The Effect of Microwave Pre-treatment on the Magnetic Properties of Enargite and Tennantite and Their Separation from Chalcopyrite
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Microwave Pre-Treatments
2.2.2. Magnetic Susceptibility Experiments
2.2.3. X-ray Photoelectron Spectroscopy (XPS)
2.2.4. Magnetic Separation Experiments
3. Results
3.1. Magnetic Susceptibility
3.2. XRF Analysis
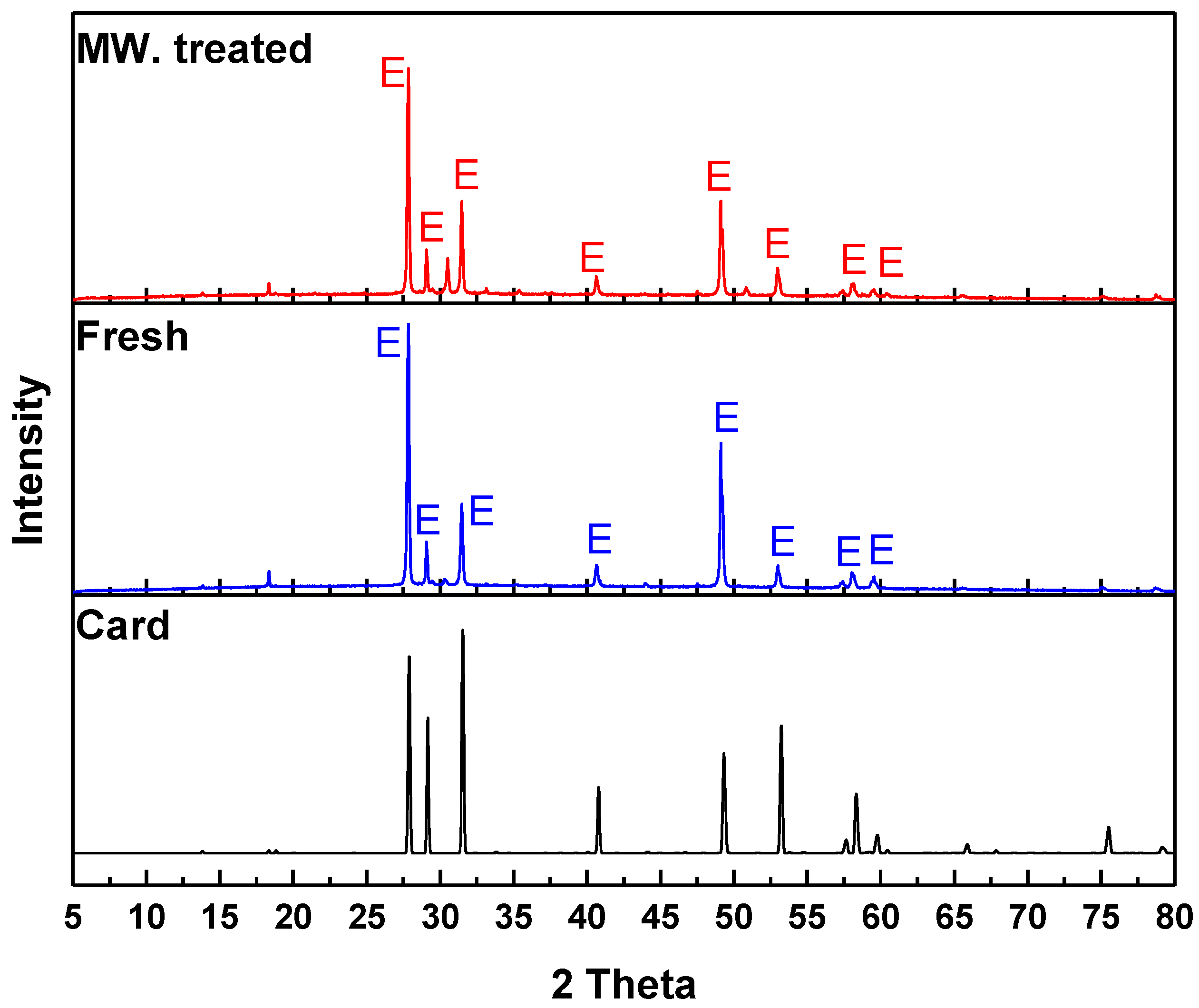
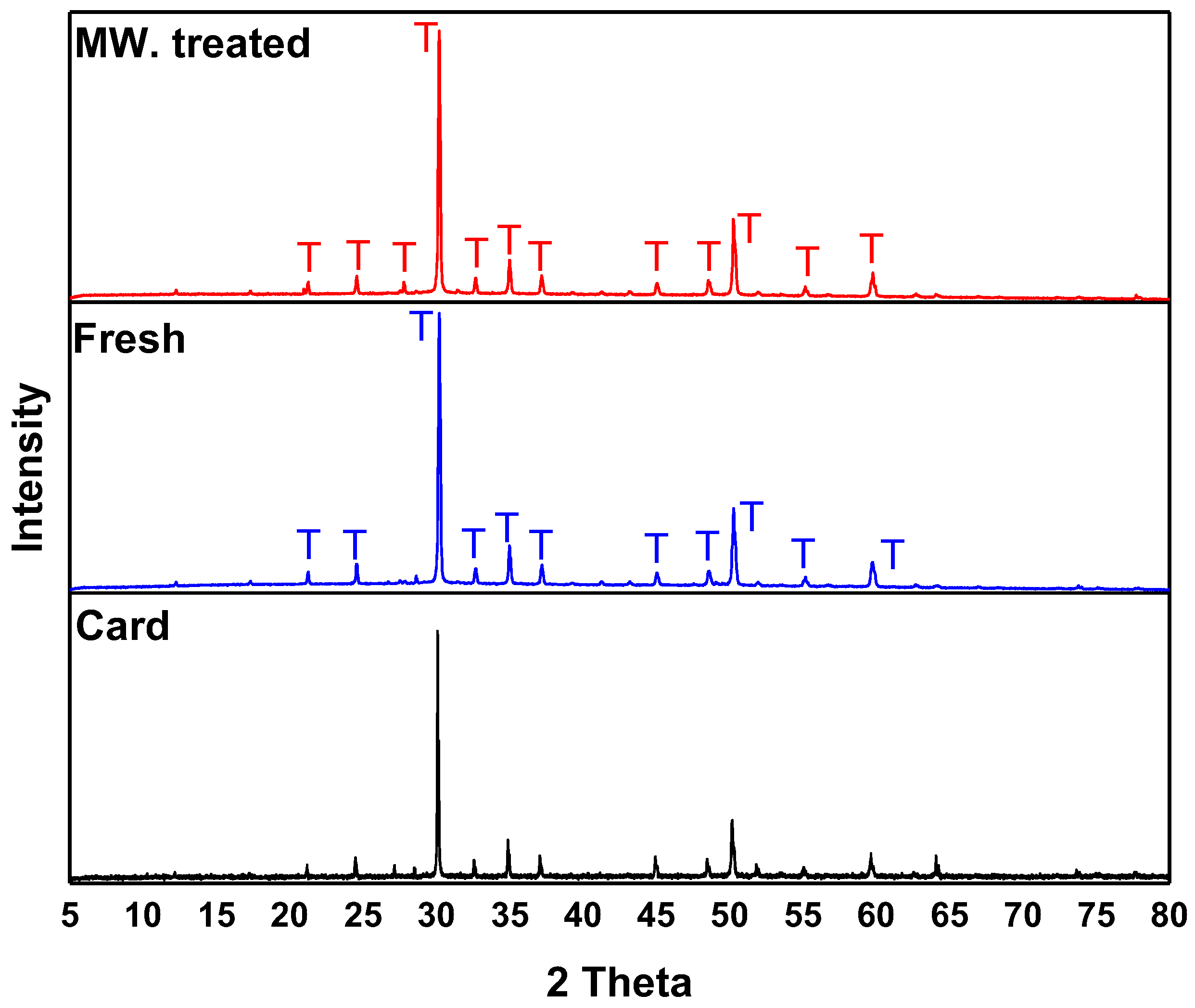
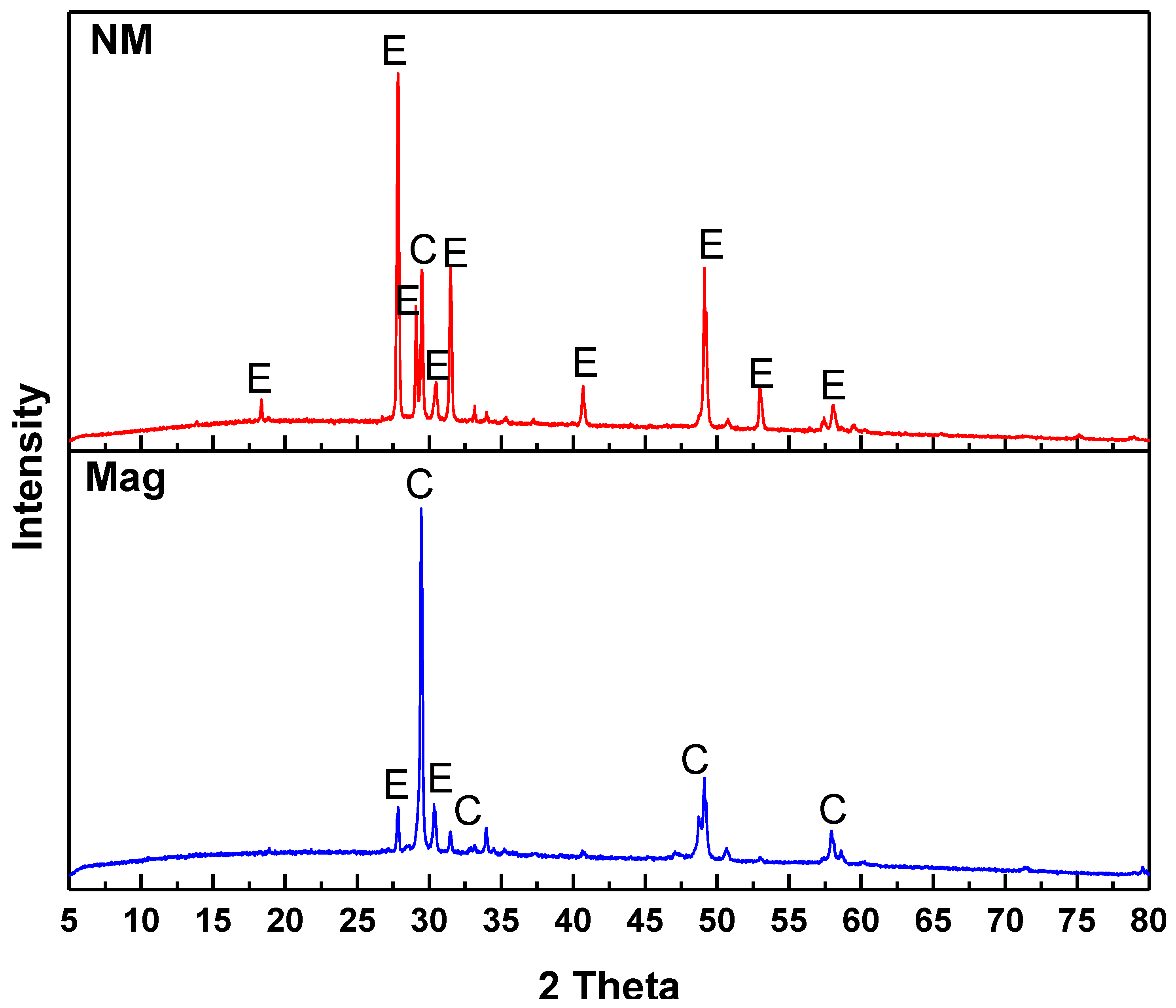
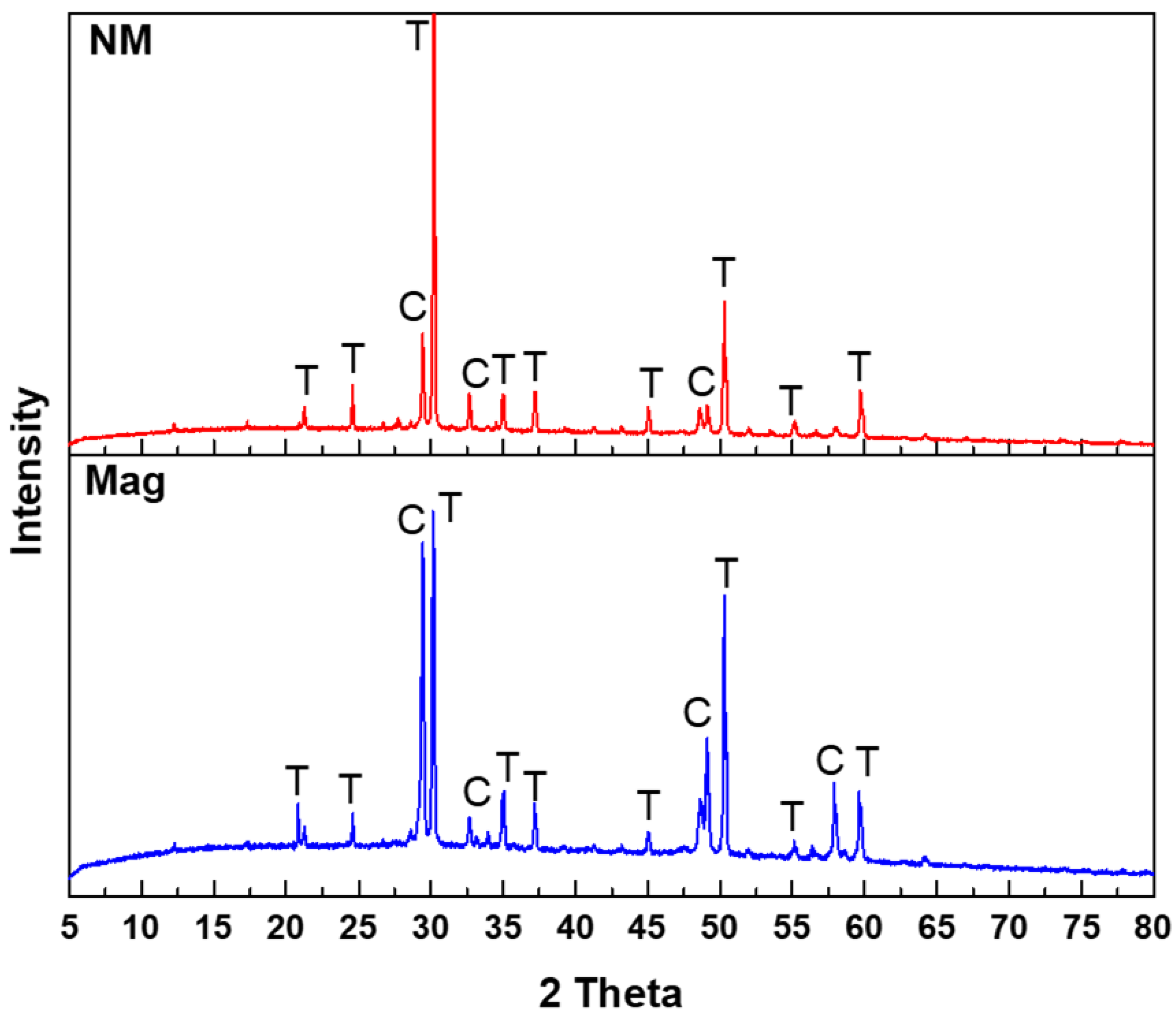
3.3. XRD Analysis
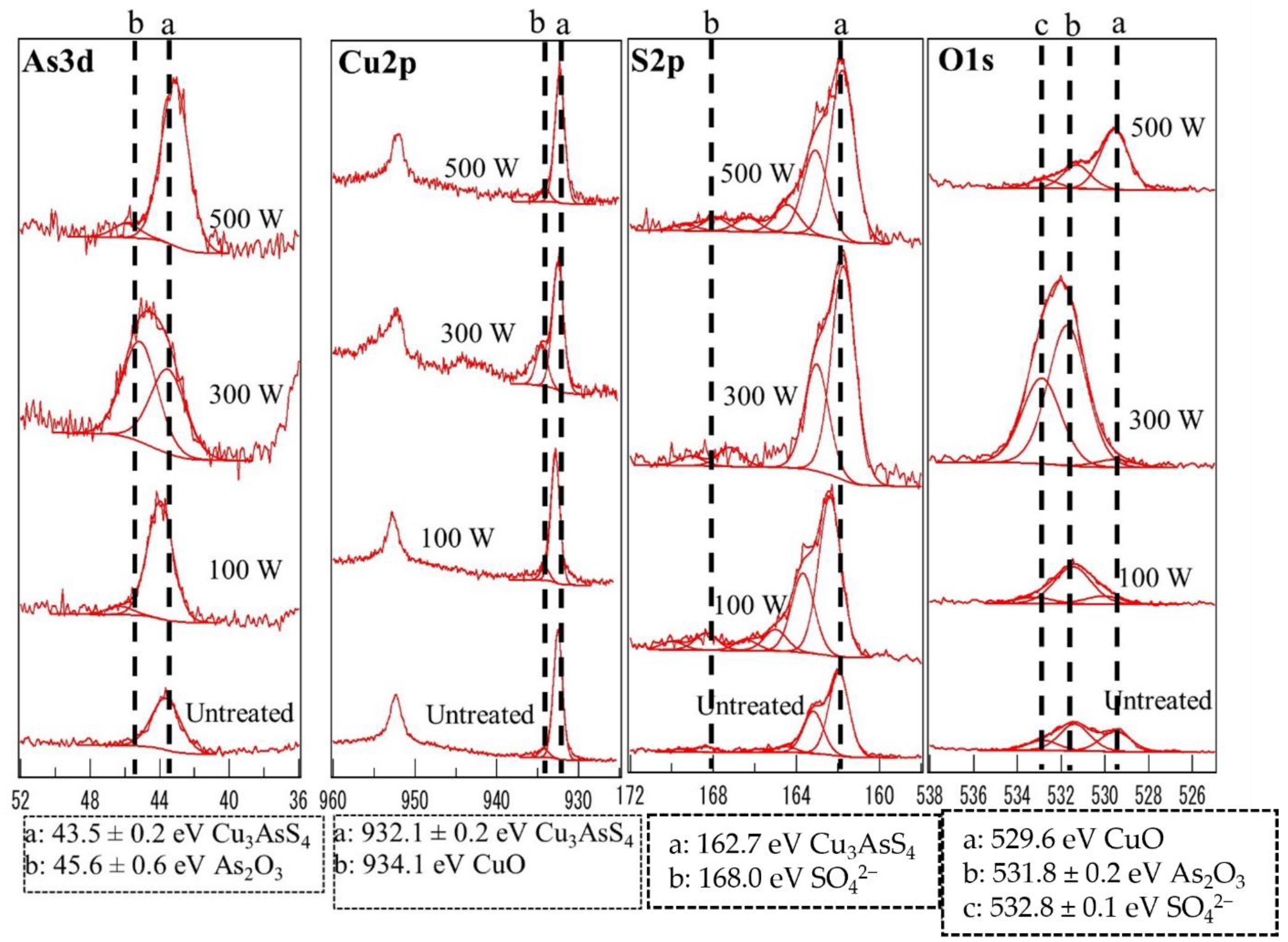
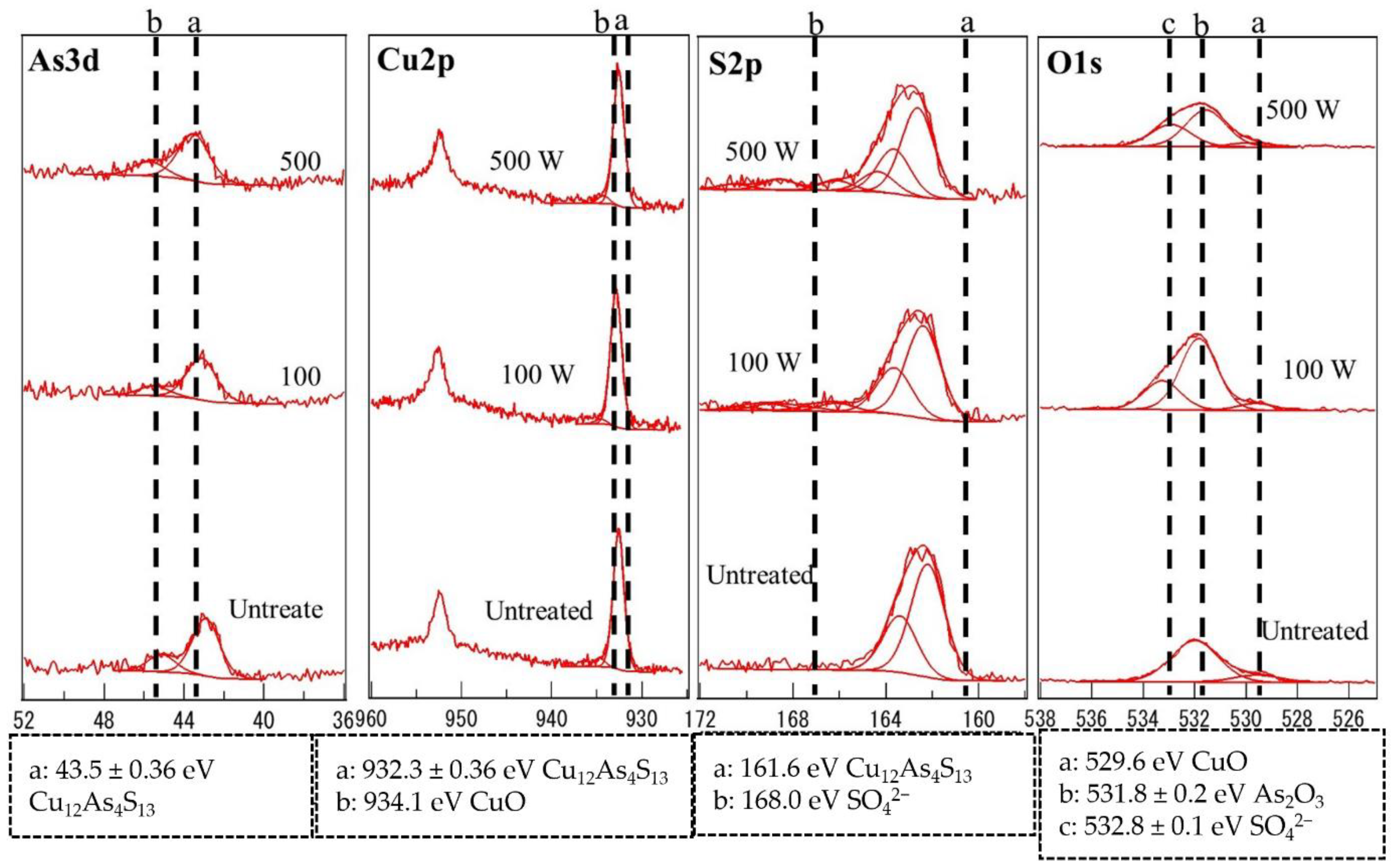
3.4. X-ray Photoelectron Spectroscopy
