A Review of Fatty Acid Collectors: Implications for Spodumene Flotation
Abstract
1. Introduction
2. Fatty Acid Properties
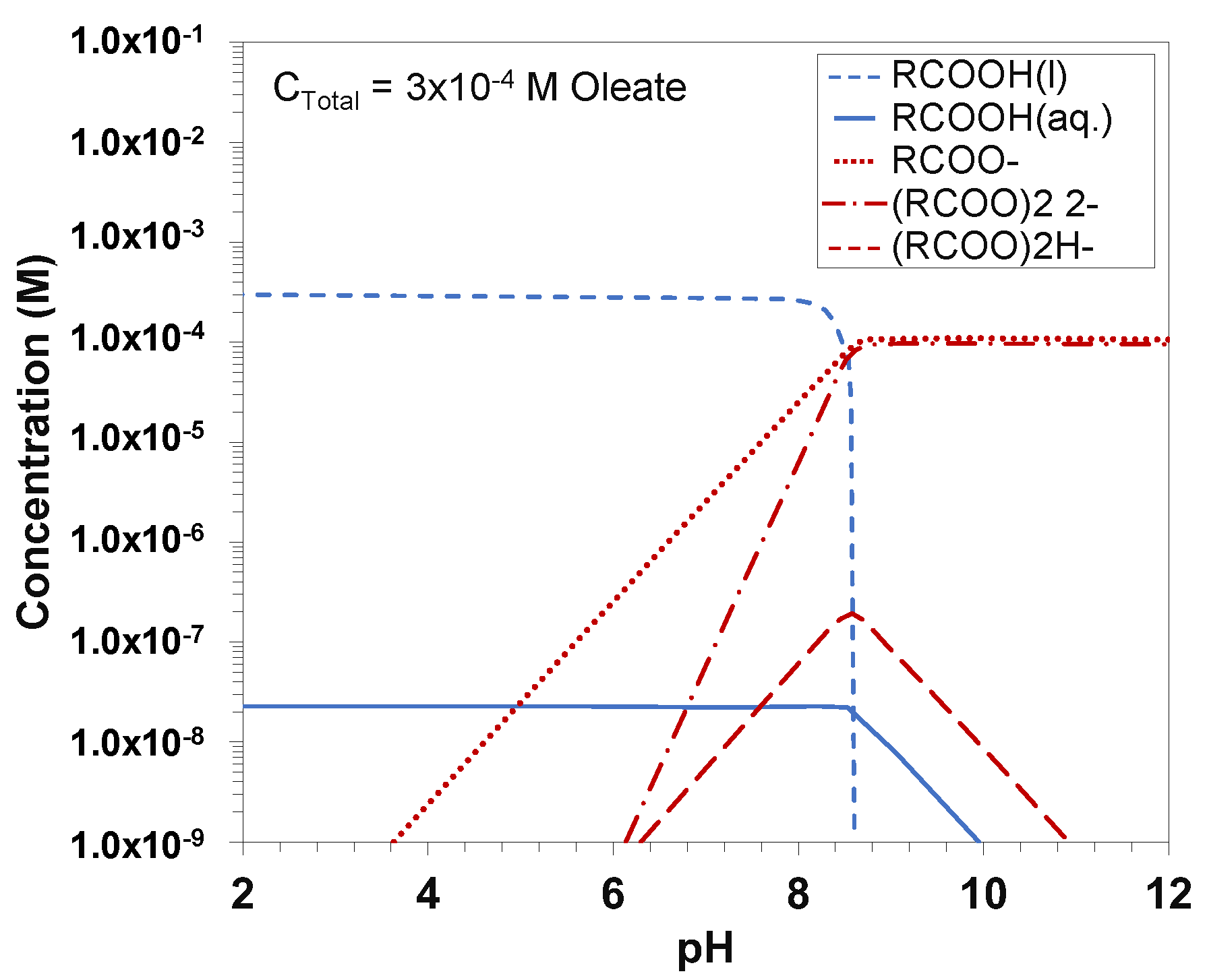
2.1. Distribution of Species in Solution
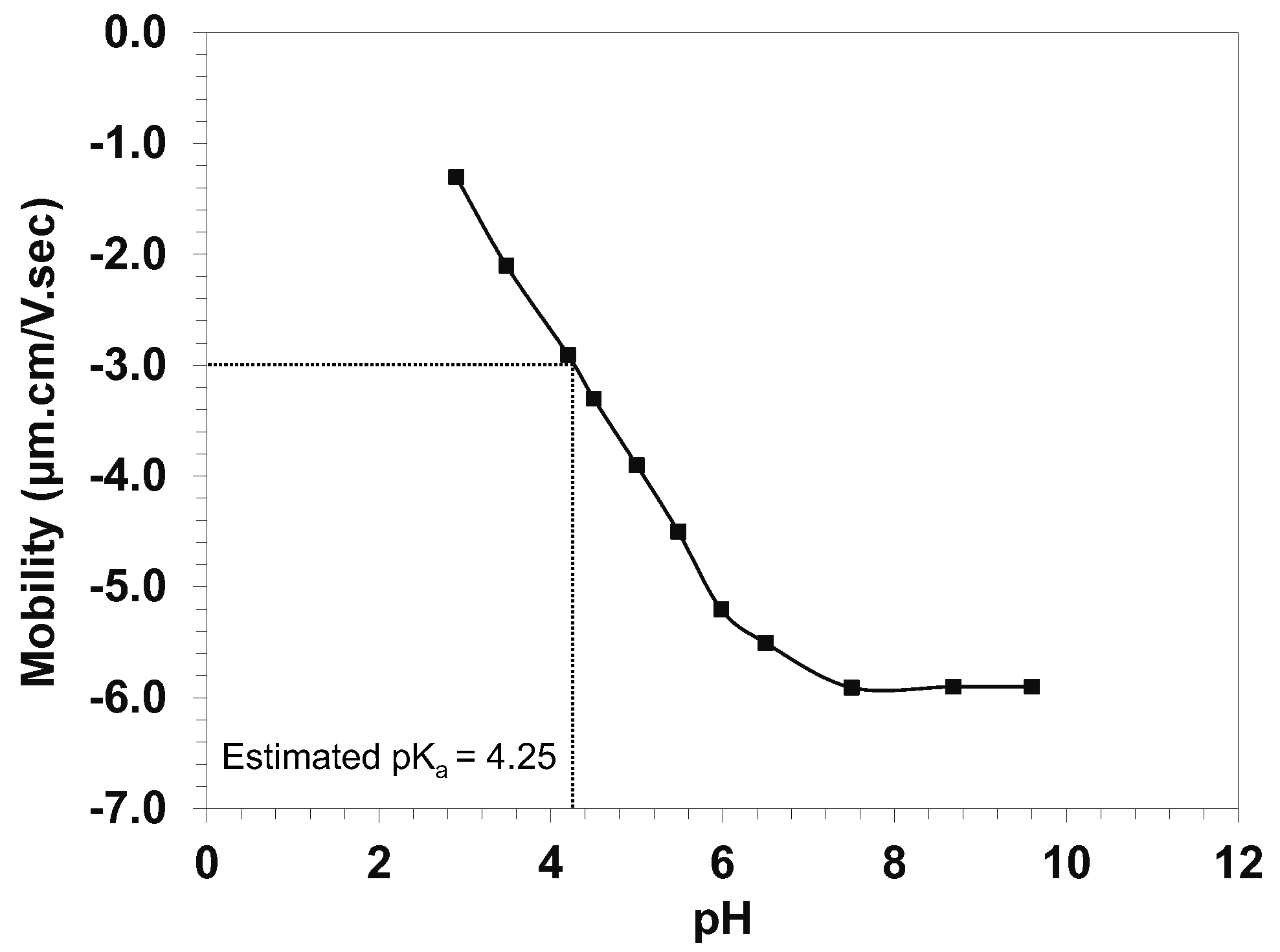
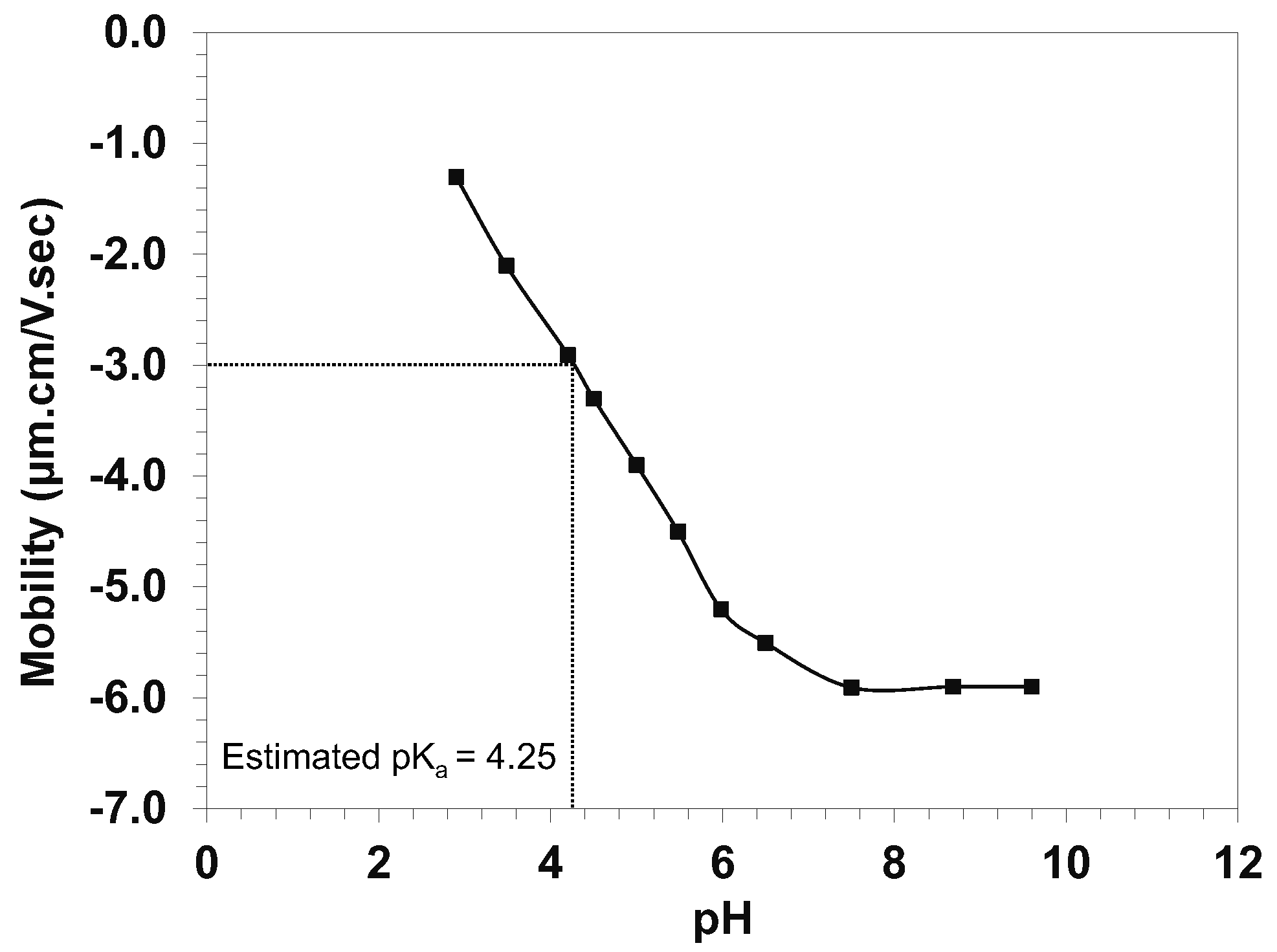
2.2. The pKa Value
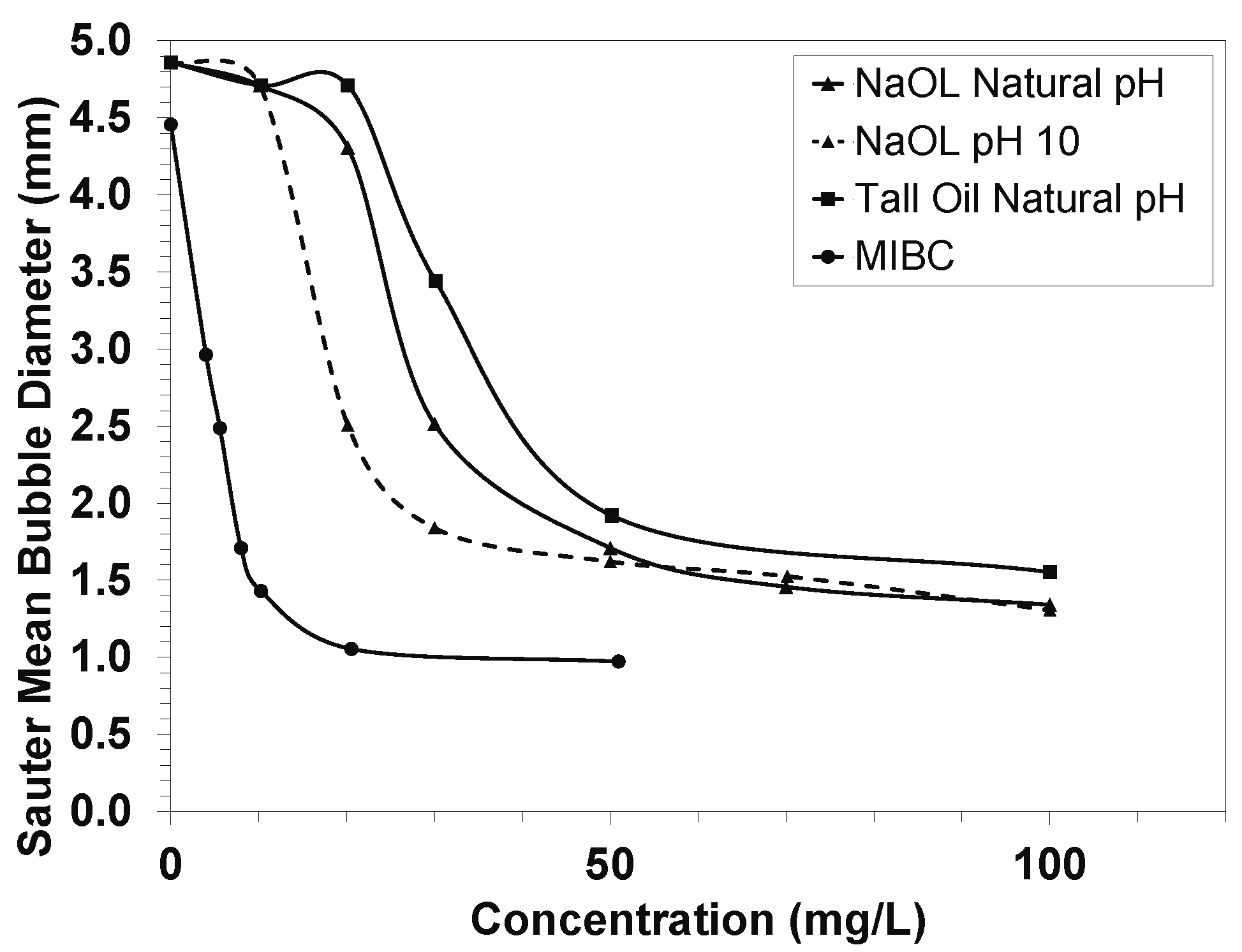
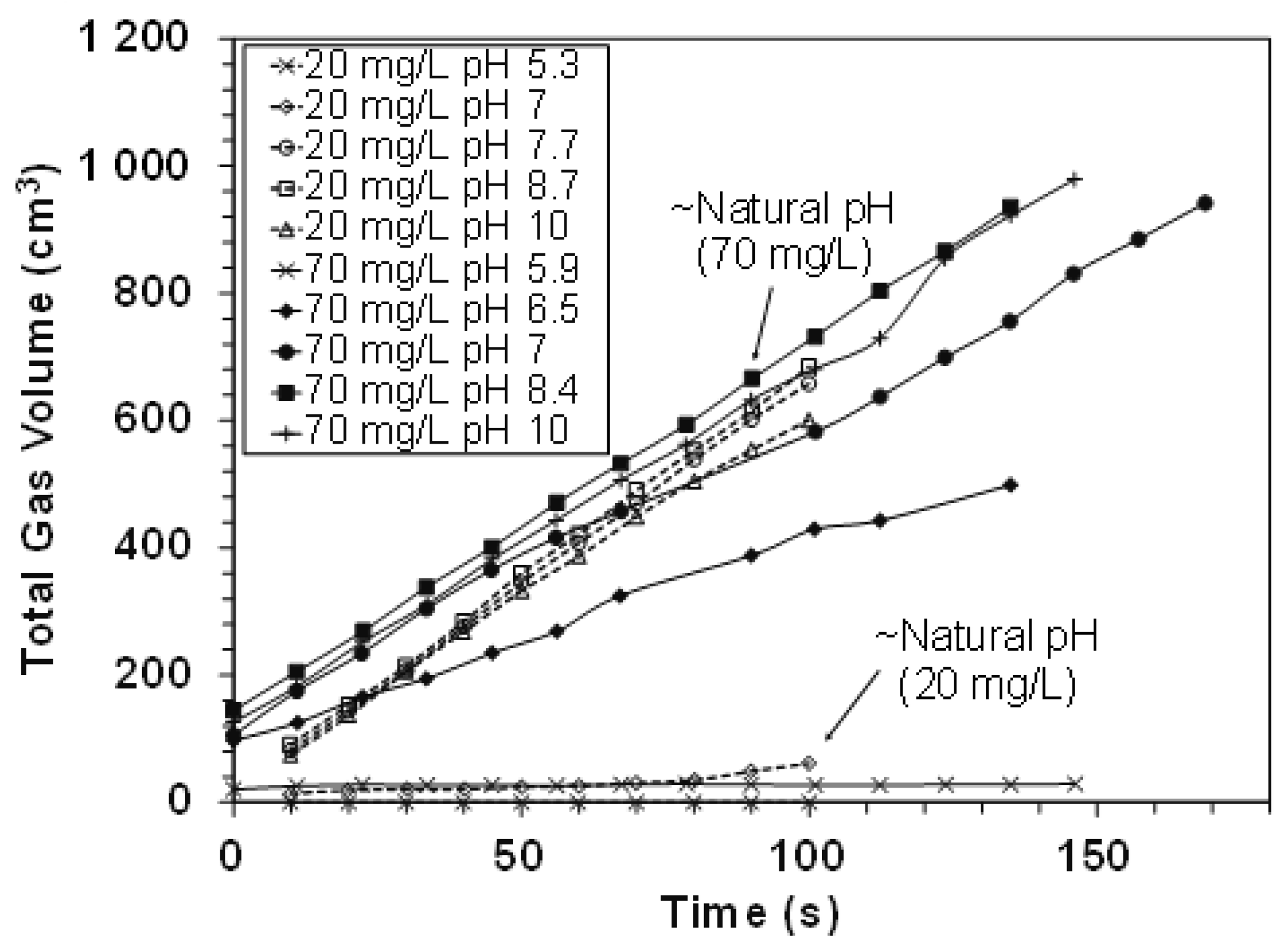
2.3. Frothing Properties
2.4. Solubility
3. Fatty Acids in Spodumene Flotation
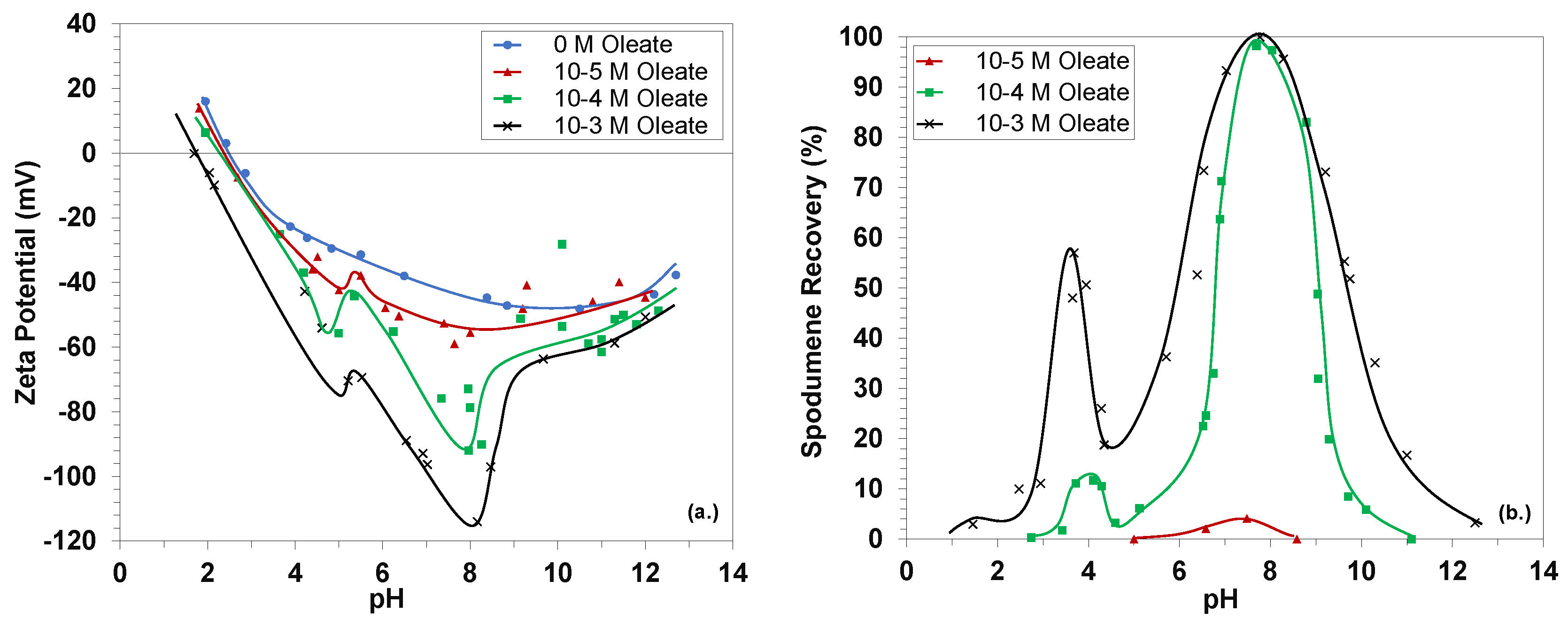
3.1. Fundamentals of the Spodumene-Oleate Flotation System
3.2. Investigations with Real Spodumene Ores
3.2.1. Historical Research (before 2000)
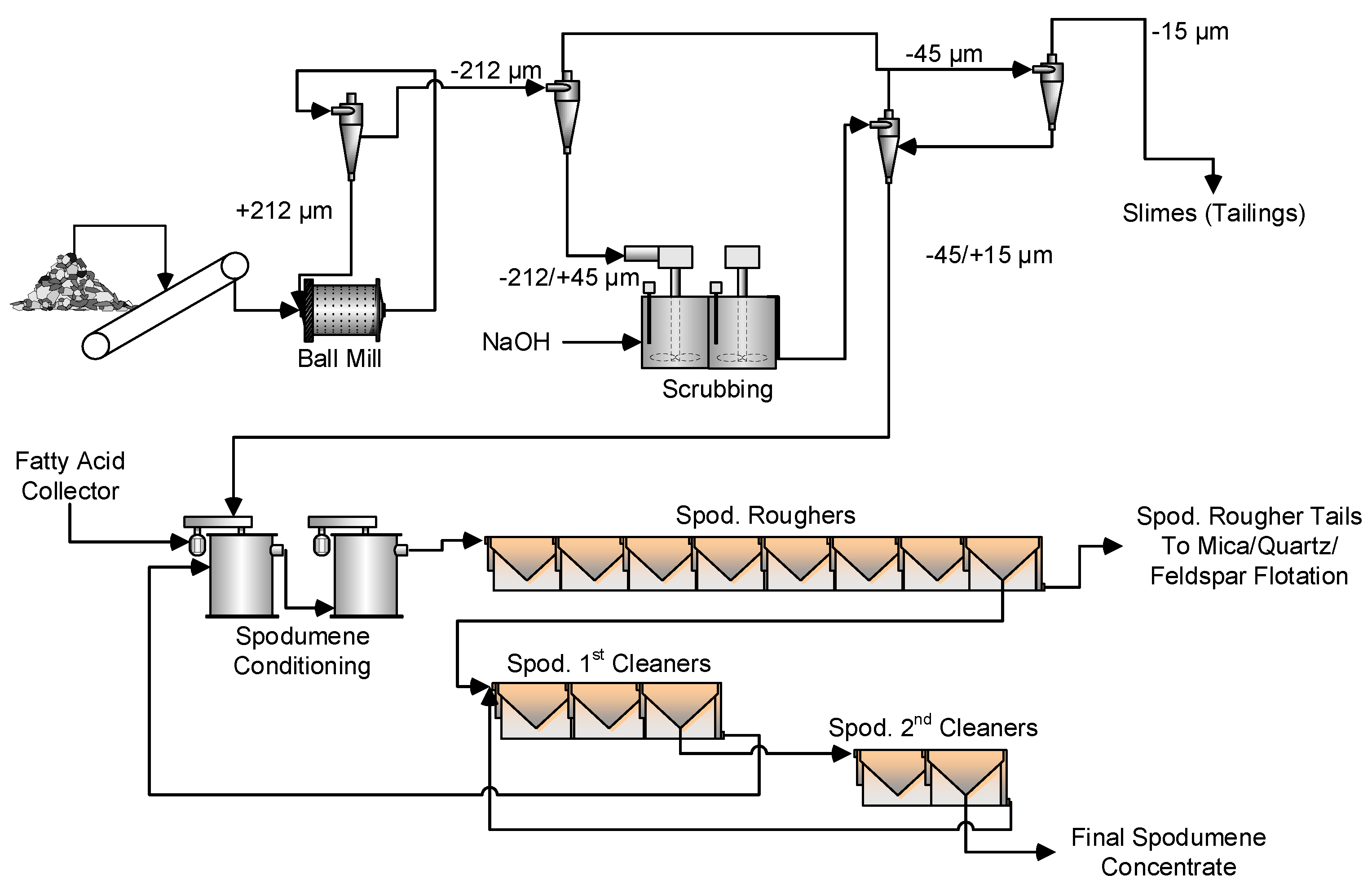
Initial Flowsheet Development and Collector Testing
Pre-Treatment with NaOH
Conditioning with Fatty Acids
Alternate Flowsheets for Spodumene Concentration
The Evolution of Industrial Spodumene Flotation Practice
3.2.2. Recent Publications (2000-Present)
Industrial Projects
Collector Testing
Flotation Optimization Studies
3.3. Fundamental Investigations to Improve Spodumene Flotation
3.3.1. Mixed Anionic/Cationic Collector Systems
3.3.2. Cationic Activators
4. Conclusions and Summary of Research Gaps
5. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedikow, M.; Zelligan, S. NI 43-101 Technical Report on the Zoro Lithium Project; Far Resources Ltd.: Vancouver, BC, Canada, 2018. [Google Scholar]
- Bowell, R.J.; Lagos, L.; de los Hoyos, C.R.; Declercq, J. Classification and Characteristics of Natural Lithium Resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- McCracken, T.; Canosa, J.; Boyko, K.; Wilson, S.; deGagne, R. NI 43-101 Technical Report PAK Property, Red Lake Mining District, Ontario; Frontier Lithium Inc.: Sudbury, ON, Canada, 2021; pp. 13–18. [Google Scholar]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T. The Beneficiation of Lithium Minerals from Hard Rock Deposits. Min. Eng. 2017, 131, 170–184. [Google Scholar]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The Beneficiation of Lithium Minerals from Hard Rock Ores: A Review. Miner. Eng. 2018, 131, 170–184. [Google Scholar] [CrossRef]
