. 1994, 50, 3–19. [Google Scholar] [CrossRef]
- Andersson, U. Petrogenesis of Some Proterozoic Granitoid Suites and Associated Basic Rocks in Sweden (Geochemistry and Isotope Geology). Doctor Dissertation, SGU, Uppsala, Sweeden, 1997; p. 217. [Google Scholar]
- Boyd, W.W.; Rämö, O.T. Mixing and mingling in the late stages of the evolution and emplacement of the Wiborg rapakivi batholith SE Finland. In Proceedings of the 7th International Symposium on Rapakivi Granites and Related Rocks, Helsinki, Finland, 24–26 July 1996; p. 10. [Google Scholar]
- Salonsaari, P.T. Hybridization in the subvolcanic Jaala-Iitti complex and its petrogenetic relation to rapakivi granites and associated mafic rocks of southern Finland. Bull. Geol. Soc. Finland 1995, 67, 104. [Google Scholar] [CrossRef]
- Dall’Agnol, R.; Teixeira, N.P.; Rämö, O.T.; Candido, M.A.V.; Macambira, M.J.B.; Oliveira, D.C. Petrogenesis of the Paleoproterozoic rapakivi A-type granites of the Archean Carajas metallogenic province, Brazil. Lithos 2005, 80, 101–129. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Malpas, S.; Marneffe, C.; Duchesne, J.-C.; Auwera, J.V.; Bolle, O. Origin of the giant Allard Lake ilmenite ore deposit (Canada) by fractional crystallization, multiple magma pulses and mixing. Lithos 2010, 117, 119–134. [Google Scholar] [CrossRef]
- Sharkov, E.V. Intraplate magmatic systems of the Mesoproterozoic by the example of anorthosite rapakivi granite complexes of baltic and ukrainian shields. Russ. J. Earth Sci. 1999, 1, 311–337. (In Russian) [Google Scholar] [CrossRef]
- Valkama, M.; Sundblad, K.; Cook, N.J.; Ivashchenko, V.I. Geochemistry and petrology of the indium-bearing polymetallic skarn ores at Pitkäranta, Ladoga Karelia, Russia. Miner. Depos. 2016, 51, 823–839. [Google Scholar] [CrossRef]
- Trüstedt, O. Die Erzlagerstätten von Pitkäranta am Ladoga-See. Bull. Comm. Géol. Finlande 1907, 19. (In German) [Google Scholar]
- Khazov, R.A. Geological Features of tin ore Mineralization of Northern Ladoga Region; Trudy of Geological Instute Karelian Branch of USSR Akademy of Science: Leningrad, Russia, 1973; p. 87. (In Russian) [Google Scholar]
- Velichkin, V.I.; Tarasov, N.N.; Andreeva, O.V.; Kiseleva, G.D.; Krylova, T.L.; Doinikova, O.A.; Golubev, V.N.; Golovin, V.A.; Kushnerenko, V.K. Geology and formation conditions of the Karku unconformity-type deposit in the northern Ladoga region (Russia). Geol. Ore Depos. 2005, 47, 87–112. [Google Scholar]
- Ivashchenko, V. Geology, geochemistry and mineralogy of indium resources at Pitkäranta Mining District, Ladoga Karelia, Russia. J. Geochem. Explor. 2022, 240, 107046. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Levkovskiy, R.Z. Geological geological structure and mechanism of rapakivi massifs formation in the northwestern part of the Kola peninsula. In Petrography of the Precambrian of the Russian Platform; Naukova dumka: Kiev, Ukraine, 1970; pp. 135–143. (In Russian) [Google Scholar]
- Gorokhov, I.M.; Kuznetsov, A.B.; Azimov, P.Y.; Dubinina, E.O.; Vasilieva, I.M.; Rizvanova, N.G. Sr and C Isotope Chemostratigraphy of the Paleoproterozoic Metacarbonate Rocks of the Sortavala Group: Fennoscandian Shield, Northern Ladoga Area. Stratigr. Geol. Correl. 2021, 29, 121–139. [Google Scholar] [CrossRef]