3.5. Magnetic Separation Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Peng, Y. The Effect of Saline Water on Mineral Flotation—A Critical Review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of H2O2 and Potassium Amyl Xanthate on Separation of Enargite and Tennantite from Chalcopyrite and Bornite Using Flotation. Miner. Eng. 2020, 152, 106371. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Zhang, B.; Jiao, F.; Qin, W. Flotation Separation of Enargite from Complex Copper Concentrates by Selective Surface Oxidation. Physicochem. Probl. Miner. Process. 2019, 55, 852–864. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, K. Pre-Concentration of Iron-Rich Sphalerite by Magnetic Separation. Minerals 2018, 8, 272. [Google Scholar] [CrossRef]
- Gholami, H.; Rezai, B.; Mehdilo, A.; Hassanzadeh, A.; Yarahmadi, M. Effect of Microwave System Location on Floatability of Chalcopyrite and Pyrite in a Copper Ore Processing Circuit. Physicochem. Probl. Miner. Process. 2020, 56, 432–448. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Dash, N.; Dhawan, N.; Rath, S.S. Microwave-Assisted Reduction Roasting—Magnetic Separation Studies of Two Mineralogically Different Low-Grade Iron Ores. Int. J. Miner. Metall. Mater. 2020, 27, 1449–1461. [Google Scholar] [CrossRef]
- Farahat, M.; Elmahdy, A.M.; Hirajima, T. Influence of Microwave Radiation on the Magnetic Properties of Molybdenite and Arsenopyrite. Powder Technol. 2017, 315, 276–281. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; Farahat, M.; Hirajima, T. Comparison between the Effect of Microwave Irradiation and Conventional Heat Treatments on the Magnetic Properties of Chalcopyrite and Pyrite. Adv. Powder Technol. 2016, 27, 2424–2431. [Google Scholar] [CrossRef]
