Geological and Geochemical Constraints on the Origin of the Sr Mineralization in Huayingshan Ore District, Chongqing, South China
Abstract
1. Introduction
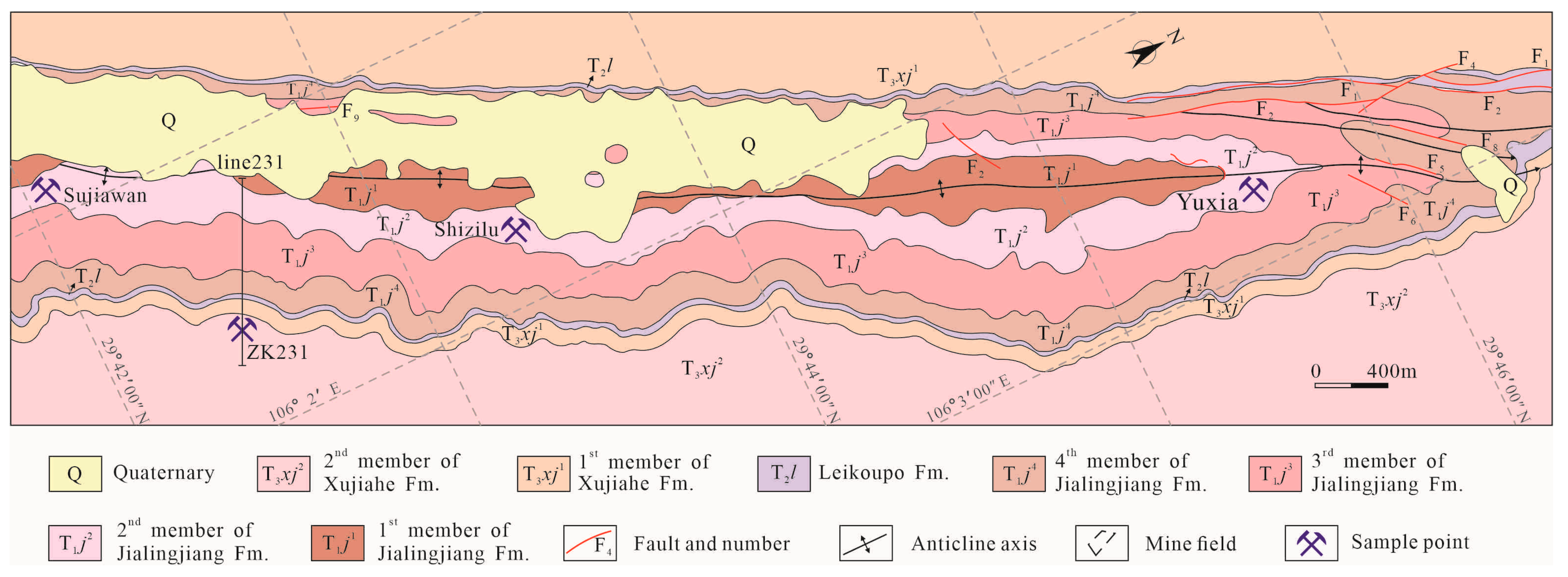
2. Regional Geology
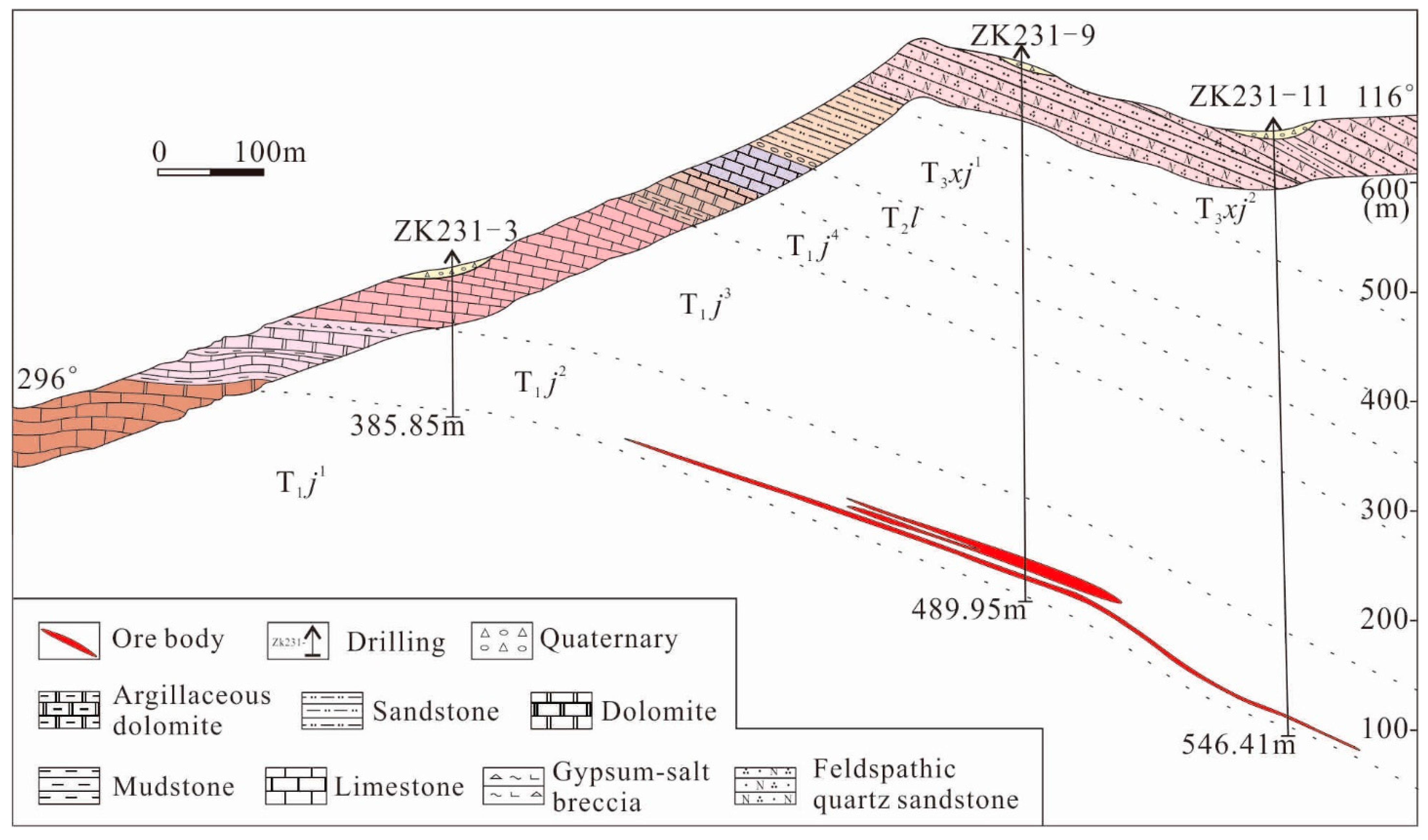
3. Deposit Geology
4. Sampling and Analytical Methods
4.1. Sampling
4.2. Fluid Inclusion Study
4.3. Strontium and Sulfur Isotope
4.4. LA–ICP–MS
5. Analytical Results
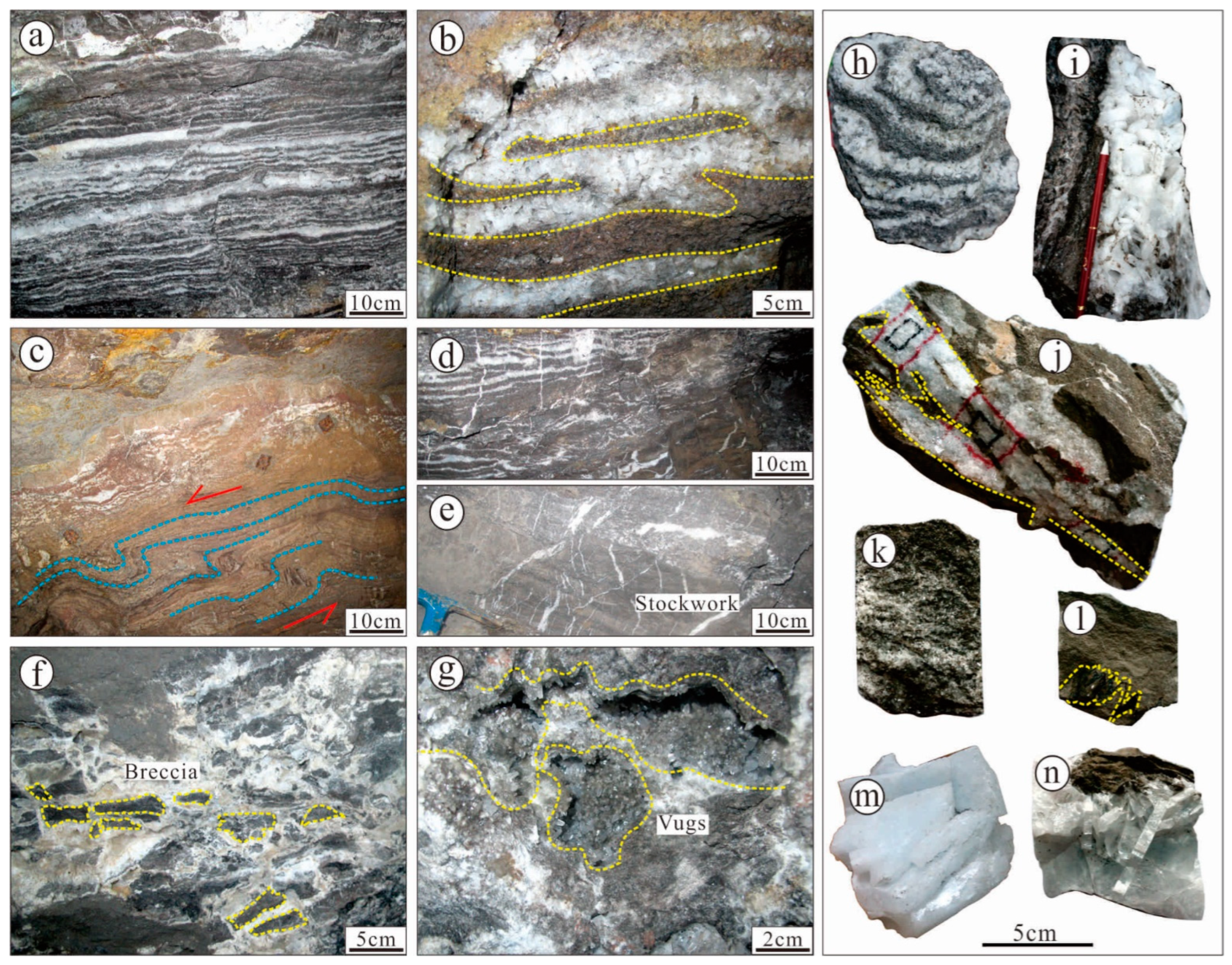
5.1. Fluid Inclusion
5.1.1. Fluid Inclusion Petrography
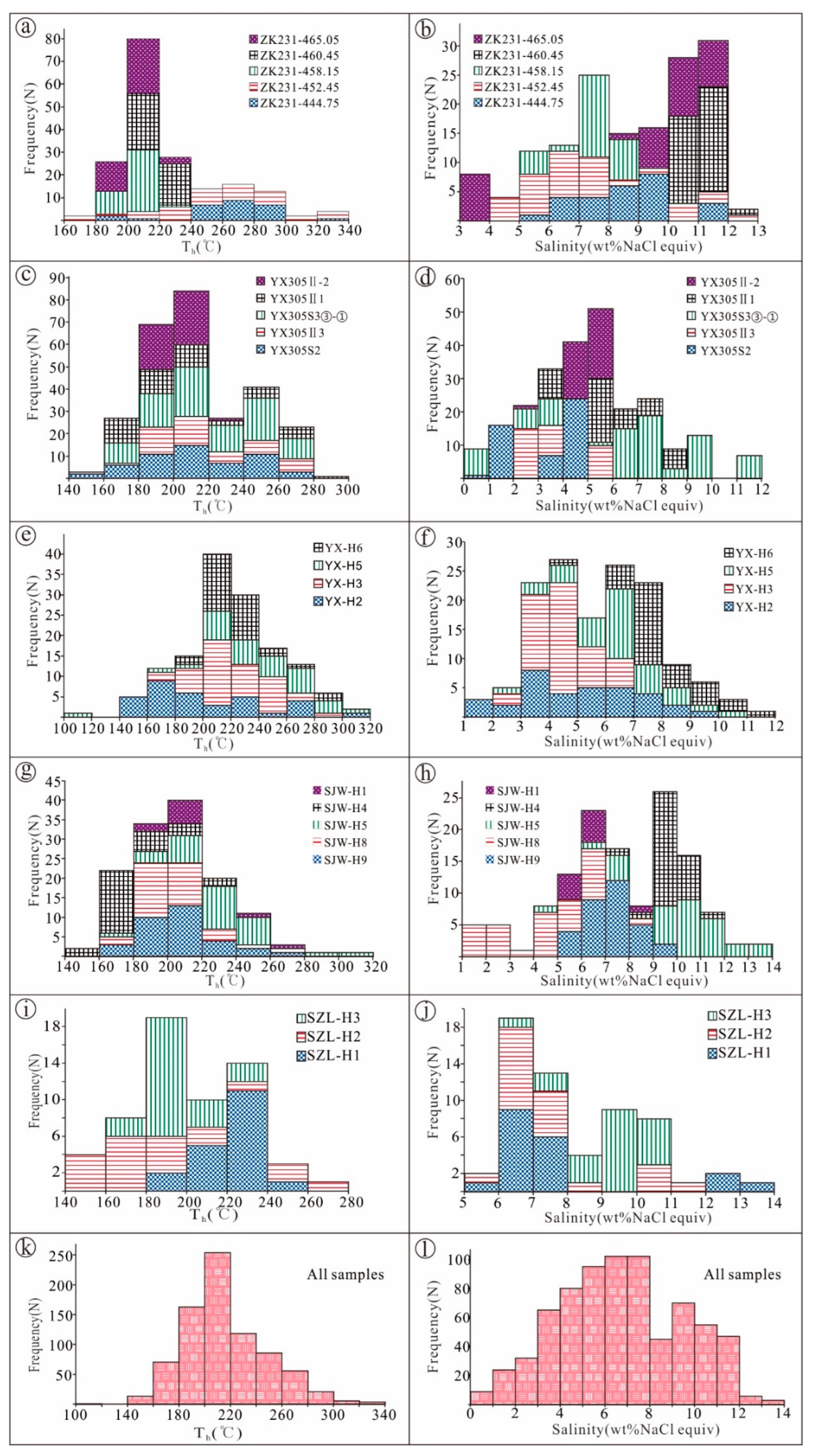
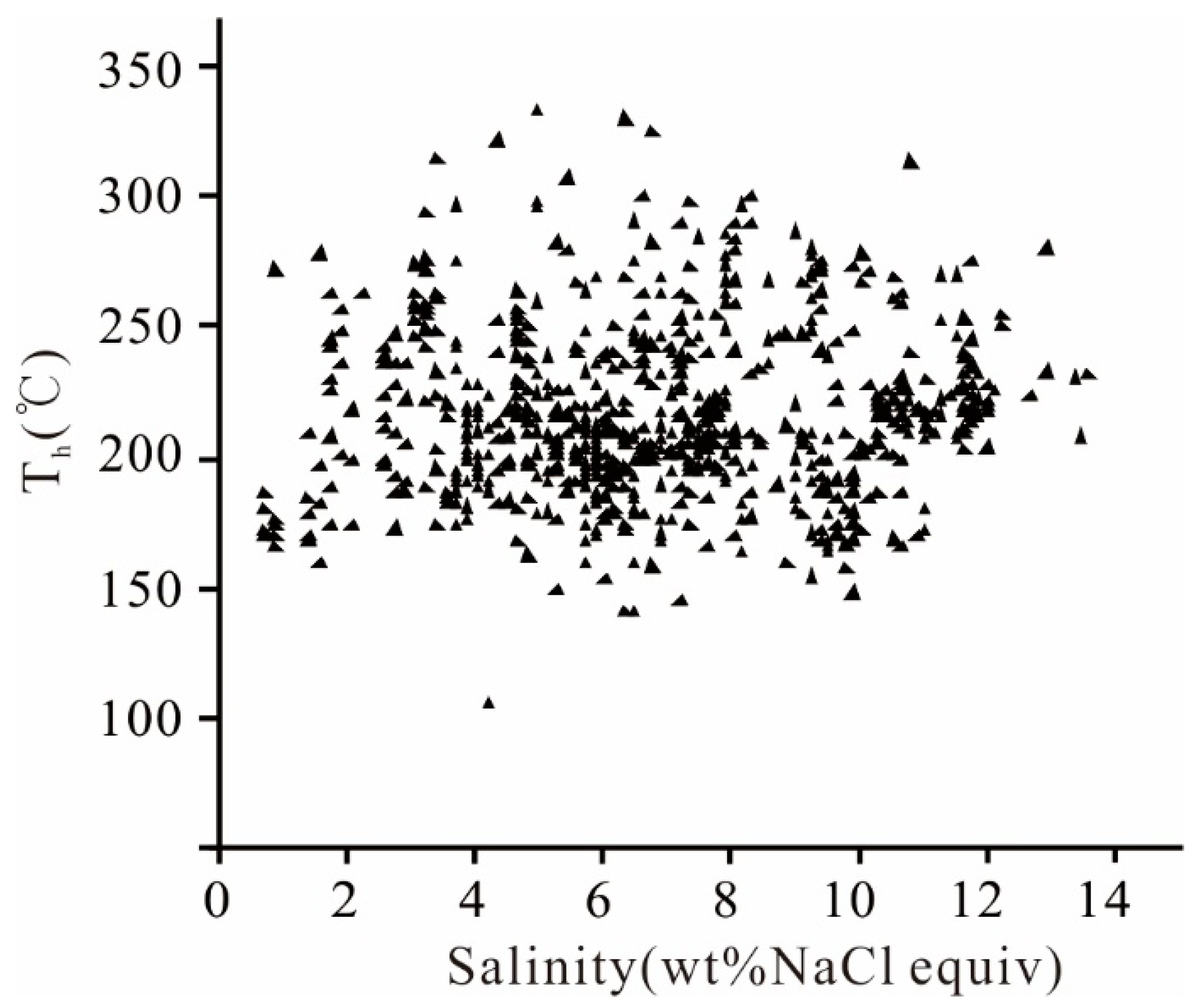
5.1.2. Microthermometry
5.1.3. Laser Raman Spectroscopy
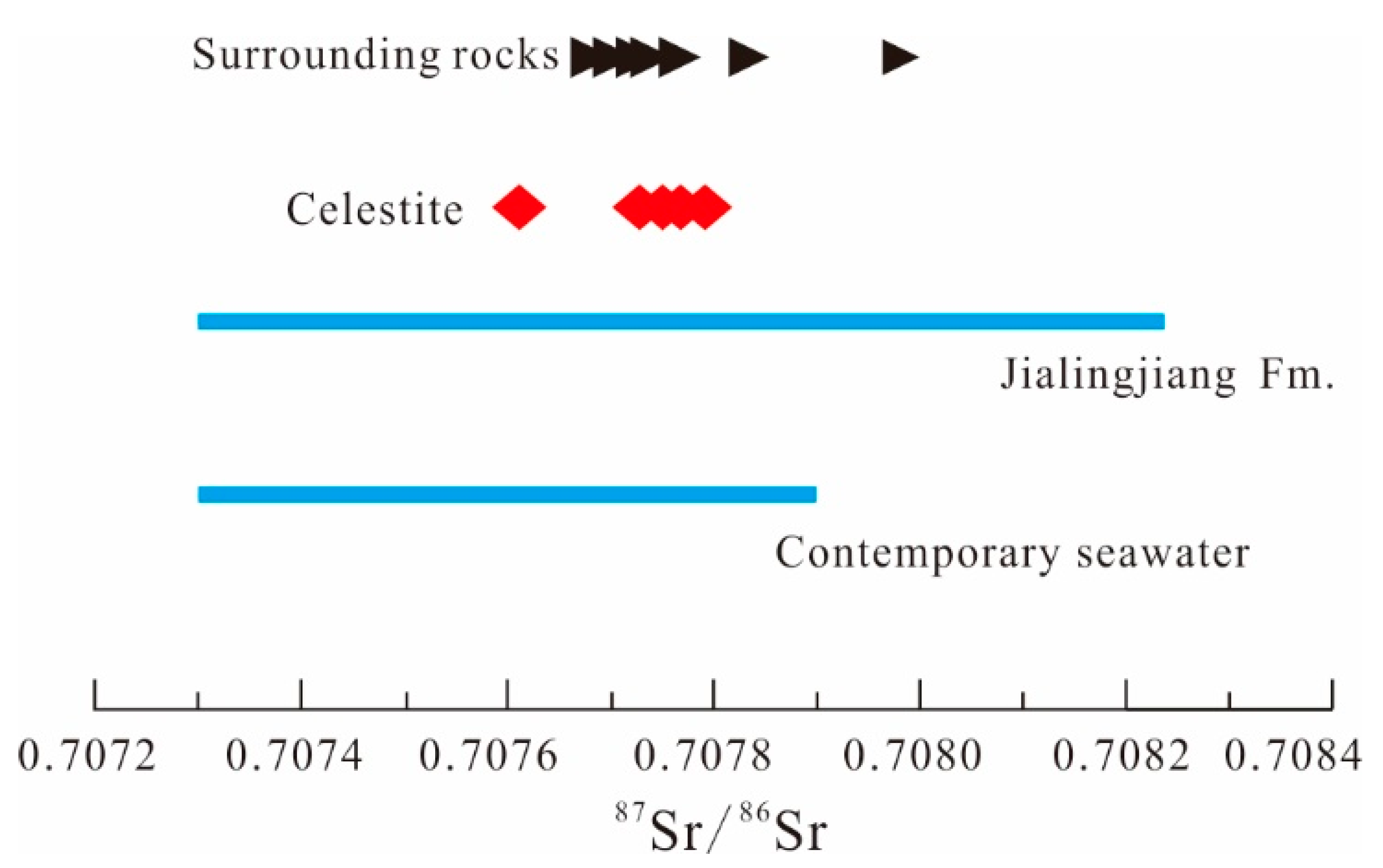
5.2. Sr-S Isotopes
| Deposit | Sample No. | Type | Sr | δ34S (‰) | Comment | |
|---|---|---|---|---|---|---|
| 87Sr/86Sr | 2σ | |||||
| Yuxia | YX305 S3 | dolomite | 0.7078 | 0.000011 | this study | |
| YX305 S1 | dolomite | 0.7077 | 0.000012 | |||
| YX305II1 | dolomite | 0.7078 | 0.000011 | |||
| YX305II2 | dolomite | 0.7077 | 0.00001 | |||
| TL-3 | massive celestine | 0.7078 | 0.000011 | 36.9 | ||
| TL-5 | massive celestine | 0.7077 | 0.000014 | 36.8 | ||
| TE-1 | celestine | 0.7087 | [34] | |||
| TE-2 | celestine | 0.7089 | ||||
| TT-4 | dolomite | 0.7088 | ||||
| TT-9 | dolomite | 0.7089 | ||||
| Xinglong | ZK231-444.75 | dolomite | 0.7080 | 0.000017 | this study | |
| ZK231-455.75 | dolomite | 0.7077 | 0.000016 | |||
| ZK231-471.25 | dolomite | 0.7077 | 0.000011 | |||
| YX305 S1 | banded celestine | 0.7078 | 0.000013 | 39.0 | ||
| YX305II3 | banded celestine | 0.7076 | 0.000011 | 36.8 | ||
| ZK231-455.75 | celestine vein | 0.7078 | 0.000009 | 36.4 | ||
| ZK231-452.45 | celestine | 36.9 | ||||
| Hechuan | HC-S27 | banded celestine | 0.7078 | [71] | ||
| HC-S2 | banded celestine | 0.7085 | 34.5 | |||
| HC-S18 | massive celestine | 0.7078 | 37.6 | |||
| HC-S24 | brecciated celestine | 0.7094 | 32.5 | |||
| HC-S22 | massive celestine | 0.7072 | ||||
| HC-1 | reticulate celestine | 0.7068 | 35.3 | |||
| Gongqiaoba | A2-1 | celestine | 0.7105 | 35.92 | [34] | |
| A3-1 | celestine | 0.7098 | 36.12 | |||
5.3. Major and Trace Elements
6. Discussion
6.1. Nature of the Mineralizing Fluid
6.2. Sources of Ore-Forming Matter
6.2.1. Source of Strontium
6.2.2. Source of Sulfur
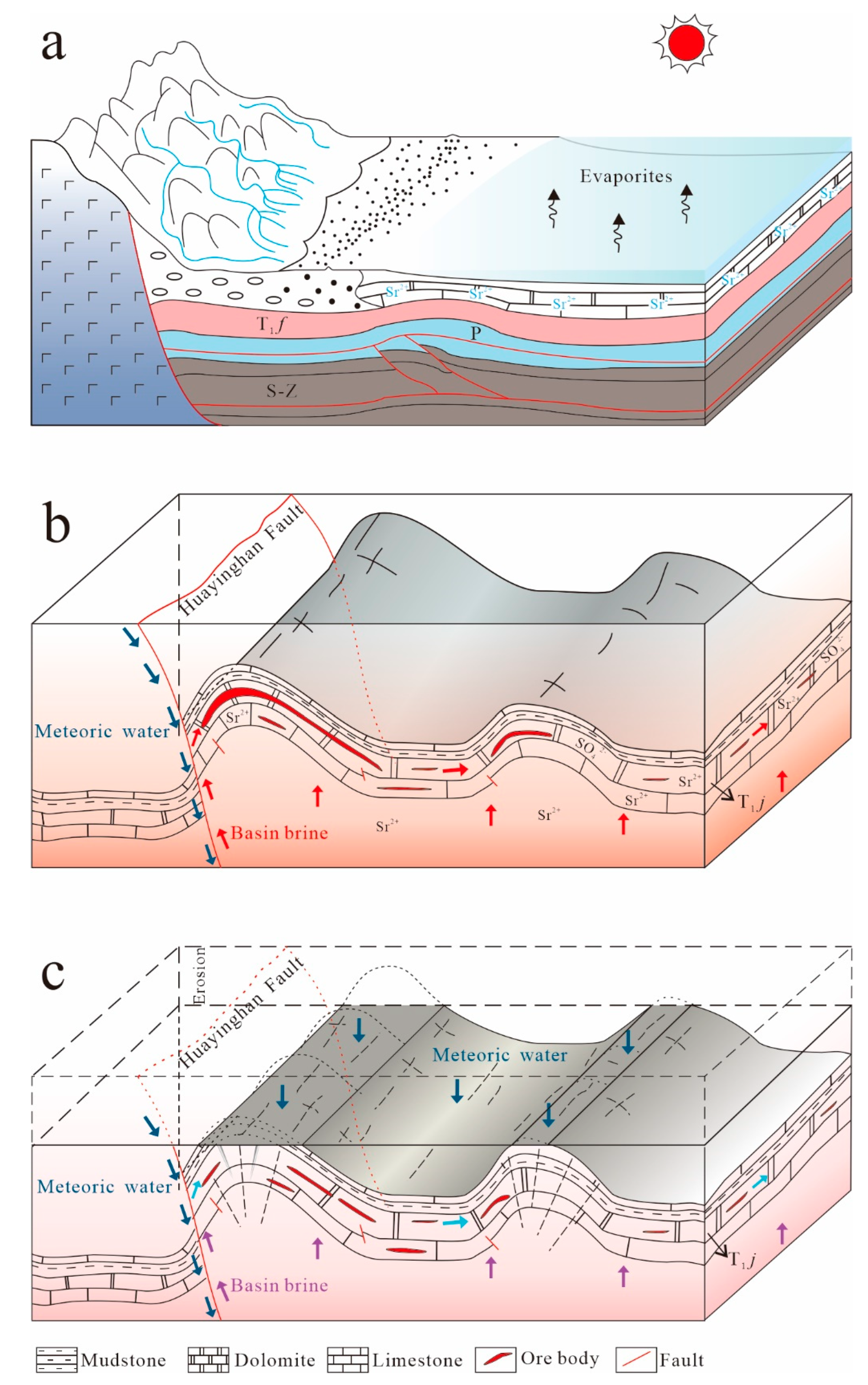
6.3. Mechanism of Mineralization
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacMillan, J.P.; Park, J.W.; Gerstenberg, R.; Wagner, H.; Köhler, K.; Wallbrecht, P. Strontium and Strontium Compounds. Ullmann’s Encycl. Ind. Chem. 2000, 34, 473–480. [Google Scholar]
- Ehya, F.; Shakouri, B.; Rafi, M. Geology, mineralogy, and isotope (Sr, S) geochemistry of the Likak celestite deposit, SW Iran. Carbonates Evaporites 2013, 28, 419–431. [Google Scholar] [CrossRef]
- Pourkaseb, H.; Zarasvandi, A.; Rezaei, M.; Mahdavi, R.; Ghanavati, F. The occurrence and origin of celestite in the Abolfares region, Iran: Implications for Sr-mineralization in Zagros fold belt (ZFB). J. Afr. Earth Sci. 2017, 134, 352–364. [Google Scholar] [CrossRef]
- Kile, D.E.; Dayvault, R.D.; Hood, W.C.; Hatch, H.S. Celestine-Bearing Geodes from Wayne and Emery Counties, Southeastern Utah: Genesis and Mineralogy. Rocks Miner. 2015, 90, 314–337. [Google Scholar] [CrossRef]
- Souissi, F.; Sassi, R.; Dandurand, J.L.; Bouhlel, S.; Hamda, S.B. Fluid inclusion microthermometry and rare earth element distribution in the celestites of the jebel doghra ore deposit (dome zone, northern tunisia): Towards a new genetic model. Bull. De La Soc. Geol. De Fr. 2007, 178, 459–471. [Google Scholar] [CrossRef]
- Wang, R.J.; Wang, D.H.; Li, J.K.; Sun, Y.; Li, D.X.; Guo, C.L.; Zhao, Z.; Yu, Y.; Huang, F.; Wang, C.H.; et al. Three-Type Rare Mineral Resources (Rare Resources, Rare Earth and Rarely Scattered Resources) and Their Utilization; Geological Publishing House: Beijing, China, 2015; p. 415. (In Chinese) [Google Scholar]
- Fishburn, M.D.; Davis, J.C. Geology of the Celestite Deposits of Brown County, Kansas. Trans. Kans. Acad. Sci. 1903 1962, 65, 47–60. [Google Scholar] [CrossRef]
- Baker, P.A.; Bloomer, S.H. The origin of celestite in deep-sea carbonate sediments. Geochim. Et Cosmochim. Acta 1988, 52, 335–339. [Google Scholar] [CrossRef]
- Piga, G.; Marmi, J.; Galobart, À.; Brunetti, A.; Lasio, B.; Malfatti, L.; Enzo, S. New data on the presence of celestite into fossil bones from the uppermost Cretaceous Molí del Baró-1 site (Spain) and an alternative hypothesis on its origin. Spectrochim. Acta Part B At. Spectrosc. 2016, 119, 41–49. [Google Scholar] [CrossRef]
