Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis
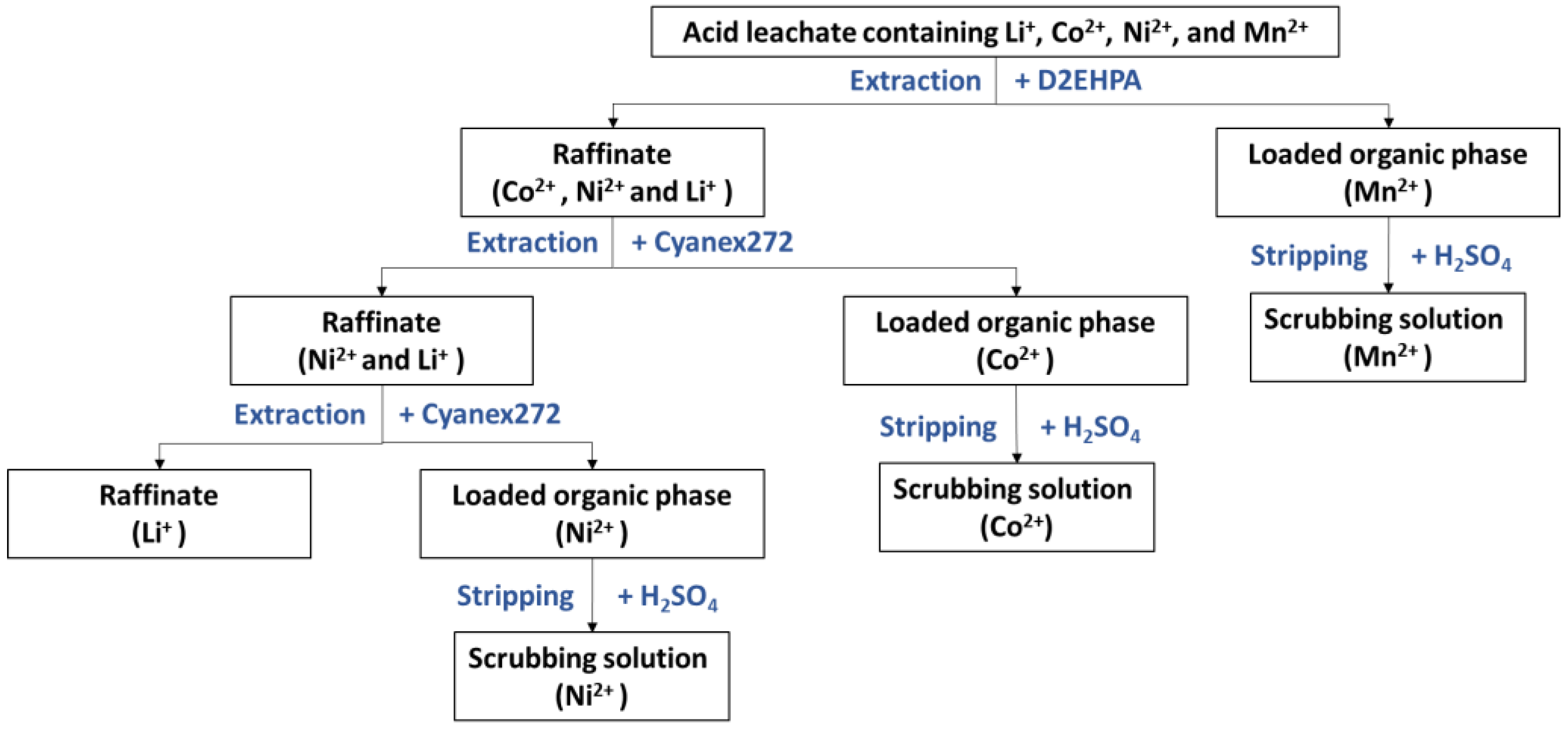
2.3. Solvent Extraction Procedure
2.4. Stripping Procedure
3. Results and Discussion
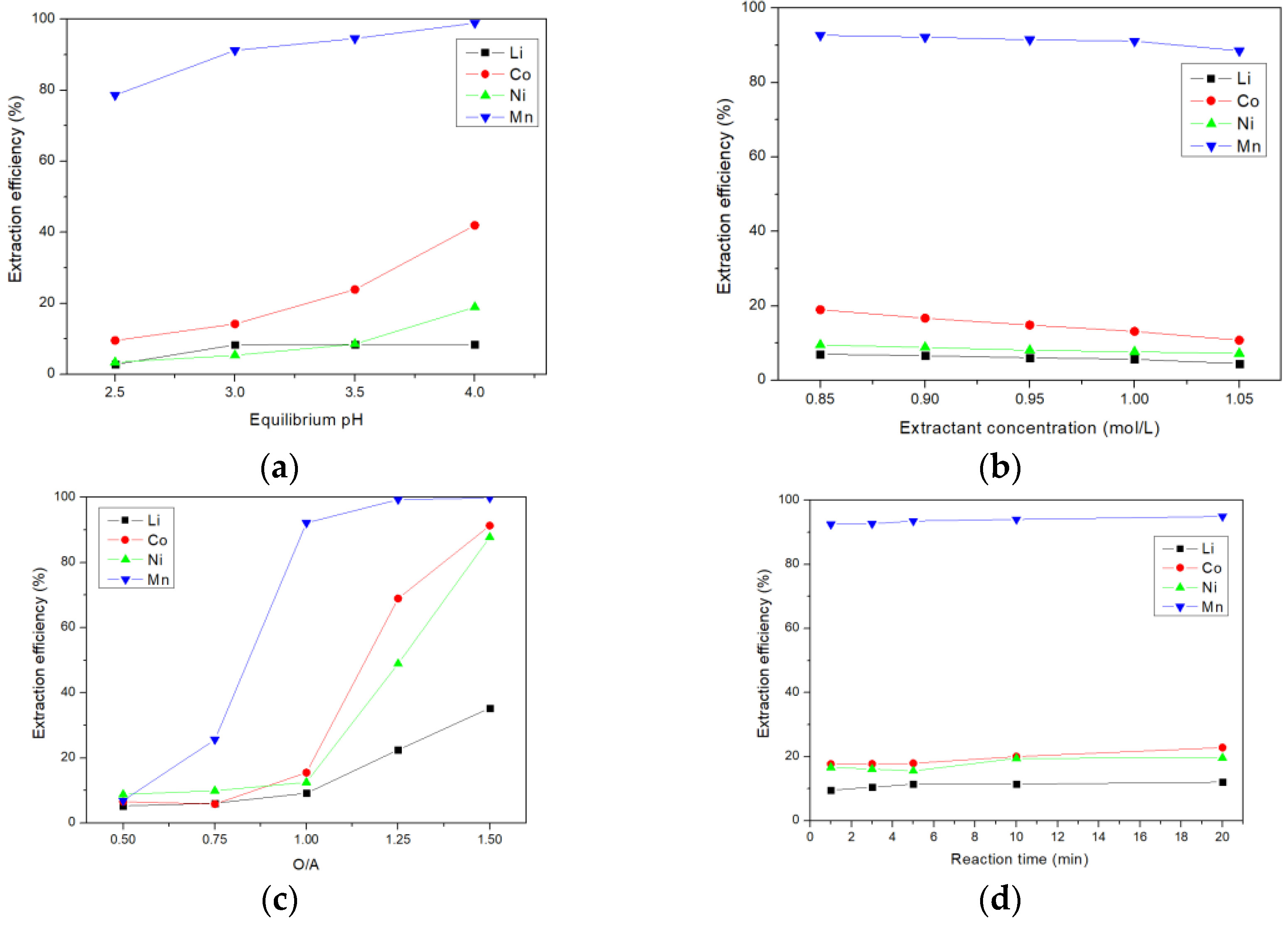
3.1. Extraction of Manganese
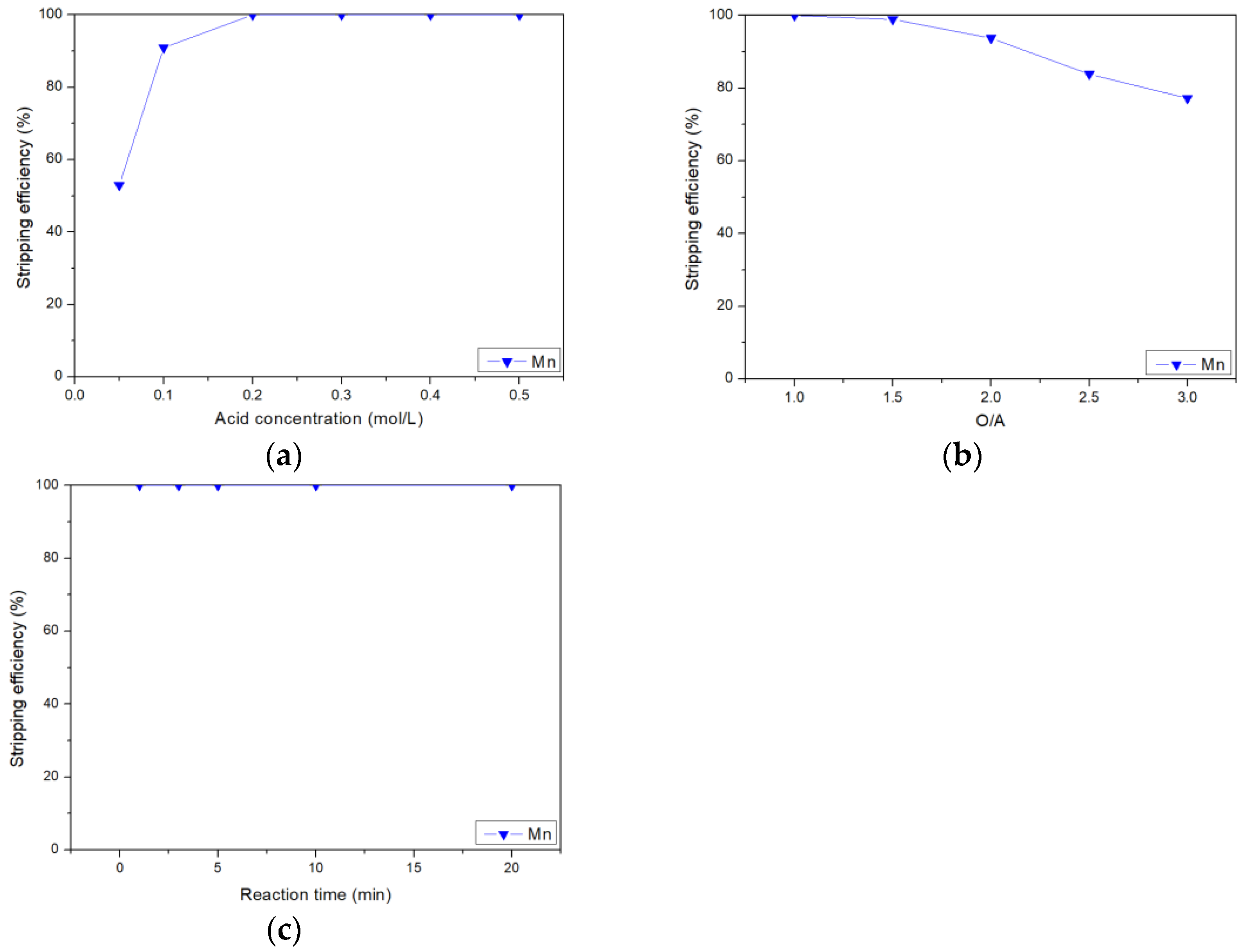
3.2. Stripping of Manganese
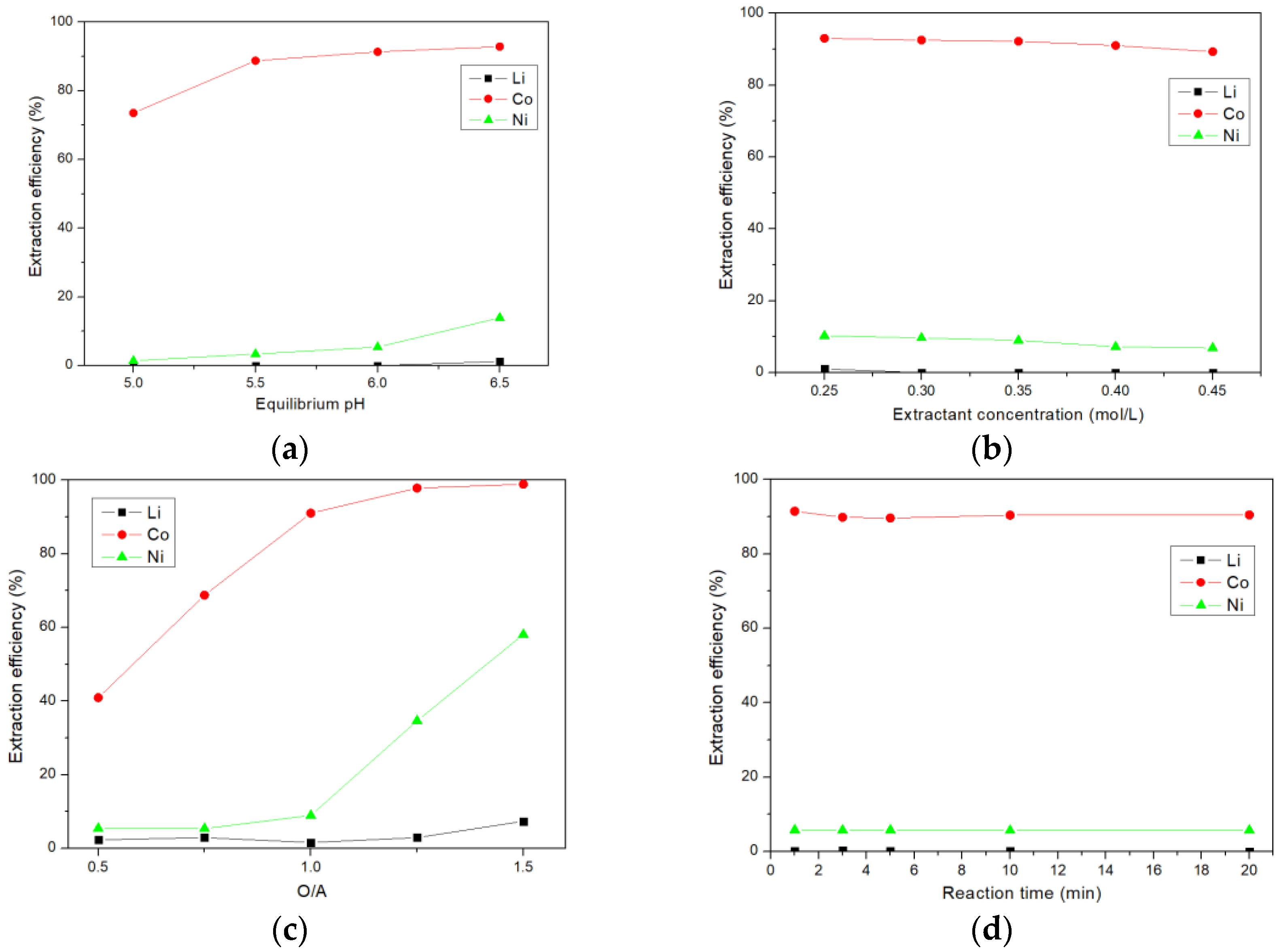
3.3. Extraction of Cobalt
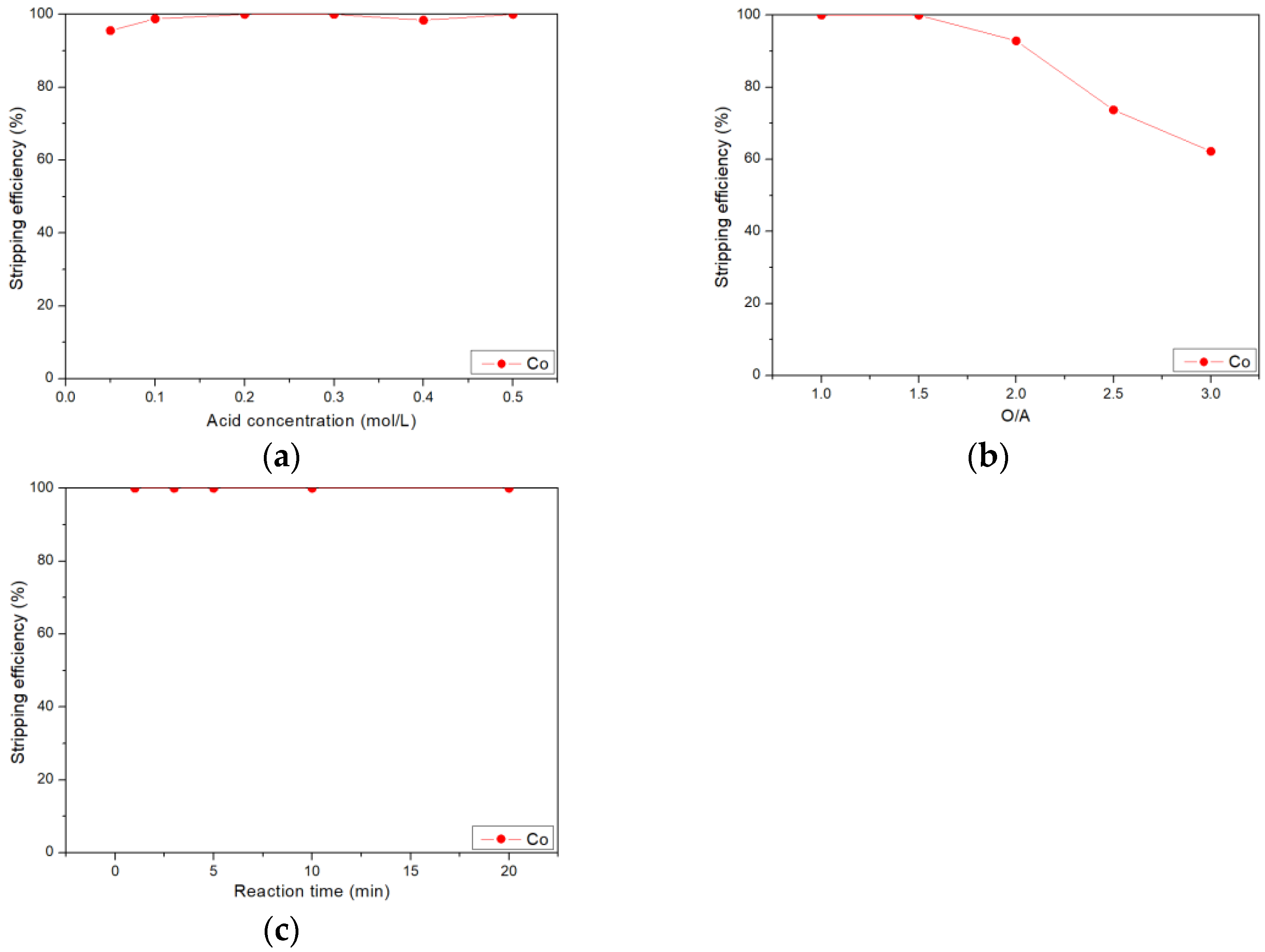
3.4. Stripping of Cobalt
3.5. Extraction of Nickel
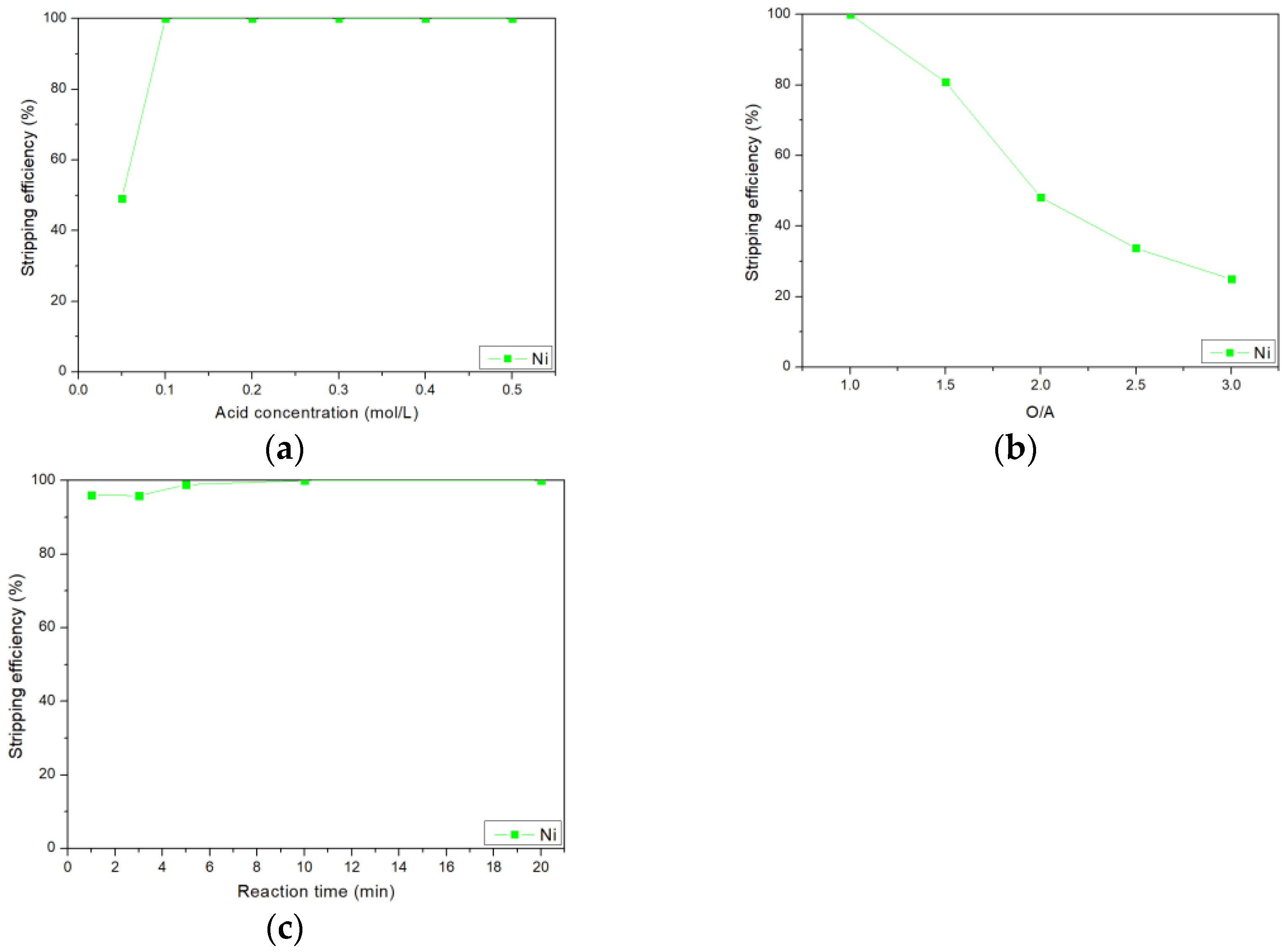
3.6. Stripping of Nickel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Li, C.; Dai, G.; Liu, R.; Wang, C.; Wang, S.; Ju, Y.; Jiang, H.; Jiao, S.; Duan, C. Separation and recovery of nickel cobalt manganese lithium from waste ternary lithium-ion batteries. Sep. Purif. Technol. 2023, 306, 122559. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, L.; Du, J.; Zhang, G.; Cao, Z.; Wu, S. Improving Extraction Performance of D2EHPA for Impurities Removal from Spent Lithium-Ion Batteries Leaching Solution by TPC[4]. ACS Sustain. Chem. Eng. 2022, 10, 4312–4322. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wang, J.Z.; Shen, Y.H. Recycling Metal from Waste Lithium-ion Batteries for Use as Electrochemical Sensor Material. Sens. Mater. 2022, 34, 2025–2035. [Google Scholar] [CrossRef]
- Meshram, P.; Abhilash; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Acid baking of spent lithium ion batteries for selective recovery of major metals: A two-step process. J. Ind. Eng. Chem. 2016, 43, 117–126. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, M.C.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Sarangi, K.; Reddy, B.R.; Das, R.P. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272.: Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Rodrigues, I.R.; Deferm, C.; Binnemans, K.; Riano, S. Separation of cobalt and nickel via solvent extraction with Cyanex-272: Batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction. Sep. Purif. Technol. 2022, 296, 121326. [Google Scholar] [CrossRef]
- Reddy, B.R.; Park, K.H. Process for the Recovery of Cobalt and Nickel from Sulphate Leach Liquors with Saponified Cyanex 272 and D2EHPA. Sep. Sci. Technol. 2007, 42, 2067–2080. [Google Scholar] [CrossRef]
- Lommelen, R.; Binnemans, K. Thermodynamic Modeling of Salting Effects in Solvent Extraction of Cobalt(II) from Chloride Media by the Basic Extractant Methyltrioctylammonium Chloride. ACS Omega 2021, 6, 11355–11366. [Google Scholar] [CrossRef]
- Qi, D. (Ed.) Extractants Used in Solvent Extraction-Separation of Rare Earths: Extraction Mechanism, Properties, and Features. In Hydrometallurgy of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–389. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; Michelis, I.D.; Prisciandaro, M.; Medici, F.; Veglio, F. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J. Power Sources 2017, 362, 202–218. [Google Scholar] [CrossRef]
- Gao, R.; Sun, C.; Xu, L.; Zhou, T.; Zhuang, L.; Xie, H. Recycling LiNi0.5Co0.2Mn0.3O2 material from spent lithium-ion batteries by oxalate co-precipitation. Vacuum 2020, 173, 109181. [Google Scholar] [CrossRef]