- Amelin, Y.V.; Larin, A.M.; Tucker, R.D. Chronology of multiphase emplacement of the Salmi rapakivi granite-anorthosite complex, Baltic Shield: Implications for magmatic evolution. Contrib. Mineral. Petrol. 1997, 127, 353–368. [Google Scholar] [CrossRef]
- Sahama, T.G. On the chemistry of the East Fennoscandian Rapakivi Granites. Bull. Comm. Geol. Finlande 1945, 136, 15–67. [Google Scholar]
- Sviridenko, L.P. Petrology of Salmi Rapakivi Granite Massif (in Karelia); Trudy of Geological Instute Karelian Branch of USSR Akademy of Science, Karelian Book Publishing House: Petrozavodsk, Russia, 1968; 116p. (In Russian) [Google Scholar]
- Nikolskaya, Z.D. New data about geology and metallogeny of Salmi rapakivi massif (Karelia). Tr. VSEGEI New Ser. 1975, 230, 52–57. (In Russian) [Google Scholar]
- Velikoslavinsky, D.A.; Birkis, A.P.; Bogatikov, O.A. Anorthosite-Rapakivi Granite Formation of the East European Platform; Mitrofanov, F.P., Ed.; Nauka: Leningrad, Russia, 1978; p. 296. (In Russian) [Google Scholar]
- Konyshev, A.A.; Chevychelov, V.Y.; Shapovalov, Y.B. Two Types of Highly Differentiated Topaz-Bearing Granites of the Salmi Batholith, Southern Karelia. Geochem. Int. 2020, 58, 11–26. [Google Scholar] [CrossRef]
- Shinkarev, A.F.; Anishchenkova, O.N. New data about composition and structure of Salmi complex. Izv. Akad. Nauk. USSR Geol. Ser. 1973, 140–144. (In Russian) [Google Scholar]
- Bantova, M.A.; Levkovskiy, R.Z.; Sharkov, E.V. Geology, material composition and age of rocks of the Salmi omplex of rapakivi granites and gabbro-anorthosites. Sov. Geol. 1975, 74–86. (In Russian) [Google Scholar]
- Larin, A. Ore mineralization. In Salmi Batholith and Pitkaranta ore Field in Soviet Karelia; Haapala, I., Rämö, O.T., Salonsaari, P.T., Eds.; University of Helsinki: Helsinki, Finland, 1991; pp. 19–34. [Google Scholar]
- Dukhovskiy, A.A.; Artamonova, N.A.; Ivanova, E.I.; Nikiforov, I.O. A volumetric model of the Salmi rapakivi granite massif and the regularities of mineralization placement. Otechestvennaya Geol. 1994, 4, 24–32. (In Russian) [Google Scholar]
- Beskin, S.M.; Lishnevskii, E.N.; Didenko, M.I. Structure of the Pitkyaranta granite massif in the Northern Ladoga region, Karelia. Izv. Akad. Nauk USSR Ser.Geol. 1983, 19–26. (In Russian) [Google Scholar]
- Bogdanov, Y.B.; Yakobson, K.E.; Guseva, E.A.; Petrov, B.V.; Amantov, A.V. Gosudarstvennaya Geological Map of the Russian Federation Scale 1:1000000 (Third Generation), Sheet P-(35),36, Map of Pre-Quaternary Formations; VSEGEI: St. Petersburg, Russia, 2015. [Google Scholar]
- Amelin, Y.V.; Heaman, L.M.; Verchogliad, M.V.; Skobelev, V.M. Geochronological constraints on the emplacement history of an anorthosite- rapakivi granite suite: U-Pb zircon and baddeleyite study of the Korosten complex, Ukraine. Contrib. Mineral. Petrol. 1994, 116, 411–419. [Google Scholar] [CrossRef]
- Bogatikov, O.A.; Birkis, A.P. Gabbro-norite-anorthosite complex of west Latvia. Izv. Akad. Nauk. USRR Geol. Ser. 1970, 23–34. (In Russian) [Google Scholar]
- Savolahti, A. On the Petrology of the Ahvenisto Massif. Bull. Comm. Geol. Finlande 1956, 174, 79. [Google Scholar]