- Hanor, J.S. A model for the origin of large carbonate-and evaporite-hosted celestine (SrSO4) deposits. J. Sediment. Res. 2004, 74, 168–175. [Google Scholar] [CrossRef]
- Hanor, J.S. Origin of saline fluids in sedimentary basins. Geol. Soc. Lond. Spec. Publ. 1994, 78, 151–174. [Google Scholar] [CrossRef]
- Hanor, J.S. Barite-celestine geochemistry and environments of formation. Rev. Mineral. Geochem. 2000, 40, 193–275. [Google Scholar] [CrossRef]
- Gu, W.S.; Li, J.; Huang, Z.Q.; Zhu, M.Z.; Tian, M. Analysis of metallogenic system of Yuxia-style Sr ore deposit. China Min. Mag. 2017, 26, 162–165, (In Chinese with English abstract). [Google Scholar]
- Li, J.; Gu, W.S.; Huang, Z.Q.; Tian, M.; Zhu, M.Z.; Luo, Y.L.; Zhang, H. Study on supergenetic corrosion of Yuxia-style Sr ore deposit. China Min. Mag. 2017, 26, 154–160, (In Chinese with English abstract). [Google Scholar]
- Xu, X.G.; Gao, Z.L.; Luo, Z.L.; Ma, Y.L. Discussion on genesis of Yuxia strontium deposit in Tongliang, Sichuan. Acta Geol. Sichuan 1990, 10, 259–266. (In Chinese) [Google Scholar]
- Xu, X.G. Genetic types and prospecting direction of strontium deposits in China. Geol. Chem. Miner. 1991, 1, 20–28. (In Chinese) [Google Scholar]
- Xu, X.G.; Gu, X.L. Strontium Deposits in China; Sichuan Science and Technology Press: Chengdu, China, 1999; p. 125, (In Chinese with English abstract). [Google Scholar]
- García-Veigas, J.; Rosell, L.; Cendón, D.I.; Gibert, L.; Martín, J.M.; Torres-Ruiz, J.; Ortí, F. Large celestine orebodies formed by early-diagenetic replacement of gypsified stromatolites (Upper Miocene, Montevive–Escúzar deposit, Granada Basin, Spain). Ore Geol. Rev. 2015, 64, 187–199. [Google Scholar] [CrossRef]
- Taberner, C.; Marshall, J.D.; Hendry, J.P.; Pierre, C.; Thirlwall, M.F. Celestite formation, bacterial sulphate reduction and carbonate cementation of Eocene reefs and basinal sediments (Igualada, NE Spain). Sedimentology 2002, 49, 171–190. [Google Scholar] [CrossRef]
- Kesler, S.E.; Jones, L.M. Sulfur- and strontium-isotopic geochemistry of celestite, barite and gypsum from the Mesozoic basins of northeastern Mexico. Chem. Geol. 1981, 31, 211–224. [Google Scholar] [CrossRef]
- Scholle, P.A.; Stemmerik, L.; Harpoth, O. Origin of major karst-associated celestite mineralization in Karstryggen, central East Greenland. J. Sediment. Res. 1990, 60, 397–410. [Google Scholar]
- Abidi, R.; Slim-Shimi, N.; Marignac, C.; Hatira, N.; Gasquet, D.; Renac, C.; Soumarin, A.; Gleeson, S. The origin of sulfate mineralization and the nature of the BaSO4–SrSO4 solid-solution series in the Ain Allega and El Aguiba ore deposits, Northern Tunisia. Ore Geol. Rev. 2012, 48, 165–179. [Google Scholar] [CrossRef]
- Brodtkorb, M.K.; Ramos, V.; Barbieri, M.; Ametrano, S. The evaporitic celestite-barite deposits of Neuquen, Argentina. Miner. Depos. 1982, 17, 423–436. [Google Scholar] [CrossRef]
- Wood, M.W.; Shaw, H.F. The geochemistry of celestites from the Yate area near Bristol (U.K.). Chem. Geol. 1976, 17, 179–193. [Google Scholar] [CrossRef]
- Carlson, E.H. Celestite replacements of evaporites in the Salina Group. Sediment. Geol. 1987, 54, 93–112. [Google Scholar] [CrossRef]
- Tekin, E.; Varol, B.; Sayili, İ.S. Indications of intermediate compositions in the BaSO4-SrSO4 solid-solution series from Baheciktepe Celestine deposit, Sivas, east-central Anatolia, Turkey. Can. Miner. 2002, 40, 895–908. [Google Scholar] [CrossRef]
- Dill, H.G.; Henjes-Kunst, F.; Berner, Z.; Stüben, D. Miocene diagenetic and epigenetic strontium mineralization in calcareous series from Cyprus and the Arabian Gulf: Metallogenic perspective on sub- and suprasalt redox-controlled base metal deposits. J. Asian Earth Sci. 2009, 34, 557–576. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.F.; Wang, R.J.; Chen, Z.Y.; Li, J.K.; Li, S.P.; Zhao, Z. Compositional characteristics of celestite in Dafengshan strontium deposit, Qinghai province. Miner. Depos. 2013, 32, 148–156, (In Chinese with English abstract). [Google Scholar]
- Li, J. Discussion on the genesis of Hechuan strontium deposit. Geol. Chem. Miner. 1989, 1, 8–15. (In Chinese) [Google Scholar]
- Li, J. Ore-forming geochemistry and formation mechanism of Hechuan celestite deposit. China Nonmet. Miner. Ind. Nonmentallic Geol. 1990, 1, 14–19+42+2, (In Chinese with English abstract). [Google Scholar]
- Cheng, M. Comment on the underground hot brine genesis of the Yuxia strontium deposit in Tongliang, Sichuan. Acta Geol. Sichuan 1992, 12, 58–61, (In Chinese with English abstract). [Google Scholar]