- Stec, W.J.; Morgan, W.E.; Albridge, R.G.; Van Wazer, J.R. Measured Binding Energy Shifts of the “3p” and “3d” Electrons1 in Arsenic Compounds. Inorg. Chem. 1972, 11, 219–225. [Google Scholar] [CrossRef]
- Rossi, A.; Atzei, D.; Da Pelo, S.; Frau, F.; Lattanzi, P.; England, K.E.R.; Vaughan, D.J. Quantitative X-ray Photoelectron Spectroscopy Study of Enargite (Cu3AsS4) Surface. Surf. Interface Anal. 2001, 31, 465–470. [Google Scholar] [CrossRef]
- Capece, F.M.; Di Castro, V.; Furlani, C.; Mattogno, G.; Fragale, C.; Gargano, M.; Rossi, M. “Copper Chromite” Catalysts: XPS Structure Elucidation and Correlation with Catalytic Activity. J. Electron Spectros. Relat. Phenom. 1982, 27, 119–128. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Cook, J.M. Interactions of NO and SO2 with Iron Deposited on Silica. J. Colloid Interface Sci. 1985, 104, 250–257. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray Photoelectron Spectroscopy Analysis of Copper and Zinc Oxides and Sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Iwasaki, H.; Nishitani, R.; Nakamura, S. Esca Studies of Ga, As, GaAs, Ga2O3, As2O3 and As2O5. J. Electron Spectros. Relat. Phenom. 1978, 14, 129–141. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS Analysis of Chalcopyrite (CuFeS2) Dissolution in Sulfuric Acid Solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsugi, K.; Ishikura, K.; Hirajima, T. Spectroscopic Study on Oxidative Dissolution of Chalcopyrite, Enargite and Tennantite at Different PH Values. Hydrometallurgy 2010, 100, 144–151. [Google Scholar] [CrossRef]