- Sviridenko, L.P.; Svetov, A.P.; Golubev, A.I.; Rybakov, S.I.; Gor`kovets, V.Y.; Lavrov, M.M.; Heiskanen, K.I.; Stepanov, V.S.; Svetova, A.I.; Rayevskaya, M.B.; et al. Precambrian Magmatism Evolution (on Karelia Example); Kulikov, V.S., Voinov, A.S., Eds.; Nauka: Leningrad, Russia, 1985; p. 256. (In Russian) [Google Scholar]
- Svetov, A.P.; Sviridenko, L.P. Riphean Volcanoplutonism of the Fennoscandian Shield; Russian Academy of Sciences, Karel. Science. Center, Institute of Geology: Petrozavodsk, Russia, 1995; p. 211. (In Russian) [Google Scholar]
- Sviridenko, L.P.; Svetov, A.P.; Golubev, A.I.; Pavlov, G.M. Topaz-containing tuffisites of the southern margin of the Baltic Shield. Dokl. USSR Acad. Sci. 1984, 276, 1449–1552. (In Russian) [Google Scholar]
- Konyshev, A.A.; Anosova, M.O.; Rusak, A.A.; Alekseev, I.A.; Yakushev, A.I.; Shapovalov, Y.B. Dikes of Quartz Porphyry and Their Role in the Formation of the Salmi Batholith (South Karelia). Dokl. Earth Sci. 2020, 491, 127–130. [Google Scholar] [CrossRef]
- Khazov, R.A. Metallogeny of the Ladoga-Bothnian Geoblock of the Baltic Shield; Nauka: Leningrad, Russia, 1982; p. 192. [Google Scholar]
- Svetov, S.A.; Stepanova, A.V.; Chazhengina, S.Y.; Svetova, E.N.; Rybnikova, Z.P.; Mikhailova, A.I.; Paramonov, A.S.; Utitsyna, V.L.; Ekhova, M.V.; Kolodey, B.S. Precision geochemical (ICP–MS, LA–ICP–MS) analysis of rock and mineral composition: The method and accuracy estimation in the case study of Early Precambrian mafic complexes. Tr. KarRC RAS 2015, 7, 54–73. [Google Scholar] [CrossRef]
- Vasil’eva, I.E.; Shabanova, E.V. Catalogue of Certified Reference Materials of Natural and Man-made Media Compositions; Vinogradov Institute of Geochemistry SB RAS: Irkutsk, Russia, 2017; p. 79. Available online: http://www.igc.irk.ru/images/Innovation/Standarts-obr/2015/CATALOGUE_OF_CRMs_IGC_SB_RAS_-2017.pdf (accessed on 6 April 2023).
- Fedotova, A.A.; Bibikova, E.V.; Simakin, S.G. Ion-microprobe geochemistry as an indicator of mineral genesis during geochronologocal studies. Geochem. Int. 2008, 46, 912–927. [Google Scholar] [CrossRef]
- Poutiainen, M.; Scherbakova, T.F. Fluid and melt inclusion evidence for the origin of idiomorphic quartz crystals in topaz–bearing granite from the Salmi batholith, Karelia, Russia. Lithos 1998, 44, 141–151. [Google Scholar] [CrossRef]
- Rub, M.G.; Hetchikov, L.N.; Kotelnikova, Z.A.; Rub, A.K. Inclusions of mineral-forming media in minerals of Precambrian tin-bearing granites of the Northern Ladoga region. Izv. Akad. Nauk USSR Ser. Geol. 1986, 30–36. (In Russian) [Google Scholar]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulfide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Fleet, M.E.; Pan, Y. Fractional crystallization of anhydrous sulfide liquid in the system Fe–Ni–S, with application to magmatic sulfide deposits. Geochim. Cosmochim. Acta 1994, 58, 3369–3377. [Google Scholar] [CrossRef]
- Kress, V. Thermochemistry of sulfide liquids I. The system O–S–Fe at 1 bar. Contr. Mineral. Petrol. 1997, 127, 176–186. [Google Scholar] [CrossRef]
