Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification
Abstract
:1. Introduction
2. Geological Background and Samples
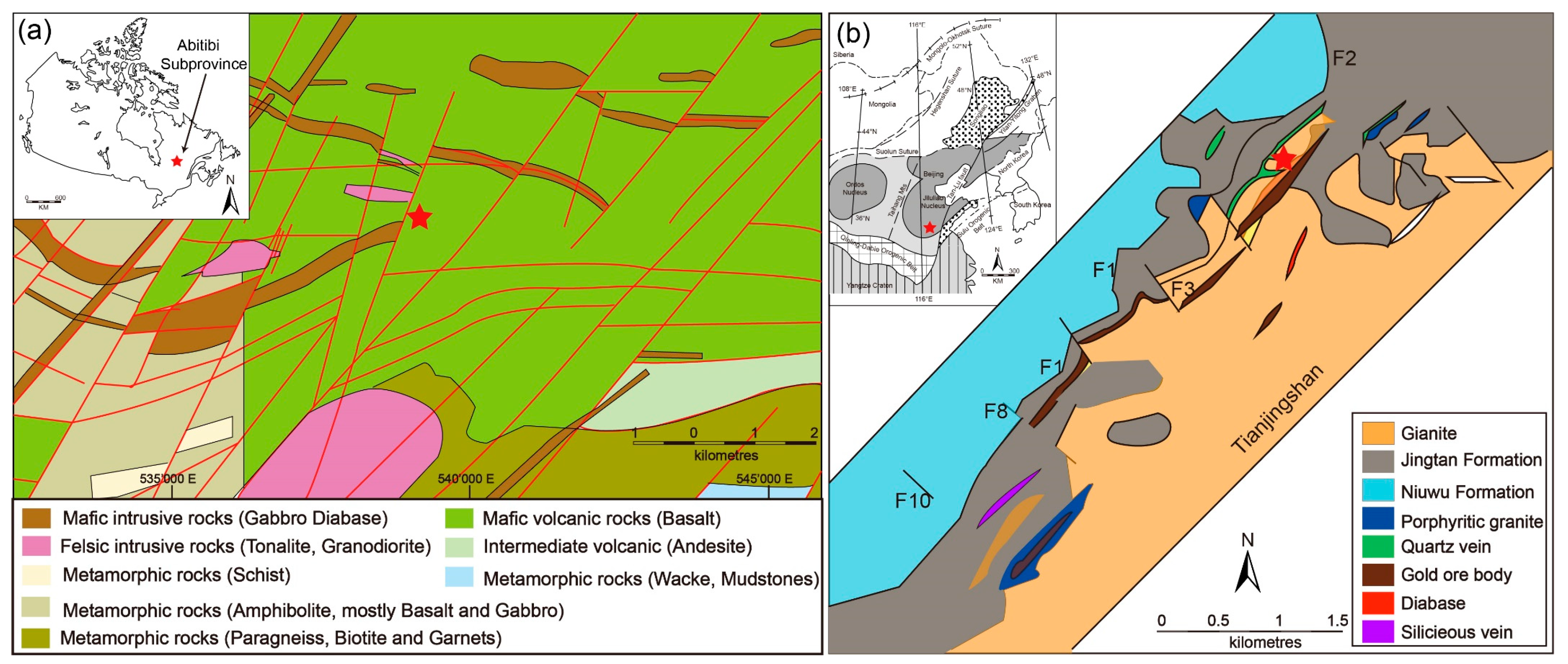
2.1. Chibougamau Quartz Vein in Canada (CBG)
2.2. Tianjingshan Quartz Vein in China (TJS)
3. Analytical Methods
3.1. Optical Microscopy Observation
3.2. Scanning Electron Microscope (SEM) and Cathodoluminescence (CL)
3.3. Raman Spectrometry Measurement
3.4. Spot Chemical Composition Analysis by LA-ICP-MS
3.5. Bulk Chemical Composition Analysis by ICP-OES
3.6. X-ray Powder Diffraction (XRD)
3.7. Purification Process for the CBG and TJS Vein Quartz
4. Results
4.1. Petrography
4.1.1. CBG Raw Vein Quartz
4.1.2. TJS Raw Vein Quartz
4.1.3. Microstructure and XRD Analysis of the CBG- and TJS-Processed Quartz Sand Samples
4.2. Cathodoluminescence of the CBG and TJS Vein Quartz
4.3. Spot Chemical Composition Analyzed by LA-ICP-MS
4.4. Bulk Chemical Composition Analyzed by ICP-OES
5. Discussion
5.1. Characteristics of Impurities in Vein Quartz
5.2. Impurity Elements Incorporation into the Raw Quartz and Processed Quartz Sand Samples
5.2.1. CBG Vein Quartz
5.2.2. TJS Vein Quartz
5.3. Economic Assessment with CBG and TJS Quartz Vein Deposits
5.4. Genetic Implications for the CBG and TJS Quartz Vein Deposits
6. Summary and Conclusions
- The petrographic investigation carried out on the CBG vein quartz reveals that the major impurities in quartz are mica, feldspar, ankerite, iron-compounds, and trace apatite, as well as silicate melt and fluids inclusions. The TJS vein quartz is characterized by a low content of mineral inclusions, being only ankerite and albite, while the silicate melt and the fluid inclusions are common in it.