- Patriot Battery Metals. Patriot Achieves 6% Li2O Spodumene Concentrate Grade in Preliminary HLS Metallurgical Test Work on the CV5 Pegmatite at the Corvette Property, Quebec; Vancouver, BC, Canada, 4 August 2022. Available online: https://www.globenewswire.com/en/news-release/2022/08/04/2492332/0/en/Patriot-Achieves-6-Li2O-Spodumene-Concentrate-Grade-in-Preliminary-HLS-Metallurgical-Test-Work-on-the-CV5-Pegmatite-at-the-Corvette-Property-Quebec.html (accessed on 16 December 2022).
- Redeker, I.H. Concentration of Spodumene From North Carolina Pegmatite Ores; Society of Mining Engineers of AIME: St. Louis, MO, USA, 1977; p. 161. [Google Scholar]
- Bulatovic, S.M. 2-Collectors. In Handbook of Flotation Reagents; Bulatovic, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 5–41. [Google Scholar] [CrossRef]
- Pearse, M.J. An Overview of the Use of Chemical Reagents in Mineral Processing. Miner. Eng. 2005, 18, 139–149. [Google Scholar] [CrossRef]
- Kraton Corporation. Sylfat 2 Tall Oil Fatty Acid Product Data Sheet. 2021. Available online: https://www.kraton.com/products/pdsDocs/SYLFAT%202LT.pdf? (accessed on 21 December 2022).
- Arbiter, N.; Abshier, J.R.; Crawford, J.W. Attritioning and Conditioning in Spodumene Flotation. Q. Colo. Sch. Mines 1961, 56, 323–332. [Google Scholar]
- Browning, J.S.; McVay, T.L. Beneficiating Spodumene From Pegmatites of Gaston County, NC; Investigation 5729; U.S. Dept of the Interior, Bureau of Mines: Washington, DC, USA, 1961; p. 16. [Google Scholar]
- Pugh, R.; Stenius, P. Solution Chemistry Studies and Flotation Behaviour of Apatite, Calcite and Fluorite Minerals with Sodium Oleate Collector. Int. J. Miner. Process. 1985, 15, 193–218. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of Crystal Chemistry and Surface Properties on Ilmenite Flotation Behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Poniatowski, A.F.; Mehta, N.R.; Shah, D.O. Cooperativity among Molecules at Interfaces in Relation to Various Technological Processes: Effect of Chain Length on the PK a of Fatty Acid Salt Solutions. Langmuir 2000, 16, 172–177. [Google Scholar] [CrossRef]