- Prabaharan, G.; Barik, S.P.; Kumar, N.; Kumar, L. Electrochemical process for electrode material of spent lithium ion batteries. Waste Manag. 2017, 68, 527–533. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Coll, M.T.; Fortuny, A.; Kedari, C.S.; Sastre, A.M. Studies on the extraction of Co(II) and Ni(II) from aqueous chloride solutions using Primene JMT-Cyanex272 ionic liquid extractant. Hydrometallurgy 2012, 125–126, 24–28. [Google Scholar] [CrossRef]
- Liang, Z.; Cai, C.; Peng, G.; Hu, J.; Hou, H.; Liu, B.; Liang, S.; Xiao, K.; Yuan, S.; Yang, J. Hydrometallurgical Recovery of Spent Lithium Ion Batteries: Environmental Strategies and Sustainability Evaluation. ACS Sustain. Chem. Eng. 2021, 9, 5750–5767. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation of divalent manganese and cobalt ions from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy 2000, 54, 117–131. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C. Manganese metallurgy review. Part II: Manganese separation and recovery from solution. Hydrometallurgy 2007, 89, 160–177. [Google Scholar] [CrossRef]
- Shuya, L.; Yang, C.; Xuefeng, C.; Wei, S.; Yaqing, W.; Yue, Y. Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, H.; Zhong, X.; Cao, H.; Wang, R.; Liu, Z. Recovery of metals from sulfate leach solutions of spent ternary lithium-ion batteries by precipitation with phosphate and solvent extraction with P507. Hydrometallurgy 2022, 210, 105861. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, S.; Song, S.; Sun, W.; Wang, L. Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries. Waste Manag. 2020, 102, 131–138. [Google Scholar] [CrossRef]
- Vieceli, N.; Reinhardt, N.; Ekberg, C.; Petranikova, M. Optimization of Manganese Recovery from a Solution Based on Lithium-Ion Batteries by Solvent Extraction with D2EHPA. Metals 2021, 11, 54. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Yin, Z. Recovery of manganese from sulfuric acid leaching liquor of spent lithium-ion batteries and synthesis of lithium ion-sieve. J. Environ. Chem. Eng. 2018, 6, 6407–6413. [Google Scholar] [CrossRef]
- Hossain, M.R.; Nash, S.; Rose, G.; Alam, S. Cobalt loaded D2EHPA for selective separation of manganese from cobalt electrolyte solution. Hydrometallurgy 2011, 107, 137–140. [Google Scholar] [CrossRef]