- Xu, Z.Z. The mechanism of tectonic control of Sr-ore belt, Huaying mountains, Sichuan. J. Chengdu Univ. Technol. 1994, 21, 79–86, (In Chinese with English abstract). [Google Scholar]
- Qin, Z. Metallogenic characteristics of the celestite type Sr deposit in Chongqing area. J. Chengdu Univ. Technol. Sci. Technol. Ed. 1998, 25, 118–126, (In Chinese with English abstract). [Google Scholar]
- Zhu, C.Y.; Wu, W.H. Stable isotopic characteristics and metallogenic mechanismof Huayingshan strontium mineralization belt. Geochimica 1999, 2, 197–202, (In Chinese with English abstract). [Google Scholar]
- Wang, J.P.; Hu, M.T.; Zhou, J.M.; Zhu, J.B.; Deng, X.L.; Yang, Q.T. Two Origins of Celestite Ores in Huaying Mountains Area. Acta Geol. Sin. 2000, 74, 325–332+386, (In Chinese with English abstract). [Google Scholar]
- Zhou, J.M.; Wang, J.P.; Yang, Q.T.; Zhu, J.B.; Deng, X.L.; Hu, M.T. Geochemical features of rare earth elements of celestite in Huaying mountains area. Geol. Chem. Miner. 2004, 26, 19–22, (In Chinese with English abstract). [Google Scholar]
- Li, K.; Cai, C.; Jiang, L.; Cai, L.; Jia, L.; Zhang, B.; Xiang, L.; Yuan, Y. Sr evolution in the Upper Permian and Lower Triassic carbonates, northeast Sichuan basin, China: Constraints from chemistry, isotope and fluid inclusions. Appl. Geochem. 2012, 27, 2409–2424. [Google Scholar] [CrossRef]
- Xu, C.H. Brief Study on the Metallogenic Geological Setting and Genesis to Celestite Deposits in Mts. Huaying, Sichuan-Chongqing; Chengdu University of Technology: Chengdu, China, 2016; pp. 1–74. [Google Scholar]
- Hu, G.C. Geological Characteristics and Discussion of Genesis to the Yuxia Celestite Mine in Tongliang of Chongqing; Chengdu university of technology: Chengdu, China, 2017; pp. 1–78. [Google Scholar]
- Song, W.Q.; Mao, X.D.; Xu, C.W. Characteristics of ore texture and structure of Yuxia celestite deposit in Chongqing and its genetic significance. J. Chengdu Univ. Technol. Sci. Technol. Ed. 2018, 45, 670–678, (In Chinese with English abstract). [Google Scholar]
- Zhu, C.Y. The extraordinary enrichment of strontium element in the Yuxia large strontium deposit, Chongqing. Bull. Mineral. Petrol. Geochem. 1998, 17, 48–50, (In Chinese with English abstract). [Google Scholar]
- Huang, S.J.; Lan, Y.F.; Huang, K.K.; Lü, J. Vug fillings and records of hydrothermal activity in the Middle Permian Qixia Formation, western Sichuan Basin. Acta Petrol. Sin. 2014, 30, 687–698, (In Chinese with English abstract). [Google Scholar]
- Wang, E.; Meng, K.; Su, Z.; Meng, Q.R.; Chu, J.J.; Chen, Z.I.; Wang, G.; Shi, X.H.; Liang, X.Q. Block rotation: Tectonic response of the Sichuan basin to the southeastward growth of the Tibetan Plateau along the Xianshuihe-Xiaojiang fault. Tectonics 2014, 33, 686–718. [Google Scholar] [CrossRef]
- Worley, B.A.; Wilson, C.J.L. Deformation partitioning and foliation reactivation during transpressional orogenesis, an example from the Central Longmen Shan, China. J. Struct. Geol. 1996, 18, 395–411. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.; Dai, J.; Zhang, S.; Zhu, G.; Liu, Z. Geochemistry and origin of sour gas accumulations in the northeastern Sichuan Basin, SW China. Org. Geochem. 2005, 36, 1703–1716. [Google Scholar] [CrossRef]
- Chen, S.F.; Wilson, C.J.L.; Worley, B.A. Tectonic transition from the Songpan-Garze Fold Belt to the Sichuan Basin, South-Western China. Basin Res. 1995, 7, 235–253. [Google Scholar] [CrossRef]
- Burchfiel, B.C.; Chen, Z.; Liu, Y.; Royden, L.H. Tectonics of the Longmen Shan and Adjacent Regions, Central China. Int. Geol. Rev. 1995, 37, 661–735. [Google Scholar] [CrossRef]
- Wen, Z.J.; Liang, J.T.; Ke, M.Y.; Gui, M.W.; Zhi, Z.L. Segmentation of the Longmen Mountains thrust belt, Western Sichuan Foreland Basin, SW China. Tectonophysics 2010, 485, 107–121. [Google Scholar]
- Zhu, M.; Chen, H.L.; Zhou, J.; Yang, S.F. Provenance change from the Middle to Late Triassic of the southwestern Sichuan basin, Southwest China: Constraints from the sedimentary record and its tectonic significance. Tectonophysics 2017, 700–701, 92–107. [Google Scholar] [CrossRef]
- Li, Y.Q.; He, D.F.; Chen, L.B.; Mei, Q.H.; Li, C.X.; Zhang, L. Cretaceous sedimentary basins in Sichuan, SW China: Restoration of tectonic and depositional environments. Cretac. Res. 2016, 57, 50–65. [Google Scholar] [CrossRef]
- Shen, C.B.; Mei, L.F.; Xu, S.H. Fission track dating of mesozoic sandstones and its tectonic significance in the Eastern Sichuan Basin, China. Radiat. Meas. 2009, 44, 945–949. [Google Scholar] [CrossRef]
