Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Elemental Analysis
2.3. Samples
2.4. Distribution Coefficients (Kd) Experiments
2.5. Speciation of U and Hg-Computational Modelling
2.6. Ion Exchange Study of U and Hg
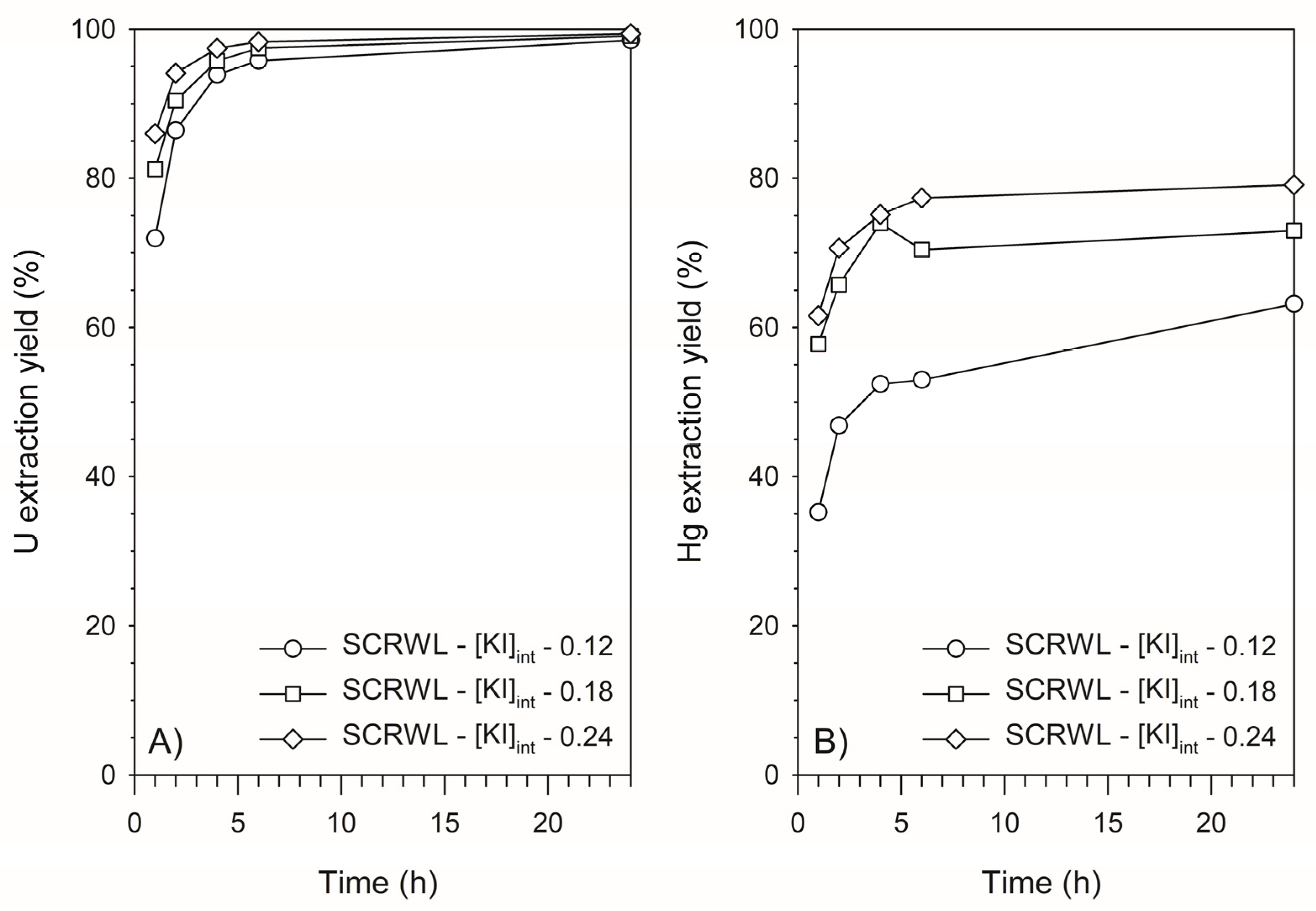
2.6.1. Kinetics Experiments
2.6.2. Continuous Mode Experiments
2.6.3. Effect of Extraction Cycles
3. Results
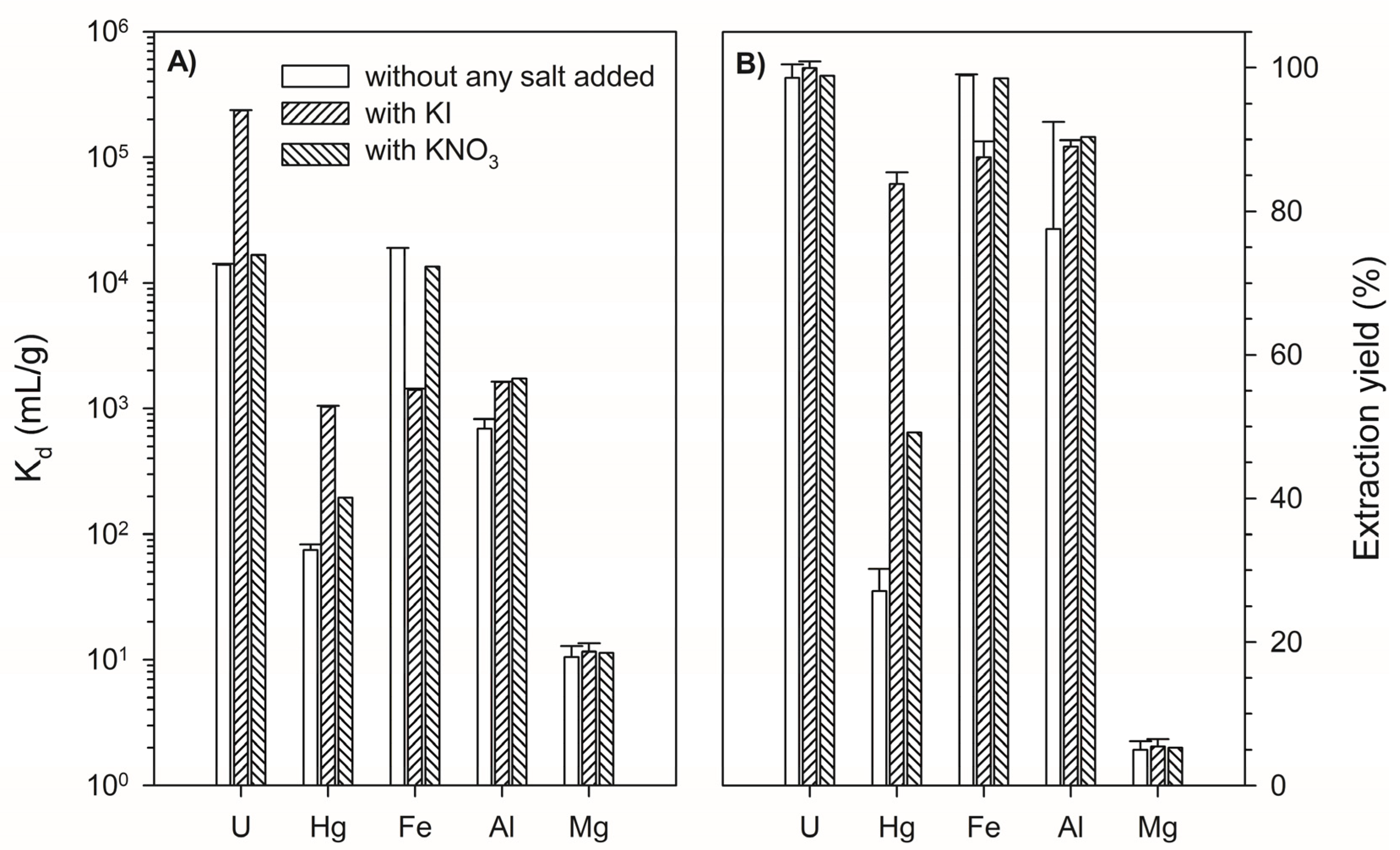
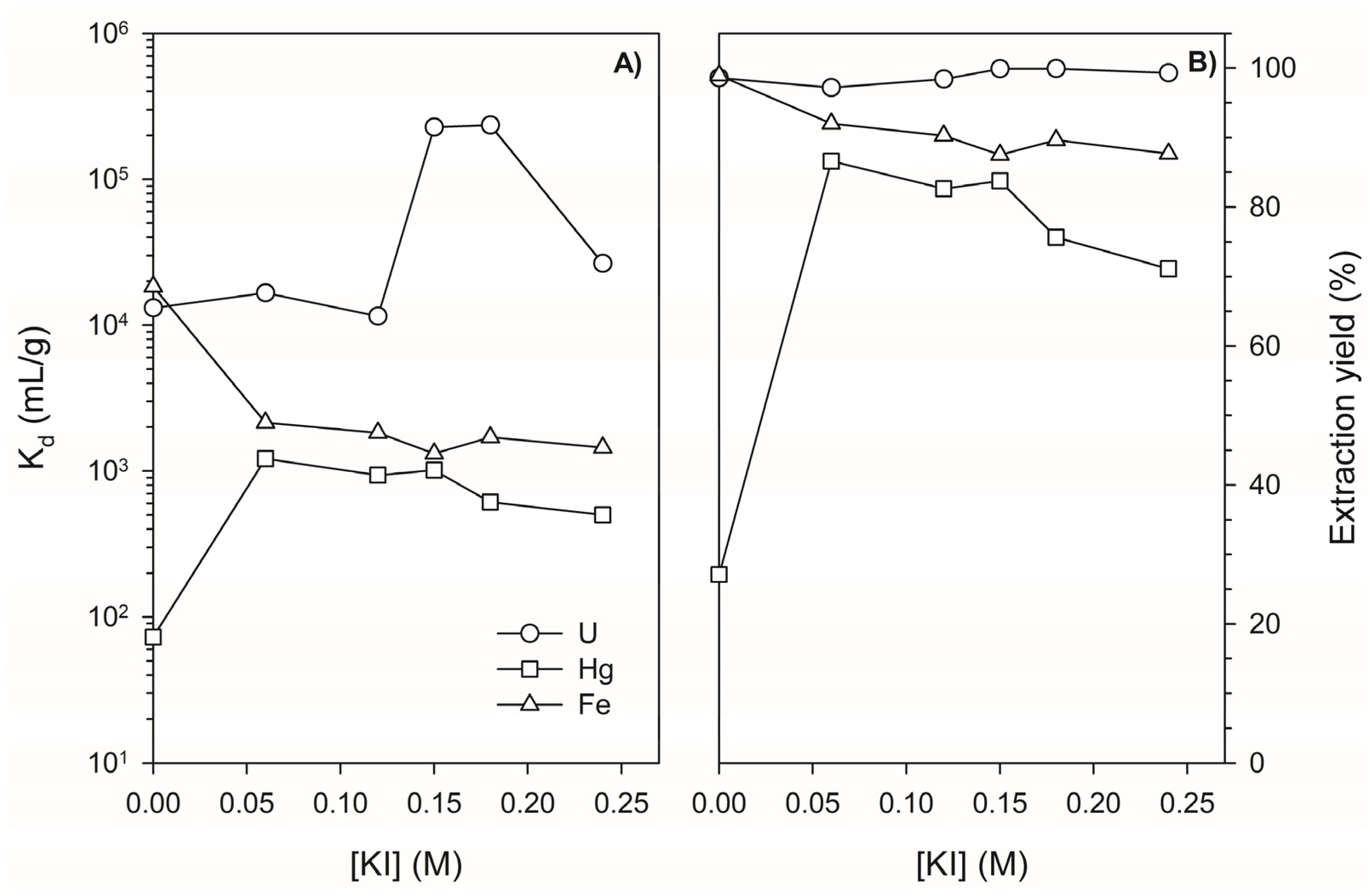
3.1. Determination of Distribution Coefficients for Major Elements, Hg, and U on Lewatit TP260 in the Absence and Presence of KI
3.2. Predicted Complexes of U and Hg at Different Concentration of KI in SCRW Leaching Solution
3.3. Retention of U and Hg from SCRW Leaching Solution by Lewatit TP260—Batch Mode Experimentation
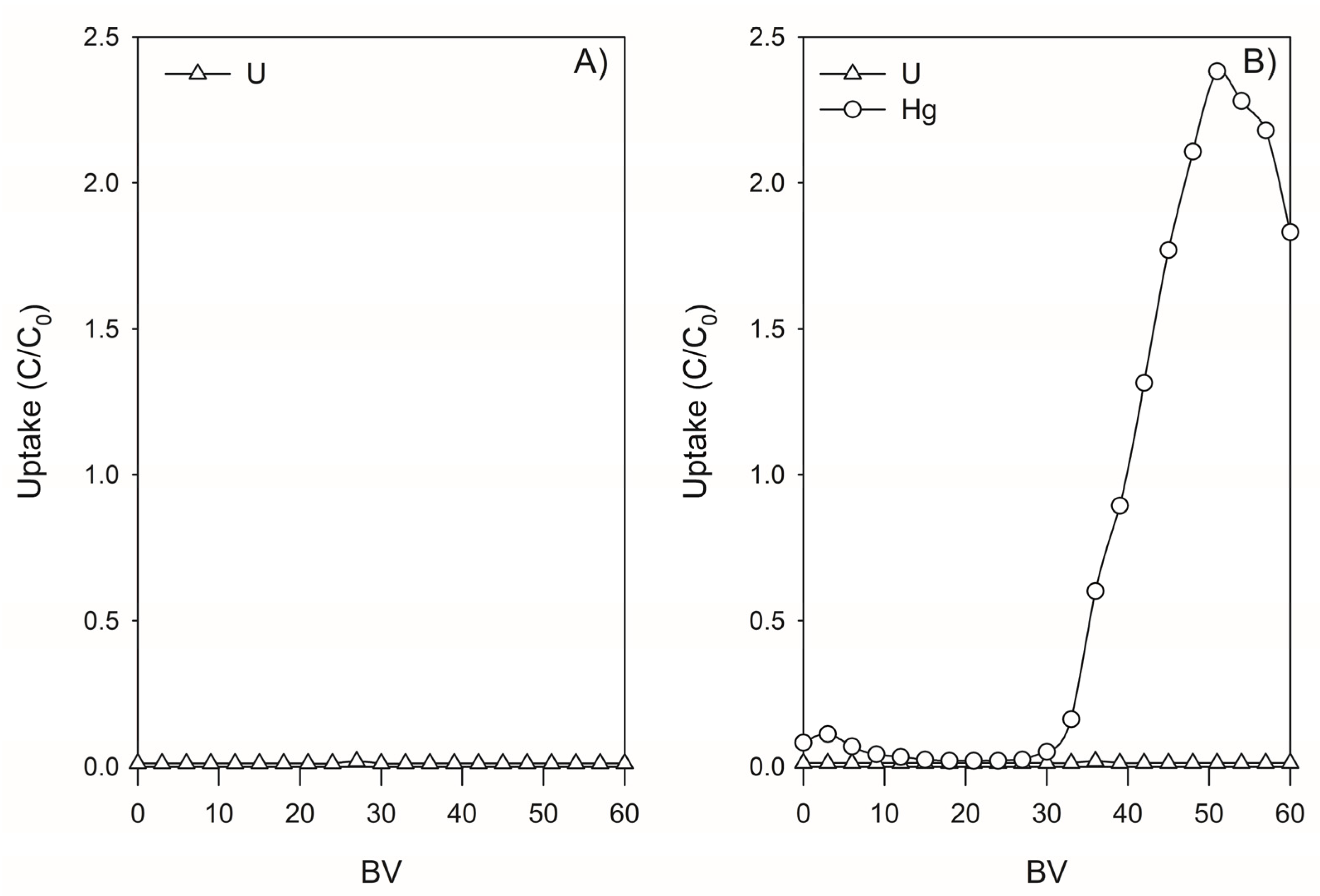
3.4. Retention of U and Hg from SCRW Leaching Solution by Lewatit TP260—Continuous Mode Experimentation
3.4.1. Assessment Using SCRW’ Leaching Solution
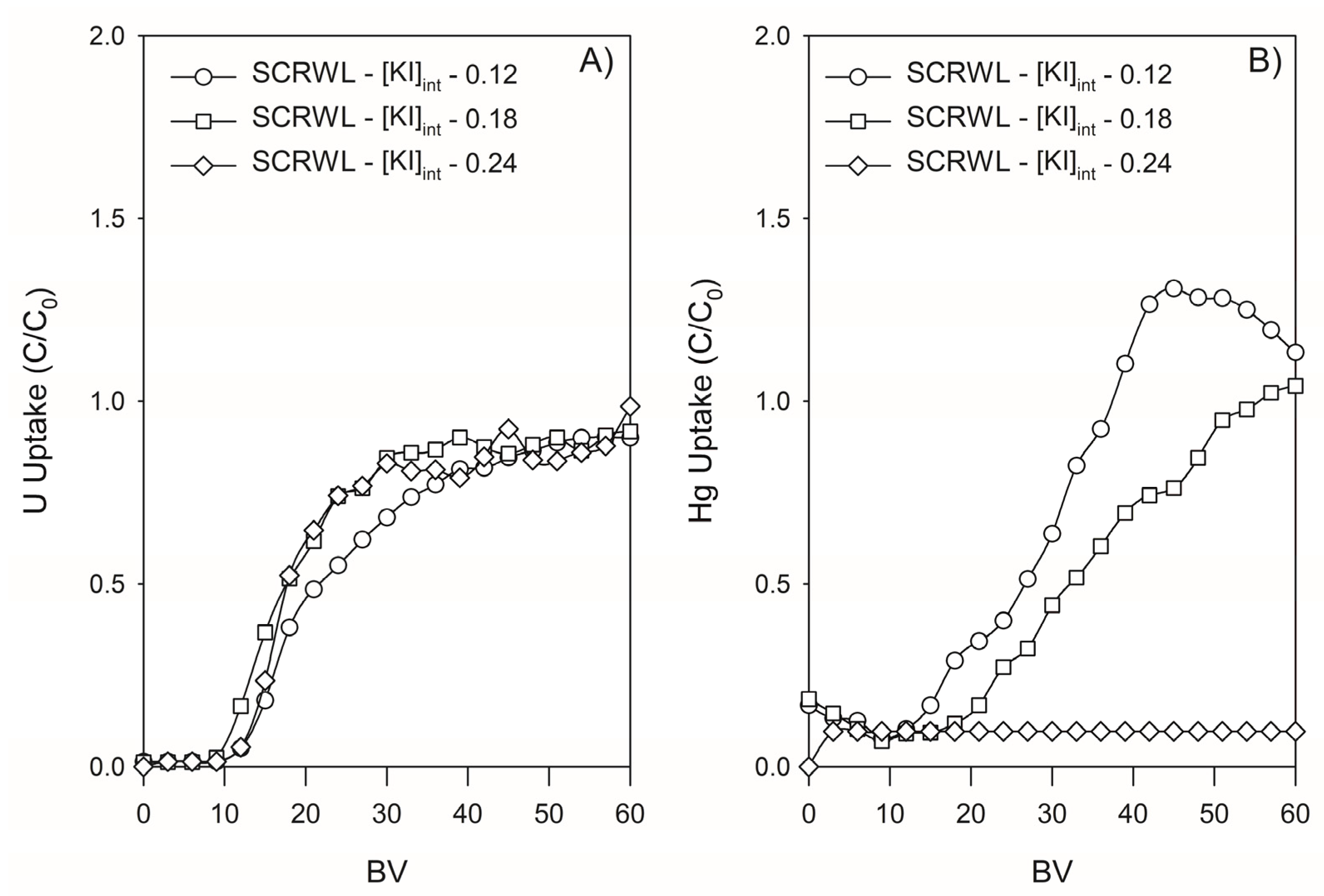
3.4.2. Retention of U and Hg by Lewatit TP260 in SCRW Leaching Solution
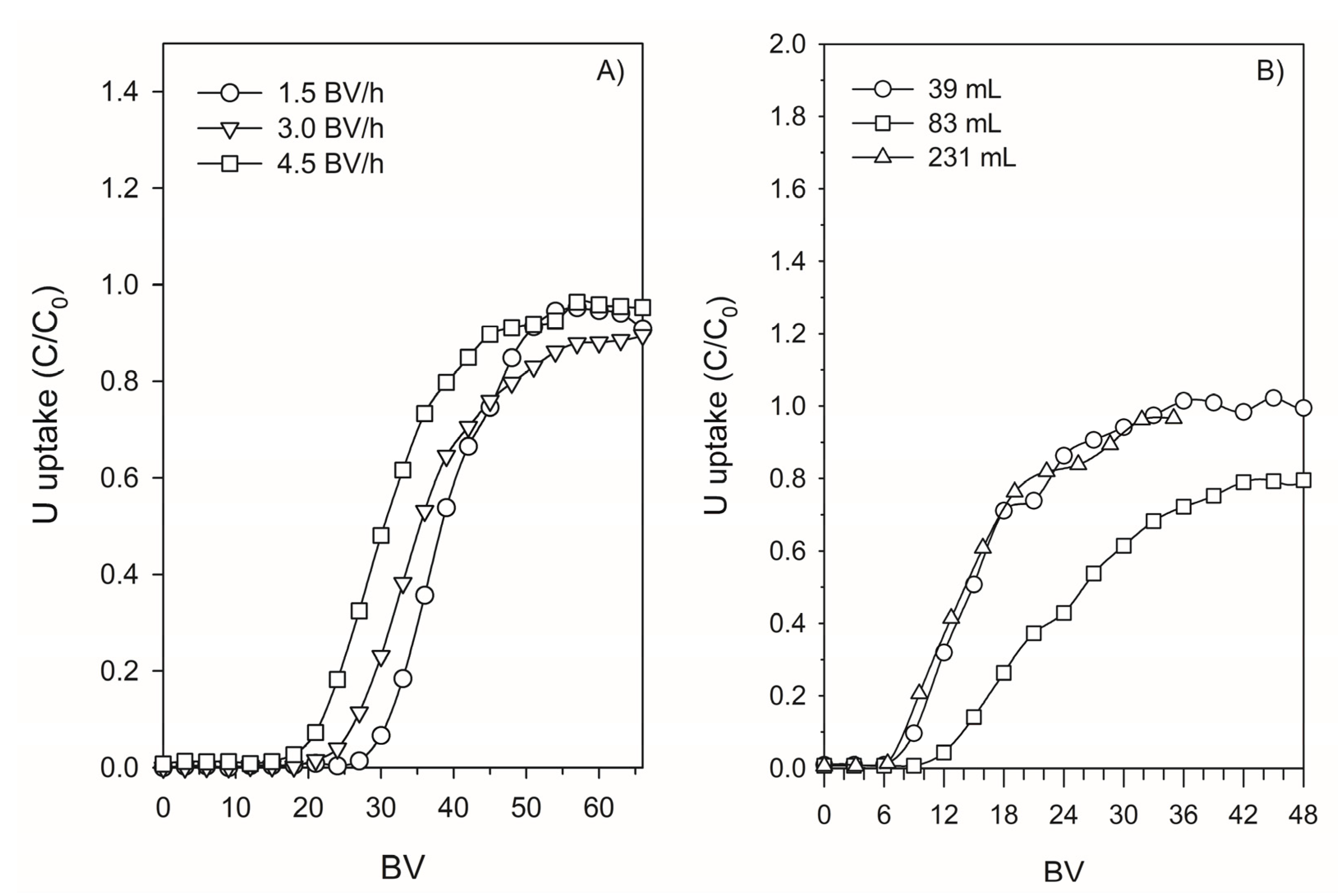
3.4.3. Effect of the Flow Rate and the Geometry
3.5. Retention of U from SCRW Leaching Solution by Lewatit TP260—Extraction Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council. Molybdenum-99/Technetium-99m Supply Reliability. Medical Isotope Production without Highly Enriched Uranium; National Academies Press: Washington, DC, USA, 2009; pp. 55–65. [Google Scholar]
- Sameh, A.H. Production Cycle for Large Scale Fission Mo-99 Separation by the Processing of Irradiated LEU Uranium Silicide Fuel Element Targets. Sci. Technol. Nucl. 2013, 14, 704846. [Google Scholar] [CrossRef]
- Wymer, R.G.; Blanco, R.E. Uranium-aluminum alloy dissolution. Ind. Eng. Chem. 1957, 49, 59–61. [Google Scholar] [CrossRef]
