Abstract
Activity models based on the ion and molecule coexistence theory have been widely used in the refining of metallurgical slags, with the SiO2 content of slag playing a crucial role in improving the mechanical properties of refining slag-based cementitious materials. In order to improve the reactivity of SiO2 in slag, this study established a SiO2 activity prediction model for the CaO-Al2O3-SiO2-MgO quaternary slag system based on the ion and molecule coexistence theory, validating the prediction results using reference values from the literature. Following this, the effects of w(SiO2), w(CaO), w(CaO)/w(Al2O3), and R(w(CaO)/w(SiO2)) on SiO2 activity were explored (where w and R represent content and alkalinity, respectively). The results show that the model could accurately predict the SiO2 activity of refining slag at 1873k. When the SiO2 content was increased from 10% to 30%, with 60% w(CaO) and a w(MgO)/w(Al2O3) ratio of 0.25, the SiO2 activity exhibited a trend of initially increasing and then decreasing, with a maximum activity value of 0.1359 reached at 17.5% w(SiO2). When slag contained 15% w(SiO2) and a w(MgO)/w(Al2O3) ratio of 0.25, the SiO2 activity decreased with increasing CaO content, reaching a maximum activity value of 0.1268 when 55% w(CaO) was present. Therefore, by controlling the ratio of w(CaO)/w(Al2O3) and w(CaO)/w(SiO2) in the slag to maintain a ratio of 3, the activity of SiO2 can be effectively increased.
1. Introduction
Geopolymers were developed by Davidovits in 1978 and have been widely utilized since then, as they exhibit properties similar to those of silicate cements [1]. The basic raw materials used in the formation of geopolymers are a class of substances containing silica-aluminate active components, with the specific nature of different raw materials affecting the final geopolymers’ performance. Industrial development has resulted in increased levels of production of industrial waste materials, such as steel slag, blast furnace slag, red mud, fly ash, and carbide slag [2,3,4,5], which typically contain silicate or silicoaluminate as a main mineral component. Therefore, these waste materials can be used as a raw material for the production of geopolymers, which can not only provide a sustainable and efficient industrial waste management solution, but also solve the environmental problems arising from the storage of these waste materials.
Refining slag is a typical type of steel slag, which is the waste slag produced during the process of steel refining. SiO2 is the main chemical component of refining slag and the further addition of pure SiO2 to slag has been shown to eliminate free calcium oxide, make the refining slag more stable and significantly improving the grindability of the refining slag [6]. When using refining slag to prepare cementitious materials, the pozzolanic activity of SiO2 consumes a large amount of Ca(OH)2. In addition, due to the crystallization nucleation and filling effect of SiO2, the hydration capacity of refining slag is enhanced, improving the mechanical properties of the final refining slag-based cementitious material [7]. Therefore, improving the reactivity of SiO2 in slag is of great importance for the preparation of gelling materials. The CaO-Al2O3-SiO2-MgO system is a typical refining slag system that is used in the process of preparing geopolymers from refining slag [8,9]. CaO, SiO2, Al2O3, and MgO are the main components of refining slag, accounting for about 90% of refining slag mass, with their relative proportions and subsequent influence, being much larger than other components (such as Fe2O3 and SO2). However, at present, there are relatively few studies on the activity of refining slag components. Therefore, establishing a calculation model for the activity of CaO-Al2O3-SiO2-MgO quaternary slag components and predicting the activity of related components are of great significance for further research on the mechanical properties of refining slag-based geopolymers.
So far, many slag thermodynamic models have been proposed, such as the Regular Solution Model (RSM) [10], ion model [11], Wilson equation [12], the new generation of solution geometric model [13], KTH model [14], molecular interaction volume model (MIVM) [15], Monte Carlo model [16], Aggregation model [17], molecular ion coexistence theoretical model [18], etc. However, most prediction models involve manually defined parameters and have a limited scope of application. All models have their advantages and disadvantages. Up to now, no model can be applied to all slag systems, so thermodynamic models need to be further developed and improved. Many scholars’ research and analysis show that [19] the theoretical model of molecular ion coexistence has certain applicability, and only the standard Gibbs free energy of relevant reactions is used in the calculation process (ΔGθ). The calculation process is simple and has a good prospect for application. Therefore, the molecular ion coexistence theory is selected in this paper to establish a prediction model for SiO2 activity of CaO-Al2O3-SiO2-MgO quaternary slag system. It is verified by the literature value in order to reduce the calculation error caused by the theoretical basic assumption. On this basis, the influence of component SiO2, CaO content, CaO/Al2O3, and alkalinity on SiO2 activity in the CaO-Al2O3-SiO2-MgO slag system is analyzed. After the rules are obtained through calculation, the conditions for preparing high-strength geopolymers can be achieved by changing the experimental parameters (alkalinity, content of acid-base oxides), which provides a prospect for preparing building materials, paving roads, and reducing environmental impact in the future.