- Theander, K.; Pugh, R.J. The Influence of PH and Temperature on the Equilibrium and Dynamic Surface Tension of Aqueous Solutions of Sodium Oleate. J. Colloid Interface Sci. 2001, 239, 209–216. [Google Scholar] [CrossRef]
- Atrafi, A.; Gomez, C.O.; Finch, J.A.; Pawlik, M. Frothing Behavior of Aqueous Solutions of Oleic Acid. Miner. Eng. 2012, 36–38, 138–144. [Google Scholar] [CrossRef]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New Reagent Formulations for Selective Flotation of Scheelite from a Skarn Ore with Complex Calcium Minerals Gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Browning, J.S.; Clemmons, B.H.; McVay, T.L. Beneficiating North Carolina Spodumene-Beryl Ores; Investigation 5750; U.S. Dept of the Interior, Bureau of Mines: Washington, DC, USA, 1961; p. 25. [Google Scholar]
- Burt, R.O.; Flemming, J. Spodumene Processing at TANCO; Canadian Institure of Mining, Metallurgy, and Petroleum: Ottawa, ON, Canada, 1986; pp. 499–514. [Google Scholar]
- Bulatovic, S.M. Beneficiation of Fluorite Ores. In Handbook of Flotation Reagents, Flotation of Industrial Minerals; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 57–76. [Google Scholar]
- Shu, K.; Xu, L.; Wu, H.; Peng, L.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z. In Situ Adsorption of Mixed Collectors BHA/DDA in Spodumene-Feldspar Flotation System. Sep. Purif. Technol. 2020, 251, 117325. [Google Scholar] [CrossRef]
- Albemarle Corporation. Spodumene Concentrate, SC 7.2 Concentrate. 2016. Available online: https://www.albemarle.com/storage/components/spodumene_72s.pdf (accessed on 22 December 2022).
- Quast, K.B. A Review of Hematite Flotation Using 12-Carbon Chain Collectors. Miner. Eng. 2000, 13, 1361–1376. [Google Scholar] [CrossRef]
- Quast, K. Flotation of Hematite Using C6–C18 Saturated Fatty Acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Quast, K. Use of Conditioning Time to Investigate the Mechanisms of Interactions of Selected Fatty Acids on Hematite. Part 1: Literature Survey. Miner. Eng. 2015, 79, 295–300. [Google Scholar] [CrossRef]