- Ethier, A.; Whynot, J.; O’Connor, N.; Briden, N. Evaluation of Potential Mercury Releases from Medical Isotope Waste. CNL Nucl. Rev. 2014, 2, 33–40. [Google Scholar] [CrossRef]
- Bond, M.J.; Silke, R.; Stuart, M.; Carr, J.; Rowan, D.J. A weight-of-evidence approach to the assessment of ecological risk from historical contamination of Ottawa river sediments near Chalk River Laboratories. CNL Nucl. Rev. 2015, 4, 155–170. [Google Scholar] [CrossRef]
- Reynier, N.; Lastra, R.; Laviolette, C.; Bouzoubaâ, N.; Chapman, M. Optimization and validation of a chemical process for uranium, mercury and cesium leaching from cemented radioactive wastes. CNL Nucl. Rev. 2015, 4, 131–139. [Google Scholar] [CrossRef]
- Abowslama, E.; Ebraheem, E.; Sam, A.K. Precipitation and purification of uranium from rock phosphate. J. Radioanal. Nucl. Chem. 2014, 299, 815–818. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, V.M.; Pranesh, S.R.; Chakravarty, A.B. Study of an improved technique for precipitation of uranium from eluted solution. Hydrometallurgy 2004, 71, 429–434. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.N.; Manchanda, V.K.; Husain, M.; Prasad, A.K.; Parmar, V.S. N,N,N′,N′-Tetraoctyl Diglycolamide (TODGA): A Promising Extractant for Actinide-Partitioning from High-Level Waste (HLW). Solvent Extr. Ion Exch. 2005, 23, 463–479. [Google Scholar] [CrossRef]
- Patil, A.B.; Pathak, P.N.; Shinde, V.S.; Alyapyshev, M.Y.; Babain, V.A.; Mohapatra, P.K. A novel solvent system containing a dipicolinamide in room temperature ionic liquids for actinide ion extraction. J. Radioanal. Nucl. Chem. 2015, 305, 521–528. [Google Scholar] [CrossRef]