2. Thermodynamic Activity Model of the CaO-SiO2-Al2O3-MgO System
2.1. Structural Units and Mass Action Concentrations in the CaO-SiO2-Al2O3-MgO System
The molecular-ion coexistence theory (IMCT) is a model used to study the structural units of slag and their interactions, to determine the relationship between slag components and properties [20]. The calculations used by the IMCT model are simple, as it only uses the thermodynamic value ΔGθ and the IMCT model can calculate the active concentration of all structural units present in slag material, unlike other comparable models such as the Van Laar equation, which cannot be used for multivariate systems. The IMCT model has been successfully applied to the analysis of slag composed of CaO-Al2O3 [21], CaO-Al2O3-Ce2O3 [22], CaO-Al2O3-SiO2 [21], CaO-Al2O3-MgO [23], CaO-SiO2-MgO-Al2O3-Na2O [24], CaO-SiO2-MgO-Al2O3-FeO [25], and CaO-SiO2-MgO-Al2O3-Cr2O3 [26]. Furthermore, the use of the IMCT model has been well established for prediction of the sulfur and phosphorus capacity of metallurgical slag, as well as for determining the sulfur partition ratio in metallurgical processes [27,28,29].
The IMCT model assumes that complex molecules are formed via chemical reactions that exhibit chemical kinetic equilibrium between simple molecules and ion pairs produced by simple ions, allowing activity values to be obtained when all components of the system are in equilibrium for all reactions. This assumption replaces activity in the traditional sense, with the mass action concentration of structural units or ion pairs in the slag system. Based on the IMCT and the phase diagram of CaO-SiO2, MgO-SiO2, CaO-Al2O3, MgO-Al2O3, CaO-SiO2-MgO, CaO-Al2O3-SiO2, and Al2O3-SiO2-MgO [30], the structural units present in the CaO-Al2O3-SiO2-MgO quaternary slag system are shown in Table 1.

Table 1.
Structural units present in the CaO-Al2O3-SiO2-MgO system.
The active concentration of each structural unit in the CaO-Al2O3-SiO2-MgO slag system can be determined using Equations (1)–(4) as follow:
where b, a1, a2, and a3 are the total moles of the components CaO, Al2O3, SiO2, and MgO, respectively, in the slag at equilibrium; indicates the total number of moles at equilibrium; and (i = 1, 2, 3,…, n) is the active concentration of each component in the refining slag, which obeys the law of mass action. The structural unit is defined as: N1 = , N2 = , N3 = , N4 = , N5 = , N6 = , N7 = , N8 = , N9 = , N10 = , N11 = , N12 = , N13 = , N14 = , N15 = , N16 = , N17 = , N18 = , N19 = , N20 = , N21 = , and N22 = .
2.2. Thermodynamic Data and Chemical Equilibrium Equation
Thermodynamic data for the formation reactions of each complex compound in slag and the respective chemical equilibrium equations were established according to the IMCT model and are listed in Table 2 [30,31,32,33,34].

Table 2.
Thermodynamic data for complex slag component chemical reactions and the corresponding chemical equilibrium equations.