- Shi, H.C.; Shi, X.B.; Glasmacher, U.A.; Yang, X.Q.; Stockli, D.F. The evolution of eastern Sichuan basin, Yangtze block since Cretaceous: Constraints from low temperature thermochronology. J. Asian Earth Sci. 2016, 116, 208–221. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Dong, S.W.; Li, J.H.; Shi, W. Mesozoic multi-directional compressional tectonics and formation-reformation of Sichuan basin. Geol. China 2011, 38, 233–250, (In Chinese with English abstract). [Google Scholar]
- Li, J.H.; Zhang, Y.Q.; Zhao, G.C.; Johnston, S.T.; Dong, S.W.; Koppers, A.; Miggins, D.P.; Sun, H.S.; Wang, W.B.; Xin, Y.J. New insights into Phanerozoic tectonics of South China: Early Paleozoic sinistral and Triassic dextral transpression in the east Wuyishan and Chencai domains, NE Cathaysia. Tectonics 2017, 36, 819–853. [Google Scholar] [CrossRef]
- Jia, X.L. Structural Geometry and Kinematics of Southeast Sichuan Basin: Insights into Tectonic Relationship with the Western Segment of Xue Feng Mountain Orogenic Belt; China University of Geosciences (Beijing): Beijing, China, 2016; p. 262. [Google Scholar]
- Zhuang, X.G.; Su, S.C.; Xiao, M.G.; Li, J.; Alastuey, A.; Querol, X. Mineralogy and geochemistry of the late permian coals in the Huayingshan coal-bearing area, Sichuan Province China. Int. J. Coal Geol. 2012, 94, 271–282. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Shao, L.Y.; Chen, Z.X.; Li, M.B.; Yan, H.; Li, Y.J.; Chen, Z.S. Depositional environments and coal-accumulation characteristics of the Upper Triassic Xujiahe Formation in the northern segment of Huaying Mountain, Sichuan Basin. J. China Coal Soc. 2016, 41, 703–711, (In Chinese with English abstract). [Google Scholar]
- Dai, S.F.; Luo, Y.B.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhao, L.; Liu, S.D.; Zhao, C.L.; Tianhe, M.; Zou, J.H. Revisiting the late Permian coal from the Huayingshan, Sichuan, southwestern China: Enrichment and occurrence modes of minerals and trace elements. Int. J. Coal Geol. 2014, 22, 110–128. [Google Scholar] [CrossRef]
- Liu, S.; Ren, X.G.; Yao, S.X.; Liu, Z.P.; Ning, M.; Wang, X.; Huang, X.H. Relationship between gas reservoir distribution and structural system of upper Triassic Xujiahe Fm in the Sichuan. Nat. Gas Ind. B 2019, 6, 220–235. [Google Scholar] [CrossRef]
- Yang, P.; Cheng, Q.; Xie, S.; Wang, J.; Chang, L.; Yu, Q.; Zhan, Z.; Chen, F. Hydrogeochemistry and geothermometry of deep thermal water in the carbonate formation in the main urban area of Chongqing, China. J. Hydrol. 2017, 549, 50–61. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Guo, T.; Zhu, G.; Cai, X.; Li, M. Petroleum geology of the Puguang sour gas field in the Sichuan Basin, SW China. Mar. Pet. Geol. 2008, 25, 357–370. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Li, Z.P.; Li, L.; Nie, Y.; Wu, J.J.; Zhou, X.G.; Tan, X.C. Syndepositional tectonic activities in the Huayingshan fracture belt during the triassic Jialing river phase and its impact on sedimentation and reservoir development. J. Stratigr. 2010, 34, 312–320, (In Chinese with English abstract). [Google Scholar]
- Wang, Z.J.; Wang, H.C.; Dong, D.; Qin, J. Review of Geophysical Results of Huayingshan Fault Zone. Earthq. Res. Sichuan 2018, 3, 6–12, (In Chinese with English abstract). [Google Scholar]
- Xu, S.R.; Xu, J.H. The new results of seismic exploitation in Huayingshan fault zone. Acta Pet. Sin. 1986, 7, 39–48, (In Chinese with English abstract). [Google Scholar]
- Ma, Y.S.; Mu, C.L.; Guo, T.L.; Tan, Q.Y.; Yu, Q. Sequence stratigraphy and reservoir distribution of Feixianguan formation in Northeastern Sichuan. J. Mineral. Petrol. 2005, 25, 73–79, (In Chinese with English abstract). [Google Scholar]
- Chongqing Institute of Geology and mineral resources. Investigation and Evaluation of Celestite Resources in Huayingshan Strontium Belt; Chongqing Institute of Geology and Mineral Resources: Chongqing, China, 2015; pp. 1–62. [Google Scholar]
- Jia, X.L.; He, D.F. Structural analysis and kinematics simulation of Xishan anticline in Southeast folded belt, Sichuan basin. Xinjiang Pet. Geol. 2014, 35, 652–658, (In Chinese with English abstract). [Google Scholar]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Et Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Liu, J.J.; Lü, Z.C.; Liu, Z.J.; Zhai, D.G.; Wang, Y.H.; Tao, Y.L. Strontium isotopic composition and its genetic significance of the Dabashan large barium metallogenic belt in southern Qinling Mountains. Earth Sci. Front. 2014, 21, 23–30, (In Chinese with English abstract). [Google Scholar]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- LI, J. Evaporite formation and celestine deposit. Geol. Chem. Miner. Chem. Geol. 1990, 2, 15–21. [Google Scholar]