- The analysis by LA-ICP-MS shows that the SiO2 contents of CBG and TJS raw quartz are very high (CBG: 99.953–99.971 wt.%, TJS: 99.969–99.976 wt.%) with very low content of the impurity elements, except for Ca, which is contained mostly in ankerite and silicate melt and fluids inclusions.
- The CBG quartz vein is produced under stable hydrothermal conditions, which resulted from the original metal-rich metamorphic fluid. However, the TJS quartz vein is formed by episodic hydrothermal activity, and the initial hydrothermal fluid has low contents of metals.
- Bulk solution ICP-OES analysis demonstrated that the SiO2 contents of the CBG and TJS processed quartz sand samples are 99.994 wt.% and 99.991 wt.%, respectively. The total impurity contents are 56.8 µg·g−1 with 13.1 µg·g−1 Al and 6.6 µg·g−1 Ti for the CBG processed quartz sand, while they were 85.2µg·g−1 with 29.4 µg·g−1 Al and 6.1 µg·g−1 Ti for the TJS quartz sand. Since the main impurities in these two quartz samples are silicate melt, fluid, and mineral inclusions, they can be removed by more advanced purification processing. Therefore, these two quartz vein deposits have the potential to be raw materials for HPQ.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rösler, H.J. Lehrbuch der Mineralogie, 2. Aufl.; VEB Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1981; p. 833. [Google Scholar]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Miner. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Ding, Y.Z.; Lu, J.W.; Yin, W.Z. Research on purifying low-grade quartz ore by flotation. Metal Mine 2009, 5, 84–87, (In Chinese with English Abstract). [Google Scholar]
- Li, X.L.; Zhang, Q.D.; Xu, H.X. Research on purification of quartz sand in Sichun. Conserv. Util. Miner. Resour. 2014, 2, 35–38, (In Chinese with English Abstract). [Google Scholar]
- Englert, A.H.; Rodrigues, R.T.; Rubio, J. Dissolved air flotation (DAF) of fine quartz particles using an amine as collector. Int. J. Miner. Process. 2009, 90, 27–34. [Google Scholar] [CrossRef]
- Haus, R. High demands on high purity. Ind. Miner. 2005, 10, 62–67. [Google Scholar]
- Haus, R.; Prinz, S.; Priess, C. Assessment of High Purity Quartz Resources. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–51. [Google Scholar]
- Moore, P. High-purity quartz. Ind. Miner. 2005, 455, 53–57. [Google Scholar]
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralisation in kyanite quartzites, Norway. Miner. Depos. 2007, 42, 523–535. [Google Scholar] [CrossRef]
- Shen, S.F. The actuality of study and manufacture in higher purity quartz. China Non-Met. Min. Ind. Her. 2006, 5, 13–16, (In Chinese with English Abstract). [Google Scholar]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and Chemical Characterisation of High-Purity Quartz Deposits with Examples from Norway. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 71–118. [Google Scholar]
- Haßler, S.; Kempe, U.; Götze, J. Trace element content of quartz from the Ehrenfriedersdorf Sn-W deposit. In Mineral Deposit Research: Meeting the Global Challenge: Proceedings of the Eighth Biennial SGA Meeting Beijing, China, 18–21 August 2005; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Lei, S.M.; Pei, Z.Y.; Zhong, L.L.; Ma, Q.L.; Huang, D.D.; Yang, Y.Y. Study on the technology and mechanism of reverse flotation and hot pressing leaching with vein quartz. Non-Met. Mines 2014, 37, 40–43. [Google Scholar]