2.3. Mass Balance Equation Construction
As the IMCT assumes that a dynamic chemical equilibrium exists between atoms and molecules in the slag system, a mass balance equation was established based on the conservation of mass before and after chemical reactions, as shown in Equation (5):
where the total number of moles of CaO, Al2O3, SiO2, and MgO before and after the reactions remained constant, as shown in Equations (6)–(9):
By combining Equations (6) and (7), it is possible to eliminate for b and a1 as described by Equation (10) as follows:
b(0.5N4 + N8 + 2N9 + N18 + N19 + N20 + N21 + N22) − a3(0.5N1 + N5 + 2N6 + 3N7 + N10 + 12N11 + 3N12 + N13 + 3N14
+ 2N15 + N16 + 3N17 + N18 + 3N19 + N20 + 2N21) = 0
+ 2N15 + N16 + 3N17 + N18 + 3N19 + N20 + 2N21) = 0
By combining Equations (7) and (9), it is possible to eliminate for a1 and a3 as described by Equation (11) as follows:
a1(0.5N4 + N8 + 2N9 + N18 + N19 + N20 + N21 + N22) − a3(N2 + N10 + 7N11 + N12 + 2N13 + 6N14 + N15 + N16 + N17 +
N22) = 0
N22) = 0
By combining Equations (8) and (9), it is possible to eliminate for a2 and a3 as described by Equation (12) as follows:
a2(0.5N4 + N8 + 2N9 + N18 + N19 + N20 + N21 + N22) − a3(N3 + N5 + N6 + N7 + N8 + N9 + N15 + 2N16 + 2N17 + N18 +
2N19 + 2N20 + 2N21) = 0
2N19 + 2N20 + 2N21) = 0
Using this approach, Equations (10)–(12) can be simplified, replacing (i = 5, 6,..., 22) with (i = 1, 2, 3, 4), allowing the system of nonlinear equations for to be collated, where Equation (5) was rectified to form Equation (13) as follows:
Equation (10) was rectified to form Equation (14) as follows:
Equation (11) was rectified to form Equation (15) as follows:
Equation (12) was rectified to form Equation (16) as follows:
Equations (13)–(16) describe the activity calculation model for the CaO-Al2O3-SiO2-MgO slag system and the above calculation models were solved using Matlab 2021a software and the fsolve function [35], allowing (i = 5, 6,..., 22) to be obtained from the equilibrium relationships, as expressed in Table 2.
2.4. CaO-Al2O3-SiO2-MgO Quaternary Slag System Activity Model Verification
In order to verify the reliability of the model, this paper compared the SiO2 activity values () obtained from the molecular-ionic coexistence theory prediction model of CaO-Al2O3-SiO2-MgO quaternary refining slag system at 1873 K with the literature-referenced SiO2 activity values () [23], and the results are shown in Figure 1. As can be seen from Figure 1, the SiO2 activity values () predicted by the molecular-ionic coexistence theory and the literature-referenced trend of SiO2 activity values () are consistent with the literature references, and their fit is 0.9086, which has a good linear relationship. Therefore, the molecule-ion coexistence theory model can be applied to calculate the activity of the tetragonal slag system group elements.
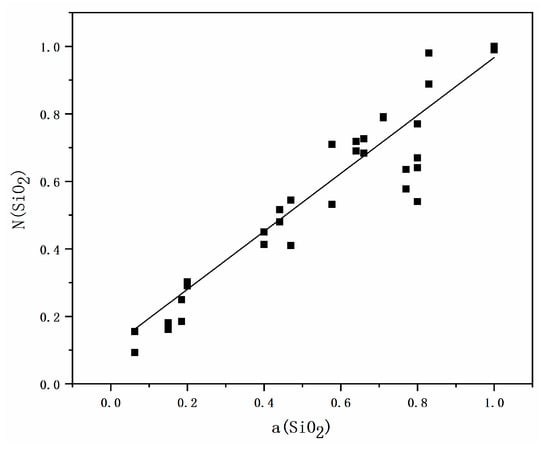
Figure 1.
Verification of the CaO-Al2O3-SiO2-MgO quaternary slag system model by the comparison of model predicted values and values reported in the literature under the same thermodynamic conditions.
2.5. Slag Sample Configuration
The slag sample used for analysis was selected in the model to explore the effect of w(SiO2), w(CaO), w(CaO)/w(Al2O3), and R(w(CaO)/w(SiO2)) on SiO2 activity in the CaO-Al2O3-SiO2-MgO slag system. The selected slag CaO-Al2O3-SiO2-MgO quaternary composition and activity values are presented in Table 3.

Table 3.