- Jensen, M.P.; Chiarizia, R.; Ulicki, J.S.; Spindler, B.D.; Murphy, D.J.; Hossain, M.M.; Roca-Sabio, A.; de Blas, A.; Rodríguez-Blas, T. Solvent Extraction Separation of Trivalent Americium from Curium and the Lanthanides. Solvent Extr. Ion Exch. 2015, 33, 329–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Huang, X.; Wang, C.; Zhu, Z.; Zhang, G. Synergistic extraction of rare earths by mixture of HDEHP and HEH/EHP in sulfuric acid medium. J. Rare Earth 2008, 26, 688–692. [Google Scholar] [CrossRef]
- Sood, D.D.; Patil, S.K. Chemistry of nuclear fuel reprocessing: Current status. J. Radioanal. Nucl. Chem. 1996, 203, 547–573. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ferella, F.; De Michelis, I.; Vegliò, F. Treatment of fluid catalytic cracking spent catalysts to recover lanthanum and cerium: Comparison between selective precipitation and solvent extraction. J. Ind. Eng. Chem. 2015, 24, 92–97. [Google Scholar] [CrossRef]
- Chan, G.Y.; Drew, M.G.; Hudson, M.J.; Iveson, P.B.; Liljenzin, J.O.; Skålberg, M.; Spjuth, L.; Madic, C. Solvent extraction of metal ions from nitric acid solution using N,N′-substituted malonamides. Experimental and crystallographic evidence for two mechanisms of extraction, metal complexation and ion-pair formation. J. Chem. Soc. Dalton 1997, 4, 649–660. [Google Scholar] [CrossRef]
- El-Nadi, Y.A.; El-Hefny, N.E.; Aly, H.F. Solvent extraction and recovery of Y(III) and Yb(III) from fluorspar mineral. Int. J. Miner. Met. Mater. 2013, 20, 713–719. [Google Scholar] [CrossRef]
- Preston, J.S.; du Preez, A.C. Solvent extraction of platinum-group metals from hydrochloric acid solutions by dialkyl sulphoxides. Solvent Extr. Ion Exch. 2002, 20, 359–374. [Google Scholar] [CrossRef]
- Shimojo, K.; Nakai, A.; Okamura, H.; Saito, T.; Ohashi, A.; Naganawa, H. Comprehensive extraction study using N, N-dioctyldiglycolamic acid. Anal. Sci. 2014, 30, 513–517. [Google Scholar] [CrossRef]