- Huang, S.J.; Huang, Y.; Lan, Y.F.; Huang, K.K. A comparative study on strontium isotope composition of dolomites andtheir coeval seawater in the late permian-early Triassic, NE Sichuan basin. Acta Petrol. Sin. 2011, 27, 3831–3842, (In Chinese with English abstract). [Google Scholar]
- Korte, C.; Kozur, H.W.; Bruckschen, P.; Veizer, J. Strontium isotope evolution of Late Permian and Triassic seawater. Geochim. Et Cosmochim. Acta 2003, 67, 47–62. [Google Scholar] [CrossRef]
- Lin, Y.T. Study on sulfur isotopes of trias marine deposit gypsum and brines in the Sichuan basin. J. Salt Lake Res. 2003, 11, 1–7, (In Chinese with English abstract). [Google Scholar]
- Zhong, Y.-J.; Chen, A.-Q.; Huang, K.-K.; Yang, S.; Xu, S.-L.; Somerville, I.D. Hydrothermal activity in the fourth member of the Triassic Leikoupo Formation in the Yuanba area, northeast Sichuan, SW China. Geol. J. 2019, 54, 266–277. [Google Scholar] [CrossRef]
- Huang, K.K.; Zhong, Y.J.; Huang, S.J.; Li, X.N.; Feng, M.S. Saddle-dolomite-bearing fracture fillings and records of hot brine activity in the Jialingjiang formation, Libixia section, Hechuan area of Chongqing city. Acta Geol. Sin. 2016, 90, 195–208. [Google Scholar]
- Tekin, E.; Varol, B.; Friedman, G.M. A prelimennary study Celestite-bearing gypsum in the Tertiary Sivas basin, central-eastern Turkey. Carbonates Evaporites 2001, 16, 93–101. [Google Scholar] [CrossRef]
- Taherzadeh, A.; Paydar, G.R. Geology, Geochemistry and Origin of Baba Mohammad Celestite Deposit, Kohgiloyeh and Boyerahmad Province, Iran. In 8th Symposium of Iranian Society of Economic Geology; Azimzadeh, D.m.A.M., Ed.; University of Zanjan: Zanjan, Iran, 2016. [Google Scholar]
- Tekin, E.; Varol, B.; Ayan, Z.; Satir, M. Epigenetic origin of celestite deposits in the Tertiary Sivas Basin: New mineralogical and geochemical evidence. Neues Jahrb. Für Mineral. Mon. 2002, 2002, 289–318. [Google Scholar] [CrossRef]
- Abidi, R.; Slim-Shimi, N.; Gasquet, D.; Hatira, N.; Somarin, A. Genesis of celestite—Bearing cap rock formation from the Ain Allega ore deposit (northern Tunisia): Contributions from microthermometric studies. Bull. De La Société Géologique De Fr. 2011, 182, 427–435. [Google Scholar] [CrossRef]
- Jabal, A.; Akhdar, A.; Ne, L.; Saad, K.; El Ebaidi, S. Geochemical and mineralogical studies of the Celestite bearing formations and associated sediments in Benghazi Formation, Ar Rajmah Group (Middle Miocene) Al Jabal Al Akhdar, NE Libya. Libyan J. Sci. Technol. 2019, 1, 1. [Google Scholar]
- Bazargani-Guilani, K.; Tak, M.A.N. Celestite ore deposit and occurrences of the Qom Formation, Oligo-Miocene, central Iran. In Proceedings of the 2nd IASME/WSEAS International Conference on GEOLOGY and SEISMOLOGY (GES ‘08), Cambridge, UK, 23–25 February 2008; pp. 48–54. [Google Scholar]
- Zhou, X.; Li, C.J.; Ju, X.M.; Du, Q.; Tong, L.H. Origin of Subsurface Brines in the Sichuan Basin. Ground Water 1997, 35, 53–58. [Google Scholar]
- Huang, S.J.; Hairuo, Q.; Hu, Z.W.; Zou, M.L.; Feng, W.L.; Wang, C.M.; Gao, X.Y.; Wang, Q.D. Influence of sulfate reduction on diagenesis of Feixianguan carbonate in Triassic, NE Sichuan basin of China. Acta Sedimentol. Sin. 2007, 25, 815–824, (In Chinese with English abstract). [Google Scholar]
- Zhou, X.; Cao, Q.; Yin, F.; Guo, J.; Wang, X.C.; Zhang, Y.; Wang, L.; Shen, Y. Characteristics of the brines and hot springs in the Triassic carbonates in the high and steep fold zone of the eastern Sichuan basin. Acta Geol. Sin. 2015, 89, 1908–1920, (In Chinese with English abstract). [Google Scholar]
- Li, K.K.; George, S.C.; Cai, C.F.; Gong, S.; Sestak, S.; Armand, S.; Zhang, X.F. Fluid inclusion and stable isotopic studies of thermochemical sulfate reduction: Upper permian and lower triassic gasfields, northeast Sichuan Basin, China. Geochim. Et Cosmochim. Acta 2019, 246, 86–108. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, X.F.; Wang, C.W.; Li, P.P.; Guo, T.L.; Zou, H.Y.; Zhu, Y.M.; Liu, J.Z.; Cai, Z.X. The fate of CO2 derived from thermochemical sulfate reduction (TSR) and effect of TSR on carbonate porosity and permeability, Sichuan Basin, China. Earth Sci. Rev. 2015, 141, 154–177. [Google Scholar] [CrossRef]
- Cai, C.F.; Xiang, L.; Yuan, Y.Y.; Xu, C.L.; He, W.X.; Tang, Y.J.; Borjigin, T. Sulfur and carbon isotopic compositions of the Permian to Triassic TSR and non-TSR altered solid bitumen and its parent source rock in NE Sichuan Basin. Org. Geochem. 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Xu, X.G. Genetic types of celestite deposits and their prospecting direciton discussed from strontium geochemistry. Geol. Rev. 1984, 30, 146–154, (In Chinese with English abstract). [Google Scholar]