Slag composition after equilibrium and the activities of components in the CaO-Al2O3-SiO2-MgO system.
3. Results and Discussion
3.1. Influence of SiO2 Content on SiO2 Activity
Figure 2a shows the slag composition of the (CaO + MgO)-SiO2-Al2O3 pseudo-ternary system, in which the proportion of w(CaO) (=60%) and the ratio of w(MgO)/w(Al2O3) (=0.25) remained constant, while the influence of w(SiO2) on the activity of SiO2 was analyzed. The pattern of variation in the activity of SiO2 is shown in Figure 2b. When w(SiO2) was increased from 10%~17.5%, SiO2 activity exhibited an upward trend, reaching a maximum value of 0.1359 at 17.5% w(SiO2). Further increases in the content of SiO2 resulted in SiO2 activity exhibiting a downward trend.
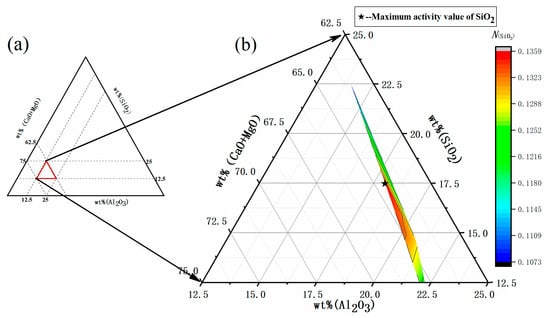
Figure 2.
(a) Slag composition of the (CaO + MgO)-SiO2-Al2O3 pseudo-ternary system. (b) Model predicted values: effect of SiO2 content on SiO2 activity in refining slag.
The reason for the initial increase and subsequent decrease in the modelled activity value was analyzed, showing that as the w(SiO2) increased from 10% to 17.5%, CaO existed in the form of a highly alkaline oxide. When w(CaO) remained constant and R(w(CaO)/w(SiO2)) decreased, the generation of dissociated O2− from the alkaline oxide was limited, inhibiting Equations (5)–(7) shown in Table 2 from proceeding and causing the SiO2 activity in slag to continually increase. When w(SiO2) exceeded 17.5%, its activity decreased accordingly. Combined with our preliminary experimental analysis in the laboratory, it can be seen that SiO2 mostly existed in a crystalline structural form, with the chemical components involved in the reaction being few and limited. At this time, the addition of a pure silicon source can improve the reaction rate to a certain extent, promoting the forward progression of Equations (5) to (7) in Table 2 and causing the SiO2 activity to decrease.
3.2. Influence of CaO Content on SiO2 Activity
The influence of w(CaO) on the activity of SiO2 was analyzed with 60% w(SiO2) and a w(MgO)/w(Al2O3) ratio of 0.25 kept constant. As shown in Figure 3, when w(CaO) = 55%, w(MgO) = 6%, and w(Al2O3) = 24%, SiO2 activity reached a maximum value of 0.1268, then exhibited a decreasing trend when the w(CaO) was increased gradually to 72.5%.
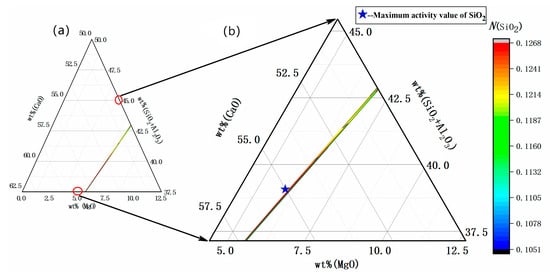
Figure 3.
(a) Slag composition of the CaO-(SiO2 + Al2O3)-MgO pseudo-ternary system. (b) Model predicted values: effect of CaO content on SiO2 activity in refining slag.
The reason for the decrease in theoretical activity was analyzed, with an increasing gradient of w(CaO) from 55% to 72.5%, while w(MgO)/w(Al2O3) remained constant at 0.25 and w(Al2O3) decreased relatively. CaO is a strongly alkaline oxide that can dissociate to produce O2− in slag (CaO → Ca2+ + O2−), with the dissociated O2− participating in the generation of silicate ions and aluminate ions. Due to the reduction of w(Al2O3), the generated O2− consumes the acidic oxide SiO2, resulting in a continual decrease in the activity of SiO2 in slag.