- Quast, K. Use of Conditioning Time to Investigate the Mechanisms of Interactions of Selected Fatty Acids on Hematite Part II Laboratory Investigations. Miner. Eng. 2015, 79, 301–305. [Google Scholar] [CrossRef]
- Quast, K. Literature Review on the Interaction of Oleate with Non-Sulphide Minerals Using Zeta Potential. Miner. Eng. 2016, 94, 10–20. [Google Scholar] [CrossRef]
- Quast, K. The Use of Zeta Potential to Investigate the Interaction of Oleate on Hematite. Miner. Eng. 2016, 85, 130–137. [Google Scholar] [CrossRef]
- Quast, K. Literature Review on the Use of Natural Products in the Flotation of Iron Oxide Ores. Miner. Eng. 2017, 108, 12–24. [Google Scholar] [CrossRef]
- Quast, K. An Investigation of the Flotation Minimum in the Oleate Flotation of Hematite under Alkaline Conditions. Miner. Eng. 2017, 113, 71–82. [Google Scholar] [CrossRef]
- Quast, K. Direct Measurement of Oleate Adsorption on Hematite and Its Consequences for Flotation. Miner. Eng. 2018, 118, 122–132. [Google Scholar] [CrossRef]
- ArrMaz Products. Mining: Innovative Solutions for Industrial Mineral Processing; ArrMaz Products: Mulberry, FL, USA, 2019. [Google Scholar]
- Moon, K.S.; Fuerstenau, D.W. Surface Crystal Chemistry in Selective Flotation of Spodumene (LiAl[SiO3]2) from Other Aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Yap, S.N.; Mishra, R.K.; Raghavan, S.; Fuerstenau, D.W. The Adsorption of Oleate from Aqueous Solution onto Hematite. In Adsorption from Aqueous Solutions; Springer: Boston, MA, USA, 1981; pp. 119–142. [Google Scholar]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of Oleic Acid Ionic−molecular Complexes in the Flotation of Spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Champion, D. Australian Resource Reviews: Lithium 2018; Australian Government: Geoscience, Australia, 2018. [Google Scholar]
- Pilbara Minerals. Pilgangoora Project. Available online: https://www.pilbaraminerals.com.au/our-company/our-projects/pilgangoora-operation/ (accessed on 23 December 2022).