- Cheng, Q.; Yang, H.L.; Zeng, M. The formation and protection of karst geothermal water resources in the main urban area of Chongqing. Carsologica Sin. 2015, 34, 217–227, (In Chinese with English abstract). [Google Scholar]
- Playà, E.; Rosell, L. The celestite problem in gypsum Sr geochemistry: An evaluation of purifying methods of gypsiferous samples. Chem. Geol. 2005, 221, 102–116. [Google Scholar] [CrossRef]
- Madsen, H.B.; Stemmerik, L. Early diagenetic celestite replacement of demosponges in Upper Cretaceous (Campanian–Maastrichtian) chalk, Stevns, Denmark. Geology 2009, 37, 355–358. [Google Scholar] [CrossRef]
- Breesch, L.; Stemmerik, L. Genetic and fluid flow model of a large-scale celestite deposit in Karstryggen, Central East Greenland. J. Geochem. Explor. 2009, 101, 9. [Google Scholar] [CrossRef]
- Mcarthur, J.M.; Burnett, J.; Hancock, J.M. Strontium isotopes at K/T boundary. Nature 1992, 355, 28. [Google Scholar] [CrossRef]
- Burke, W.H.; Denison, R.E.; Hetherington, E.A.; Koepnick, R.B.; Nelson, H.F.; Otto, J.B. Variation of seawater 87Sr/86Sr throughout Phanerozoic time. Geology 1982, 10, 516. [Google Scholar] [CrossRef]
- Huang, S.J.; Hairuo, Q.; Huang, P.P.; Huo, Z.W.; Wang, Q.D.; Zou, M.L.; Liu, H.N. Strontium isotopic composition and evolution of seawater from Late Permian to Early Triassic-Based on the Results of Marine Carbonate in Zhongliang Mountain, Chongqing. Sci. China Earth Sci. 2008, 38, 273–283. (In Chinese) [Google Scholar]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Ebneth, S.; Godderis, Y. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 1586. [Google Scholar] [CrossRef]
- Prokoph, A.; Shields, G.A.; Veizer, J. Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth Sci. Rev. 2008, 87, 113–133. [Google Scholar] [CrossRef]
- Joachimski, M.; Lai, X.L.; Shen, S.Z.; Jiang, H.; Luo, G.M.; Chen, B.; Chen, J.; Sun, Y.D. Climate warming in the latest Permian and the Permian-Triassic mass extinction. Geology 2012, 40, 195–198. [Google Scholar] [CrossRef]
- Lin, L.B.; Chen, H.D.; Zhu, L.D.; Xu, S.L.; Zhong, Y.J. The sequence-based lithofacies-paleogeography of Jialingjiang Formation and Leikoupo Formation in eastern Sichuan Basin, China. J. Chengdu Univ. Technol. 2010, 37, 446–451, (In Chinese with English abstract). [Google Scholar]
- Canfield, D.; Raiswell, R. Carbonate precipitation and dissolution. In Taphonomy—Releasing the Data Locked in the Fossil Record; Allison, A., Briggs, D.E.G., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 411–453. [Google Scholar]
- Wolf, K.H. Carbonate sediments and their diagenesis. Earth Sci. Rev. 1973, 9, 152–157. [Google Scholar] [CrossRef]
- Zherebtsova, I.K.; Volkova, N.N. Experimental study of behavior of trace elements in the process of natural solar evaporation of Black Sea water and Sasyk-Sivash brine. Geokhimiya 1966, 3, 832–845. [Google Scholar]
- Katz, A.; Sass, E.; Starinsky, A.; Holland, H.D. Strontium behavior in the aragonite-calcite transformation: An experimental study at 40–98 °C. Geochim. Et Cosmochim. Acta 1972, 36, 481–496. [Google Scholar] [CrossRef]
- Helz, G.R.; Holland, H.D. The solubility and geologic occurrence of strontianite. Geochim. Et Cosmochim. Acta 1965, 29, 1303–1315. [Google Scholar] [CrossRef]
- Sayles, F.L.; Manheim, F.T. Interstitial solutions and diagenesis in deeply buried marine sediments: Results from the Deep Sea Drilling Project. Geochim. Et Cosmochim. Acta 1975, 39, 103–127. [Google Scholar] [CrossRef]
- Huang, S.J.; Qing, H.R.; Hu, Z.W.; Zou, M.L.; Feng, W.L.; Wang, C.M.; Gao, X.Y.; Wang, Q.D. Closed-system dolomitization and the significance for petroleum and economic geology: An example from Feixianguan carbonates, Triassic NE Sichuan basin of China. Acta Geol. Sin. 2007, 23, 2955–2962, (In Chinese with English abstract). [Google Scholar]
- Poonoosamy, J.; Curti, E.; Kosakowski, G.; Grolimund, D.; Van Loon, L.R.; Mäder, U. Barite precipitation following celestite dissolution in a porous medium: A SEM/BSE and μ-XRD/XRF study. Geochim. Et Cosmochim. Acta 2016, 182, 131–144. [Google Scholar] [CrossRef]
- Canfield, D.E. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim. Et Cosmochim. Acta 2011, 65, 1117–1124. [Google Scholar] [CrossRef]
- Chen, J.S.; Chu, X.L. Sulfur isotope composition of triassic marine sulfates of South China. Chem. Geol. Isot. Geosci. Sect. 1988, 72, 155–161. [Google Scholar]