3.3. Influence of w(CaO)/w(Al2O3) on SiO2 Activity
The influence of w(CaO)/w(Al2O3) on the activity of SiO2 in slag was analyzed, with 60% w(SiO2) and 2% w(MgO) remaining constant. The red triangle area shown in Figure 4 indicates the area used for the reference slag selection value, while Figure 5 shows the variation in SiO2 activity with varying ratios of w(CaO)/w(Al2O3). When the w(CaO)/w(Al2O3) ratio was 1, SiO2 activity reached only 0.0185, while increasing the w(CaO)/w(Al2O3) from 1 to 3 resulted in an increase in N(SiO2), reaching a peak value of 0.1248 when w(CaO)/w(Al2O3) = 3. With further increases in w(CaO)/w(Al2O3), the activity of SiO2 exhibited a decreasing trend (Figure 5).
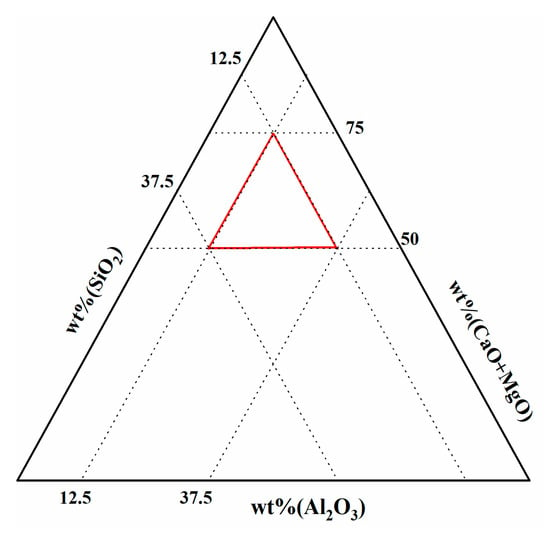
Figure 4.
Red area shows the Slag composition of the (CaO + MgO)-SiO2-Al2O3 pseudo-ternary system.
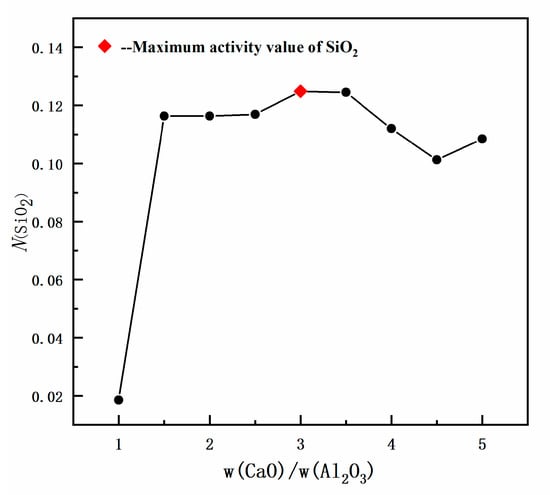
Figure 5.
Model predicted values: effect of w(CaO)/w(Al2O3) on SiO2 activity in slag.
The reason for the observed reduction in SiO2 activity value when w(CaO)/w(Al2O3) was increased to >3, is that the content of CaO gradually increases with higher w(CaO)/w(Al2O3) ratios, causing the generated Ca2+ + O2− ion pair to react with SiO2 and Al2O3 to form calcium silicate and calcium aluminate complexes, such as CaO-SiO2, CaO-2SiO2, CaO-Al2O3, and CaO-2Al2O3. In addition, the content of Al2O3 was found to decrease with the increase in w(CaO)/w(Al2O3), with the reaction between the Ca2+ + O2− ion pair and Al2O3 (as shown in Equations (10) to (14) in Table 2) being weakened, reducing the activity of calcium aluminate complex molecules, such as CaO-Al2O3 and CaO-2Al2O3. Therefore, as shown in Equations (5)–(7) in Table 2, CaO consumes more SiO2 to generate calcium silicate composite molecules, which consequently reduces the activity of SiO2.