- Su, D.; Liu, Y.; Li, S.; Ding, S.; Jin, Y.; Wang, Z.; Hu, X.; Zhang, L. Selective Extraction of Americium(III) over Europium(III) Ions with Pyridylpyrazole Ligands: Structure–Property Relationships. Eur. J. Inorg. Chem. 2017, 3, 651–658. [Google Scholar] [CrossRef]
- Yuezhoua, W.; Ruiqina, L.; Yana, W.; Jianhuaa, Z.; Xinpenga, W.; Zia, C. Chromatographic Separation of Actinides and Fission Products. Energy Procedia 2013, 39, 110–119. [Google Scholar]
- Sharma, B.K.; Rajamani, P.; Mathur, P.K. Use of type-II strong base anion exchange resins for ion exchange chromatographic separation of isotopes of boron. Indian J. Chem. Technol. 1997, 4, 308–316. [Google Scholar]
- McGarvey, F.X.; Ungar, J. The Influence of Resin Functional Group on the Ion- Exchange Recovery of Uranium. J. S. Afr. I Min. Metall. 1981, 81, 93–100. [Google Scholar]
- Strelow, F.W.E.; Bothma, C.J.C. Anion Exchange and a Selectivity Scale for Elements in Sulfuric Acid Media with a Strongly Basic Resin Anal. Chem. 1967, 39, 595–599. [Google Scholar]
- Hashikin, N.A.A.; Yeong, C.H.; Abdullah, B.J.J.; Ng, K.H.; Chung, L.Y.; Dahalan, R.; Perkins, A.C. Neutron Activated Samarium-153 Microparticles for Transarterial Radioembolization of Liver Tumour with Post-Procedure Imaging Capabilities. PLoS ONE 2015, 10, e0138106. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Nelson, F. Anion-exchange Studies. XXV. The Rare Earth in Nitrate Solutions. J. Phys. Chem. 1959, 63, 77–79. [Google Scholar] [CrossRef]
- Kadous, A.; Didi, M.A.; Villemin, D. Removal of uranium(VI) from acetate medium using Lewatit TP 260 resin. J. Radioanal. Nucl. Chem. 2011, 288, 553–561. [Google Scholar] [CrossRef]
- Esma, B.; Omar, A.; Amine, D.M. Comparative study on lanthanum(III) sorption onto Lewatit TP 207 and Lewatit TP 260. J. Radioanal. Nucl. Chem. 2014, 299, 439–446. [Google Scholar] [CrossRef]