- Bottrell, S.H.; Newton, R.J. Reconstruction of changes in global sulfur cycling from marine sulfate isotopes. Earth Sci. Rev. 2006, 75, 59–83. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Henjes-Kunst, E.; Raith, J.G.; Boyce, A.J. Micro-scale sulfur isotope and chemical variations in sphalerite from the Bleiberg Pb-Zn deposit, Eastern Alps, Austria. Ore Geol. Rev. 2017, 90, 52–62. [Google Scholar] [CrossRef]
- Fazli, S.; Taghipour, B.; Moore, F.; Lentz, D.R. Fluid inclusions, S isotopes, and Pb isotopes characteristics of the Kuh-e-Surmeh carbonate-hosted Zn–Pb deposit in the Zagros Fold Belt, southwest Iran: Implications for the source of metals and sulfur and MVT genetic model. Ore Geol. Rev. 2019, 109, 615–629. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, W.; Zhang, J.; Wang, J.; Zhang, X. Formation of Pb–Zn deposits in the Sichuan–Yunnan–Guizhou triangle linked to the Youjiang foreland basin: Evidence from Rb–Sr age and in situ sulfur isotope analysis of the Maoping Pb–Zn deposit in northeastern Yunnan Province, southeast China. Ore Geol. Rev. 2019, 107, 780–800. [Google Scholar] [CrossRef]
- Sheng, X.; Bi, X.; Hu, R.; Tang, Y.; Lan, Q.; Xiao, J.; Tao, Y.; Huang, M.; Peng, J.; Xu, L. The mineralization process of the Lanuoma Pb-Zn-Sb deposit in the Sanjiang Tethys region: Constraints from in situ sulfur isotopes and trace element compositions. Ore Geol. Rev. 2019, 111, 102941. [Google Scholar] [CrossRef]
- Yan, J.X.; Carlson, E.H. Nodular celestite in the Chihsia Formation (Middle Permian) of south China. Sedimentology 2003, 50, 265–278. [Google Scholar] [CrossRef]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and thermochemical sulfate reduction in diagenetic settings-old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Cai, C.F.; Li, K.K.; Zhu, Y.M.; Xiang, L.; Jiang, L.; Cai, X.Y.; Cai, L.L. TSR origin of sulfur in Permian and Triassic reservoir bitumen, East Sichuan Basin, China. Org. Geochem. 2010, 41, 871–878. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zhang, S.C.; Liang, Y.B.; Dai, J.X. Stable sulfur isotopic composition of hydrogen sulfide and its genesis in Sichuan basin. Geochimica 2006, 35, 333–345, (In Chinese with English abstract). [Google Scholar]
- Cross, M.M.; Bottrell, S.H. Reconciling experimentally observed sulphur isotope fractionation during thermochemical sulphate reduction (TSR) with field data: A ‘steadystate’ model of isotopic behaviour. Goldschmidt J. Conf. Abstr. 2000, 5, 325. [Google Scholar]
- Hryniv, S.; Peryt, T.M. Strontium distribution and celestite occurrence in Zechstein (Upper Permian) anhydrites of West Poland. Chem. Der Erde Geochem. 2010, 70, 137–147. [Google Scholar] [CrossRef]
- Rosenberg, Y.O.; Sade, Z.; Ganor, J. The precipitation of gypsum, celestine, and barite and coprecipitation of radium during seawater evaporation. Geochim. Et Cosmochim. Acta 2018, 233, 50–65. [Google Scholar] [CrossRef]
- Shi, H.Y.; Miao, W.L.; Zhang, X.Y.; Li, W.X.; Tang, Q.L.; Li, Y.S. Geochemical characteristics and ore-forming material source of celestite deposits in Dafeng mountain, Northwestern Qaidam basin. Acta Geol. Sin. 2018, 92, 1733–1752. [Google Scholar]
- Shan, Y.W.; Xiao, Y.F.; Juan, H.J.; Zhang, J.; Xu, C.; Guo, X.Z.; Li, X.R. Characteristics and metallogenic conditions of the Huayingshan strontium belt in Southeastern Sichuan. Geol. J. China Univ. 2013, 19, 267–268, (In Chinese with English abstract). [Google Scholar]
- Turianicova, E. CO2 utilization for mechanochemical carbonation of celestine. Geosci. Eng. 2015, 61, 20–23. [Google Scholar] [CrossRef]


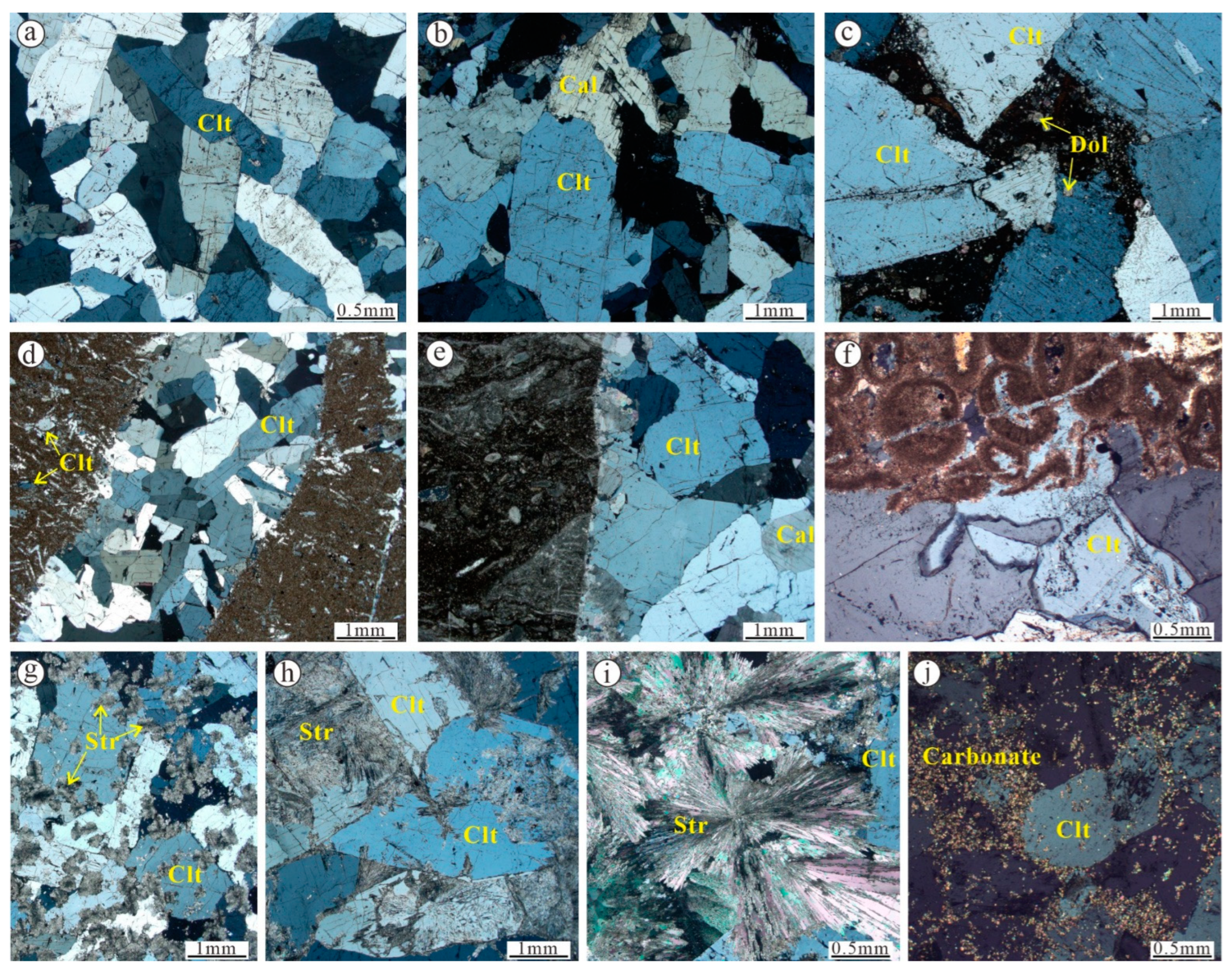
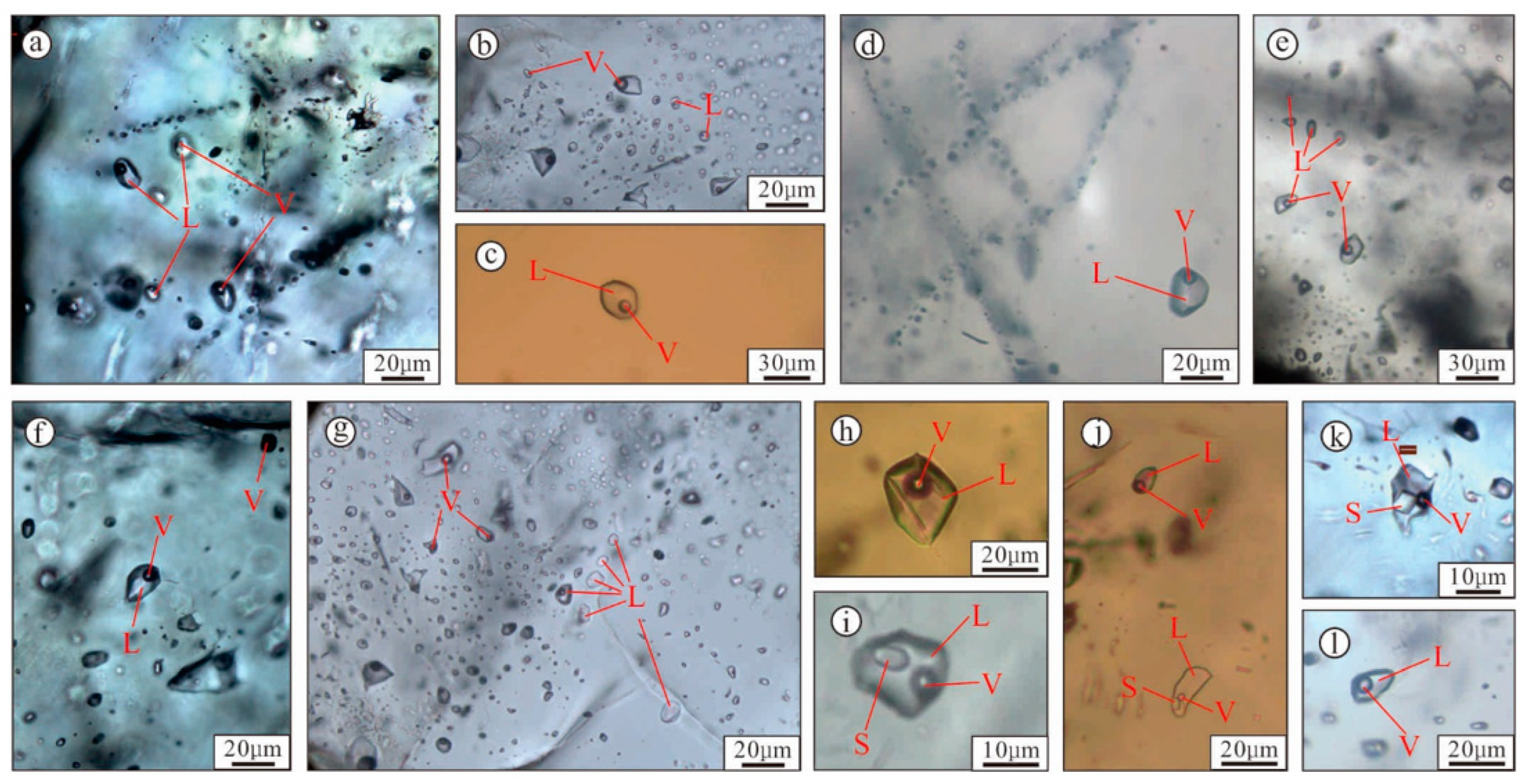
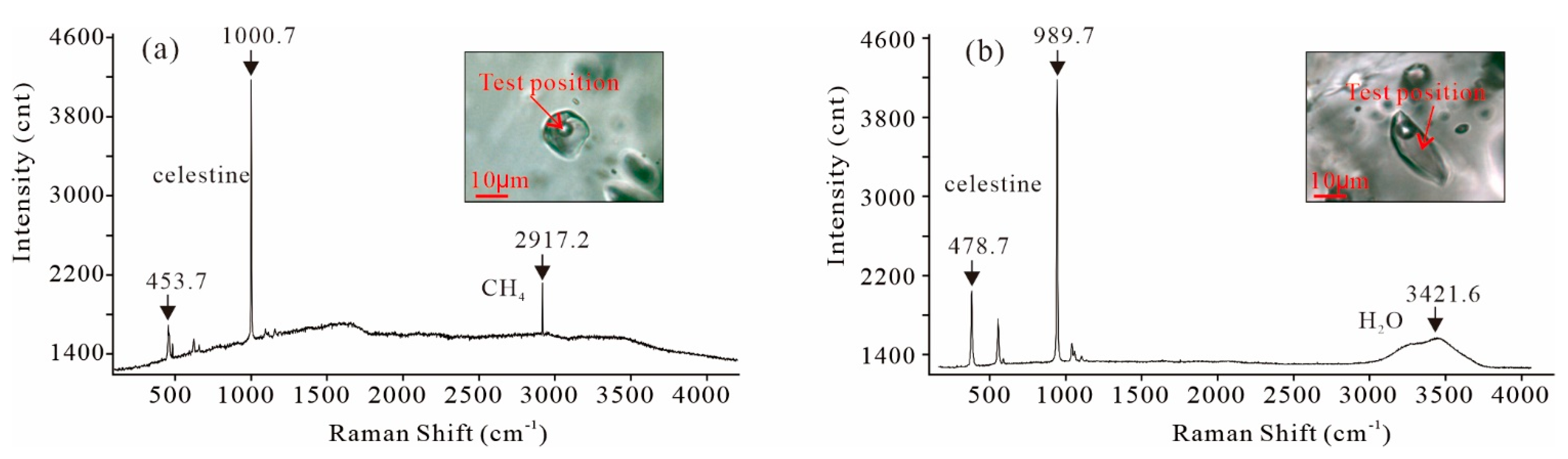


| Deposit/Mining Area | Location | Coastal Carbonates and Evaporites | Age of Host Rock | Hydrothermal Activity | Fluid Properties | Celestine 87Sr/86Sr | Coeval Seawater 87Sr/86Sr | Interpreted Sr Source | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Montevive | Spain | yes | Miocene | yes | basinal brines and meteoric water | 0.7089–0.7086 | basinal brines | [18] | |
| Igualada | Spain | yes | Eocene | yes | basinal brines and meteoric water | 0.7078 | 0.7078 | evaporites | [19] |
| Paila | Mexico | yes | Cretaceous | minor | groundwater | 0.7077 | 0.7072 | limestones | [20] |
| Karstryggen | Greenland | yes | Permian | minor | basinal brines | 0.7134 | 0.7068 | redbeds | [21] |
| Ain Allega | Tunisia | yes | Triassic | yes | basinal brined and magmatic-meteoric fluid | carbonates | [22] | ||