3.4. Influence of R(w(CaO)/w(SiO2)) on SiO2 Activity
Table 4 shows the slag sample configurations used to study the effect of R on SiO2 activity. The influence of w(CaO)/w(Al2O3) on SiO2 activity was analyzed with 60% w(SiO2) and 2% w(MgO) kept constant. The law of variation of theoretical SiO2 activity under varying alkalinity conditions is shown in Figure 6. Results show that when w(CaO)/w(SiO2) was increased from 1 to 3, N(SiO2) continually increased, reaching a maximum activity value of 0.1326 when w(CaO)/w(SiO2) = 3. When w(CaO)/w(SiO2) was increased to >3, the SiO2 activity exhibited a downward trend, which is consistent with the results presented in Section 3.1 and Section 3.2.

Table 4.
Effect of alkalinity on the activity of components in the CaO-Al2O3-SiO2-MgO system.
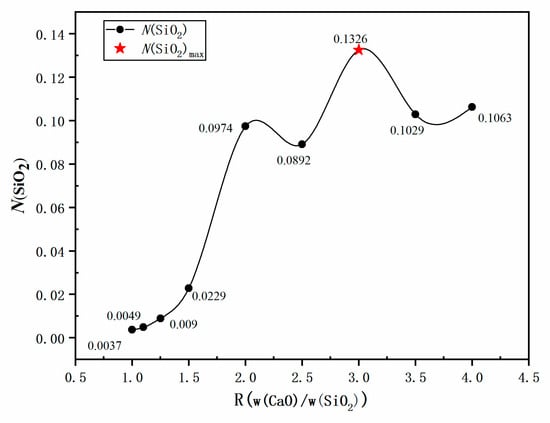
Figure 6.
Model predicted values: effect of R(w(CaO)/w(SiO2)) on SiO2 activity in slag.
The reason for the initial increase and subsequent decrease in theoretical activity value was that as R increased from 1 to 3, the relative increase in w(CaO) enhanced the generation of Ca2+ + O2− ion pairs, which reacted with SiO2 and Al2O3 to form calcium silicate and calcium aluminate phases, such as CaO-SiO2, CaO-2SiO2, CaO-Al2O3, and CaO-2Al2O3. Simultaneously, SiO2 decreased with the increase in R, weakening the reaction between the Ca2+ + O2− ion pair and SiO2, as shown in Equations (10) to (12), which reduced the activity of calcium silicate composite molecules, such as CaO-SiO2 and CaO-2SiO2, increasing the activity of SiO2 molecules. When R was increased further to >3 and the content of Al2O3 in the reference slag remained constant, the generation of aluminate ions by the reaction between Al2O3 and O2− (dissociated from CaO) was weakened and the generated O2− consumed more acidic oxide SiO2, resulting in a continuous decrease in SiO2 activity.
4. Conclusions
Based on the molecular ion coexistence theory, an activity calculation model of the CaO-SiO2-MgO-Al2O3 slag system was established and the influence of varying slag composition on SiO2 activity was calculated, allowing the following conclusions to be obtained:
- (1)
- According to the degree of coincidence between the theoretical prediction value and the literature value, the activity prediction model based on the molecule-ion coexistence theory is feasible, using simple model calculations to generate reasonable and high-precision predictions.
- (2)
- When w(CaO) = 60% and w(MgO)/w(Al2O3) = 0.25, an increased w(SiO2) caused the SiO2 activity to increase initially and then decrease gradually, with SiO2 activity reaching a maximum value of 0.1359 when w(SiO2) was 17.5%.
- (3)
- When w(SiO2) = 15% and w(MgO)/w(Al2O3) = 0.25, the w(CaO) in slag was negatively correlated with SiO2 activity. With increasing w(CaO), SiO2 activity gradually decreased, reaching a maximum value of 0.1268 when the w(CaO) was 55%.
- (4)
- SiO2 activity can be maintained at a high level by controlling w(CaO)/w(Al2O3) and w(CaO)/w(SiO2). When w(SiO2) was 15%, it was found that controlling the w(CaO)/w(Al2O3) to ≤3 can effectively improve SiO2 activity. In addition, when w(MgO) was 2%, w(Al2O3) was 8%, and w(CaO)/w(SiO2) was 3, the highest level of SiO2 activity was obtained, while further increases in w(CaO)/w(SiO2) from 3 to 4 caused the activity of SiO2 to decrease from 0.1326 to 0.1063.
