The System KCl–CaCO3–MgCO3 at 3 GPa
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The System KCl–CaCO3
3.2. The System KCl–MgCO3
3.3. The System KCl–CaCO3–MgCO3
4. Discussion
4.1. Phase Relations in the KCl–CaCO3 and KCl–MgCO3 Binaries at 3 and 6 GPa
4.2. Phase Relations in the KCl–CaCO3–MgCO3 Ternary at 3 and 6 GPa
4.3. Comparison with the K2CO3–CaCO3–MgCO3 System
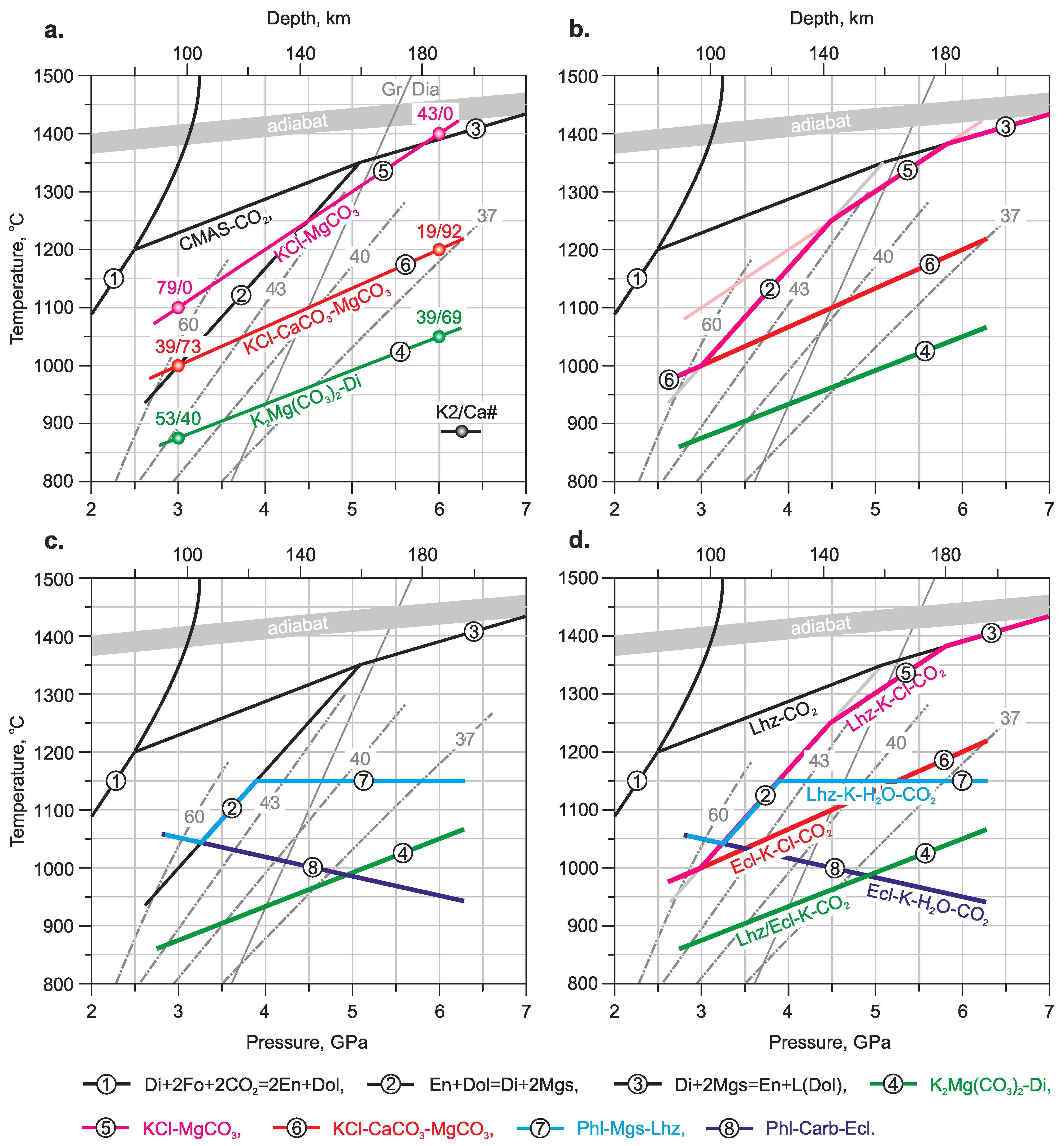
4.4. Implications for Peridotite and Eclogite Solidi
5. Conclusions
- The KCl–CaCO3 binary has eutectic type T-X diagrams. At 900 °C, the subsolidus assemblage is represented by KCl + aragonite, while at 1000 °C by KCl + calcite. The KCl–calcite eutectic is situated at 1000 °C and K2# 56, where K2# = 2KCl/(2KCl + CaCO3 + MgCO3) × 100 mol%. The KCl–MgCO3 binary has eutectic type T-X diagrams. At 900–1000 °C, the subsolidus assemblage is represented by KCl + magnesite. The KCl-magnesite eutectic is situated at 1100 °C and K2# 79.