- Wang, L.; Li, C.X.; Wang, Y.; Yin, D.Q. China technologies present of high-purity quartz processing and the development propositions. J. Mineral. Petrol. 2011, 31, 110–114, (In Chinese with English Abstract). [Google Scholar]
- Larsen, R.B.; Polve’, M.; Juve, G. Granite pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for high-purity quartz formation. Nor. Geol. Undersøkelse Bull. 2000, 436, 57–65. [Google Scholar]
- Ibrahim, S.S.; Shahien, M.M.; Seliem, A.Q.; Abukhadra, M.R.; Zayed, A.M. Marwit Rod El Leqah Quartz Deposits as a Strategic Source of High Purity Quartz. J. Geosci. Environ. Prot. 2015, 3, 41–47. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xie, Z.F.; Wang, C.L.; Hu, Y.F. Trace Element Concentrations and Mineralogy of Quartz Vein Deposits from Southeastern Hubei Province, China. Minerals 2022, 12, 814. [Google Scholar] [CrossRef]
- Müller, A.; Ihlen, P.M.; Snook, B.; Larsen, R.B.; Flem, B.; Bingen, B.; Williamson, B.J. The Chemistry of Quartz in Granitic Pegmatites of Southern Norway: Petrogenetic and Economic Implications. Econ. Geol. 2015, 110, 1737–1757. [Google Scholar] [CrossRef]
- Goodwin, A.M.; Ridler, R.H. The Abitibi orogenic belt. In Symposium on Basins and Geosynclines of the Canadian Shield; Baer, A.J., Ed.; Geological Association of Canada: Wininipeg, MB, Canada, 1970; Volume 10–15, pp. 1–31. [Google Scholar]
- Leclerc, F.; Harris, L.B.; Bédard, J.H.; Breemen, O.V.; Goulet, N. Structural and Stratigraphic Controls on Magmatic, Vol-canogenic, and Shear Zone-Hosted Mineralization in the Chapais-Chibougamau Mining Camp, Northeastern Abitibi, Canada. Econ. Geol. 2012, 107, 963–989. [Google Scholar] [CrossRef]
- Mathieu, L. Detecting magmatic-derived fluids using pyrite chemistry: Example of the Chibougamau area, Abitibi Subprovince, Québec. Ore. Geol. Rev. 2019, 114, 103–127. [Google Scholar] [CrossRef]
- Fayol, N.; Jébrak, M. Archean Sanukitoid Gold Porphyry Deposits: A New Understanding and Genetic Model from the Lac Bachelor Gold Deposit, Abitibi, Canada. Econ. Geol. 2017, 112, 1913–1936. [Google Scholar] [CrossRef]
- Dimroth, E.; Rocheleau, M.; Mueller, W. Paleogeography, isostasy and crustal evolution of the Archean Abitibi Belt: A com-parison between the Rouyn-Noranda and the Chibougamau-Chapais areas. Can. Inst. Min. Metall. Spec. 1984, 34, 73–91. [Google Scholar]
- Daigneault, R.; St-Julien, P.; Allard, G.O. Tectonic evolution of the northeast portion of the Archean Abitibi greenstone belt, Chibougamau area, Quebec. Can. J. Earth Sci. 1990, 27, 1714–1736. [Google Scholar] [CrossRef]
- Chown, E.H.; Daigneault, R.; Mueller, W.; Mortensen, J.K. Tectonic evolution of the Northern volcanic zone, Abitibi belt, Quebec. Can. J. Earth Sci. 1992, 29, 2211–2225. [Google Scholar] [CrossRef]
- Chown, E.H.; Harrap, R.; Moukhsil, A. The role of granitic intrusions in the evolution of the Abitibi belt, Canada. Precambrian Res. 2002, 115, 291–310. [Google Scholar] [CrossRef]
- Beauregard, A.J.; Gaudreault, D. NI 43-101 Technical Report on the Joe Mann Mining Property; Legault Metal Inc.: Val-D’Or, QC, Canada, 2012. [Google Scholar]
- Xing, F.M.; Xu, X.; Chen, J.F.; Zhou, T.X.; Foland, K.A. The late Proterozoic continental accretionary history of the south-eastern margin of the Yangtze Platform. Acta Geol. Sin. 1992, 66, 59–72, (In Chinese with English Abstract). [Google Scholar]