- Peng, C.; Chang, C.; Wang, Z.; Wilson, B.P.; Liu, F.; Lundstrom, M. Recovery of High-Purity MnO2 from the Acid Leaching Solution of Spent Li-Ion Batteries. JOM 2020, 72, 790–799. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Keller, A.; Hlawitschka, M.W.; Bart, H.J. Application of saponified D2EHPA for the selective extraction of manganese from spend lithium-ion batteries. Chem. Eng. Process. Process Intensif. 2022, 171, 108552. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Wang, F.; He, F.; Zhao, J.; Sui, N.; Xu, L.; Liu, H. Extraction and separation of cobalt(II), copper(II) and manganese(II) by Cyanex272, PC-88A and their mixtures. Sep. Purif. Technol. 2012, 93, 8–14. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, G.; Luo, M.; Zeng, M. Separation of Co and Mn from acetic acid leaching solution of spent lithium-ion battery by Cyanex272. J. Environ. Chem. Eng. 2022, 10, 108250. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Chen, A.; Zhang, J.; Wu, X.; Xu, J. Comprehensive recovery of NCM cathode materials for spent lithium-ion batteries by microfluidic device. Sep. Purif. Technol. 2022, 294, 121185. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Shen, X.; Perera, J.M.; Stevens, G.W. The effect of DTAC on the extraction of Co(II) with Cyanex272 in a neutral micellar phase. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 109–114. [Google Scholar] [CrossRef]

| Element | Li | Co | Ni | Mn | Fe | Al |
|---|---|---|---|---|---|---|
| wt% | 8.04 | 21.46 | 22.27 | 15.09 | 0.127 | 0.122 |
| Element | Li | Co | Ni | Mn |
|---|---|---|---|---|
| Concentration (ppm) | 1459 | 3333 | 3878 | 2965 |
| Equilibrium pH | 2.5 | 3.0 | 3.5 | 4.0 |
|---|---|---|---|---|
| DMn | 2132 | 1184 | 1491 | 3.6 |
| DCo | 1.1 | 0.4 | 5.0 | 0.9 |
| βMn/Co | 1878 | 2716 | 297 | 4.0 |
| Extractant Concentration | 0.85 | 0.90 | 0.95 | 1.00 | 1.05 |
|---|---|---|---|---|---|
| DMn | 12.70 | 11.82 | 10.79 | 10.29 | 7.73 |
| DCo | 0.23 | 0.20 | 0.18 | 0.15 | 0.21 |
| βMn/Co | 54.24 | 59.00 | 61.84 | 67.88 | 64.08 |
| Extractant Concentration | 0.25 | 0.30 | 0.35 | 0.40 | 0.45 |