- In the KCl–CaCO3–MgCO3 ternary subsolidus assemblage consists of KCl and Ca-Mg carbonates. Just below solidus, the system is divided into two partial ternaries: KCl + magnesite + dolomite and KCl + calcite–dolomite solid solutions. The melting of both partial ternaries was established at 1000 °C. The system has one eutectic situated at K2#/Ca# 39/73 and two peritectics: KCl + dolomite = magnesite + liquid at K2#/Ca# 40/68 and Ca-dolomite + magnesite = dolomite + liquid, where Ca# = 100∙Ca/(Ca + Mg) × 100 mol%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Navon, O.; Hutcheon, I.; Rossman, G.; Wasserburg, G. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Brine inclusions in diamonds: A new upper mantle fluid. Earth Planet. Sci. Lett. 2001, 187, 323–332. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Wirth, R.; Fedorova, E.N.; Sobolev, N.V. Nanometre-sized mineral and fluid inclusions in cloudy Siberian diamonds: New insights on diamond formation. Eur. J. Mineral. 2008, 20, 317–331. [Google Scholar]
- Kamenetsky, V.S.; Maas, R.; Kamenetsky, M.B.; Paton, C.; Phillips, D.; Golovin, A.V.; Gornova, M.A. Chlorine from the mantle: Magmatic halides in the Udachnaya-East kimberlite, Siberia. Earth Planet. Sci. Lett. 2009, 285, 96–104. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: New minerals in the carbonate-halide association. Can. Mineral. 2013, 51, 669–688. [Google Scholar] [CrossRef]
- Weiss, Y.; McNeill, J.; Pearson, D.G.; Nowell, G.M.; Ottley, C.J. Highly saline fluids from a subducting slab as the source for fluid-rich diamonds. Nature 2015, 524, 339–342. [Google Scholar]
- Jablon, B.M.; Navon, O. Most diamonds were created equal. Earth Planet. Sci. Lett. 2016, 443, 41–47. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Griffin, W.L. Diamond formation during metasomatism of mantle eclogite by chloride-carbonate melt. Contrib. Mineral. Petrol. 2018, 173, 84. [Google Scholar]
- Weiss, Y.; Czas, J.; Navon, O. Fluid inclusions in fibrous diamonds. Rev. Mineral. Geochem. 2022, 88, 475–532. [Google Scholar]
- Sharygin, I.S.; Golovin, A.V.; Tarasov, A.A.; Dymshits, A.M.; Kovaleva, E. Confocal Raman spectroscopic study of melt inclusions in olivine of mantle xenoliths from the Bultfontein kimberlite pipe (Kimberley cluster, South Africa): Evidence for alkali-rich carbonate melt in the mantle beneath Kaapvaal Craton. J. Raman Spectrosc. 2021, 53, 508–524. [Google Scholar] [CrossRef]
- Abersteiner, A.; Golovin, A.; Chayka, I.; Kamenetsky, V.S.; Goemann, K.; Rodemann, T.; Ehrig, K. Carbon Compounds in the West Kimberley Lamproites (Australia): Insights from Melt and Fluid Inclusions. Gondwana Res. 2022, 109, 536–557. [Google Scholar] [CrossRef]
- Abersteiner, A.; Giuliani, A.; Kamenetsky, V.S.; Phillips, D. Petrographic and melt-inclusion constraints on the petrogenesis of a magmaclast from the Venetia kimberlite cluster, South Africa. Chem. Geol. 2017, 455, 331–341. [Google Scholar] [CrossRef]
- Kopylova, M.; Navon, O.; Dubrovinsky, L.; Khachatryan, G. Carbonatitic mineralogy of natural diamond-forming fluids. Earth Planet. Sci. Lett. 2010, 291, 126–137. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Fluid and mineral inclusions in cloudy diamonds from Koffiefontein, South Africa. Geochim. Et Cosmochim. Acta 2004, 68, 2561–2575. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, V.V.; Pokhilenko, N.P.; Mal’kovets, V.G.; Kolesov, B.A.; Sobolev, N.V. Secondary melt inclusions in olivine from unaltered kimberlites of the Udachnaya-East pipe, Yakutia. Dokl. Earth Sci. 2003, 388, 93–96. [Google Scholar]
- Tarasov, A.A.; Golovin, A.V.; Sharygin, I.S. Alkali-containing minerals within melt inclusions in olivine of mantle xenoliths from bultfontein kimberlite pipe (Kaapvaal craton): Evidence on high concentrations of alkalis in kimberlite melts. Geodyn. Tectonophys. 2022, 13, 0662. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Ryabchikov, I.D.; Wirth, R. A primary natrocarbonatitic association in the Deep Earth. Mineral. Petrol. 2016, 110, 387–398. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R.; Matsyuk, S.; Schreiber, A.; Thomas, R. Nyerereite and nahcolite inclusions in diamond: Evidence for lower-mantle carbonatitic magmas. Mineral. Mag. 2009, 73, 797–816. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Maas, R.; Danyushevsky, L.V.; Thomas, R.; Pokhilenko, N.P.; Sobolev, N.V. Kimberlite melts rich in alkali chlorides and carbonates: A potent metasomatic agent in the mantle. Geology 2004, 32, 845–848. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Golovin, A.V.; Maas, R.; Giuliani, A.; Kamenetsky, M.B.; Weiss, Y. Towards a new model for kimberlite petrogenesis: Evidence from unaltered kimberlites and mantle minerals. Earth-Sci. Rev. 2014, 139, 145–167. [Google Scholar]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V.; Kamenetsky, V.S.; Abersteiner, A. Can primitive kimberlite melts be alkali-carbonate liquids: Composition of the melt snapshots preserved in deepest mantle xenoliths. J. Raman Spectrosc. 2020, 51, 1849–1867. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Golovin, A.; Sharygin, I.; Kamenetsky, V.; Korsakov, A.; Yaxley, G. Alkali-carbonate melts from the base of cratonic lithospheric mantle: Links to kimberlites. Chem. Geol. 2018, 483, 261–274. [Google Scholar] [CrossRef]
- Safonov, O.G.; Perchuk, L.L.; Litvin, Y.A. Melting relations in the chloride-carbonate-silicate systems at high-pressure and the model for formation of alkalic diamond-forming liquids in the upper mantle. Earth Planet. Sci. Lett. 2007, 253, 112–128. [Google Scholar] [CrossRef]
- Butvina, V.G.; Safonov, O.G.; Litvin, Y.A. Experimental study of eclogite melting with participation of the H2O-CO2-KCl fluid at 5 GPa. Dokl. Earth Sci. 2009, 427, 956–960. [Google Scholar]
- Safonov, O.G.; Chertkova, N.V.; Perchuk, L.L.; Litvin, Y.A. Experimental model for alkalic chloride-rich liquids in the upper mantle. Lithos 2009, 112, 260–273. [Google Scholar] [CrossRef]
- Safonov, O.G.; Perchuk, L.L.; Yapaskurt, V.O.; Litvin, Y.A. Immiscibility of carbonate-silicate and chloride-carbonate melts in the kimberlite-CaCO3–Na2CO3–KCl system at 4.8 GPa. Dokl. Earth Sci. 2009, 424, 388–392. [Google Scholar] [CrossRef]
- Litasov, K.D.; Safonov, O.G.; Ohtani, E. Origin of Cl-bearing silica-rich melt inclusions in diamonds: Experimental evidence for an eclogite connection. Geology 2010, 38, 1131–1134. [Google Scholar] [CrossRef]
- Litasov, K.D.; Sharygin, I.S.; Shatskiy, A.F.; Ohtani, E.; Pokhilenko, N.P. Experimental constraints on the role of chloride in the origin and evolution of kimberlitic magma. Dokl. Earth Sci. 2010, 435, 1641–1646. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. The KCl−CaCO3−MgCO3 system at 6 GPa: A link between saline and carbonatitic diamond-forming fluids. Chem. Geol. 2022, 604, 120931. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. The systems KCl–CaCO3 and KCl–MgCO3 at 6 GPa. High Press. Res. 2022, 42, 245–258. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Fedoraeva, A.S.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. The NaCl-CaCO3 and NaCl-MgCO3 systems at 6 GPa: Link between saline and carbonatitic diamond forming melts. Am. Mineral. 2023; in press. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. Genetic link between saline and carbonatitic mantle fluids: The system NaCl-CaCO3-MgCO3±H2O±Fe0 at 6 GPa. Geosci. Front. 2022, 13, 101431. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Terasaki, H.; Katsura, T.; Ohtani, E. Performance of semi-sintered ceramics as pressure-transmitting media up to 30 GPa. High Press. Res. 2010, 30, 443–450. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Minin, D.A.; Chanyshev, A.D.; Litasov, K.D. Revision of the CaCO3–MgCO3 phase diagram at 3 and 6 GPa. Am. Mineral. 2018, 103, 441–452. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Pistorius, C.W.F.T. Melting curves of the potassium halides at high pressures. J. Phys. Chem. Solids 1965, 26, 1543–1548. [Google Scholar]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Rashchenko, S.V.; Chanyshev, A.D.; Litasov, K.D. The system K2CO3-CaCO3 at 3 GPa: Link between phase relations and variety of K-Ca double carbonates at ≤ 0.1 and 6 GPa. Phys. Chem. Miner. 2019, 46, 229–244. [Google Scholar] [CrossRef]
- Pistorius, C.W.F.T. Polymorphic transitions of the alkali chlorides at high pressures to 200 °C. J. Phys. Chem. Solids 1964, 25, 1477–1481. [Google Scholar] [CrossRef]