Author Contributions
Y.L. established the prediction model, calculated and verified most of the thermodynamic formulas, and prepared a manuscript. Y.Y. provided guidance for the ideas and contents of the article, and participated in the writing and editing of the manuscript. M.F. validated the model data analysis. W.M. supervised the work. W.L. supervised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (51868072) and Xinjiang Natural Science Foundation (2022D01C388).
Data availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Correspondence and requests for materials should be addressed to Y.Y.
Acknowledgments
Thanks a lot for the support of the Natural Science Foundation of China (51868072) and Xinjiang Natural Science Foundation (2022D01C388).
Conflicts of Interest
The authors declare no competing interests.
References
- Davidovits, J.; Cordi, S.A. Synthesis of new high temperature geo-polymers for reinforced plastics/composites. Fourth Annu. Pac. Tech. Conf. Tech. Disp. 1979, 79, 151–154. [Google Scholar]
- Huang, F.; Zhou, Z.; Zhang, J.; Cheng, X. Preparation of geopolymer materials using steel slag and fly ash by pouring process. In Proceedings of the 8th International Symposium on Cement & Concrete, Bangkok, Thailand, 19–21 September 2013; Volume 48. [Google Scholar]
- Liang, X.; Ji, Y. Experimental study on durability of red mud-blast furnace slag geopolymer mortar. Constr. Build. Mater. 2021, 267, 120942. [Google Scholar] [CrossRef]
- Xiong, L.; Wan, Z.; Zhang, Y.; Wang, F.; Wang, J.; Kang, Y. Fly ash particle size effect on pore structure and strength of fly ash foamed geopolymer. Adv. Polym. Tech. 2019, 2019, 1098027. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Wang, X.; Pan, H.; Zhao, Q.; Wang, D. Utilization of municipal solid waste incineration fly ash with red mud-carbide slag for eco-friendly geopolymer preparation. J. Clean. Prod. 2022, 340, 130820. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, S.; Dong, Y.; Liu, H. The role of SiO2 in the modification of steel slag. In 2010 National Metallurgical Physical Chemistry Academic Conference Album; Journal of Rare Earths: Beijing, China, 2010; pp. 50–53. [Google Scholar]
- Wu, S.; Dai, S.B.; Ma, B.G.; Zhang, T.; Yang, H. Effect of nano-SiO2 on steel slag/cement-based cementitious materials. Bull. Chin. Ceram Soc. 2021, 40, 1594–1600. [Google Scholar]
- Zhang, X.; Guo, H.F.; Tang, Z.Q.; He, Z.W.; Song, L.T.; Yu, M.Q. Properties and microstructure of steel slag powder-fly ash based geopolymer. Bull. Chin. Ceram. Soc. 2021, 40, 4044–4051. [Google Scholar]
- Guo, X.; Pan, X. Mechanical properties and mechanisms of fiber reinforced fly ash–steel slag based geopolymer mortar. Constr. Build. Mater. 2018, 179, 633–641. [Google Scholar] [CrossRef]
- Tao, D.P. The universal characteristics of a thermodynamic model to conform to the Gibbs-Duhem equation. Sci. Rep. 2016, 6, 35792. [Google Scholar] [CrossRef]
- Tijskens, E.; Viaene, W.A.; Geerlings, P. The ionic model: Extension to spatial charge distributions, derivation of an interaction potential for silica polymorphs. Phys. Chem. Miner. 1995, 22, 186–199. [Google Scholar] [CrossRef]
- Ezati, F.; Sepehr, E.; Ahmadi, F. The efficiency of nano-TiO2 and γ-Al2O3 in copper removal from aqueous solution by characterization and adsorption study. Sci. Rep. 2021, 11, 18831. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhou, G. Improvement and application of new generation solution geometric model. Nonferr. Met. Sci. Eng. 2020, 11, 7–19. [Google Scholar]
- She, Y.; Ju, J.; Guo, Y.; Xie, X. Study of the composition optimization of CaO-SiO2-Al2O3-MgO slag based on the KTH model. Bull. Chin. Ceram. Soc. 2014, 33, 3166–3170. [Google Scholar]