- Fullston, D.; Fornasiero, D.; Ralston, J. Oxidation of Synthetic and Natural Samples of Enargite and Tennantite: 1. Dissolution and Zeta Potential Study. Langmuir 1999, 15, 4524–4529. [Google Scholar] [CrossRef]
| Power, Watt | Tennantite | Enargite |
|---|---|---|
| 0 | −4.43 × 10−7 | −4.59 × 10−7 |
| 100 | −4.45 × 10−7 | 2.49 × 10−7 |
| 300 | −4.50 × 10−7 | 1.35 × 10−7 |
| 500 | −4.74 × 10−7 | 1.45 × 10−7 |
| Element/Mineral | Enargite | Tennantite | Chalcopyrite |
|---|---|---|---|
| Cu | 46.37 | 40.2 | 39.7 |
| As | 22.4 | 15.42 | 0.01 |
| Fe | 2.28 | 1.56 | 27.12 |
| Product | Wt., % | Assay, % | Distribution, % | ||||
|---|---|---|---|---|---|---|---|
| Cu | As | Fe | Cu | As | Fe | ||
| Feed mixture | 100 | 44.0 | 11.4 | 14.1 | - | - | - |
| Mag | 38 | 42.0 | 4.8 | 21.6 | 36.3 | 15.8 | 60.3 |
| N-Mag | 62 | 45.1 | 15.7 | 8.72 | 63.7 | 84.2 | 39.7 |
| Calculated feed | 100 | 43.9 | 11.6 | 13.6 | 100 | 100 | 100 |
| Product | Wt., % | Assay, % | Distribution, % | ||||
|---|---|---|---|---|---|---|---|
| Cu | As | Fe | Cu | As | Fe | ||
| Feed mixture | 100 | 40.5 | 7.4 | 14.5 | - | - | - |
| Mag | 49 | 38.4 | 3.6 | 22.3 | 49.4 | 23.7 | 74.3 |
| N-Mag | 51 | 37.8 | 11.1 | 7.4 | 50.6 | 76.3 | 25.7 |
| Calculated feed | 100 | 38.1 | 7.4 | 14.7 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmahdy, A.M.; Miki, H.; Sasaki, K.; Farahat, M. The Effect of Microwave Pre-treatment on the Magnetic Properties of Enargite and Tennantite and Their Separation from Chalcopyrite. Minerals 2023, 13, 334. https://doi.org/10.3390/min13030334
Elmahdy AM, Miki H, Sasaki K, Farahat M. The Effect of Microwave Pre-treatment on the Magnetic Properties of Enargite and Tennantite and Their Separation from Chalcopyrite. Minerals. 2023; 13(3):334. https://doi.org/10.3390/min13030334
Chicago/Turabian StyleElmahdy, Ahmed M., Hajime Miki, Keiko Sasaki, and Mohsen Farahat. 2023. "The Effect of Microwave Pre-treatment on the Magnetic Properties of Enargite and Tennantite and Their Separation from Chalcopyrite" Minerals 13, no. 3: 334. https://doi.org/10.3390/min13030334
APA StyleElmahdy, A. M., Miki, H., Sasaki, K., & Farahat, M. (2023). The Effect of Microwave Pre-treatment on the Magnetic Properties of Enargite and Tennantite and Their Separation from Chalcopyrite. Minerals, 13(3), 334. https://doi.org/10.3390/min13030334