- Druzhbin, D.; Rashchenko, S.; Shatskiy, A.; Crichton, W. New high-pressure, high-temperature CaCO3 polymorph. ACS Earth Space Chem. 2022, 6, 1506–1513. [Google Scholar] [CrossRef]
- Niggli, P. Gleichgewichte zwischen TiO2 und CO2, sowie SiO2 und CO2 in Alkali-, Kalk-Alkali und Alkali-Aluminatschmelzen. Z. Anorg. Und Allg. Chem. 1916, 98, 241–326. [Google Scholar] [CrossRef]
- Eitel, W.; Skaliks, W. Some double carbonates of alkali and earth alkali. Z. Anorg. Und Allg. Chem. 1929, 183, 263–286. [Google Scholar] [CrossRef]
- Cooper, A.F.; Gittins, J.; Tuttle, O.F. The system Na2CO3–K2CO3–CaCO3 at 1 kilobar and its significance in carbonatite petrogenesis. Am. J. Sci. 1975, 275, 534–560. [Google Scholar] [CrossRef]
- Shatskiy, A.; Sharygin, I.S.; Gavryushkin, P.N.; Litasov, K.D.; Borzdov, Y.M.; Shcherbakova, A.V.; Higo, Y.; Funakoshi, K.-i.; Palyanov, Y.N.; Ohtani, E. The system K2CO3-MgCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2013, 98, 1593–1603. [Google Scholar] [CrossRef]
- Shatskiy, A.; Sharygin, I.S.; Litasov, K.D.; Borzdov, Y.M.; Palyanov, Y.N.; Ohtani, E. New experimental data on phase relations for the system Na2CO3–CaCO3 at 6 GPa and 900–1400 °C. Am. Mineral. 2013, 98, 2164–2171. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Sharygin, I.S.; Palyanov, Y.N.; Ohtani, E. Phase relationships in the system K2CO3-CaCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2015, 100, 223–232. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Litasov, K.D. Phase relations in the system Na2CO3–CaCO3–MgCO3 at 3 GPa with implications for carbonatite genesis and evolution. Lithos 2019, 330–331, 74–89. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Litasov, K.D. The K2CO3–CaCO3–MgCO3 system at 6 GPa: Implications for diamond forming carbonatitic melts. Minerals 2019, 9, 558. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. New data on the system Na2CO3–CaCO3–MgCO3 at 6 GPa with implications to the composition and stability of carbonatite melts at the base of continental lithosphere. Chem. Geol. 2019, 515, 50–60. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Shatskiy, A.; Wirth, R.; Tomilenko, A.A.; Ugap’eva, S.S.; Sobolev, N.V. Carbonatite melt in type Ia gem diamond. Lithos 2019, 342–343, 463–467. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Pokhilenko, N.P. Melt pockets in sheared garnet lherzolite xenoliths from the Udachnaya-East kimberlite pipe (Yakutia, Russia). In Proceedings of the 9th International Kimberlite Conference, Frankfurt, Germany, 10–15 August 2008. Extended Abstract No. 9IKC-A-00213. [Google Scholar]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Weiss, Y.; Navon, O.; Nielsen, T.F.D.; Mernagh, T.P. How unique is the Udachnaya-East kimberlite? Comparison with kimberlites from the Slave Craton (Canada) and SW Greenland. Lithos 2009, 112, 334–346. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V. Origin of alkaline carbonates in kimberlites of the Siberian craton: Evidence from melt inclusions in mantle olivine of the Udachnaya-East pipe. Chem. Geol. 2017, 455, 357–375. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Behtenova, A.; Litasov, K.D. The system K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for carbonatite melt compositions in the subcontinental lithospheric mantle. Minerals 2019, 9, 296. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Litasov, K.D. Melting and subsolidus phase relations in the system K2CO3-MgCO3 at 3 GPa. High Press. Res. 2018, 38, 422–439. [Google Scholar] [CrossRef]
- Rashchenko, S.V.; Shatskiy, A.F.; Ignatov, M.A.; Arefiev, A.V.; Litasov, K.D. High-pressure synthesis and crystal structure of non-centrosymmetric K2Ca3(CO3)4. CrystEngComm 2021, 23, 6675–6681. [Google Scholar] [CrossRef]
- Golubkova, A.; Merlini, M.; Schmidt, M.W. Crystal structure, high-pressure, and high-temperature behavior of carbonates in the K2Mg(CO3)2–Na2Mg(CO3)2 join. Am. Mineral. 2015, 100, 2458–2467. [Google Scholar] [CrossRef]
- Pabst, A. Synthesis, properties, and structure of K2Ca(CO3)2, buetschliite. Am. Mineral. 1974, 59, 353–358. [Google Scholar]