- Yang, S.C. Geological characteristics and prospecting direction of fluorite deposit in Huangshan area, Anhui Province. China Sci. Technol. Inf. 2009, 1, 18–19, (In Chinese with English Abstract). [Google Scholar]
- Duan, L.A.; Yang, X.Y.; Sun, W.D.; Fang, S.M.; Yu, L.F.; Fan, Z.P. Geological and geochemical characteristics and prospecting prospect of Tianjingshan gold deposit in southern Anhui Province. Acta Geol. Sin. 2011, 85, 6, (In Chinese with English Abstract). [Google Scholar]
- Wu, R.X.; Zheng, Y.F.; Wu, Y.B. Zircon U-Pb age and isotope geochemistry of Neoproterozoic Jingtan volcanics in South Anhui. Geol. J. China Univ. 2007, 13, 282–296, (In Chinese with English Abstract). [Google Scholar]
- Ning, S.; Wang, F.; Xue, W.; Zhou, T. Geochemistry of the Baoshan pluton in the Tongling region of the Lower Yangtze River Belt. Geochimica 2017, 46, 397–412. [Google Scholar]
- Wang, F.; Ge, C.; Ning, S.; Nie, L.; Zhong, G.; White, N. A new approach to LA-ICP-MS mapping and application in geology. Acta Petrol. Sin. 2017, 33, 3422–3436. [Google Scholar]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Z.Y.; Wei, Y.; Liu, B.; Meng, Y.; Qiu, H.; Lei, S.M.; Zhang, X.; Li, Y.B. A Critical Review on the Mineralogy and Processing for High-Grade Quartz. Min. Metall. Explor. 2020, 37, 1627–1639. [Google Scholar] [CrossRef]
- Demir, C.; Gülgonul, I.; Bentli, I.; Çelik, M. Differential separation of albite from microcline by monovalent salts in HF medium. Miner. Metall. Proc. 2003, 20, 120–124. [Google Scholar] [CrossRef]
- Rusk, B.; Lowers, H.A.; Reed, M.H. Trace elements in hydrothermal quartz: Relationships to cathodoluminescent textures and insights into vein formation. Geology 2008, 36, 547–550. [Google Scholar] [CrossRef]
- Rusk, B. Cathodoluminescent textures and trace elements in hydrothermal quartz. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2012; pp. 307–330. [Google Scholar]
- Pan, X.D.; Li, S.Q.; Li, Y.K.; Guo, P.H.; Zhao, X.; Cai, Y.S. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Frezzotti, M.L. Silicate-melt inclusions in magmatic rocks: Applications to petrology. Lithos 2001, 55, 273–299. [Google Scholar] [CrossRef]
- Leeder, O.; Thomas, R.; Klemm, W. Einschlüsse in Mineralen; VEB Deutscher Grundstoffverlag: Leipzig, Germany, 1987. [Google Scholar]
- Webster, J.D. Melt Inclusions in Plutonic Rocks; Short Course Series 36; Mineralogical Association of Canada: Montreal, QC, Canada, 2006; p. 237. [Google Scholar]
- Roedder, E. Fluid Inclusions. Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Van den Kerkhof, A.M.; Hein, U.F. Fluid inclusion petrography. Lithos 2001, 55, 27–47. [Google Scholar] [CrossRef]
- Blankenburg, H.J.; Götze, J.; Schulz, H. Quarzrohstoffe; Deutscher Verlag für Grundstof-Findustrie: Leipzig-Stuttgart, Germany, 1994; p. 296. [Google Scholar]
- Götze, J.; Plötze, M.; Graupner, T.; Hallbauer, D.K.; Bray, C. Trace element incorporation into quartz: A combined study by ICP-MS, electron spin resonance, cathodoluminescence, capillary ion analysis and gas chromatography. Geochim. Cosmochim. Acta 2004, 68, 3741–3759. [Google Scholar] [CrossRef]
- Gemeinert, M.; Gaber, M.; Hager, I.; Willfahrt, M.; Bortschuloun, D. On correlation of gas-liquid-inclusion’s properties and melting behaviour of different genetic quartzes for production of transparent fused silica. Neues Jahrb. Mineral. Abh. 1992, 165, 19–27. [Google Scholar]