- Sigma Lithium Resources. Our Project. Available online: https://www.sigmalithiumresources.com/project/ (accessed on 23 December 2022).
- Frontier Lithium. Resource and Assets. Available online: https://www.frontierlithium.com/resource-assets (accessed on 23 December 2022).
- Richard, P.-L.; Frenette, P.; Baril, F.; Roberge, P.R.; Poirier, E.; Joyal, O. Rose Lithium-Tantalum Project Feasibility Study NI 43 101 Technical Report; NI 43 101 Rev. 1; Critical Elements Corporation: Montreal, QC, Canada, 2017; p. 115. [Google Scholar]
- Maguran, D.; Dupere, M.; Gagnon, R.; Anson, J.; Boyd, A.; Gravel, A.-F. NI 43-101 Technical Report on the Estimate to Complete for the Whabouchi Lithium Mine and Shawinigan Electrochemical Plant Nemaska Project; Nemaska Lithium Inc.: James Bay, QC, Canada, 2019; p. 129. [Google Scholar]
- Foremost Lithium Resources. Properties. Available online: https://foremostlithium.com (accessed on 23 December 2022).
- Green Technology Metals. Mining Exploration Flagship Seymour Project. Available online: https://www.greentm.com.au/seymour-project (accessed on 23 December 2022).
- Sayona Mining Ltd.; North American Lithium (NAL). Available online: https://sayonamining.com.au/projects/nal-project/ (accessed on 23 December 2022).
- Sinomine. Sinomine Tanco’s Technical Renovation & Restoration for Spodumene Processing System Officially Put into Operation in Canada. Available online: http://en.sinomine.cn/xwdti/176.html (accessed on 5 January 2023).
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Argus Media. China’s Rongda Resumes Output at Jiajika Spodumene Mine. Available online: https://www.argusmedia.com/en/news/1919877-chinas-rongda-resumes-output-at-jiajika-spodumene-mine (accessed on 12 June 2019).
- Mining Technology. Ewoyaa Lithium Project, Ghana. Available online: https://www.mining-technology.com/projects/ewoyaa-lithium-project-ghana/ (accessed on 5 January 2023).
- Albemarle Corporation. Albemarle—Lithium Resources. Available online: https://www.albemarle.com/businesses/lithium/resources--recycling/lithium-resources (accessed on 16 December 2022).
- Redeker, I.H. Concentration of Spodumene from North Carolina Ores. Mining Eng. April 1979, 395–398. [Google Scholar]
- Piedmont Lithium. Projects—Carolina Lithium. Available online: https://piedmontlithium.com/projects/carolina-lithium/ (accessed on 16 December 2022).
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the Impurities on Lithium Extraction from β-Spodumene in the Sulfuric Acid Process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Norman, J.; Gieseke, E.W. Beneficiation of Spodumene Rock by Froth Flotation; American Institute of Mining and Metallurgical Engineers: Wilkes-Barre, PA, USA, 1940. [Google Scholar]
- McDaniel, W. Flotation of Spodumene and Iron Bearing Minerals from LCA Samples; Confidential Report 80-6; NCSU Archives: Raleigh, NC, USA, 1980. [Google Scholar]
- McDaniel, W. Continued Flotation Study on High-Iron Spodumene Ore; Confidential Report 81-10; NCSU Archives: Raleigh, NC, USA, 1981. [Google Scholar]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective Flotation Separation of Spodumene from Feldspar Using New Mixed Anionic/Cationic Collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, Y.; Zheng, X.; Wang, Y.; Zheng, H.; Lu, D. Understanding the Role of Sodium Hydroxide in the Selective Flotation Separation of Spodumene from Feldspar and Quartz. Miner. Eng. 2020, 159, 106648. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of Lithium from Mineral Resources: State-of-the-Art and Perspectives—A Review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A Review on Methods for Liberating Lithium from Pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Yelatontsev, D.; Mukhachev, A. Processing of Lithium Ores: Industrial Technologies and Case Studies—A Review. Hydrometallurgy 2021, 201, 105578. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Shumin, Z. Research Status of Spodumene Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2021, 42, 321–334. [Google Scholar] [CrossRef]
- Brown, W.H. Carboxylic Acid. In Encyclopedia Britannica; Encyclopaedia Britannica: London, UK, 2022. [Google Scholar]
- Logan, R.L. Tall Oil Fatty Acids. J. Am. Oil Chem. Soc. 1979, 56 Pt 2, 777A–779A. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Somasundaran, P. Acid-Soap Formation in Aqueous Oleate Solutions. J. Colloid Interface Sci. 1988, 122, 104–109. [Google Scholar] [CrossRef]