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth Planet. Sci. Lett. 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Katsura, T. A revised adiabatic temperature profile for the mantle. J. Geophys. Res. Solid Earth 2022, 127, e2021JB023562. [Google Scholar]
- Eggler, D.H. Peridotite-Carbonate Relations in the System CaO-MgO-SiO2-CO2. Carnegie Inst. Wash. Yearb. 1975, 74, 468–474. [Google Scholar]
- Wyllie, P.J.; Huang, W.L. Inflence of mantle CO2 ingeneration of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Brey, G.; Brice, W.R.; Ellis, D.J.; Green, D.H.; Harris, K.L.; Ryabchikov, I.D. Pyroxene-carbonate reactions in the upper mantle. Earth Planet. Sci. Lett. 1983, 62, 63–74. [Google Scholar] [CrossRef]
- Dalton, J.A.; Presnall, D.C. Carbonatitic melts along the solidus of model lherzolite in the system CaO-MgO-Al2O3-SiO2-CO2 from 3 to 7 GPa. Contrib. Mineral. Petrol. 1998, 131, 123–135. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Bekhtenova, A.; Vinogradova, Y.G.; Stepanov, K.M.; Litasov, K.D. Pyroxene-carbonate reactions in the CaMgSi2O6 ± NaAlSi2O6 + MgCO3 ± Na2CO3 ± K2CO3 system at 3-6 GPa: Implications for partial melting of carbonated peridotite. Contrib. Mineral. Petrol. 2021, 176, 34. [Google Scholar] [CrossRef]
- Shatskiy, A.; Bekhtenova, A.; Arefiev, A.V.; Podborodnikov, I.V.; Vinogradova, Y.G.; Rezvukhin, D.I.; Litasov, K.D. Solidus and melting of carbonated phlogopite peridotite at 3-6.5 GPa: Implications for mantle metasomatism. Gondwana Res. 2022, 101, 156–174. [Google Scholar] [CrossRef]
- Shatskiy, A.; Bekhtenova, A.; Podborodnikov, I.V.; Arefiev, A.V.; Vinogradova, Y.G.; Litasov, K.D. Solidus of carbonated phlogopite eclogite at 3-6 GPa: Implications for mantle metasomatism and ultra-high pressure metamorphism. Gondwana Res. 2022, 103, 188–204. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 1975, 3, 621–624. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V. Melting of K-rich carbonated peridotite at 6-10 GPa and the stability of K-phases in the upper mantle. Chem. Geol. 2011, 281, 333–342. [Google Scholar] [CrossRef]
- Bekhtenova, A.; Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D. Phase relations in carbonate component of carbonatized eclogite and peridotite along subduction and continental geotherms. Gondwana Res. 2021, 94, 186–200. [Google Scholar]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E.; Yaxley, G.M. The solidus of alkaline carbonatite in the deep mantle. Geology 2013, 41, 79–82. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D.; Chanyshev, A.D.; Sharygin, I.S.; Karmanov, N.S.; Ohtani, E. Effect of alkalis on the reaction of clinopyroxene with Mg-carbonate at 6 GPa: Implications for partial melting of carbonated lherzolite. Am. Mineral. 2017, 102, 1934–1946. [Google Scholar] [CrossRef]
- Enggist, A.; Luth, R.W. Phase relations of phlogopite and pyroxene with magnesite from 4 to 8 GPa: KCMAS–H2O and KCMAS–H2O–CO2. Contrib. Mineral. Petrol. 2016, 171, 88. [Google Scholar] [CrossRef]








| # | Phases | K2# | Ca# | Cl2# | n | MgO | CaO | K2O | Cl2 | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | Bulk | 20 | 100 | 20 | - | - | 40.0 | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 100(0) | 6 | b.d.l. | b.d.l. | 50.2(2) | 49.8(3) | - | |
| Arg | - | 100(0) | - | 6 | b.d.l. | 50.0(2) | b.d.l. | b.d.l. | 50.0(2) | |
| 1-2 | Bulk | 80 | 100 | 80 | - | - | 10.0 | 40.0 | 40.0 | 10.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.2(2) | 49.8(3) | - | |
| Arg | - | 100(0) | - | 5 | b.d.l. | 48.8(8) | 1.26(80) | 1.19(80) | 48.8(8) | |
| 1-3 | Bulk | 5 | 95 | 5 | - | 2.38 | 45.1 | 2.50 | 2.50 | 47.5 |
| KCl | 99(0) | - | 100(0) | 5 | b.d.l. | 0.64(21) | 50.0(1) | 49.1(2) | - | |
| Cal | - | 97(2) | - | 9 | 1.64(93) | 48.3(9) | b.d.l. | b.d.l. | 50.0(0) | |
| 4-3 | Bulk | 40 | 92 | 40 | - | 2.40 | 27.6 | 20.0 | 20.0 | 30.0 |
| KCl | 100 | - | 100 | 4 | b.d.l. | b.d.l. | 50.3(2) | 49.6(1) | b.d.l. | |
| Cal | - | 94 | - | 4 | 3.09 | 46.6 | 0.32 | 0.23 | 49.8 | |
| 3-4 | Bulk | 60 | 80 | 60 | - | 4.00 | 16.0 | 30.0 | 30.0 | 20.0 |
| KCl | 100 | - | 100 | 4 | b.d.l. | b.d.l. | 50.3(2) | 49.7(1) | - | |
| Dol | - | 81(2) | - | 7 | 9.50(1.22) | 40.0(1.3) | 0.46(14) | 0.40(15) | 49.6(2) | |
| 1-4 | Bulk | 8 | 75 | 8 | - | 11.5 | 34.5 | 4.0 | 4.0 | 46.0 |
| KCl | 99(0) | - | 100(0) | 5 | b.d.l. | 0.42(12) | 50.1(1) | 49.3(1) | - | |