- Yuchi, A.; Sato, T.; Morimoto, Y.; Mizuno, H.; Wada, H. Adsorption Mechanism of Trivalent Metal Ions on Chelating Resins Containing Iminodiacetic Acid Groups with Reference to Selectivity. Anal. Chem. 1997, 69, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rösch, F. Processing of Generator-Produced 68Ga for Medical Application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Torralvo, F.A.; Fernández-Pereira, C. Recovery of germanium from real fly ash leachates by ion-exchange extraction. Miner. Eng. 2011, 24, 35–41. [Google Scholar] [CrossRef]
- Reynier, N.; Lastra, R.; Laviolette, C.; Fiset, J.F.; Bouzoubaâ, N.; Chapman, M. Comparison of Uranium Recovery by Ion Exchange from Sulfuric Acid Leaching solution in Iodide and Chloride Media. Solvent Extr. Ion Exch. 2016, 34, 188–200. [Google Scholar] [CrossRef]
- Lewatit MonoPlus TP260. Product Information. Edition: 2021-08-31. Available online: https://lanxess.com/en/Products-and-Solutions/Products/l/LEWATIT-MonoPlus-TP-260 (accessed on 6 March 2023).
- Pappas, R.S. Sample Preparation Problem Solving for Inductively Coupled Plasma-Mass Spectrometry with Liquid Introduction Systems I. Solubility, Chelation, and Memory Effects. Spectroscopy 2012, 27, 20–31. [Google Scholar] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Denver, CO, USA, 2013; Volume 6, pp. 1–497. [Google Scholar]
- Subramonian, S.; Clifford, D.; Vijjeswarapu, W. Evaluating Ion Exchange for Removing Radium From Groundwater. J. Am. Water Works Ass. 1990, 82, 61–70. [Google Scholar] [CrossRef]
- Trambouze, P. Countercurrent two-phase flow fixed bed catalytic reactors. Chem. Eng. Sci. 1990, 45, 2269–2275. [Google Scholar] [CrossRef]