- Kitamura, R.; Pilon, L.; Jonasz, M. Optical constants of silica glass from extreme ultraviolet to far infrared at near room tem-perature. Appl. Opt. 2007, 46, 8118–8133. [Google Scholar] [CrossRef]
- Dos Santos, M.F.M.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Processing of quartz lumps rejected by silicon industry to obtain a raw material for silica glass. Int. J. Miner. Process. 2015, 135, 65–70. [Google Scholar] [CrossRef]
- Jourdan, A. Elemental and isotopic zoning in natural Alpine quartz crystals. Transplantation 2008, 29, 405–408. [Google Scholar]
- Lehmann, K.; Berger, A.; Götte, T.; Ramseyer, K.; Wiedenbeck, M. Growth related zonations in authigenic and hydrothermal quartz characterized by SIMS-, EPMA-, SEM-CL- and SEM-CC-imaging. Miner. Mag. 2009, 73, 633–644. [Google Scholar] [CrossRef]
- Müller, A.; Wiedenbeck, M.; Van den Kerkhof, A.M.; Kronz, A.; Simon, K. Trace elements in quartz—A combined electron microprobe, secondary ion mass spectrometry, laser-ablation ICP-MS, and cathodoluminescence study. Eur. J. Miner. 2003, 15, 747–763. [Google Scholar] [CrossRef]
- Miyoshi, N.; Yamaguchi, Y.; Makino, K. Successive zoning of Al and H in hydrothermal vein quartz. Am. Miner. 2005, 90, 310–315. [Google Scholar] [CrossRef]
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Miner. Mag. 2009, 73, 569–584. [Google Scholar] [CrossRef]
- Dhamrin, M.; Saitoh, T.; Kamisako, K.; Yamada, K.; Araki, N.; Yamaga, I.; Sugimoto, H.; Tajima, M. Technology development of high-quality n-type multicrystalline silicon for next-generation ultra-thin crystalline silicon solar cells. Sol. Energ. Mat. Sol. Cells 2009, 93, 1139–1142. [Google Scholar] [CrossRef]
- Griscom, D.L. Self-trapped holes in pure-silica glass: A history of their discovery and characterization and an example of their critical significance to industry. J. Non-Cryst. Solids 2006, 352, 2601–2617. [Google Scholar] [CrossRef]
- Minami, T.; Maeda, S.; Higasa, M.; Kashima, K. In-situ observation of bubble formation at silicon melt–silica glass interface. J. Cryst. Growth 2011, 318, 196–199. [Google Scholar] [CrossRef]
- Dos Santos, M.F.M.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Quartz resources in the Serra de Santa Helena formation, Brazil: A geochemical and technological study. J. S. Am. Earth Sci. 2014, 56, 328–338. [Google Scholar] [CrossRef]
- Dos Santos, M.F.M.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Quartz sand resources in the Santa Maria Eterna formation, Bahia, Brazil: A geochemical and morphological study. J. S. Am. Earth Sci. 2015, 62, 176–185. [Google Scholar] [CrossRef]
- Götze, J. Mineralogy, geochemistry and cathodoluminescence of authigenic quartz from different sedimentary rocks. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–306. [Google Scholar]
- Fruth, M.; Blankenburg, H.J. Charaketerisierung von authigenen idiomorphen Kohle-und Salinarquartzen durch Ein-schlussuntersuchungen. Neues Jahrb. Mineral. Abh. 1992, 165, 53–64. [Google Scholar]
- Götze, J.; Pan, Y.; Müller, A.; Kotova, E.; Cerin, D. Trace Element Compositions and Defect Structures of High-Purity Quartz from the Southern Ural Region, Russia. Minerals 2017, 7, 189. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.Y.; Li, Y.B.; Liu, Y.Y.; Wei, Z.L.; Lei, S.M. Separation mechanism of lattice-bound trace elements from quartz by KCl-doping calcination and pressure leaching. Miner. Eng. 2018, 125, 42–49. [Google Scholar] [CrossRef]