- Haapala, I. Magmatic and Postmagmatic Processes in Tin-mineralized Granites: Topaz-bearing Leucogranite in the Eurajoki Rapakivi Granite Stock, Finland. J. Petrol. 1997, 38, 1645–1659. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace Element Distribution Diagramms for the Tectonic Interpretation of Granitic Rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivision of A-type granitoids: Petrogenetic and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Zaraisky, G.P.; Aksyuk, A.M.; Devyatova, V.N.; Udoratina, O.V.; Chevychelov, V.Y. Zr/Hf ratio as an indicator of fractionation of rare-metal granites by the example of the Kukulbei complex, eastern Transbaikalia. Petrology 2008, 16, 710–736. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Irber, W. The lanthanide tetrad effect and its correlation with K/Rb, Eu/Eu*, Sr/Eu, Y/Ho, and Zr/Hf of evolving peraluminous granite suites. Geochim. Comochim. Acta 1999, 63, 489–508. [Google Scholar] [CrossRef]
- Hofman, A.W. Chemical differentiation of the Earth: The relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 1988, 90, 297–314. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. The Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier-Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar] [CrossRef]
- Veksler, V.I.; Dorfman, A.M.; Kamenetsky, M.; Dulski, P.; Dingwell, D.B. Partitioning of lanthanides and Y between immiscible silicate and fluoride melts, fluorite and cryolite and the origin of the lanthanide tetrad effect in igneous rocks. Geochim. Cosmochim. Acta 2005, 69, 2847–2860. [Google Scholar] [CrossRef]
- Corfu, F.; Hanchar, J.M.; Hoskin, P.W.O.; Kinny, P. Atlas of Zircon Textures. Rev. Mineral. Geochem. 2003, 53, 468–500. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The Composition of zircon and igneous and metamorphic petrogenesis. Rev. Mineral. Geochem. 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Grønvold, F.; Stølen, S.J. Thermodynamics of iron sulfides II. Heat capacity and thermodynamic properties of FeS and of Fe0.875S at temperatures from 298.15 K to 1000 K, of Fe0.98S from 298.15 K to 800 K, and of Fe0.89S from 298.15 K to about 650 K. Thermodynamics of formation. J. Chem. Thermodyn. 1992, 24, 913–936. [Google Scholar] [CrossRef]
- Linnen, R.L. The solubility of Nb-Ta-Zr-Hf-W in granitic melt with Li and Li + F: Constrains for mineralization in rare metal granite and pegmatite. Econ. Geol. 1998, 93, 1013–1025. [Google Scholar] [CrossRef]
- Aseri, A. Effects of Fluorine on the Solubilities of Nb, Ta, Zr and Hf Minerals in Highly Fluxed Water-Saturated Haplogranitic Melts. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Alferyeva, Y.O.; Gramenitskii, E.N.; Shchekina, T.I. Changes in the Ta/Nb Ratio in Successively Formed Differentiates of Granite Melt (Calculations Based on Experimental Data). Russ. Geol. Geophys. 2020, 61, 26–35. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Savina, E.A. Tetrad Effects in the Rare Earth Element Patterns of Granitoid Rocks as an Indicator of Fluoride-Silicate Liquid Immiscibility in Magmatic Systems. Petrology 2010, 18, 514–543. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yoshida, H.; Sato, N.; Hama, K.; Yusa, Y.; Shimizu, H. W- and M- type tetrad effect in REE patterns for water-rock systems in the Tono uranium deposit, central Japan. Chem. Geol. 2002, 184, 311–335. [Google Scholar] [CrossRef]
- Jahn, B.; Wu, F.; Capdevila, R.; Martineau, F.; Zhenhua, Z.; Wang, Y. Highly evolved juvenile granites with tetrad REE patterns: The Woule and Baerzhe granites from the Great Xing’an Mountains in NE China. Lithos 2001, 59, 171–198. [Google Scholar] [CrossRef]