- Kou, J.; Tao, D.; Xu, G. Fatty Acid Collectors for Phosphate Flotation and Their Adsorption Behavior Using QCM-D. Int. J. Miner. Process. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Atrafi, A. Frothing Properties of Fatty Acid Collectors. Ph.D. Thesis, University of British Colombia, Vancouver, BC, Canada, 2015. [Google Scholar]
- Shen, L.; Liu, L.; Zhu, J.; Qiao, E. Effect of Oleic Acid on Froth Properties and Reverse Flotation Performance of Thermal Coal. Trans. Indian Inst. Met. 2018, 71, 1841–1846. [Google Scholar] [CrossRef]
- PubChem. Oleic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445639 (accessed on 16 December 2022).
- Morrison, R.T. Organic Chemistry, 4th ed.; Allyn and Bacon: Toronto, ON, Canada, 1983. [Google Scholar]
- Ananthapadmanabhan, K.; Somasundaran, P.; Healy, T.W. Chemistry of Oleate and Amine Solutions in Relation to Flotation. Trans. SME-AIME 1980, 266, 2003–2009. [Google Scholar]
- Ananthapadmanabhan, K.P.; Somasundaran, P. Surface Precipitation of Inorganics and Surfactants and Its Role in Adsorption and Flotation. Colloids Surf. 1985, 13, 151–167. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of Degree, Type, and Position of Unsaturation on the PKa of Long-Chain Fatty Acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W. Chapter 4: Structure-Function Relationships of Long Chain Collectors. In Challenges in Mineral Processing; SME Inc.: Littleton, CO, USA, 1989; pp. 51–89. [Google Scholar]
- King, R.P. Principles of Flotation; South African Institute of Mining and Metallurgy Monograph Series; South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1982. [Google Scholar]
- Growney, G.; Lewis, W.C.M. Electrophoresis of Complex Particles as a Function of PH: Effect of Stearic Acid in Ester Particles. Trans. Faraday Soc. 1941, 37, 148–151. [Google Scholar] [CrossRef]
- Mehrishi, J.N.; Seaman, G.V.F. Electrokinetic Properties of Dispersions of Model Compounds of Biological Interest. Trans. Faraday Soc. 1968, 64, 3152–3157. [Google Scholar] [CrossRef]
- Usui, S.; Healy, T.W. Zeta Potential of Stearic Acid Monolayer at the Air–Aqueous Solution Interface. J. Colloid Interface Sci. 2002, 250, 371–378. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of Premicellar Aggregation on the PKa of Fatty Acid Soap Solutions. Langmuir 2003, 19, 2034–2038. [Google Scholar] [CrossRef]
- Christodoulou, A.P.; Rosano, H.L. Effect of PH and Nature of Monovalent Cations on Surface Isotherms of Saturated C16 to C22 Soap Monolayers. In Molecular Association in Biological and Related Systems; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1968; Volume 84, pp. 210–234. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Deamer, D.W.; Cornwell, D.G. Solution of Fatty Acids from Monolayers Spread at the Air-Water Interface: Identification of Phase Transformations and the Estimation of Surface Charge. J. Lipid Res. 1970, 11, 195–200. [Google Scholar] [CrossRef]
- Wellen, B.A.; Lach, E.A.; Allen, H.C. Surface PKa of Octanoic, Nonanoic, and Decanoic Fatty Acids at the Air–Water Interface: Applications to Atmospheric Aerosol Chemistry. Phys. Chem. Chem. Phys. 2017, 19, 26551–26558. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P. Classification of Flotation Frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Sodium Oleate Product Number O 7501; Sigma-Aldrich: Burlington, MA, USA, 2021. [Google Scholar]
- Pradip; Fuerstenau, D.W. Design and Development of Novel Flotation Reagents for the Beneficiation of Mountain Pass Rare-Earth Ore. Miner. Metall. Process. 2013, 30, 1–9. [Google Scholar] [CrossRef]
- Ralston, A.W.; Hoerr, C.W. The Solubilities of the Normal Saturated Fatty Acids. J. Org. Chem. 1942, 7, 546–555. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Miller, J.D.; Kuhn, M.C. Chemistry of Flotation; Society of Mining Engineers of the America Institute of Mining, Metallurgical and Petroleum Engineers, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Eggenberger, D.N.; Broome, F.K.; Ralston, A.W.; Harwood, H.J. The Solubilities of the Normal Saturated Fatty Acids in Water. J. Org. Chem. 1949, 14, 1108–1110. [Google Scholar] [CrossRef]
- Munson, G.A.; Erickson, K.L. Studies on the Flotation of Spodumene from the Edison Mine, Keystone, South Dakota; Report of Investigations 3892; U.S. Dept of the Interior, Bureau of Mines: Washington D.C., USA, 1946. [Google Scholar]
- Weir, L.J.; Moskovits, E.E. Flotation of Lithium and Berylium Pegmatites. AIMM Proc. 1963, 206, 143–153. [Google Scholar]
- Bale, M.D.; May, A.V. Processing of Ores to Produce Tantalum and Lithium. Miner. Eng. 1989, 2, 299–320. [Google Scholar] [CrossRef]