| Jebel Doghra | Tunisia | yes | Triassic | yes | basinal brines | feldspar-rich series | [5] | ||
| Neuquen | Argentina | yes | Cretaceous | yes | 0.7072 | 0.7072 | seawater | [23] | |
| Yate | Great Britain | yes | Triassic | no | 0.7105 | 0.7076 | limestones | [24] | |
| Ohio basin | United States | yes | Silurian | some | basinal brines | evaporites | [25] | ||
| Sivas basin | Turkey | yes | Miocene | yes | magmatic–hydrothermal fluids and meteoric water | 0.7078 | 0.7083–0.7087 | evaporites | [26] |
| Abolfares | Iran | yes | Oligo-Miocene | yes | diagenetic brines | carbonates | [3] | ||
| Likak | Iran | yes | Miocene | yes | basinal brines | 0.7087–0.7088 | 0.7083–0.7090 | evaporites | [2] |
| Cyprus | Cyprus | yes | Miocene | yes | basinal brines | 0.7089 | carbonates | [27] | |
| Huyingshan | China | yes | Triassic | yes | basinal brines | 0.7076–0.7078 | 0.7073–0.7080 | carbonates and evaporites | this study |
| Dafengshan | China | no | Pliocene | no | granite | [28] |
| Sample No. | Type | Characteristics of Inclusions | Salinity (wt% NaCl Equiv.) | Th (°C) | |
|---|---|---|---|---|---|
| Size (μm) | Vapor Volume (%) | ||||
| ZK231-444.75 | vein | 3–24 | 5–15 | 5.6–11.7 (26) | 190–330 (27) |
| ZK231-452.45 | vein | 3–27 | 5–20 | 4.3–12.6 (34) | 173–345 (37) |
| ZK231-458.15 | vein | 2–15 | 5–20 | 5.3–11.1 (26) | 192–221 (38) |
| ZK231-460.45 | massive | 5–17 | 10–20 | 10.2–12.0 (35) | 211–232 (43) |
| ZK231-465.05 | striped | 4–19 | 5–20 | 3.6–11.6 (34) | 181–229 (40) |
| YX305 S2 | massive | 3–21 | 5–25 | 0.9–4.8 (48) | 157–278 (55) |
| YX305II3 | striped | 5–18 | 5–20 | 2.1–5.4 (34) | 148–276 (44) |
| YX305 S3③ | vein | 3–15 | 5–15 | 5.9–8.1 (22) | 192–242 (26) |
| YX305 S3② | vein | 3–17 | 5–15 | 2.6–9.1 (26) | 177–258 (26) |
| YX305 S3① | vein | 4–20 | 5–15 | 0.7–11.7 (32) | 165–277 (34) |
| YX305II1 | striped | 3–16 | 5–15 | 3.7–8.0 (45) | 167–283 (45) |
| YX305II-2 | striped | 3–19 | 5–20 | 2.9–5.9 (39) | 182–224 (45) |
| YX-H2 | striped | 7–23 | 5–15 | 1.6–9.7 (33) | 152–315 (35) |
| YX-H3 | striped | 5–20 | 5–20 | 2.7–6.7 (46) | 172–297 (44) |
| YX-H5 | striped | 6–22 | 10–20 | 2.6–10.5 (33) | 106–300 (31) |
| YX-H6 | striped | 3–23 | 5–15 | 4.0–11.9 (30) | 180–297 (32) |
| SJW-H9 | striped | 5–21 | 5–20 | 5.1–10.0 (32) | 169–263 (33) |
| SJW-H8 | striped | 3–25 | 5–20 | 1.4–8.3 (32) | 173–262 (32) |
| SJW-H5 | striped | 3–20 | 5–20 | 4.7–13.5 (31) | 174–313 (31) |
| SJW-H4 | striped | 3–15 | 5–25 | 7.9–11.7 (28) | 147–221 (28) |
| SJW-H1 | massive | 3–10 | 10–25 | 5.3–9.0 (10) | 199–262 (10) |
| SZL-H1 | massive | 4–14 | 10–20 | 5.7–13.4 (20) | 195–300 (20) |
| SZL-H2 | striped | 3–17 | 5–25 | 5.7–11.6 (20) | 140–271 (20) |
| SZL-H3 | striped | 5–15 | 5–20 | 6.9–10.9 (20) | 166–222 (20) |
| Spot | CaO (wt. %) | Sr (ppm) |
|---|---|---|
| yx305 s-2_1 | 32.4 | 226,725 |
| yx305 s-2_2 | 40.7 | 145,667 |
| yx305 s-2_3 | 32.3 | 227,958 |
| zk231-465-1 | 30.9 | 249,758 |
| zk231-465-2 | 43.6 | 107,998 |
| zk231-465-3 | 30.1 | 249,852 |
| zk231-465-4 | 29.0 | 265,361 |
| zk231-465-5 | 29.6 | 262,730 |
| zk231-465-6 | 30.0 | 254,193 |
| zk231-465-7 | 31.3 | 242,441 |
| zk231-465-8 | 29.4 | 258,119 |
| zk231-458-1 | 30.9 | 250,319 |
| zk231-458-2 | 30.2 | 257,271 |
| zk231-458-3 | 30.6 | 253,011 |
| zk231-458-4 | 30.2 | 256,737 |
| zk231-468-2 | 25.1 | 284,900 |
| zk231-468-3 | 31.3 | 247,194 |
| zk231-468-4 | 29.3 | 261,366 |
| yx305 II1-1 | 30.4 | 251,458 |
| yx305 II1-2 | 29.5 | 263,088 |
| yx305 II3-1 | 28.8 | 270,634 |
| yx305 II3-2 | 27.4 | 255,012 |
| yx305 II3-3 | 28.7 | 249,488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Sun, Y.; Wang, D.; Chen, B.; Gu, W. Geological and Geochemical Constraints on the Origin of the Sr Mineralization in Huayingshan Ore District, Chongqing, South China. Minerals 2023, 13, 279. https://doi.org/10.3390/min13020279
Gao Y, Sun Y, Wang D, Chen B, Gu W. Geological and Geochemical Constraints on the Origin of the Sr Mineralization in Huayingshan Ore District, Chongqing, South China. Minerals. 2023; 13(2):279. https://doi.org/10.3390/min13020279
Chicago/Turabian StyleGao, Yun, Yan Sun, Denghong Wang, Bailin Chen, and Wenshuai Gu. 2023. "Geological and Geochemical Constraints on the Origin of the Sr Mineralization in Huayingshan Ore District, Chongqing, South China" Minerals 13, no. 2: 279. https://doi.org/10.3390/min13020279
APA StyleGao, Y., Sun, Y., Wang, D., Chen, B., & Gu, W. (2023). Geological and Geochemical Constraints on the Origin of the Sr Mineralization in Huayingshan Ore District, Chongqing, South China. Minerals, 13(2), 279. https://doi.org/10.3390/min13020279