- Dal Martello, E.; Tranell, G.; Gaal, S.; Raaness, O.S.; Tang, K.; Arnberg, L. Study of Pellets and Lumps as Raw Materials in Silicon Production from Quartz and Silicon Carbide. Metall. Mater. Trans. B 2011, 42, 939–950. [Google Scholar] [CrossRef]
- Belashev, B.Z.; Skamnitskaya, L.S. Irradiation methods for removal of fluid inclusion from minerals. RMZ–Mater. Geoenviron. 2009, 56, 138–147. [Google Scholar]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Miner. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Monecke, T.; Kempe, U.; Götze, J. Genetic significance of the trace element content in metamorphic and hydrothermal quartz: A reconnaissance study. Earth Planet. Sci. Lett. 2002, 202, 709–724. [Google Scholar] [CrossRef]








| Sample | Classification | SiO2 Content (wt.%) | Al | Ti | Na | Ca | K | Li | Fe | P | B | Sum | Analytical Instrument |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBG | spot | 99.971 | 10.7 | 1.9 | 14.0 | 219.1 | 2.3 | dbl | 34.2 | dbl | 4.8 | 286.9 | LA-ICP-MS |
| 99.953 | 10.8 | 1.5 | 10.2 | 279.2 | dbl | dbl | 158.4 | 12.0 | 2.0 | 474.1 | |||
| 99.966 | 11.5 | 2.0 | 10.5 | 207.6 | 30.1 | 0.6 | 1.7 | 71.2 | 2.7 | 337.9 | |||
| 99.969 | 9.5 | 2.0 | 51.2 | 226.4 | 9.0 | 0.7 | dbl | 5.4 | 3.3 | 307.5 | |||
| raw | 99.866 | 470.1 | 7.9 | 125.3 | 384.8 | 132.1 | dbl | 212.4 | 5.1 | 5.5 | 1343.1 | ICP-OES | |
| processed | 99.994 | 13.1 | 6.6 | <11.2 | 6.3 | dbl | dbl | 12.9 | 3.3 | 3.3 | 56.8 | ||
| TJS | spot | 99.975 | 16.6 | 0.5 | 18.3 | 199.6 | dbl | dbl | 0.0 | 6.2 | 4.5 | 245.7 | LA-ICP-MS |
| 99.973 | 19.6 | 0.6 | 19.9 | 196.5 | dbl | 0.1 | 12.8 | 13.8 | 3.3 | 266.6 | |||
| 99.969 | 14.3 | 2.8 | 7.9 | 219.2 | dbl | 0.1 | 41.7 | 23.9 | 3.4 | 313.3 | |||
| 99.976 | 11.5 | 1.2 | 9.0 | 214.1 | 1.3 | 0.1 | dbl | 0.1 | 2.7 | 240.0 | |||
| raw | 99.980 | 97.4 | 6.4 | 42.3 | 14.3 | 20.2 | dbl | 6.8 | 4.0 | 5.3 | 196.8 | ICP-OES | |
| processed | 99.991 | 29.4 | 6.1 | 33.1 | 0.9 | <5.7 | 2.0 | dbl | 3.6 | 4.5 | 85.2 | ||
| HPQ standard | 99.995 | <30.0 | <10.0 | <8.0 | <5.0 | <8.0 | <5.0 | <3.0 | <2.0 | <1.0 | <50.0 | ICP-OES | |
| Refined HPQ powder | Iota STD | 99.998 | 16.2 | 1.3 | 0.9 | 0.5 | 0.6 | 0.9 | 0.2 | 0.1 | 0.1 | 20.8 | |
| Iota 8 | 99.999 | 7.0 | 1.2 | 0.0 | 0.5 | <0.04 | <0.02 | <0.03 | 0.1 | <0.08 | 9.0 | ||
| Drag NC1 | 99.996 | 26.0 | 4.0 | 2.7 | 0.6 | 0.7 | 4.0 | 0.5 | dbl | <0.4 | 38.9 | ||
| Drag NCA | 99.999 | 7.0 | 4.0 | 0.7 | 0.1 | 0.3 | 0.7 | 0.1 | dbl | dbl | 12.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, M.; Sun, C.; Yang, X.; Chen, J. Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification. Minerals 2023, 13, 261. https://doi.org/10.3390/min13020261
Xia M, Sun C, Yang X, Chen J. Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification. Minerals. 2023; 13(2):261. https://doi.org/10.3390/min13020261
Chicago/Turabian StyleXia, Mei, Chao Sun, Xiaoyong Yang, and Jian Chen. 2023. "Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification" Minerals 13, no. 2: 261. https://doi.org/10.3390/min13020261
APA StyleXia, M., Sun, C., Yang, X., & Chen, J. (2023). Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification. Minerals, 13(2), 261. https://doi.org/10.3390/min13020261