- Davidson, D.; Elliott, A.; Kingsnorth, D.J. Spodumene: A Mineral Source of Lithium; Perth: Kalgoorie, WA, USA, 1989; pp. 103–110. [Google Scholar]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene Flotation Mechanism. Minerals 2019, 9, 372. [Google Scholar] [CrossRef]
- Jie, Z.; Weiqing, W.; Jing, L.; Yang, H.; Qiming, F.; Hong, Z. Fe(III) as an Activator for the Flotation of Spodumene, Albite, and Quartz Minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The Effects of Ca(II) and Mg(II) Ions on the Flotation of Spodumene Using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption Mechanism of New Mixed Anionic/Cationic Collectors in a Spodumene-Feldspar Flotation System. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Fang, S.; Zhang, Z.; Chi, R. Flotation and Adsorption of a New Mixed Anionic/Cationic Collector in the Spodumene-Feldspar System. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, X.; Yu, F.; Miller, J.D. States of Coadsorption for Oleate and Dodecylamine at Selected Spodumene Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 313–321. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Wang, X.; Li, Y. Differential Collecting Performance of a New Complex of Decyloxy-Propyl-Amine and α-Bromododecanoic Acid on Flotation of Spodumene and Feldspar. Miner. Eng. 2020, 153, 106377. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Li, Y.; Han, Y. Flotation Behavior and Mechanism of a New Mixed Collector on Separation of Spodumene from Feldspar. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124932. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. A Self-Assembly Mixed Collector System and the Mechanism for the Flotation Separation of Spodumene from Feldspar and Quartz. Miner. Eng. 2021, 171, 107082. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Effects of Metal Ions on the Flotation Separation of Spodumene from Feldspar and Quartz. Miner. Eng. 2021, 168, 106931. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Flotation Behavior and Mechanism of α-Bromododecanoic Acid as Collector on the Flotation Separation of Spodumene from Feldspar and Quartz. J. Mol. Liq. 2021, 336, 116303. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Wu, H.; Fang, S.; Deng, W.; Peng, T.; Sun, W.; Hu, Y. A Novel Approach for Flotation Recovery of Spodumene, Mica and Feldspar from a Lithium Pegmatite Ore. J. Clean. Prod. 2018, 174, 625–633. [Google Scholar] [CrossRef]
- Falconer, S.A. Pretreatment of Mineral Surfaces for Froth Flotation. Min. Trans. 1949, 184, 247–255. [Google Scholar]
- Kulkarni, R.D.; Somasundaran, P. Flotation Chemistry of Hematite/Oleate System. Colloids Surf. 1980, 1, 387–405. [Google Scholar] [CrossRef]
- Munson, G.A.; Clarke, F.F. Mining and Concentrating Spodumene in the Black Hills, South Dakota. Min. Eng. 1955, 202, 1041–1045. [Google Scholar]
- Bhappu, R.B.; Fuerstenau, M.C. Recovery of Valuable Minerals From Pegmatitic Ores; American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc.: New York, NY, USA, 1964. [Google Scholar]
- Purcell, G.; Bhappu, R.B. Flotation of Some Silicates; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Banks, M.K.; McDaniel, W.T.; Sales, P.N. A Method for Concentration of North Carolina Spodumene Ores. Mining Eng. 1953, 181–186. [Google Scholar]
- Goter, E.R.; Hudspeth, W.R.; Rainey, D.L. Mining and Milling of Lithium Pegmatites at King’s Mountain, N.C. Mining Eng. 1953, September 1953, 890–893. [Google Scholar]
- Redeker, I.H. Flotation of Feldspar, Spodumene, Quartz and Mica from Pegmatites in North Carolina, USA. In Proceedings of the 13th CMP Annual Meeting, Ottawa, ON, Canada, 20 January 1981. Report No. 8. [Google Scholar]
- Karim, N. Historic Tanco Mine in Manitoba Producing Lithium Again—But This Time under China’s Sinomine. Available online: https://www.canadianminingjournal.com/news/historic-tanco-mine-in-manitoba-producing-lithium-again-but-this-time-under-chinas-sinomine/ (accessed on 29 April 2022).
- Mining Technology. North American Lithium Project, Quebec, Canada. Available online: https://www.mining-technology.com/projects/north-american-lithium-project-quebec-canada/ (accessed on 7 September 2022).
- Amarante, M.M.; de Sousa, A.B.; Leite, M.M. Processing a Spodumene Ore to Obtain Lithium Concentrates for Addition to Glass and Ceramic Bodies. Miner. Eng. 1999, 12, 433–436. [Google Scholar] [CrossRef]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T. The Removal of Iron-Bearing Silicate Minerals From a Hard Rock Lithium Ore; Canadian Institure of Mining, Metallurgy, and Petroleum: Ottawa, ON, Canada, 2017. [Google Scholar]