| Dol | - | 75(14) | - | 12 | 12.4(7.1) | 37.6(7.0) | b.d.l. | b.d.l. | 50.0(0) | |
| 4-1 | Bulk | 50 | 75 | 50 | - | 6.3 | 18.8 | 25.0 | 25.0 | 25.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.3(1) | 49.7(1) | - | |
| Dol | - | 75(6) | - | 5 | 12.5(3.2) | 37.1(3.4) | 0.44(19) | 0.38(26) | 49.6(3) | |
| 4-2 | Bulk | 10 | 60 | 10 | - | 18.0 | 27.0 | 5.0 | 5.0 | 45.0 |
| KCl | 100 | - | 100 | 1 | b.d.l. | b.d.l. | 50.2 | 49.8 | - | |
| Ca-Dol | - | 71(14) | - | 10 | 14.6(6.9) | 35.3(6.9) | b.d.l. | b.d.l. | 49.9(1) | |
| Dol | - | 54(1) | - | 9 | 23.0(4) | 27.0(4) | b.d.l. | b.d.l. | 50.0(1) | |
| 2-1 | Bulk | 43 | 54 | 43 | - | 13.1 | 15.4 | 21.5 | 21.5 | 28.5 |
| KCl | 100(0) | - | 100(0) | 9 | b.d.l. | b.d.l. | 50.2(1) | 49.4(2) | - | |
| Dol | - | 53 | - | 1 | 23.5 | 26.5 | b.d.l. | b.d.l. | 50.0 | |
| 2-2 | Bulk | 8 | 42 | 8 | - | 26.7 | 19.3 | 4.00 | 4.00 | 46.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.3(1) | 49.7(1) | - | |
| Mgs | - | 1(0) | - | 5 | 49.7(0) | 0.27(3) | b.d.l. | b.d.l. | 50.0(0) | |
| 2-3 | Bulk | 40 | 33 | 40 | - | 20.1 | 9.90 | 20.0 | 20.0 | 30.0 |
| KCl | 100(1) | - | 100(0) | 7 | b.d.l. | b.d.l. | 50.2(2) | 49.8(5) | - | |
| Dol | - | - | - | + | - | - | - | - | - | |
| Mgs | - | 1(1) | - | 5 | 49.5(4) | 0.41(34) | b.d.l. | b.d.l. | 50.0(0) | |
| 2-4 | Bulk | 20 | 37 | 20 | - | 25.2 | 14.8 | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 100(0) | 8 | b.d.l. | b.d.l. | 50.1(2) | 49.9(4) | - | |
| Dol | - | - | - | + | - | - | - | - | - | |
| Mgs | - | 1(0) | - | 6 | 49.7(0) | 0.27(3) | b.d.l. | b.d.l. | 50.0(0) | |
| 4-4 | Bulk | 10 | 25 | 10 | - | 33.8 | 11.2 | 5.00 | 5.00 | 45.0 |
| KCl | 100(1) | - | 100(1) | 5 | b.d.l. | b.d.l. | 50.3(3) | 49.7(3) | - | |
| Dol | - | 49 | - | 2 | 25.5 | 24.5 | b.d.l. | b.d.l. | 50.0 | |
| Mgs | - | 1(0) | - | 5 | 49.6(3) | 0.44(29) | b.d.l. | b.d.l. | 50.0(0) | |
| 2-1 | Bulk | 30 | 50 | 30 | - | 17.5 | 17.5 | 15.0 | 15.0 | 35.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.1(0) | 49.9(0) | - | |
| Dol | - | 52(1) | - | 5 | 24.1(6) | 25.9(6) | b.d.l. | b.d.l. | 50.0(0) | |
| 3-1 | Bulk | 20 | 0 | 20 | - | 40.0 | b.d.l. | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.3(1) | 49.7(1) | - | |
| Mgs | - | 0 | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) | |
| 3-2 | Bulk | 70 | 0 | 70 | - | 15.0 | b.d.l. | 35.0 | 35.0 | 15.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.3(1) | 49.7(1) | - | |
| Mgs | - | 0 | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) |
| # | Phases | K2# | Ca# | Cl2# | n | MgO | CaO | K2O | Cl2 | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 3-5 | Bulk | 20 | 100 | 20 | - | - | 40.0 | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.2(2) | 49.8(2) | - | |
| Cal | - | 100(0) | - | 5 | b.d.l. | 50.0(0) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 56 | 100 | 56 | 1 | b.d.l. | 22.0 | 28.0 | 28.0 | 22.0 | |
| 1-1 | Bulk | 20 | 97 | 20 | - | 1.20 | 38.8 | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.1(1) | 49.9(1) | - | |
| Cal | - | 98(0) | - | 5 | 0.93(07) | 49.1(1) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 57(0) | 93(0) | 61(1) | 5 | 1.56(3) | 19.9(2) | 28.2(3) | 30.7(4) | 19.3(4) | |
| 1-2 | Bulk | 80 | 90 | 80 | - | 1.00 | 9.00 | 40.0 | 40.0 | 10.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.0(0) | 50.0(1) | - | |
| Cal | - | 98(1) | - | 5 | 1.01(29) | 49.0(5) | b.d.l. | b.d.l. | 50.0(5) | |
| L | 53(1) | 90(0) | 55(1) | 5 | 2.47(9) | 21.3(4) | 26.3(5) | 27.7(4) | 22.3(4) | |
| 1-3 | Bulk | 5 | 95 | 5 | - | 2.38 | 45.1 | 2.50 | 2.50 | 47.5 |
| Cal | - | 97(0) | - | 9 | 1.75(15) | 48.3(1) | b.d.l. | b.d.l. | 50.0(0) | |
| L | - | - | - | + | - | - | - | - | - | |
| 4-3 | Bulk | 40 | 92 | 40 | - | 2.40 | 27.6 | 20.0 | 20.0 | 30.0 |
| KCl | 100(0) | - | 99(0) | 5 | b.d.l. | b.d.l. | 50.2(2) | 49.8(2) | b.d.l. | |
| Cal | - | 90(2) | - | 6 | 5.02(99) | 44.8(1.0) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 53(1) | 83(0) | 57(1) | 5 | 3.93(8) | 19.6(7) | 26.4(7) | 28.3(3) | 21.7(3) | |
| 3-4 | Bulk | 60 | 80 | 60 | - | 4.00 | 16.0 | 30.0 | 30.0 | 20.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.0(0) | 50.0(1) | - | |