- Jakobsen, J.K.; Veksler, I.V.; Tegner, C.; Brooks, C.K. Crystallization of the Skaergaard Intrusion from an Emulsion of Immiscible Iron- and Silica-rich Liquids: Evidence from Melt Inclusions in Plagioclase. J. Petrol. 2011, 52, 345–373. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon saturation revisited: Temperature and composition effects in a variety of crustal magma types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Eklund, O.; Shebanov, A.D. The origin of rapakivi texture by sub-isothermal decompression. Precam. Res. 1999, 95, 129–146. [Google Scholar] [CrossRef]
- Shebanov, A.D.; Belyaev, A.M.; Savatenkov, V.M. The signicance of residual source material (restite) in rapakivi granite petrogenesis: An example from the Salmi batholith, Russian Karelia (abstract). In Proceedings of the 7th International Conference Rapakivi Granites Related Rocks, Helsinki, Finland, 24–26 July 1996. [Google Scholar]
- Frost, B.R. Introduction to oxygen fugacity and its petrologic importance. In Oxide Minerals: Petrologic and Magnetic Significance, Reviews in Mineralogy; Lindsley, D.H., Ed.; Stony Brook: New York, NY, USA, 1991; Volume 25, pp. 1–10. [Google Scholar]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Schlegel, H.; Schüller, A. Das Zustandsbild Kupfer-Eisen-Schwefel. Int. J. Mater. Res. 1952, 43, 421–428. [Google Scholar] [CrossRef]
- Tsujimura, T.; Kitakaze, A. New phase relations in the Cu-Fe-S system at 800°C; constraint of fractional crystallization of a sulfide liquid. Neues Jahrb. Mineral. Mon. 2004, 10, 433–444. [Google Scholar] [CrossRef]
- Belyaev, A.M. Regularities of the distribution of ore elements in pegmatites, pegmatoid granites and granitogneisses of the Northern Ladoga region. In Patterns of Concentration of Ore Elements in Granitoid Formations of the Karelian–Kola Region; USSR Academy of Sciences, Kirov Kola Branch, Geological Institute: Apatity, Russia, 1985; pp. 89–96. (In Russian) [Google Scholar]
- Kullerud, G.; Yund, R.A.; Moh, G.H. Phase relations in the Cu-Fe-S, Cu-Ni-S and Fe-Ni-S systems. In Magmatic Ore Deposits; Wilson, H.D.B., Ed.; Society of Economic Geologists: Stanford, UK, 1969; Volume 4, pp. 323–343. [Google Scholar] [CrossRef]
- Craig, J.R.; Kullerud, G. The Cu-Fe-Pb-S system. Year Book Carnegie Inst. Wash. 1967, 65, 344–352. [Google Scholar]
- Freidrich, K. The binary systems FeS-ZnS and FeS-PbS. Metallurgie 1907, 4, 479–485. [Google Scholar]
- Mavrogenes, J.A.; MacIntosh, I.W.; Ellis, D.J. Partial melting of the Broken Hill galena-sphalerite ore experimental studies in the system PbS-FeS-ZnS-(Ag2S). Econ. Geol. 2001, 96, 205–210. [Google Scholar] [CrossRef]
- Stevens, G.; Pinz, S.; Rozendaal, A. Partial melting of the assemblage sphalerite + galena + pyrrhotite + chalcopyrite + sulfur: Implications for high-grade metamorphosed massive sulfide deposits. Econ. Geol. 2005, 100, 781–786. [Google Scholar] [CrossRef]
- Clark, L.A. The Fe-As-S system: Phase relations and applications. Econ. Geol. 1960, 55, 1345–1381. [Google Scholar] [CrossRef]
- Barton, P.B., Jr. Thermochemical study of the system Fe-As-S. Geochim. Cosmochim. Acta 1969, 33, 841–857. [Google Scholar] [CrossRef]