- Alguacil, F.J.A. Kinetic study of Cadmium(II) Adsorption on Lewatit TP260 Resin. J. Chem. Res. 2003, 3, 144–146. [Google Scholar] [CrossRef]
- Cerpa, A.; Alguacil, F.J.; Lado, I.; Lopez, A.; Lopez, F.A. Removal of Ni (II) and Co (II) ions from acidic solutions by Lewatit TP-260 resin. Desalin. Water Treat. 2017, 70, 169–174. [Google Scholar] [CrossRef]
- Clever, H.L.; Johnson, S.A.; Derrick, M.E. The solubility of mercury and some sparingly soluble mercury salts in water and aqueous electrolyte solutions. J. Phys. Chem. Ref. Data 1985, 14, 631–680. [Google Scholar] [CrossRef]
- Sawicki, M.; Lecerclé, D.; Grillon, G.; Le Gall, B.; Sérandour, A.L.; Poncy, J.L.; Taran, F. Bisphosphonate sequestering agents. Synthesis and preliminary evaluation for in vitro and in vivo uranium(VI) chelation. Eur. J. Med. Chem. 2008, 43, 2768–2777. [Google Scholar] [CrossRef]
- Vukovic, S.; Hay, B.P.; Bryantsev, V.S. Predicting stability constants for uranyl complexes using density functional theory. Inorg. Chem. 2015, 54, 3995–4001. [Google Scholar] [CrossRef]
- Fan, L.; Huang, G.; Yang, S.; Xie, Y.; Liu, W.; Shi, J. Preparation of phosphate-functionalized biopolymer/graphene oxide gels for enhanced selective adsorption of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2021, 329, 555–564. [Google Scholar] [CrossRef]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Lapidus, L.; Amundson, N.R. Mathematics of adsorption in beds. The effect of longitudinal diffusion in ion exchange and chromatographic columns. J. Phys. Chem 1952, 56, 984–988. [Google Scholar] [CrossRef]
- Nesterenko, P.N.; Shaw, M.J.; Hill, S.J.; Jones, P. Aminophosphonate-functionalized silica: A versatile chromatographic stationary phase for high-performance chelation ion chromatography. Microchem. J. 1999, 62, 58–69. [Google Scholar] [CrossRef]