| Dol | - | 76(1) | - | 5 | 11.8(3) | 38.1(4) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 53(0) | 78(0) | 54(0) | 5 | 5.19(13) | 18.6(1) | 26.3(2) | 27.2(2) | 22.8(2) | |
| 1-4 | Bulk | 8 | 82 | 8 | - | 8.28 | 37.7 | 4.00 | 4.00 | 46.0 |
| Cal | - | 83(0) | - | 5 | 8.40(22) | 41.6(3) | b.d.l. | b.d.l. | 50.0(1) | |
| L | 33(1) | 75(1) | 35(1) | 5 | 8.23(29) | 25.1(3) | 16.6(5) | 17.3(4) | 32.7(4) | |
| 4-1 | Bulk | 57 | 75 | 57 | - | 5.38 | 16.1 | 28.5 | 28.5 | 21.5 |
| KCl | 100(0) | - | 99(1) | 5 | b.d.l. | b.d.l. | 50.5(5) | 49.5(5) | - | |
| Dol | - | 74(2) | - | 5 | 13.0(1.1) | 36.8(1.1) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 44(1) | 73(1) | 45(1) | 5 | 7.51(4) | 20.7(7) | 21.8(6) | 22.7(5) | 27.3(5) | |
| 4-2 | Bulk | 7 | 62 | 7 | - | 17.7 | 28.8 | 3.50 | 3.50 | 46.5 |
| Dol | - | 61(1) | - | 5 | 19.5(4) | 30.5(3) | b.d.l. | b.d.l. | 50.0(1) | |
| L | 36(2) | 67(1) | 37(1) | 5 | 107.(5) | 21.3(4) | 17.9(9) | 18.5(7) | 31.5(7) | |
| 2-1 | Bulk | 30 | 50 | 30 | - | 17.5 | 17.5 | 15.0 | 15.0 | 35.0 |
| KCl | 100 | - | 99 | 3 | b.d.l. | b.d.l. | 50.0 | 50.0 | - | |
| Dol | - | 52 | - | 2 | 23.7 | 26.2 | b.d.l. | b.d.l. | 50.1 | |
| Mgs | - | 8(0) | - | 5 | 46.2(1) | 3.80(15) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 40(4) | 63(1) | 41(4) | 5 | 11.2(8) | 18.8(1.4) | 20.0(2.2) | 20.3(2.2) | 29.7(2.2) | |
| 2-2 | Bulk | 8 | 42 | 8 | - | 26.7 | 19.3 | 4.00 | 4.00 | 46.0 |
| Dol | - | 50(1) | - | 5 | 24.8(4) | 25.2(4) | b.d.l. | b.d.l. | 50.0(0) | |
| Mgs | - | 7(1) | - | 5 | 46.4(4) | 3.60(40) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 28(0) | 61(0) | 28(0) | 5 | 14.3(0) | 21.9(1) | 13.8(0) | 14.2(1) | 35.8(1) | |
| 2-3 | Bulk | 40 | 33 | 40 | - | 20.1 | 9.90 | 20.0 | 20.0 | 30.0 |
| KCl | 100(0) | - | 99(1) | 5 | b.d.l. | b.d.l. | 50.1(0) | 49.9(3) | - | |
| Dol | - | 51(1) | - | 8 | 24.4(5) | 25.5(7) | b.d.l. | b.d.l. | 50.0(4) | |
| Mgs | - | 8(0) | - | 5 | 46.1(1) | 3.81(13) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 50 | 57 | 51 | 1 | 10.7 | 14.3 | 25.0 | 25.6 | 24.4 | |
| 2-4 | Bulk | 20 | 37 | 20 | - | 25.2 | 14.8 | 10.0 | 10.0 | 40.0 |
| KCl | 100 | - | 100 | 2 | b.d.l. | b.d.l. | 50.1(0) | 49.9(0) | - | |
| Dol | - | - | - | + | - | - | - | - | - | |
| Mgs | - | 7 | - | 2 | 46.4 | 3.55(34) | b.d.l. | b.d.l. | 50.0 | |
| L | 42 | 61 | 42 | 1 | 11.4 | 17.8 | 20.8 | 21.1 | 28.9 | |
| 4-4 | Bulk | 14 | 25 | 14 | - | 32.3 | 10.8 | 7.00 | 7.00 | 43.0 |
| KCl | 100 | - | 99 | 1 | b.d.l. | b.d.l. | 50.2 | 49.8 | - | |
| Dol | - | 50 | - | 2 | 24.9 | 25.1 | b.d.l. | b.d.l. | 50.0 | |
| Mgs | - | 6 | - | 2 | 46.8 | 3.20 | b.d.l. | b.d.l. | 50.0 | |
| L | 40 | 64 | 40 | 2 | 10.9 | 19.1 | 20.0 | 20.1 | 29.9 | |
| 3-3 | Bulk | 30 | 20 | 30 | - | 28.0 | 7.00 | 15.0 | 15.0 | 35.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.0(0) | 50.0(0) | - | |
| Dol | - | 50(4) | - | 5 | 25.2(2.2) | 24.8(2.2) | b.d.l. | b.d.l. | 50.0(0) | |
| Mgs | - | 5 | - | 1 | 47.3 | 2.74 | b.d.l. | b.d.l. | 50.0 | |
| L | 48 | 64 | 51 | 1 | 9.17 | 16.6 | 24.2 | 25.4 | 24.6 | |
| 3-1 | Bulk | 20 | 0 | 20 | - | 40.0 | b.d.l. | 10.0 | 10.0 | 40.0 |
| KCl | 100(0) | - | 99(1) | 5 | b.d.l. | b.d.l. | 50.3(1) | 49.7(3) | - | |
| Mgs | - | 0(0) | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) | |
| 3-2 | Bulk | 70 | 0 | 70 | - | 15.0 | b.d.l. | 35.0 | 35.0 | 15.0 |
| KCl | 100(0) | - | 100(0) | 5 | b.d.l. | b.d.l. | 50.0(0) | 50.0(0) | - | |
| Mgs | - | 0 | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) |
| # | Phases | K2# | Ca# | Cl2# | n | MgO | CaO | K2O | Cl2 | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | Bulk | 20 | 100 | 20 | - | - | 40.0 | 10.0 | 10.0 | 40.0 |
| Cal | - | 100(0) | - | 5 | b.d.l. | 50.0(0) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 50 | 100 | 50 | 1 | b.d.l. | 25.0 | 25.0 | 25.0 | 25.0 | |
| 4-4 | Bulk | 20 | 99 | 20 | - | 0.40 | 39.6 | 10.0 | 10.0 | 40.0 |
| Cal | - | 100(0) | - | 5 | b.d.l. | 50.0(0) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 47(1) | 97(0) | 47(1) | 5 | 0.72(11) | 25.9(2) | 23.4(3) | 23.7(3) | 26.3(3) | |
| 1-2 | Bulk | 80 | 100 | 80 | - | - | 10.0 | 40.0 | 40.0 | 10.0 |
| KCl | 100 | - | 100 | 2 | b.d.l. | b.d.l. | 50.0 | 50.0 | - | |
| L | 77 | 100 | 77 | 3 | b.d.l. | 11.6 | 38.4 | 38.4 | 11.6 | |