- Menéndez, M.; Vidal, J.; Toraño, J.; Gent, M. Optimisation of Spodumene Flotation. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 130–135. [Google Scholar]
- Cao, M.; Bu, H.; Li, S.; Meng, Q.; Gao, Y.; Ou, L. Impact of Differing Water Hardness on the Spodumene Flotation. Miner. Eng. 2021, 172, 107159. [Google Scholar] [CrossRef]
- Yu, F.-S.; Wang, Y.-H.; Wang, J.-M.; Xie, Z.-F.; Zhang, L. First-Principle Investigation on Mechanism of Ca Ion Activating Flotation of Spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Gao, J.; Sun, W.; Lyu, F. Understanding the Activation Mechanism of Ca2+ Ion in Sodium Oleate Flotation of Spodumene: A New Perspective. Chem. Eng. Sci. 2021, 244, 116742. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Cao, Y.; Luo, X.; Xie, F.; Zhang, B.; Yang, S. Activation Mechanism of Calcium Hydrolysate on the Spodumene Surface and Its Effect on the Adsorption of Collector. Miner. Eng. 2021, 174, 107221. [Google Scholar] [CrossRef]
- Quezada, G.R.; Toledo, P.G. Complexation of Alkali and Alkaline-Earth Metal Cations at Spodumene-Saltwater Interfaces by Molecular Simulation: Impact on Oleate Adsorption. Minerals 2021, 11, 12. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, W.; Deng, J.; Chen, B.; Yan, W.; Xiong, S.; Huang, Y.; Liu, J. Flotation Behaviors of Ilmenite, Titanaugite, and Forsterite Using Sodium Oleate as the Collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Wang, W.; Xiong, S.; Wang, D.; Yan, W.; Deng, J. Flotation Characteristics of Two Different Types of Ilmenite with Sodium Oleate. Mineral. Petrol. 2015, 109, 299–307. [Google Scholar] [CrossRef]

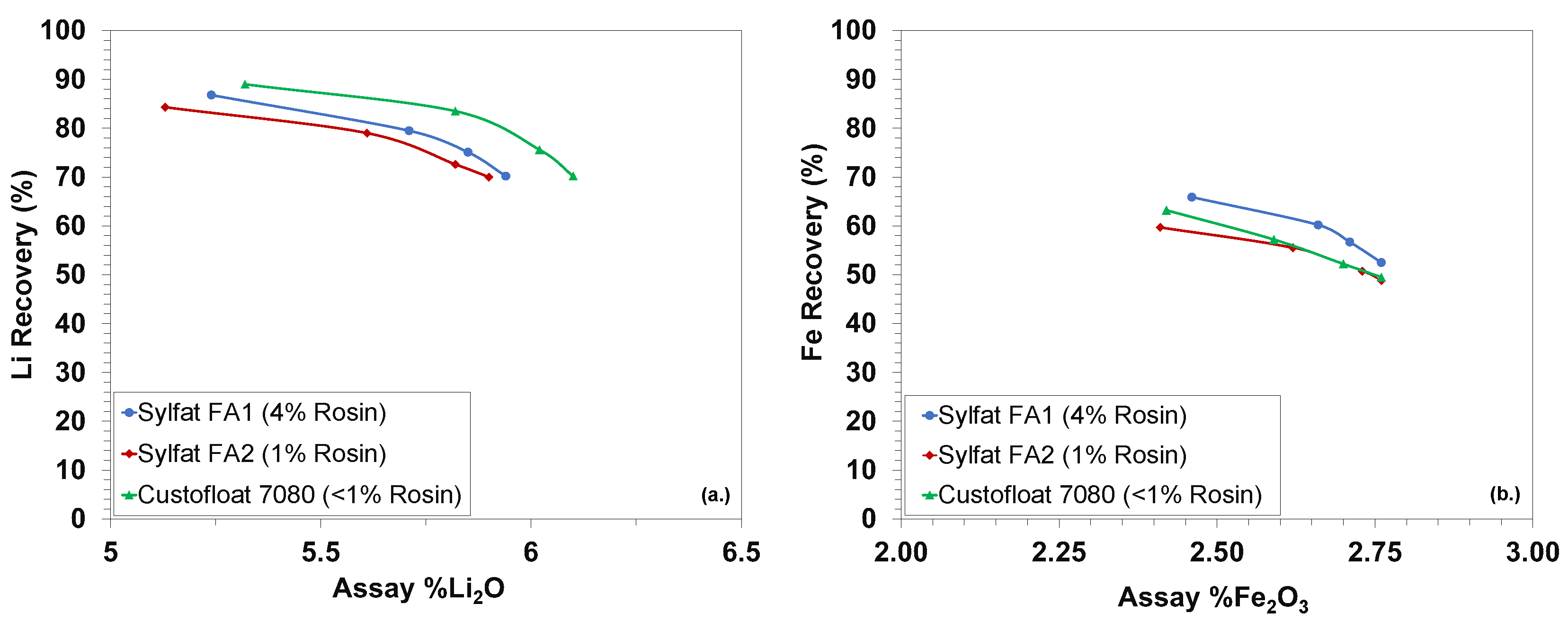
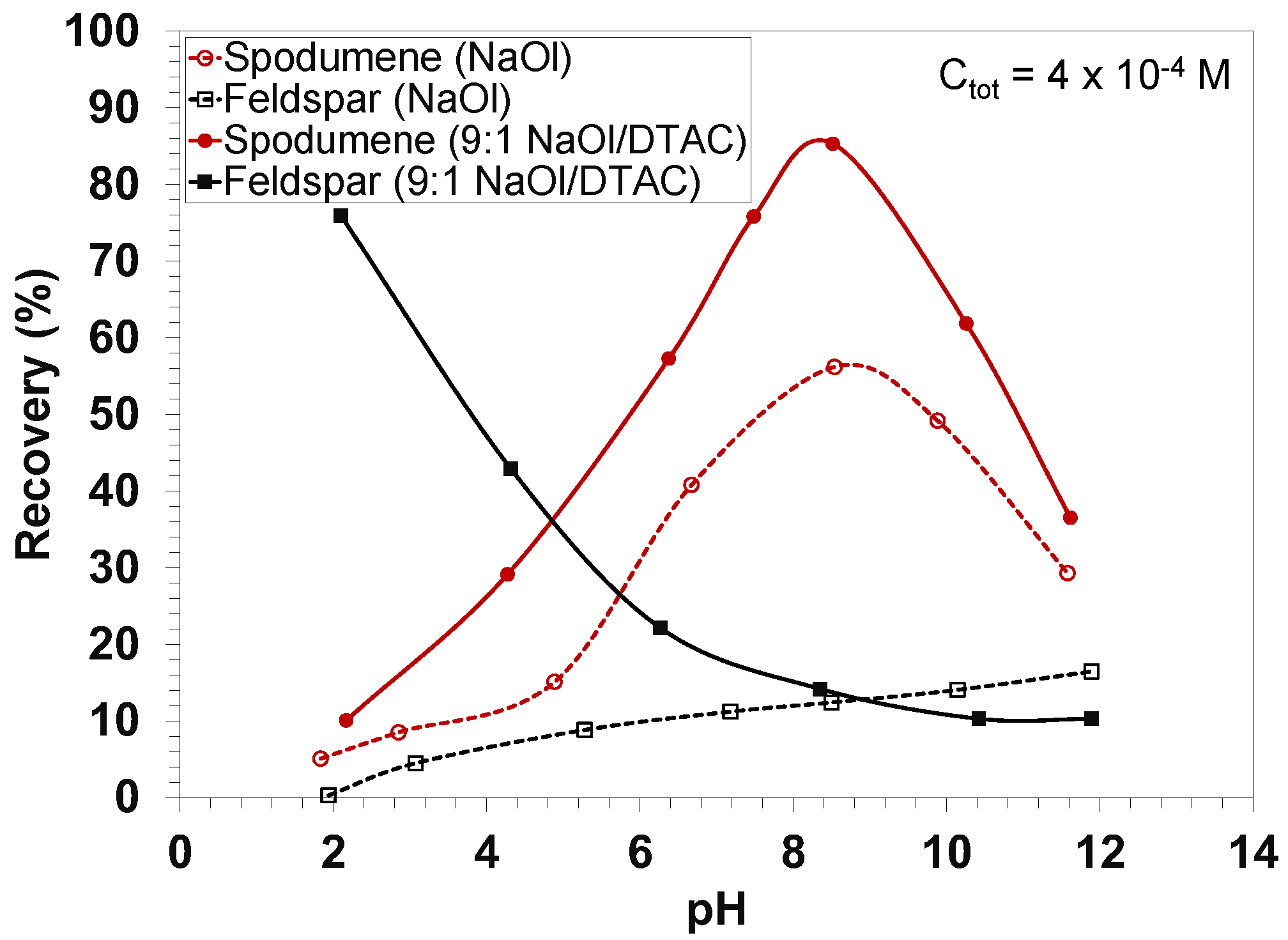
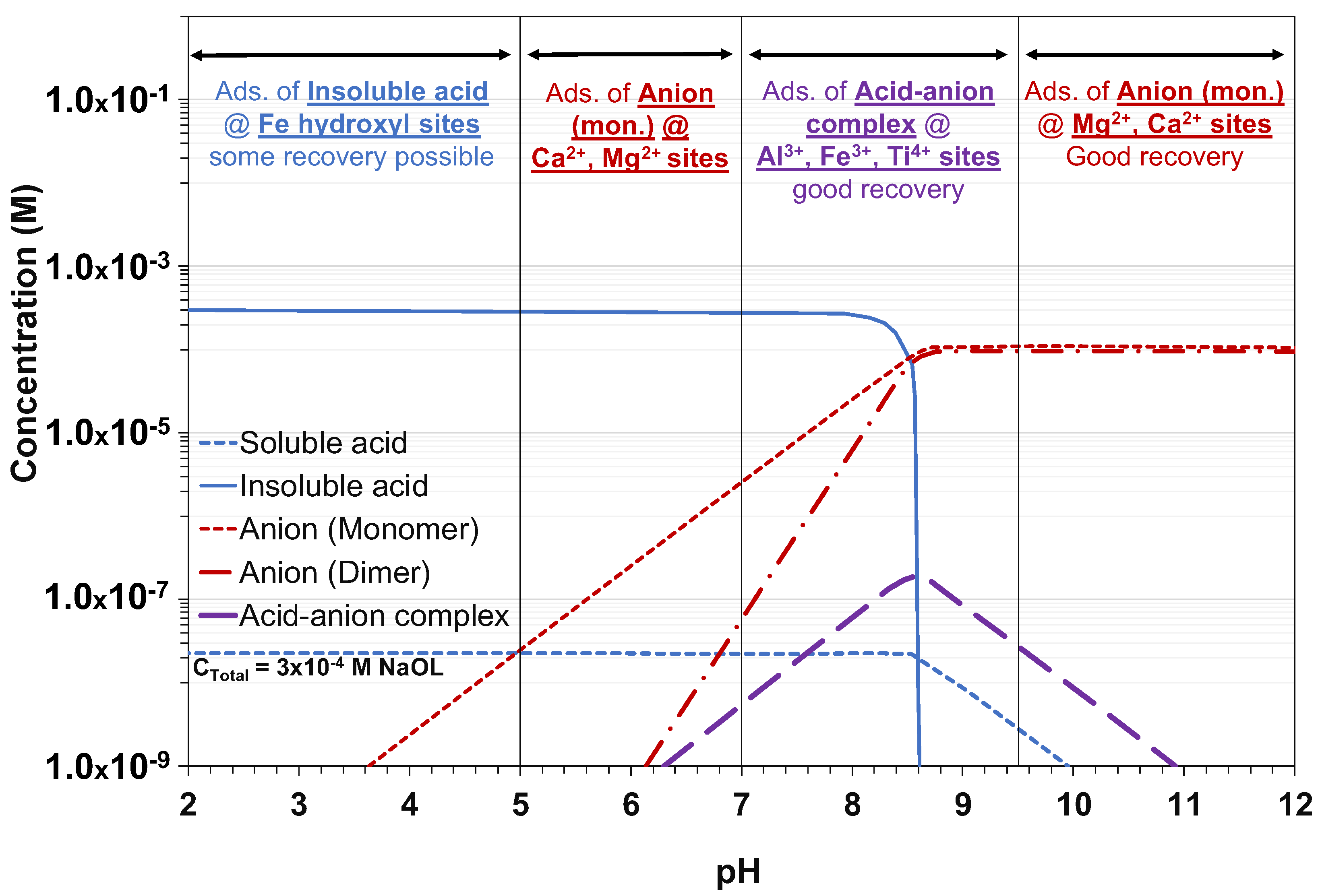
| Owner—Operation/Project | Country | Operating Status | Avg. Grade (% Li2O) | Reference |
|---|---|---|---|---|
| Alliance Minerals—Bald Hill | Australia | Operating | 1.00 | [38] |
| Covalent Lithium—Mt. Holland | Australia | In Development | 1.50 | [38] |
| Talison/Albemarle—Greenbushes | Australia | Operating | 2.80 | [38] |
| Galaxy Resources—Mt. Cattlin | Australia | Operating | 1.08 | [38] |
| Mineral Resources—Mt. Marion | Australia | Operating | 1.37 | [38] |
| Pilbara Minerals—Pilgangoora Project | Australia | Operating | 1.19 | [39] |
| Albemarle/Mineral Resources—Wodgina | Australia | Operating | 1.02 | [38] |
| Sigma Lithium (Several Projects) | Brazil | In Development | 1.55 | [40] |
| Frontier Lithium—PAK | Canada | In Development | 2.06 | [41] |
| Frontier Lithium—Spark | Canada | In Development | 1.37 | [41] |
| Critical Elements—Rose Li-Ta Project | Canada | In Development | 1.15 | [42] |
| Nemaska—Whabouchi | Canada | In Development | 1.55 | [43] |
| Foremost Lithium—Snow Lake | Canada | In Development | 0.91 | [44] |
| Green Technology Metals—Seymour Project | Canada | Exploration/Dev. | 1.04 | [45] |
| Patriot Battery Metals—Corvette Property | Canada | In Development | 1.30 | [7] |
| Sayona/Piedmont—North American Lithium | Canada | In Development | 1.06 | [46] |
| Sayona Mining Ltd.—Moblan Project | Canada | In Development | 1.40 | [46] |
| Sinomine—TANCO | Canada | Operating | 2.44 | [47] |
| Jiajika Mine—Rongda Lithium | China | Operating | 1.33 | [48,49] |
| Atlantic Lithium/Piedmont—Ewoyaa | Ghana | In Development | 1.26 | [50] |
| Albemarle—Kings Mountain | USA | Recommissioning | 1.50 | [51,52] |
| Piedmont Lithium—Carolinas Project | USA | In Development | 1.11 | [53] |
| Acid Type | Chemical Name | Chemical Formula |
|---|---|---|
| Saturated | Lauric Acid | CH3(CH2)10COOH |
| Palmetic Acid | CH3(CH2)14COOH | |
| Stearic Acid | CH3(CH2)16COOH | |
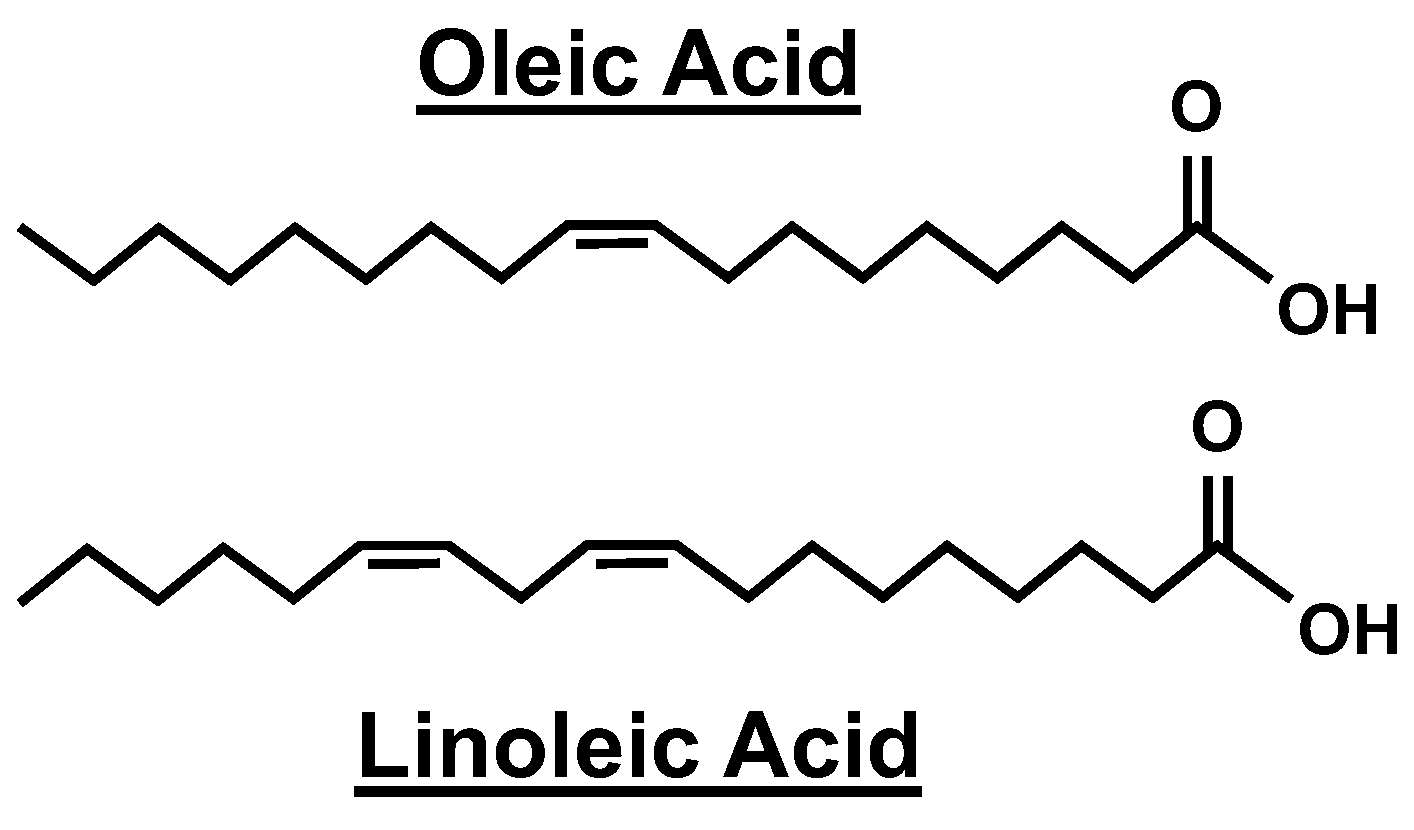
| Unsaturated | Oleic Acid | CH3(CH2)7CH ═ CH(CH2)7COOH |
| Linoleic Acid | CH3(CH2)4CH ═ CHCH2CH ═ CH(CH2)7COOH | |
| Linolenic Acid | CH3(CH2)2CH ═ CHCH2CH ═ CHCH2CH ═ CH(CH2)7COOH | |
| Rosin | Abietic Acid | C20H30O2 |
| Palustric Acid * | C20H30O2 | |
| Neoabietic Acid * | C20H30O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, B.K.; Gibson, C.E. A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals 2023, 13, 212. https://doi.org/10.3390/min13020212
Cook BK, Gibson CE. A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals. 2023; 13(2):212. https://doi.org/10.3390/min13020212
Chicago/Turabian StyleCook, Brian Kawenski, and Charlotte E. Gibson. 2023. "A Review of Fatty Acid Collectors: Implications for Spodumene Flotation" Minerals 13, no. 2: 212. https://doi.org/10.3390/min13020212
APA StyleCook, B. K., & Gibson, C. E. (2023). A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals, 13(2), 212. https://doi.org/10.3390/min13020212