| Instrumental Parameters | ICP-OES | ICP-MS |
|---|---|---|
| RF Power (W) | 1250 | 1500 |
| Plasma gas flow (L/min) | 15 | 14 |
| Auxiliary gas flow (L/min) | 1.5 | 0.8 |
| Nebulizer gas flow (L/min) | 0.75 | 0.78 |
| Replicates | 3 | 3 |
| Replicate Time (s) | 10 | 30 |
| Stabilization Time (s) | 30 | 10 |
| Sample flow rate (rpm) | 14 | 15 |
| Wavelength (nm)/Isotope | Mg(279.553), Al(237.312), Ca(422.673), Fe(238.204), In(190.794), Hg(184.887), U(385.957), Tl(190.794), In(230.606), Ar(737.212) | 24Mg, 27Al, 44Ca, 56Fe, 200Hg, 202Hg 232Th, 238U 103Rh, 115In |
| SCRW Leaching Solution | KI a (M) | KI a (g/L) | U (mg/L) | Hg (mg/L) | ||
|---|---|---|---|---|---|---|
| Kinetics | Column | Kinetics | Column | |||
| SCRWL-[KI]int—0.12 | 0.12 | 20 | 137 | 164 | 72 | 74 |
| SCRWL-[KI]int—0.15 | 0.15 | 25 | - | 152 | - | 87 |
| SCRWL-[KI]int—0.18 | 0.18 | 30 | 134 | 186 | 118 | 82 |
| SCRWL-[KI]int—0.24 | 0.24 | 40 | 107 | 168 | 104 | 62 |
| Parameter | Concentration (mol/L) | Concentration (mg/L) |
|---|---|---|
| U(VI) | 0.0004 | 95.2 |
| Hg2+ | 0.0005 | 100 |
| Fe3+ | 0.013 | 726 |
| Al3+ | 0.056 | 1511 |
| Mg2+ | 0.0061 | 148 |
| K+ | 0.015; 0.15; 0.30 | 587; 5865; 11,730 |
| I− | 0.015; 0.15; 0.30 | 1904; 19,036; 38,071 |
| N(V) | 0.072 | 1008.5 |
| S(VI) | 1 | 32,660 |
| Ca2+ | 0.5 * | 20,039 |
| pH | 1.82 | 1.82 |
| Sample Identification | Concentration | |||
|---|---|---|---|---|
| U (mg/L) | Hg (mg/L) | Ca (mg/L) | KI (M) | |
| SCRWL’-0.15-U | 115 | - | 351 | 0.15 |
| SCRWL’-0.15-U.Hg | 100 | 97 | 317 | 0.15 |
| U Extraction (%) | Hg Extraction (%) | |||
|---|---|---|---|---|
| Bed Volume | Mean Uptake (C/C0) | Standard Deviation | Mean Uptake (C/C0) | Standard Deviation |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5 | 0.37 | 0.09 | 10.32 | 1.39 |