|---|---|---|---|---|---|
| DCo | 13.32 | 12.40 | 11.83 | 10.14 | 8.35 |
| DNi | 0.11 | 0.10 | 0.09 | 0.07 | 0.07 |
| βCo/Ni | 116.18 | 116.01 | 120.12 | 130.60 | 113.07 |
| Extractant Concentration | 0.3 | 0.35 | 0.4 | 0.45 | 0.5 |
|---|---|---|---|---|---|
| DNi | 24.06 | 32.11 | 46.17 | 45.94 | 45.95 |
| DLi | 0.01 | 0.002 | 0.0001 | 0.0001 | 0.0001 |
| βNi/Li | 2312 | 15,259 | 461,651 | 459,437 | 459,437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-C.; Wang, J.-Z.; Shen, Y.-H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. https://doi.org/10.3390/min13020285
Tang Y-C, Wang J-Z, Shen Y-H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals. 2023; 13(2):285. https://doi.org/10.3390/min13020285
Chicago/Turabian StyleTang, Yi-Chin, Jian-Zhi Wang, and Yun-Hwei Shen. 2023. "Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction" Minerals 13, no. 2: 285. https://doi.org/10.3390/min13020285
APA StyleTang, Y.-C., Wang, J.-Z., & Shen, Y.-H. (2023). Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals, 13(2), 285. https://doi.org/10.3390/min13020285