| 4-3 | Bulk | 82 | 96 | 82 | - | 0.36 | 8.64 | 41.0 | 41.0 | 9.00 |
| KCl | 100(0) | - | 100(0) | 7 | b.d.l. | b.d.l. | 50.4(2) | 49.6(1) | - | |
| L | 77(1) | 96(1) | 78(1) | 5 | 0.49(12) | 11.0(5) | 38.6(6) | 39.0(6) | 11.0(6) | |
| 1-3 | Bulk | 7 | 95 | 7 | - | 2.33 | 44.2 | 3.50 | 3.50 | 46.5 |
| Cal | - | 98(0) | - | 5 | 1.14(12) | 48.7(1) | b.d.l. | b.d.l. | 49.8 | |
| L | 28(0) | 89(0) | 28(1) | 5 | 4.00(3) | 32.1(2) | 13.9(2) | 14.1(4) | 35.9(4) | |
| 3-4 | Bulk | 60 | 64 | 60 | - | 7.20 | 12.8 | 30.0 | 30.0 | 20.0 |
| L | 59(0) | 64(0) | 61(1) | 5 | 7.29(8) | 13.1(1) | 29.6(1) | 30.6(3) | 19.4(3) | |
| 1-4 | Bulk | 10 | 79 | 10 | - | 9.45 | 35.6 | 5.00 | 5.00 | 45.0 |
| Cal | - | 84(0) | - | 5 | 8.19(14) | 41.8(2) | b.d.l. | b.d.l. | 50.0(1) | |
| L | 21(0) | 73(0) | 20(0) | 5 | 10.6(1) | 28.9(2) | 10.5(1) | 10.1(2) | 39.9(2) | |
| 4-1 | Bulk | 60 | 72 | 60 | - | 5.60 | 14.4 | 30.0 | 30.0 | 20.0 |
| L | 59 | 72 | 61 | 3 | 5.65 | 14.7 | 29.6 | 30.5 | 19.5 | |
| 2-1 | Bulk | 30 | 50 | 30 | - | 17.5 | 17.5 | 15.0 | 15.0 | 35.0 |
| Mgs | - | 7(1) | - | 6 | 46.4(6) | 3.56(59) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 32(0) | 56(0) | 33(0) | 5 | 15.0(3) | 18.9(0) | 16.1(2) | 16.4(2) | 33.6(2) | |
| 2-2 | Bulk | 8 | 42 | 8 | - | 26.7 | 19.3 | 4.00 | 4.00 | 46.0 |
| Dol | - | 48(1) | - | 6 | 25.9(6) | 24.1(4) | b.d.l. | b.d.l. | 50.0(4) | |
| Mgs | - | 9(1) | - | 7 | 45.5(3) | 4.48(26) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 19(0) | 58(0) | 20(0) | 5 | 17.0(1) | 23.3(2) | 9.74(23) | 9.82(17) | 40.2(2) | |
| 2-4 | Bulk | 21 | 32 | 21 | - | 26.9 | 12.6 | 10.5 | 10.5 | 39.5 |
| Mgs | - | 7(1) | - | 5 | 46.3(3) | 3.68(28) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 34(1) | 55(0) | 34(1) | 5 | 14.8(3) | 18.4(3) | 16.8(6) | 17.2(6) | 32.8(6) | |
| 2-3 | Bulk | 50 | 40 | 50 | - | 15.0 | 10.0 | 25.0 | 25.0 | 25.0 |
| Mgs | - | 4(0) | - | 6 | 48.2(2) | 1.76(21) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 55(0) | 49(1) | 56(0) | 5 | 11.5(3) | 11.1(2) | 27.3(2) | 27.8(2) | 22.2(2) | |
| 3-3 | Bulk | 21 | 1 | 21 | - | 39.3 | 0.20 | 10.5 | 10.5 | 39.5 |
| Mgs | - | 0(0) | - | 5 | 49.8(1) | 0.21(7) | b.d.l. | b.d.l. | 50.0(0) | |
| L | 78(1) | 6(0) | 85(2) | 5 | 10.4(7) | 0.61(4) | 39.0(7) | 42.3(1.1) | 7.7(1.1) | |
| 3-1 | Bulk | 20 | 0 | 20 | - | 40.0 | 0 | 10.0 | 10.0 | 40.0 |
| Mgs | - | 0(0) | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) | |
| L | 79 | 0 | 79 | 1 | 10.5 | b.d.l. | 39.5 | 39.5 | 10.5 | |
| 4-2 | Bulk | 70 | 0 | 70 | - | 15.0 | - | 35.0 | 35.0 | 15.0 |
| KCl | 100 | - | 100 | 1 | b.d.l. | b.d.l. | 50.0 | 50.0 | - | |
| L | 79 | - | 79 | 1 | 10.5 | b.d.l. | 39.5 | 39.5 | 10.5 | |
| 3-2 | Bulk | 79 | 5 | 79 | - | 10.0 | 0.53 | 39.5 | 39.5 | 10.5 |
| L | 79(1) | 5(1) | 83(2) | 5 | 10.0(4) | 0.54(11) | 39.5(4) | 41.6(8) | 8.44(77) |
| # | Phases | K2# | Ca# | Cl2# | n | MgO | CaO | K2O | Cl2 | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 2-1 | Bulk | 85 | 100 | 85 | - | - | 7.50 | 42.5 | 42.5 | 7.50 |
| L | 85(0) | 100(0) | 88(1) | 5 | b.d.l. | 7.28 | 42.7 | 44.2 | 5.79 | |
| 2-2 | Bulk | 97 | 100 | 97 | - | - | 1.50 | 48.5 | 48.5 | 1.50 |
| KCl | 100 | - | 100 | 3 | b.d.l. | b.d.l. | 50.0(0) | 50.0(0) | - | |
| L | 93(1) | 100(0) | 94(1) | 5 | b.d.l. | 3.56(42) | 46.4(4) | 47.2(6) | 2.75(63) | |
| 3-1 | Bulk | 20 | 0 | 20 | - | 40.0 | 0 | 10.0 | 10.0 | 40.0 |
| Mgs | - | 0(0) | - | 5 | 50.0(0) | b.d.l. | b.d.l. | b.d.l. | 50.0(0) | |
| L | 45(0) | 1(0) | 48(0) | 5 | 27.4(1) | b.d.l. | 22.6(1) | 23.8(1) | 26.2(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D. The System KCl–CaCO3–MgCO3 at 3 GPa. Minerals 2023, 13, 248. https://doi.org/10.3390/min13020248
Shatskiy A, Podborodnikov IV, Arefiev AV, Litasov KD. The System KCl–CaCO3–MgCO3 at 3 GPa. Minerals. 2023; 13(2):248. https://doi.org/10.3390/min13020248
Chicago/Turabian StyleShatskiy, Anton, Ivan V. Podborodnikov, Anton V. Arefiev, and Konstantin D. Litasov. 2023. "The System KCl–CaCO3–MgCO3 at 3 GPa" Minerals 13, no. 2: 248. https://doi.org/10.3390/min13020248
APA StyleShatskiy, A., Podborodnikov, I. V., Arefiev, A. V., & Litasov, K. D. (2023). The System KCl–CaCO3–MgCO3 at 3 GPa. Minerals, 13(2), 248. https://doi.org/10.3390/min13020248