| 10 | 0.75 | 0.18 | 41.69 | 5.87 |
| 15 | 6.74 | 4.06 | 90.51 | 2.23 |
| 20 | 16.51 | 1.97 | 105.02 | 3.20 |
| 25 | 29.84 | 3.59 | 107.62 | 1.28 |
| 30 | 42.49 | 4.47 | 104.97 | 5.88 |
| 35 | 55.31 | 3.34 | 102.76 | 4.45 |
| 40 | 60.54 | 5.52 | 107.42 | 5.64 |
| 45 | 69.72 | 3.36 | 101.76 | 2.83 |
| 50 | 73.91 | 3.09 | 100.86 | 4.10 |
| Cycles | Steps | U (% ± STD) | Hg (% ± STD) | Fe (% ± STD) |
|---|---|---|---|---|
| 1 | Adsorption | 87 ± 1% | 64 ± 1% | 18 ± 2% |
| Desorption | 82 ± 5% | 5 ± 1% | 0% | |
| 2 | Adsorption | 75 ± 2% | 56 ± 4% | 54 ± 4% |
| Desorption | 80 ± 13% | 5 ± 1% | 0% | |
| 3 | Adsorption | 62 ± 2% | 29 ± 9% | 56 ± 1% |
| Desorption | 82 ± 14% | 6 ± 2% | 0% | |
| 4 | Adsorption | 58 ± 1% | 26 ± 1% | 44 ± 6% |
| Desorption | 84 ± 3% | 6 ± 1% | 0% | |
| 5 | Adsorption | 54 ± 1% | 22 ± 1% | 50 ± 1% |
| Desorption | 84 ± 7% | 5 ± 1% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courchesne, M.; Couture, R.-M.; Basque, J.; Reynier, N.; Larivière, D. Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media. Minerals 2023, 13, 405. https://doi.org/10.3390/min13030405
Courchesne M, Couture R-M, Basque J, Reynier N, Larivière D. Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media. Minerals. 2023; 13(3):405. https://doi.org/10.3390/min13030405
Chicago/Turabian StyleCourchesne, Maxime, Raoul-Marie Couture, Justine Basque, Nicolas Reynier, and Dominic Larivière. 2023. "Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media" Minerals 13, no. 3: 405. https://doi.org/10.3390/min13030405
APA StyleCourchesne, M., Couture, R.-M., Basque, J., Reynier, N., & Larivière, D. (2023). Co-Extraction of Uranium and Mercury Using Ion Exchange from Cemented Radioactive Waste Sulfuric Leachate in Iodide Media. Minerals, 13(3), 405. https://doi.org/10.3390/min13030405