- Botcharnikov, R.E.; Holtz, F.; Mungall, J.E.; Beermann, O.; Linnen, R.L.; Garbe-Schönberg, D. Behavior of gold in a magma at sulfide-sulfate transition: Revisited. Am. Miner. 2013, 98, 1459–1464. [Google Scholar] [CrossRef]
- Polferov, D.V. On the relationships of sulfides and silicates in copper-nickel ores. Geol. Ore Depos. 1996, 36, 49–62. (In Russian) [Google Scholar]
- Robinson, D.J.; Hutchinson, R.W. Evidence for a volcanogenic-exhalative origin of a massive nickel sulphide deposit at Redstone, Timmins, Ontario. In Precambrian Sulphide Deposits; Geological Association of Canada Special Paper: St. Johns, NL, Canada, 1982; pp. 211–254. [Google Scholar]
- Li, C.; Naldrett, A.J.; Coats, C.J.C.; Johannessen, P. Platinum, palladium, gold, and copper-rich stringers at the Strathcona mine, Sudbmy: Their enrichment by fractionation of a sulfide liquid. Econ. Geol. 1992, 87, 1584–1598. [Google Scholar] [CrossRef]
- Farrow, C.E.G.; Watkinson, D.H. Alteration and the role of fluids in Ni, Cu and platinum-group element deposition, Sudbury Igneous Complex contact, Onaping-Levack area, Ontario. Mineral. Petrol. 1992, 46, 67–83. [Google Scholar] [CrossRef]
- Li, C.; Naldrett, A.J. High chlorine alteration minerals and calcium rich brines in fluid inclusions from the Strathcona Deep Copper zone, Sudbury, Ontario. Econ. Geol. 1993, 88, 1780–1796. [Google Scholar] [CrossRef]
- Zelenski, M.; Kamenetsky, V.S.; Nekrylov, N.; Abersteiner, A.; Ehrig, K.; Khanin, D. Textural, morphological and compositional varieties of modern arc sulfide melts: A case study of the Tolbachik volcano, Kamchatka. Lithos 2018, 318–319, 14–29. [Google Scholar] [CrossRef]
- Konnikov, E.G. Experimental studies of the melting processes in a pyrrhotine-water system. Geol. Ore Depos. 1997, 39, 474–479. (In Russian) [Google Scholar]
- Wykes, J.L.; Mavrogenes, J.A. Hydrous sulfide melting: Experimental evidence for the solubility oh H2O in sulfide melts. Econ. Geol. 2005, 100, 157–164. [Google Scholar] [CrossRef]
- Mungall, J.E.; Brenan, J.M. Experimental evidence for the chalcophile behavior of the halogens. Can. Mineral. 2003, 41, 207–220. [Google Scholar] [CrossRef]
- Tomkins, A.G.; Mavrogenes, J.A. Generation of metal-rich felsic magmas during crustal anatexis. Geology 2003, 31, 765–768. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konyshev, A. Natural Experiment on the Extraction and Quenching of Rapakivi-like Magmas: Traces of Interaction with the Mafic Melts and Their Derivatives, Salmi Batholith (Karelia, Russia). Minerals 2023, 13, 527. https://doi.org/10.3390/min13040527
Konyshev A. Natural Experiment on the Extraction and Quenching of Rapakivi-like Magmas: Traces of Interaction with the Mafic Melts and Their Derivatives, Salmi Batholith (Karelia, Russia). Minerals. 2023; 13(4):527. https://doi.org/10.3390/min13040527
Chicago/Turabian StyleKonyshev, Artem. 2023. "Natural Experiment on the Extraction and Quenching of Rapakivi-like Magmas: Traces of Interaction with the Mafic Melts and Their Derivatives, Salmi Batholith (Karelia, Russia)" Minerals 13, no. 4: 527. https://doi.org/10.3390/min13040527
APA StyleKonyshev, A. (2023). Natural Experiment on the Extraction and Quenching of Rapakivi-like Magmas: Traces of Interaction with the Mafic Melts and Their Derivatives, Salmi Batholith (Karelia, Russia). Minerals, 13(4), 527. https://doi.org/10.3390/min13040527