- Tao, D.P. Correct expressions of enthalpy of mixing and excess entropy from MIVM and their simplified forms. Metall. Mater. Trans. B 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Gonçalves, D.R.R.; Fontes, W.C.; Mendes, J.C.; Silva, G.J.B.; Peixoto, R.A.F. Evaluation of the economic feasibility of a processing plant for steelmaking slag. Waste Manag. Res. 2016, 34, 107–112. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Cheng, Z. A new polymerization model applying to liquid silicate system CaO-SiO2. Acta Metall. Sin. 1986, 22, A425–A433. [Google Scholar]
- Zhao, M.; Li, G.; Li, Z.; Wang, Q.; He, S. Study of thermodynamic for low-reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O mold flux based on the model of ion and molecular coexistence theory. Metals 2022, 12, 1099. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, G.; Wang, Y.; Zhang, X.; Tan, B.; Yuan, C.; Miao, H. A thermodynamic model of the titanium distribution behaviour between slag and molten steel during LF refining process: Description and application. Ironmak. Steelmak. 2022, 49, 679–692. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Lv, N.; Yu, J.; Su, C.; Wang, H. Activity calculation for the components in CaO-Al2O3 and CaO-SiO2-Al2O3 slags. J. Northeast. Univ. Nat. Sci. 2013, 34, 1743–1746. [Google Scholar]
- Zheng, X.; Liu, C. Thermodynamic properties assessment of CaO-Al2O3-Ce2O3 system. Metall. Mater. Trans. B 2021, 52, 3183–3192. [Google Scholar] [CrossRef]
- Kume, K.; Morita, K.; Miki, T.; Sano, N. Activity measurement of CaO–SiO2–AlO1.5–MgO slags equilibrated with molten silicon alloys. ISIJ Int. 2000, 40, 561–566. [Google Scholar] [CrossRef]
- Lei, J.; Zhao, D.; Zhu, H.; Jiang, Y. A thermodynamic model of desulfurization for CaO-SiO2-MgO-Al2O3-Na2O slag based on IMCT theory. Iron Steel 2019, 54, 35–41. [Google Scholar]
- Lei, J.; Li, D.; Zhu, H. Calculation model of optimal basicity for the refining slag of bearing steel based on IMCT theory. Iron Steel Van. Tit. 2019, 40, 107–111. [Google Scholar]
- Guo, H.; Hu, Y.T.; Jin, Y.; Wang, L.X.; Cheng, X.L.; Bai, H.; Zong, Y.B. Calculation model of mass action concentrations for CaO–MgO–SiO2–Al2O3–Cr2O3 penta-slag. Chin. Chem. Lett. 2010, 21, 229–233. [Google Scholar] [CrossRef]
- Shi, C.-B.; Yang, X.-M.; Jiao, J.-S.; Li, C.; Guo, H.-J. A sulphide capacity prediction model of CaO–SiO2–MgO–Al2O3 ironmaking slags based on the ion and molecule coexistence theory. ISIJ Int. 2010, 50, 1362–1372. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Deng, X.X.; Barati, M.; McLean, A. Inclusion evolution during refining and continuous casting of 316L stainless steel. Ironmak. Steelmak. 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Yang, X.-M.; Shi, C.-B.; Zhang, M.; Duan, J.-P.; Zhang, J. A thermodynamic model of phosphate capacity for CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags equilibrated with molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 951–977. [Google Scholar] [CrossRef]
- Allibert, M.; Eisenhüttenleute, V.D. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Liang, Y.; Che, Y. Handbook of Thermodynamic Data of Inorganic Compounds; Northeast University Press: Shenyang, China, 1993. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Tian, Y.; Zhai, X.; Liu, K. A Concise Course of Metallurgical Physical Chemistry, 2nd ed.; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Zheng, H.; Wang, S.; Guo, Y.; Jiang, X.; Gao, Q.; Sheng, F. Thermodynamic model for calculating sulfur distribution ratio between CaO-SiO2-MgO-Al2O3 slag and hot metal. Iron Steel 2021, 56, 16–23. [Google Scholar]
- Zhang, D.; Cao, S.-Z. Series method for solving nonlinear control system based on MATLAB state equation and its realization. In Proceedings of the 2013 3rd International Conference on Computer Science and Network Technology, Dalian, China, 12–13 October 2013; pp. 249–252. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).